Role of Humic Acid Chemical Structure Derived from Different Biomass Feedstocks on Fe(III) Bioreduction Activity: Implication for Sustainable Use of Bioresources
Abstract
1. Introduction
2. Results and Discussion
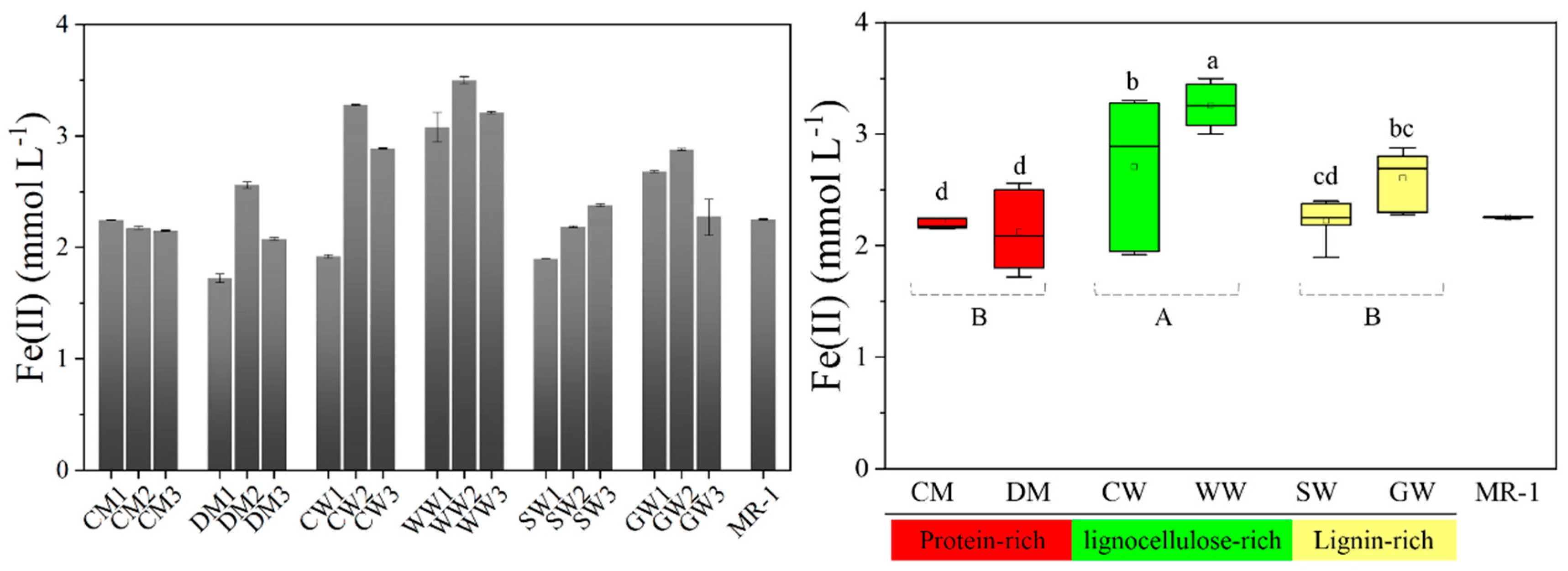
2.1. Bioreduction of Dissimilatory Fe(III) Mediated by Different HAs
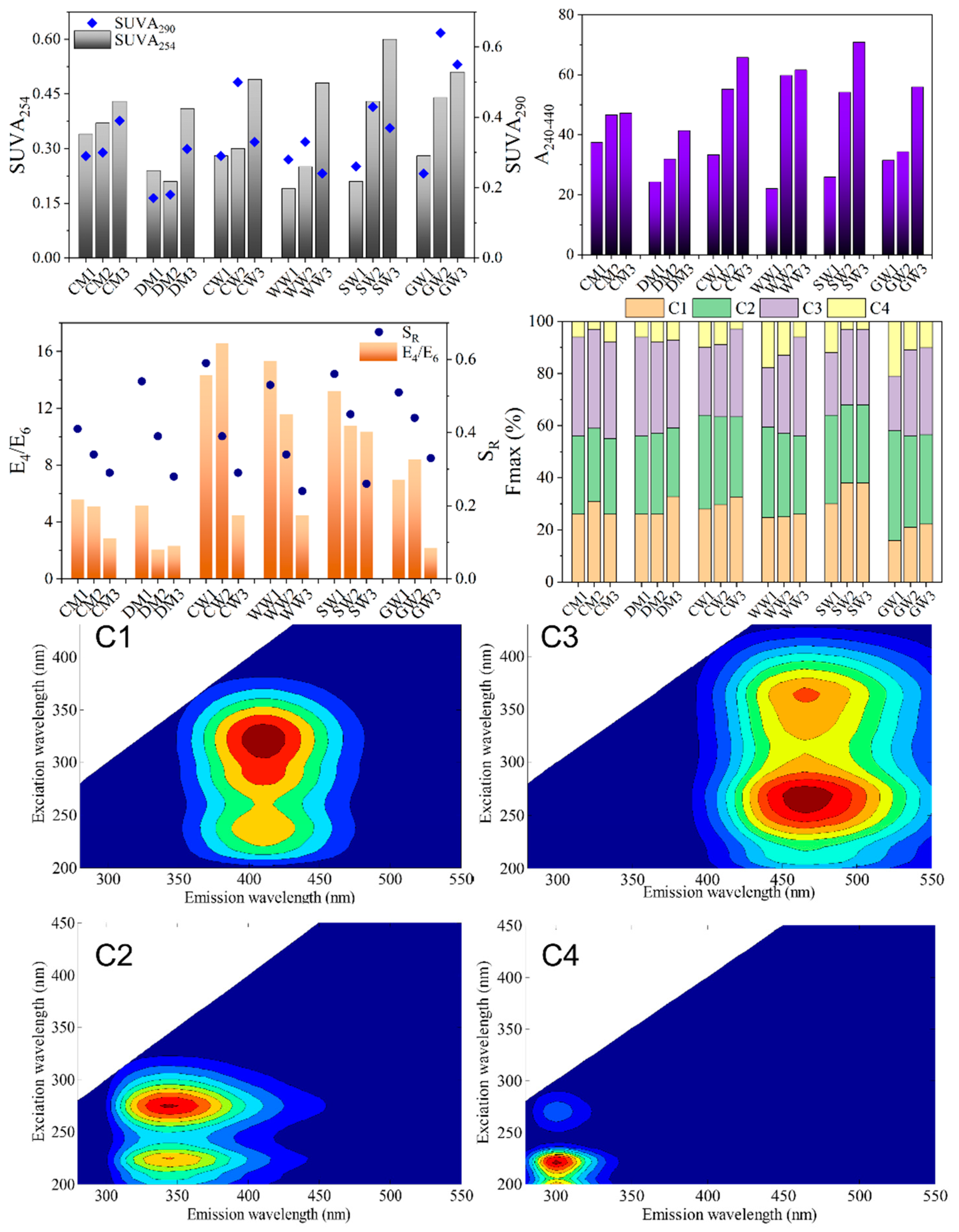
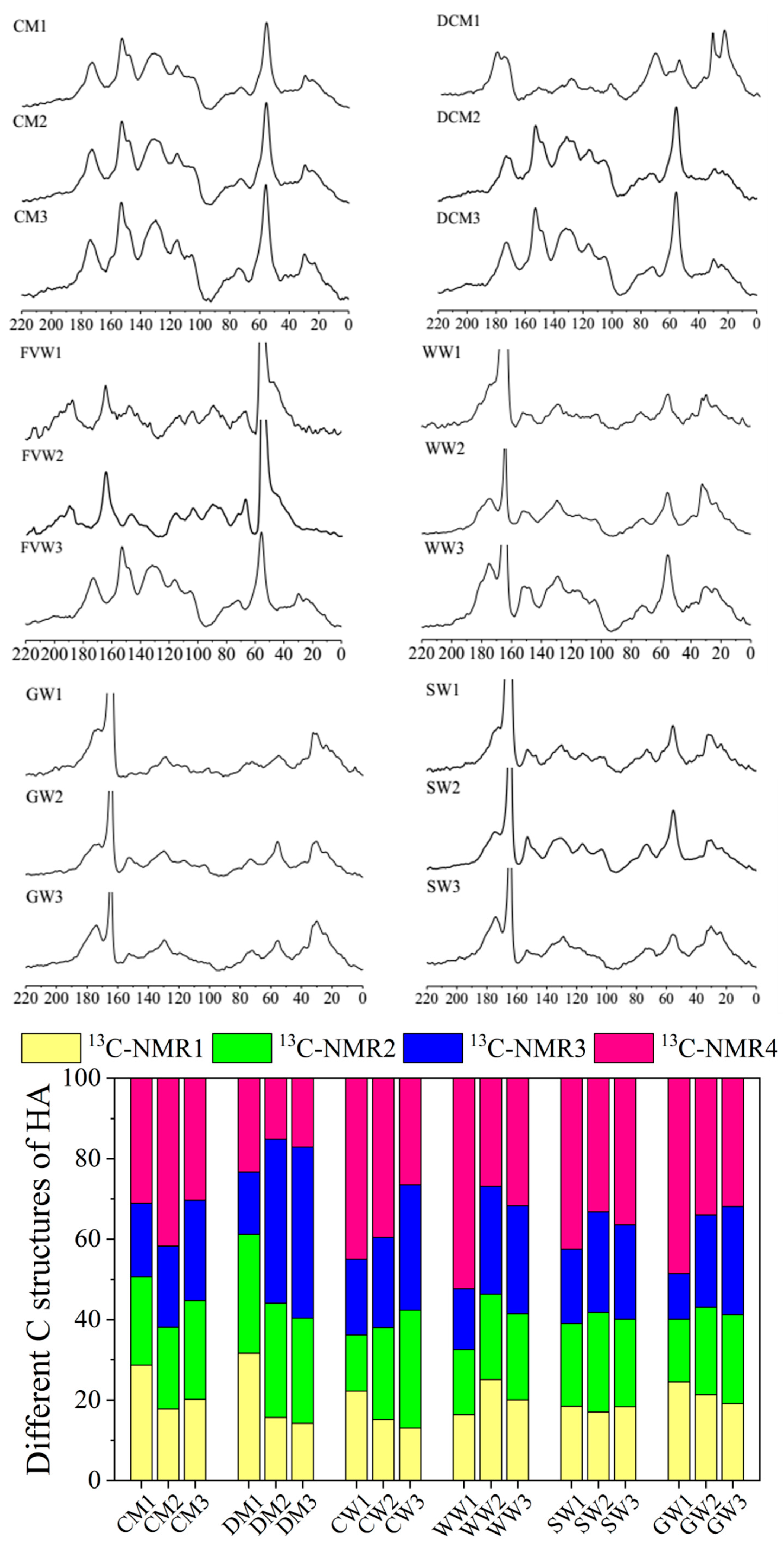
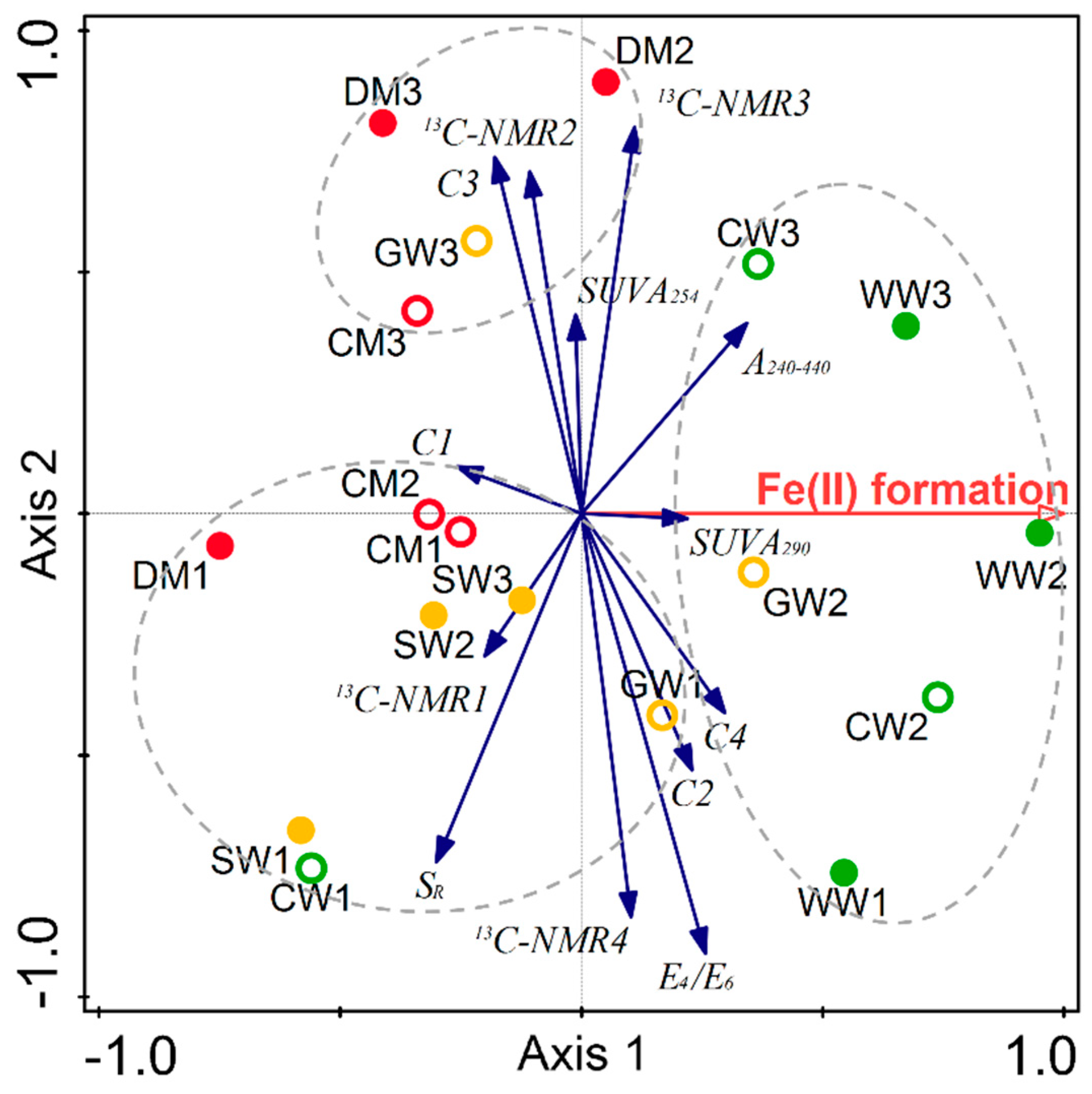
2.2. Structural Characteristics of Different HAs
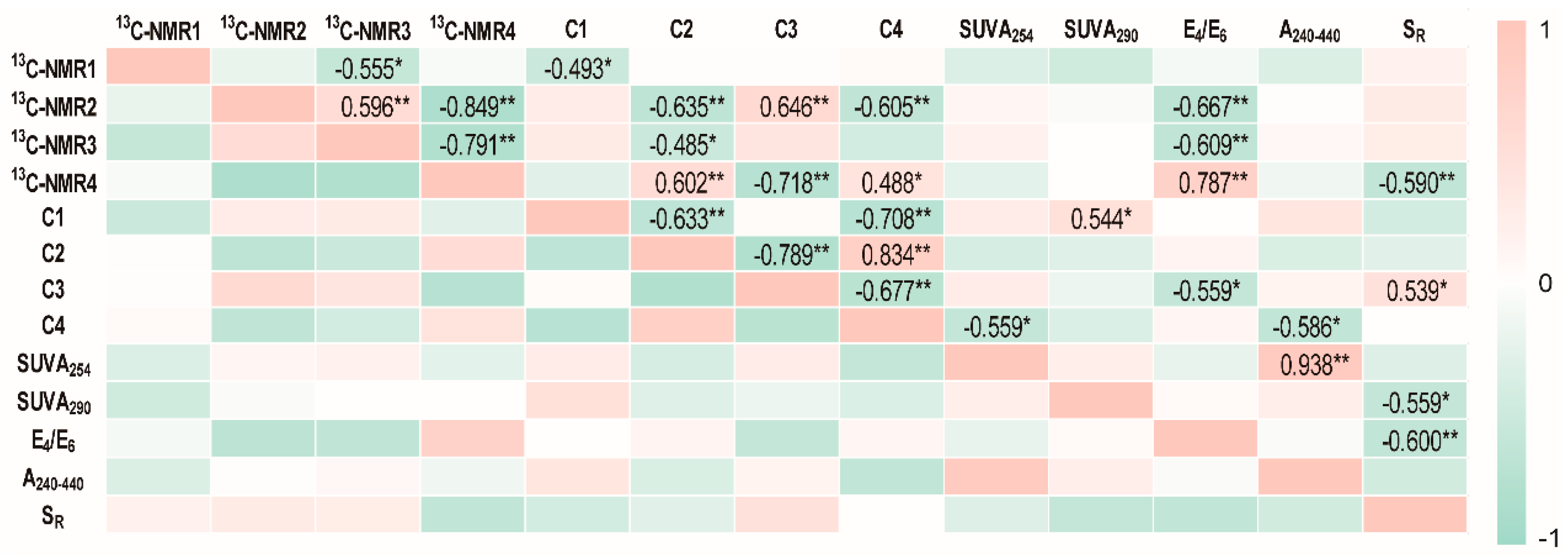
2.3. The Potential Role of HA in Dissimilatory Fe(III) Bioreduction
3. Materials and Methods
3.1. Preparation of Shewanella oneidensis MR-1
3.2. Extraction and analysis of HA from Different Composting
3.3. Batch Experiments of Dissimilatory Fe(III) Reduction
3.4. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, G.; Qiu, S.; Liu, B.; Pu, Y.; Gao, Z.; Wang, J.; Jin, R.; Zhou, J. Microbial reduction of Fe (III)-bearing clay minerals in the presence of humic acids. Sci. Rep. 2017, 7, 45354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tan, W.; Dang, Q.; Li, R.; Xi, B. Enhanced biotic contributions to the dechlorination of pentachlorophenol by humus respiration from different compostable environments. Chem. Eng. J. 2019, 361, 1565–1575. [Google Scholar] [CrossRef]
- Klüpfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195. [Google Scholar] [CrossRef]
- Stern, N.; Mejia, J.; He, S.; Yang, Y.; Ginder-Vogel, M.; Roden, E.E. Dual role of humic substances as electron donor and shuttle for dissimilatory iron reduction. Environ. Sci. Technol. 2018, 52, 5691–5699. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tu, Q.; Dong, D.; Strong, P.; Wang, H.; Sun, B.; Wu, W. Spectroscopic evidence for biochar amendment promoting humic acid synthesis and intensifying humification during composting. J. Hazard. Mater. 2014, 280, 409–416. [Google Scholar] [CrossRef]
- Gao, X.; Tan, W.; Zhao, Y.; Wu, J.; Sun, Q.; Qi, H.; Xie, X.; Wei, Z. Diversity in the Mechanisms of Humin Formation during Composting with Different Materials. Environ. Sci. Technol. 2019, 53, 3653–3662. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Y.; Zhao, W.; Yang, T.; Zhang, X.; Xie, X.; Cui, H.; Wei, Z. Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour. Technol. 2017, 226, 191–199. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Yu, H.; Wei, D.; Yang, T.; Wei, Z.; Lu, Q.; Zhang, X. Effects of aeration rates on the structural changes in humic substance during co-composting of digestates and chicken manure. Sci. Total Environ. 2019, 658, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Zhao, X.; He, X.; Huang, C.; Tan, W.; Gao, R.; Zhang, H.; Li, D. Successions and diversity of humic-reducing microorganisms and their association with physical-chemical parameters during composting. Bioresour. Technol. 2016, 219, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Le, T.H.T.; Idemoto, Y.; Abe, M.; Rollon, A.P. Comparison of organic matter degradation and microbial community during thermophilic composting of two different types of anaerobic sludge. Bioresour. Technol. 2009, 100, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.V.; Mladenov, N.; McKnight, D.M.; Zheng, Y.; Kirk, M.F.; Nemergut, D.R. Dissolved fulvic acids from a high arsenic aquifer shuttle electrons to enhance microbial iron reduction. Sci. Total Environ. 2018, 615, 1390–1395. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Yuan, Y.; He, X.; Xi, B.; Li, D.; Gao, R.; Tan, W.; Zhang, H.; Yang, C.; Zhao, X. Polarity and molecular weight of compost-derived humic acid affect Fe(III) oxides reduction. Chemosphere 2018, 208, 77–83. [Google Scholar] [CrossRef]
- Zhao, X.; He, X.; Xi, B.; Gao, R.; Tan, W.; Zhang, H.; Huang, C.; Li, D.; Li, M. Response of humic-reducing microorganisms to the redox properties of humic substance during composting. Waste Manag. 2017, 70, 37–44. [Google Scholar] [CrossRef]
- Tan, W.; Xi, B.; Wang, G.; Jiang, J.; He, X.; Mao, X.; Gao, R.; Huang, C.; Zhang, H.; Li, D. Increased electron-accepting and decreased electron-donating capacities of soil humic substances in response to increasing temperature. Environ. Sci. Technol. 2017, 51, 3176–3186. [Google Scholar] [CrossRef]
- Ratasuk, N.; Nanny, M.A. Characterization and Quantification of Reversible Redox Sites in Humic Substances. Environ. Sci. Technol. 2007, 41, 7844–7850. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Meth. 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.G.; Martin-Neto, L.; Saab, S.C.; Novotny, E.H.; Milori, D.M.; Bagnato, V.S.; Colnago, L.A.; Melo, W.J.; Knicker, H. Characterization of humic acids from a Brazilian Oxisol under different tillage systems by EPR, 13C NMR, FTIR and fluorescence spectroscopy. Geoderma 2004, 118, 181–190. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Evaluation of humic substances during cocomposting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Smilek, J.; Sedlácˇek, P.; Kalina, M.; Klucáková, M. On the role of humic acids’ carboxyl groups in the binding of charged organic compounds. Chemosphere 2015, 138, 503–510. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [PubMed]
- Xu, Y.; He, Y.; Feng, X.; Liang, L.; Xu, J.; Brookes, P.C.; Wu, J. Enhanced abiotic and biotic contributions to dechlorination of pentachlorophenol during Fe(III) reduction by an iron-reducing bacterium Clostridium beijerinckii Z. Sci. Total Environ. 2014, 473–474, 215–223. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Fan, Y.; Zhang, F.; Tan, W.; He, X.; Xi, B. Roles of bacterial community in the transformation of dissolved organic matter for the stability and safety of material during sludge composting. Bioresour. Technol. 2018, 267, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Zhao, Y.; Chen, Y.N.; Zhang, X.; Wang, X.Q.; Lu, Q.; Jia, L.M.; Wei, Z.M. Assessment of phytotoxicity grade during composting based on EEM/PARAFAC combined with projection pursuit regression. J. Hazard. Mater. 2017, 326, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhao, X.; Zhu, C.; Xi, B.; Zhao, Y.; Yu, X. Assessment of humification degree of dissolved organic matter from different composts using fluorescence spectroscopy technology. Chemosphere 2014, 95, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Jouraiphy, A.; Meddich, A.; El Gharous, M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef]
- Trubetskoj, O.A.; Hatcher, P.G.; Trubetskaya, O.E. 1H-NMR and 13C-NMR spectroscopy of chernozem soil humic acid fractionated by combined size-exclusion chromatography and electrophoresis. Chem. Ecol. 2010, 26, 315–325. [Google Scholar] [CrossRef]
- Tan, W.; Li, R.; Yu, H.; Zhao, X.; Dang, Q.; Jiang, J.; Wang, L.; Xi, B. Prominent Conductor Mechanism-Induced Electron Transfer of Biochar Produced by Pyrolysis of Nickel-Enriched Biomass. Catalysts 2018, 8, 573. [Google Scholar] [CrossRef]

| kred (h−1) | R | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| CM | 0.0158 ± 0.0032 | 0.0271 ± 0.0060 | 0.0213 ± 0.0040 | 0.9564 | 0.9384 | 0.9583 |
| DM | 0.0126 ± 0.0037 | 0.0183 ± 0.0030 | 0.0309 ± 0.0091 | 0.9365 | 0.9705 | 0.8917 |
| CW | 0.0260 ± 0.0056 | 0.0354 ± 0.0026 | 0.0094 ± 0.0031 | 0.9421 | 0.9709 | 0.9296 |
| WW | 0.0141 ± 0.0039 | 0.0432 ± 0.0166 | 0.0072 ± 0.0022 | 0.9263 | 0.8293 | 0.9539 |
| SW | 0.0367 ± 0.0108 | 0.0209 ± 0.0048 | 0.0356 ± 0.0118 | 0.8918 | 0.9378 | 0.8664 |
| GW | 0.0208 ± 0.0041 | 0.0182 ± 0.0051 | 0.0368 ± 0.0129 | 0.9565 | 0.9120 | 0.8517 |
| CK1 | 0.0227 ± 0.0044 | 0.9568 | ||||
| CK2 | 0.0310 ± 0.0030 | 0.9841 | ||||
| Treatment | Sodium Lactate and Fe(III)-citrate | Shewanella oneidensis MR-1 | Humic Acids | ||
|---|---|---|---|---|---|
| Mesophilic Phase | Thermophilic Phase | Mature Phase | |||
| CK1 | + | − | − | − | − |
| CK2 | + | + | − | − | − |
| HA(X1) | + | + | + | − | − |
| HA(X2) | + | + | − | + | − |
| HA(X3) | + | + | − | − | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Wei, Z.; Zhang, F.; Li, X.; Tan, W.; Xi, B. Role of Humic Acid Chemical Structure Derived from Different Biomass Feedstocks on Fe(III) Bioreduction Activity: Implication for Sustainable Use of Bioresources. Catalysts 2019, 9, 450. https://doi.org/10.3390/catal9050450
Wei Y, Wei Z, Zhang F, Li X, Tan W, Xi B. Role of Humic Acid Chemical Structure Derived from Different Biomass Feedstocks on Fe(III) Bioreduction Activity: Implication for Sustainable Use of Bioresources. Catalysts. 2019; 9(5):450. https://doi.org/10.3390/catal9050450
Chicago/Turabian StyleWei, Yuquan, Zimin Wei, Fang Zhang, Xiang Li, Wenbing Tan, and Beidou Xi. 2019. "Role of Humic Acid Chemical Structure Derived from Different Biomass Feedstocks on Fe(III) Bioreduction Activity: Implication for Sustainable Use of Bioresources" Catalysts 9, no. 5: 450. https://doi.org/10.3390/catal9050450
APA StyleWei, Y., Wei, Z., Zhang, F., Li, X., Tan, W., & Xi, B. (2019). Role of Humic Acid Chemical Structure Derived from Different Biomass Feedstocks on Fe(III) Bioreduction Activity: Implication for Sustainable Use of Bioresources. Catalysts, 9(5), 450. https://doi.org/10.3390/catal9050450





