The Impact of CeO2 Loading on the Activity and Stability of PdO/γ-AlOOH/γ-Al2O3 Monolith Catalysts for CH4 Oxidation
Abstract
1. Introduction
2. Results
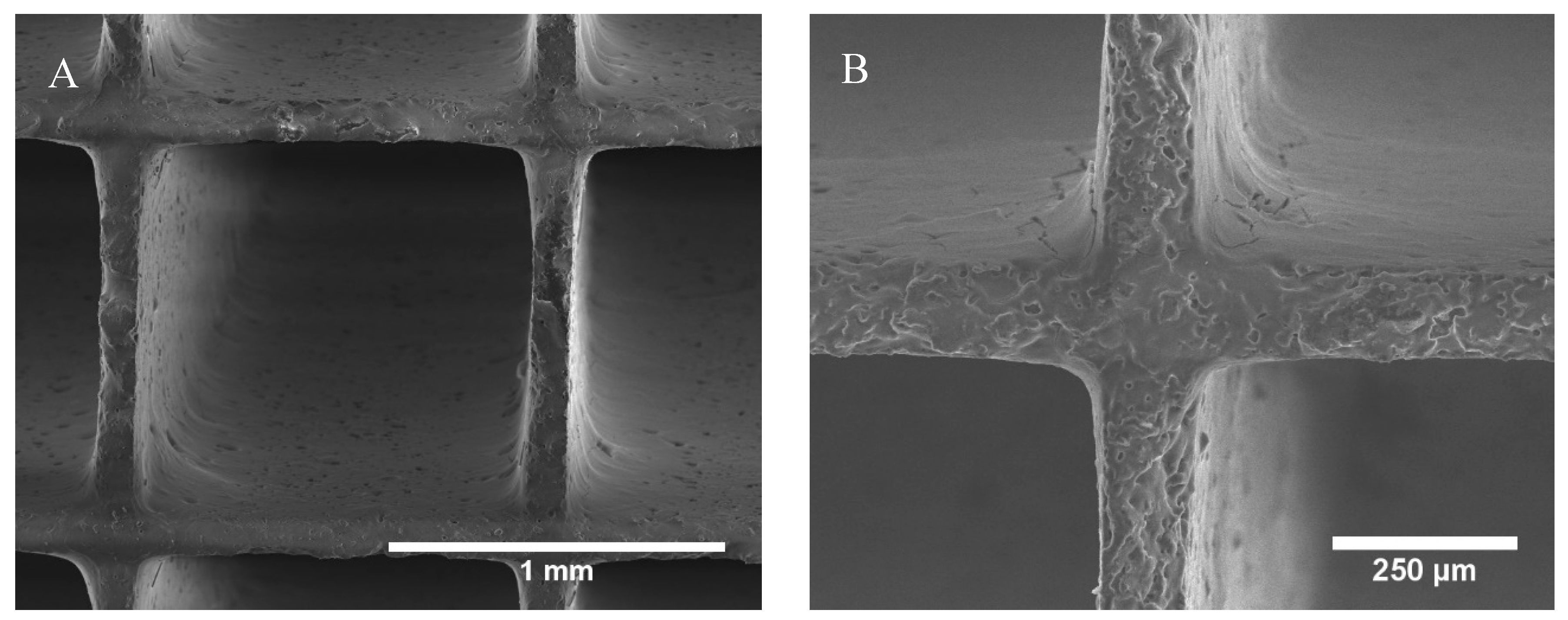
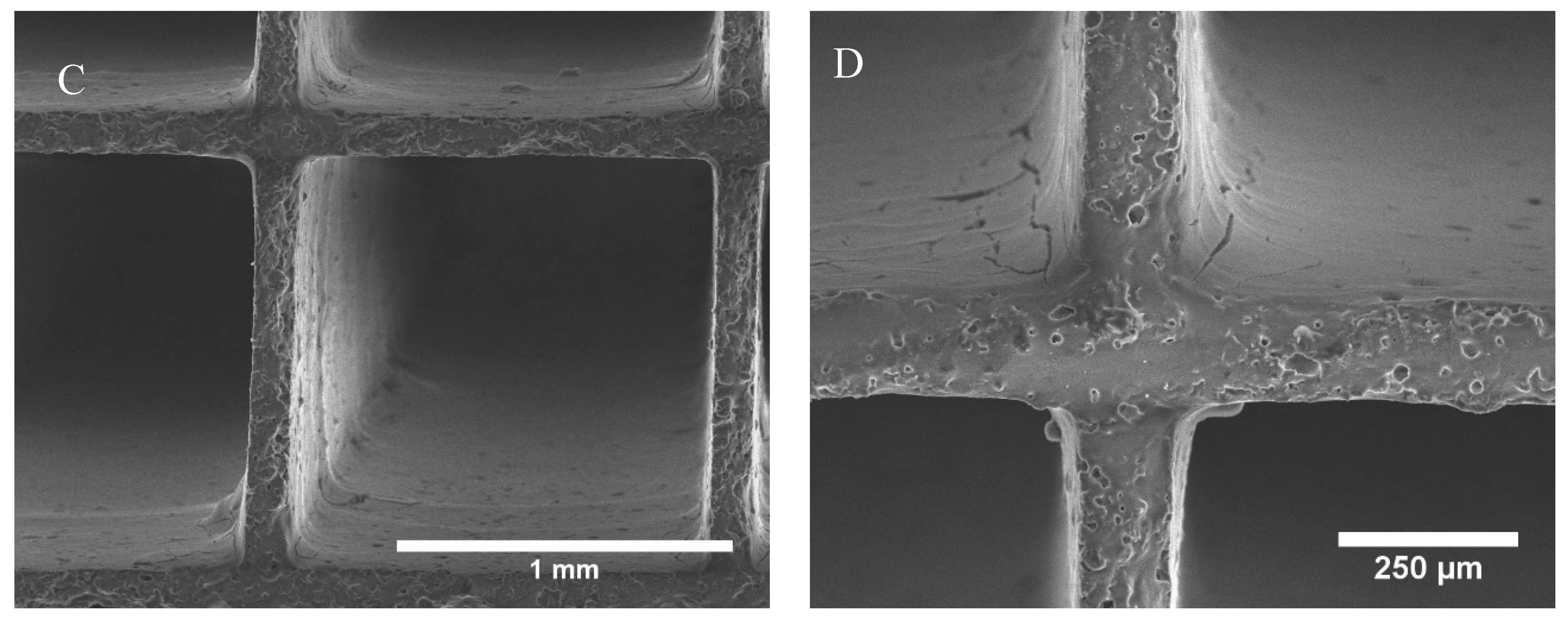
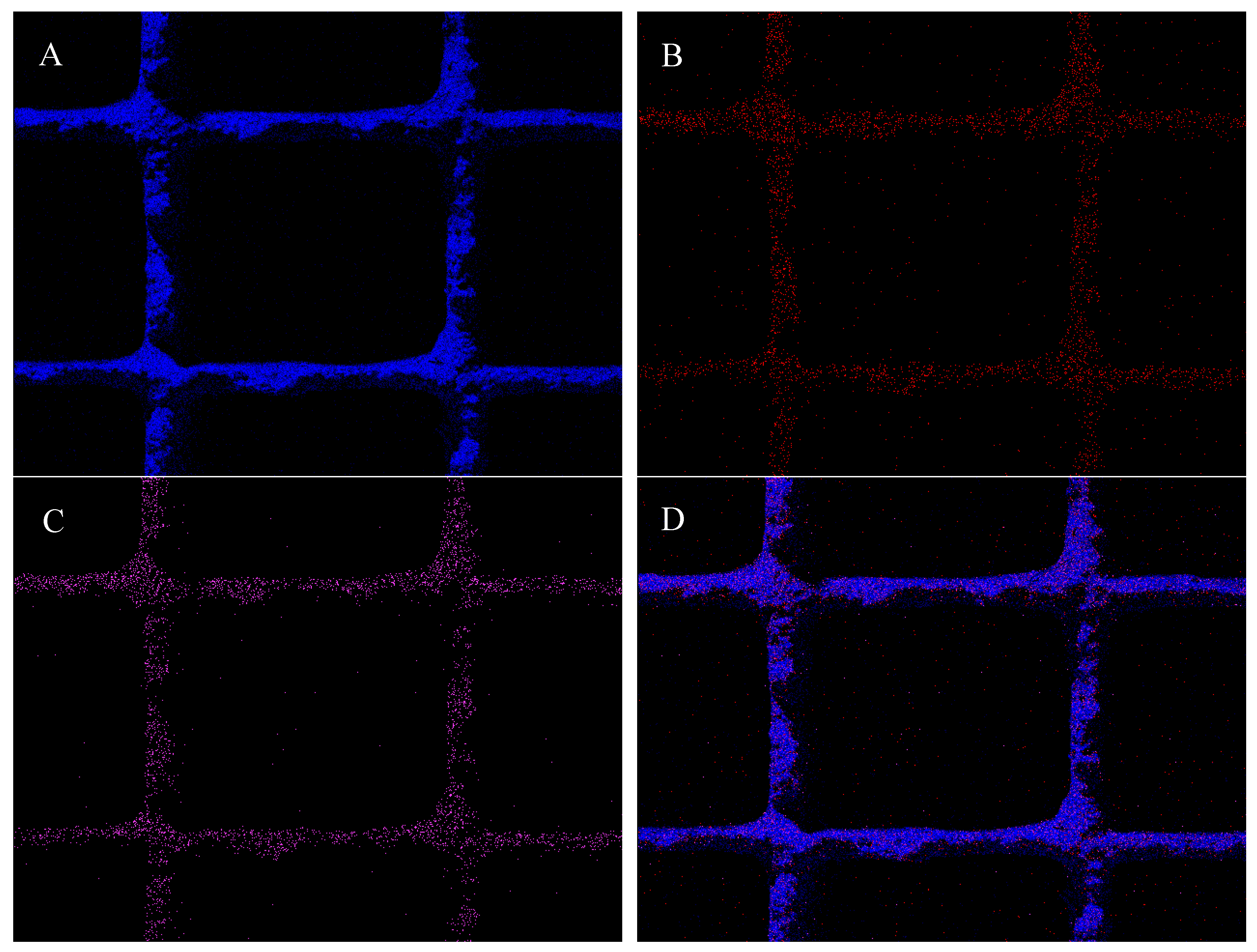
2.1. Monolith Characterization
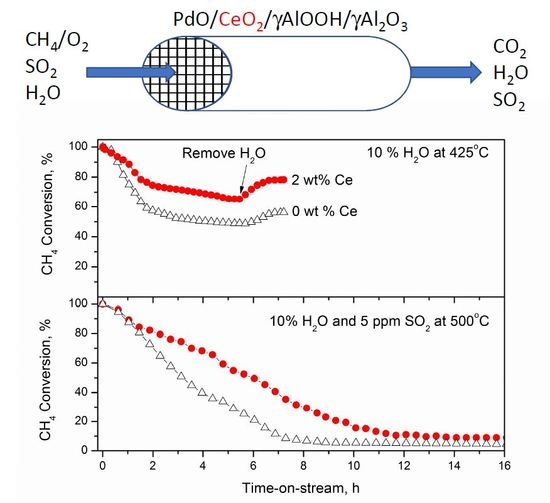
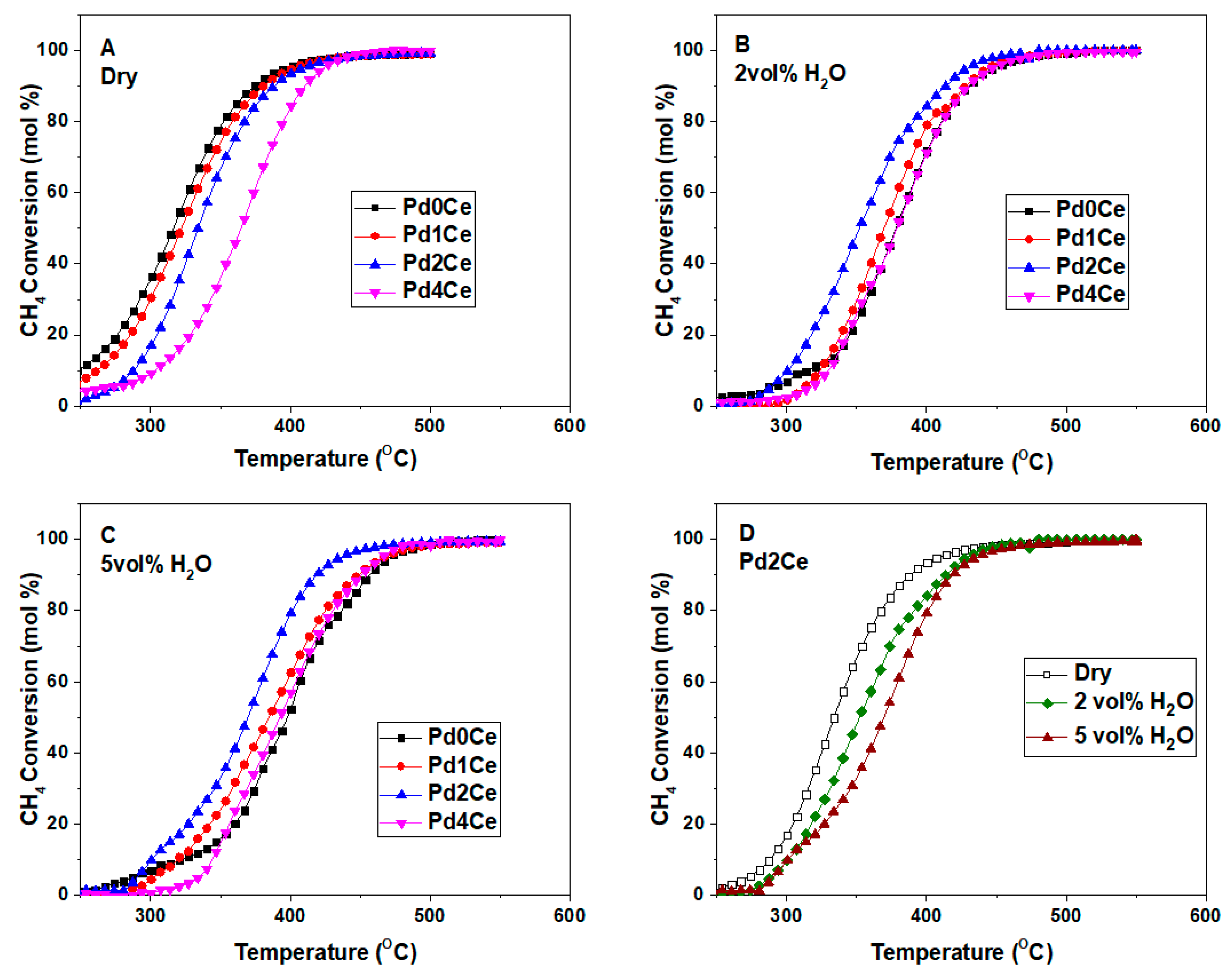
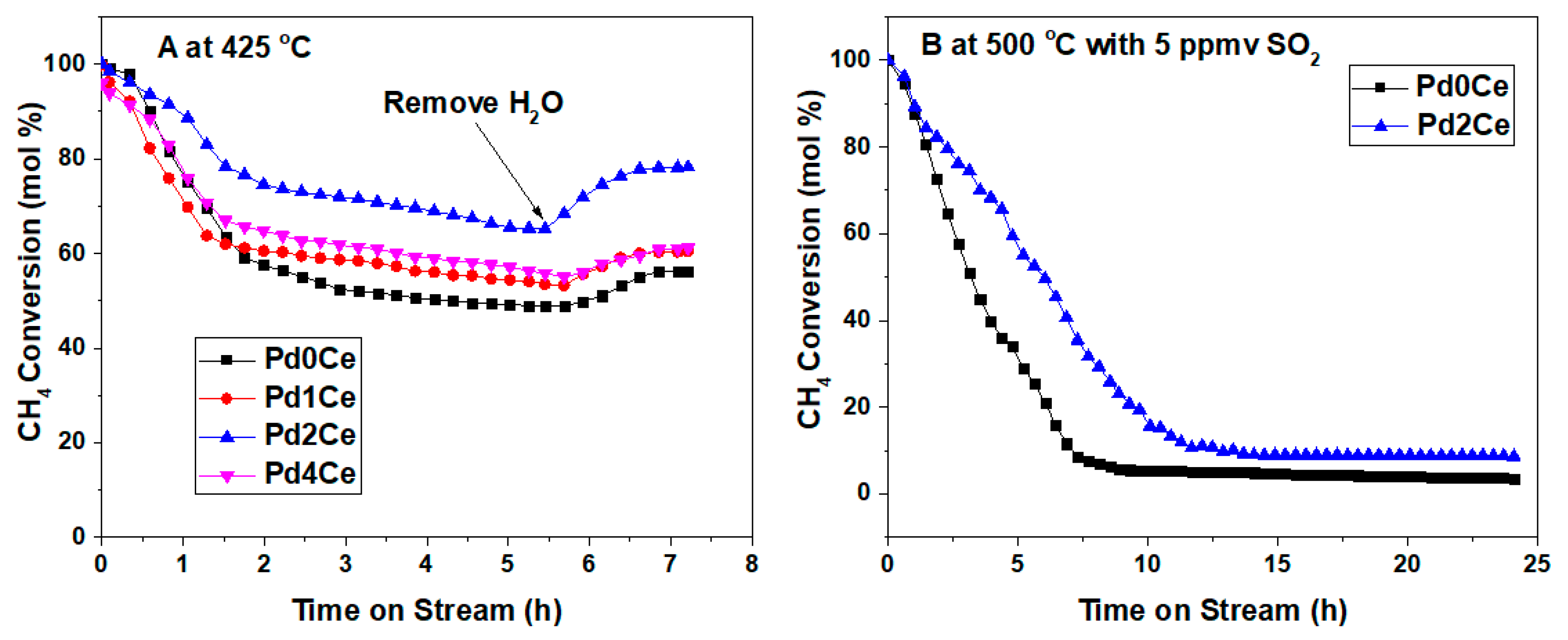
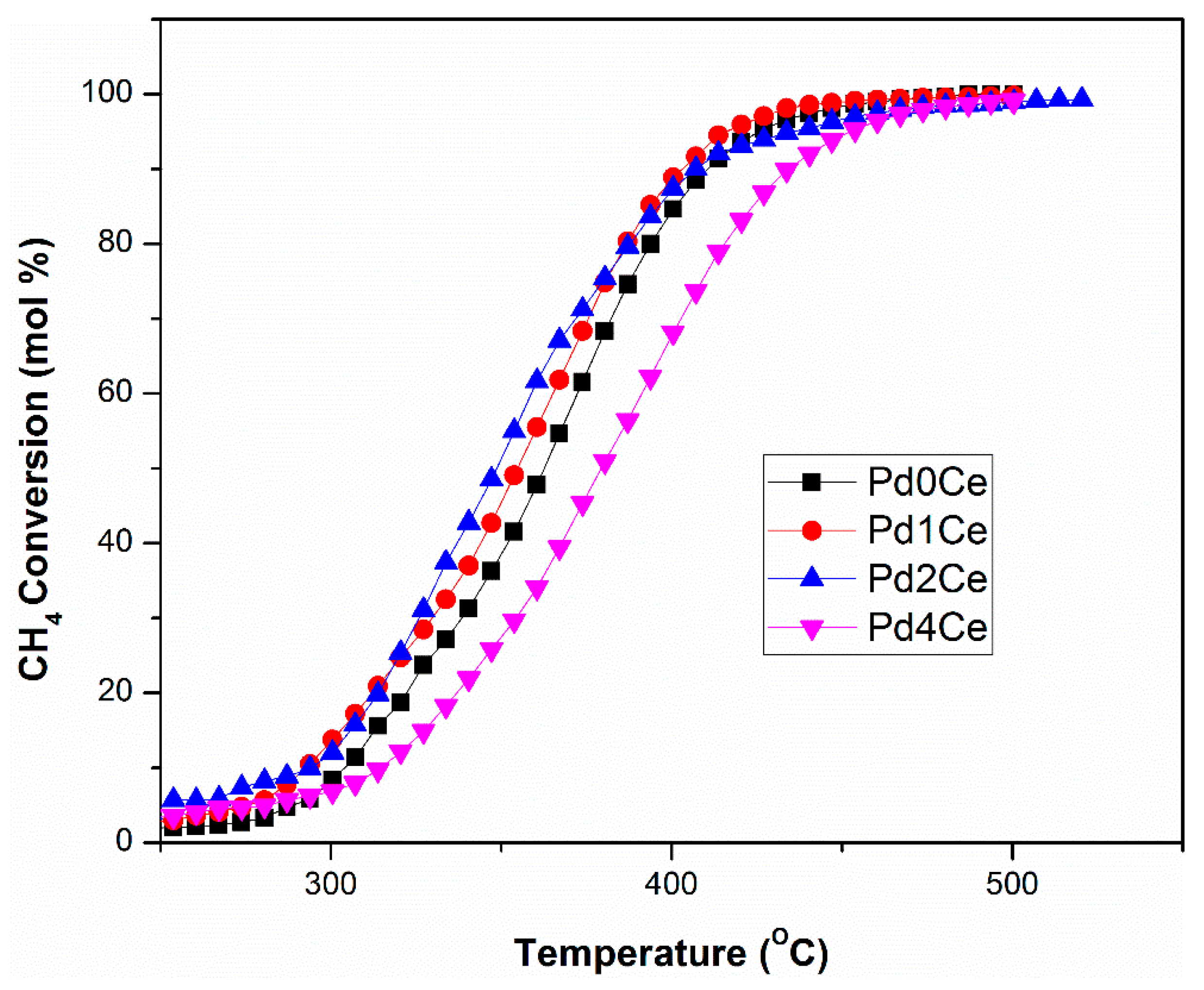
2.2. Catalysts Activity and Stability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Monolith Catalyst
4.3. Monolith Characterization
4.4. Reaction System and Activity Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbasi, R.; Huang, G.; Istratescu, G.M.; Wu, L.; Hayes, R.E. Methane oxidation over Pt, Pt: Pd, and Pd based catalysts: Effects of pre-treatment. Can. J. Chem. Eng. 2015, 93, 1474–1482. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Adamowska, M.; Da Costa, P. Structured Pd/γ-Al2O3 Prepared by Washcoated Deposition on a Ceramic Honeycomb for Compressed Natural Gas Applications. J. Nanoparticles 2015, 3, 9–18. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Banerjee, S.; Choudhary, V.R. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Cargnello, M.; Jaén, J.J.D.; Garrido, J.C.H.; Bakhmutsky, K.; Montini, T.; Gámez, J.J.C.; Gorte, R.J.; Fornasiero, P. Exceptional Activity for Methane Combustion over Modular Pd@CeO2 Subunits on Functionalized Al2O3. Science 2012, 337, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Han, J.; Zemlyanov, D.Y.; Ribeiro, F.H. The Turnover Rate for the Catalytic Combustion of Methane over Palladium Is Not Sensitive to the Structure of the Catalyst. J. Am. Chem. Soc. 2004, 126, 9896–9897. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, L.J.; Praliaud, H.; Primet, M. Catalytic combustion of methane over palladium supported on alumina and silica in presence of hydrogen sulfide. Appl. Catal. A Gen. 1993, 98, 125–138. [Google Scholar] [CrossRef]
- Lampert, J.K.; Kazi, M.S.; Farrauto, R.J. Palladium catalyst performance for methane emissions abatement from lean burn natural gas vehicles. Appl. Catal. B Environ. 1997, 14, 211–223. [Google Scholar] [CrossRef]
- Gholami, R.; Alyani, M.; Smith, K.J. Deactivation of Pd Catalysts by Water during Low Temperature Methane Oxidation Relevant to Natural Gas Vehicle Converters. Catalysts 2015, 5, 561. [Google Scholar] [CrossRef]
- Mowery, D.L.; McCormick, R.L. Deactivation of alumina supported and unsupported PdO methane oxidation catalyst: The effect of water on sulfate poisoning. Appl. Catal. B Environ. 2001, 34, 287–297. [Google Scholar] [CrossRef]
- Li, Z.; Hoflund, G.B. A review on complete oxidation of methane at low temperatures. J. Nat. Gas Chem. 2003, 12, 153–160. [Google Scholar]
- Gandhi, H.S.; Graham, G.W.; McCabe, R.W. Automotive exhaust catalysis. J. Catal. 2003, 216, 433–442. [Google Scholar] [CrossRef]
- Narui, K.; Furuta, K.; Yata, H.; Nishida, A.; Kohtoku, Y.; Matsuzaki, T. Catalytic activity of PdO/ZrO2 catalyst for methane combustion. Catal. Today 1998, 45, 173–178. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Lampert, J.K.; Hobson, M.C.; Waterman, E.M. Thermal decomposition and reformation of PdO catalysts; support effects. Appl. Catal. B Environ. 1995, 6, 263–270. [Google Scholar] [CrossRef]
- Haneda, M.; Mizushima, T.; Kakuta, N.; Ueno, A.; Sato, Y.; Matsuura, S.; Kasahara, K.; Sato, M. Structural Characterization and Catalytic Behavior of Al2O3-Supported Cerium Oxides. Bull. Chem. Soc. Jpn. 1993, 66, 1279–1288. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.H.; Kim, Y.J.; Kim, E.S.; Han, H.S.; Shin, C. Hydrothermal stability of Pd/ZrO2 catalysts for high temperature methane combustion. Appl. Catal. B Environ. 2014, 160, 135–143. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Cristiani, C.; Lietti, L.; Groppi, G. The influence of ceria and other rare earth promoters on palladium-based methane combustion catalysts. Catal. Today 2012, 180, 124–130. [Google Scholar] [CrossRef]
- Ramírez-López, R.; Elizalde-Martinez, I.; Balderas-Tapia, L. Complete catalytic oxidation of methane over Pd/CeO2–Al2O3: The influence of different ceria loading. Catal. Today 2010, 150, 358–362. [Google Scholar] [CrossRef]
- Stasinska, B.; Gac, W.; Ioannides, T.; Machocki, A. Complete oxidation of methane over palladium supported on alumina modified with calcium, lanthanum, and cerium ions. J. Nat. Gas Chem. 2007, 16, 342–348. [Google Scholar] [CrossRef]
- Groppi, G.; Cristiani, C.; Lietti, L.; Ramella, C.; Valentini, M.; Forzatti, P. Effect of ceria on palladium supported catalysts for high temperature combustion of CH4 under lean conditions. Catal. Today 1999, 50, 399–412. [Google Scholar] [CrossRef]
- Fan, X.; Wang, F.; Zhu, T.; He, H. Effects of Ce on catalytic combustion of methane over Pd-Pt/Al2O3 catalyst. J. Environ. Sci. 2012, 24, 507–511. [Google Scholar] [CrossRef]
- Persson, K.; Ersson, A.; Colussi, S.; Trovarelli, A.; Järås, S.G. Catalytic combustion of methane over bimetallic Pd–Pt catalysts: The influence of support materials. Appl. Catal. B Environ. 2006, 66, 175–185. [Google Scholar] [CrossRef]
- Ciuparu, D.; Perkins, E.; Pfefferle, L. In situ DR-FTIR investigation of surface hydroxyls on γ-Al2O3 supported PdO catalysts during methane combustion. Appl. Catal. A Gen. 2004, 263, 145–153. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Farnesi Camellone, M.; Boaro, M.; Llorca, J.; Fabris, S.; Trovarelli, A. Nanofaceted Pd-O Sites in Pd-Ce Surface Superstructures: Enhanced Activity in Catalytic Combustion of Methane. Angew. Chem. Int. Ed. 2009, 48, 8481–8484. [Google Scholar] [CrossRef] [PubMed]
- Escandón, L.S.; Niño, D.; Díaz, E.; Ordóñez, S.; Díez, F.V. Effect of hydrothermal ageing on the performance of Ce-promoted PdO/ZrO2 for methane combustion. Catal. Commun. 2008, 9, 2291–2296. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K.; Urbano, F.J. 1st World Conference Environmental Catalysis for a Better World and Life Some aspects of hydrocarbon activation on platinum group metal combustion catalysts. Catal. Today 1996, 27, 243–248. [Google Scholar] [CrossRef]
- Gélin, P.; Urfels, L.; Primet, M.; Tena, E. Complete oxidation of methane at low temperature over Pt and Pd catalysts for the abatement of lean-burn natural gas fuelled vehicles emissions: Influence of water and sulphur containing compounds. Catal. Today 2003, 83, 45–57. [Google Scholar] [CrossRef]
- Alyani, M.; Smith, K.J. A kinetic analysis of the inhibition of CH4 oxidation by H2O on PdO/Al2O3 and CeO2/PdO/Al2O3 catalysts. Ind Eng Chem Res 2016, 55, 8309–8318. [Google Scholar] [CrossRef]
- Gomathi, A.; Vickers, S.M.; Gholami, R.; Alyani, M.; Man, R.W.; MacLachlan, M.J.; Smith, K.J.; Wolf, M.O. Nanostructured Materials Prepared by Surface-Assisted Reduction: New Catalysts for Methane Oxidation. Acs Appl. Mater. Interfaces 2015, 7, 19268–19273. [Google Scholar] [CrossRef]
- Toso, A.; Colussi, S.; Llorca, J.; Trovarelli, A. The dynamics of PdO-Pd phase transformation in the presence of water over Si-doped Pd/CeO2 methane oxidation catalysts. Appl. Catal. A Gen. 2019, 574, 79–86. [Google Scholar] [CrossRef]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Monolithic catalysts — non-uniform active phase distribution by impregnation. Appl. Catal. A Gen. 2001, 213, 179–187. [Google Scholar] [CrossRef]
- Geus, J.W.; Van Giezen, J.C. Monoliths in catalytic oxidation. Catal. Today 1999, 47, 169–180. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. Sci. Eng. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Hayes, R.E.; Awdry, S.; Kolaczkowski, S.T. Catalytic combustion of methane in a monolith washcoat: Effect of water inhibition on the effectiveness factor. Can. J. Chem. Eng. 1999, 77, 688–697. [Google Scholar] [CrossRef]
- Thevenin, P.O.; Menon, P.G.; Järås, S.G. Catalytic Total Oxidation of Methane. Part II. Catalytic Processes to Convert Methane: Partial or Total Oxidation. CATTECH 2003, 7, 10–22. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Tylus, W.; Kȩpiński, L. Pd-based monolithic catalysts on metal supports for catalytic combustion of methane. Appl. Catal. B Environ. 2004, 49, 27–37. [Google Scholar] [CrossRef]
- Ordóñez, S.; Hurtado, P.; Sastre, H.; Díez, F.V. Methane catalytic combustion over Pd/Al2O3 in presence of sulphur dioxide: Development of a deactivation model. Appl. Catal. A Gen. 2004, 259, 41–48. [Google Scholar] [CrossRef]
- Kikuchi, R.; Maeda, S.; Sasaki, K.; Wennerström, S.; Eguchi, K. Low-temperature methane oxidation over oxide-supported Pd catalysts: Inhibitory effect of water vapor. Appl. Catal. A Gen. 2002, 232, 23–28. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Järås, S.G. Stability of palladium-based catalysts during catalytic combustion of methane: The influence of water. Appl. Catal. B Environ. 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Zhu, G.; Han, J.; Zemlyanov, D.Y.; Ribeiro, F.H. Temperature dependence of the kinetics for the complete oxidation of methane on palladium and palladium oxide. J. Phys. Chem. B 2005, 109, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Stasinska, B.; Machocki, A.; Antoniak, K.; Rotko, M.; Figueiredo, J.L.; Gonçalves, F. Importance of palladium dispersion in Pd/Al2O3 catalysts for complete oxidation of humid low-methane–air mixtures. Catal. Today 2008, 137, 329–334. [Google Scholar] [CrossRef]
- Roth, D.; Gélin, P.; Primet, M.; Tena, E. Catalytic behaviour of Cl-free and Cl-containing Pd/Al2O3 catalysts in the total oxidation of methane at low temperature. Appl. Catal. A Gen. 2000, 203, 37–45. [Google Scholar] [CrossRef]
- Burch, R.; Urbano, F.; Loader, P. Methane combustion over palladium catalysts: The effect of carbon dioxide and water on activity. Appl. Catal. A Gen. 1995, 123, 173–184. [Google Scholar] [CrossRef]
- Burch, R. Low NOx options in catalytic combustion and emission control. Catal. Today 1997, 35, 27–36. [Google Scholar] [CrossRef]
- Lucena, P.; Vadillo, J.M.; Laserna, J.J. Mapping of platinum group metals in automotive exhaust three-way catalysts using laser-induced breakdown spectrometry. Anal. Chem. 1999, 71, 4385–4391. [Google Scholar] [CrossRef]
- Muraki, H.; Shinjoh, H.; Fujitani, Y. Effect of lanthanum on the no reduction over palladium catalysts. Appl. Catal. 1986, 22, 325–335. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Hu, Z.; Yao, M.; Li, Y. A review on the Pd-based three-way catalyst. Catal. Rev. 2015, 57, 79–144. [Google Scholar] [CrossRef]
- Di Monte, R.; Fornasiero, P.; Kašpar, J.; Graziani, M.; Gatica, J.; Bernal, S.; Gómez-Herrero, A. Stabilisation of nanostructured Ce0. 2Zr0. 8O2 solid solution by impregnation on Al2O3: A suitable method for the production of thermally stable oxygen storage/release promoters for three-way catalysts. Chem. Commun. 2000, 2167–2168. [Google Scholar] [CrossRef]
- Yao, H.; Yao, Y.Y. Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Su, E.; Montreuil, C.; Rothschild, W. Oxygen storage capacity of monolith three-way catalysts. Appl. Catal. 1985, 17, 75–86. [Google Scholar] [CrossRef]
- Hailstone, R.; DiFrancesco, A.; Leong, J.; Allston, T.; Reed, K. A study of lattice expansion in CeO2 nanoparticles by transmission electron microscopy. J. Phys. Chem. C 2009, 113, 15155–15159. [Google Scholar] [CrossRef]
- Ozawa, M.; Kimura, M. Effect of cerium addition on the thermal stability of gamma alumina support. J. Mater. Sci. Lett. 1990, 9, 291–293. [Google Scholar] [CrossRef]
- Piras, A.; Trovarelli, A.; Dolcetti, G. Remarkable stabilization of transition alumina operated by ceria under reducing and redox conditions. Appl. Catal. B Environ. 2000, 28, L77–L81. [Google Scholar] [CrossRef]
- Jiang, P.; Lu, G.; Guo, Y.; Guo, Y.; Zhang, S.; Wang, X. Preparation and properties of a γ-Al2O3 washcoat deposited on a ceramic honeycomb. Surf. Coat. Technol. 2005, 190, 314–320. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-Ray Photoelectron Spectroscopy; National Institute of Informatics: Tokyo, Japan, 1995; pp. 230–232. [Google Scholar]
- Cullis, C.; Willatt, B. Oxidation of methane over supported precious metal catalysts. J. Catal. 1983, 83, 267–285. [Google Scholar] [CrossRef]
- Haneda, M.; Mizushima, T.; Kakuta, N. Synergistic effect between Pd and nonstoichiometric cerium oxide for oxygen activation in methane oxidation. J. Phys. Chem. B 1998, 102, 6579–6587. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Vesselli, E.; Baraldi, A.; Comelli, G.; Groppi, G.; Llorca, J. Structure and morphology of Pd/Al2O3 and Pd/CeO2/Al2O3 combustion catalysts in Pd–PdO transformation hysteresis. Appl. Catal. A Gen. 2010, 390, 1–10. [Google Scholar] [CrossRef]
- Fouladvand, S.; Schernich, S.; Libuda, J.; Grönbeck, H.; Pingel, T.; Olsson, E.; Skoglundh, M.; Carlsson, P. Methane oxidation over Pd supported on ceria–alumina under rich/lean cycling conditions. Top. Catal. 2013, 56, 410–415. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ribeiro, F.H.; Avalos-Borja, M.; Iglesia, E. Structure and reactivity of PdOx/ZrO2 catalysts for methane oxidation at low temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef]
- Yao, Y.Y. Oxidation of alkanes over noble metal catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 293–298. [Google Scholar] [CrossRef]
- Oh, S.H.; Mitchell, P.J.; Siewert, R.M. Methane oxidation over alumina-supported noble metal catalysts with and without cerium additives. J. Catal. 1991, 132, 287–301. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, K.; Xu, X.; Li, X. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition–precipitation method. Catal. Commun. 2005, 6, 796–801. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of Methane over palladium-based catalysts: Catalytic deactivation and Role of the Support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Hobson, M.; Kennelly, T.; Waterman, E. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A Gen. 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Shyu, J.; Otto, K.; Watkins, W.; Graham, G.; Belitz, R.; Gandhi, H. Characterization of Pd/γ-alumina catalysts containing ceria. J. Catal. 1988, 114, 23–33. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Groppi, G.; Llorca, J. The effect of CeO2 on the dynamics of Pd–PdO transformation over Pd/Al2O3 combustion catalysts. Catal. Commun. 2007, 8, 1263–1266. [Google Scholar] [CrossRef]
- Cullis, C.; Nevell, T.; Trimm, D. Role of the catalyst support in the oxidation of methane over palladium. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1972, 68, 1406–1412. [Google Scholar] [CrossRef]
- Araya, P.; Guerrero, S.; Robertson, J.; Gracia, F.J. Methane combustion over Pd/SiO2 catalysts with different degrees of hydrophobicity. Appl. Catal. A Gen. 2005, 283, 225–233. [Google Scholar] [CrossRef]
- Okumura, K.; Shinohara, E.; Niwa, M. Pd loaded on high silica beta support active for the total oxidation of diluted methane in the presence of water vapor. Catal. Today 2006, 117, 577–583. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Fraga, M.A.; Pastore, H.O. Methane combustion over Pd supported on MCM-41. Appl. Catal. B Environ. 2007, 76, 115–122. [Google Scholar] [CrossRef]
- Carchini, G.; García-Melchor, M.; Łodziana, Z.; López, N. Understanding and tuning the intrinsic hydrophobicity of rare-earth oxides: A DFT U study. Acs Appl. Mater. Interfaces 2015, 8, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, M.; Wang, J.; Kärkkäinen, M.; Huuhtanen, M.; Jiang, H.; Kallinen, K.; Keiski, R.L.; Akola, J.; Vippola, M. Regeneration of sulfur-poisoned Pd-based catalyst for natural gas oxidation. J. Catal. 2018, 358, 253–265. [Google Scholar] [CrossRef]
- Almohamadi, H.; Smith, K.J. Beneficial Effect of Adding g-AlOOH to the g-Al2O3 Washcoat of a PdO Catalyst for Methane Oxidation. Can. J. Chem. Eng. 2019. accepted. [Google Scholar]

| Sample | Ce Content | BET Area | Pore Volume | Average Pore Diameter | CO Uptake |
|---|---|---|---|---|---|
| wt.% | m2/g | cm3/g | nm | µmol/gcat | |
| Pd0Ce | 0 | 55 | 0.24 | 9 | 19.4 |
| Pd1Ce | 1 | 51 | 0.17 | 13 | 13.5 |
| Pd2Ce | 2 | 49 | 0.14 | 11 | 11.5 |
| Pd4Ce | 4 | 46 | 0.12 | 10 | 8.5 |
| Pd0Ce—used | 0 | 45 | 0.12 | 10 | 10 |
| Pd2Ce—used | 2 | 42 | 0.11 | 10 | 8.8 |
| O, wt.% | Al, wt.% | Ce, wt.% | Pd, wt.% | |
|---|---|---|---|---|
| Pd0Ce | 53.2 ± 1 | 45.1 ± 2.1 | 0 | 1.7 ± 0.2 |
| Pd1Ce | 50.1 ± 1.9 | 45.2 ± 2.4 | 3.2 ± 0.8 | 1.5 ± 0.4 |
| Pd2Ce | 48.2 ± 1.6 | 44.3 ± 2.1 | 5.9 ± 0.7 | 1.6 ± 0.3 |
| Pd4Ce | 45.9 ± 1.9 | 41.1 ± 1.5 | 11.3 ± 0.5 | 1.7 ± 0.2 |
| Monolith Catalyst | Pd B.E. | Surface Composition | |||||
|---|---|---|---|---|---|---|---|
| 3d5/2 | 3d3/2 | Pd | Ce | Al | O | Pd/Al | |
| eV | eV | atom % | % | ||||
| Pd0Ce | 336.6 | 342.1 | 0.2 | 0 | 39.1 | 60.7 | 0.51 |
| Pd1Ce | 337.1 | 342.3 | 0.28 | 0.29 | 35.13 | 64.3 | 0.80 |
| Pd2Ce | 337.1 | 342.3 | 0.30 | 0.45 | 31.25 | 68 | 0.96 |
| Pd4Ce | 336.9 | 342.1 | 0.69 | 0.51 | 35.4 | 63.4 | 1.95 |
| Pd0Ce-Used | 336.9 | 342.2 | 0.17 | 0 | 37.5 | 62.3 | 0.45 |
| Pd2Ce-Used | 337.7 | 342.9 | 0.26 | 0.64 | 35.7 | 63.4 | 0.73 |
| Monolith | T50 Dry Gas | T50 Wet Gas 2 vol.% H2O | T50 Wet Gas 5 vol.% H2O |
|---|---|---|---|
| Pd0Ce | 317 | 379 | 401 |
| Pd1Ce | 322 | 370 | 385 |
| Pd2Ce | 333 | 352 | 370 |
| Pd4Ce | 362 | 377 | 390 |
| Sample | Catalyst Composition, wt.% | ||||
|---|---|---|---|---|---|
| Pd | Ce | γ-AlOOH | γ-Al2O3 | Cordierite | |
| Pd0Ce | 0.5 | 0 | 5.3 | 21.2 | 73.0 |
| Pd1Ce | 0.5 | 1.0 | 5.1 | 20.4 | 73.0 |
| Pd2Ce | 0.5 | 2.0 | 4.9 | 19.6 | 73.0 |
| Pd4Ce | 0.5 | 4.0 | 4.5 | 18.0 | 73.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMohamadi, H.; Smith, K.J. The Impact of CeO2 Loading on the Activity and Stability of PdO/γ-AlOOH/γ-Al2O3 Monolith Catalysts for CH4 Oxidation. Catalysts 2019, 9, 557. https://doi.org/10.3390/catal9060557
AlMohamadi H, Smith KJ. The Impact of CeO2 Loading on the Activity and Stability of PdO/γ-AlOOH/γ-Al2O3 Monolith Catalysts for CH4 Oxidation. Catalysts. 2019; 9(6):557. https://doi.org/10.3390/catal9060557
Chicago/Turabian StyleAlMohamadi, Hamad, and Kevin J. Smith. 2019. "The Impact of CeO2 Loading on the Activity and Stability of PdO/γ-AlOOH/γ-Al2O3 Monolith Catalysts for CH4 Oxidation" Catalysts 9, no. 6: 557. https://doi.org/10.3390/catal9060557
APA StyleAlMohamadi, H., & Smith, K. J. (2019). The Impact of CeO2 Loading on the Activity and Stability of PdO/γ-AlOOH/γ-Al2O3 Monolith Catalysts for CH4 Oxidation. Catalysts, 9(6), 557. https://doi.org/10.3390/catal9060557