1. Introduction
The removal of sulfur from transportation fuel and petrochemicals is gaining more attention due to the increased awareness of the adverse effects of burning sulfur containing oils on human health and the environment [
1]. Hence, desulfurization of petrol fuels or waste biomass derived fuel oils has become a critical component of the oil refining industries [
2].
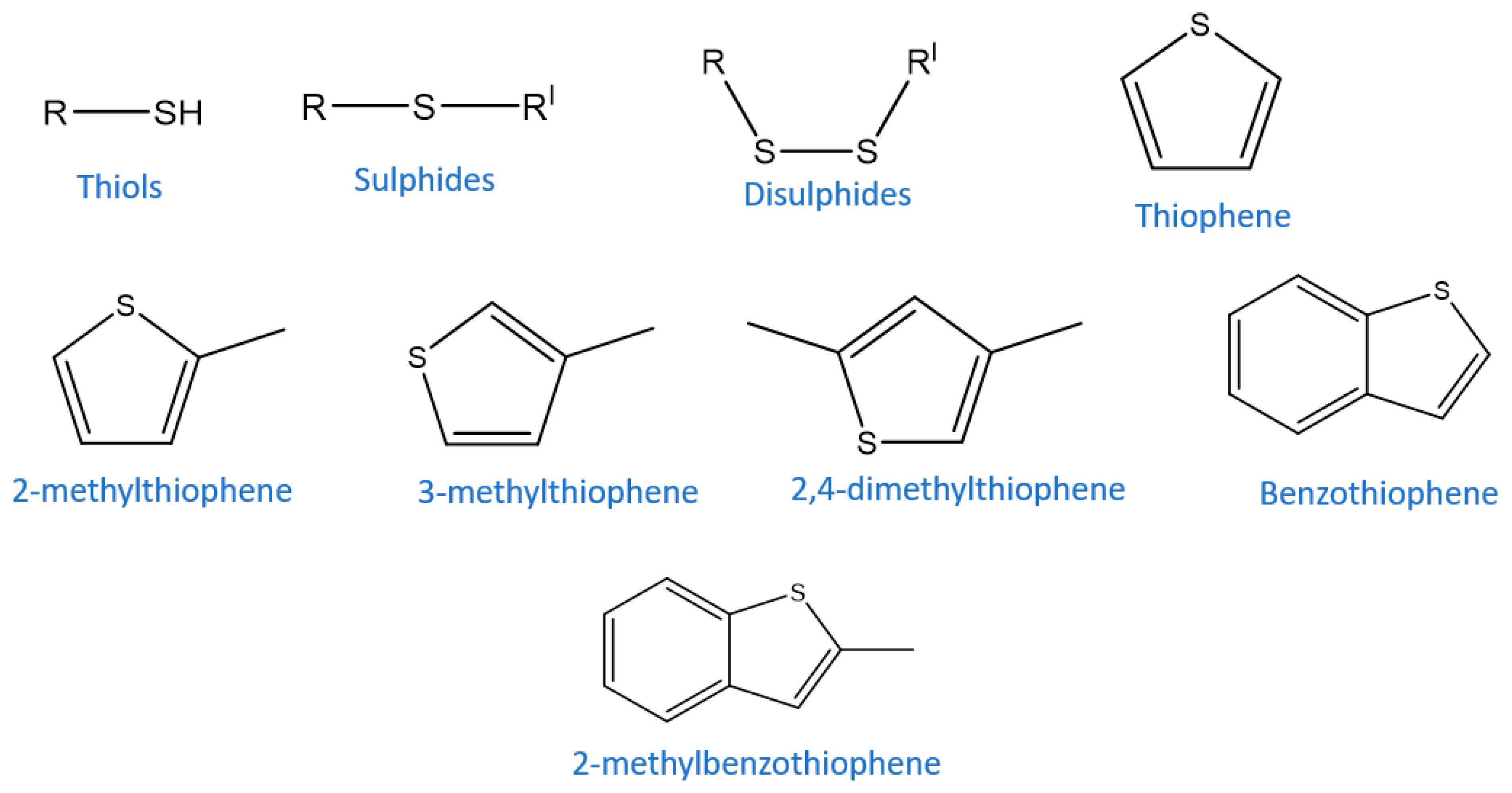
Sulfur containing compounds (
Figure 1) in fuel oil, such as mercaptans, thiophenes (T), benzothiophenes (BT), and dibenzothiophenes (DBT), produce sulfur oxide (SOx) upon combustion, which are the main sources of acid rain and air pollution. These compounds also cause corrosion and deactivation of the catalyst during the desulfurization process of fuel oil in refining industries [
3,
4,
5]. Therefore, removal of such sulfur-containing compounds is imperative for the production of green fuel oils and to meet the new standards of sulfur content (10–15 ppm) as per the recommendations of the United State Protection Agency (USEPA), given the environmental concerns surrounding sulfur [
1,
2,
6,
7].
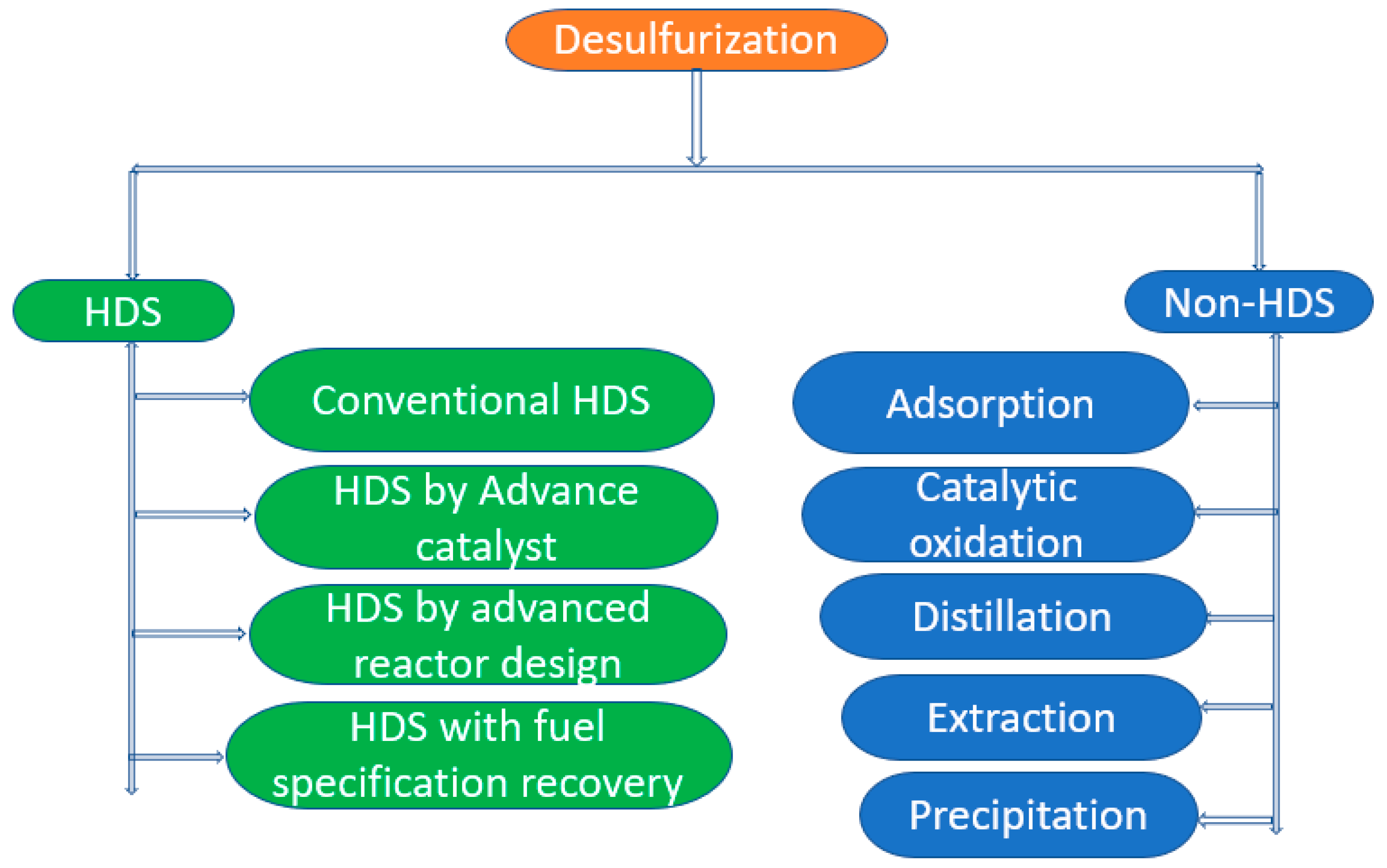
Several techniques, such as hydrodesulfurization (HDS), biodesulfurization (BDS), adsorption, and oxidative desulfurization (ODS), have been applied to remove sulfur-containing compounds from fuel oil [
8]. At present, the conventional HDS process is a well-established method at the industrial level. Hydrodesulfurizarion reactions are carried out in the presence of transition metallic (Ni, Co, and Mo) catalysts at high temperature (up to 400 °C) and pressure (up to 100 atm) using hydrogen gas [
8,
9,
10]. It is a well-known process for removal of aliphatic and acyclic sulfur-containing organic compounds. HDS process is very effective to remove T, BT, and DBT, but the more refractory S compounds found in heaviest cuts require high reaction conditions (high temperature and pressure) and high operational costs [
11,
12,
13].
Research shows that there are several other methods (
Figure 2) for obtaining ultra-low sulfur fuel, which are alternatives to HDS, such as biodesulfurization [
14,
15,
16], selective adsorption [
17,
18], and catalytic oxidative desulfurization [
2,
3,
19,
20]. However, these alternative methods are still in the development stage. At the same time, amongst all the aforementioned alternative desulfurization methods, the ODS process has received a lot of attention as a promising technique due to its mild reaction conditions (low temperature and pressure) and higher selectivity in removing aromatic sulfur compounds compared to the HDS process. Additionally, it does not require expensive hydrogen [
19,
20,
21,
22].
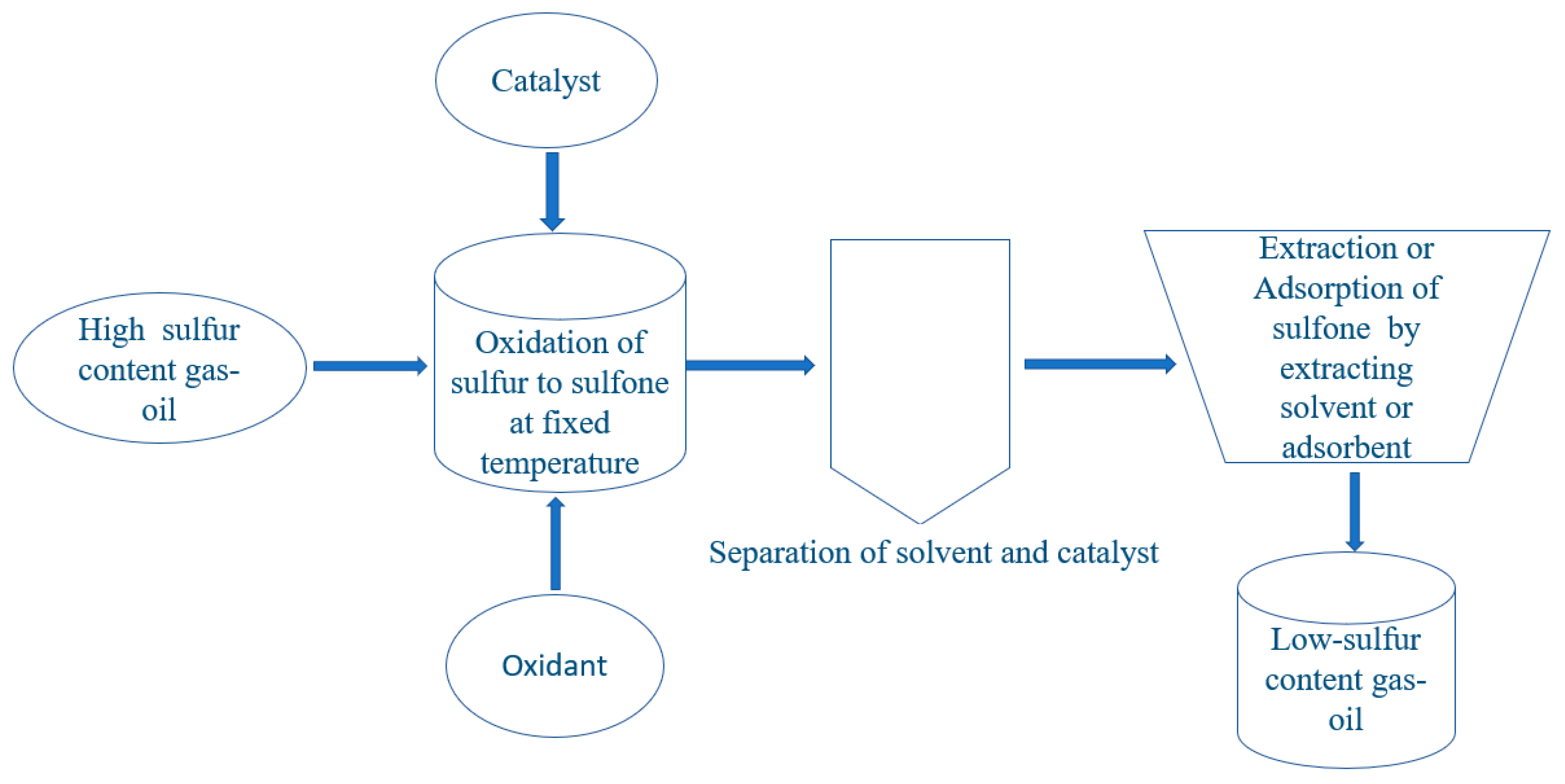
The oxidative desulfurization reaction is a two-step process (
Figure 3). In the first step, the sulfur containing compounds in fuel oil are oxidized to their respective sulfones or sulfoxides in the presence of oxidizing agents. In the second step, these oxidized sulfur compounds are removed from the reaction mixtures by adsorption or liquid/liquid extraction method.
The primary aim of this review article is to provide a comparative analysis of all the existing catalytic oxidation systems applied in the oxidation desulfurization method, and evaluate the difficulties arising in the process. Based on the analyses, this review proposes recommendations for the successful production of green fuel oils.
2. Catalytic Oxidation System
2.1. Acetic Acid/Formic Acid Catalytic Oxidation System
The oxidative desulfurization method has evolved as a viable alternative and a supplement to the HDS method, thereby creating newer avenues in the refinery industries. In the recent past, ODS has been receiving more attention due to its higher removal rate of thiophene derivatives of sulfur compounds compared to the HDS method.
Farsi et al. studied sulfur removal from heavy oil using an oxidation-extraction system, with acetic acid (CH
3COOH) as the catalyst and hydrogen peroxide (H
2O
2) as the oxidant. The experiment was conducted in a glass batch reactor. The results showed that the oxidation process was optimized under the following reaction conditions: the oxidant and catalyst to sulfur content molar ratio in the range of 5–10, reaction time of 90 min, and optimized reaction temperature as 60 °C at a stirring rate of 750 rpm. This ODS method showed that the sulfur content in heavy oils can be lowered from 2.75 wt % to 1.14 wt % [
23].
Similarly, Zannikos et al. investigated the desulfurization of model gas oil using H
2O
2 as oxidant and acetic acid as catalyst. The sulfur removal efficiency improved to 90% under reaction conditions of temperature 90 °C and time 30 min [
24].
Mamaghani et al. worked on oxidative desulfurization of model fuels containing benzothiophene, dibenzothiophene, and DBT-derivatives, wherein formic acid (HCOOH) was used as a catalyst and H
2O
2 as an oxidizing agent. For extraction of the oxidized sulfur compounds, acetonitrile was used as an extracting solvent. The sulfur removal efficiency was found to be 100% under the optimal reaction conditions of formic acid to sulfur molar ratio as 222:1, H
2O
2 to sulfur as 2:1, temperature = 65 °C, and reaction time = 56 min [
25]. In addition, there are studies that have reported oxidative desulfurization of fuel oil through HCOOH-CH
3COOH/H
2O
2 oxidation system [
26,
27].
The ODS process via H2O2-CH3COOH/HCOOH organic acid system is a mild reaction condition system, wherein the peroxide-organic acid mixture has strong oxidizing ability for achieving high sulfur removal efficiency. Despite the evident benefits of this liquid acid-peroxide oxidation system, the ODS process has certain disadvantages. The use of oil soluble organic acid as a catalyst in the reaction mixture causes separation problems, which greatly affects the fuel properties of diesel oil. Furthermore, oil soluble organic acid catalysts are not fully recoverable and are non-renewable. Therefore, recoverable and recyclable catalysts are better alternatives for the fuel oil refining industries.
2.2. Heteropolyacid (HPA) Catalytic Oxidation System
Heteropolyacids are multifunctional heterogeneous acid catalysts, which have been used for developing green technologies and as replacements for conventional liquid homogeneous acid catalysts. They are a special type of polyoxometalate catalysts and are formed by the condensation of various metallic oxyanions (Mo, W, V, and Nb). In recent times, heteropolyacids have been recognized for their versatile function (acid-base characteristics and redox power). The most important role of heteropolyacids has been their use for oxidizing organic substrates under mild reaction conditions [
28,
29,
30].
Li et al. studied the oxidative desulfurization of model fuel oil using macroporous supported heteropolyacids. The model fuel was made by dissolving DBT in normal octane, and oxygen was used as the oxidant. Under optimum conditions of temperature at 60 °C, the conversion rate of fuel oil reached nearly 100% in 120 min [
31].
Rafiee et al. applied the Keggin type heteropolyacid catalysts for oxidative desulfurization of model oils containing DBT under mild reaction conditions. Hydrogen peroxide was used as an oxidizing agent in a biphasic system. The process removed more than 95% of the sulfur under optimum conditions. Most importantly, the catalyst was found to be recyclable and reusable multiple times, with negligible loss in activity [
32].
Tang et al. also applied the HPA catalyst (HPW-TUD-1) for oxidative desulfurization of model fuel oil, using H
2O
2 as the oxidant. The catalyst was observed to be highly effective in the desulfurization of benzothiophene derivatives of sulfur compounds. The achieved sulfur reduction rate was 98% under optimum conditions, and the catalyst could be recycled three times [
33].
2.3. Ionic Liquid Catalytic Oxidation System
The reactivity of sulfur refractory compounds in fuel oil during the oxidation process decreases in the order of 4,6-dimethyldibenzothiophene (4,6-DMDBT) > 4-methylbenzothiophene (4-MDBT) > DBT > T. In the ODS process, the sulfur compounds are oxidized to their respective sulfones, which are then extracted by various organic solvents [
34]. In recent years, ionic liquids as extractants have gained attention in the refinery industry due to their remarkable characteristics. Ionic liquids could be used in the ODS process as both catalyst and extractive agent and achieved ultra-low desulfurization of the model fuel [
35].
Wang et al. applied a Lewis acidic ionic liquid (ILs) [ODBU]Cl/nZnCl
2 (n = 1, 2, 3, 4, and 5) for oxidative desulfurization of model and diesel fuel oils, with H
2O
2 as the oxidant. The model oil was prepared by dissolving S-compounds (such as BT, DBT, and 4,6-DMDBT) in n-octane in desired concentrations. The results revealed that the catalyst was highly reactive towards the reduction of sulfur compounds in fuel oil, wherein the amount of sulfur reduced by 99% in a single step process, under mild reaction conditions. The catalyst could also be successfully reused up to six times without any change in its structure [
36].
Dharaskar et al. studied the ODS process using 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid as a catalyst for removing sulfur compounds from fuel oil. The model fuel oil was prepared by dissolving sulfur compounds (BT, T, and 3-methylthiphene (3-MT) in dodecane. The conversion rate was obtained to be 73.02% at 30 °C and 10 min under mild reaction conditions. The catalyst was recyclable up to four times without regeneration [
37]. Thus, to date, numerous research studies have been conducted using ionic liquid in catalytic ODS of fuel oil [
38,
39,
40,
41,
42].
2.4. Molecular Sieve Catalytic Oxidation System
The performance of the catalyst in a reaction depends on its number and the nature of active sites. In addition, catalytic support material plays a crucial role in determining the active sites of catalysts. A number of studies have shown that the use of molecular sieves significantly improves the performance of catalysts in the removal of sulfur. Molecular sieve type of the catalyst has also been drawing interest because of its large pore size and surface area, and for being environmentally friendly [
43].
Zhao et al. studied different phthalocyanine molecular sieve catalysts, such as Cu
2(PcAN)
2–W-HZSM-5 and Cu
2(PcTN)
2/W-HZSM-5, for oxidative desulfurization of fuel oil. The sample fuel oil was formed by dissolving different sulfur compounds, such as T, BT, and DBT, while the molecular oxygen supplied to the reactor was used as an oxidant. The copper phthalocyanine molecular sieve catalyst was successfully modified by HZSM-5 zeolite. The catalyst showed superior activity under optimum reaction conditions of temperature 60 °C, reaction time 3 h, and catalyst load of 0.1 g. The sulfur removal efficiencies for T, BT, and DBT were obtained as 93.82%, 91.23%, and 87.32%, respectively [
44].
Yang et al. investigated the catalytic ODS process using catalyst of molybdenum supported on 4A molecular sieve. The sulfur refractory compounds selected for the model fuel oil were BT and DBT. The solvent cyclohexanone peroxide (CYHPO) was chosen as the oxidant. To obtain optimum results, the experiment was designed based on the Box–Behnken (BBD) method. The removal of DBT and the residual sulfur content was achieved at 99% under optimum catalytic conditions of Mo loading of 6 wt %, volume mass ratio of model gasoline to catalyst MoO
3/4A of 100, oxidant (CYHPO)/DBT molar ratio of 2.5, and temperature of 100 °C over 30 min [
45].
Shiraishi et al. studied vanadosilicate molecular sieve as a catalyst for oxidative desulfurization of light oil. The oil feedstock content of sulfur compounds (BT and DBT) was dissolved in acetonitrile, with H
2O
2 as the oxidant. The sulfur refractory compounds were catalyzed successfully by the vanadosilicate molecular sieve and the amount of sulfur was reduced to ultra-low levels of less than 50 ppm from 450 ppm. In addition, the reduction in the sulfur content was accompanied by reduction in the nitrogen content of the light oil. However, one of the disadvantages of this process is that the recovered vanadosilicate cannot be used further because of the dissolution of the vanadium present on the silica surface into the reaction media [
46].
Shifu et al. examined the oxidative desulfurization of model light oil using Ti-MWW catalyst and H
2O
2 oxidant. The model oil was made with BT and DBT sulfur compounds, and their oxidized forms were removed through extraction using acetonitrile. Consequently, the conversion of BT and DBT were attained as 100% and 95%, respectively [
47].
2.5. Polyoxometalates Catalytic Oxidation System
Polyoxometalates are a cluster of early transitional metal ions and polyoxoanions. They are versatile catalysts due to their multifunctional active sites, such as protons, oxygen atoms, and metals. Of late, polyoxometalate catalysts in the ODS process have attracted significant attention as green catalysts in combination with H
2O
2 oxidant [
48].
Trakarnpruk et al. applied various polyoxometalate catalysts based on molybdenum and tungsten transitional metals for desulfurization of model gas oil containing sulfur refractory compounds (such as BT, DBT, and 4,6-DMBT) in a H
2O
2/acetic acid system. The results showed that the W-based polyoxometalate catalysts were more active than the Mo-based catalysts in desulfurization of fuel oil. In this study, the extraction stage was combined with the oxidation stage, and the sulfur removal efficiency was achieved as 98% under mild reaction conditions, along with 90% recovery of the gas oil [
49].
Julião et al. investigated the removal of sulfur from model diesel oil using zinc-substituted polyoxotungstate anion, TBA
4.2H
0.8[PW
11Zn (H
2O) O
39] (denoted PW
11Zn). The model diesel oil was prepared by dissolving various sulfur compounds, such as BT, 1-DBT, and 4,6-DMBT in n-octane, while H
2O
2 was used as the oxidant. The oxidized oil was extracted in a biphasic system using ionic liquid as a solvent, as well as another solvent, acetonitrile. Complete desulfurization of the diesel oil was achieved within two hours of using ionic liquid as an extracting solvent compared to the acetonitrile. However, it was found that when acetonitrile was used as the extraction solvent, the catalyst composite was more stable, easily recoverable from the reaction mixture, and reusable several times [
50].
Although these polyoxometalate catalysts have received notable attention for their high reactivity in the ODS process, their low surface area that resulted in longer reaction time proved to be their major limitation.
2.6. Titanium Catalytic Oxidation System
The examination of titanium silicate (TS) and modified titanium catalysts for oxidative desulfurization of fuel oil revealed that these catalysts had remarkable oxidation ability and yielded better results when used alongside eco-friendly peroxide solvent as an oxidant [
51,
52,
53].
Bazyari et al. applied titania-silica nanocomposite catalysts for ultra-deep desulfurization of model fuel oil. The catalysts were prepared by a sol-gel method with different TiO
2 wt % loadings, and the synthesized catalysts were further evaluated by several analytical techniques. The model fuel oil was prepared by dissolving DBT in an isooctane solvent, while tert-butyl hydroperoxide (TBHP) was added into the reactor as the oxidant. The various effects of titanium loading, reaction temperature, and calcination temperature were investigated. It was found that the catalyst with 50 wt % TiO
2 loading at high acidity showed high catalytic performance and was capable of reducing nearly 98% of DBT within 20 min [
54].
Yang et al. studied the catalytic oxidative desulfurization of mesoporous titanium silicalite-1 (TS-1) using a hybrid SiO
2–TiO
2 xerogel for removal of sulfur refractory compounds from model oil, which was dissolved in n-octane. The sulfur compounds (BT, DBT, and 4,6-DMDBT) were oxidized in a H
2O
2/acetic acid oxidant system, and the reaction was run in a 100 mL glass batch reactor at 300 rpm. It was observed that the modified TS-1 hybrid, i.e., Mesoporous TS-1 (M-TS-1), was more active than the conventional TS-1. The conversion rate for DBT using the former was 98%, whereas the conversion was 5.6% upon usage of conventional TS-1 catalyst [
55].
Zhao et al. examined desulfurization of diesel oil using Ti-containing zeolite photocatalysts. The diesel oil was prepared by dissolving DBT sulfur compounds in n-octane solvent. The optimal condition for the oxidative desulfurization process was obtained as 30 wt % H
2O
2 oxidant system. The achieved rate of sulfur reduction for DBT was 90% and the catalyst was found to be reusable several times [
56].
2.7. Ultrasound-Assisted Oxidation System
The ODS method has gained higher recognition in the desulfurization of fuel oil compared to the hydrodesulfurization (HDS) process, because of its high selectivity under mild operating conditions. Furthermore, in recent years, an effective and innovative ultrasound-assisted oxidative desulfurization (UADO) method has been introduced in the desulfurization process, which has created a new dimension in the petrochemical industry. The UADO method has several benefits over the conventional mixing-assisted oxidative desulfurization process. The use of ultrasound probe in UADO increases the rate of desulfurization because of its smoother dispersion capability. In the UADO process, the reaction kinetics is better due to increased mass transfer in the heterogeneous reaction system. The reaction rate and mass transfer are also enhanced because of the simultaneous physical and mechanical effects [
57,
58,
59].
Gildo et al. studied ultrasound-assisted oxidative desulfurization of simulated fuel using activated carbon-supported phosphotungstic acid catalyst. Simulated fuel oil containing sulfur refractory compounds (T, BT, and DBT) was dissolved in toluene, and H
2O
2 was added in the reaction mixture as an oxidizing agent. The 24 factorial design and face centered method was applied for optimization of the experiment. The oxidation of sulfur compounds was attained as 94.74% at reaction conditions of 25.52 wt.% of H
2O
2 concentration, catalyst loading of 983.9 mg, and sonication time of 76.36 min. The study also found that 99% sulfur was removed from kerosene oil by applying optimized UADO parameters in a 4-cycle extraction process, using acetonitrile as the extracting solvent [
60].
Similarly, Jalali et al. applied ultrasound irradiation in the oxidative desulfurization process of gas oil, with sulfur content at 0.221 wt %, H
2O
2 as the oxidant, and formic acid as the catalyst. The experiment was optimized using response surface methodology (RSM) and Box–Behnken design. The various effects of reaction parameters, such as sonication time, temperature, peroxide to sulfur molar ratio, formic acid to oxidant molar ratio, and sonication power per gas oil volume were investigated in the desulfurization of gas oil. It was observed that maximum desulfurization was achieved under the following optimum reaction conditions: temperature of 50 °C, sonication time of 19.81 min, oxidant to sulfur molar ratio of 46.36, formic acid to oxidant molar ratio of 3.22, and sonication power of 7.78 W/mL. The sulfur removal efficiency obtained was 96.2% after the fourth extraction cycle using acetonitrile solvent. The author also found that higher desulfurization efficiency was obtained within a shorter reaction time as compared to the traditional mixing ODS process [
61]. Some studies have also reported the successful result of ultrasound assisted oxidative desulfurization of fuel oil performed under mild reaction conditions [
62,
63,
64,
65,
66,
67]. One summary table for oxidative catalytic desulfurization of liquid fuel oil has been presented at the end (
Table 1).
Despite having several advantages, the UADO process involves certain difficulties. It consumes high energy and requires a sono-reactor, amplifier, and generator that increase the investment costs. Therefore, for industrial applications, cost and energy-effective alternatives to UADO need to be researched.
3. Conclusions, Future Challenges, and Recommendations
Hydrodesulfurization is a well-established and conventional technique used in oil refinery industries. However, it involves certain constraints, such as higher investment costs. In order to meet the new standards specified for sulfur, the HDS process needs to perform under high reaction conditions, such as high temperature (~400 °C), high pressure (~100 atm), and in a large reaction vessel, which raises the amount of investment costs. Therefore, newer techniques, such as extractive desulfurization, biodesulfurization, extraction with ionic liquids, selective adsorption, and oxidative desulfurization have been proposed in addition, or as alternatives, to HDS. Amongst these alternative methods, the oxidative desulfurization process has received more attention due to its mild operating condition and high sulfur removal efficiency. However, there still exists many issues with the ODS process, such as high loading of the oxidizing agent, deactivation of the catalyst, increasing investment costs with increasing sulfur concentration in the feedstock, and waste management of the oxidized sulfur compounds. Several initiatives have been proposed to address these drawbacks found in the ODS method, which include developing a cost effective, high efficiency, and recyclable catalyst, and developing an environment friendly and cheap oxidizing agent. Eventually, to meet the revised sulfur standards recommended by USEPA and the public demand for safer fuels, effective catalytic oxidative desulfurization of fuel oil is required by improvising the ODS process with technological innovations. Until now, the hydrotreating activity of different oxidative catalysts is limited to model sulfur compounds with short chains. On the contrary, the catalyst’s activity in the case of real feedstock with heavy sulfur compounds would vary from the model feedstock, which has not yet been studied in-depth. Therefore, another challenge that remains for researchers is to examine the application of the catalyst in the oxidative desulfurization of real feedstock of heavy oil, such as waste tire pyrolysis oil. Besides these challenges, the more critical challenge is the commercialization of the catalytic oxidative desulfurization process due to some major obstacles, such as low selectivity for the sulfides present in fuel feedstock, recovery, and separation of the used catalysts after the reaction.
Author Contributions
Conceptualization, M.N.H. and H.S.C.; writing—original draft preparation, M.N.H.; writing—review and editing, M.N.H.; H.C.P. and H.S.C.; visualization, H.C.P.; supervision, H.S.C.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT, and Future Planning (MSIP) of Korea (NRF-2017R1A2B4009340). This work has also been supported by the R&D Program of Forest Science Technology (Project No. 2017052C10-1819-BB02), provided by the Korea Forest Service (Korea Forestry Promotion Institute).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, L.-J.; Li, F.-T. Oxidative desulfurization of model gasoline over modified titanium silicalite. Pet. Sci. Technol. 2015, 33, 196–202. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Zhang, Q. Design rules of ionic liquids tasked for highly efficient fuel desulfurization by mild oxidative extraction. Fuel 2017, 189, 334–339. [Google Scholar] [CrossRef]
- González-García, O.; Cedeño-Caero, L. V-Mo based catalysts for oxidative desulfurization of diesel fuel. Catal. Today 2009, 148, 42–48. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Aghbolagh, Z.S.; Babaei, R. Oxidative desulfurization of gasoline catalyzed by IMID@PMA@CS nanocomposite as a high-performance amphiphilic nano catalyst. Environ. Prog. Sustain. Energy 2018, 37, 1891–1900. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Akbarzadeh, F.; Khandan, S. Deep oxidative desulfurization of gasoline induced by PMoCu@MgCu2O4- PVA composite as a high-performance heterogeneous nanocatalyst. Chem. Eng. J. 2018, 333, 537–544. [Google Scholar] [CrossRef]
- Bakar, W.A.W.A.; Ali, R.; Kadir, A.A.A.; Mokhtar, W.N.A.W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process. Technol. 2012, 101, 78–84. [Google Scholar] [CrossRef]
- Subhan, S.; Rahman, A.U.; Yaseen, M.; Haroon, H.U.; Ishaq, M.; Sahibzada, M.; Tong, Z. Ultra-fast and highly efficient catalytic oxidative desulfurization of dibenzothiophene at ambient temperature over low Mn loaded Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using NaClO as oxidant. Fuel 2019, 237, 793–805. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.I.; Naeem, K.; Humayun, M.; Sebt-E-Zaeem, F.F. Oxidative desulfurization of tire pyrolysis oil. Chem. Ind. Chem. Eng. Q. 2016, 22, 249–254. [Google Scholar] [CrossRef]
- Ismagilov, Z.; Yashnik, S.; Kerzhentsev, M.; Parmon, V.; Bourane, A.; Al-Shahrani, F.M.; Hajji, A.A.; Koseoglu, O.R. Oxidative desulfurization of hydrocarbon fuels. Catal. Rev. Sci. Eng. 2011, 53, 199–255. [Google Scholar] [CrossRef]
- Muhammad, Y.; Shoukat, A.; Rahman, A.U.; Rashid, H.U.; Ahmad, W. Oxidative desulfurization of dibenzothiophene over Fe promoted Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using hydrogen peroxide and formic acid as oxidants. Chin. J. Chem. Eng. 2018, 26, 593–600. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Asli, M.A.; Khandan, S.; Mousavi, H.; Aghbolagh, Z.S. Synthesis and characterization of new nanocomposite CTAB-PTA@CS as an efficient heterogeneous catalyst for oxidative desulfurization of gasoline. Chem. Eng. J. 2017, 312, 243–251. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, H.; Zhang, L.; Zhang, R.; Sun, Y.; Huang, Y. Efficient oxidative desulfurization of diesel fuel using amide-based ionic liquids. Chem. Eng. J. 2016, 283, 89–96. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Perez-Presas, P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef]
- Dinamarca, M.A.; Ibacache-Quiroga, C.; Baeza, P.; Galvez, S.; Villarroel, M.; Olivero, P.; Ojeda, J. Biodesulfurization of gas oil using inorganic supports biomodified with metabolically active cells immobilized by adsorption. Bioresour. Technol. 2010, 101, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Bhasarkar, J.B.; Dikshit, P.K.; Moholkar, V.S. Ultrasound assisted biodesulfurization of liquid fuel using free and immobilized cells of Rhodococcus rhodochrous MTCC 3552: A mechanistic investigation. Bioresour. Technol. 2015, 187, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Davoodi-Dehaghani, F.; Vosoughi, M.; Ziaee, A.A. Biodesulfurization of dibenzothiophene by a newly isolated Rhodococcus erythropolis strain. Bioresour. Technol. 2010, 101, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Tan, Q.; Yu, G.X.; Chen, L.F.; Wang, J.A.; Novaro, O. Removal of dibenzothiophene in diesel oil by oxidation over a promoted activated carbon catalyst. Kinet. Catal. 2009, 50, 543–549. [Google Scholar] [CrossRef]
- Miao, G.; Ye, F.; Wu, L.; Ren, X.; Xiao, J.; Li, Z.; Wang, H. Selective adsorption of thiophenic compounds from fuel over TiO2/SiO2 under UV-irradiation. J. Hazard. Mater. 2015, 300, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ali Rezvani, M.; Khandan, S.; Sabahi, N. Oxidative desulfurization of gas oil catalyzed by (TBA)4PW11Fe@PbO as an efficient and recoverable heterogeneous phase-transfer nanocatalyst. Energy Fuels 2017, 31, 5472–5481. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, Y.; Zhao, D.; Zhang, H. An oxidative desulfurization method using ultrasound/Fenton’s reagent for obtaining low and/or ultra-low sulfur diesel fuel. Fuel Process. Technol. 2008, 89, 927–932. [Google Scholar] [CrossRef]
- Betiha, M.A.; Rabie, A.M.; Ahmed, H.S.; Abdelrahman, A.A.; El-Shahat, M.F. Oxidative desulfurization using graphene and its composites for fuel containing thiophene and its derivatives: An update review. Egypt. J. Pet. 2018, 27, 715–730. [Google Scholar] [CrossRef]
- Abdelrahman, A.A.; Betiha, M.A.; Rabi, A.M.; Ahmed, H.S.; Elshahat, M.F. Removal of refractory organosulfur compounds using an efficient and recyclable {Mo132} nanoball supported graphene oxide. J. Mol. Liq. 2018, 252, 121–132. [Google Scholar] [CrossRef]
- Farshi, A.; Shiralizadeh, P. Sulfur reduction of heavy fuel oil by oxidative desulfurization (Ods) method. Pet. Coal 2015, 57, 295–302. [Google Scholar]
- Joskić, R.; Margeta, D.; Sertić-Bionda, K. Oxidative desulfurization of model diesel fuel with hydrogen peroxide. Goriva i Maziva 2014, 53, 11–18. [Google Scholar]
- Mamaghani, A.H.; Fatemi, S.; Asgari, M. Investigation of influential parameters in deep oxidative desulfurization of dibenzothiophene with hydrogen peroxide and formic acid. Int. J. Chem. Eng. 2013. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Yu, G.; Lu, S.; Chen, H.; Zhu, Z. Diesel fuel desulfurization with hydrogen peroxide promoted by formic acid and catalyzed by activated carbon. Carbon 2005, 43, 2285–2294. [Google Scholar] [CrossRef]
- da Silva, M.J.; Teixeira, M.G. An unexpected behaviour of H3PMo12O40 heteropolyacid catalyst on the biphasic hydrolysis of vegetable oils. RSC Adv. 2017, 7, 8192–8199. [Google Scholar] [CrossRef]
- da Silva, M.J.; Santos, L.F.D. Novel oxidative desulfurization of a model fuel with H2O2 Catalyzed by AlPMo12O40 under phase transfer catalyst-free conditions. J. Appl. Chem. 2013. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heteropolyacid catalysts. Curr. Opin. Solid State Mater. Sci. 1997, 2, 84–89. [Google Scholar] [CrossRef]
- Li, S.W.; Gao, R.; Zhang, W.; Zhang, Y.; Zhao, J. Heteropolyacids supported on macroporous materials POM@MOF-199@LZSM-5: Highly catalytic performance in oxidative desulfurization of fuel oil with oxygen. Fuel 2018, 221, 1–11. [Google Scholar] [CrossRef]
- Rafiee, E.; Nobakht, N. Keggin type heteropolyacid, encapsulated in metal-organic framework: A heterogeneous and recyclable nanocatalyst for selective oxidation of sulfides and deep desulfurization of model fuels. J. Mol. Catal. A Chem. 2015, 398, 17–25. [Google Scholar] [CrossRef]
- Tang, L.; Luo, G.; Zhu, M.; Kang, L.; Dai, B. Preparation, characterization and catalytic performance of HPW-TUD-1 catalyst on oxidative desulfurization. J. Ind. Eng. Chem. 2013, 19, 620–626. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, S.; Liu, X.; Nie, Y.; Zhang, X.; Zhang, S. Extractive desulfurization of fuel using N-butylpyridinium-based ionic liquids. RSC Adv. 2015, 5, 30234–30238. [Google Scholar] [CrossRef]
- Lu, L.; Cheng, S.; Gao, J.; Gao, G.; He, M. Deep oxidative desulfurization of fuels catalyzed by ionic liquid in the presence of H2O2. Energy Fuels 2007, 21, 383–384. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Sun, Y.; Jiang, B.; Chen, Y.; Gao, X.; Yang, H. Deep catalytic oxidative desulfurization of fuels by novel Lewis acidic ionic liquids. Fuel Process. Technol. 2018, 177, 81–88. [Google Scholar] [CrossRef]
- Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C. Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab. J. Chem. 2016, 9, 578–587. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhou, F.; Wang, Y.; Yuan, X.; Wang, H. Oxidative desulfurization of dibenzothiophene and diesel by hydrogen peroxide: Catalysis of H3PMo12O40 immobilized on the ionic liquid modified SiO2. Mol. Catal. 2018, 452, 93–99. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Sun, Y.; Jiang, B.; Yang, H. Deep oxidative desulfurization of fuels by superbase-derived Lewis acidic ionic liquids. Chem. Eng. J. 2017, 328, 445–453. [Google Scholar] [CrossRef]
- Francisco, M.; Arce, A.; Soto, A. Ionic liquids on desulfurization of fuel oils. Fluid Phase Equilib. 2010, 294, 39–48. [Google Scholar] [CrossRef]
- Jiang, W.; Zhua, W.; Chang, Y.; Chao, Y.; Yin, S.; Liu, H.; Zhu, F.; Li, H. Ionic liquid extraction and catalytic oxidative desulfurization of fuels using dialkylpiperidinium tetrachloroferrates catalyst. Chem. Eng. J. 2014, 250, 48–54. [Google Scholar] [CrossRef]
- Li, F. Deep extractive and oxidative desulfurization of dibenzothiophene with C5H9NO·SnCl2 coordinated ionic liquid. J. Hazard. Mater. 2012, 205, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Chica, A.; Corma, A.; Dómine, M.E. Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixed-bed reactor. J. Catal. 2006, 242, 299–308. [Google Scholar] [CrossRef]
- Zhao, N.; Li, S.; Wang, J.; Zhang, R.; Gao, R.; Zhao, J.; Wang, J. Synthesis and application of different phthalocyanine molecular sieve catalyst for oxidative desulfurization. J. Solid-State Chem. 2015, 225, 347–353. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, K.; Cheng, Y.; Zeng, G.; Zhang, M.; Shao, J. Catalytic oxidative desulfurization of BT and DBT from n-octane using cyclohexanone peroxide and catalyst of molybdenum supported on 4A molecular sieve. Sep. Purif. Technol. 2016, 163, 153–161. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Naito, T.; Hirai, T. Vanadosilicate molecular sieve as a catalyst for oxidative desulfurization of light oil. Ind. Eng. Chem. Res. 2003, 42, 6034–6039. [Google Scholar] [CrossRef]
- Cheng, S. Catalytic oxidation of benzothiophene and dibenzothiophene in model light oil Ti-MWW. Chin. J. Catal. 2006, 27, 547–549. [Google Scholar] [CrossRef]
- Wang, S.; Yang, G. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Trakarnpruk, W.; Rujiraworawut, K. Oxidative desulfurization of Gas oil by polyoxometalates catalysts. Fuel Process. Technol. 2009, 90, 411–414. [Google Scholar] [CrossRef]
- Julião, D.; Gomes, A.C.; Pillinger, M.; Cunha-Silva, L.; de Castro, B.; Gonçalves, I.S.; Balula, S.S. Desulfurization of model diesel by extraction/oxidation using a zinc-substituted polyoxometalate as catalyst under homogeneous and heterogeneous (MIL-101(Cr) encapsulated) conditions. Fuel Process. Technol. 2015, 131, 78–86. [Google Scholar] [CrossRef]
- Fraile, J.M.; Gil, C.; Mayoral, J.A.; Muel, B.; Roldán, L.; Vispe, E.; Calderón, S.; Puente, F. Heterogeneous titanium catalysts for oxidation of dibenzothiophene in hydrocarbon solutions with hydrogen peroxide: On the road to oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 680–686. [Google Scholar] [CrossRef]
- Jin, C.; Li, G.; Wang, X.; Wang, Y.; Zhao, L.; Sun, D. A titanium containing micro/mesoporous composite and its catalytic performance in oxidative desulfurization. Microporous Mesoporous Mater. 2008, 111, 236–242. [Google Scholar] [CrossRef]
- Sengupta, A.; Kamble, P.D.; Basu, J.K.; Sengupta, S. Kinetic study and optimization of oxidative desulfurization of benzothiophene using mesoporous titanium silicate-1 catalyst. Ind. Eng. Chem. Res. 2012, 51, 147–157. [Google Scholar] [CrossRef]
- Bazyari, A.; Khodadadi, A.A.; Mamaghani, A.H.; Beheshtian, J.A.; Thompson, L.T.; Mortazavi, Y. Microporous titania–silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 65–77. [Google Scholar] [CrossRef]
- Yang, S.; Jeong, K.; Jeong, S.; Ahn, W. Synthesis of mesoporous TS-1 using a hybrid SiO2–TiO2 xerogel for catalytic oxidative desulfurization. Mater. Res. Bull. 2012, 47, 4398–4402. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Wang, J.; Liang, W.; Li, H. Photocatalytic oxidation desulfurization of diesel oil using Ti-containing zeolite. Pet. Sci. Technol. 2009, 27, 1–11. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Roces, S.; Dugos, N.; Wan, M. Oxidation by H2O2 of bezothiophene and dibenzothiophene over different polyoxometalate catalysts in the frame of ultrasound and mixing assisted oxidative desulfurization. Fuel 2016, 180, 127–136. [Google Scholar] [CrossRef]
- Wu, Z.; Ondruschka, B. Ultrasound-assisted oxidative desulfurization of liquid fuels and its industrial application. Ultrason. Sonochem. 2010, 17, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Afzalinia, A.; Mirzaie, A.; Nikseresht, A.; Musabeygi, T. Ultrasound-assisted oxidative desulfurization process of liquid fuel by phosphotungstic acid encapsulated in a interpenetrating amine-functionalized Zn (II)-based MOF as catalyst. Ultrason. Sonochem. 2017, 34, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Gildo, P.J.; Dugos, N.; Wan, S.R.M. Optimized ultrasound-assisted oxidative desulfurization process of simulated fuels over activated carbon-supported phosphotungstic acid. MATEC Web Conf. 2018, 156, 03045. [Google Scholar] [CrossRef]
- Jalali, M.R.; Sobati, M.A. Intensification of oxidative desulfurization of gas oil by ultrasound irradiation: Optimization using Box–Behnken design (BBD). Appl. Therm. Eng. 2017, 111, 1158–1170. [Google Scholar] [CrossRef]
- Margeta, D.; Sertić-Bionda, K.; Foglar, L. Ultrasound assisted oxidative desulfurization of model diesel fuel. Appl. Acoust. 2016, 103, 202–206. [Google Scholar] [CrossRef]
- Akbari, A.; Omidkhah, M.; Darian, J.T. Facilitated and selective oxidation of thiophenic sulfur compoundsusing MoOx/Al2O3–H2O2 system under ultrasonic irradiation. Ultrason. Sonochem. 2015, 23, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.E.S.; Roces, S.; Dugos, N.; Futalan, C.M.; Lin, S.; Wan, M. Optimization of ultrasound-assisted oxidative desulfurization of model sulfur compounds using commercial ferrate (VI). J. Taiwan Inst. Chem. Eng. 2014, 45, 2935–2942. [Google Scholar] [CrossRef]
- Tang, Q.; Lin, S.; Cheng, Y.; Liu, S.; Xiong, J. Ultrasound-assisted oxidative desulfurization of bunker-C oil using tert-butyl hydroperoxide. Ultrason. Sonochem. 2013, 20, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.A.; Mello, P.D.; Bizzi, C.A.; Nunes, M.A.G.; Moreira, E.M.; Alencar, M.S.; Motta, H.N.; Dressler, V.L.; Flores, É.M.M. Sulfur removal from hydrotreated petroleum fractions using ultrasound-assisted oxidative desulfurization process. Fuel 2011, 90, 2158–2164. [Google Scholar] [CrossRef]
- Mello, P.D.; Duarte, F.A.; Nunes, M.A.G.; Alencar, M.S.; Moreira, E.M.; Korn, M.; Dressler, V.L.; Flores, É.M.M. Ultrasound-assisted oxidative process for sulfur removal from petroleum product feedstock. Ultrason. Sonochem. 2009, 16, 732–736. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).