Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. TiO2 P25
2.2. g-C3N4
2.3. CdS
2.4. Photolysis
2.5. TOC Analysis
3. Experimental
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K.J. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.; Kuo, D.-H.; Chen, X. High efficient noble metal free Zn (O, S) nanoparticles for hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 5638–5648. [Google Scholar] [CrossRef]
- Agegnehu, A.K.; Pan, C.-J.; Tsai, M.-C.; Rick, J.; Su, W.-N.; Lee, J.-F.; Hwang, B.-J. Visible light responsive noble metal-free nanocomposite of V-doped TiO2 nanorod with highly reduced graphene oxide for enhanced solar H2 production. Int. J. Hydrogen Energy 2016, 41, 6752–6762. [Google Scholar] [CrossRef]
- Alharbi, A.; Alarifi, I.M.; Khan, W.S.; Asmatulu, R. Synthesis and analysis of electrospun SrTiO3 nanofibers with NiO nanoparticles shells as photocatalysts for water splitting. In Proceedings of the 14th Brazilian Polymer Conference, São Paulo, Brazil, 22–26 October 2017; 22-26. [Google Scholar]
- Al-Mayman, S.I.; Al-Johani, M.S.; Mohamed, M.M.; Al-Zeghayer, Y.S.; Ramay, S.M.; Al-Awadi, A.S.; Soliman, M.A. TiO2 ZnO photocatalysts synthesized by sol–gel auto-ignition technique for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 5016–5025. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.; Wang, L.; Ye, L. Bismuth-rich Bi4O5X2 (X = Br, and I) nanosheets with dominant {101} facets exposure for photocatalytic H2 evolution. Chem. Eng. J. 2016, 304, 454–460. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Gasparotto, A.; Maccato, C.; Warwick, M.E.; Toniato, E.; Gombac, V.; Sada, C.; Turner, S.; Van Tendeloo, G. Iron–Titanium Oxide Nanocomposites Functionalized with Gold Particles: From Design to Solar Hydrogen Production. Adv. Mater. Interfaces 2016, 3, 1600348. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Palmisano, L. Photocatalytic formation of H2 and value-added chemicals in aqueous glucose (Pt)-TiO2 suspension. Int. J. Hydrogen Energy 2016, 41, 5934–5947. [Google Scholar] [CrossRef]
- Beltram, A.; Romero-Ocana, I.; Jaen, J.J.D.; Montini, T.; Fornasiero, P. Photocatalytic valorization of ethanol and glycerol over TiO2 polymorphs for sustainable hydrogen production. Appl. Catal. A Gen. 2016, 518, 167–175. [Google Scholar] [CrossRef]
- Betzler, S.B.; Podjaski, F.; Beetz, M.; Handloser, K.; Wisnet, A.; Handloser, M.; Hartschuh, A.; Lotsch, B.V.; Scheu, C. Titanium Doping and Its Effect on the Morphology of Three-Dimensional Hierarchical Nb3O7 (OH) Nanostructures for Enhanced Light-Induced Water Splitting. Chem. Mater 2016, 28, 7666–7672. [Google Scholar] [CrossRef]
- Cargnello, M.; Montini, T.; Smolin, S.Y.; Priebe, J.B.; Jaén, J.J.D.; Doan-Nguyen, V.V.; McKay, I.S.; Schwalbe, J.A.; Pohl, M.-M.; Gordon, T.R. Engineering titania nanostructure to tune and improve its photocatalytic activity. Proc. Natl. Acad. Sci. 2016, 113, 3966–3971. [Google Scholar] [CrossRef]
- Cha, G.; Altomare, M.; Truong Nguyen, N.; Taccardi, N.; Lee, K.; Schmuki, P. Double-Side Co-Catalytic Activation of Anodic TiO2 Nanotube Membranes with Sputter-Coated Pt for Photocatalytic H2 Generation from Water/Methanol Mixtures. Chem. Asian J. 2017, 12, 314–323. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, D.; Zhang, N.; Ye, W.; Ju, H.; Shi, L.; Long, R.; Zhu, J.; Xiong, Y. Noble-Metal-Free Janus-like Structures by Cation Exchange for Z-Scheme Photocatalytic Water Splitting under Broadband Light Irradiation. Angew. Chem. 2017, 129, 4270–4274. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Q.; Hou, L.; Zhang, C.; Lu, Y.; Wang, X.; Long, J. Molecular p–n heterojunction-enhanced visible-light hydrogen evolution over a N-doped TiO2 photocatalyst. Catal. Sci. Technol. 2017, 7, 2039–2049. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Li, X.; Liu, S.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W.; Fang, W.; Liu, Y. Polymerizable complex synthesis of SrTiO3:(Cr/Ta) photocatalysts to improve photocatalytic water splitting activity under visible light. Appl. Catal. B Environ. 2016, 192, 145–151. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical Layered WS2/Graphene-Modified CdS Nanorods for Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2016, 9, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Y.; Wang, Q.; Wang, Z.; He, L.; Lei, Y.; Wang, Z. Oxygen-rich carbon-nitrogen quantum dots as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanofibers. Prog. Nat. Sci. Mater. Int. 2017, 37, 333–337. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Luciani, G.; Di Somma, I.; Andreozzi, R.; Marotta, R. In situ photodeposited nanoCu on TiO2 as a catalyst for hydrogen production under UV/visible radiation. Appl. Catal. A Gen. 2016, 518, 142–149. [Google Scholar] [CrossRef]
- Dhanalaxmi, K.; Yadav, R.; Kundu, S.K.; Reddy, B.M.; Amoli, V.; Sinha, A.K.; Mondal, J. MnFe2O4 Nanocrystals Wrapped in a Porous Organic Polymer: A Designed Architecture for Water-Splitting Photocatalysis. Chem. Eur. J. 2016, 22, 15639–15644. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ming, J.; Lu, D.; Wu, W.; Liu, M.; Zhao, X.; Li, C.; Yang, M.; Fang, P. Study of the enhanced visible-light-sensitive photocatalytic activity of Cr2O3-loaded titanate nanosheets for Cr (vi) degradation and H2 generation. Catal. Sci. Technol. 2017, 7, 2283–2297. [Google Scholar] [CrossRef]
- Elbanna, O.; Kim, S.; Fujitsuka, M.; Majima, T. TiO2 mesocrystals composited with gold nanorods for highly efficient visible-NIR-photocatalytic hydrogen production. Nano Energy 2017, 35, 1–8. [Google Scholar] [CrossRef]
- Escobedo, S.; Serrano, B.; Calzada, A.; Moreira, J.; de Lasa, H. Hydrogen production using a platinum modified TiO2 photocatalyst and an organic scavenger. Kinetic modeling. Fuel 2016, 181, 438–449. [Google Scholar] [CrossRef]
- Feng, N.; Liu, F.; Huang, M.; Zheng, A.; Wang, Q.; Chen, T.; Cao, G.; Xu, J.; Fan, J.; Deng, F. Unravelling the Efficient Photocatalytic Activity of Boron-induced Ti3+ Species in the Surface Layer of TiO2. Sci. Rep. 2016, 6, 34765. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Bellardita, M.; D’Urso, L.; Compagnini, G.; Palmisano, L.; Scirè, S. Au/TiO2-CeO2 Catalysts for Photocatalytic Water Splitting and VOCs Oxidation Reactions. Catalysts 2016, 6, 121. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Conesa, J.C.; Fernández-García, M.; Kubacka, A. UV and visible hydrogen photo-production using Pt promoted Nb-doped TiO2 photo-catalysts: Interpreting quantum efficiency. Appl. Catal. B Environ. 2017, 216, 133–145. [Google Scholar] [CrossRef]
- Fornari, A.M.D.; de Araujo, M.B.; Duarte, C.B.; Machado, G.; Teixeira, S.R.; Weibel, D.E. Photocatalytic reforming of aqueous formaldehyde with hydrogen generation over TiO2 nanotubes loaded with Pt or Au nanoparticles. Int. J. Hydrogen Energy 2016, 41, 11599–11607. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.-Q. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Guerrero-Araque, D.; Acevedo-Peña, P.; Ramírez-Ortega, D.; Calderon, H.A.; Gómez, R. Charge transfer processes involved in photocatalytic hydrogen production over CuO/ZrO2–TiO2 materials. Int. J. Hydrogen Energy 2017, 42, 9744–9753. [Google Scholar] [CrossRef]
- Gullapelli, S.; Scurrell, M.S.; Valluri, D.K. Photocatalytic H2 production from glycerol–water mixtures over Ni/γ-Al2O3 and TiO2 composite systems. Int. J. Hydrogen Energy 2017, 42, 15031–15043. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dash, S.; Tyagi, A. TiO2 modification by gold (Au) for photocatalytic hydrogen (H2) production. Renew. Sustain. Energy Rev. 2016, 58, 1366–1375. [Google Scholar] [CrossRef]
- Gusain, R.; Singhal, N.; Singh, R.; Kumar, U.; Khatri, O.P. Ionic-Liquid-Functionalized Copper Oxide Nanorods for Photocatalytic Splitting of Water. ChemPlusChem 2016, 81, 489–495. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ohta, H.; Nasu, H.; Ishihara, A. Preparation and photocatalytic activity of porous Bi2O3 polymorphisms. Int. J. Hydrogen Energy 2016, 41, 7388–7392. [Google Scholar] [CrossRef]
- He, G.-L.; Zhong, Y.-H.; Chen, M.-J.; Li, X.; Fang, Y.-P.; Xu, Y.-H. One-pot hydrothermal synthesis of SrTiO3-reduced graphene oxide composites with enhanced photocatalytic activity for hydrogen production. J. Mol. Catal. A Chem. 2016, 423, 70–76. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Wang, L.; Shi, J.; Chen, Y. Surface treatment effect on the photocatalytic hydrogen generation of CdS/ZnS core-shell microstructures. Chin. J. Catal. 2017, 38, 489–497. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Hernández-Gordillo, A.; Zanella, R.; Rodríguez-González, V. Renewable hydrogen harvest process by hydrazine as scavenging electron donor using gold TiO2 photocatalysts. Catal. Today 2016, 266, 2–8. [Google Scholar] [CrossRef]
- Hou, H.-J.; Zhang, X.-H.; Huang, D.-K.; Ding, X.; Wang, S.-Y.; Yang, X.-L.; Li, S.-Q.; Xiang, Y.-G.; Chen, H. Conjugated microporous poly (benzothiadiazole)/TiO2 heterojunction for visible-light-driven H2 production and pollutant removal. Appl. Catal. B Environ. 2017, 203, 563–571. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Zhang, P.; Liang, C.; Shao, G. Construction of solid-state Z-scheme carbon-modified TiO2/WO3 nanofibers with enhanced photocatalytic hydrogen production. J. Power Sources 2016, 328, 28–36. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, L.; Jian, X.; Zhou, W. Synthesis of mesoporous silica-embedded TiO2 loaded with Ag nanoparticles for photocatalytic hydrogen evolution from water splitting. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 67–75. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Palma, V. Enhanced photocatalytic hydrogen production from glucose aqueous matrices on Ru-doped LaFeO3. Appl. Catal. B Environ. 2017, 207, 182–194. [Google Scholar] [CrossRef]
- Jeong, S.; Chung, K.-H.; Lee, H.; Park, H.; Jeon, K.-J.; Park, Y.-K.; Jung, S.-C. Enhancement of Hydrogen Evolution from Water Photocatalysis Using Liquid Phase Plasma on Metal Oxide-Loaded Photocatalysts. ACS Sustain. Chem. Eng. 2017, 5, 3659–3666. [Google Scholar] [CrossRef]
- Jiang, C.; Lee, K.Y.; Parlett, C.M.; Bayazit, M.K.; Lau, C.C.; Ruan, Q.; Moniz, S.J.; Lee, A.F.; Tang, J. Size-controlled TiO2 nanoparticles on porous hosts for enhanced photocatalytic hydrogen production. Appl. Catal. A Gen. 2016, 521, 133–139. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, L.; Bi, J.; Liang, S.; Liu, M. Design and Synthesis of TiO2 Hollow Spheres with Spatially Separated Dual Cocatalysts for Efficient Photocatalytic Hydrogen Production. Nanomaterials 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Hart, J.N.; Boensch, D.; Scott, J.; Ng, Y.H.; Amal, R. Hydrogen evolution via glycerol photoreforming over Cu–Pt nanoalloys on TiO2. Appl. Catal. A Gen. 2016, 518, 221–230. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Scott, J.; Ng, Y.H.; Jiang, Y.; Amal, R. Exploring Cu oxidation state on TiO2 and its transformation during photocatalytic hydrogen evolution. Appl. Catal. A Gen. 2016, 521, 190–201. [Google Scholar] [CrossRef]
- Kang, H.W.; Park, S.B. Effects of Mo sources on Mo doped SrTiO3 powder prepared by spray pyrolysis for H2 evolution under visible light irradiation. Mater. Sci. Eng. B 2016, 211, 67–74. [Google Scholar] [CrossRef]
- Kang, H.W.; Park, S.B. Improved performance of tri-doped photocatalyst SrTiO3: Rh/Ta/F for H2 evolution under visible light irradiation. Int. J. Hydrogen Energy 2016, 41, 13970–13978. [Google Scholar] [CrossRef]
- Khan, M.; Sinatra, L.; Oufi, M.; Bakr, O.M.; Idriss, H. Evidence of plasmonic induced photocatalytic hydrogen production on Pd/TiO2 upon deposition on thin films of gold. Catal. Lett. 2017, 147, 811–820. [Google Scholar] [CrossRef]
- Khore, S.K.; Tellabati, N.V.; Apte, S.K.; Naik, S.D.; Ojha, P.; Kale, B.B.; Sonawane, R.S. Green sol–gel route for selective growth of 1D rutile N–TiO2: A highly active photocatalyst for H2 generation and environmental remediation under natural sunlight. RSC Adv. 2017, 7, 33029–33042. [Google Scholar] [CrossRef]
- Kim, Y.K.; Seo, H.-J.; Kim, S.; Hwang, S.-H.; Park, H.; Lim, S.K. Effect of ZnO Electrodeposited on Carbon Film and Decorated with Metal Nanoparticles for Solar Hydrogen Production. J. Mater. Sci. Technol. 2016, 32, 1059–1065. [Google Scholar] [CrossRef]
- Kumar, D.P.; Reddy, N.L.; Karthik, M.; Neppolian, B.; Madhavan, J.; Shankar, M. Solar light sensitized p-Ag2O/n-TiO2 nanotubes heterojunction photocatalysts for enhanced hydrogen production in aqueous-glycerol solution. Sol. Energy Mater. Sol. Cells 2016, 154, 78–87. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Lu, D.; Wu, W.; Ding, J.; Zhao, X.; Xiong, R.; Yang, M.; Wu, P.; Chen, F. Visible-light-driven water splitting from dyeing wastewater using Pt surface-dispersed TiO2-based nanosheets. J. Alloys Compd. 2017, 699, 183–192. [Google Scholar] [CrossRef]
- Li, F.; Wangyang, P.; Zada, A.; Humayun, M.; Wang, B.; Qu, Y. Synthesis of hierarchical Mn2O3 microspheres for photocatalytic hydrogen production. Mater. Res. Bull. 2016, 84, 99–104. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Li, W.; Li, X.; Tian, H.; Wei, X.; Ren, Z.; Han, G. Ultrathin Anatase TiO2 Nanosheets for High-Performance Photocatalytic Hydrogen Production. Small 2017, 13, 1604115. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Li, C. Spatial distribution of active sites on a ferroelectric PbTiO3 photocatalyst for photocatalytic hydrogen production. Faraday Discuss. 2017, 198, 463–472. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Liu, J.; Yu, X.; Li, Z. Facile synthesis of Ti3+ doped Ag/AgI TiO2 nanoparticles with efficient visible-light photocatalytic activity. Int. J. Hydrogen Energy 2017, 42, 13031–13038. [Google Scholar] [CrossRef]
- Liang, J.; Chai, Y.; Li, L. Facile fabrication of rod-shaped Zn2GeO4 nanocrystals as photocatalyst for hydrogen production. Cryst. Res. Technol. 2017, 52, 1700022. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Zheng, P.X.; Liu, Z.Q.; Lei, J.L.; Wu, H.; Li, N.; Ma, T.Y. Embedding Au quantum dots in rimous cadmium sulfide nanospheres for enhanced photocatalytic hydrogen evolution. Small 2016, 12, 6735–6744. [Google Scholar] [CrossRef]
- Peng, B.; Liu, X.; Li, R. Preparation of a Single-Walled Carbon Nanotube/Cd0.8Zn0.2S Nanocomposite and Its Enhanced Photocatalytic Hydrogen Production Activity. Eur. J. Inorg. Chem. 2016, 2016, 3204–3212. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, J.; Yang, Y.; Luo, S.; Zhang, S.; Li, Y.; Liang, S.; Wu, L. HNbxTa1-x WO6 monolayer nanosheets solid solutions: Tunable energy band structures and highly enhanced photocatalytic performances for hydrogen evolution. Appl. Catal. B Environ. 2017, 203, 798–806. [Google Scholar] [CrossRef]
- Lozano-Sánchez, L.; Méndez-Medrano, M.; Colbeau-Justin, C.; Rodríguez-López, J.; Hernández-Uresti, D.; Obregón, S. Long-lived photoinduced charge-carriers in Er3+ doped CaTiO3 for photocatalytic H2 production under UV irradiation. Catal. Commun. 2016, 84, 36–39. [Google Scholar] [CrossRef]
- Lucchetti, R.; Onotri, L.; Clarizia, L.; Di Natale, F.; Di Somma, I.; Andreozzi, R.; Marotta, R. Removal of nitrate and simultaneous hydrogen generation through photocatalytic reforming of glycerol over “in situ” prepared zero-valent nano copper/P25. Appl. Catal. B Environ. 2017, 202, 539–549. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.; Sánchez-Martínez, D.; Cruz, M.A. Cu2O precipitation-assisted with ultrasound and microwave radiation for photocatalytic hydrogen production. Int. J. Hydrogen Energy 2017, 42, 12997–13010. [Google Scholar] [CrossRef]
- Luna, A.L.; Novoseltceva, E.; Louarn, E.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Synergetic effect of Ni and Au nanoparticles synthesized on titania particles for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 191, 18–28. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Badshah, A.; Kanodarwala, F.K.; Ali, H.; Khan, M.A.; Stride, J.A.; Nadeem, M.A. Titania supported MOF-199 derived Cu–Cu2O nanoparticles: Highly efficient non-noble metal photocatalysts for hydrogen production from alcohol–water mixtures. Catal. Sci. Technol. 2017, 7, 677–686. [Google Scholar] [CrossRef]
- Manjunath, K.; Souza, V.; Ramakrishnappa, T.; Nagaraju, G.; Scholten, J.; Dupont, J. Heterojunction CuO-TiO2 nanocomposite synthesis for significant photocatalytic hydrogen production. Mater. Res. Express 2016, 3, 115904. [Google Scholar] [CrossRef]
- Manjunath, K.; Souza, V.S.; Ganganagappa, N.; Scholten, J.D.; Teixeira, S.R.; Dupont, J.; Thippeswamy, R. Effect of the magnetic core of (MnFe)2O3@Ta2O5 nanoparticles on photocatalytic hydrogen production. New J. Chem. 2017, 41, 326–334. [Google Scholar] [CrossRef]
- Mansingh, S.; Padhi, D.; Parida, K. Enhanced photocatalytic activity of nanostructured Fe doped CeO2 for hydrogen production under visible light irradiation. Int. J. Hydrogen Energy 2016, 41, 14133–14146. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Kozlova, E.A.; Cherepanova, S.V.; Saraev, A.A.; Gerasimov, E.Y.; Parmon, V.N. Synthesis of Pt/Zn(OH)2/Cd0.3Zn0.7S for the Photocatalytic Hydrogen Evolution from Aqueous Solutions of Organic and Inorganic Electron Donors Under Visible Light. Top. Catal. 2016, 59, 1297–1304. [Google Scholar] [CrossRef]
- Melián, E.P.; López, C.R.; Santiago, D.E.; Quesada-Cabrera, R.; Méndez, J.O.; Rodríguez, J.D.; Díaz, O.G. Study of the photocatalytic activity of Pt-modified commercial TiO2 for hydrogen production in the presence of common organic sacrificial agents. Appl. Catal. A Gen. 2016, 518, 189–197. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Rau, S.; Colbeau-Justin, C.; Rodríguez-López, J.; Remita, H. Surface Modification of TiO2 with Au Nanoclusters for Efficient Water Treatment and Hydrogen Generation under Visible Light. J. Phys. Chem. C 2016, 120, 25010–25022. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Michal, R.; Dworniczek, E.; Caplovicova, M.; Monfort, O.; Lianos, P.; Caplovic, L.; Plesch, G. Photocatalytic properties and selective antimicrobial activity of TiO2 (Eu)/CuO nanocomposite. Appl. Surf. Sci. 2016, 371, 538–546. [Google Scholar] [CrossRef]
- Miseki, Y.; Fujiyoshi, S.; Gunji, T.; Sayama, K. Photocatalytic Z-scheme water splitting for independent H2/O2 production via a stepwise operation employing a vanadate redox mediator under visible light. J. Phys. Chem. C 2017, 121, 9691–9697. [Google Scholar] [CrossRef]
- Moon, S.Y.; Naik, B.; Park, J.Y. Photocatalytic activity of metal-decorated SiO2@TiO2 hybrid photocatalysts under water splitting. Korean J. Chem. Eng. 2016, 33, 2325–2329. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Al-Oufi, M.; Wahab, A.K.; Anjum, D.; Idriss, H. Hydrogen Production on Ag-Pd/TiO2 Bimetallic Catalysts: Is there a Combined Effect of Surface Plasmon Resonance with Schottky Mechanism on the Photo-Catalytic Activity? ChemistrySelect 2017, 2, 2754–2762. [Google Scholar] [CrossRef]
- Narendranath, S.B.; Thekkeparambil, S.V.; George, L.; Thundiyil, S.; Devi, R.N. Photocatalytic H2 evolution from water–methanol mixtures on InGaO3(ZnO) m with an anisotropic layered structure modified with CuO and NiO cocatalysts. J. Mol. Catal. A Chem. 2016, 415, 82–88. [Google Scholar] [CrossRef]
- Niu, F.; Shen, S.; Guo, L. A noble-metal-free artificial photosynthesis system with TiO2 as electron relay for efficient photocatalytic hydrogen evolution. J. Catal. 2016, 344, 141–147. [Google Scholar] [CrossRef]
- Nsib, M.F.; Saafi, S.; Rayes, A.; Moussa, N.; Houas, A. Enhanced photocatalytic performance of Ni–ZnO/Polyaniline composite for the visible-light driven hydrogen generation. J. Energy Inst. 2016, 89, 694–703. [Google Scholar] [CrossRef]
- Obregón, S.; Lee, S.; Rodríguez-González, V. Loading effects of silver nanoparticles on hydrogen photoproduction using a Cu-TiO2 photocatalyst. Mater. Lett. 2016, 173, 174–177. [Google Scholar] [CrossRef]
- Pai, M.R.; Banerjee, A.M.; Rawool, S.A.; Singhal, A.; Nayak, C.; Ehrman, S.H.; Tripathi, A.K.; Bharadwaj, S.R. A comprehensive study on sunlight driven photocatalytic hydrogen generation using low cost nanocrystalline Cu-Ti oxides. Sol. Energy Mater. Sol. Cells 2016, 154, 104–120. [Google Scholar] [CrossRef]
- Patra, K.K.; Gopinath, C.S. Bimetallic and Plasmonic Ag–Au on TiO2 for Solar Water Splitting: An Active Nanocomposite for Entire Visible-Light-Region Absorption. ChemCatChem 2016, 8, 3294–3311. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Hernández-Gordillo, A.; Morales-Mendoza, G.; Lartundo-Rojas, L.; Mantilla, Á.; Gómez, R. Enhancing the H2 evolution from water–methanol solution using Mn2+–Mn+3–Mn4+ redox species of Mn-doped TiO2 sol–gel photocatalysts. Catal. Today 2016, 266, 9–16. [Google Scholar] [CrossRef]
- Polliotto, V.; Morra, S.; Livraghi, S.; Valetti, F.; Gilardi, G.; Giamello, E. Electron transfer and H2 evolution in hybrid systems based on [FeFe]-hydrogenase anchored on modified TiO2. Int. J. Hydrogen Energy 2016, 41, 10547–10556. [Google Scholar] [CrossRef]
- Preethi, L.; Antony, R.P.; Mathews, T.; Loo, S.; Wong, L.H.; Dash, S.; Tyagi, A. Nitrogen doped anatase-rutile heterostructured nanotubes for enhanced photocatalytic hydrogen production: Promising structure for sustainable fuel production. Int. J. Hydrogen Energy 2016, 41, 5865–5877. [Google Scholar] [CrossRef]
- Priebe, J.B.; Radnik, J.; Kreyenschulte, C.; Lennox, A.J.; Junge, H.; Beller, M.; Brückner, A. H2 Generation with (Mixed) Plasmonic Cu/Au-TiO2 Photocatalysts: Structure–Reactivity Relationships Assessed by in situ Spectroscopy. ChemCatChem 2017, 9, 1025–1031. [Google Scholar] [CrossRef]
- Qiu, Y.; Ouyang, F. Fabrication of TiO2 hierarchical architecture assembled by nanowires with anatase/TiO2 (B) phase-junctions for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2017, 403, 691–698. [Google Scholar] [CrossRef]
- Qiu, Y.; Ouyang, F.; Zhu, R. A facile nonaqueous route for preparing mixed-phase TiO2 with high activity in photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2017, 42, 11364–11371. [Google Scholar] [CrossRef]
- Ramasami, A.K.; Ravishankar, T.N.; Nagaraju, G.; Ramakrishnappa, T.; Teixeira, S.R.; Balakrishna, R.G. Gel-combustion-synthesized ZnO nanoparticles for visible light-assisted photocatalytic hydrogen generation. Bull. Mater. Sci. 2017, 40, 345–354. [Google Scholar] [CrossRef]
- Rather, R.A.; Singh, S.; Pal, B. Visible and direct sunlight induced H2 production from water by plasmonic Ag-TiO2 nanorods hybrid interface. Sol. Energy Mater. Sol. Cells 2017, 160, 463–469. [Google Scholar] [CrossRef]
- Rather, R.A.; Singh, S.; Pal, B. A Cu+1/Cu0-TiO2 mesoporous nanocomposite exhibits improved H2 production from H2O under direct solar irradiation. J. Catal. 2017, 346, 1–9. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Vaz, M.D.O.; Khan, S.; Ramakrishnappa, T.; Teixeira, S.R.; Balakrishna, G.; Nagaraju, G.; Dupont, J. Ionic Liquid Assisted Hydrothermal Syntheses of TiO2/CuO Nano-Composites for Enhanced Photocatalytic Hydrogen Production from Water. ChemistrySelect 2016, 1, 2199–2206. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Vaz, M.D.O.; Ramakrishnappa, T.; Teixeira, S.R.; Dupont, J. Ionic liquid assisted hydrothermal syntheses of Au doped TiO2 NPs for efficient visible-light photocatalytic hydrogen production from water, electrochemical detection and photochemical detoxification of hexavalent chromium (Cr6+). RSC Adv. 2017, 7, 43233–43244. [Google Scholar] [CrossRef]
- Reddy, N.L.; Emin, S.; Valant, M.; Shankar, M. Nanostructured Bi2O3@TiO2 photocatalyst for enhanced hydrogen production. Int. J. Hydrogen Energy 2017, 42, 6627–6636. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Manvitha, C.; Reddy, P.V.L.; Kim, K.-H.; Kumari, V.D. Enhanced hydrogen production activity over BiOX TiO2 under solar irradiation: Improved charge transfer through bismuth oxide clusters. J. Energy Chem. 2017, 26, 390–397. [Google Scholar] [CrossRef]
- Ren, X.; Hou, H.; Liu, Z.; Gao, F.; Zheng, J.; Wang, L.; Li, W.; Ying, P.; Yang, W.; Wu, T. Shape-Enhanced Photocatalytic Activities of Thoroughly Mesoporous ZnO Nanofibers. Small 2016, 12, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Rico-Oller, B.; Boudjemaa, A.; Bahruji, H.; Kebir, M.; Prashar, S.; Bachari, K.; Fajardo, M.; Gómez-Ruiz, S. Photodegradation of organic pollutants in water and green hydrogen production via methanol photoreforming of doped titanium oxide nanoparticles. Sci. Total Environ. 2016, 563, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, G.; Valluri, D.K.; Scurrell, M.S. Highly stabilized Ag2O-loaded nano TiO2 for hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrogen Energy 2017, 42, 807–820. [Google Scholar] [CrossRef]
- Sakata, Y.; Miyoshi, Y.; Maeda, T.; Ishikiriyama, K.; Yamazaki, Y.; Imamura, H.; Ham, Y.; Hisatomi, T.; Kubota, J.; Yamakata, A. Photocatalytic property of metal ion added SrTiO3 to Overall H2O splitting. Appl. Catal. A Gen. 2016, 521, 227–232. [Google Scholar] [CrossRef]
- Salgado, S.Y.A.; Zamora, R.M.R.; Zanella, R.; Peral, J.; Malato, S.; Maldonado, M.I. Photocatalytic hydrogen production in a solar pilot plant using a Au/TiO2 photo catalyst. Int. J. Hydrogen Energy 2016, 41, 11933–11940. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Oliveira, J.W.; Sombrio, C.I.; Baptista, D.L.; Teixeira, S.R.; Carabineiro, S.A.; Silva, C.G.; Faria, J.L. Photocatalytic performance of Au/ZnO nanocatalysts for hydrogen production from ethanol. Appl. Catal. A Gen. 2016, 518, 198–205. [Google Scholar] [CrossRef]
- Siddiqi, G.; Mougel, V.; Copéret, C. Highly Active Subnanometer Au Particles Supported on TiO2 for Photocatalytic Hydrogen Evolution from a Well-Defined Organogold Precursor, [Au5(mesityl)5]. Inorg. Chem. 2016, 55, 4026–4033. [Google Scholar] [CrossRef]
- Song, T.; Zhang, P.; Zeng, J.; Wang, T.; Ali, A.; Zeng, H. Boosting the photocatalytic H2 evolution activity of Fe2O3 polymorphs (α-, γ-and β-Fe2O3) by fullerene [C60]-modification and dye-sensitization under visible light irradiation. RSC Adv. 2017, 7, 29184–29192. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Shen, C.; Xu, X. Efficient Photocatalytic Hydrogen Production over Rh-Doped Inverse Spinel Zn2TiO4. ChemCatChem 2016, 8, 2289–2295. [Google Scholar] [CrossRef]
- Taylor, S.; Mehta, M.; Barbash, D.; Samokhvalov, A. One-pot photoassisted synthesis, in situ photocatalytic testing for hydrogen generation and the mechanism of binary nitrogen and copper promoted titanium dioxide. Photochem. Photobiol. Sci. 2017, 16, 916–924. [Google Scholar] [CrossRef]
- Tian, H.; Wang, S.; Zhang, C.; Veder, J.-P.; Pan, J.; Jaroniec, M.; Wang, L.; Liu, J. Design and synthesis of porous ZnTiO3/TiO2 nanocages with heterojunctions for enhanced photocatalytic H2 production. J. Mater. Chem. A 2017, 5, 11615–11622. [Google Scholar] [CrossRef]
- Tiwari, A.; Mondal, I.; Ghosh, S.; Chattopadhyay, N.; Pal, U. Fabrication of mixed phase TiO2 heterojunction nanorods and their enhanced photoactivities. Phys. Chem. Chem. Phys. 2016, 18, 15260–15268. [Google Scholar] [CrossRef] [PubMed]
- Vebber, M.; Faria, A.; Dal’Acqua, N.; Beal, L.; Fetter, G.; Machado, G.; Giovanela, M.; Crespo, J. Hydrogen production by photocatalytic water splitting using poly (allylamine hydrochloride)/poly (acrylic acid)/TiO2/copper chlorophyllin self-assembled thin films. Int. J. Hydrogen Energy 2016, 41, 17995–18004. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Chen, Y.; Cheng, Z.; Lin, P.; Yang, Z.; Sun, S. Improved Hydrogen Production by Glycerol Photoreforming over Ag2O-TiO2 Nanocomposite Mixed Oxide Synthesized by a Sol-gel Method. Energy Procedia 2017, 105, 1657–1664. [Google Scholar] [CrossRef]
- Wang, P.; Sun, F.; Kim, J.H.; Kim, J.H.; Yang, J.; Wang, X.; Lee, J.S. Synthesis of high-purity, layered structured K2Ta4O11 intermediate phase nanocrystals for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2016, 18, 25831–25836. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, D.; Wu, Y.; Guo, H.; She, H.; Li, J.; Zhong, J.; Wang, F.; Tong, J. Carbon doped solid solution Bi0.5Dy0.5VO4 for efficient photocatalytic hydrogen evolution from water. Int. J. Hydrogen Energy 2016, 41, 16032–16039. [Google Scholar] [CrossRef]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Song, Y.; Wei, S.; Rong, Y.; Lu, C.; Chen, Y.; Wang, J.; Zhang, Z. Enhanced visible-light photocatalytic hydrogen evolution activity of Er3+:Y3Al5O12/PdS–ZnS by conduction band co-catalysts (MoO2, MoS2 and MoSe2). Int. J. Hydrogen Energy 2016, 41, 12826–12835. [Google Scholar] [CrossRef]
- Zhang, K.; Qian, S.; Kim, W.; Kim, J.K.; Sheng, X.; Lee, J.Y.; Park, J.H. Double 2-dimensional H2-evoluting catalyst tipped photocatalyst nanowires: A new avenue for high-efficiency solar to H2 generation. Nano Energy 2017, 34, 481–490. [Google Scholar] [CrossRef]
- Wu, B.-H.; Liu, W.-T.; Chen, T.-Y.; Perng, T.-P.; Huang, J.-H.; Chen, L.-J. Plasmon-enhanced photocatalytic hydrogen production on Au/TiO2 hybrid nanocrystal arrays. Nano Energy 2016, 27, 412–419. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Z.; Cheng, F.; Zhang, P.; Mai, W.; Tong, Y. Ceria and ceria-based nanostructured materials for photoenergy applications. Nano Energy 2017, 34, 313–337. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Liang, S.; Zhang, S.; Li, Y.; Wu, L. Insights into the role of Cu in promoting photocatalytic hydrogen production over ultrathin HNb3O8 nanosheets. J. Catal. 2016, 342, 98–104. [Google Scholar] [CrossRef]
- Xu, D.; Hai, Y.; Zhang, X.; Zhang, S.; He, R. Bi2O3 cocatalyst improving photocatalytic hydrogen evolution performance of TiO2. Appl. Surf. Sci. 2017, 400, 530–536. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Z.; Zhang, Z.; Ao, D. Highly efficient photocatalytic H2 evolution from water over CdLa2S4/mesoporous gC3N4 hybrids under visible light irradiation. Appl. Catal. B Environ. 2016, 192, 234–241. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Tu, H.; Jia, Q.; Gong, H.; Guan, J. Synchronous etching-epitaxial growth fabrication of facet-coupling NaTaO3/Ta2O5 heterostructured nanofibers for enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 184, 309–319. [Google Scholar] [CrossRef]
- Xu, X.; Lv, M.; Sun, X.; Liu, G. Role of surface composition upon the photocatalytic hydrogen production of Cr-doped and La/Cr-codoped SrTiO3. J. Mater. Sci. 2016, 51, 6464–6473. [Google Scholar] [CrossRef]
- Yan, X.; Xue, C.; Yang, B.; Yang, G. Novel three-dimensionally ordered macroporous Fe3+-doped TiO2 photocatalysts for H2 production and degradation applications. Appl. Surf. Sci. 2017, 394, 248–257. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, P.; Wang, Y.; Sha, L.; Ren, X.; Zhang, J.; Yang, P.; Wu, T.; Chen, Y.; Li, X. A simple and efficient hydrogen production-storage hybrid system (Co/TiO2) for synchronized hydrogen photogeneration with uptake. J. Mater. Chem. A 2017, 5, 9198–9203. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Irvine, J.T.; Cheng, H.M. Enhanced Photocatalytic H2 Production in Core–Shell Engineered Rutile TiO2. Adv. Mater. 2016, 28, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, Y.; He, Y.; Xia, Q.; Jiang, Z. Synthesis of hierarchical dendritic micro–nano structure ZnFe2O4 and photocatalytic activities for water splitting. Chin. J. Chem. Eng. 2016, 24, 1112–1116. [Google Scholar] [CrossRef]
- Yu, H.; Sun, D.; Liu, J.; Fang, Y.; Li, C.C. Monodisperse mesoporous Ta2O5 colloidal spheres as a highly effective photocatalyst for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 17225–17232. [Google Scholar] [CrossRef]
- Yu, X.; Wei, Y.; Li, Z.; Liu, J. One-step synthesis of the single crystal Ta2O5 nanowires with superior hydrogen production activity. Mater. Lett. 2017, 191, 150–153. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, H.; Zhou, J.; Cui, Z.; Liu, T.; Wang, Y.; Wang, Y.; Zou, Z. Confinement effect of monolayer MoS2 quantum dots on conjugated polyimide and promotion of solar-driven photocatalytic hydrogen generation. Dalton Trans. 2017, 46, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Z.; Gao, Z.; Ge, H.; Zhao, S.; Chen, C.; Chen, S.; Tong, X.; Wang, M.; Zheng, Z. Porous TiO2 nanotubes with spatially separated platinum and CoOx cocatalysts produced by atomic layer deposition for photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2017, 56, 816–820. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, R.; Li, Y.; Shi, Q.; Xie, L.; Chen, J.; Xu, X.; Shi, H.; Zhao, W. High H2 evolution from quantum Cu (II) nanodot-doped two-dimensional ultrathin TiO2 nanosheets with dominant exposed {001} facets for reforming glycerol with multiple electron transport pathways. J. Phys. Chem. C 2016, 120, 10746–10756. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, R.; Li, Z.; Li, A.; Wang, S.; Liang, Z.; Liao, S.; Li, C. The dependence of photocatalytic activity on the selective and nonselective deposition of noble metal cocatalysts on the facets of rutile TiO2. J. Catal. 2016, 337, 36–44. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, L.; Kang, L.; Yang, M.Y.; Zhang, K. A new CaWO4/alkali-activated blast furnace slag-based cementitious composite for production of hydrogen. Int. J. Hydrogen Energy 2017, 42, 3690–3697. [Google Scholar] [CrossRef]
- Shen, C.-C.; Liu, Y.-N.; Zhou, X.; Guo, H.-L.; Zhao, Z.-W.; Liang, K.; Xu, A.-W. Large improvement of visible-light photocatalytic H2-evolution based on cocatalyst-free Zn0.5Cd0.5S synthesized through a two-step process. Catal. Sci. Technol. 2017, 7, 961–967. [Google Scholar] [CrossRef]
- Zhu, F.; Li, C.; Ha, M.N.; Liu, Z.; Guo, Q.; Zhao, Z. Molten-salt synthesis of Cu–SrTiO3/TiO2 nanotube heterostructures for photocatalytic water splitting. J. Mater. Sci. 2016, 51, 4639–4649. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, X.; Fujitsuka, M.; Zhang, J.; Majima, T. Au/La2Ti2O7 Nanostructures Sensitized with Black Phosphorus for Plasmon-Enhanced Photocatalytic Hydrogen Production in Visible and Near-Infrared Light. Angew. Chem. 2017, 129, 2096–2100. [Google Scholar] [CrossRef]
- Zhu, W.; Han, D.; Niu, L.; Wu, T.; Guan, H. Z-scheme Si/MgTiO3 porous heterostructures: Noble metal and sacrificial agent free photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2016, 41, 14713–14720. [Google Scholar] [CrossRef]
- Zhu, Z.; Kao, C.-T.; Tang, B.-H.; Chang, W.-C.; Wu, R.-J. Efficient hydrogen production by photocatalytic water-splitting using Pt-doped TiO2 hollow spheres under visible light. Ceram. Int. 2016, 42, 6749–6754. [Google Scholar] [CrossRef]
- Zhuang, B.; Xiangqing, L.; Ge, R.; Kang, S.; Qin, L.; Li, G. Assembly and electron transfer mechanisms on visible light responsive 5, 10, 15, 20-meso-tetra (4-carboxyphenyl) porphyrin/cuprous oxide composite for photocatalytic hydrogen production. Appl. Catal. A Gen. 2017, 533, 81–89. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, Y.; Chu, Z.; Long, J.; An, X.; Zhang, H.; Lin, H.; Zhang, Z.; Wang, X. Synergy of metal and nonmetal dopants for visible-light photocatalysis: A case-study of Sn and N co-doped TiO2. Phys. Chem. Chem. Phys. 2016, 18, 9636–9644. [Google Scholar] [CrossRef] [PubMed]
- Zywitzki, D.; Jing, H.; Tüysüz, H.; Chan, C.K. High surface area, amorphous titania with reactive Ti 3+ through a photo-assisted synthesis method for photocatalytic H2 generation. J. Mater. Chem. A 2017, 5, 10957–10967. [Google Scholar] [CrossRef]
- Bharatvaj, J.; Preethi, V.; Kanmani, S. Hydrogen production from sulphide wastewater using Ce3+–TiO2 photocatalysis. Int. J. Hydrogen Energy 2018, 43, 3935–3945. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial hydrogen production over TiO2-based photocatalysts: Polyols, carboxylic acids, and saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- Maldonado, M.; López-Martín, A.; Colón, G.; Peral, J.; Martínez-Costa, J.; Malato, S. Solar pilot plant scale hydrogen generation by irradiation of Cu/TiO2 composites in presence of sacrificial electron donors. Appl. Catal. B Environ. 2018, 229, 15–23. [Google Scholar] [CrossRef]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic Hydrogen Generation Using Metal-Decorated TiO2: Sacrificial Donors vs True Water Splitting. ACS Energy Lett. 2018, 3, 542–545. [Google Scholar] [CrossRef]
- Ravi, P.; Rao, V.N.; Shankar, M.; Sathish, M. CuOCr2O3 core-shell structured co-catalysts on TiO2 for efficient photocatalytic water splitting using direct solar light. Int. J. Hydrogen Energy 2018, 43, 3976–3987. [Google Scholar] [CrossRef]
- Camacho, S.Y.T.; Rey, A.; Hernández-Alonso, M.D.; Llorca, J.; Medina, F.; Contreras, S. Pd/TiO2-WO3 photocatalysts for hydrogen generation from water-methanol mixtures. Appl. Surf. Sci. 2018, 455, 570–580. [Google Scholar] [CrossRef]
- Huang, J.; Li, G.; Zhou, Z.; Jiang, Y.; Hu, Q.; Xue, C.; Guo, W. Efficient photocatalytic hydrogen production over Rh and Nb codoped TiO2 nanorods. Chem. Eng. J. 2018, 337, 282–289. [Google Scholar] [CrossRef]
- Li, Y.; Kuang, L.; Xiao, D.; Badireddy, A.R.; Hu, M.; Zhuang, S.; Wang, X.; Lee, E.S.; Marhaba, T.; Zhang, W. Hydrogen production from organic fatty acids using carbon-doped TiO2 nanoparticles under visible light irradiation. Int. J. Hydrogen Energy 2018, 43, 4335–4346. [Google Scholar] [CrossRef]
- Castañeda, C.; Tzompantzi, F.; Rodríguez-Rodríguez, A.; Sánchez-Dominguez, M.; Gómez, R. Improved photocatalytic hydrogen production from methanol/water solution using CuO supported on fluorinated TiO2. J. Chem. Technol. Biotechnol. 2018, 93, 1113–1120. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, J.; Fujitsuka, M.; Majima, T. Graphitic-C3N4 hybridized N-doped La2Ti2O7 two-dimensional layered composites as efficient visible-light-driven photocatalyst. Appl. Catal. B Environ. 2017, 202, 191–198. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, J.; Zhu, B.; Yu, J. Shape-dependent photocatalytic hydrogen evolution activity over a Pt nanoparticle coupled gC3N4 photocatalyst. Phys. Chem. Chem. Phys. 2016, 18, 19457–19463. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. Carbon-based H2-production photocatalytic materials. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 72–99. [Google Scholar] [CrossRef]
- Chang, D.W.; Baek, J.B. Nitrogen-Doped Graphene for Photocatalytic Hydrogen Generation. Chem. Asian J. 2016, 11, 1125–1137. [Google Scholar] [CrossRef]
- Chen, F.; Yang, H.; Wang, X.; Yu, H. Facile synthesis and enhanced photocatalytic H2-evolution performance of NiS2-modified gC3N4 photocatalysts. Chin. J. Catal. 2017, 38, 296–304. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Jin, B.; Luo, J.; Xu, X.; Zhang, L.; Hong, Y. Heterojunctions in gC3N4/B-TiO2 nanosheets with exposed {001} plane and enhanced visible-light photocatalytic activities. Int. J. Hydrogen Energy 2016, 41, 7292–7300. [Google Scholar] [CrossRef]
- Chen, L.-C.; Yeh, T.-F.; Lee, Y.-L.; Teng, H. Incorporating nitrogen-doped graphene oxide dots with graphene oxide sheets for stable and effective hydrogen production through photocatalytic water decomposition. Appl. Catal. A Gen. 2016, 521, 118–124. [Google Scholar] [CrossRef]
- Chen, T.; Quan, W.; Yu, L.; Hong, Y.; Song, C.; Fan, M.; Xiao, L.; Gu, W.; Shi, W. One-step synthesis and visible-light-driven H2 production from water splitting of Ag quantum dots/gC3N4 photocatalysts. J. Alloys Compd. 2016, 686, 628–634. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Guan, J.; Zhen, J.; Sun, Z.; Du, P.; Lu, Y.; Yang, S. A facile mechanochemical route to a covalently bonded graphitic carbon nitride (gC3N4) and fullerene hybrid toward enhanced visible light photocatalytic hydrogen production. Nanoscale 2017, 9, 5615–5623. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, J.; Hu, Y.; Guo, L. WO3/g-C3N4 composites: One-pot preparation and enhanced photocatalytic H2 production under visible-light irradiation. Nanotechnology 2017, 28, 164002. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, L.; Fan, X.; Wang, M.; Li, M.; Shi, J. One-step construction of FeOx modified gC3N4 for largely enhanced visible-light photocatalytic hydrogen evolution. Carbon 2016, 101, 62–70. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A Facile One-Step Synthesis of Fe-Doped g-C3N4 Nanosheets and Their Improved Visible-Light Photocatalytic Performance. ChemCatChem 2017, 9, 1708–1715. [Google Scholar] [CrossRef]
- Gholipour, M.R.; Béland, F.; Do, T.-O. Graphitic Carbon Nitride-Titanium Dioxide Nanocomposite for Photocatalytic Hydrogen Production under Visible Light. Int. J. Chem. React. Eng. 2016, 14, 851–858. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Kan, C.; Zhu, J.; Tong, T.; Ke, S.; Choy, W.C.; Wei, B. Au Multimer@ MoS2 hybrid structures for efficient photocatalytical hydrogen production via strongly plasmonic coupling effect. Nano Energy 2016, 30, 549–558. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Qu, L. Graphitic Carbon Nitride/Nitrogen-Rich Carbon Nanofibers: Highly Efficient Photocatalytic Hydrogen Evolution without Cocatalysts. Angew. Chem. 2016, 128, 11007–11011. [Google Scholar] [CrossRef]
- Hao, R.; Guo, S.; Wang, X.; Feng, T.; Feng, Q.; Li, M.; Jiang, B. Two-dimensional assembly structure of graphene and TiO2 nanosheets from titanic acid with enhanced visible-light photocatalytic performance. Chem. Phys. Lett. 2016, 653, 190–195. [Google Scholar] [CrossRef]
- Han, W.; Li, Z.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. The Promoting Role of Different Carbon Allotropes Cocatalysts for Semiconductors in Photocatalytic Energy Generation and Pollutants Degradation. Front. Chem. 2017, 5, 84. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Yang, Z.; Shen, R.; Fang, Y.; Ma, S.; Chen, X.; Li, X. Earth-abundant WC nanoparticles as an active noble-metal-free co-catalyst for the highly boosted photocatalytic H2 production over gC3N4 nanosheets under visible light. Catal. Sci. Technol. 2017, 7, 1193–1202. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Li, J.; Mao, M.; Yan, Z.; Wang, W.; Wang, J. Hydrilla derived ZnIn2S4 photocatalyst with hexagonal-cubic phase junctions: A bio-inspired approach for H2 evolution. Catal. Commun. 2016, 87, 1–5. [Google Scholar] [CrossRef]
- Hong, Y.; Fang, Z.; Yin, B.; Luo, B.; Zhao, Y.; Shi, W.; Li, C. A visible-light-driven heterojunction for enhanced photocatalytic water splitting over Ta2O5 modified gC3N4 photocatalyst. Int. J. Hydrogen Energy 2017, 42, 6738–6745. [Google Scholar] [CrossRef]
- Huang, Q.-Z.; Wang, J.-C.; Wang, P.-P.; Yao, H.-C.; Li, Z.-J. In-situ growth of mesoporous Nb2O5 microspheres on gC3N4 nanosheets for enhanced photocatalytic H2 evolution under visible light irradiation. Int. J. Hydrogen Energy 2017, 42, 6683–6694. [Google Scholar] [CrossRef]
- Huo, J.; Liu, X.; Li, X.; Qin, L.; Kang, S.-Z. An efficient photocatalytic system containing Eosin Y, 3D mesoporous graphene assembly and CuO for visible-light-driven H2 evolution from water. Int. J. Hydrogen Energy 2017, 42, 15540–15550. [Google Scholar] [CrossRef]
- Kalyani, R.; Gurunathan, K. PTh-rGO-TiO2 nanocomposite for photocatalytic hydrogen production and dye degradation. J. Photochem. Photobiol. A Chem. 2016, 329, 105–112. [Google Scholar] [CrossRef]
- Karthik, P.; Vinoth, R.; Selvam, P.; Balaraman, E.; Navaneethan, M.; Hayakawa, Y.; Neppolian, B. A visible-light active catechol–metal oxide carbonaceous polymeric material for enhanced photocatalytic activity. J. Mater. Chem. A 2017, 5, 384–396. [Google Scholar] [CrossRef]
- Lau, V.W.H.; Klose, D.; Kasap, H.; Podjaski, F.; Pignié, M.C.; Reisner, E.; Jeschke, G.; Lotsch, B.V. Dark Photocatalysis: Storage of Solar Energy in Carbon Nitride for Time-Delayed Hydrogen Generation. Angew. Chem. Int. Ed. 2017, 56, 510–514. [Google Scholar] [CrossRef]
- Lei, Y.; Shi, Q.; Han, C.; Wang, B.; Wu, N.; Wang, H.; Wang, Y. N-doped graphene grown on silk cocoon-derived interconnected carbon fibers for oxygen reduction reaction and photocatalytic hydrogen production. Nano Res. 2016, 9, 2498–2509. [Google Scholar] [CrossRef]
- Litke, A.; Weber, T.; Hofmann, J.P.; Hensen, E.J. Bottlenecks limiting efficiency of photocatalytic water reduction by mixed Cd-Zn sulfides/Pt-TiO2 composites. Appl. Catal. B Environ. 2016, 198, 16–24. [Google Scholar] [CrossRef]
- Liu, B.; Su, S.; Zhou, W.; Wang, Y.; Wei, D.; Yao, L.; Ni, Y.; Cao, M.; Hu, C. Photo-reduction assisted synthesis of W-doped TiO2 coupled with Au nanoparticles for highly efficient photocatalytic hydrogen evolution. CrystEngComm 2017, 19, 675–683. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, G.; Zhou, W.; Liu, Y.; Pang, H.; Zhang, H.; Hao, D.; Meng, X.; Li, P.; Kako, T. In situ bond modulation of graphitic carbon nitride to construct p–n homojunctions for enhanced photocatalytic hydrogen production. Adv. Funct. Mater. 2016, 26, 6822–6829. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, B.; Yang, Y.; Huang, E.; Ge, Z.; Zhang, T.; Zhang, S.; Li, L.; Guan, N.; Ma, Y. High activity of hot electrons from bulk 3D graphene materials for efficient photocatalytic hydrogen production. Nano Res. 2017, 10, 1662–1672. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Lin, K.; Li, J.; Zhan, H.; Liu, W.; Li, C. Mo2C as Non-Noble Metal Co-Catalyst in Mo2C/CdS Composite for Enhanced Photocatalytic H2 Evolution under Visible Light Irradiation. ChemSusChem 2016, 9, 820–824. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, J.; Yang, Y.; Wang, D.; Bie, L.; Fahlman, B.D. Novel g-C3N4/CoO nanocomposites with significantly enhanced visible-light photocatalytic activity for H2 evolution. ACS Appl. Mater. Interfaces 2017, 9, 12427–12435. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Esteve-Adell, I.; Albero, J.; Royo, J.F.S.; Primo, A.; Garcia, H. 111 oriented gold nanoplatelets on multilayer graphene as visible light photocatalyst for overall water splitting. Nat. Commun. 2016, 7, 11819. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Pooja, D.; Thakur, A.; Basu, S. Enhanced photocatalytic water splitting by gold carbon dot core shell nanocatalyst under visible/sunlight. New J. Chem. 2017, 41, 4573–4581. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Vu, N.N.; Chabot, S.; Kaliaguine, S.; Do, T.O. Role of CxNy-Triazine in Photocatalysis for Efficient Hydrogen Generation and Organic Pollutant Degradation Under Solar Light Irradiation. Sol. RRL 2017, 1, 1700012. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Pan, Z.; Zheng, Y.; Guo, F.; Niu, P.; Wang, X. Decorating CoP and Pt nanoparticles on graphitic carbon nitride nanosheets to promote overall water splitting by conjugated polymers. ChemSusChem 2017, 10, 87–90. [Google Scholar] [CrossRef]
- Putri, L.K.; Ng, B.-J.; Ong, W.-J.; Lee, H.W.; Chang, W.S.; Chai, S.-P. Heteroatom nitrogen-and boron-doping as a facile strategy to improve photocatalytic activity of standalone reduced graphene oxide in hydrogen evolution. ACS Appl. Mater. Interfaces 2017, 9, 4558–4569. [Google Scholar] [CrossRef]
- Qiao, S.; Mitchell, R.W.; Coulson, B.; Jowett, D.V.; Johnson, B.R.; Brydson, R.; Isaacs, M.; Lee, A.F.; Douthwaite, R.E. Pore confinement effects and stabilization of carbon nitride oligomers in macroporous silica for photocatalytic hydrogen production. Carbon 2016, 106, 320–329. [Google Scholar] [CrossRef]
- Qu, A.; Xu, X.; Xie, H.; Zhang, Y.; Li, Y.; Wang, J. Effects of calcining temperature on photocatalysis of gC3N4/TiO2 composites for hydrogen evolution from water. Mater. Res. Bull. 2016, 80, 167–176. [Google Scholar] [CrossRef]
- Raevskaya, A.E.; Panasiuk, Y.V.; Korzhak, G.V.; Stroyuk, O.L.; Kuchmiy, S.Y.; Dzhagan, V.M.; Zahn, D.R. Photocatalytic H2 production from aqueous solutions of hydrazine and its derivatives in the presence of nitric-acid-activated graphitic carbon nitride. Catal. Today 2017, 284, 229–235. [Google Scholar] [CrossRef]
- Rather, R.A.; Singh, S.; Pal, B. Core–shell morphology of Au-TiO2@ graphene oxide nanocomposite exhibiting enhanced hydrogen production from water. J. Ind. Eng. Chem. 2016, 37, 288–294. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-free synthesis of 2D porous ultrathin nonmetal-doped gC3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Shen, L.; Xing, Z.; Zou, J.; Li, Z.; Wu, X.; Zhang, Y.; Zhu, Q.; Yang, S.; Zhou, W. Black TiO2 nanobelts/g-C3N4 nanosheets Laminated Heterojunctions with Efficient Visible-Light-Driven Photocatalytic Performance. Sci. Rep. 2017, 7, 41978. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, C.; Tang, L.; Wei, S.; Song, Y.; Wang, J. Efficient photocatalytic hydrogen evolution from methanol/water splitting over Tm3+, Yb3+:NaYF4–Er3+:Y3Al5O12/MoS2–NaTaO3 nanocomposite particles under infrared–visible light irradiation. Sol. Energy Mater. Sol. Cells 2016, 149, 128–136. [Google Scholar] [CrossRef]
- Shin, S.R.; Park, J.H.; Kim, K.-H.; Choi, K.M.; Kang, J.K. Network of Heterogeneous Catalyst Arrays on the Nitrogen-Doped Graphene for Synergistic Solar Energy Harvesting of Hydrogen from Water. Chem. Mater. 2016, 28, 7725–7730. [Google Scholar] [CrossRef]
- Song, K.; Xiao, F.; Zhang, L.; Yue, F.; Liang, X.; Wang, J.; Su, X. W18O49 nanowires grown on gC3N4 sheets with enhanced photocatalytic hydrogen evolution activity under visible light. J. Mol. Catal. A Chem. 2016, 418, 95–102. [Google Scholar] [CrossRef]
- Sun, J.; Schmidt, B.V.; Wang, X.; Shalom, M. Self-Standing Carbon Nitride-Based Hydrogels with High Photocatalytic Activity. ACS Appl. Mater. Interfaces 2017, 9, 2029–2034. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, P.; Yu, H.; Wang, X. In situ hydrothermal synthesis and enhanced photocatalytic H2-evolution performance of suspended rGO/gC3N4 photocatalysts. J. Mol. Catal. A Chem. 2016, 424, 369–376. [Google Scholar] [CrossRef]
- Tay, Q.; Wang, X.; Zhao, X.; Hong, J.; Zhang, Q.; Xu, R.; Chen, Z. Enhanced visible light hydrogen production via a multiple heterojunction structure with defect-engineered gC3N4 and two-phase anatase/brookite TiO2. J. Catal. 2016, 342, 55–62. [Google Scholar] [CrossRef]
- Thaweesak, S.; Lyu, M.; Peerakiatkhajohn, P.; Butburee, T.; Luo, B.; Chen, H.; Wang, L. Two-dimensional gC3N4/Ca2Nb2TaO10 nanosheet composites for efficient visible light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2017, 202, 184–190. [Google Scholar] [CrossRef]
- Virca, C.N.; Winter, H.; Goforth, A.M.; Mackiewicz, M.R.; McCormick, T.M. Photocatalytic water reduction using a polymer coated carbon quantum dot sensitizer and a nickel nanoparticle catalyst. Nanotechnology 2017, 28, 195402. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, G.; Hou, Y.; Wang, X. Layering MoS2 on soft hollow gC3N4 nanostructures for photocatalytic hydrogen evolution. Appl. Catal. A Gen. 2016, 521, 2–8. [Google Scholar] [CrossRef]
- Wang, J.; Feng, K.; Chen, B.; Li, Z.-J.; Meng, Q.-Y.; Zhang, L.-P.; Tung, C.-H.; Wu, L.-Z. Polymer-modified hydrophilic graphene: A promotor to photocatalytic hydrogen evolution for in situ formation of core@ shell cobalt nanocomposites. J. Photochem. Photobiol. A Chem. 2016, 331, 247–254. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Wu, L.; Li, X.; Shu, J. MnO2 and carbon nanotube co-modified C3N4 composite catalyst for enhanced water splitting activity under visible light irradiation. Int. J. Hydrogen Energy 2016, 41, 22743–22750. [Google Scholar] [CrossRef]
- Wang, P.; Sinev, I.; Sun, F.; Li, H.; Wang, D.; Li, Q.; Wang, X.; Marschall, R.; Wark, M. Rational fabrication of a graphitic-C3N4/Sr2KNb5O15 nanorod composite with enhanced visible-light photoactivity for degradation of methylene blue and hydrogen production. RSC Adv. 2017, 7, 42774–42782. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, J.; Ma, Q.; Zhang, S.; He, J.; Zhong, J.; Li, J.; Huang, H.; Su, B. Preparation of carbon spheres supported CdS photocatalyst for enhancement its photocatalytic H2 evolution. Catal. Today 2017, 281, 662–668. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, W.; Hong, J.; Zhang, W.; Xu, R. Molybdenum carbide microcrystals: Efficient and stable catalyst for photocatalytic H2 evolution from water in the presence of dye sensitizer. J. Mater. 2016, 2, 344–349. [Google Scholar] [CrossRef]
- Zhang, H.; Xin, C.; Wang, X.; Wang, K. Facile synthesis of Cd0.2Zn0.8 S-ethylenediamine hybrid solid solution and its improved photocatalytic performance. Int. J. Hydrogen Energy 2016, 41, 12019–12028. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Shen, R.; Li, X.; Luo, X.; Zhang, H.; Zhang, A.; Bi, G. Markedly enhanced visible-light photocatalytic H2 generation over gC3N4 nanosheets decorated by robust nickel phosphide (Ni12P5) cocatalysts. Dalton Trans. 2017, 46, 1794–1802. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Fan, W.; Li, Z.; Wang, L.; Li, X.; Wang, Y.; Wang, R.; Zheng, J.; Wu, M. Remedying defects in carbon nitride to improve both photooxidation and H2 generation efficiencies. ACS Catal. 2016, 6, 3365–3371. [Google Scholar] [CrossRef]
- Xie, L.; Ai, Z.; Zhang, M.; Sun, R.; Zhao, W. Enhanced Hydrogen Evolution in the Presence of Plasmonic Au-Photo-Sensitized g-C3N4 with an Extended Absorption Spectrum from 460 to 640 nm. PLoS ONE 2016, 11, e0161397. [Google Scholar] [CrossRef]
- Xing, W.; Chen, G.; Li, C.; Sun, J.; Han, Z.; Zhou, Y.; Hu, Y.; Meng, Q. Construction of Large-Scale Ultrathin Graphitic Carbon Nitride Nanosheets by a Hydrogen-Bond-Assisted Strategy for Improved Photocatalytic Hydrogen Production and Ciprofloxacin Degradation Activity. ChemCatChem 2016, 8, 2838–2845. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, L.; Zhang, Y.; Yang, Y.; Fang, Z.; Wang, B.; Zhang, S.; Liu, P. Near-infrared-activated NaYF4:Yb3+, Er3+/Au/CdS for H2 production via photoreforming of bio-ethanol: Plasmonic Au as light nanoantenna, energy relay, electron sink and co-catalyst. J. Mater. Chem. A 2017, 5, 10311–10320. [Google Scholar] [CrossRef]
- Yan, M.; Hua, Y.; Zhu, F.; Sun, L.; Gu, W.; Shi, W. Constructing nitrogen doped graphene quantum dots-ZnNb 2 O 6/gC3N4 catalysts for hydrogen production under visible light. Appl. Catal. B Environ. 2017, 206, 531–537. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Qin, J.; Shen, X.; Yu, R.; Ma, M.; Liu, R. Porous carbon-doped TiO2 on TiC nanostructures for enhanced photocatalytic hydrogen production under visible light. J. Catal. 2017, 347, 36–44. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Shi, L.; Cao, L.; Yu, Q.; Jie, Y.; Fei, J.; Ouyang, H.; Ye, J. A surface modification resultant thermally oxidized porous g-C3N4 with enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 204, 335–345. [Google Scholar] [CrossRef]
- Ye, P.; Liu, X.; Iocozzia, J.; Yuan, Y.; Gu, L.; Xu, G.; Lin, Z. A highly stable non-noble metal Ni2P co-catalyst for increased H2 generation by gC3N4 under visible light irradiation. J. Mater. Chem. A 2017, 5, 8493–8498. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Zhang, J.; Cheng, B.; Yu, J. Enhanced photocatalytic H2 production on CdS nanorod using cobalt-phosphate as oxidation cocatalyst. Appl. Surf. Sci. 2016, 389, 775–782. [Google Scholar] [CrossRef]
- Yue, X.; Yi, S.; Wang, R.; Zhang, Z.; Qiu, S. A novel architecture of dandelion-like Mo2C/TiO2 heterojunction photocatalysts towards high-performance photocatalytic hydrogen production from water splitting. J. Mater. Chem. A 2017, 5, 10591–10598. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Chen, J.; Jiang, Y.; Kiani, M.; Li, B.; Wang, R. Fabrication of high-activity hybrid NiTiO3/gC3N4 heterostructured photocatalysts for water splitting to enhanced hydrogen production. Ceram. Int. 2016, 42, 12297–12305. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, K.; Wei, K.; Dai, Y.; Yan, L.; Guo, H.; Luo, X. Fabrication of porous gC3N4 and supported porous gC3N4 by a simple precursor pretreatment strategy and their efficient visible-light photocatalytic activity. Chin. J. Catal. 2017, 38, 498–507. [Google Scholar] [CrossRef]
- Zha, D.-W.; Li, L.-F.; Pan, Y.-X.; He, J.-B. Coconut shell carbon nanosheets facilitating electron transfer for highly efficient visible-light-driven photocatalytic hydrogen production from water. Int. J. Hydrogen Energy 2016, 41, 17370–17379. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Zhang, P.; Song, T.; Wang, T.; Zeng, H. In-situ synthesis of Cu nanoparticles hybridized with carbon quantum dots as a broad spectrum photocatalyst for improvement of photocatalytic H2 evolution. Appl. Catal. B Environ. 2017, 206, 328–335. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Zhang, H.; Tu, W. One-pot hydrothermal synthesis of CdS/NiS photocatalysts for high H2 evolution from water under visible light. Int. J. Hydrogen Energy 2017, 42, 11199–11205. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, L.; Zhang, M.; Ai, Z.; Xi, H.; Li, Y.; Shi, Q.; Chen, J. Enhanced photocatalytic activity of all-solid-state gC3N4/Au/P25 Z-scheme system for visible-light-driven H2 evolution. Int. J. Hydrogen Energy 2016, 41, 6277–6287. [Google Scholar] [CrossRef]
- Zou, J.-P.; Wang, L.-C.; Luo, J.; Nie, Y.-C.; Xing, Q.-J.; Luo, X.-B.; Du, H.-M.; Luo, S.-L.; Suib, S.L. Synthesis and efficient visible light photocatalytic H2 evolution of a metal-free gC3N4/graphene quantum dots hybrid photocatalyst. Appl. Catal. B Environ. 2016, 193, 103–109. [Google Scholar] [CrossRef]
- Chu, J.; Han, X.; Yu, Z.; Du, Y.; Song, B.; Xu, P. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production on CdS/Cu7S4/g-C3N4 Ternary Heterostructures. ACS Appl. Mater. Interfaces 2018, 10, 20404–20411. [Google Scholar] [CrossRef]
- Hua, S.; Qu, D.; An, L.; Jiang, W.; Wen, Y.; Wang, X.; Sun, Z. Highly efficient p-type Cu3P/n-type g-C3N4 photocatalyst through Z-scheme charge transfer route. Appl. Catal. B Environ. 2019, 240, 253–261. [Google Scholar] [CrossRef]
- Wang, P.; Guan, Z.; Li, Q.; Yang, J. Efficient visible-light-driven photocatalytic hydrogen production from water by using Eosin Y-sensitized novel g-C3N4/Pt/GO composites. J. Mater. Sci. 2018, 53, 774–786. [Google Scholar] [CrossRef]
- Liu, J.; Jia, Q.; Long, J.; Wang, X.; Gao, Z.; Gu, Q. Amorphous NiO as co-catalyst for enhanced visible-light-driven hydrogen generation over g-C3N4 photocatalyst. Appl. Catal. B Environ. 2018, 222, 35–43. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- de Castro, S.; da Silva, A.F.; Felix, J.F.; Piton, M.R.; Galeti, H.V.A.; Rodrigues, A.D.G.; Gobato, Y.G.; Al Saqri, N.; Henini, M.; Albadri, A.M.; et al. Effect of growth techniques on the structural, optical and electrical properties of indium doped TiO2 thin films. J. Alloys Compd. 2018, 766, 194–203. [Google Scholar]
- Yu, Y.; Yan, W.; Wang, X.; Li, P.; Gao, W.; Zou, H.; Wu, S.; Ding, K. Surface Engineering for Extremely Enhanced Charge Separation and Photocatalytic Hydrogen Evolution on g-C3N4. Adv. Mater. 2018, 30, 1705060. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Li, D.; Wang, X.; Lin, K. Molybdenum-Based Co-catalysts in Photocatalytic Hydrogen Production: Categories, Structures, and Roles. ChemSusChem 2018, 11, 3871–3881. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Kandiel, T.A.; Dillert, R.; Bahnemann, D.W. Photocatalytic hydrogen production from biomass-derived compounds: A case study of citric acid. Environ. Technol. 2016, 37, 2687–2693. [Google Scholar] [CrossRef]
- Almahdi, M.; Dincer, I.; Rosen, M. Analysis and assessment of methanol production by integration of carbon capture and photocatalytic hydrogen production. Int. J. Greenh. Gas Control 2016, 51, 56–70. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, Z.; Zhang, Q.; Li, B.; Shen, Z. Fullerene (C60)/CdS nanocomposite with enhanced photocatalytic activity and stability. Appl. Surf. Sci. 2017, 403, 151–158. [Google Scholar] [CrossRef]
- Cai, X.; Li, G.; Yang, Y.; Zhang, C.; Yang, X. Cobalt thiolate complexes catalyst in noble-metal-free system for photocatalytic hydrogen production. Russ. J. Appl. Chem. 2016, 89, 1506–1511. [Google Scholar] [CrossRef]
- Chang, C.-J.; Chu, K.-W. ZnS/polyaniline composites with improved dispersing stability and high photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2016, 41, 21764–21773. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lee, Z.; Chu, K.-W.; Wei, Y.-H. CoFe2O4@ ZnS core–shell spheres as magnetically recyclable photocatalysts for hydrogen production. J. Taiwan Ins. Chem. Eng. 2016, 66, 386–393. [Google Scholar] [CrossRef]
- Chang, K.; Hai, X.; Ye, J. Transition Metal Disulfides as Noble-Metal-Alternative Co-Catalysts for Solar Hydrogen Production. Adv. Energy Mater. 2016, 6, 1502555. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, D.; Sun, Z.; Irfan, R.M.; Zhang, L.; Du, P. Cobalt nitride as an efficient cocatalyst on CdS nanorods for enhanced photocatalytic hydrogen production in water. Catal. Sci. Technol. 2017, 7, 1515–1522. [Google Scholar] [CrossRef]
- Chen, T.; Song, C.; Fan, M.; Hong, Y.; Hu, B.; Yu, L.; Shi, W. In-situ fabrication of CuS/gC3N4 nanocomposites with enhanced photocatalytic H2-production activity via photoinduced interfacial charge transfer. Int. J. Hydrogen Energy 2017, 42, 12210–12219. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, G.; Zhou, W.; Xiao, Y.; Wang, J.; Zhang, X.; Fu, H. Enhanced photogenerated carrier separation in CdS quantum dot sensitized ZnFe2O4/ZnIn2S4 nanosheet stereoscopic films for exceptional visible light photocatalytic H2 evolution performance. Nanoscale 2017, 9, 5912–5921. [Google Scholar] [CrossRef]
- Chu, J.; Han, X.; Yu, Z.; Du, Y.; Song, B.; Xu, P. Fabrication of H-TiO2/CdS/Cu2-xS Ternary Heterostructures for Enhanced Photocatalytic Hydrogen Production. ChemistrySelect 2017, 2, 2684–2689. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, P.; Jiang, C.; Cheng, B.; Yu, J. First-principles investigation of Cu-doped ZnS with enhanced photocatalytic hydrogen production activity. Chem. Phys. Lett. 2017, 668, 1–6. [Google Scholar] [CrossRef]
- Du, R.; Zhang, Y.; Li, B.; Yu, X.; Liu, H.; An, X.; Qu, J. Biomolecule-assisted synthesis of defect-mediated Cd1−xZnxS/MoS2/graphene hollow spheres for highly efficient hydrogen evolution. Phys. Chem. Chem. Phys. 2016, 18, 16208–16215. [Google Scholar] [CrossRef]
- Fang, X.; Song, J.; Shi, H.; Kang, S.; Li, Y.; Sun, G.; Cui, L. Enhanced efficiency and stability of Co0.5Cd0.5S/gC3N4 composite photo-catalysts for hydrogen evolution from water under visible light irradiation. Int. J. Hydrogen Energy 2017, 42, 5741–5748. [Google Scholar] [CrossRef]
- Feng, W.; Fang, Z.; Wang, B.; Zhang, L.; Zhang, Y.; Yang, Y.; Huang, M.; Weng, S.; Liu, P. Grain boundary engineering in organic–inorganic hybrid semiconductor ZnS (en) 0.5 for visible-light photocatalytic hydrogen production. J. Mater. Chem. A 2017, 5, 1387–1393. [Google Scholar] [CrossRef]
- García-Mendoza, C.; Oros-Ruiz, S.; Hernández-Gordillo, A.; López, R.; Jácome-Acatitla, G.; Calderón, H.A.; Gómez, R. Suitable preparation of Bi2S3 nanorods–TiO2 heterojunction semiconductors with improved photocatalytic hydrogen production from water/methanol decomposition. J. Chem. Technol. Biotechnol. 2016, 91, 2198–2204. [Google Scholar] [CrossRef]
- Gu, Q.; Sun, H.; Xie, Z.; Gao, Z.; Xue, C. MoS2-coated microspheres of self-sensitized carbon nitride for efficient photocatalytic hydrogen generation under visible light irradiation. Appl. Surf. Sci. 2017, 396, 1808–1815. [Google Scholar] [CrossRef]
- Guo, H.-L.; Du, H.; Jiang, Y.-F.; Jiang, N.; Shen, C.-C.; Zhou, X.; Liu, Y.-N.; Xu, A.-W. Artificial Photosynthetic Z-scheme Photocatalyst for Hydrogen Evolution with High Quantum Efficiency. J. Phys. Chem. C 2016, 121, 107–114. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Qin, Z.; Wang, M.; Guo, L. One-step hydrothermal synthesis of ZnxCd1−xS/ZnO heterostructures for efficient photocatalytic hydrogen production. Int. J. Hydrogen Energy 2016, 41, 15208–15217. [Google Scholar] [CrossRef]
- Ha, E.; Liu, W.; Wang, L.; Man, H.-W.; Hu, L.; Tsang, S.C.E.; Chan, C.T.-L.; Kwok, W.-M.; Lee, L.Y.S.; Wong, K.-Y. Cu2ZnSnS4/MoS2-Reduced Graphene Oxide Heterostructure: Nanoscale Interfacial Contact and Enhanced Photocatalytic Hydrogen Generation. Sci. Rep. 2017, 7, 39411. [Google Scholar] [CrossRef]
- Hai, X.; Zhou, W.; Chang, K.; Pang, H.; Liu, H.; Shi, L.; Ichihara, F.; Ye, J. Engineering the crystallinity of MoS 2 monolayers for highly efficient solar hydrogen production. J. Mater. Chem. A 2017, 5, 8591–8598. [Google Scholar] [CrossRef]
- Hong, S.; Kumar, D.P.; Reddy, D.A.; Choi, J.; Kim, T.K. Excellent photocatalytic hydrogen production over CdS nanorods via using noble metal-free copper molybdenum sulfide (Cu2MoS4) nanosheets as co-catalysts. Appl. Surf. Sci. 2017, 396, 421–429. [Google Scholar] [CrossRef]
- Hu, P.; Ngaw, C.K.; Yuan, Y.; Bassi, P.S.; Loo, S.C.J.; Tan, T.T.Y. Bandgap engineering of ternary sulfide nanocrystals by solution proton alloying for efficient photocatalytic H2 evolution. Nano Energy 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, M. Enhanced Solar Hydrogen Generation by a Heterojunction of Perovskite-type La2Ti2O7 Nanosheets Doped with CdS Quantum Dots. ChemPlusChem 2016, 81, 1202–1208. [Google Scholar] [CrossRef]
- Huang, E.; Yao, X.; Wang, W.; Wu, G.; Guan, N.; Li, L. SnS2 Nanoplates with Specific Facets Exposed for Enhanced Visible-Light-Driven Photocatalysis. ChemPhotoChem 2017, 1, 60–69. [Google Scholar] [CrossRef]
- Huang, H.; Dai, B.; Wang, W.; Lu, C.; Kou, J.; Ni, Y.; Wang, L.; Xu, Z. Oriented Built-in Electric Field Introduced by Surface Gradient Diffusion Doping for Enhanced Photocatalytic H2 Evolution in CdS Nanorods. Nano Lett. 2017, 17, 3803–3808. [Google Scholar] [CrossRef]
- Huang, T.; Chen, W.; Liu, T.-Y.; Hao, Q.-L.; Liu, X.-H. Hybrid of AgInZnS and MoS2 as efficient visible-light driven photocatalyst for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 12254–12261. [Google Scholar] [CrossRef]
- Huang, T.; Chen, W.; Liu, T.-Y.; Hao, Q.-L.; Liu, X.-H. ZnIn2S4 hybrid with MoS2: A non-noble metal photocatalyst with efficient photocatalytic activity for hydrogen evolution. Powder Technol. 2017, 315, 157–162. [Google Scholar] [CrossRef]
- Irfan, R.M.; Jiang, D.; Sun, Z.; Lu, D.; Du, P. Enhanced photocatalytic H2 production on CdS nanorods with simple molecular bidentate cobalt complexes as cocatalysts under visible light. Dalton Trans. 2016, 45, 12897–12905. [Google Scholar] [CrossRef]
- Jiang, F.; Pan, B.; You, D.; Zhou, Y.; Wang, X.; Su, W. Visible light photocatalytic H2-production activity of epitaxial Cu2ZnSnS4/ZnS heterojunction. Catal. Commun. 2016, 85, 39–43. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, J.; Gao, M.; Fan, X.; Zhang, L.; Zhang, J. Assembling Polyoxo-Titanium Clusters and CdS Nanoparticles to a Porous Matrix for Efficient and Tunable H2-Evolution Activities with Visible Light. Adv. Mater. 2017, 29, 1603369. [Google Scholar] [CrossRef]
- Jo, W.-K.; Selvam, N.C.S. Fabrication of photostable ternary CdS/MoS2/MWCNTs hybrid photocatalysts with enhanced H2 generation activity. Appl. Catal. A Gen. 2016, 525, 9–22. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Takanabe, K. Solvent-induced deposition of Cu–Ga–In–S nanocrystals onto a titanium dioxide surface for visible-light-driven photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 184, 264–269. [Google Scholar] [CrossRef]
- Kaur, M.; Nagaraja, C. Template-Free Synthesis of Zn1–xCdxS Nanocrystals with Tunable Band Structure for Efficient Water Splitting and Reduction of Nitroaromatics in Water. ACS Sustain. Chem. Eng. 2017, 5, 4293–4303. [Google Scholar] [CrossRef]
- Kim, Y.G.; Jo, W.-K. Photodeposited-metal/CdS/ZnO heterostructures for solar photocatalytic hydrogen production under different conditions. Int. J. Hydrogen Energy 2017, 42, 11356–11363. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lim, S.K.; Park, H.; Hoffmann, M.R.; Kim, S. Trilayer CdS/carbon nanofiber (CNF) mat/Pt-TiO2 composite structures for solar hydrogen production: Effects of CNF mat thickness. Appl. Catal. B Environ. 2016, 196, 216–222. [Google Scholar] [CrossRef]
- Kimi, M.; Yuliati, L.; Shamsuddin, M. Preparation and characterization of In and Cu co-doped ZnS photocatalysts for hydrogen production under visible light irradiation. J. Energy Chem. 2016, 25, 512–516. [Google Scholar] [CrossRef]
- Kong, Z.; Yuan, Y.-J.; Chen, D.; Fang, G.; Yang, Y.; Yang, S.; Cao, D. Noble-metal-free MoS2 nanosheet modified-InVO4 heterostructures for enhanced visible-light-driven photocatalytic H2 production. Dalton Trans. 2017, 46, 2072–2076. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Hong, S.; Reddy, D.A.; Kim, T.K. Ultrathin MoS2 layers anchored exfoliated reduced graphene oxide nanosheet hybrid as a highly efficient cocatalyst for CdS nanorods towards enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 212, 7–14. [Google Scholar] [CrossRef]
- Leo, I.M.; Soto, E.; Vaquero, F.; Mota, N.; Navarro, R.; Fierro, J. Influence of the reduction of graphene oxide (rGO) on the structure and photoactivity of CdS-rGO hybrid systems. Int. J. Hydrogen Energy 2017, 42, 13691–13703. [Google Scholar]
- Li, M.; Zhang, L.; Fan, X.; Wu, M.; Du, Y.; Wang, M.; Kong, Q.; Zhang, L.; Shi, J. Dual synergetic effects in MoS2/pyridine-modified gC3N4 composite for highly active and stable photocatalytic hydrogen evolution under visible light. Appl. Catal. B Environ. 2016, 190, 36–43. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Liu, S.; Zhang, J.; Chen, W.; Huang, C.; Mao, L. Effect of Pt–Pd hybrid nano-particle on CdS’s activity for water splitting under visible light. Int. J. Hydrogen Energy 2016, 41, 23015–23021. [Google Scholar] [CrossRef]
- Li, Y.; Hou, Y.; Fu, Q.; Peng, S.; Hu, Y.H. Oriented growth of ZnIn2S4/In(OH)3 heterojunction by a facile hydrothermal transformation for efficient photocatalytic H2 production. Appl. Catal. B Environ. 2017, 206, 726–733. [Google Scholar] [CrossRef]
- Li, Y.; Jin, R.; Xing, Y.; Li, J.; Song, S.; Liu, X.; Li, M.; Jin, R. Macroscopic Foam-Like Holey Ultrathin g-C3N4 Nanosheets for Drastic Improvement of Visible-Light Photocatalytic Activity. Adv. Energy Mater. 2016, 6, 1601273. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Shangguan, W.; Su, Y.; Liu, Y.; Dong, X.; Sharma, P.; Zhang, Y. Prickly Ni3S2 nanowires modified CdS nanoparticles for highly enhanced visible-light photocatalytic H2 production. Int. J. Hydrogen Energy 2017, 42, 6618–6626. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Li, H.; Wang, X. Multi-node CdS hetero-nanowires grown with defect-rich oxygen-doped MoS2 ultrathin nanosheets for efficient visible-light photocatalytic H2 evolution. Nano Res. 2017, 10, 1377–1392. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Z.; Zhang, Z.; Ao, D. Novel visible-light driven Mn0.8Cd0.2S/gC3N4 composites: Preparation and efficient photocatalytic hydrogen production from water without noble metals. Appl. Catal. A Gen. 2016, 518, 150–157. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Su, J.; Shi, J.; Wang, X.; Guo, L. Photocatalytic hydrogen production using twinned nanocrystals and an unanchored NiSx co-catalyst. Nat. Energy 2016, 1, 16151. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black N-TiO2-x@ MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2017, 201, 119–127. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, C. Enhancement of photocatalytic H2 evolution over TiO2 nano-sheet films by surface loading NiS nanoparticles. Russ. J. Phys. Chem. A 2016, 90, 1042–1048. [Google Scholar] [CrossRef]
- Lu, D.; Wang, H.; Zhao, X.; Kondamareddy, K.K.; Ding, J.; Li, C.; Fang, P. Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen. ACS Sustain. Chem. Eng. 2017, 5, 1436–1445. [Google Scholar] [CrossRef]
- Ma, L.; Chen, K.; Nan, F.; Wang, J.H.; Yang, D.J.; Zhou, L.; Wang, Q.Q. Improved Hydrogen Production of Au–Pt–CdS Hetero-Nanostructures by Efficient Plasmon-Induced Multipathway Electron Transfer. Adv. Funct. Mater. 2016, 26, 6076–6083. [Google Scholar] [CrossRef]
- Ma, X.; Li, J.; An, C.; Feng, J.; Chi, Y.; Liu, J.; Zhang, J.; Sun, Y. Ultrathin Co (Ni)-doped MoS- nanosheets as catalytic promoters enabling efficient solar hydrogen production. Nano Res. 2016, 9, 2284–2293. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Hussain, E.; Badshah, A.; Gilani, R.; Nadeem, M.A. Effect of deposition method on metal loading and photocatalytic activity of Au/CdS for hydrogen production in water electrolyte mixture. Int. J. Hydrogen Energy 2017, 42, 3006–3018. [Google Scholar] [CrossRef]
- Malekshoar, G.; Ray, A.K. In-situ grown molybdenum sulfide on TiO2 for dye-sensitized solar photocatalytic hydrogen generation. Chem. Eng. Sci. 2016, 152, 35–44. [Google Scholar] [CrossRef]
- Mancipe, S.; Tzompantzi, F.; Gómez, R. Synthesis of CdS/MgAl layered double hydroxides for hydrogen production from methanol-water decomposition. Appl. Clay Sci. 2017, 136, 67–74. [Google Scholar] [CrossRef]
- Manjunath, K.; Souza, V.; Nagaraju, G.; Santos, J.M.L.; Dupont, J.; Ramakrishnappa, T. Superior activity of the CuS–TiO2/Pt hybrid nanostructure towards visible light induced hydrogen production. New J. Chem. 2016, 40, 10172–10180. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, M.; Schneider, J.; Wang, W.; Zhang, N.; Su, Y.; Chen, B.; Wang, S.; Rogach, A.L.; Pan, F. Hexagonal Zn1−xCdxS (0.2 ≤ x ≤ 1) solid solution photocatalysts for H2 generation from water. Catal. Sci. Technol. 2017, 7, 982–987. [Google Scholar] [CrossRef]
- Nandy, S.; Goto, Y.; Hisatomi, T.; Moriya, Y.; Minegishi, T.; Katayama, M.; Domen, K. Synthesis and Photocatalytic Activity of La5Ti2Cu (S1−xSex)5O7 Solid Solutions for H2 Production under Visible Light Irradiation. ChemPhotoChem 2017, 1, 265–272. [Google Scholar] [CrossRef]
- Núñez, J.; Fresno, F.; Collado, L.; Jana, P.; Coronado, J.M.; Serrano, D.P.; Víctor, A. Photocatalytic H2 production from aqueous methanol solutions using metal-co-catalysed Zn2SnO4 nanostructures. Appl. Catal. B Environ. 2016, 191, 106–115. [Google Scholar] [CrossRef]
- Oros-Ruiz, S.; Hernández-Gordillo, A.; García-Mendoza, C.; Rodríguez-Rodríguez, A.A.; Gomez, R. Comparative activity of CdS nanofibers superficially modified by Au, Cu, and Ni nanoparticles as co-catalysts for photocatalytic hydrogen production under visible light. J. Chem. Technol. Biotechnol. 2016, 91, 2205–2210. [Google Scholar] [CrossRef]
- Park, H.; Ou, H.-H.; Kim, M.; Kang, U.; Han, D.S.; Hoffmann, M.R. Photocatalytic H2 production on trititanate nanotubes coupled with CdS and platinum nanoparticles under visible light: Revisiting H2 production and material durability. Faraday Discuss. 2017, 198, 419–431. [Google Scholar] [CrossRef]
- Qiu, F.; Han, Z.; Peterson, J.J.; Odoi, M.Y.; Sowers, K.L.; Krauss, T.D. Photocatalytic hydrogen generation by CdSe/CdS nanoparticles. Nano Lett. 2016, 16, 5347–5352. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Davey, K.; Qiao, S.Z. Counteracting Blueshift Optical Absorption and Maximizing Photon Harvest in Carbon Nitride Nanosheets Photocatalyst. Small 2017, 13, 1700376. [Google Scholar] [CrossRef]
- Rahmawati, F.; Yuliati, L.; Alaih, I.S.; Putri, F.R. Carbon rod of zinc-carbon primary battery waste as a substrate for CdS and TiO2 photocatalyst layer for visible light driven photocatalytic hydrogen production. J. Environ. Chem. Eng. 2017, 5, 2251–2258. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Rao, H.; Yu, W.-Q.; Zheng, H.-Q.; Bonin, J.; Fan, Y.-T.; Hou, H.-W. Highly efficient photocatalytic hydrogen evolution from nickel quinolinethiolate complexes under visible light irradiation. J. Power Sources 2016, 324, 253–260. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Hong, S.; Kumar, D.P.; Kim, T.K. Hydrazine-assisted formation of ultrathin MoS2 nanosheets for enhancing their co-catalytic activity in photocatalytic hydrogen evolution. J. Mater. Chem. A 2017, 5, 6981–6991. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Ma, R.; Kumar, D.P.; Lim, M.; Kim, T.K. Heterostructured WS2-MoS2 Ultrathin Nanosheets Integrated on CdS Nanorods to Promote Charge Separation and Migration and Improve Solar-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 2017, 10, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Dong, X.; Dang, H. Facile fabrication of novel red phosphorus-CdS composite photocatalysts for H2 evolution under visible light irradiation. Int. J. Hydrogen Energy 2016, 41, 5908–5915. [Google Scholar] [CrossRef]
- Sola, A.; Homs, N.; de la Piscina, P.R. Photocatalytic H2 production from ethanol (aq) solutions: The effect of intermediate products. Int. J. Hydrogen Energy 2016, 41, 19629–19636. [Google Scholar] [CrossRef]
- Souza, E.A.; Silva, L.A. Energy recovery from tannery sludge wastewaters through photocatalytic hydrogen production. J. Environ. Chem. Eng. 2016, 4, 2114–2120. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Li, Y.; Chen, Y.; Liu, M. Photocatalytic activities of copper doped cadmium sulfide microspheres prepared by a facile ultrasonic spray-pyrolysis method. Molecules 2016, 21, 735. [Google Scholar] [CrossRef]
- Vaquero, F.; Navarro, R.; Fierro, J. Evolution of the nanostructure of CdS using solvothermal synthesis at different temperature and its influence on the photoactivity for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 11558–11567. [Google Scholar] [CrossRef]
- Sun, S.; Gao, P.; Yang, Y.; Yang, P.; Chen, Y.; Wang, Y. N-doped TiO2 nanobelts with coexposed (001) and (101) facets and their highly efficient visible-light-driven photocatalytic hydrogen production. ACS Appl. Mater. Interfaces 2016, 8, 18126–18131. [Google Scholar] [CrossRef]
- Wang, F.; Jin, Z.; Jiang, Y.; Backus, E.H.; Bonn, M.; Lou, S.N.; Turchinovich, D.; Amal, R. Probing the charge separation process on In2S3/Pt-TiO2 nanocomposites for boosted visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 198, 25–31. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Shu, D.; Chen, X.; Liu, X.; Wang, X.; Zhang, J.; Wang, H. CoPtx-loaded Zn0.5Cd0.5S nanocomposites for enhanced visible light photocatalytic H2 production. Int. J. Energy Res. 2016, 40, 1280–1286. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhou, W.; Tian, G.; Xiao, Y.; Fu, H.; Fu, H. Cubic quantum dot/hexagonal microsphere ZnIn2S4 heterophase junctions for exceptional visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. A 2017, 5, 8451–8460. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhu, Z. Synergetic effect of Ni(OH)2 cocatalyst and CNT for high hydrogen generation on CdS quantum dot sensitized TiO2 photocatalyst. Appl. Catal. B Environ. 2017, 204, 577–583. [Google Scholar] [CrossRef]
- Wang, L.; Di, Q.; Sun, M.; Liu, J.; Cao, C.; Liu, J.; Xu, M.; Zhang, J. Assembly-promoted photocatalysis: Three-dimensional assembly of CdSxSe1−x (x = 0–1) quantum dots into nanospheres with enhanced photocatalytic performance. J. Mater. 2017, 3, 63–70. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Jiang, Z.; Jiang, G.; Zhao, Z.; Wu, Q.; Liu, Y.; Xu, Q.; Duan, A.; Xu, C. Controlled fabrication and enhanced visible-light photocatalytic hydrogen production of Au@ CdS/MIL-101 heterostructure. Appl. Catal. B Environ. 2016, 185, 307–314. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Liu, J.; Jiang, W.; Zhou, Y.; An, C.; Zhang, J. Synthesis of AgInS2-xAg2S-yZnS-zIn6S7 (x, y, z = 0, or 1) Nanocomposites with Composition-Dependent Activity towards Solar Hydrogen Evolution. Materials 2016, 9, 329. [Google Scholar] [CrossRef]
- Wu, A.; Tian, C.; Jiao, Y.; Yan, Q.; Yang, G.; Fu, H. Sequential two-step hydrothermal growth of MoS2/CdS core-shell heterojunctions for efficient visible light-driven photocatalytic H2 evolution. Appl. Catal. B Environ. 2017, 203, 955–963. [Google Scholar] [CrossRef]
- Wu, L.; Gong, J.; Ge, L.; Han, C.; Fang, S.; Xin, Y.; Li, Y.; Lu, Y. AuPd bimetallic nanoparticles decorated Cd0.5Zn0.5S photocatalysts with enhanced visible-light photocatalytic H2 production activity. Int. J. Hydrogen Energy 2016, 41, 14704–14712. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Q.; Lv, K.; Tang, D.; Li, M. Superiority of graphene over carbon analogs for enhanced photocatalytic H2-production activity of ZnIn2S4. Appl. Catal. B Environ. 2017, 206, 344–352. [Google Scholar] [CrossRef]
- Xin, Y.; Lu, Y.; Han, C.; Ge, L.; Qiu, P.; Li, Y.; Fang, S. Novel NiS cocatalyst decorating ultrathin 2D TiO2 nanosheets with enhanced photocatalytic hydrogen evolution activity. Mater. Res. Bull. 2017, 87, 123–129. [Google Scholar] [CrossRef]
- Xing, Z.; Zong, X.; Zhu, Y.; Chen, Z.; Bai, Y.; Wang, L. A nanohybrid of CdTe@ CdS nanocrystals and titania nanosheets with p–n nanojunctions for improved visible light-driven hydrogen production. Catal. Today 2016, 264, 229–235. [Google Scholar] [CrossRef]
- Yan, J.; Li, X.; Yang, S.; Wang, X.; Zhou, W.; Fang, Y.; Zhang, S.; Peng, F.; Zhang, S. Design and preparation of CdS/H-3D-TiO2/Pt-wire photocatalysis system with enhanced visible-light driven H2 evolution. Int. J. Hydrogen Energy 2017, 42, 928–937. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, A.; Yan, H.; Dong, Y.; Tian, C.; Jiang, B.; Fu, H. Gelatin-assisted synthesis of ZnS hollow nanospheres: The microstructure tuning, formation mechanism and application for Pt-free photocatalytic hydrogen production. CrystEngComm 2017, 19, 461–468. [Google Scholar] [CrossRef]
- Yang, L.; Guo, S.; Li, X. Au nanoparticles@ MoS2 core-shell structures with moderate MoS2 coverage for efficient photocatalytic water splitting. J. Alloys Compd. 2017, 706, 82–88. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Fang, Z.; Zhang, L.; Zheng, Z.; Wang, Z.; Feng, W.; Weng, S.; Zhang, S.; Liu, P. Simultaneous Realization of Enhanced Photoactivity and Promoted Photostability by Multilayered MoS2 Coating on CdS Nanowire Structure via Compact Coating Methodology. ACS Appl. Mater. Interfaces 2017, 9, 6950–6958. [Google Scholar] [CrossRef]
- Yu, X.; Shi, J.; Wang, L.; Wang, W.; Bian, J.; Feng, L.; Li, C. A novel Au NPs-loaded MoS2/RGO composite for efficient hydrogen evolution under visible light. Mater. Lett. 2016, 182, 125–128. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Chen, D.Q.; Huang, Y.W.; Yu, Z.T.; Zhong, J.S.; Chen, T.T.; Tu, W.G.; Guan, Z.J.; Cao, D.P.; Zou, Z.G. MoS2 Nanosheet-Modified CuInS2 Photocatalyst for Visible-Light-Driven Hydrogen Production from Water. ChemSusChem 2016, 9, 1003–1009. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Tu, J.-R.; Ye, Z.-J.; Chen, D.-Q.; Hu, B.; Huang, Y.-W.; Chen, T.-T.; Cao, D.-P.; Yu, Z.-T.; Zou, Z.-G. MoS2-graphene/ZnIn2S4 hierarchical microarchitectures with an electron transport bridge between light-harvesting semiconductor and cocatalyst: A highly efficient photocatalyst for solar hydrogen generation. Appl. Catal. B Environ. 2016, 188, 13–22. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, A.; Zhang, C.; Huang, J.; Zhu, M.; Du, Y.; Yang, P. Noble-metal-free hetero-structural CdS/Nb2O5/N-doped-graphene ternary photocatalytic system as visible-light-driven photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2017, 201, 202–210. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, W.; Huang, C.; Shi, P.; Xu, Q. High efficiency and stable tungsten phosphide cocatalysts for photocatalytic hydrogen production. J. Mater. Chem. A 2017, 5, 12513–12519. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, D.; Cai, B.; Wang, S.; Niu, F.; Qin, L.; Huang, Y. Facile synthesis of CdS ZnWO4 composite photocatalysts for efficient visible light driven hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 1962–1969. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Zeng, Y.; Xu, Y.; Tang, Y.; Luo, S.; Liu, Y.; Liu, C. CdS-Nanoparticles-Decorated Perpendicular Hybrid of MoS2 and N-Doped Graphene Nanosheets for Omnidirectional Enhancement of Photocatalytic Hydrogen Evolution. ChemCatChem 2016, 8, 2557–2564. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Wang, C.; Wang, W.; Ling, T.; Yang, J.; Dong, C.; Lin, F.; Du, X.-W. Zinc-Blende CdS Nanocubes with Coordinated Facets for Photocatalytic Water Splitting. ACS Catal. 2017, 7, 1470–1477. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, R.; Li, X.; Sun, X. Enhanced photocatalytic activity for hydrogen evolution from water by Zn0.5Cd0.5S/WS2 heterostructure. Mater. Sci. Semicond. Process. 2017, 59, 68–75. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, J.; Zhang, H.; Sun, H.; Tu, W. Controlled synthesis of CdS nanoparticles and their surface loading with MoS 2 for hydrogen evolution under visible light. Int. J. Hydrogen Energy 2016, 41, 14758–14767. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, X.; Zhang, Z.; Zhang, L.; Wang, Y.; Sun, Z.; Irfan, R.M.; Du, P. Highly efficient simultaneous hydrogen evolution and benzaldehyde production using cadmium sulfide nanorods decorated with small cobalt nanoparticles under visible light. J. Catal. 2018, 357, 147–153. [Google Scholar] [CrossRef]
- Kumar, D.P.; Park, H.; Kim, E.H.; Hong, S.; Gopannagari, M.; Reddy, D.A.; Kim, T.K. Noble metal-free metal-organic framework-derived onion slice-type hollow cobalt sulfide nanostructures: Enhanced activity of CdS for improving photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 224, 230–238. [Google Scholar] [CrossRef]
- Lv, J.-X.; Zhang, Z.-M.; Wang, J.; Lu, X.-L.; Zhang, W.; Lu, T.-B. In situ synthesis of CdS/graphdiyne heterojunction for enhanced photocatalytic activity of hydrogen production. ACS Appl. Mater. Interfaces 2019, 11, 2655–2661. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Hou, J.; Li, J.; Li, X.; Xu, L.; Sun, M.; Zeng, R. Effectively enhanced photocatalytic hydrogen production performance of one-pot synthesized MoS2 clusters/CdS nanorod heterojunction material under visible light. Chem. Eng. J. 2018, 345, 404–413. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Liu, W.; Shang, Y.; Zhu, A.; Tan, P.; Xiong, X.; Pan, J. Facet and morphology dependent photocatalytic hydrogen evolution with CdS nanoflowers using a novel mixed solvothermal strategy. J. Colloid Interface Sci. 2018, 513, 222–230. [Google Scholar] [CrossRef]
- Wang, L.; Xu, N.; Pan, X.; He, Y.; Wang, X.; Su, W. Cobalt lactate complex as a hole cocatalyst for significantly enhanced photocatalytic H2 production activity over CdS nanorods. Catal. Sci. Technol. 2018, 8, 1599–1605. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Ahmed, A.Y.; Kandiel, T.A.; Ivanova, I.; Bahnemann, D. Photocatalytic and photoelectrochemical oxidation mechanisms of methanol on TiO2 in aqueous solution. Appl. Surf. Sci. 2014, 319, 44–49. [Google Scholar] [CrossRef]
- Al-Ahmed, A.; Mukhtar, B.; Hossain, S.; Javaid Zaidi, S.; Rahman, S. Application of Titanium dioxide (TiO2) based photocatalytic nanomaterials in Solar and Hydrogen Energy: A Short Review. Mater. Sci. Forum 2012, 712, 25–47. [Google Scholar] [CrossRef]
- Amao, Y. Solar fuel production based on the artificial photosynthesis system. ChemCatChem 2011, 3, 458–474. [Google Scholar] [CrossRef]
- An, X.; Jimmy, C.Y. Graphene-based photocatalytic composites. RSC Adv. 2011, 1, 1426–1434. [Google Scholar] [CrossRef]
- Ashokkumar, M. An overview on semiconductor particulate systems for photoproduction of hydrogen. Int. J. Hydrogen Energy 1998, 23, 427–438. [Google Scholar] [CrossRef]
- Babu, V.J.; Vempati, S.; Uyar, T.; Ramakrishna, S. Review of one-dimensional and two-dimensional nanostructured materials for hydrogen generation. Phys. Chem. Chem. Phys. 2015, 17, 2960–2986. [Google Scholar] [CrossRef]
- Bai, S.; Yin, W.; Wang, L.; Li, Z.; Xiong, Y. Surface and interface design in cocatalysts for photocatalytic water splitting and CO2 reduction. RSC Adv. 2016, 6, 57446–57463. [Google Scholar] [CrossRef]
- Bowker, M. Sustainable hydrogen production by the application of ambient temperature photocatalysis. Green Chem. 2011, 13, 2235–2246. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Colón, G. Towards the hydrogen production by photocatalysis. Appl. Catal. A Gen. 2016, 518, 48–59. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Fornasiero, P.; Christoforidis, K.C. Photocatalytic Hydrogen production: A rift into the future energy supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Gholipour, M.R.; Dinh, C.-T.; Béland, F.; Do, T.-O. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Guo, L.; Jing, D.; Liu, M.; Chen, Y.; Shen, S.; Shi, J.; Zhang, K. Functionalized nanostructures for enhanced photocatalytic performance under solar light. Beilstein J. Nanotechnol. 2014, 5, 994–1004. [Google Scholar] [CrossRef]
- Han, B.; Hu, Y.H. MoS2 as a co-catalyst for photocatalytic hydrogen production from water. Energy Sci. Eng. 2016, 4, 285–304. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Shen, W.; Rahman, Z.U.; Wang, D. Recent progress in red semiconductor photocatalysts for solar energy conversion and utilization. Nanotechnol. Rev. 2016, 5, 135–145. [Google Scholar] [CrossRef]
- Junge, H.; Rockstroh, N.; Fischer, S.; Brückner, A.; Ludwig, R.; Lochbrunner, S.; Kühn, O.; Beller, M. Light to Hydrogen: Photocatalytic Hydrogen Generation from Water with Molecularly-Defined Iron Complexes. Inorganics 2017, 5, 14. [Google Scholar] [CrossRef]
- Kagkoura, A.; Skaltsas, T.; Tagmatarchis, N. Transition metal chalcogenides/graphene ensembles for light-induced energy applications. Chem. Eur. J. 2017, 23, 12967–12979. [Google Scholar] [CrossRef]
- Kitano, M.; Tsujimaru, K.; Anpo, M. Hydrogen production using highly active titanium oxide-based photocatalysts. Top. Catal. 2008, 49, 4. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sundaramurthy, J.; Sundarrajan, S.; Babu, V.J.; Singh, G.; Allakhverdiev, S.I.; Ramakrishna, S. Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ. Sci. 2014, 7, 3192–3222. [Google Scholar] [CrossRef]
- Leung, D.Y.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.; Wang, X.; Fu, X. Hydrogen Production over Titania-Based Photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-free photocatalysts for various applications in energy conversion and environmental purification. Green Chem. 2017, 19, 882–899. [Google Scholar] [CrossRef]
- Francesco, P.; Fabrizio, S.; Marco, M.; Claudio, M.; Valter, M. The Role of Surface Texture on the Photocatalytic H2Production on TiO2. Catalysts 2019, 9, 32. [Google Scholar]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B Environ. 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in photocatalysis: A review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.-L.; Sa, B.; Ahuja, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Martha, S.; Sahoo, P.C.; Parida, K. An overview on visible light responsive metal oxide based photocatalysts for hydrogen energy production. RSC Adv. 2015, 5, 61535–61553. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kitano, M.; Takeuchi, M.; Tsujimaru, K.; Anpo, M.; Thomas, J.M. Photocatalysis for new energy production: Recent advances in photocatalytic water splitting reactions for hydrogen production. Catal. Today 2007, 122, 51–61. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M. Design of graphene-based TiO2 photocatalysts—A review. Environ. Sci. Pollut. Res. 2012, 19, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Phan, T.-D.; Baber, A.E.; Rodriguez, J.A.; Senanayake, S.D. Au and Pt nanoparticle supported catalysts tailored for H2 production: From models to powder catalysts. Appl. Catal. A Gen. 2016, 518, 18–47. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Nurlaela, E.; Ziani, A.; Takanabe, K. Tantalum nitride for photocatalytic water splitting: Concept and applications. Mater. Renew. Sustain. Energy 2016, 5, 18. [Google Scholar] [CrossRef]
- Pasternak, S.; Paz, Y. On the similarity and dissimilarity between photocatalytic water splitting and photocatalytic degradation of pollutants. ChemPhysChem 2013, 14, 2059–2070. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production. Mater. Sci. Semicond. Process. 2013, 16, 561–575. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; García, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef]
- Protti, S.; Albini, A.; Serpone, N. Photocatalytic generation of solar fuels from the reduction of H2O and CO2: A look at the patent literature. Phys. Chem. Chem. Phys. 2014, 16, 19790–19827. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Samokhvalov, A. Hydrogen by photocatalysis with nitrogen codoped titanium dioxide. Renew. Sustain. Energy Rev. 2017, 72, 981–1000. [Google Scholar] [CrossRef]
- Serrano, D.P.; Coronado, J.M.; Víctor, A.; Pizarro, P.; Botas, J.Á. Advances in the design of ordered mesoporous materials for low-carbon catalytic hydrogen production. J. Mater. Chem. A 2013, 1, 12016–12027. [Google Scholar] [CrossRef]
- Sharma, P.; Kolhe, M.L. Review of sustainable solar hydrogen production using photon fuel on artificial leaf. Int. J. Hydrogen Energy 2017, 42, 22704–22712. [Google Scholar] [CrossRef]
- Shi, J.; Guo, L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mater. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Stroyuk, A.; Kryukov, A.; Kuchmii, S.Y.; Pokhodenko, V. Semiconductor photocatalytic systems for the production of hydrogen by the action of visible light. Theor. Exp. Chem. 2009, 45, 209. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Peng, X.; Zeng, G.; Zhong, H.; Liang, J.; Ren, M. Graphene-based materials: Fabrication, characterization and application for the decontamination of wastewater and wastegas and hydrogen storage/generation. Adv. Colloid Interface Sci. 2013, 195, 19–40. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Wang, L. Strategies for efficient solar water splitting using carbon nitride. Chem. Asian J. 2017, 12, 1421–1434. [Google Scholar]
- Wang, M.; Han, K.; Zhang, S.; Sun, L. Integration of organometallic complexes with semiconductors and other nanomaterials for photocatalytic H2 production. Coord. Chem. Rev. 2015, 287, 1–14. [Google Scholar] [CrossRef]
- Watanabe, M. Dye-sensitized photocatalyst for effective water splitting catalyst. Sci. Technol. Adv. Mater. 2017, 18, 705–723. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kuwahara, Y.; An, T.; Yamashita, H. Design and architecture of metal organic frameworks for visible light enhanced hydrogen production. Appl. Catal. B Environ. 2017, 218, 555–569. [Google Scholar] [CrossRef]
- Xiao, F.X.; Miao, J.; Tao, H.B.; Hung, S.F.; Wang, H.Y.; Yang, H.B.; Chen, J.; Chen, R.; Liu, B. One-Dimensional Hybrid Nanostructures for Heterogeneous Photocatalysis and Photoelectrocatalysis. Small 2015, 11, 2115–2131. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, K.; Guo, B.; Liu, Q.; Fang, L.; Gong, J.R. Graphene-Based Materials for Hydrogen Generation from Light-Driven Water Splitting. Adv. Mater. 2013, 25, 3820–3839. [Google Scholar] [CrossRef]
- Xing, J.; Fang, W.Q.; Zhao, H.J.; Yang, H.G. Inorganic photocatalysts for overall water splitting. Chem. Asian J. 2012, 7, 642–657. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R. Nickel-based cocatalysts for photocatalytic hydrogen production. Appl. Surf. Sci. 2015, 351, 779–793. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Hydrogen photogeneration from water on the biomimetic hybrid artificial photocatalytic systems of semiconductors and earth-abundant metal complexes: Progress and challenges. Catal. Sci. Technol. 2015, 5, 3084–3096. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A review on gC3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Yin, S.; Han, J.; Zhou, T.; Xu, R. Recent progress in gC3N4 based low cost photocatalytic system: Activity enhancement and emerging applications. Catal. Sci. Technol. 2015, 5, 5048–5061. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Lu, H.W.; Yu, Z.T.; Zou, Z.G. Noble-Metal-Free Molybdenum Disulfide Cocatalyst for Photocatalytic Hydrogen Production. ChemSusChem 2015, 8, 4113–4127. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef]
- Zhang, Q.; Gangadharan, D.T.; Liu, Y.; Xu, Z.; Chaker, M.; Ma, D. Recent advancements in plasmon-enhanced visible light-driven water splitting. J. Mater. 2017, 3, 33–50. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, P.; Long, M. Electro- and Photocatalytic Hydrogen Production by Molecular Cobalt Complexes with Pentadentate Ligands. Comments Inorg. Chem. 2017, 37, 238–270. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Shwetharani, R.; Sakar, M.; Fernando, C.; Binas, V.; Balakrishna, R.G. Recent advances and strategies to tailor the energy levels, active sites and electron mobility in titania and its doped/composite analogues for hydrogen evolution in sunlight. Catal. Sci. Technol. 2019, 9, 12–46. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Chen, D.; Yu, Z.-T.; Zou, Z.-G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A 2018, 6, 11606–11630. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Ye, Z.-J.; Lu, H.-W.; Hu, B.; Li, Y.-H.; Chen, D.-Q.; Zhong, J.-S.; Yu, Z.-T.; Zou, Z.-G. Constructing anatase TiO2 nanosheets with exposed (001) facets/layered MoS2 two-dimensional nanojunctions for enhanced solar hydrogen generation. ACS Catal. 2015, 6, 532–541. [Google Scholar] [CrossRef]
- Liu, S.-H.; Tang, W.-T.; Lin, W.-X. Self-assembled ionic liquid synthesis of nitrogen-doped mesoporous TiO2 for visible-light-responsive hydrogen production. Int. J. Hydrogen Energy 2017, 42, 24006–24013. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X.; Zhu, C.; Shi, C. Chromium-modified Bi4Ti3O12 photocatalyst: Application for hydrogen evolution and pollutant degradation. Appl. Catal. B Environ. 2016, 199, 241–251. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Yu, H.; Zhang, S.; Fang, Y.; Zhang, S.; Peng, F. A facile fabrication of hierarchical Ag nanoparticles-decorated N-TiO2 with enhanced photocatalytic hydrogen production under solar light. Int. J. Hydrogen Energy 2016, 41, 3446–3455. [Google Scholar] [CrossRef]
- Silva, L.A.; Ryu, S.Y.; Choi, J.; Choi, W.; Hoffmann, M.R. Photocatalytic hydrogen production with visible light over Pt-interlinked hybrid composites of cubic-phase and hexagonal-phase CdS. J. Phys. Chem. C 2008, 112, 12069–12073. [Google Scholar] [CrossRef]
- Qin, N.; Xiong, J.; Liang, R.; Liu, Y.; Zhang, S.; Li, Y.; Li, Z.; Wu, L. Highly efficient photocatalytic H2 evolution over MoS2/CdS-TiO2 nanofibers prepared by an electrospinning mediated photodeposition method. Appl. Catal. B Environ. 2017, 202, 374–380. [Google Scholar] [CrossRef]
- Gopannagari, M.; Kumar, D.P.; Reddy, D.A.; Hong, S.; Song, M.I.; Kim, T.K. In situ preparation of few-layered WS2 nanosheets and exfoliation into bilayers on CdS nanorods for ultrafast charge carrier migrations toward enhanced photocatalytic hydrogen production. J. Catal. 2017, 351, 153–160. [Google Scholar] [CrossRef]
- Wang, M.; Na, Y.; Gorlov, M.; Sun, L. Light-driven hydrogen production catalysed by transition metal complexes in homogeneous systems. Dalton Trans. 2009, 6458–6467. [Google Scholar] [CrossRef] [PubMed]
- Linkous, C.A.; Huang, C.; Fowler, J.R. UV photochemical oxidation of aqueous sodium sulfide to produce hydrogen and sulfur. J. Photochem. Photobiol. A Chem. 2004, 168, 153–160. [Google Scholar] [CrossRef]
- Huang, C.; Linkous, C.A.; Adebiyi, O.; T-Raissi, A. Hydrogen production via photolytic oxidation of aqueous sodium sulfite solutions. Environ. Sci. Technol. 2010, 44, 5283–5288. [Google Scholar] [CrossRef]
- Li, C.; Hu, P.; Meng, H.; Jiang, Z. Role of Sulfites in the Water Splitting Reaction. J. Solut. Chem. 2016, 45, 67–80. [Google Scholar] [CrossRef]
- Husin, H.; Adisalamun, S.Y.; Asnawi, T.M.; Hasfita, F. Pt nanoparticle on La0.02Na0.98TaO3 catalyst for hydrogen evolution from glycerol aqueous solution. AIP Conf. Proc. 2017, 1788, 030073. [Google Scholar]
- López-Tenllado, F.; Hidalgo-Carrillo, J.; Montes, V.; Marinas, A.; Urbano, F.; Marinas, J.; Ilieva, L.; Tabakova, T.; Reid, F. A comparative study of hydrogen photocatalytic production from glycerol and propan-2-ol on M/TiO2 systems (M = Au, Pt, Pd). Catal. Today 2017, 280, 58–64. [Google Scholar] [CrossRef]
- Li, F.; Gu, Q.; Niu, Y.; Wang, R.; Tong, Y.; Zhu, S.; Zhang, H.; Zhang, Z.; Wang, X. Hydrogen evolution from aqueous-phase photocatalytic reforming of ethylene glycol over Pt/TiO2 catalysts: Role of Pt and product distribution. Appl. Surf. Sci. 2017, 391, 251–258. [Google Scholar] [CrossRef]
- Oscar, Q.C.; Socorro, O.R.; Solís-Gómezb, A.; Rosendo, L.; Ricardo, G. Enhanced photocatalytic hydrogen production by CdS nanofibers modified with graphene oxide and nickel nanoparticles under visible light. Fuel 2019, 237, 227–235. [Google Scholar]
- Andrea, S.; Francesca, G.; Federica, M.; Michela, S.; Daniele, D.; Lorenzo, M.; Antonella, P. Photocatalytic hydrogen evolution assisted by aqueous (waste)biomass under simulated solar light: Oxidized g-C3N4 vs. P25 titanium dioxide. Int. J. Hydrogen Energy 2019, 44, 4072–4078. [Google Scholar]
- Tao, C.; Jie, M.; Qingyun, L.; Xiao, W.; Jixue, L.; Ze, Z. One-step synthesis of hollow BaZrO3 nanocrystals with oxygen vacancies for photocatalytic hydrogen evolution from pure water. J. Alloys Compd. 2019, 780, 498–503. [Google Scholar]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Pedrono, F. New insights into the mechanism of photocatalytic reforming on Pd/TiO2. Appl. Catal. B Environ. 2011, 107, 205–209. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Leung, D.Y.; Gu, Q.; Chen, S.; Huang, H. Photocatalytic reforming of C3-polyols for H2 production: Part (I). Role of their OH groups. Appl. Catal. B Environ. 2011, 106, 681–688. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Sauer, M.C.; Gosztola, D. Efficient, rapid photooxidation of chemisorbed polyhydroxyl alcohols and carbohydrates by TiO2 nanoparticles in an aqueous solution. J. Phys. Chem. B 2004, 108, 12512–12517. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Sauer, M.C. Hole Scavenging and Photo-Stimulated Recombination of Electron—Hole Pairs in Aqueous TiO2 Nanoparticles. J. Phys. Chem. B 2004, 108, 12497–12511. [Google Scholar] [CrossRef]
- Du, M.-H.; Feng, J.; Zhang, S. Photo-oxidation of polyhydroxyl molecules on TiO2 surfaces: From hole scavenging to light-induced self-assembly of TiO2-cyclodextrin wires. Phys. Rev. Lett. 2007, 98, 066102. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Huang, F. Enhanced visible light photocatalytic H2 evolution of metal-free g-C3N4/SiC heterostructured photocatalysts. Appl. Surf. Sci. 2017, 391, 449–456. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Lu, L.; Si, Y.; Zhang, S.; Chen, Y.; Dai, K.; Duan, P.; Duan, L.; Liu, J. Graphitic carbon nitride nanosheet for photocatalytic hydrogen production: The impact of morphology and element composition. Appl. Surf. Sci. 2017, 391, 369–375. [Google Scholar] [CrossRef]
- Fang, L.J.; Wang, X.L.; Li, Y.H.; Liu, P.F.; Wang, Y.L.; Zeng, H.D.; Yang, H.G. Nickel nanoparticles coated with graphene layers as efficient co-catalyst for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2017, 200, 578–584. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.T.; et al. Liquid exfoliation of g-C3N4 nanosheets to construct 2D-2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity. Appl. Catal. B Environ. 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Cai, J.; Shen, J.; Zhang, X.; Ng, Y.H.; Huang, J.; Guo, W.; Lin, C.; Lai, Y. Light-Driven Sustainable Hydrogen Production Utilizing TiO2 Nanostructures: A Review. Small 2019, 3, 1800184. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Jin, J.; Liu, J.; Li, Y.; Wu, M.; Chen, L.; Wang, B.; Yang, X.; Su, B.-L. Probing effective photocorrosion inhibition and highly improved photocatalytic hydrogen production on monodisperse PANI@ CdS core-shell nanospheres. Appl. Catal. B Environ. 2016, 188, 351–359. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.; Sun, R.; Li, X.; Sun, D. An efficient hydrogen evolution catalyst composed of palladium phosphorous sulphide (PdP ~ 0.33 S ~ 1.67) and twin nanocrystal Zn0.5Cd0.5S solid solution with both homo-and hetero-junctions. Energy Environ. Sci. 2017, 10, 225–235. [Google Scholar] [CrossRef]
- Ma, S.; Xie, J.; Wen, J.; He, K.; Li, X.; Liu, W.; Zhang, X. Constructing 2D layered hybrid CdS nanosheets/MoS2 heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2017, 391, 580–591. [Google Scholar] [CrossRef]
- Cheng, F.; Yin, H.; Xiang, Q. Low-temperature solid-state preparation of ternary CdS/g-C3N4/CuS nanocomposites for enhanced visible-light photocatalytic H2-production activity. Appl. Surf. Sci. 2017, 391, 432–439. [Google Scholar] [CrossRef]
- Tian, F.; Hou, D.; Hu, F.; Xie, K.; Qiao, X.; Li, D. Pouous TiO2 nanofibers decorated CdS nanoparticles by SILAR method for enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2017, 391, 295–302. [Google Scholar] [CrossRef]
- Vignesh, K.; Suganthi, A.; Min, B.-K.; Kang, M. Photocatalytic activity of magnetically recoverable MnFe2O4/g-C3N4/TiO2 nanocomposite under simulated solar light irradiation. J. Mol. Catal. A Chem. 2014, 395, 373–383. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Yang, B.; Wu, Y.; Li, Z.; Lu, G. The enhancement of CdS photocatalytic activity for water splitting via anti-photocorrosion by coating Ni2P shell and removing nascent formed oxygen with artificial gill. Appl. Catal. B Environ. 2018, 221, 243–257. [Google Scholar] [CrossRef]
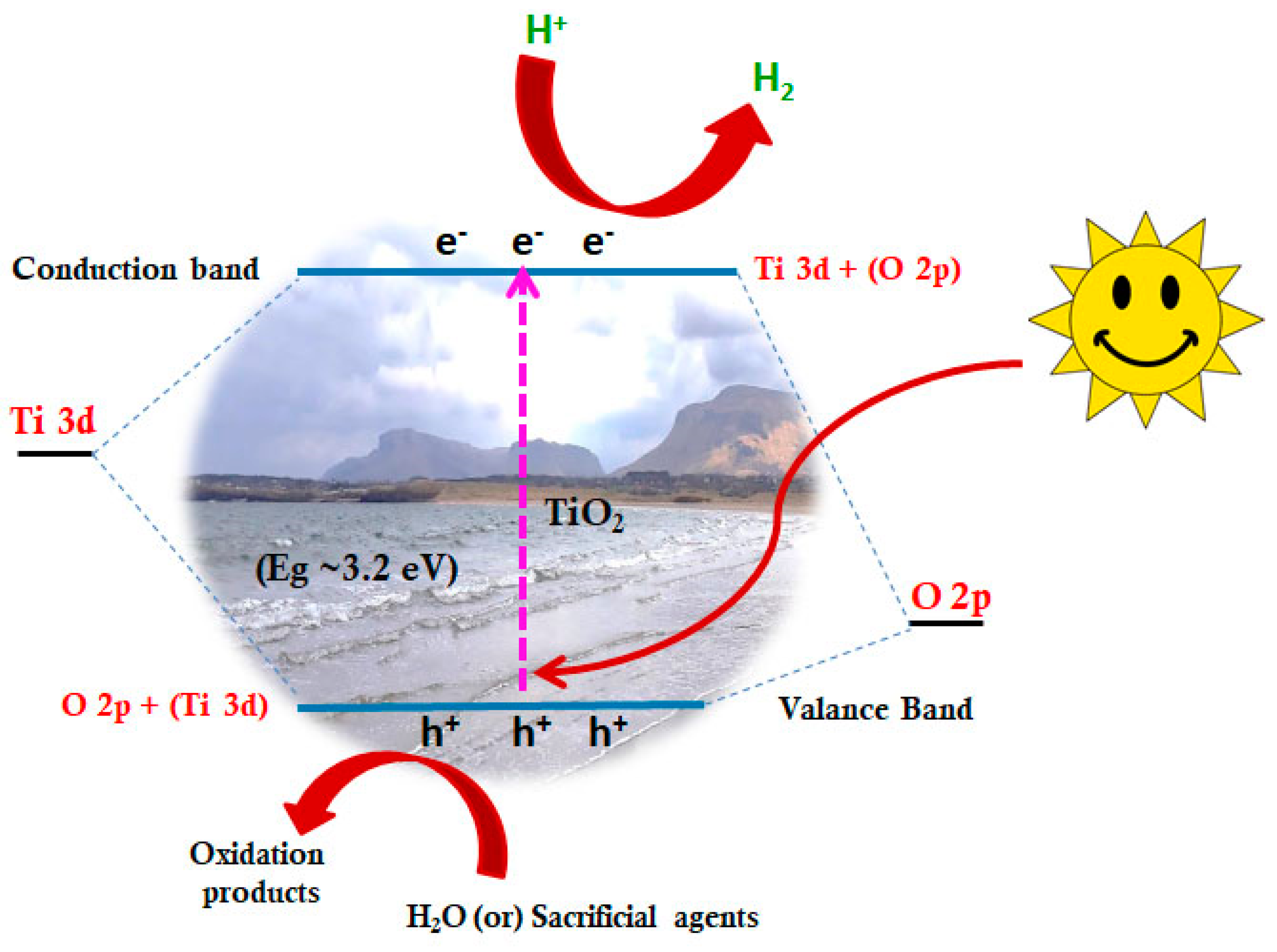


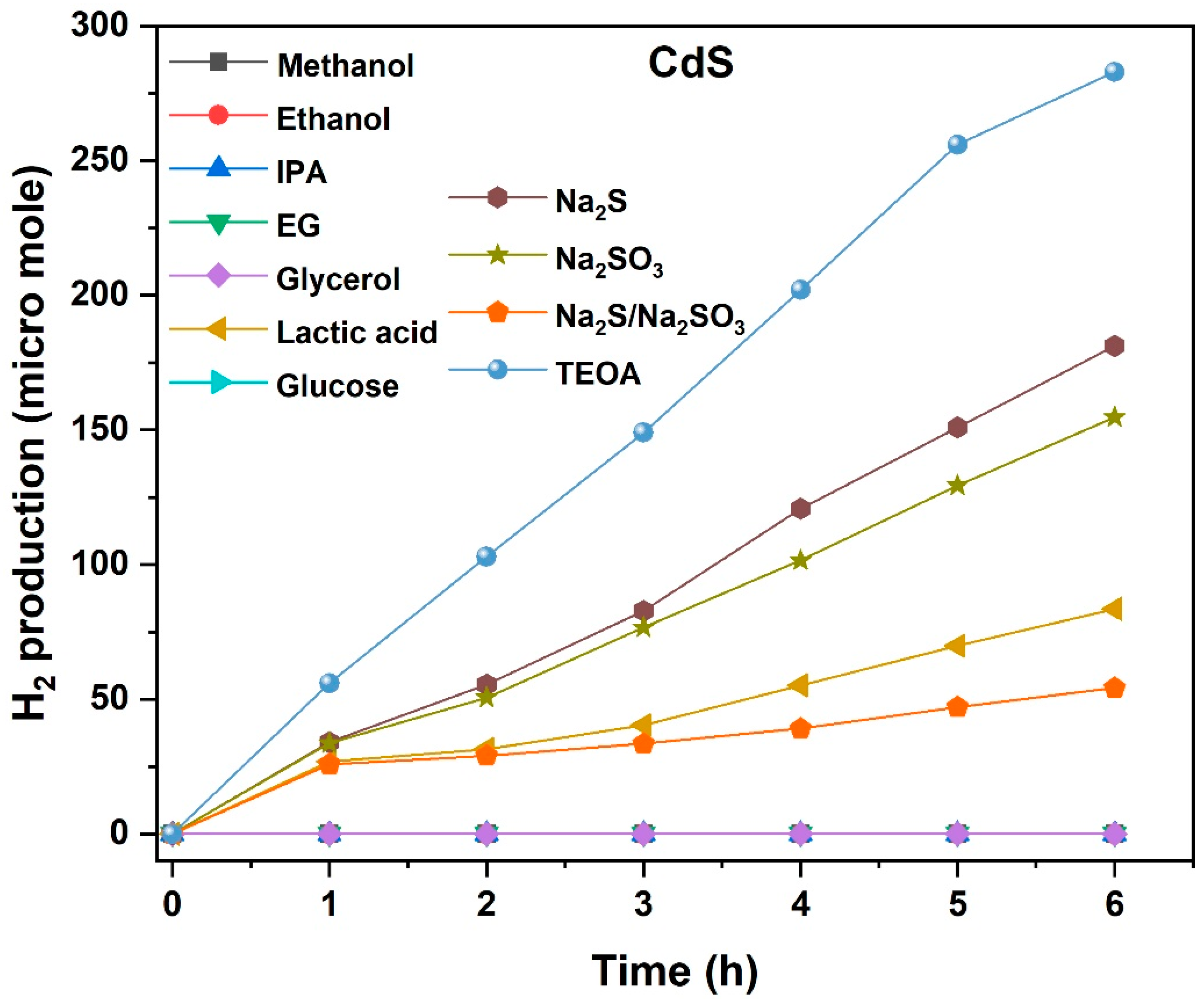
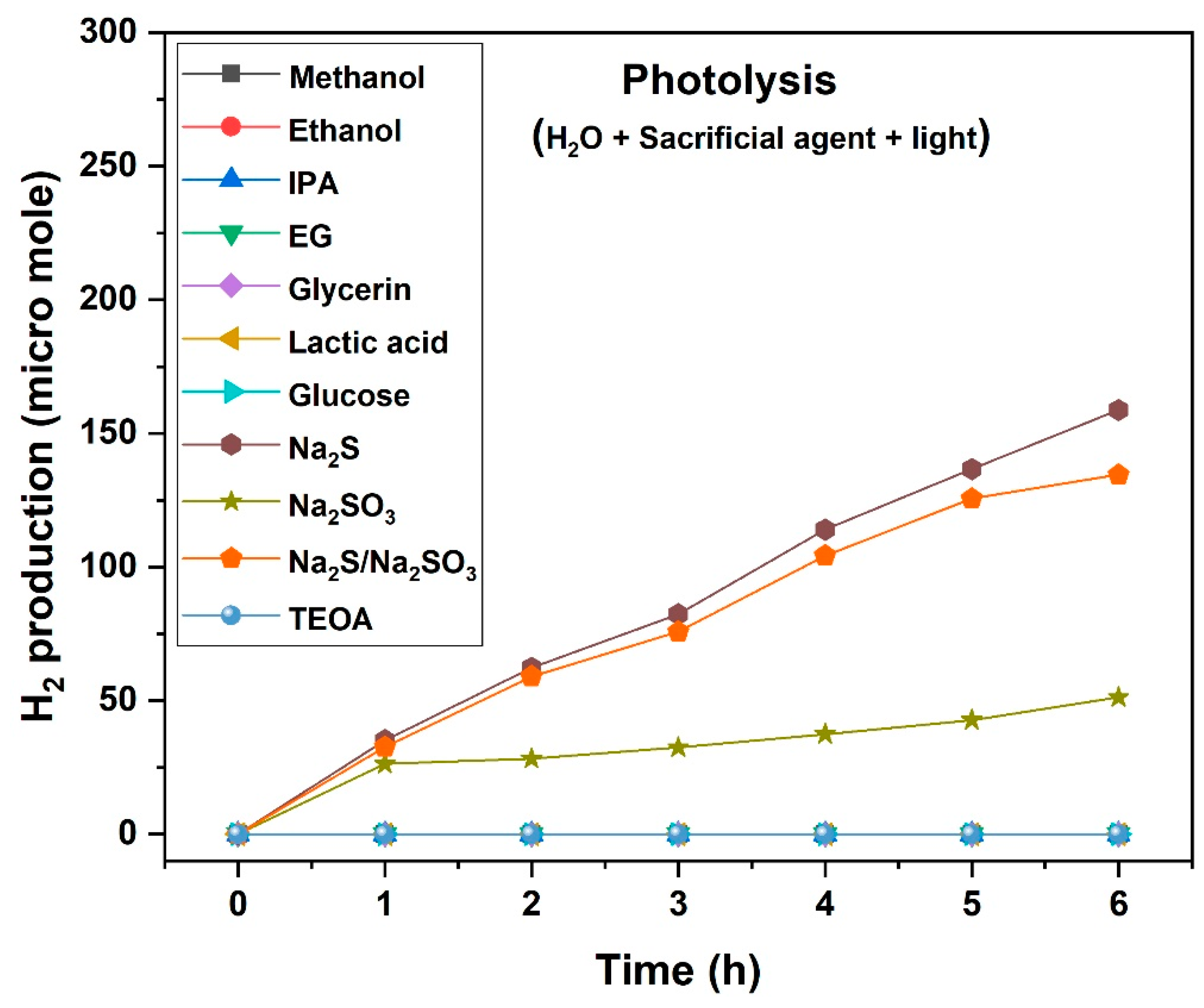
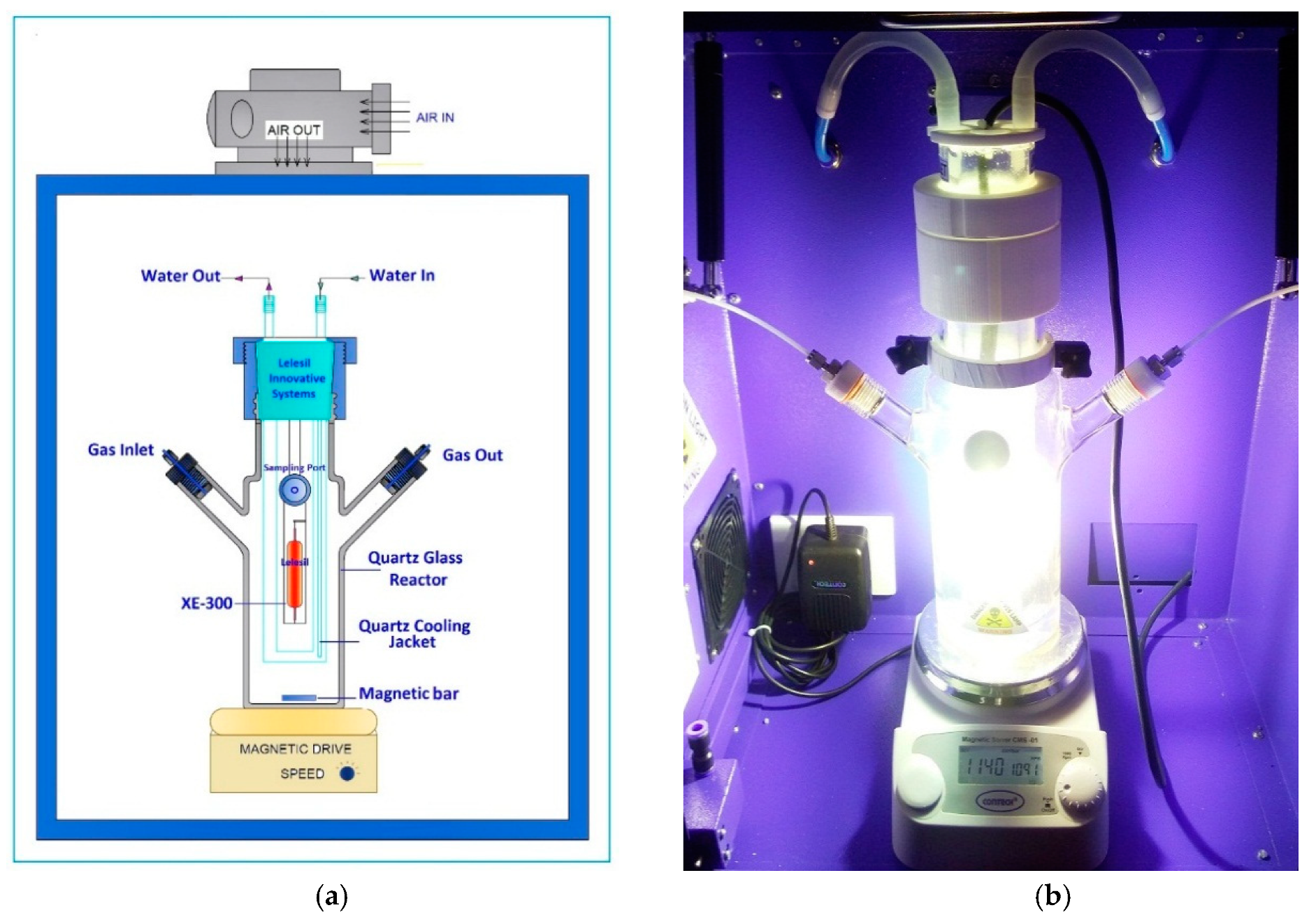
| Catalyst Amount (g/L) | Concentration of Methanol (%) | Light Source | H2 Production Efficiency (μmol/g/h) | Reference |
|---|---|---|---|---|
| 1 | 10 | 300 W of Xe (without UV cutoff filter) | 42.00 | [420] |
| 0.6 | 16.66 | 300 W of Xe (with UV cutoff filter) | 18.47 | [217] |
| 0.5 | 20 | 300 W of Xe (with UV cutoff filter) | ~20.00 | [194] |
| 1.29 | 25.8 | 300 W of Xe (with UV cutoff filter) | ~2.00 | [421] |
| Sample | TOC (mg/L) | |
|---|---|---|
| Blank (Before Light Irradiation) | TiO2-P25 | |
| Water | 8.659 | - |
| Methanol | 30,450 | 15,530 |
| Ethanol | 43,680 | 17,070 |
| Isopropanol | 55,010 | 17,770 |
| Glycerol | 54,220 | 18,700 |
| Ethylene glycol | 59,080 | 17,930 |
| Glucose | 7699 | 11,500 |
| Lactic acid | 47,310 | 20,440 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. https://doi.org/10.3390/catal9030276
Kumaravel V, Imam MD, Badreldin A, Chava RK, Do JY, Kang M, Abdel-Wahab A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts. 2019; 9(3):276. https://doi.org/10.3390/catal9030276
Chicago/Turabian StyleKumaravel, Vignesh, Muhammad Danyal Imam, Ahmed Badreldin, Rama Krishna Chava, Jeong Yeon Do, Misook Kang, and Ahmed Abdel-Wahab. 2019. "Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts" Catalysts 9, no. 3: 276. https://doi.org/10.3390/catal9030276
APA StyleKumaravel, V., Imam, M. D., Badreldin, A., Chava, R. K., Do, J. Y., Kang, M., & Abdel-Wahab, A. (2019). Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts, 9(3), 276. https://doi.org/10.3390/catal9030276








