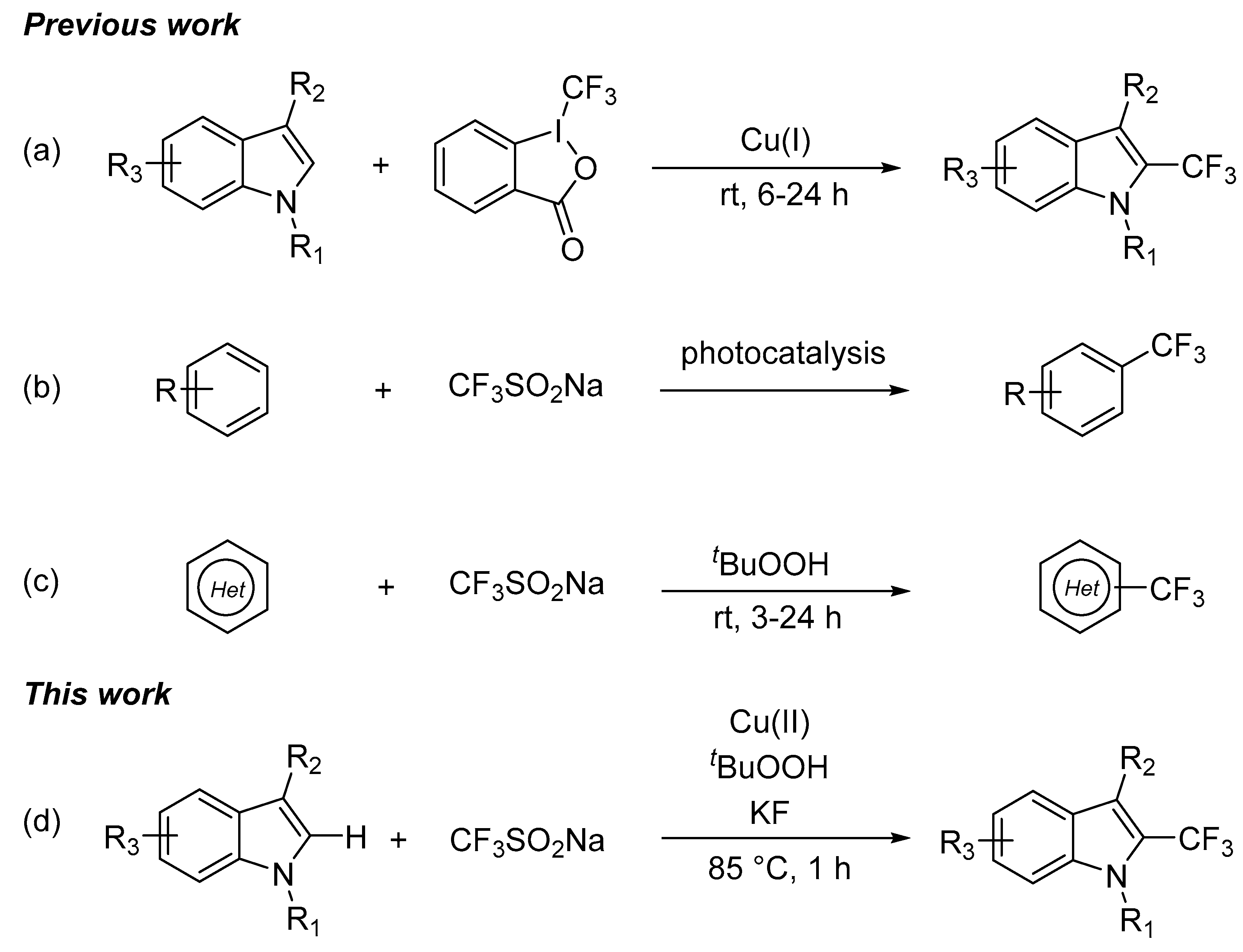
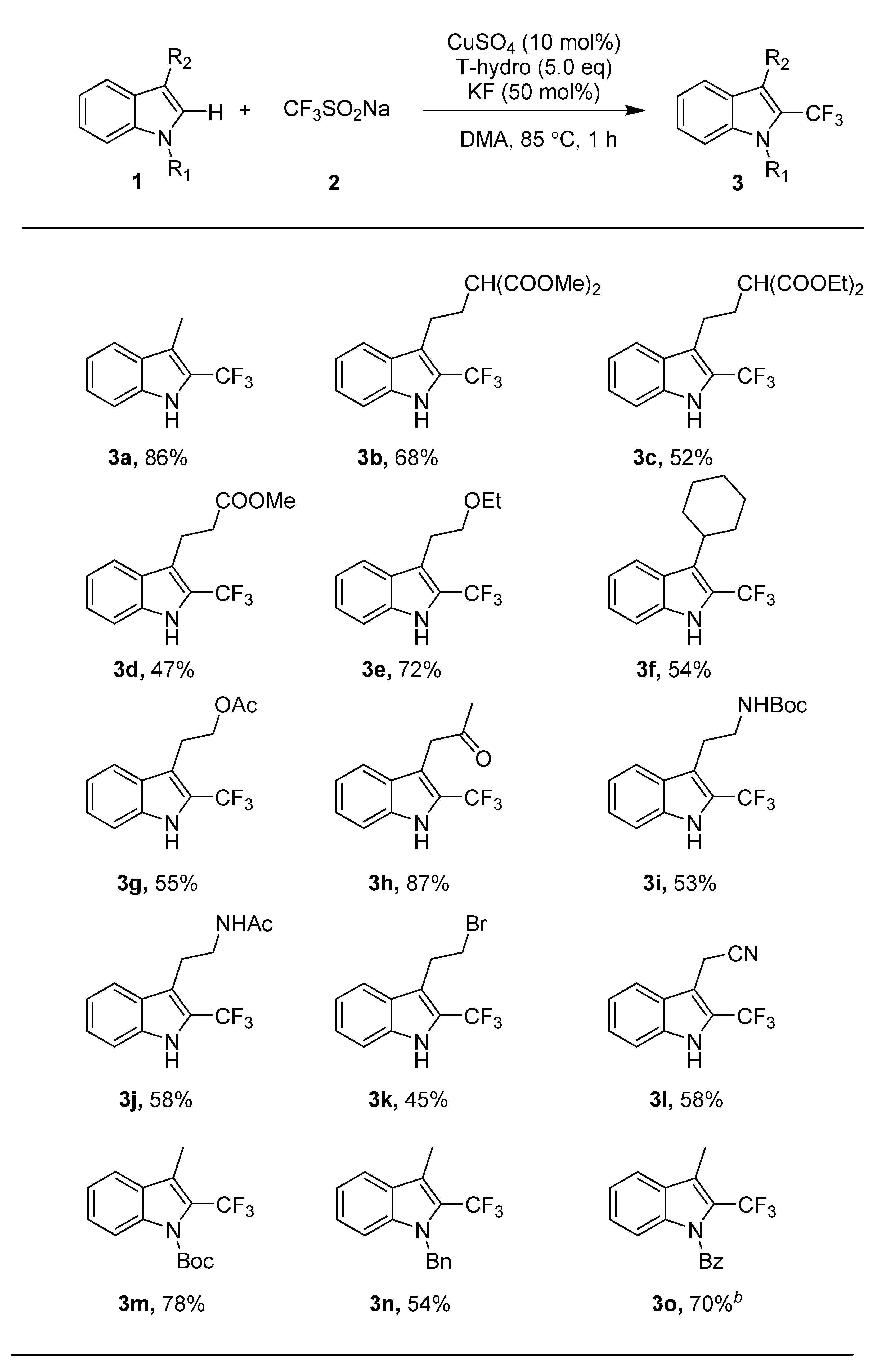
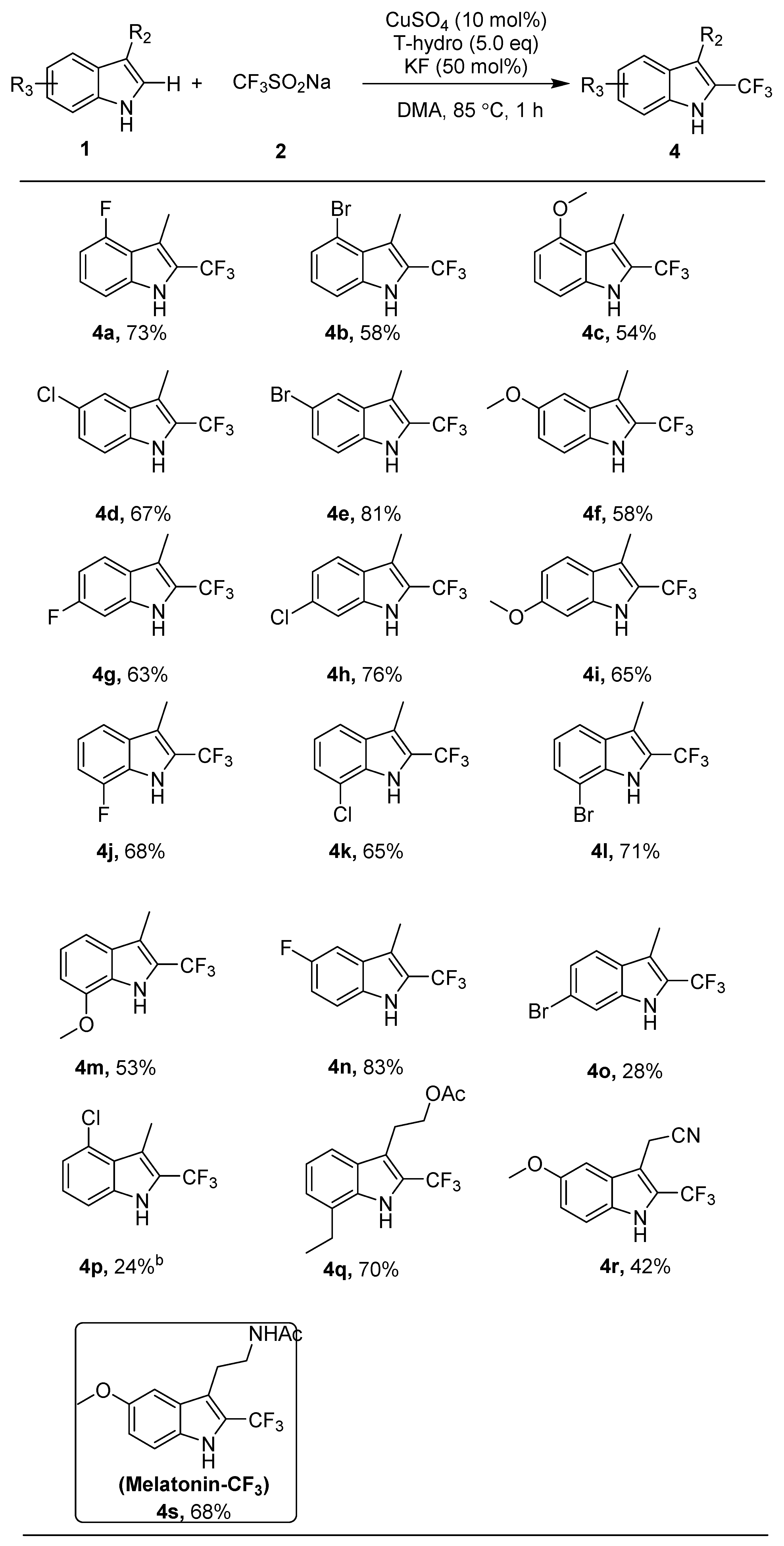
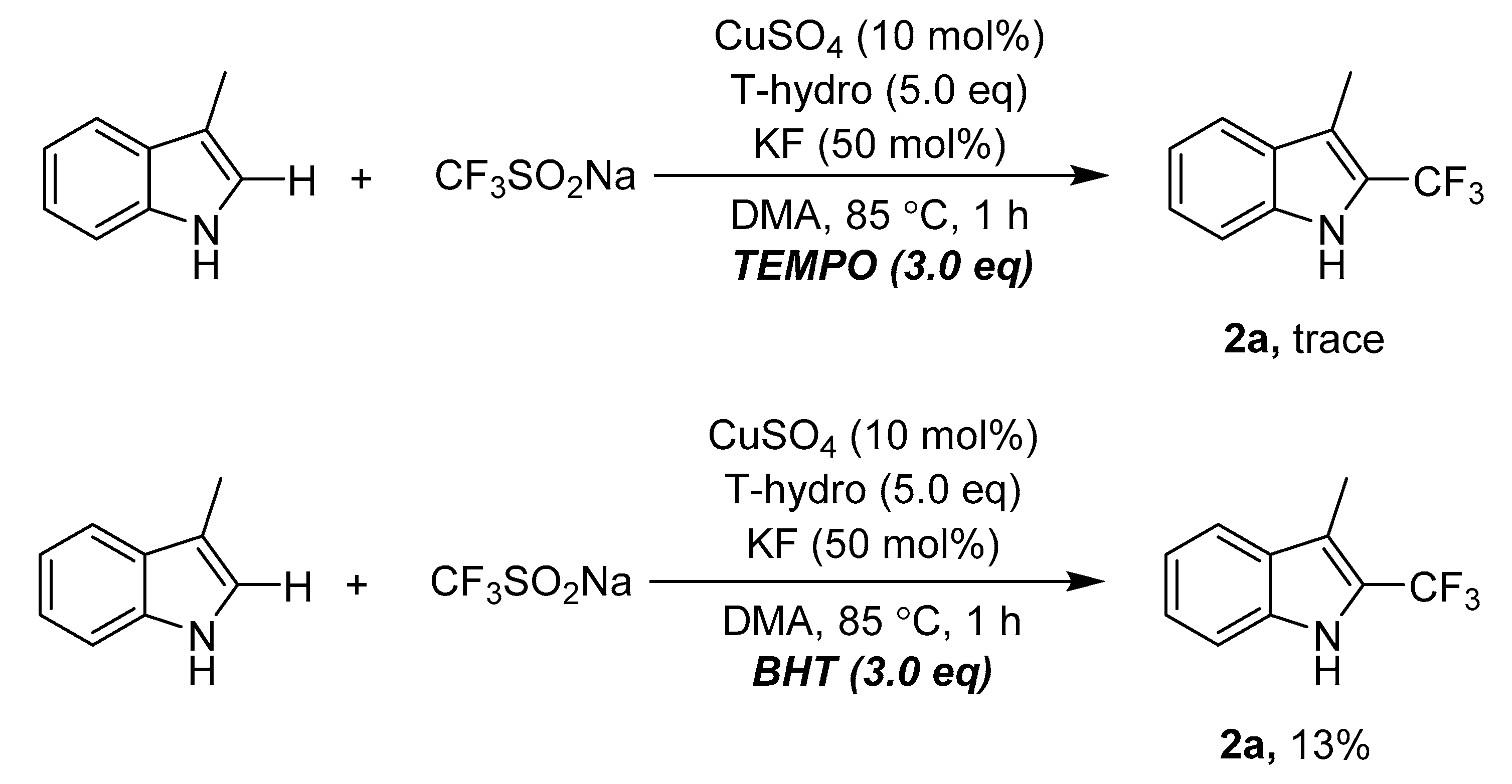
3. Materials and Methods
1H NMR spectra were recorded on Bruker 500 MHz spectrometer and the chemical shifts were reported in parts per million (
δ) relative to internal standard TMS (0 ppm) for CDCl
3. The peak patterns are indicated as follows: s, singlet; d, doublet; dd, doublet of doublet; t, triplet; m, multiplet; q, quartet. The coupling constants,
J, are reported in Hertz (Hz).
13C NMR spectra were obtained at Bruker 125 MHz and referenced to the internal solvent signals (central peak is 77.0 ppm in CDCl
3). The NMR yield was determined by
1H NMR using CH
2Br
2 as an internal standard. APEX II (Bruker Inc.) was used for ESI-HRMS.
19F NMR spectra were recorded on Bruker 470 MHz spectrometer (see
Supplementary Materials). IR spectra were recorded by a Nicolet 5MX-S infrared spectrometer. Flash column chromatography was performed over silica gel 200–300. All reagents were weighed and handled in air at room temperature. All chemical reagents were purchased from Alfa, Acros, Aldrich, and TCI, J&K and used without further purification.
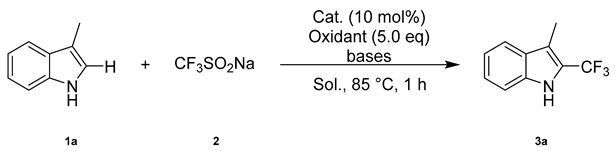
A dry Schlenk tube was charged with 1 (0.5 mmol), 2 (1.5 mmol), CuSO4 (12.5 mg, 10 mol%) and KF (14.7 mg, 50 mol%). DMA (dimethylacetamide, 3.0 mL) was added under argon, and the mixture was stirred at room temperature. tert-Butyl hydroperoxide (tBuOOH, 70% solution in H2O, 2.5 mmol) was dropped into the mixture under argon at room temperature. The resulting mixture was stirred at 85 °C for 1 h. Once the mixture was cooled to room temperature, the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography on silica gel (ethyl acetate/petroleum ether) to give product 3 or 4.
3-Methyl-2-(trifluoromethyl)-1H-indole (3a) (86 mg, 86%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 3391, 2921, 2803, 1452, 1257, 1077, 1030, 754, 715 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.20 (s, 1H), 7.65 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.35 (t, J = 7.5 Hz, 1H), 7.20 (t, 1H), 2.45 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 135.2, 128.0, 124.7, 124.3 (q, JC-F = 262.5 Hz), 121.6 (q, J2 = 37.5 Hz), 120.4, 120.1, 114.0 (q, J3 = 3.0 Hz) 111.5, 8.3; 19F NMR (470 MHz, CDCl3) δ −58.6 (d, J = 1.1 Hz); HRMS (ESI) calcd. for C10H7NF3 [M − H]−, 198.0536; found: 198.0538.
Dimethyl 2-(2-(2-(trifluoromethyl)-1H-indol-3-yl)ethyl)malonate (3b). (117 mg, 68%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:40, Rf = 0.3); IR (neat): νmax 3394, 2923, 2843, 1724, 1260, 1111, 1078, 908, 730 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.45 (s, 1H), 7.70 (d, J = 8.1 Hz, 1H), 7.40 (d, J = 8.3 Hz, 1H), 7.30 (t, J = 7.60 Hz, 1H), 7.20 (t, 1H), 3.75 (s, 6H), 3.45 (t, J = 7.3 Hz, 1H), 2.30–2.95 (m, 2H), 2.35–2.25 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 169.6, 135.3, 127.1, 124.8, 122.0 (q, JC-F = 267.3 Hz), 121.7 (q, J2 = 36.8 Hz), 120.6, 120.1, 116.7 (q, J3 = 3.0 Hz), 111.8, 52.5, 51.1, 29.6, 21.5; 19F NMR (470 MHz, CDCl3) δ −58.3 (s); HRMS (ESI) calcd. for C16H15O4NF3 [M − H]−, 342.0959; found: 342.0956; HRMS (ESI) calcd. for C16H15O4NF3 [M − H]−, 342.0959; found: 342.0956.
Diethyl 2-(2-(2-(trifluoromethyl)-1H-indol-3-yl)ethyl)malonate (3c). (97 mg, 52%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:40, Rf = 0.3); IR (neat): νmax 3371, 2993, 2863, 1721, 1260, 1161, 1118, 908, 732 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.40 (s, 1H), 7.70 (d, J = 8.1 Hz, 1H), 7.40 (d, J = 8.3 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.20 (t, 1H), 4.30–4.15 (m, 4H), 3.45 (t, J = 7.3 Hz, 1H), 3.00–2.90 (m, 2H), 2.35–2.20 (m, 2H), 1.30 (t, J = 7.1 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 169.28, 135.27, 127.16, 124.78, 121.9 (q, JC-F = 267.4 Hz), 121.6 (q, J2 = 36.8 Hz), 120.57, 120.17, 116.9 (q, J3 = 2.6 Hz), 111.73, 61.47, 51.57, 29.60, 21.57, 13.98; 19F NMR (470 MHz, CDCl3) δ −58.3 (s); HRMS (ESI) calcd. for C18H19O4NF3 [M − H]−, 370.1272; found: 370.1280.
Methyl 3-(2-(trifluoromethyl)-1H-indol-3-yl)propanoate (3d). (61 mg, 47%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 3386, 2943, 2822, 1324, 1261, 1106, 1076, 747, 725 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.65 (s, 1H), 7.65 (d, J = 8.1 Hz, 1H), 7.35–7.25 (m, 2H), 7.25–7.20 (m, 1H), 3.95 (s, 2H), 3.70 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.2, 135.1, 127.2, 124.9, 123.8 (q, JC-F = 267.4 Hz), 122.8 (q, J2 = 36.9 Hz), 121.0, 120.0, 111.8, 110.2 (q, J3 = 2.8 Hz), 52.2, 29.6; 19F NMR (470 MHz, CDCl3) δ −58.6 (s); HRMS (ESI) calcd. for C12H9O2NF3 [M − H]−, 256.0591; found: 256.0588.
Ethyl 3-(2-(trifluoromethyl)-1H-indol-3-yl)propanoate (3e). (93 mg, 72%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:40, Rf = 0.3); IR (neat): νmax 3321, 2823, 1323, 1146, 1097, 1052, 741, 726 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.45 (s, 1H), 7.70 (d, J = 8.1 Hz, 1H), 7.40 (d, J = 8.3 Hz, 1H), 7.35 (t, J = 7.4 Hz, 1H), 7.20 (t, 1H), 3.70 (t, J = 7.7 Hz, 2H), 3.55 (q, J = 7.0 Hz, 2H), 3.25–3.15 (m, 2H), 1.20 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 135.2, 127.6, 124.7, 122.1 (q, J2 = 36.5 Hz), 122.0 (q, JC-F = 267.4 Hz), 120.5, 120.3, 114.9 (q, J3 = 3.0 Hz), 111.7, 70.6, 66.2, 24.6, 15.1; 19F NMR (470 MHz, CDCl3) δ −58.3 (s); HRMS (ESI) calcd. for C12H9O2NF3 [M − H]−, 256.0955; found: 256.0954.
3-Cyclohexyl-2-(trifluoromethyl)-1H-indole (3f). (72 mg, 54%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:40, Rf = 0.3); IR (neat): νmax 3387, 2922, 2823, 1318, 1249, 1115, 1082, 740, 705 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.20 (s, 1H), 7.90 (d, J = 8.2 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.15 (t, J = 7.6 Hz, 1H), 3.00 (t, J = 12.1 Hz, 1H), 2.00–1.80 (m, 8H), 1.50–1.40 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 135.4, 126.3, 124.3, 123.9 (q, J3 = 3.0 Hz), 122.2, 122.1 (q, JC-F = 267.3 Hz), 120.5 (q, J2 = 36.0 Hz), 120.0, 111.9, 36.2, 32.8, 27.0, 26.2; 19F NMR (470 MHz, CDCl3) δ −57.5 (s); HRMS (ESI) calcd. for C15H15NF3 [M − H]−, 266.1162; found: 266.1163.
2-(2-(Trifluoromethyl)-1H-indol-3-yl)ethyl acetate (3g). (75 mg, 55%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:40, Rf = 0.3); IR (neat): νmax 3361, 2954, 1719, 1160, 1086, 1037, 907, 731 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 7.70 (d, J = 8.1 Hz, 1H), 7.40 (d, J = 8.3, 0.6 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.20 (t, 1H), 4.30 (t, J = 6.9 Hz, 2H), 3.25 (t, 2H), 2.00 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.2, 135.2, 127.3, 124.8, 122.4 (q, J2 = 36.5 Hz), 121.9 (q, JC-F = 267.4 Hz), 120.7, 113.8 (q, J3 = 2.9 Hz), 111.8, 64.1, 23.4, 20.9; 19F NMR (470 MHz, CDCl3) δ −58.3 (s); HRMS (ESI) calcd. for C13H11O2NF3 [M − H]−, 270.0747; found: 270.0745.
1-(2-(Trifluoromethyl)-1H-indol-3-yl)propan-2-one (3h). (105 mg, 87%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:10, Rf = 0.3); IR (neat): νmax 3389, 2933, 2823, 1703, 1253, 1109, 1074, 740, 725 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.83 (s, 1H), 7.57 (d, J = 8.1 Hz, 1H), 7.36 (d, J = 8.2 Hz, 1H), 7.34–7.29 (m, 1H), 7.20–7.15 (m, 1H), 3.98 (s, 2H), 2.19 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 205.9, 135.3, 127.2, 126.0, 125.1, 124.0 (q, JC-F = 267.5 Hz), 123.1 (q, J3 = 3.4 Hz), 122.7 (q, J2 = 36.8 Hz), 121.1, 120.0, 112.0, 39.3, 28.9; 19F NMR (470 MHz, CDCl3) δ −58.3 (s); HRMS (ESI) calcd. for C12H9ONF3 [M − H]−, 240.0642; found: 240.0639.
Tert-butyl (2-(2-(trifluoromethyl)-1H-indol-3-yl)ethyl)carbamate (3i). (87 mg, 53%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:5, Rf = 0.3); IR (neat): νmax 3351, 2961, 2853, 1688, 1250, 1111, 1083, 732 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.85 (s, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.30 (t, J = 7.3 Hz, 1H), 7.15 (d, J = 6.6 Hz, 1H), 4.65 (s, 1H), 3.45 (s, 2H), 3.10 (s, 2H), 1.45 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 156.0, 135.4, 127.4, 124.7, 122.3 (q, J2 = 36.4 Hz), 122.0 (q, JC-F = 267.5 Hz), 120.5, 120.2, 115.0 (q, J3 = 3.0 Hz), 111.8, 79.3, 41.1, 28.3, 24.4; 19F NMR (470 MHz, CDCl3) δ −57.9 (s); HRMS (ESI) calcd. for C16H18O2N2F3 [M − H]−, 327.1326; found: 327.1322.
N-(2-(2-(trifluoromethyl)-1H-indol-3-yl)ethyl)acetamide (3j). (78 mg, 58%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:1, Rf = 0.3); IR (neat): νmax 3309, 2923, 2813, 1051, 1024, 1005, 820, 757 cm−1; 1H NMR (500 MHz, DMSO) δ 11.90 (s, 1H), 8.00 (t, J = 5.8 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.30 (t, J = 7.5 Hz, 1H), 5.75 (s, 1H), 3.30–3.25 (m, 2H), 3.00 (t, J = 7.1 Hz, 2H), 1.75 (s, 3H); 13C NMR (125 MHz, DMSO) δ 169.2, 135.8, 126.9, 124.4, 122.4 (q, JC-F = 267.4 Hz), 121.2 (q, J2 = 36.0 Hz), 120.0, 117.9, 114.6 (q, J3 = 2.8 Hz), 112.4, 24.0, 22.7, 22.6; 19F NMR (470 MHz, DMSO) δ −56.5 (s); HRMS (ESI) calcd. for C13H14ON2F3 [M + H]+, 271.1053; found: 271.1057.
3-(2-Bromoethyl)-2-(trifluoromethyl)-1H-indole (3k). (66 mg, 45%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 3388, 2932, 2860, 1313, 1251, 1109, 1068, 741, 727 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.35 (s, 1H), 7.70 (d, J = 8.1 Hz, 1H), 7.40 (d, J = 8.3 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.25–7.20 (m, 1H), 3.60–3.55 (m, 2H), 3.50–3.45 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 135.1, 126.9, 125.1, 122.2 (q, J2 = 36.9 Hz), 121.7 (q, JC-F = 267.5 Hz), 120.9, 119.9, 115.3 (q, J3 = 2.8 Hz), 111.8, 31.3, 27.7; 19F NMR (470 MHz, CDCl3) δ −58.3 (s); HRMS (ESI) calcd. for C11H8NBrF3 [M − H]−, 289.9798; found: 289.9792.
2-(2-(Trifluoromethyl)-1H-indol-3-yl)acetonitrile (3l). (65 mg, 58%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:10, Rf = 0.3); IR (neat): νmax 3396, 2995, 2873, 1328, 1161, 1133, 1073, 740, 618 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.80 (s, 1H), 7.75 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.40 (t, J = 7.6 Hz, 1H), 7.30–7.25 (m, 1H), 4.00 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 135.0, 125.9, 125.6, 122.8 (q, J2 = 37.5 Hz), 121.7, 121.2 (q, JC-F = 267.6 Hz),119.4, 116.8, 112.2, 105.5 (q, J3 = 2.5 Hz), 12.7; 19F NMR (470 MHz, CDCl3) δ −58.5 (s); HRMS (ESI) calcd. for C11H6N2F3 [M − H]−, 223.0489; found: 223.0489.
Tert-butyl 3-methyl-2-(trifluoromethyl)-1H-indole-1-carboxylate (3m). (117 mg, 78%). Isolated by flash column chromatography (petroleum ether, Rf = 0.3); IR (neat): νmax 2921, 2873, 1738, 1324, 1126, 1087, 743, 731 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.20 (d, J = 8.5 Hz, 1H), 7.60 (d, J = 7.9 Hz, 1H), 7.45–7.40 (m, 1H), 7.35–7.30 (m, 1H), 2.45 (q, J = 2.9 Hz, 3H), 1.65 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 149.0, 136.8, 128.6, 127.2, 123.0, 122.2 (q, J2 = 36.9 Hz), 121.9 (q, JC-F = 267.9 Hz), 119.9, 115.4, 111.5 (q, J3 = 3.0 Hz), 85.0, 29.7, 27.8; 19F NMR (470 MHz, CDCl3) δ −54.1 (d, J = 2.9 Hz); HRMS (ESI) calcd. for C15H16O2NF3K [M + K]+, 338.0770; found: 338.0773.
1-Benzyl-3-methyl-2-(trifluoromethyl)-1H-indole (3n). (78 mg, 54%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 2911, 1428, 1270, 1163, 1098, 1046, 737, 695 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.70 (d, J = 7.9 Hz, 1H), 7.30–7.25 (m, 3H), 7.25–7.20 (m, 3H), 7.00 (d, J = 7.2 Hz, 2H), 5.45 (s, 2H), 2.55–2.50 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 137.6, 137.4, 128.8, 128.6, 127.3, 125.8, 125.5, 124.9, 122.7 (q, JC-F = 268.3 Hz),122.7 (q, J2 = 34.9 Hz), 120.3, 114.9 (q, J3 = 2.9 Hz), 114.9, 110.5, 48.1, 29.7; 19F NMR (470 MHz, CDCl3) δ −55.1 (d, J = 1.6 Hz); HRMS (ESI) calcd. for C17H15NF3 [M + H]+, 290.1078; found: 290.1070.
(3-Methyl-2-(trifluoromethyl)-1H-indol-1-yl)(phenyl)methanone (3o). (106 mg, 70%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 2925, 2886, 1708, 1322, 1125, 1020, 711, 665 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.90–7.85 (m, 2H), 7.70 (t, J = 7.5 Hz, 1H), 7.65 (d, J = 7.9 Hz, 1H), 7.55 (t, J = 7.8 Hz, 2H), 7.25–7.20 (m, 1H), 7.15 (t, J = 7.7 Hz, 1H), 6.75 (d, J = 8.5 Hz, 1H), 2.55–2.50 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 168.5, 136.8, 134.1, 133.9, 130.3, 129.0, 128.8, 126.2, 124.3 (q, J2 = 37.0 Hz), 123.8 (q, JC-F = 271.1 Hz), 122.9 (q, J3 = 2.8 Hz), 122.7, 120.3, 113.7, 29.7; 19F NMR (470 MHz, CDCl3) δ −54.4 (d, J = 2.1 Hz); HRMS (ESI) calcd. for C17H11ONF3 [M − H]−, 302.0798; found: 302.0793.
4-Fluoro-3-methyl-2-(trifluoromethyl)-1H-indole (4a). (79 mg, 73%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 2204, 1165, 1118, 1054, 1027, 909, 731 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.25 (s, 1H), 7.20–7.15 (m, 1H), 7.15 (d, J = 8.2 Hz, 1H), 6.80 (dd, J = 11.0, 7.9 Hz, 1H), 2.55 (d, J = 1.2 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 159.2, 157.2, 137.5 (d, J = 10.9 Hz), 125.3 (d, J = 8.0 Hz), 121.7 (q, JC-F = 267.3 Hz), 121.6 (q, J2 = 36.5 Hz), 113.0 (q, J3 = 3.0 Hz), 107.6 (d, J = 4.0 Hz), 105.5 (d, J = 19.1 Hz), 9.9 (d, J = 2.9 Hz); 19F NMR (470 MHz, CDCl3) δ −58.8 (d, J = 1.3 Hz), -123.3 (dd, J = 11.1, 4.7 Hz); HRMS (ESI) calcd. for C10H6NF4 [M − H]−, 216.0442; found: 216.0443.
N-benzyl-N-methyl-3-phenylprop-2-yn-1-amine (4b). (81 mg, 58%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 1253, 1181, 1162, 1120, 1027, 908, 734 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.25 (s, 1H), 7.35–7.30 (m, 2H), 7.10 (t, J = 7.9 Hz, 1H), 2.70 (dd, J = 3.3, 1.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 136.2, 125.9, 125.3, 125.1, 122.9 (q, J2 = 36.1 Hz), 121.8 (q, JC-F = 267.5 Hz),115.9, 115.2 (q, J3 = 2.9 Hz), 111.0, 10.7; 19F NMR (470 MHz, CDCl3) δ −58.6 (d, J = 0.9 Hz); HRMS (ESI) calcd. for C10H6NBrF3 [M − H]−, 275.9641; found: 275.9644.
4-Methoxy-3-methyl-2-(trifluoromethyl)-1H-indole (4c). (62 mg, 54%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 3402, 2927, 2813, 1311, 1251, 1165, 1120 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.10 (s, 1H), 7.20 (t, J = 8.0 Hz, 1H), 6.95 (d, J = 8.2 Hz, 1H), 6.50 (d, J = 7.8 Hz, 1H), 3.90 (s, 3H), 2.60 (dd, J = 3.6, 1.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 156.1, 136.7, 125.5, 121.1 (q, JC-F = 266.8 Hz), 120.1 (q, J2 = 36.4 Hz), 118.1, 114.9 (q, J3 = 3.1 Hz), 104.5, 100.1, 55.1, 10.5; 19F NMR (470 MHz, CDCl3) δ −58.4 (d, J = 1.3 Hz); HRMS (ESI) calcd. for C11H9ONF3 [M − H]−, 228.0642; found: 228.0641.
5-Chloro-3-methyl-2-(trifluoromethyl)-1H-indole (4d). (78 mg, 67%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 3396, 2995, 2874, 1445, 1251, 1094, 1025, 797, 720, 592 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.25 (s, 1H), 7.60 (d, J = 1.2 Hz, 1H), 7.30–7.25 (m, 2H), 2.40 (dd, J = 3.4, 1.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 133.43, 129.06, 126.12, 125.17, 122.8 (q, J2 = 36.9 Hz), 121.7 (q, JC-F = 267.4 Hz), 119.57, 113.6 (q, J3 = 2.9 Hz), 112.69, 8.2; 19F NMR (470 MHz, CDCl3) δ −58.9 (d, J = 1.3 Hz); HRMS (ESI) calcd. for C10H6NClF3 [M − H]−, 232.0146; found: 232.0148.
5-Bromo-3-methyl-2-(trifluoromethyl)-1H-indole (4e). (113 mg, 81%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 3520, 2988, 2873, 1317, 1105, 1047, 1023, 793, 718 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.25 (s, 1H), 7.75 (s, 1H), 7.40 (dd, J = 8.7, 1.4 Hz, 1H), 7.25 (d, J = 8.7 Hz, 1H), 2.40 (d, J = 1.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 133.7, 129.7, 127.7, 122.8, 122.3 (q, J2 = 36.8 Hz), 121.7 (q, JC-F = 272.9 Hz), 113.6, 113.5 (q, J3 = 3.1 Hz), 113.1, 8.2; 19F NMR (470 MHz, CDCl3) δ −58.9 (d, J = 1.3 Hz); HRMS (ESI) calcd. for C10H6NBrF3 [M − H]−, 275.9641; found: 275.9645.
5-Methoxy-3-methyl-2-(trifluoromethyl)-1H-indole (4f). (66 mg, 58%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 3367, 2911, 2823, 1469, 1165, 1067, 1018, 840, 798, 725, 701, 624 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.10 (s, 1H), 7.30–7.25 (m, 1H), 7.00 (s, 1H), 7.00 (d, J = 8.8 Hz, 1H), 3.90 (d, J = 0.9 Hz, 3H), 2.40 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 154.5, 130.3, 128.4, 122.1 (q, J2 = 36.8 Hz), 122.0 (q, JC-F = 267.1 Hz), 115.6, 113.5 (q, J3 = 3.1 Hz), 112.5, 100.9, 55.8, 8.4; 19F NMR (470 MHz, CDCl3) δ −58.7 (d, J = 1.3 Hz); HRMS (ESI) calcd. for C11H9ONF3 [M − H]−, 228.0642; found: 228.0640.
6-Fluoro-3-methyl-2-(trifluoromethyl)-1H-indole (4g). (68 mg, 63%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 1318, 1258, 1118, 1027, 734 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.20 (s, 1H), 7.55 (dd, J = 8.7, 5.3 Hz, 1H), 7.05 (dd, J = 9.3, 2.1 Hz, 1H), 7.00–6.90 (m, 1H), 2.40 (dd, J = 3.6, 1.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 161.4 (d, J = 239.8 Hz), 135.2 (d, J = 13.3 Hz), 124.7, 121.9 (q, JC-F = 266.9 Hz), 121.8 (q, J2 = 36.6 Hz), 121.2 (d, J = 10.4 Hz), 114.3 (q, J3 = 2.8 Hz), 109.6 (d, J = 24.9 Hz), 97.7 (d, J = 26.4 Hz), 8.3; 19F NMR (470 MHz, CDCl3) δ −58.8 (d, J = 1.0 Hz), -117.1 (td, J = 9.4, 5.3 Hz); HRMS (ESI) calcd. for C10H6NF4 [M − H]−, 216.0442; found: 216.0441.
6-Chloro-3-methyl-2-(trifluoromethyl)-1H-indole (4h). (89 mg, 76%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 2223, 1052, 1025, 1007, 751, 726 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.15 (s, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.35 (d, J = 1.5 Hz, 1H), 7.15 (dd, J = 8.5, 1.8 Hz, 1H), 2.40 (dd, J = 3.6, 1.8 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 135.4, 130.7, 126.6, 121.8 (q, JC-F = 267.1 Hz), 122.1 (q, J2 = 36.8 Hz), 121.3, 121.1, 114.2 (q, J3 = 3.0 Hz), 111.4, 8.2; 19F NMR (470 MHz, CDCl3) δ −58.9 (d, J = 1.3 Hz); HRMS (ESI) calcd. for C10H6NClF3 [M − H]−, 232.0146; found: 232.0146.
6-Methoxy-3-methyl-2-(trifluoromethyl)-1H-indole (4i). (74 mg, 65%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 2343, 1053, 1025, 1006, 819, 757, 727 cm−1; 1H NMR (500 MHz, DMSO) δ 11.60 (s, 1H), 7.50 (d, J = 8.7 Hz, 1H), 6.85 (s, 1H), 6.75 (dd, J = 8.8, 1.9 Hz, 1H), 3.80 (s, 3H), 2.35 (s, 3H); 13C NMR (125 MHz, DMSO) δ 157.7, 136.7, 122.7 (q, JC-F = 266.5 Hz), 121.6, 120.8, 119.2 (q, J2 = 35.8 Hz), 112.7 (q, J3 = 3.1 Hz), 110.9, 94.1, 55.3, 8.3; 19F NMR (470 MHz, DMSO) δ −56.4 (d, J = 1.5 Hz); HRMS (ESI) calcd. for C11H9ONF3 [M − H]−, 228.0642; found: 228.0641.
7-Fluoro-3-methyl-2-(trifluoromethyl)-1H-indole (4j). (74 mg, 68%). Isolated by flash column chromatography (petroleum ether, Rf = 0.3); IR (neat): νmax 2287, 1052, 1026, 1007, 908, 723 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.35 (s, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.15–7.05 (m, 1H), 7.05 (dd, J = 10.8, 7.8 Hz, 1H), 2.45 (dd, J = 3.5, 1.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 149.5 (d, J = 244.4 Hz), 131.6 (d, J = 4.9 Hz), 123.9 (q, JC-F = 267.5 Hz), 122.4 (q, J2 = 36.6 Hz), 120.7 (d, J = 5.9 Hz), 115.8 (d, J = 3.6 Hz), 114.7 (q, J3 = 2.8 Hz), 109.3 (d, J = 15.6 Hz), 107.8 (d, J = 16.3 Hz), 8.5; 19F NMR (470 MHz, CDCl3) δ −59.0 (d, J = 1.0 Hz), -134.7 (dd, J = 11.0, 4.7 Hz); HRMS (ESI) calcd. for C10H6NF4 [M − H]−, 216.0442; found: 216.0442.
7-Chloro-3-methyl-2-(trifluoromethyl)-1H-indole (4k). (76 mg, 65%). Isolated by flash column chromatography (petroleum ether, Rf = 0.3); IR (neat): νmax 1313, 1164, 1119, 1027, 734 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.35 (s, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.15 (t, J = 7.8 Hz, 1H), 2.45 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 132.6, 129.3, 124.0, 122.3 (q, J2 = 37.0 Hz), 121.7 (q, JC-F = 267.4 Hz), 121.2, 118.7, 117.0, 115.1 (q, J3 = 2.9 Hz), 8.5; 19F NMR (470 MHz, CDCl3) δ −58.9 (d, J = 1.2 Hz); HRMS (ESI) calcd. for C10H6NClF3 [M − H]−, 232.0146; found: 232.0146.
7-Bromo-3-methyl-2-(trifluoromethyl)-1H-indole (4l). (99 mg, 71%). Isolated by flash column chromatography (petroleum ether, Rf = 0.3); IR (neat): νmax 3471, 2931, 2813, 1580, 1328, 1115, 908, 779, 731 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.30 (s, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 7.6 Hz, 1H), 7.10 (t, J = 7.8 Hz, 1H), 2.45 (dd, J = 3.4, 1.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 134.0, 129.0, 127.0, 122.2 (q, J2 = 36.8 Hz), 121.7 (q, JC-F = 267.3 Hz), 121.5, 119.3, 115.2 (q, J3 = 3.0 Hz), 105.0, 8.6; 19F NMR (470 MHz, CDCl3) δ −58.8 (d, J = 1.1 Hz); HRMS (ESI) calcd. for C10H6NBrF3 [M − H]−, 275.9641; found: 275.9643.
7-Methoxy-3-methyl-2-(trifluoromethyl)-1H-indole (4m). (61 mg, 53%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 3411, 2918, 2806, 1265, 1161, 1109, 733 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.40 (s, 1H), 7.25 (d, J = 8.1 Hz, 1H), 7.10 (t, J = 7.9 Hz, 1H), 6.75 (d, J = 7.7 Hz, 1H), 3.95 (s, 3H), 2.45–2.40 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 146.3, 129.3, 126.1, 122.1 (q, JC-F = 267.0 Hz), 121.3 (q, J2 = 36.8 Hz), 120.8, 114.3 (q, J3 = 2.9 Hz), 112.4, 103.9, 55.4, 8.5; 19F NMR (470 MHz, CDCl3) δ −58.7 (s); HRMS (ESI) calcd. for C11H9ONF3 [M − H]−, 228.0642; found: 228.0646.
5-Fluoro-3-methyl-2-(trifluoromethyl)-1H-indole (4n). (90 mg, 83%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 3393, 2921, 2863, 1325, 1169, 1109, 1026, 852, 796, 728 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.20 (s, 1H), 7.31 (dd, J = 8.9, 4.2 Hz, 1H), 7.27 (dd, J = 8.9, 2.5 Hz, 1H), 7.08 (td, J = 9.0, 2.4 Hz, 1H), 2.41–2.38 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 159.0, 157.1, 131.6, 128.5, 128.4, 123.2 (q, J2 = 36.8 Hz), 121.8 (q, JC-F = 267.4 Hz), 114.0 (q, J3 = 3.0 Hz), 113.7, 113.5, 112.6, 112.5, 104.9, 104.7, 8.3; 19F NMR (470 MHz, CDCl3) δ −58.96 (d, J = 1.4 Hz), -123.0 (td, J = 9.1, 4.2 Hz); HRMS (ESI) calcd. for C10H6NF4 [M − H]−, 216.0442; found: 216.0442.
6-Bromo-3-methyl-2-(trifluoromethyl)-1H-indole (4o). (39 mg, 28%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:100, Rf = 0.3); IR (neat): νmax 3390, 2901, 2862, 1325, 1172, 1109, 1022, 842, 728 cm−1; 1H NMR (500 MHz, CDCl3) 1H NMR (500 MHz, cdcl3) δ 8.18 (s, 1H), 7.53 (d, J = 1.4 Hz, 1H), 7.49 (d, J = 8.5 Hz, 1H), 7.29 (dd, J = 8.5, 1.6 Hz, 1H), 2.41 (s, 3H); 13C NMR (125 MHz, CDCl3) 13C NMR (126 MHz, cdcl3) δ 135.77, 126.94, 123.89, 122.0 (q, J2 = 36.9 Hz), 121.8 (q, JC-F = 267.3 Hz) 121.39, 118.39, 114.45, 114.2 (q, J3 = 2.9 Hz), 8.24; 19F NMR (470 MHz, CDCl3) δ −58.96 (d, J = 1.4 Hz), -123.0 (td, J = 9.1, 4.2 Hz); HRMS (ESI) calcd. for C10H6NF4 [M − H]−, 275.9641; found: 275.964.
2-(7-Ethyl-2-(trifluoromethyl)-1H-indol-3-yl)ethyl acetate (4q). (105 mg, 70%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:50, Rf = 0.3); IR (neat): νmax 3397, 2998, 2883, 1723, 1253, 1163, 1117, 908, 732 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.30 (s, 1H), 7.60–7.55 (m, 1H), 7.20–7.15 (m, 2H), 4.30 (dd, J = 9.1, 4.8 Hz, 2H), 3.25 (td, J = 6.9, 1.1 Hz, 2H), 2.90 (q, J = 7.6 Hz, 2H), 2.00 (s, 3H), 1.40 (t, J = 7.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.2, 134.3, 129.8, 127.2, 123.2, 122.1 (q, J2 = 36.6 Hz), 122.0 (q, JC-F = 267.3 Hz), 121.1, 117.7, 114.4 (q, J3 = 2.8 Hz), 65.6, 64.1, 23.6, 20.9, 13.6; 19F NMR (470 MHz, CDCl3) δ −58.1 (s); HRMS (ESI) calcd. for C15H15O2NF3 [M − H]−, 298.1060; found: 298.1055.
2-(5-Methoxy-2-(trifluoromethyl)-1H-indol-3-yl)acetonitrile (4r). (54 mg, 42%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:10, Rf = 0.3); IR (neat): νmax 3389, 1052, 1024, 1005, 820, 757, 624, 558 cm−1; 1H NMR (500 MHz, DMSO) δ 12.30 (s, 1H), 7.40 (d, J = 8.9 Hz, 1H), 7.35 (s, 1H), 7.00 (d, J = 8.9 Hz, 1H), 4.25 (s, 2H), 3.80 (s, 3H); 13C NMR (125 MHz, DMSO) δ 154.6, 130.7, 126.2, 122.2 (q, J2 = 36.8 Hz), 121.8 (q, JC-F = 267.5 Hz), 118.5, 116.3, 113.7, 105.4 (q, J3 = 2.3 Hz), 100.4, 55.6, 12.0; 19F NMR (470 MHz, DMSO) δ −56.9 (s); HRMS (ESI) calcd. for C12H8ON2F3 [M − H]−, 253.0594; found: 253.0591.
N-(2-(5-methoxy-2-(trifluoromethyl)-1H-indol-3-yl)ethyl)acetamide (4s) [24]. (102 mg, 68%). Isolated by flash column chromatography (ethyl acetate/petroleum ether = 1:10, R
f = 0.3);
1H NMR (500 MHz, DMSO) δ 12.30 (s, 1H), 7.40 (d,
J = 8.9 Hz, 1H), 7.35 (s, 1H), 7.00 (d,
J = 8.9 Hz, 1H), 4.25 (s, 2H), 3.80 (s, 3H);
13C NMR (125 MHz, CDCl
3) δ 170.5, 154.7, 130.6, 127.7, 122.7 (q,
J2 = 36.4 Hz), 121.9 (q,
JC-F = 267.3 Hz), 116.0, 114.3 (q,
J3 = 2.8 Hz), 112.9, 100.5, 55.7, 40.0, 23.9, 23.2;
19F NMR (470 MHz, DMSO) δ −57.9 (s); HRMS (ESI) calcd. for C
14H
14O
2N
2F
3 [M − H]
−, 299.1013; found: 299.1009.