Marine Algae-Derived Porous Carbons as Robust Electrocatalysts for ORR
Abstract
:1. Introduction
2. Results and Discussion
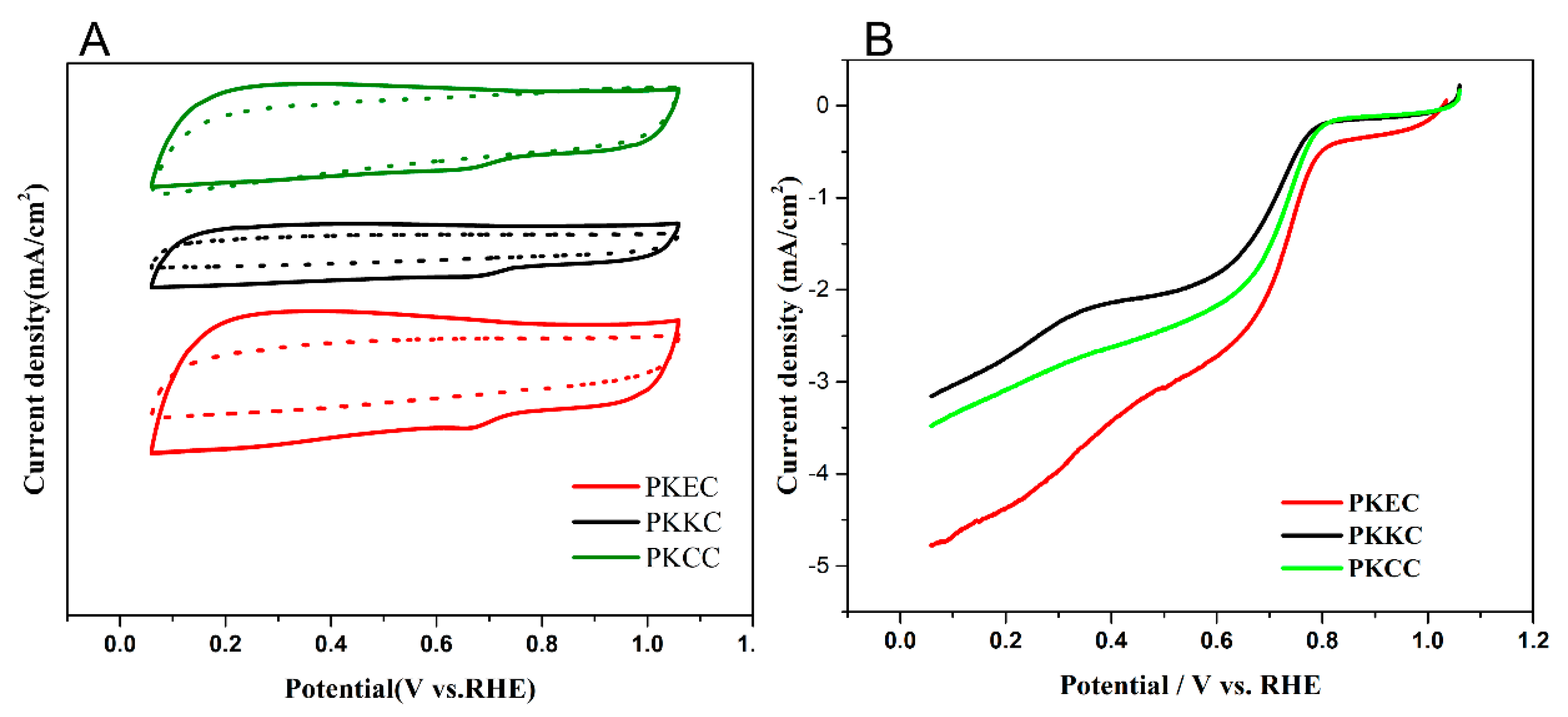
2.1. ORR Performance of PKEC, PKCC and PKKC
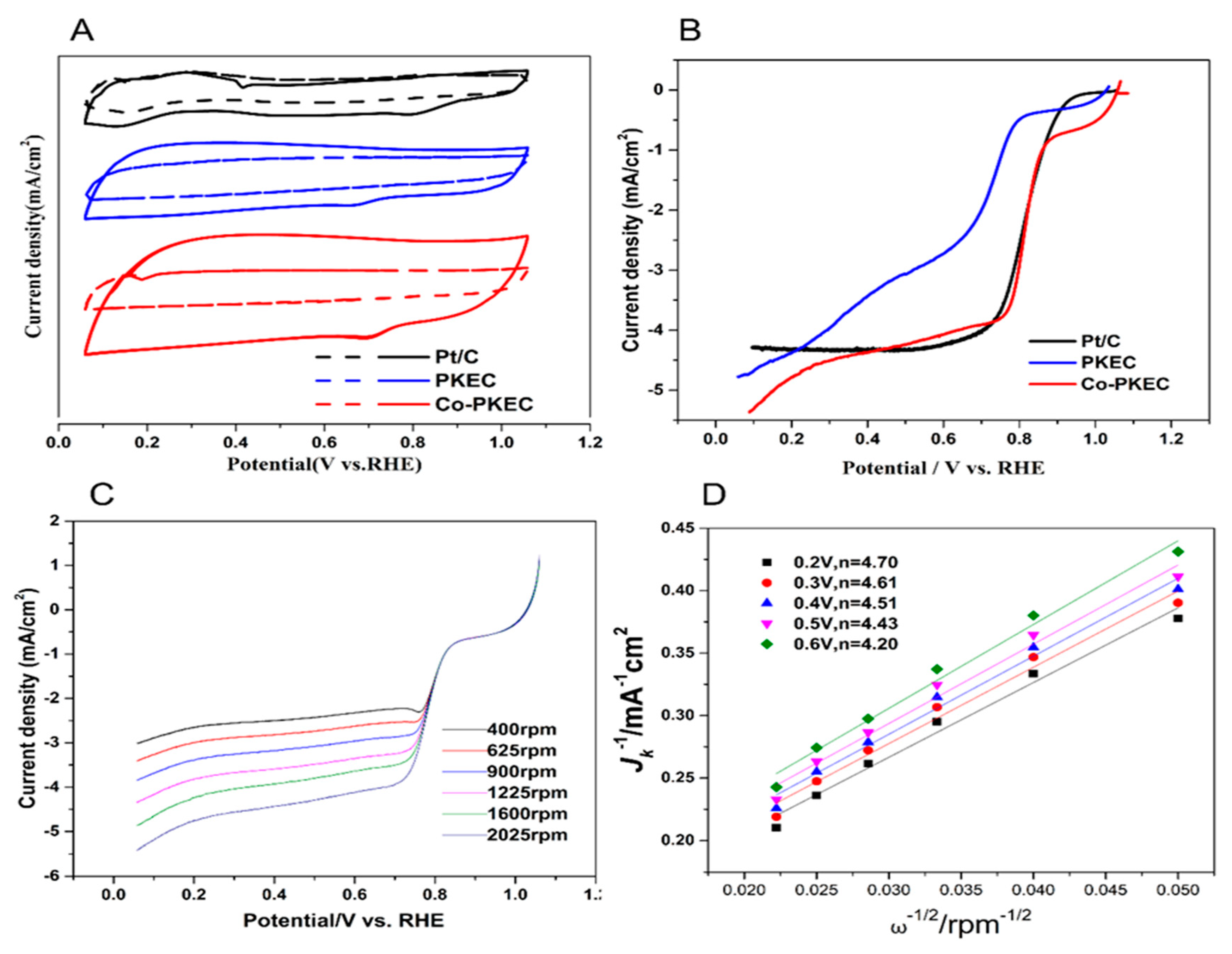
2.2. ORR Performance of Co-PKEC
2.2.1. CV and LSV
2.2.2. Stability Test and Methanol Poisoning Resistance Test
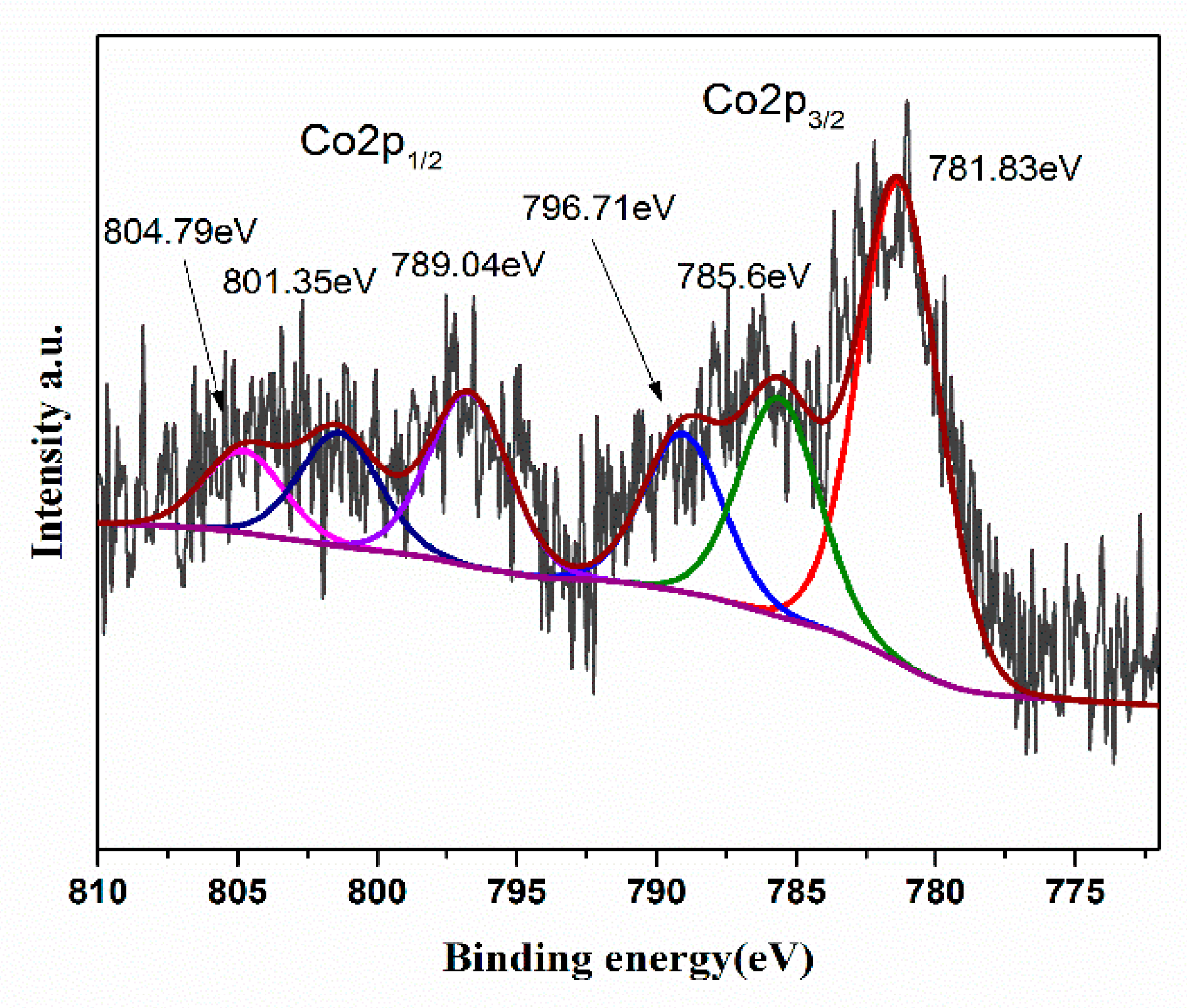
2.3. Material Characterization
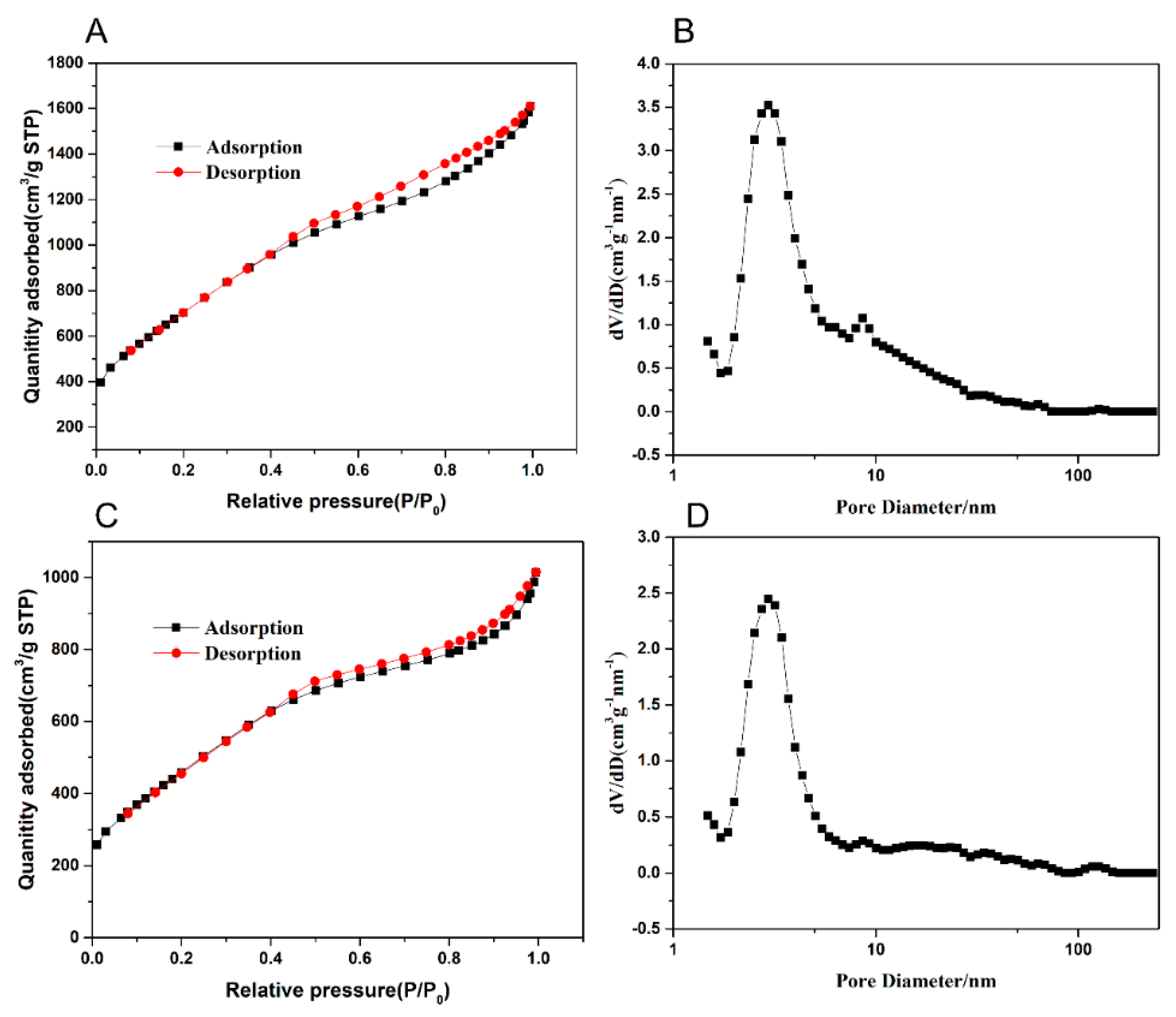
2.3.1. SEM Characterization and Pore Structure Analysis
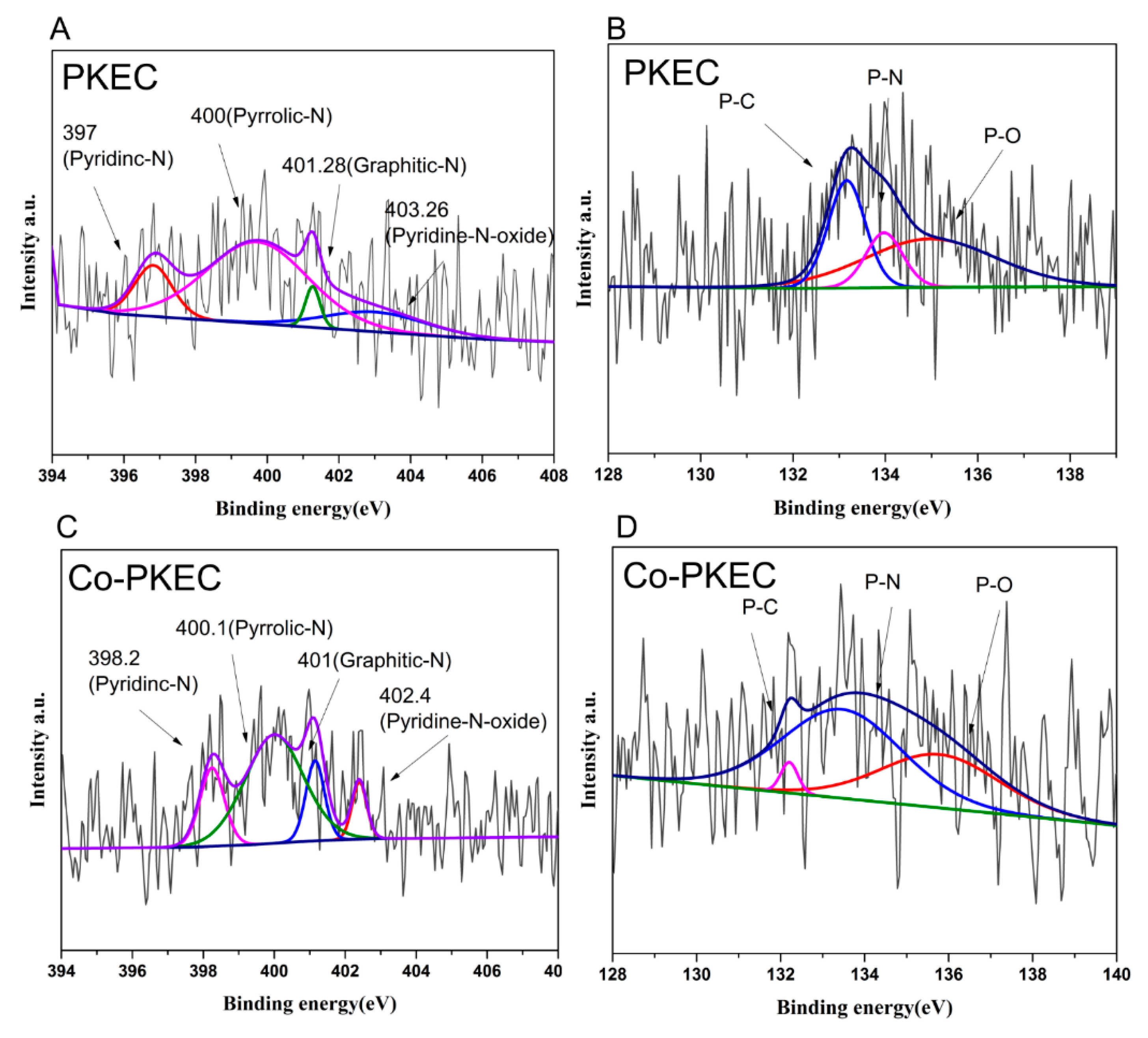
2.3.2. Structural Characterization of Materials
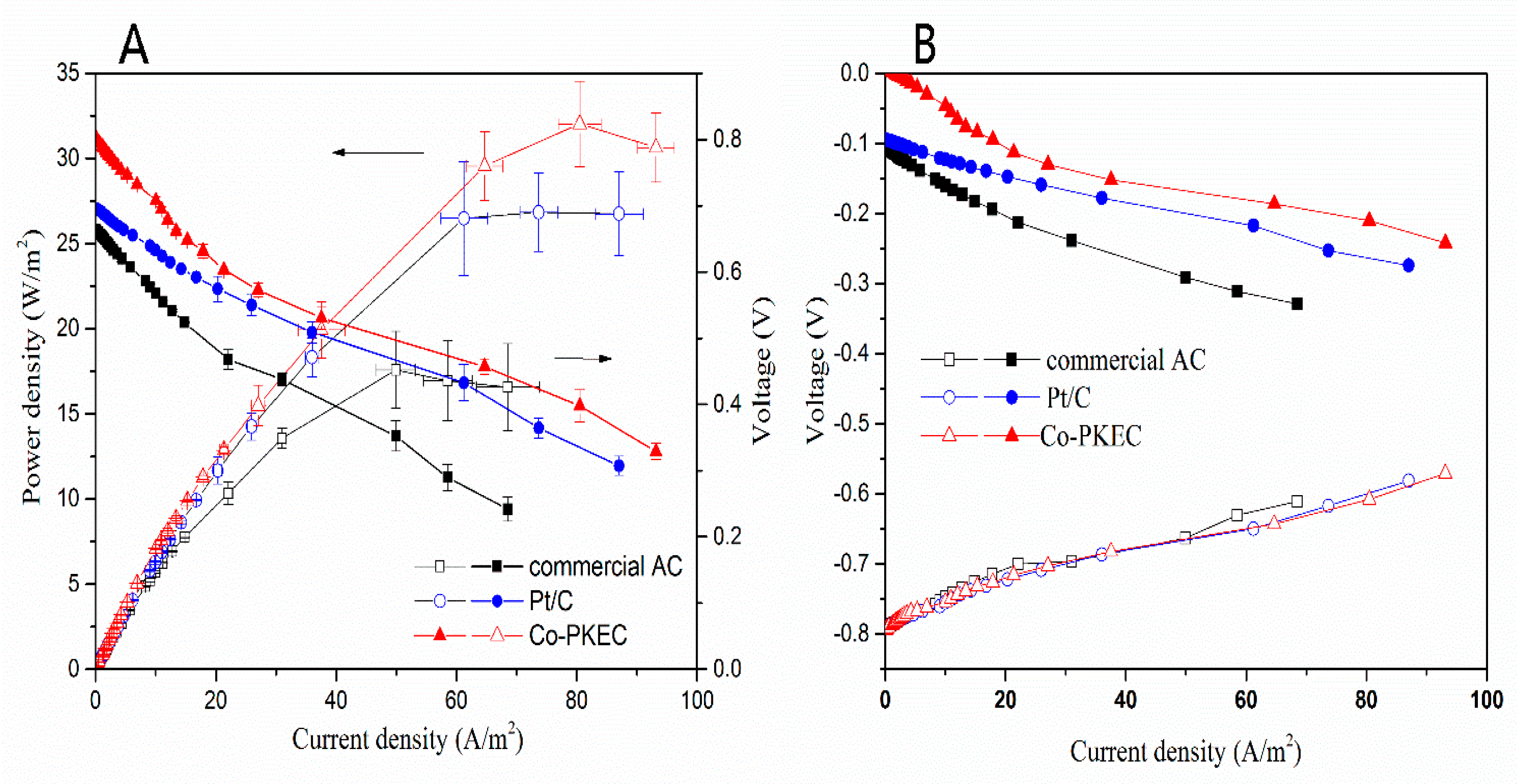
2.4. Performance of Co-PKEC in Fuel Cell
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Electrochemical and Physical Measurements
3.4. Fuel Cell Assembly and Performance Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Liu, X.; Wang, Y.; Zhang, P. Electricity generation from macroalgae Enteromorpha prolifera hydrolysates using an alkaline fuel cell. Bioresour. Technol. 2016, 222, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Savage, P.E.; Deng, D. Trash to Treasure: From Harmful Algal Blooms to High-Performance Electrodes for Sodium-Ion Batteries. Environ. Sci. Technol. 2015, 49, 12543–12550. [Google Scholar] [CrossRef] [PubMed]
- Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, M.; Feng, M.; Zhang, L.; Zhao, Y.; Du, X.; Wang, G. A One-compartment direct glucose alkaline fuel cell with methyl viologen as electron mediator. Appl. Energy 2013, 106, 176–183. [Google Scholar] [CrossRef]
- Hao, M.; Liu, X.; Feng, M.; Zhang, P.; Wang, G. Generating power from cellulose in an alkaline fuel cell enhanced by methyl viologen as an electron-transfer catalyst. J. Power Sources 2014, 251, 222–228. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A. Advances in stationary and portable fuel cell applications. Int. J. Hydrog. Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef] [Green Version]
- You, P.Y.; Kamarudin, S.K. Recent progress of carbonaceous materials in fuel cell applications: An overview. Chem. Eng. J. 2017, 309, 489–502. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Higgins, D.C.; Chen, Z. Recent progress in non-precious metal catalysts for PEM fuel cell applications. Can. J. Chem. Eng. 2013, 91, 1881–1895. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Tang, C.; He, G.; Qiu, K.; Binions, R.; Parkin, I.P.; Zhang, Q.; Guo, Z.X.; Titirici, M. Graphene/nitrogen-doped porous carbon sandwiches for the metal-free oxygen reduction reaction: Conductivity versus active sites. J. Mater. Chem. A 2016, 4, 12658–12666. [Google Scholar] [CrossRef]
- Deng, H.; Li, Q.; Liu, J.; Wang, F. Active sites for oxygen reduction reaction on nitrogen-doped carbon nanotubes derived from polyaniline. Carbon 2017, 112, 219–229. [Google Scholar] [CrossRef]
- Zeng, L.; Cui, X.; Chen, L.; Ye, T.; Huang, W.; Ma, R.; Zhang, X.; Shi, J. Non-noble bimetallic alloy encased in nitrogen-doped nanotubes as a highly active and durable electrocatalyst for oxygen reduction reaction. Carbon 2017, 114, 347–355. [Google Scholar] [CrossRef]
- Qiao, M.; Meysami, S.S.; Ferrero, G.A.; Xie, F.; Meng, H.; Grobert, N.; Titirici, M.-M. Low-Cost Chitosan-Derived N-Doped Carbons Boost Electrocatalytic Activity of Multiwall Carbon Nanotubes. Adv. Funct. Mater. 2018, 28, 1707284. [Google Scholar] [CrossRef]
- Gao, T.; Jin, Z.; Zhang, Y.; Tan, G.; Yuan, H.; Xiao, D. Coupling cobalt-iron bimetallic nitrides and N-doped multi-walled carbon nanotubes as high-performance bifunctional catalysts for oxygen evolution and reduction reaction. Electrochim. Acta 2017, 258, 51–60. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Ng, E.P.; Chang, J.-S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Dou, M.; Ji, J.; Wang, F. Fe–Nx moiety-modified hierarchically porous carbons derived from porphyra for highly effective oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 1526–1532. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, X.; Yang, Q.; Chen, K.; Guo, J.; Zhou, D.; Feng, L.; Slanina, Z. Highly porous defective carbons derived from seaweed biomass as efficient electrocatalysts for oxygen reduction in both alkaline and acidic media. Carbon 2018, 137, 93–103. [Google Scholar] [CrossRef]
- Chatterjee, K.; AshokKumar, M.; Gullapalli, H.; Gong, Y.; Vajtai, R.; Thanikaivelan, P.; Ajayan, P.M. Nitrogen-rich carbon nano-onions for oxygen reduction reaction. Carbon 2018, 130, 645–651. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Borghei, M.; Laocharoen, N.; Kibena-Põldsepp, E.; Johansson, L.-S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N, P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Li, L.; Wei, X. A versatile biomass derived carbon material for oxygen reduction reaction, supercapacitors and oil/water separation. Nano Energy 2017, 33, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Peng, H.; Qiao, X.; Fu, Z.; Huang, P.; Liao, S. High-performance doped carbon electrocatalyst derived from soybean biomass and promoted by zinc chloride. Int. J. Hydrog. Energy 2014, 39, 10128–10134. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, D.; Guo, W.; He, X.; Ma, X. Nitrogen-and sulfur-doped carbon nanoplatelets via thermal annealing of alkaline lignin with urea as efficient electrocatalysts for oxygen reduction reaction. RSC Adv. 2016, 6, 104183–104192. [Google Scholar] [CrossRef]
- Zhao, L. Natural phosphorus-doped honeycomb carbon materials as oxygen reduction catalysts derived from Pulsatilla chinensis (Bunge) Regel. RSC Adv. 2017, 7, 13904–13910. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Gao, X.; Dou, M.; Ji, J.; Wang, F. Biomass Derived N-Doped Porous Carbon Supported Single Fe Atoms as Superior Electrocatalysts for Oxygen Reduction. Small 2017, 13, 1604290. [Google Scholar] [CrossRef]
- Wan, W.; Wang, Q.; Zhang, L.; Liang, H.-W.; Chen, P.; Yu, S.-H. N-, P- and Fe-tridoped nanoporous carbon derived from plant biomass: An excellent oxygen reduction electrocatalyst for zinc-air batteries. J. Mater. Chem. A 2016, 4, 8602–8609. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Zhou, T.; Key, J.; Ma, Y.; Zhang, Z.; Wang, Q.; Ji, S. The enhanced electrocatalytic activity of okara-derived N-doped mesoporous carbon for oxygen reduction reaction. J. Power Sources 2015, 274, 741–747. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Chen, X.; Cheng, K.; Pan, M.; Mu, S. An animal liver derived non-precious metal catalyst for oxygen reduction with high activity and stability. RSC Adv. 2014, 4, 32811–32816. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, C.; Chen, C.; Fan, M.; Gong, J.; Zhang, Y.; Zhao, T.; Sun, Y.; Xu, X.; Li, M.; et al. High content of pyridinic-and pyrrolic-nitrogen-modified carbon nanotubes derived from blood biomass for the electrocatalysis of oxygen reduction reaction in alkaline medium. Electrochim. Acta 2015, 168, 386–393. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, H.; Yu, H.; Peng, F. From chicken feather to nitrogen and sulfur co-doped large surface bio-carbon flocs: An efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2016, 213, 273–282. [Google Scholar] [CrossRef]
- Kundu, S.; Nagaiah, T.C.; Xia, W.; Wang, Y.; Van Dommele, S.; Bitter, J.H.; Santa, M.; Grundmeier, G.; Bron, M.; Schuhmann, W.; et al. Electrocatalytic Activity and Stability of Nitrogen-Containing Carbon Nanotubes in the Oxygen Reduction Reaction. J. Phys. Chem. C 2009, 113, 14302–14310. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, L.; Li, K.; Chen, Z.; Deng, S. The high-performance and mechanism of P-doped activated carbon as a catalyst for air-cathode microbial fuel cells. J. Mater. Chem. A 2015, 3, 21149–21158. [Google Scholar] [CrossRef]
- Lin, G.; Ma, R.; Zhou, Y.; Liu, Q.; Dong, X.; Wang, J. KOH activation of biomass-derived nitrogen-doped carbons for supercapacitor and electrocatalytic oxygen reduction. Electrochim. Acta 2018, 261, 49–57. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhöfer, G.; Pohl, M.-M.; Radnik, J.; Surkus, A.-E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Wang, W.; Huang, Y.; Liu, X.; Miao, S.; Liu, J.; Zhang, T. Co–N–C Catalyst for C–C Coupling Reactions: On the Catalytic Performance and Active Sites. ACS Catal. 2015, 5, 6563–6572. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.-K.; Wang, G.; Gao, M.-R.; Ge, J.; Yuan, W.-J.; Shen, Y.-H.; Xie, A.-J.; Yu, S.-H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 4095–4103. [Google Scholar] [CrossRef]
- Zhang, J. Pem Fuel Cell Electrocatalysts and Catalyst Layers Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2008; pp. 655–714. [Google Scholar]
- Yang, Y.-L.; Liu, X.-H.; Hao, M.-Q.; Zhang, P.-P. Performance of a low-cost direct glucose fuel cell with an anion-exchange membrane. Int. J. Hydrog. Energy 2015, 40, 10979–10984. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.; Dong, F.; Lin, Q.; Tong, Y.; Li, Y.; Zhang, P. Electricity generation from banana peels in an alkaline fuel cell with a Cu2O-Cu modified activated carbon cathode. Sci. Total Environ. 2018, 631, 849–856. [Google Scholar] [CrossRef] [PubMed]





| Biomass | Doped Elements | Activator | Specific Surface Area () | Pore Volume | EOnset (V vs. RHE) | E1/2 (V vs. RHE) | Jd | Electron Transfer Number (n) | Article |
|---|---|---|---|---|---|---|---|---|---|
| Enteromorpha | Co,N,P | H3PO4 KOH | 1783.83 | 1.57 | 1.05 | 0.81 | 4.4 | 4.5 | This study |
| Coconut shell | N,P | H3PO4 | 1071 | 0.9 | 0.95 | 0.76 | 5.3 | 3.7 | [23] |
| Eggplant | N | NH3 | 1969 | - | 0.935 | 0.636 | 5.5 | 3.87 | [24] |
| Soybean | N,S | ZnCl2 | 949 | 0.42 | 0.95 | 0.84 | 5 | 4 | [25] |
| Pomelo peel | Co,N | KOH | 2091 | 1.28 | 0.88 | 0.97 | 5.2 | 3.9 | [26] |
| Pulsatilla chinensis | P | - | - | - | 0.94 | 0.773 | 4.36 | 3.6-4.6 | [27] |
| Laver | Fe | KOH | 1070 | 0.95 | - | 0.87 | 5 | 4 | [28] |
| Corn silk | Fe,N | NH3 | 592 | 0.54 | 0.957 | 0.927 | 5 | 3.87-4 | [29] |
| Bean dregs | Fe | - | 517 | 0.43 | 0.98 | 0.87 | 4 | 3.5 | [30] |
| Pork liver | Fe,N | - | 700 | - | 0.985 | 0.82 | 5 | 3.7 | [31] |
| Porcine blood | N | - | - | - | 0.915 | 0.81 | 3.4 | 3.7 | [32] |
| Chicken feather | N,S,Zn | NH3 | 829 | - | 1.0 | 0.94 | 3.4 | 3.98 | [33] |
| Sample | Carbon (At. %) | Oxygen (At. %) | N Total (At. %) | P Total (At. %) | Co (At. %) |
|---|---|---|---|---|---|
| PKEC | 88.34 | 9.72 | 1.6 | 0.34 | — |
| Co-PKEC | 88.16 | 9.51 | 1.45 | 0.38 | 0.5 |
| At. % | Pyridinc-N | Pyrrolic-N | Graphitic-N | Pyridinc-N-oxide |
|---|---|---|---|---|
| PKEC | 10.89 | 63.15 | 6.23 | 19.74 |
| Co-PKEC | 16.54 | 43.44 | 10.35 | 29.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, X.; Wang, J.; Yang, L.; Chen, X.; Wang, X.; Zhang, P. Marine Algae-Derived Porous Carbons as Robust Electrocatalysts for ORR. Catalysts 2019, 9, 730. https://doi.org/10.3390/catal9090730
Li Y, Liu X, Wang J, Yang L, Chen X, Wang X, Zhang P. Marine Algae-Derived Porous Carbons as Robust Electrocatalysts for ORR. Catalysts. 2019; 9(9):730. https://doi.org/10.3390/catal9090730
Chicago/Turabian StyleLi, Yang, Xianhua Liu, Jiao Wang, Li Yang, Xiaochen Chen, Xin Wang, and Pingping Zhang. 2019. "Marine Algae-Derived Porous Carbons as Robust Electrocatalysts for ORR" Catalysts 9, no. 9: 730. https://doi.org/10.3390/catal9090730
APA StyleLi, Y., Liu, X., Wang, J., Yang, L., Chen, X., Wang, X., & Zhang, P. (2019). Marine Algae-Derived Porous Carbons as Robust Electrocatalysts for ORR. Catalysts, 9(9), 730. https://doi.org/10.3390/catal9090730








