Acidic and Catalytic Properties of Zeolites Modified by Zinc in the Conversion Process of Lower C3–C4 Alkanes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Catalysts
2.2. Catalytic Activity
2.3. Acid Propertis of Catalysts
3. Material and Methods
3.1. Catalysts Preparation
3.2. Characterization Techniques
3.3. Acid Properties of Catalysts
3.4. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choudhary, V.R.; Mantri, K.; Sivadinarayana, C. Influence of zeolite factors affecting zeolitic acidity on the propane aromatization activity and selectivity of Ga/H-ZSM-5. Microporous Mesoporous Mater. 2000, 37, 1–8. [Google Scholar] [CrossRef]
- Erofeev, V.I.; Trofimova, A.S.; Koval, L.M.; Ryabov, Y.V. Acidity and catalytic properties of Cu-ZSM-5 in conversion of lover alkanes. Russ. J. Appl. Chem. 2000, 73, 2057–2061. [Google Scholar]
- Trofimova, A.S.; Koval, L.M.; Erofeev, V.I. Synthesis of Lower Olefins from C3–C4 Alkanes on ZSM-5 Zeolites Modified with Alkali Metals. Russ. J. Phys. Chem. 2000, 74, S537–S540. [Google Scholar]
- Xiao, H.; Zhang, J.; Wang, P.; Zhang, Z.; Zhang, Q.; Xie, H.; Yang, G.; Han, Y.; Tan, Y. Mechanistic insight to acidity effects of Ga/HZSM-5 on its activity for propane aromatization. RSC Adv. 2015, 112, 92222–92233. [Google Scholar] [CrossRef]
- Choudhary, C.V.R.; Panjala, D.; Banerjee, S. Aromatization of propene and n-butene over H-galloaluminosilicate (ZSM-5 type) zeolite. Appl. Catal. A Gen. 2002, 231, 243–251. [Google Scholar] [CrossRef]
- Trofimova, A.S.; Erofeev, V.I.; Koval, L.M. The Preparation of the lower olefins from C3–C4 Alkanes on ZSM-5 Zeolites modified by Lithium. Russ. J. Phys. Chem. 2002, 76, 922–925. [Google Scholar]
- Erofeev, V.I.; Adyaeva, L.V. Transformations of straight-run naphthas on indium-modified pentasils. Russ. J. Appl. Chem. 2003, 76, 1083–1088. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Zheng, L.; Zheng, X. Degydrogenation and aromatization of propane over rhenium-modified HZSM-5 catalyst. J. Mol. Catal. A Chem. 2005, 239, 222–227. [Google Scholar] [CrossRef]
- Caeiro, G.; Carvalho, R.H.; Wang, X.; Wang, X.; Lemos, M.A.N.D.A.; Lemos, F.; Guisnet, M.; Ribeiro, F.R. Activation of C2–C4 alkanes over acid bifunctional zeolite catalysts. J. Mol. Catal. A Chem. 2006, 255, 131–158. [Google Scholar] [CrossRef]
- Chang, F.; Wei, Y.; Liu, X.; Zxao, Y.; Xu, L.; Sun, Y.; Zhang, D.; He, Y.; Liu, Z. A mechanistic investigation of the coupled reaction of n-hexane and methanol over HZSM-5. Appl. Catal. A Gen. 2007, 328, 163–173. [Google Scholar] [CrossRef]
- Asachenko, E.V.; Rodina, O.V.; Ordomskii, V.V.; Gurev, Y.V.; Ivanova, I.I. Specifics of the deactivation of acid and zinc-containing propane aromatization catalysts. Petroleum Chem. 2008, 48, 100–104. [Google Scholar] [CrossRef]
- Vosmerikova, L.N.; Barbashin, Y.E.; Vosmerikov, A.V. Catalytic aromatization of ethane on zinc-modified zeolites of various framework types. Pet. Chem. 2014, 54, 420–425. [Google Scholar] [CrossRef]
- Bai, L.Y.; Zhou, Y.M.; Zhang, Y.W.; Liu, H.; Tang, M.H. Influence of Calcium Addition on Catalytic Properties of PtSn/ZSM-5 Catalyst for Propane Dehydrogenation. Catal. Lett. 2009, 129, 449–456. [Google Scholar] [CrossRef]
- Mentzel, U.V.; Rovik, A.K.; Christensen, C.H. Co–conversion of ethane and methanol into higher hydrocarbons over Ga/H-ZSM-5, Mo/H-ZSM-5 and Ga-Mo/H-ZSM-5. Catal. Lett. 2009, 127, 44–48. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Arzumanov, S.S.; Stepanov, A.G.; Freude, D. Propane aromatization on Zn-modified zeolite BEA studied by solid–state NMR in situ. J. Phys. Chem. C 2010, 114, 12681–12688. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Eon, J.-G.; Faro, A.C., Jr. Correlations between dispersion, acidity, reducibility, and propane aromatization activity of gallium species supported on HZSM5 zeolites. J. Phys. Chem. C 2010, 114, 4557–4567. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Wang, X.; Zhang, Q.; Xie, H.; Han, Y.; Tan, Y. A highly efficient Ga/ZSM-5 catalyst prepared by formic acid impregnation and in situ treatment for propane aromatization. Catal. Sci. Technol. 2015, 5, 4081–4090. [Google Scholar] [CrossRef]
- Asaftei, I.V.; Lungu, N.C.; Birsa, M.L.; Sarbu, L.G.; Ignat, M.; Sandu, I.G. Conversion of light hydrocarbons from petroleum refining processes over Zn-HZSM-5 (nitrate) and Zn-HZSM-5 (acetate) catalyst a comparative study. Rev. Chimie 2016, 67, 1523–1528. [Google Scholar]
- Choi, S.-W.; Kim, W.-G.; So, J.-S.; Sievers, C.; Sholl, D.S.; Nair, S.; Jones, C.W.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G. Propane dehydrogenation catalyzed by gallosilicate MFI zeolites with perturbed acidity. J. Catal. 2017, 345, 113–123. [Google Scholar] [CrossRef]
- Liu, R.-L.; Zhu, H.-Q.; Wu, Z.-W.; Qin, Z.-F.; Fan, W.-B.; Wang, J.-G. Aromatization of propane over Ga-modified ZSM-5 Catalysts. Ranliao Huaxue Xuebao 2015, 43, 961–969. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Erofeev, V.I.; Koval, L.M. Synthetic Zeolite and Method for Production Thereof. RU. Patent 2313486, 27 December 2007. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves; Publisher World: Moscow, Russia, 1976. [Google Scholar]
- Bogdankova, L.A.; Chuhleb, D.M. The Method of Producing Metal Sulfide. RU. Patent 2525174, 8 October 2014. [Google Scholar]
- Erofeev, V.I.; Khomyakhov, I.S.; Egorova, L.A. Production of high-octane Gasoline from straight-run Gasoline on ZSM-5 modified Zeolites. Theor. Found. Chem. Eng. 2014, 48, 71–76. [Google Scholar] [CrossRef]
- Pidko, E.A.; Santen, R.A.V. Activation of light alkanes over zinc species stabilized in ZSM-5: A comprehensive DFT study. J. Phys. Chem. C 2007, 111, 2643–2655. [Google Scholar] [CrossRef]
- Bhan, A.; Delgass, W.N. Propane aromatization over HZSM-5 and Ga/HZSM-5 catalysts. Catal. Rev. Sci. Eng. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Tshabalala, T.E.; Scurrell, M.S. Aromatization of n-hexane over Ga, Mo and Zn modified H-ZSM-5 zeolite catalysts. Catal. Commun. 2015, 72, 49–52. [Google Scholar] [CrossRef]
- Wan, H.; Pallavi, C. Catalytic conversion of propane to BTX over Ga, Zn, Mo and Re impregnated ZSM-5 catalysys. J. Anal. Appl. Pyrolysis 2016, 121, 369–375. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Faro Junior, A.C. On catalyst activation and reaction mechanisms in propane aromatization on Ga/HZSM5 catalysts. Appl. Cat. A Gen. 2012, 435–436, 68–77. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Vasconcellos, F.J., Jr.; Faro Junior, A.C. Mechanistic studies through H-D ehchange reactions: Propane aromatization in HZSM5 catalysts. J. Catal. 2016, 344, 252–262. [Google Scholar] [CrossRef]
- Framework Type MFI. Database of Zeolite Structures. Structure Commission of the International Zeolite Association (IZA-SC). Available online: http://europe.iza-structure.org/IZA-SC/framework.php?STC=MFI (accessed on 1 July 2007).
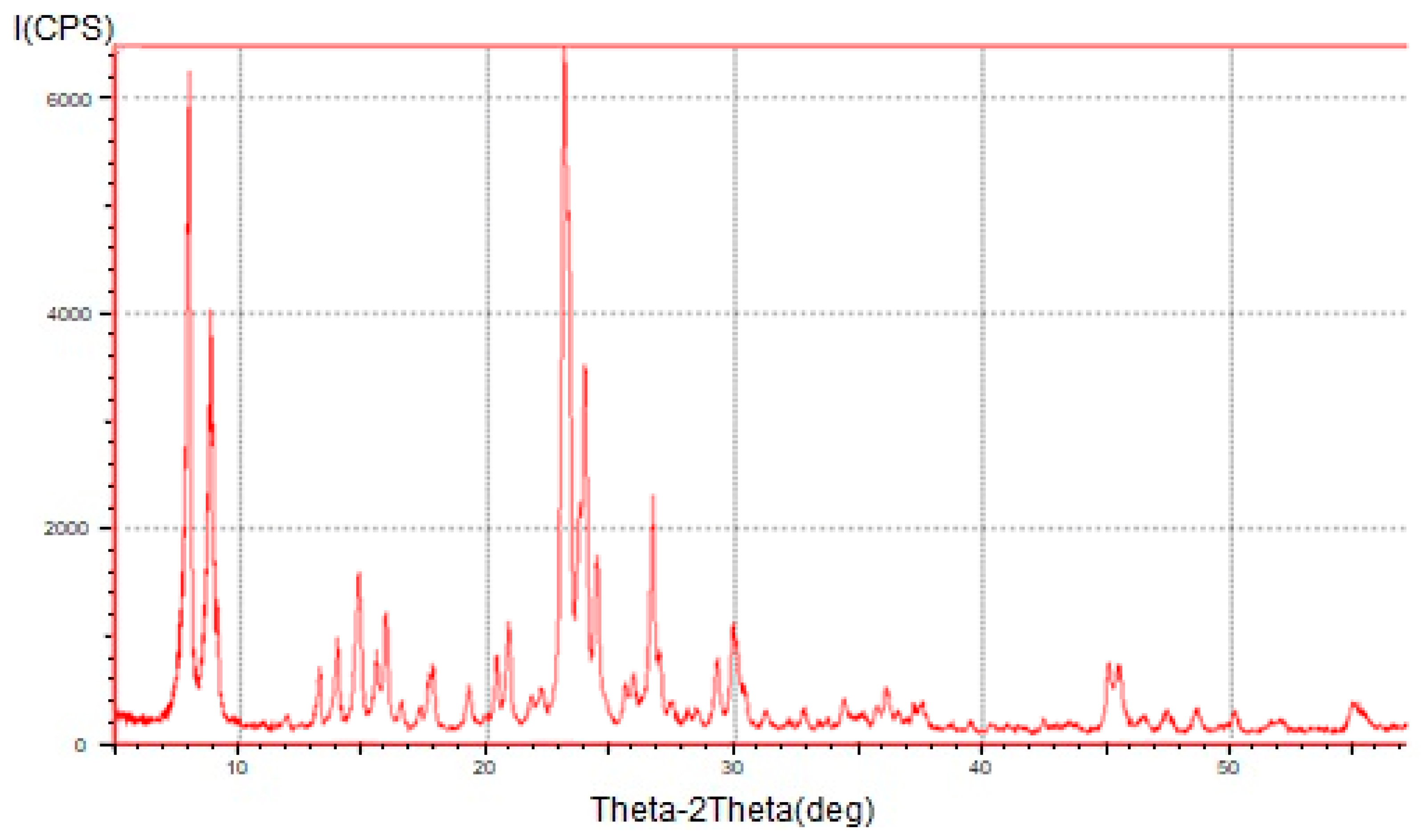
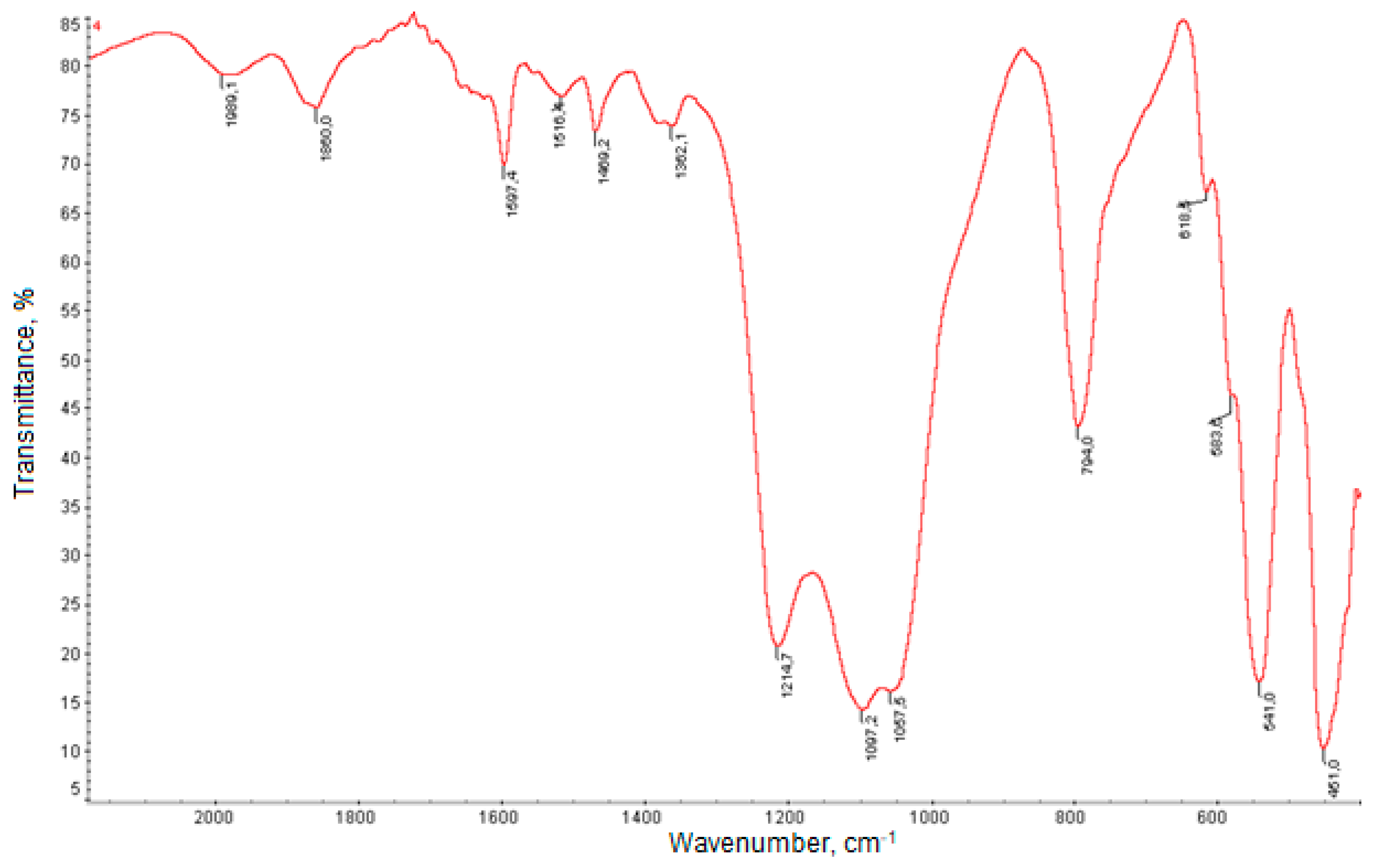

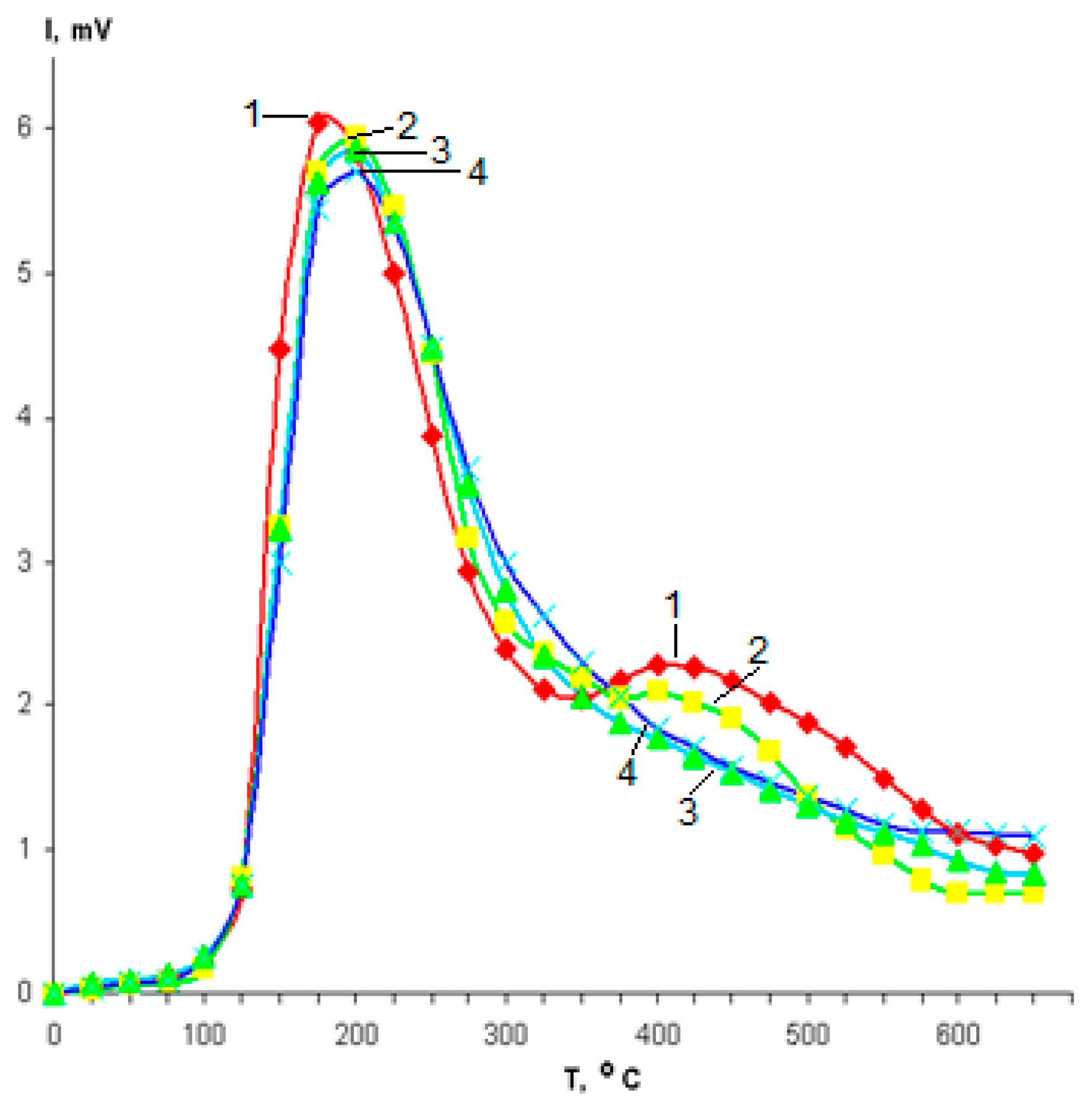
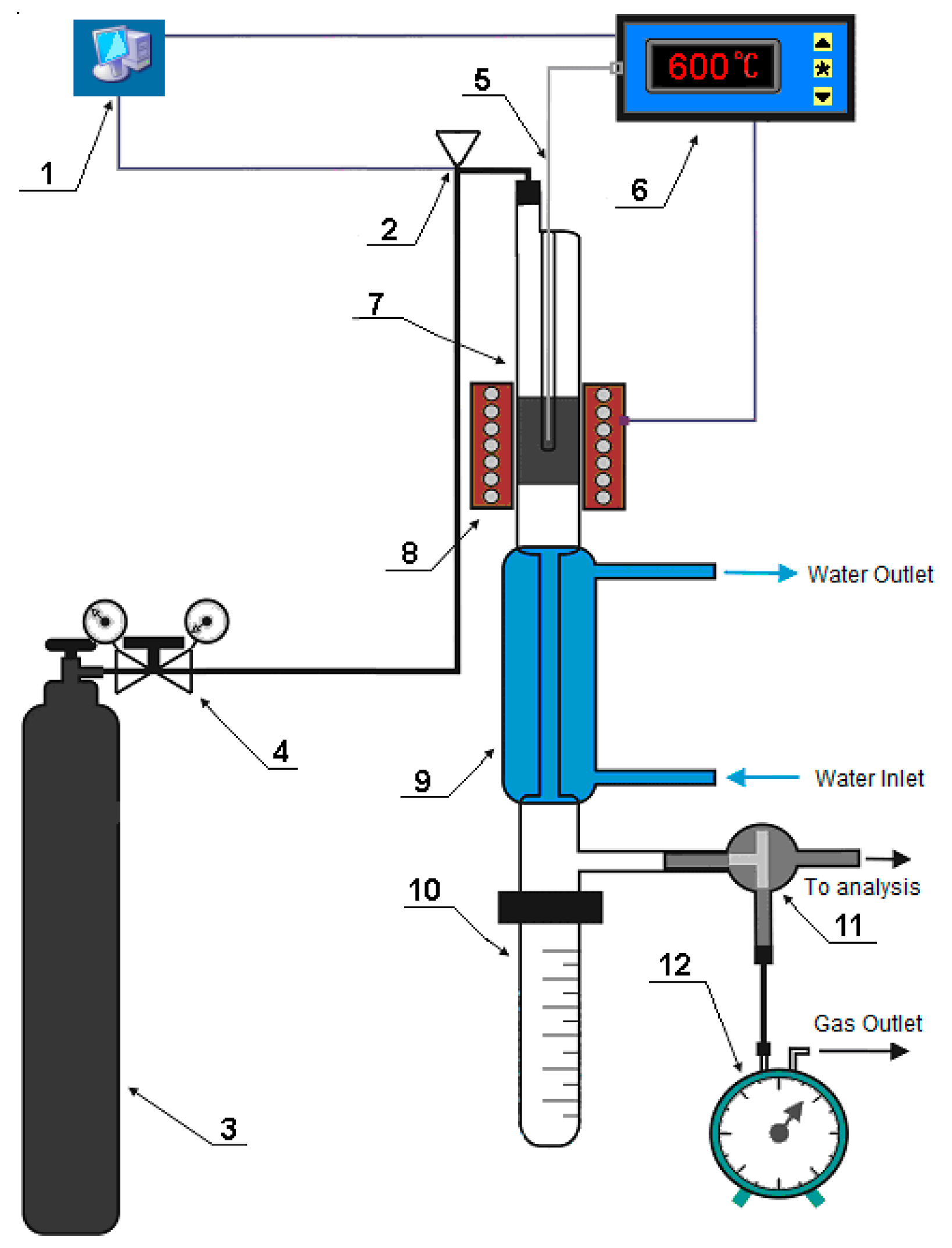
| Catalyst | H-ZKE-AF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modifier | Initial H-ZKE-AF | 1% ZnO | 3% ZnO | 5% ZnO | ||||||||
| Temperature, °C | 550 | 575 | 600 | 550 | 575 | 600 | 550 | 575 | 600 | 550 | 575 | 600 |
| Conversion, % | 82.3 | 86.2 | 88.8 | 79.5 | 84.9 | 84.9 | 76.0 | 83.4 | 85.2 | 80.4 | 81.1 | 77.1 |
| Yield of gas products, % | 53.9 | 51.9 | 48.6 | 48.1 | 45.3 | 43.2 | 49.3 | 46.7 | 45.3 | 51.2 | 50.8 | 50.1 |
| Yield of liquid products, % | 46.1 | 48.1 | 51.4 | 51.9 | 54.7 | 56.8 | 50.7 | 53.3 | 54.7 | 48.8 | 49.2 | 49.9 |
| Composition of gas products, normalized by wt. % | ||||||||||||
| Methane | 34.4 | 38.5 | 40.9 | 31.2 | 33.5 | 36.5 | 18.7 | 23.5 | 23.9 | 20.9 | 20.8 | 16.5 |
| Ethane | 24.1 | 23.5 | 22.0 | 20.3 | 21.7 | 23.0 | 27.6 | 34.7 | 33.6 | 34.1 | 31.8 | 22.5 |
| Ethylene | 3.7 | 5.6 | 7.9 | 3.2 | 4.2 | 5.7 | 1.6 | 1.8 | 2.7 | 1.3 | 2.1 | 3.4 |
| Propane | 31.6 | 25.9 | 22.4 | 41.6 | 34.6 | 32.7 | 47.5 | 34.9 | 32.0 | 37.5 | 36.5 | 42.9 |
| Propylene | 2.9 | 3.8 | 4.7 | 2.0 | 2.4 | 3.5 | 2.6 | 3.8 | 6.4 | 4.2 | 6.5 | 11.1 |
| Isobutane | 1.0 | 0.7 | 0.3 | 0.5 | 0.2 | 0.1 | 0.4 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 |
| Butane | 1.4 | 1.0 | 0.6 | 1.0 | 0.5 | 0.5 | 1.3 | 0.7 | 0.7 | 1.2 | 1.2 | 1.8 |
| Composition of liquid products, normalized by wt. % | ||||||||||||
| Benzene | 13.5 | 14.5 | 16.4 | 17.8 | 20.7 | 21.0 | 18.6 | 21.0 | 21.6 | 18.8 | 20.7 | 19.2 |
| Toluene | 36.9 | 37.6 | 39.8 | 40.9 | 40.4 | 37.8 | 42.2 | 41.5 | 39.0 | 40.0 | 40.0 | 38.9 |
| Ethylbenzene | 2.4 | 2.2 | 2.3 | 2.2 | 1.9 | 1.6 | 1.2 | 0.9 | 0.9 | 1.0 | 1.1 | 1.6 |
| m-Xylene | 11.3 | 10.8 | 10.5 | 10.6 | 8.8 | 7.4 | 10.9 | 8.9 | 7.8 | 8.9 | 8.1 | 7.4 |
| p-Xylene | 5.1 | 4.8 | 4.7 | 4.8 | 4.1 | 3.4 | 5.2 | 4.4 | 4.2 | 5.7 | 5.7 | 6.0 |
| o-Xylene | 5.3 | 5.1 | 5.0 | 4.9 | 4.2 | 3.5 | 4.7 | 3.8 | 3.2 | 3.5 | 3.2 | 3.1 |
| Pseudocumene | 1.2 | 1.1 | 1.0 | 1.0 | 0.7 | 0.6 | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Naphthalene | 8.5 | 7.8 | 7.7 | 7.0 | 8.3 | 9.5 | 8.0 | 9.8 | 10.1 | 11.1 | 9.4 | 7.5 |
| α-methylnaphthalene | 2.1 | 1.9 | 1.7 | 1.6 | 1.7 | 2.2 | 1.1 | 1.0 | 1.1 | 0.4 | 0.3 | 0.6 |
| β-methylnaphthalene | 5.4 | 4.7 | 4.2 | 4.0 | 4.3 | 5.9 | 4.1 | 4.7 | 6.2 | 6.3 | 5.7 | 5.7 |
| Catalyst | H-ZKE-AF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modifier | Initial H-ZKE-AF | 1% ZnS | 3% ZnS | 5% ZnS | ||||||||
| Temperature, °C | 550 | 575 | 600 | 550 | 575 | 600 | 550 | 575 | 600 | 550 | 575 | 600 |
| Conversion, % | 82.3 | 86.2 | 88.8 | 76.6 | 85.5 | 87.6 | 77.3 | 82.1 | 87.0 | 73.3 | 81.8 | 89.9 |
| Yield of gas products, % | 53.8 | 51.2 | 48.6 | 54.7 | 44.8 | 40.2 | 49.5 | 44.9 | 39.4 | 48.8 | 43.9 | 39.5 |
| Yield of liquid products, % | 46.1 | 48.8 | 51.4 | 45.3 | 55.2 | 59.8 | 50.5 | 55.1 | 60.6 | 51.2 | 56.1 | 60.5 |
| The composition of gas products, normalized by wt. % | ||||||||||||
| Methane | 34.4 | 38.5 | 40.9 | 34.6 | 44.0 | 44.8 | 33.8 | 36.9 | 36.9 | 24.2 | 27.2 | 31.8 |
| Ethane | 24.1 | 23.5 | 22.0 | 17.0 | 17.0 | 16.1 | 14.4 | 16.4 | 21.9 | 15.0 | 24.7 | 34.6 |
| Ethylene | 3.7 | 5.6 | 7.9 | 2.4 | 4.0 | 5.7 | 3.3 | 3.9 | 4.4 | 3.4 | 3.2 | 3.5 |
| Propane | 31.6 | 25.9 | 22.4 | 41.4 | 31.8 | 30.4 | 45.0 | 39.4 | 32.6 | 53.8 | 40.9 | 25.3 |
| Propylene | 2.9 | 3.8 | 4.7 | 1.8 | 2.1 | 2.4 | 1.9 | 2.3 | 3.5 | 2.0 | 3.0 | 4.2 |
| Isobutane | 1.0 | 0.7 | 0.3 | 0.8 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 |
| Butane | 1.4 | 1.0 | 0.6 | 1.4 | 0.5 | 0.3 | 0.9 | 0.5 | 0.3 | 0.9 | 0,5 | 0.2 |
| The composition of liquid products, normalized by wt. % | ||||||||||||
| Benzene | 13.5 | 14.5 | 16.4 | 15.8 | 20.6 | 23.9 | 18.3 | 20.9 | 23.7 | 18.7 | 21.8 | 23.5 |
| Toluene | 36.9 | 37.6 | 39.8 | 39.6 | 40.8 | 38.0 | 41.4 | 40.0 | 39.8 | 41.0 | 40.9 | 39.0 |
| Ethylbenzene | 2.4 | 2.2 | 2.3 | 2.5 | 2.3 | 2.0 | 2.4 | 1.9 | 1.2 | 2.1 | 1.3 | 0.8 |
| m-Xylene | 11.3 | 10.8 | 10.5 | 11.7 | 9.7 | 7.3 | 10.5 | 8.5 | 7.3 | 10.4 | 8.6 | 7.3 |
| p-Xylene | 5.1 | 4.8 | 4.7 | 5.1 | 4.3 | 3.3 | 4.7 | 4.1 | 3.3 | 4.6 | 3.8 | 3.3 |
| o-Xylene | 5.3 | 5.1 | 5.0 | 5.4 | 4.5 | 3.5 | 5.0 | 4.3 | 3.5 | 4.9 | 4.1 | 3.6 |
| Pseudocumene | 1.2 | 1.1 | 1.0 | 1.3 | 1.0 | 0.7 | 1.1 | 0.7 | 0.4 | 1.0 | 0.5 | 0.3 |
| Naphthalene | 8.5 | 7.8 | 7.7 | 5.6 | 6.2 | 8.0 | 6.1 | 8.2 | 8.7 | 6.1 | 7.9 | 10.2 |
| α-methylnaphthalene | 5.4 | 4.7 | 4.2 | 3.9 | 3.5 | 5.6 | 3.4 | 4.1 | 4.2 | 3.6 | 3.9 | 4.7 |
| β- methylnaphthalene | 2.1 | 1.9 | 1.7 | 1.6 | 1.5 | 2.0 | 1.5 | 1.8 | 1.8 | 1.6 | 1.7 | 1.9 |
| Catalyst | Tpeak, °C | Concentration, μmol/g | |||
|---|---|---|---|---|---|
| TI | TII | CI | CII | CΣ | |
| H-ZKE-AF | 180 | 435 | 607 | 213 | 820 |
| 1% ZnO | 195 | 420 | 561 | 171 | 732 |
| 3% ZnO | 185 | 425 | 613 | 130 | 743 |
| 5% ZnO | 190 | 420 | 638 | 129 | 767 |
| 1% ZnS | 190 | 415 | 593 | 202 | 795 |
| 3% ZnS | 180 | 435 | 586 | 132 | 718 |
| 5% ZnS | 185 | 420 | 571 | 110 | 681 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erofeev, V.I.; Khasanov, V.V.; Dzhalilova, S.N.; Reschetilowski, W.P.; Syskina, A.A.; Bogdankova, L.A. Acidic and Catalytic Properties of Zeolites Modified by Zinc in the Conversion Process of Lower C3–C4 Alkanes. Catalysts 2019, 9, 421. https://doi.org/10.3390/catal9050421
Erofeev VI, Khasanov VV, Dzhalilova SN, Reschetilowski WP, Syskina AA, Bogdankova LA. Acidic and Catalytic Properties of Zeolites Modified by Zinc in the Conversion Process of Lower C3–C4 Alkanes. Catalysts. 2019; 9(5):421. https://doi.org/10.3390/catal9050421
Chicago/Turabian StyleErofeev, Vladimir I., Vyacheslav V. Khasanov, Sofia N. Dzhalilova, Wladimir P. Reschetilowski, Anna A. Syskina, and Lyubov A. Bogdankova. 2019. "Acidic and Catalytic Properties of Zeolites Modified by Zinc in the Conversion Process of Lower C3–C4 Alkanes" Catalysts 9, no. 5: 421. https://doi.org/10.3390/catal9050421
APA StyleErofeev, V. I., Khasanov, V. V., Dzhalilova, S. N., Reschetilowski, W. P., Syskina, A. A., & Bogdankova, L. A. (2019). Acidic and Catalytic Properties of Zeolites Modified by Zinc in the Conversion Process of Lower C3–C4 Alkanes. Catalysts, 9(5), 421. https://doi.org/10.3390/catal9050421






