Investigation of Various Pd Species in Pd/BEA for Cold Start Application
Abstract
1. Introduction
2. Results
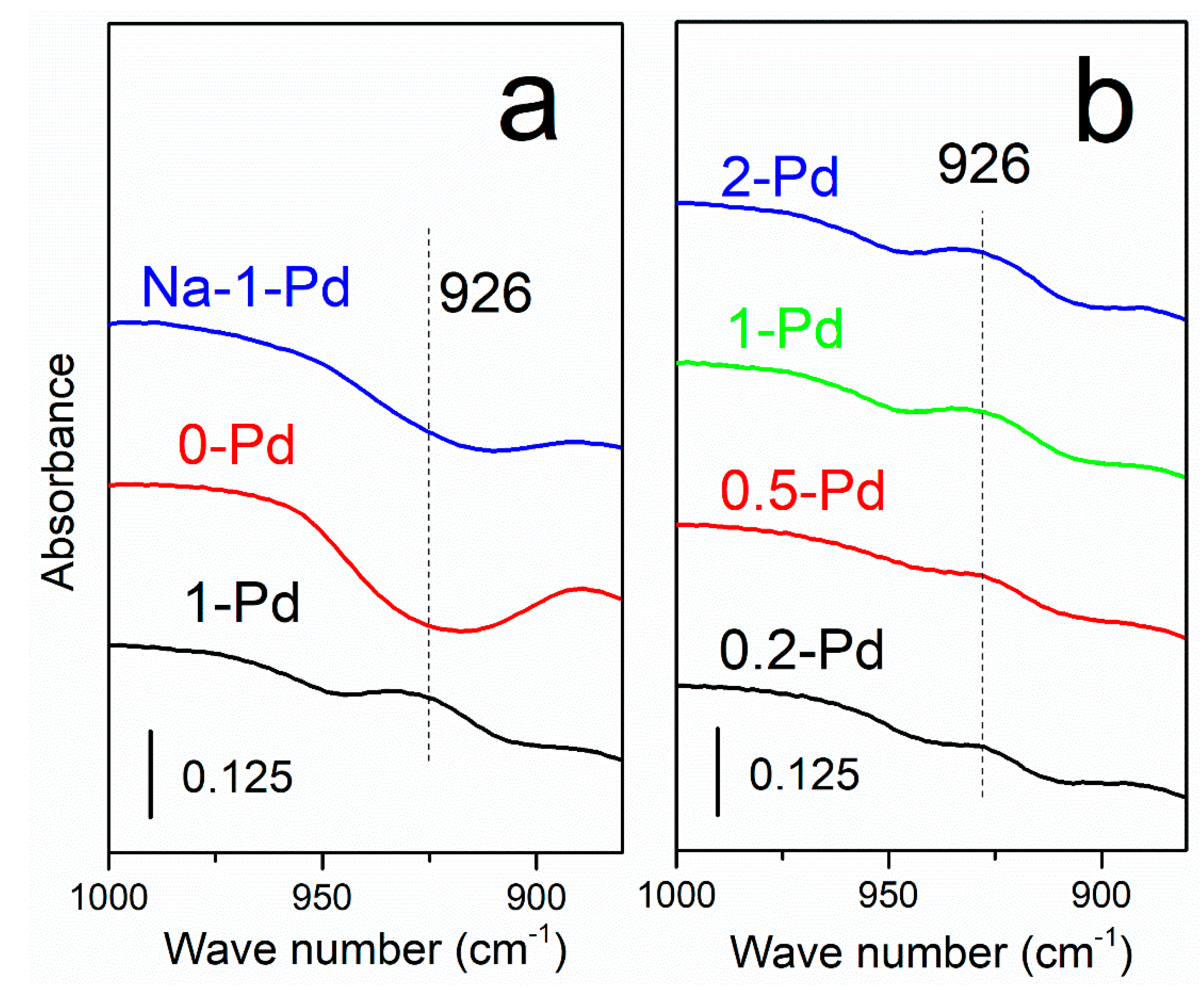
2.1. Ex-Situ FTIR
2.2. Na+ Titration
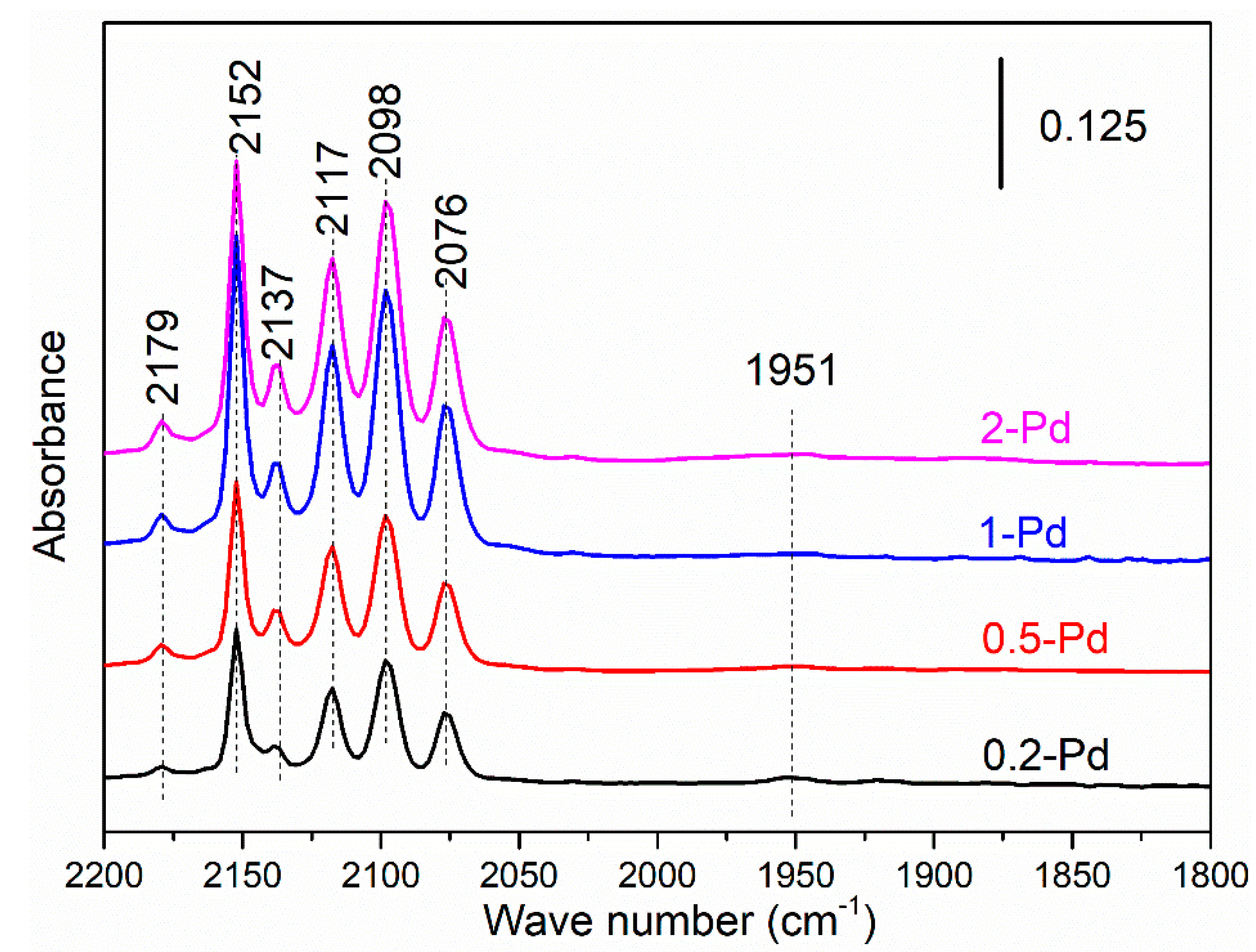
2.3. CO In Situ FTIR
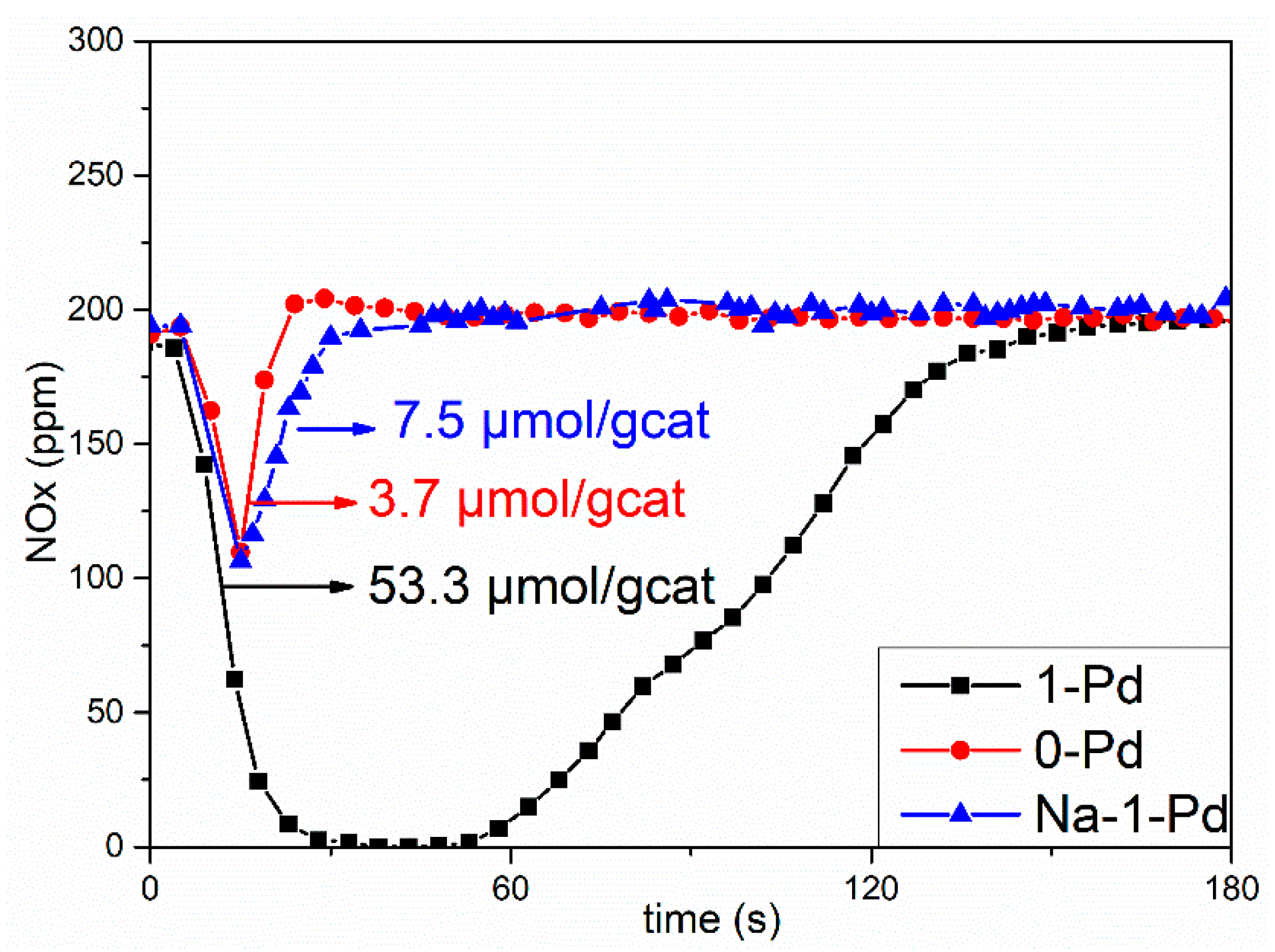
2.4. Catalyst Evaluation
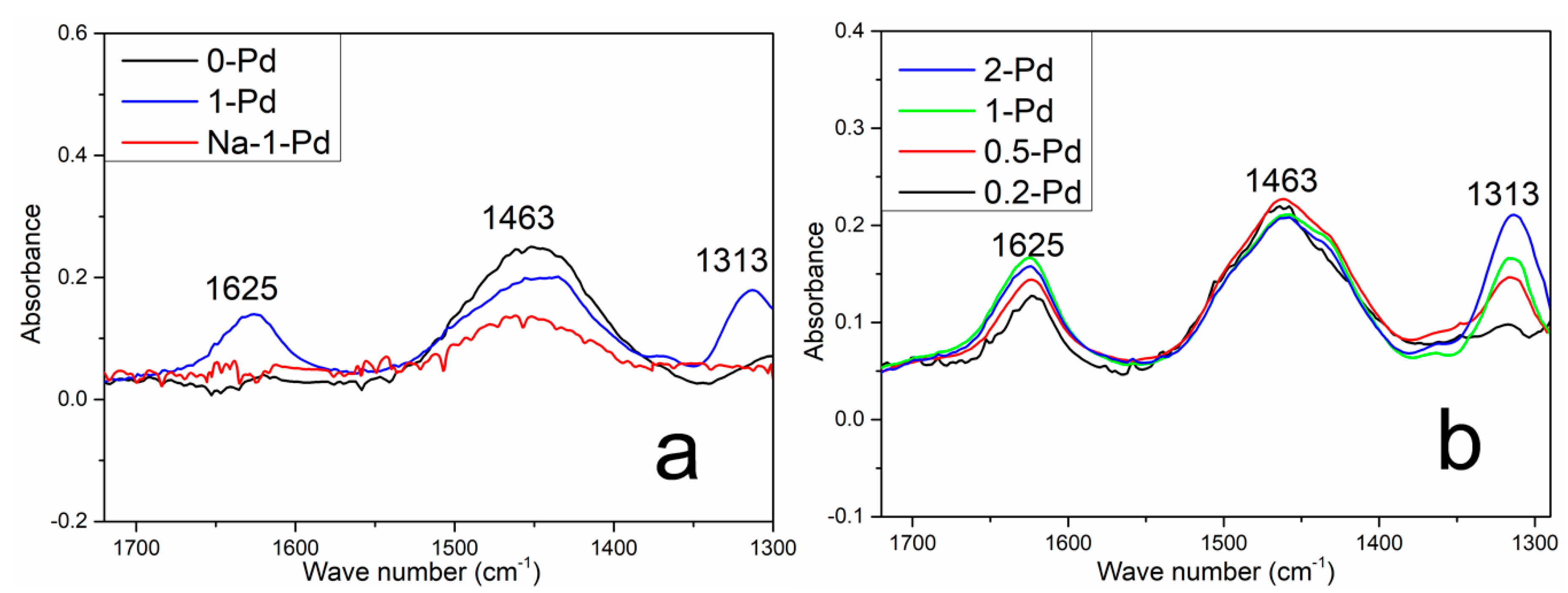
2.5. NH3 In Situ FTIR
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, T.; Joshi, A. Review of vehicle engine efficiency and emissions. SAE Int. J. Engines 2018, 11, 1307–1330. [Google Scholar]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn–Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Kubiak, L.; Matarrese, R.; Castoldi, L.; Lietti, L.; Daturi, M.; Forzatti, P. Study of N2O Formation over Rh- and Pt-Based LNT Catalysts. Catalysts 2016, 6, 36. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Collier, J.E.; Liu, D.; Mantarosie, L.; Durán-Martín, D.; Novák, V.; Rajaram, R.R.; Thompsett, D. Low temperature NO storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Mulla, S.; Weigert, E.; Camm, K.; Ballinger, T.; Cox, J.; Blakeman, P. Cold Start Concept (CSC™) A Novel Catalyst for Cold Start Emission Control. SAE Int. J. Fuels Lubr. 2013, 6, 372–381. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Activation of Pd/SSZ-13 catalyst by hydrothermal aging treatment in passive NO adsorption performance at low temperature for cold start application. Appl. Catal. B 2017, 212, 140–149. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Hwang, S.; Kim, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Comparative study of the mobility of Pd species in SSZ-13 and ZSM-5, and its implication for their activity as passive NOx adsorbers (PNAs) after hydro-thermal aging. Catal. Sci. Technol. 2019, 9, 163–173. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Lee, H.; Kim, C.H.; Kim, D.H. Effect of various activation conditions on the low temperature NO adsorption performance of Pd/SSZ-13 passive NOx adsorber. Catal. Today 2019, 320, 175–180. [Google Scholar] [CrossRef]
- Vu, A.; Luo, J.; Li, J.; Epling, W.S. Effects of CO on Pd/BEA Passive NOx Adsorbers. Catal. Lett. 2017, 147, 745–750. [Google Scholar] [CrossRef]
- Zheng, Y.; Kovarik, L.; Engelhard, M.H.; Wang, Y.; Wang, Y.; Gao, F.; Szanyi, J. Low-Temperature Pd/Zeolite Passive NOx Adsorbers: Structure, Performance, and Adsorption Chemistry. J. Phys. Chem. C 2017, 121, 15793–15803. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Investigation of the active sites and optimum Pd/Al of Pd/ZSM–5 passive NO adsorbers for the cold-start application: Evidence of isolated-Pd species obtained after a high-temperature thermal treatment. Appl. Catal. B 2018, 226, 71–82. [Google Scholar] [CrossRef]
- Khivantsev, K.; Gao, F.; Kovarik, L.; Wang, Y.; Szanyi, J. Molecular Level Understanding of How Oxygen and Carbon Monoxide Improve NOx Storage in Palladium/SSZ-13 Passive NOx Adsorbers: The Role of NO+ and Pd (II)(CO)(NO) Species. J. Phys. Chem. C 2018, 122, 10820–10827. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Hanson, J.C.; Tao, F.; Tang, Y.; Zhang, X.; Koleva, I.Z.; Aleksandrov, H.A.; Vayssilov, G.N.; et al. Achieving Atomic Dispersion of Highly Loaded Transition Metals in Small-Pore Zeolite SSZ-13: High-Capacity and High-Efficiency Low-Temperature CO and Passive NOx Adsorbers. Angew. Chem. 2018, 130, 16914–16919. [Google Scholar] [CrossRef]
- Mei, D.; Gao, F.; Szanyi, J.; Wang, Y. Mechanistic insight into the passive NOx adsorption in the highly dispersed Pd/HBEA zeolite. Appl. Catal. A 2019, 569, 181–189. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Prodinger, S.; Derewinski, M.A.; Wang, Y.; Gao, F.; Szanyi, J. Palladium/Beta zeolite passive NOx adsorbers (PNA): Clarification of PNA chemistry and the effects of CO and zeolite crystallite size on PNA performance. Appl. Catal. A Gen. 2019, 569, 141–148. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Kim, Y.; Hwang, S.; Lee, H.; Kim, C.H.; Kim, D.H. Effect of reduction treatments (H2 vs. CO) on the NO adsorption ability and the physicochemical properties of Pd/SSZ-13 passive NOx adsorber for cold start application. Appl. Catal. A 2019, 569, 28–34. [Google Scholar] [CrossRef]
- Porta, A.; Pellegrinelli, T.; Castoldi, L.; Matarrese, R.; Morandi, S.; Dzwigaj, S.; Lietti, L. Low Temperature NOx Adsorption Study on Pd-Promoted Zeolites. Top. Catal. 2018, 61, 2021–2034. [Google Scholar] [CrossRef]
- Theis, J.R.; Lambert, C.K. Mechanistic assessment of low temperature NOx adsorbers for cold start NOx control on diesel engines. Catal. Today 2019, 320, 181–195. [Google Scholar] [CrossRef]
- Newsam, J.; Treacy, M.M.; Koetsier, W.; De Gruyter, C. Structural characterization of zeolite beta. Proc. R. Soc. Lond. A 1988, 420, 375–405. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H. Effects of Si/Al ratio on Cu/SSZ-13 NH 3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Giordanino, F.; Vennestrøm, P.N.; Lundegaard, L.F.; Stappen, F.N.; Mossin, S.; Beato, P.; Bordiga, S.; Lamberti, C. Characterization of Cu-exchanged SSZ-13: A comparative FTIR, UV-Vis, and EPR study with Cu-ZSM-5 and Cu-β with similar Si/Al and Cu/Al ratios. Dalton Trans. 2013, 42, 12741–12761. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Peden, C.H.F. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. [Google Scholar]
- Ogura, M.; Hayashi, M.; Kage, S.; Matsukata, M.; Kikuchi, E. Determination of active palladium species in ZSM-5 zeolite for selective reduction of nitric oxide with methane. Appl. Catal. B 1999, 23, 247–257. [Google Scholar] [CrossRef]
- Kumar, A.; Buttry, D.A. Influence of Halide Ions on Anodic Oxidation of Ethanol on Palladium. Electrocatalysis 2016, 7, 201–206. [Google Scholar] [CrossRef]
- Yuan, N.; Pascanu, V.; Huang, Z.; Valiente, A.; Heidenreich, N.; Leubner, S.; Inge, A.K.; Gaar, J.; Stock, N.; Persson, I. Probing the evolution of palladium species in Pd@ MOF catalysts during the Heck coupling reaction: An operando X-ray absorption spectroscopy study. J. Am. Chem. Soc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Palazov, A.; Chang, C.; Kokes, R. The infrared spectrum of carbon monoxide on reduced and oxidized palladium. J. Catal. 1975, 36, 338–350. [Google Scholar] [CrossRef]
- Aylor, A.W.; Lobree, L.J.; Reimer, J.A.; Bell, A.T. Investigations of the Dispersion of Pd in H-ZSM-5. J. Catal. 1997, 172, 453–462. [Google Scholar] [CrossRef]
- Okumura, K.; Amano, J.; Yasunobu, N.; Niwa, M. X-ray absorption fine structure study of the formation of the highly dispersed PdO over ZSM-5 and the structural change of Pd induced by adsorption of NO. J. Phys. Chem. B 2000, 104, 1050–1057. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401–1407. [Google Scholar] [CrossRef]
- Pawelec, B.; Campos-Martin, J.; Cano-Serrano, E.; Navarro, R.; Thomas, S.; Fierro, J. Removal of PAH compounds from liquid fuels by Pd catalysts. Environ. Sci. Technol. 2005, 39, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Centeno, M.; Carrizosa, I.; Odriozola, J. NO–NH3 coadsorption on vanadia/titania catalysts: Determination of the reduction degree of vanadium. Appl. Catal. B 2001, 29, 307–314. [Google Scholar] [CrossRef]
- Niwa, M.; Nishikawa, S.; Katada, N. IRMS–TPD of ammonia for characterization of acid site in β-zeolite. Microporous Mesoporous Mater. 2005, 82, 105–112. [Google Scholar] [CrossRef]
- Datka, J.; Gil, B.; Kubacka, A. Acid properties of NaH-mordenites: Infrared spectroscopic studies of ammonia sorption. Zeolites 1995, 15, 501–506. [Google Scholar] [CrossRef]
- Ding, K.; Gulec, A.; Johnson, A.M.; Schweitzer, N.M.; Stucky, G.D.; Marks, L.D.; Stair, P.C. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 2015, 350, 189. [Google Scholar] [CrossRef] [PubMed]
- Bare, S.R.; Kelly, S.D.; Sinkler, W.; Low, J.J.; Modica, F.S.; Valencia, S.; Corma, A.; Nemeth, L.T. Uniform catalytic site in Sn-β-Zeolite determined using x-ray absorption fine structure. J. Am. Chem. Soc. 2005, 127, 12924–12932. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.J.; Iglesia, E. The strength of Brønsted acid sites in microporous aluminosilicates. ACS Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Higgins, J.; LaPierre, R.B.; Schlenker, J.; Rohrman, A.; Wood, J.; Kerr, G.; Rohrbaugh, W. The framework topology of zeolite beta. Zeolites 1988, 8, 446–452. [Google Scholar] [CrossRef]
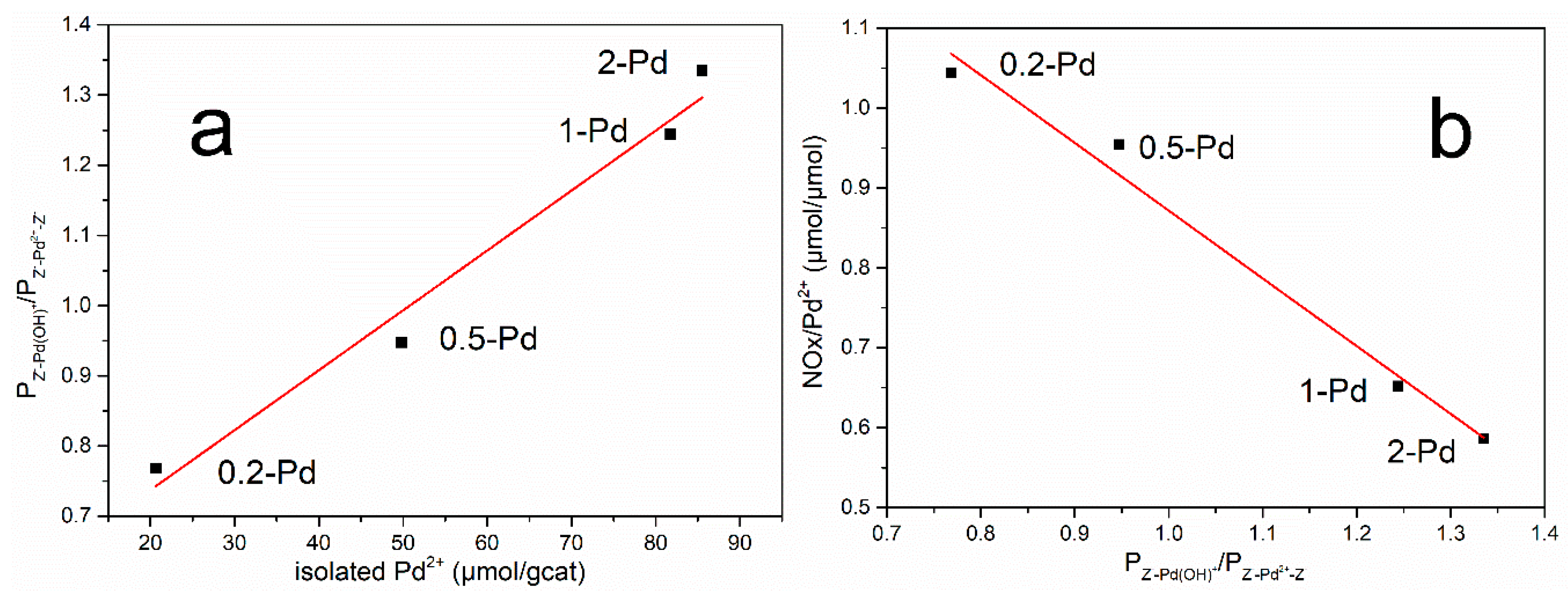
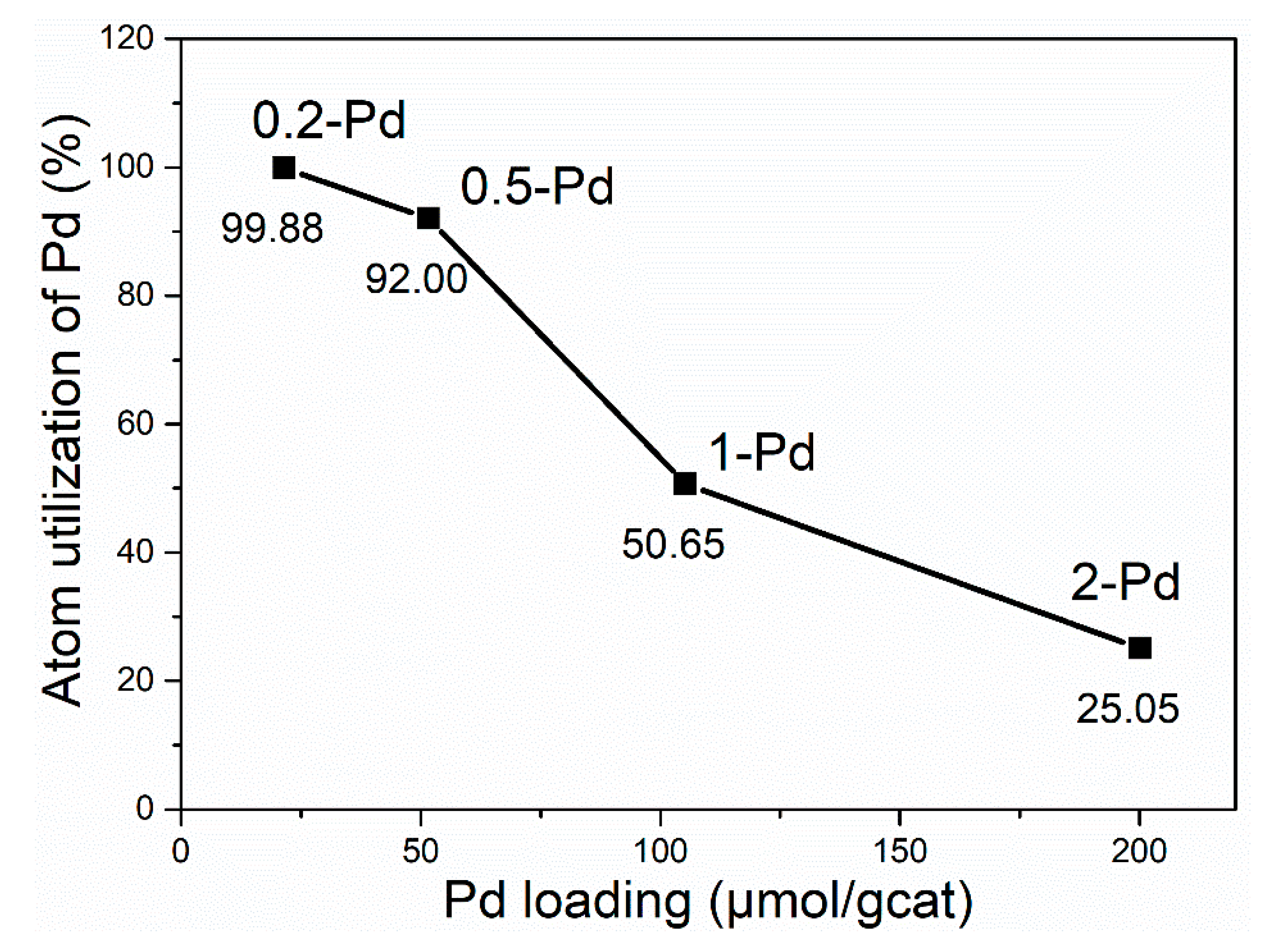
| Catalyst | 0-Pd | 0.2-Pd | 0.5-Pd | 1-Pd | 2-Pd |
|---|---|---|---|---|---|
| Isolated Pd (wt %) | 0.00 | 0.22 | 0.53 | 0.87 | 0.91 |
| Isolated Pd/Pd loading (%) | 0.00 | 95.7 | 96.4 | 77.7 | 42.7 |
| Catalyst | 0-Pd | 0.2-Pd | 0.5-Pd | 1-Pd | 2-Pd |
|---|---|---|---|---|---|
| NOx storage capacity (μmol/gcat) | 3.7 | 21.6 | 47.5 | 53.3 | 50.1 |
| Catalyst | 0-Pd | 0.2-Pd | 0.5-Pd | 1-Pd | 2-Pd | Na-1-Pd |
|---|---|---|---|---|---|---|
| ICP Pd (wt %) | 0 | 0.23 | 0.55 | 1.12 | 2.13 | 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Shen, M.; Wang, J.; Wang, J.; Wang, J. Investigation of Various Pd Species in Pd/BEA for Cold Start Application. Catalysts 2019, 9, 247. https://doi.org/10.3390/catal9030247
Zhang B, Shen M, Wang J, Wang J, Wang J. Investigation of Various Pd Species in Pd/BEA for Cold Start Application. Catalysts. 2019; 9(3):247. https://doi.org/10.3390/catal9030247
Chicago/Turabian StyleZhang, Beibei, Meiqing Shen, Jianqiang Wang, Jiaming Wang, and Jun Wang. 2019. "Investigation of Various Pd Species in Pd/BEA for Cold Start Application" Catalysts 9, no. 3: 247. https://doi.org/10.3390/catal9030247
APA StyleZhang, B., Shen, M., Wang, J., Wang, J., & Wang, J. (2019). Investigation of Various Pd Species in Pd/BEA for Cold Start Application. Catalysts, 9(3), 247. https://doi.org/10.3390/catal9030247






