Alkali and Alkaline Earth Cation-Decorated TiO2 Nanotube-Supported Rh Catalysts for Vinyl Acetate Hydroformylation
Abstract
:1. Introduction
2. Results and Discussion
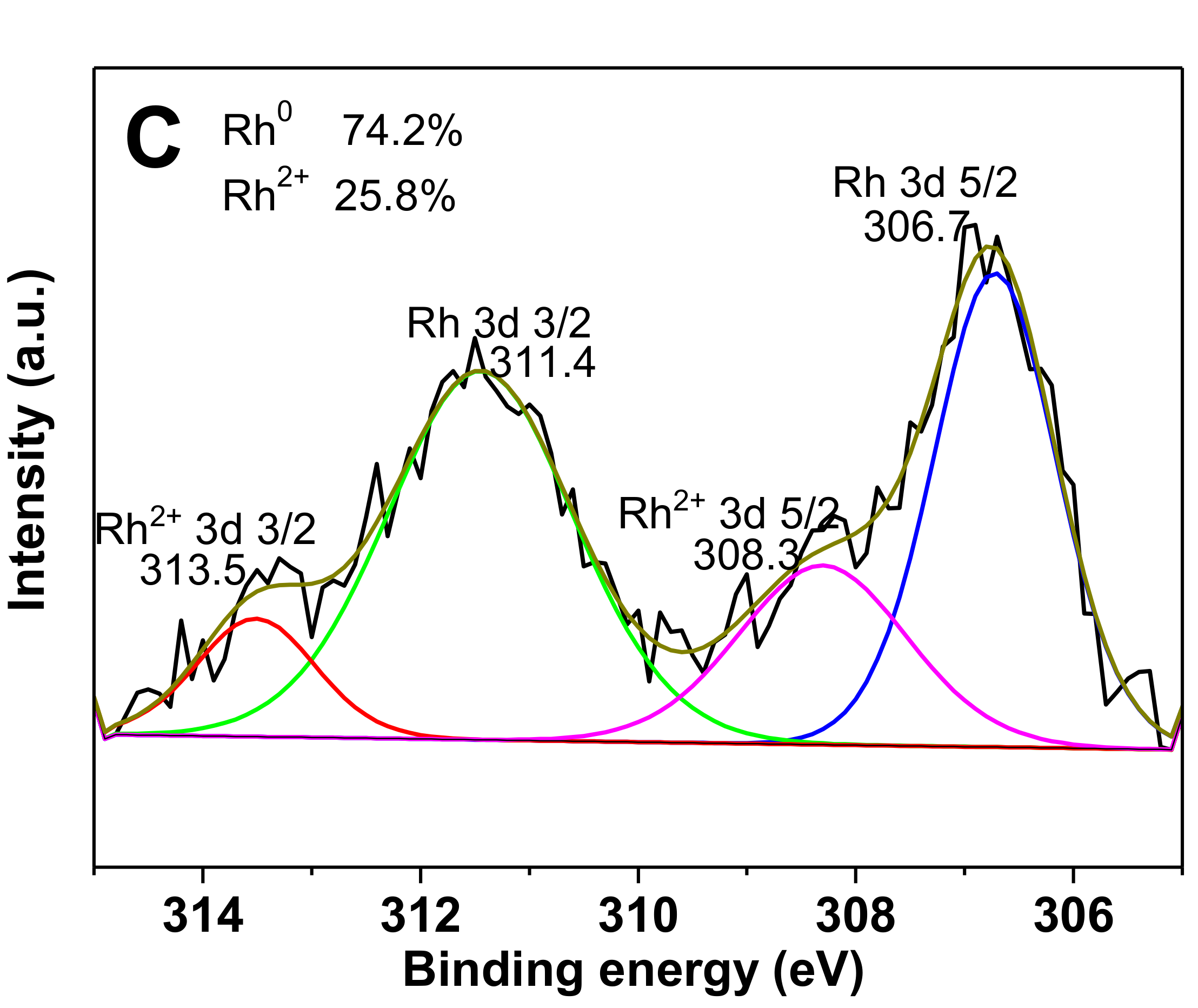
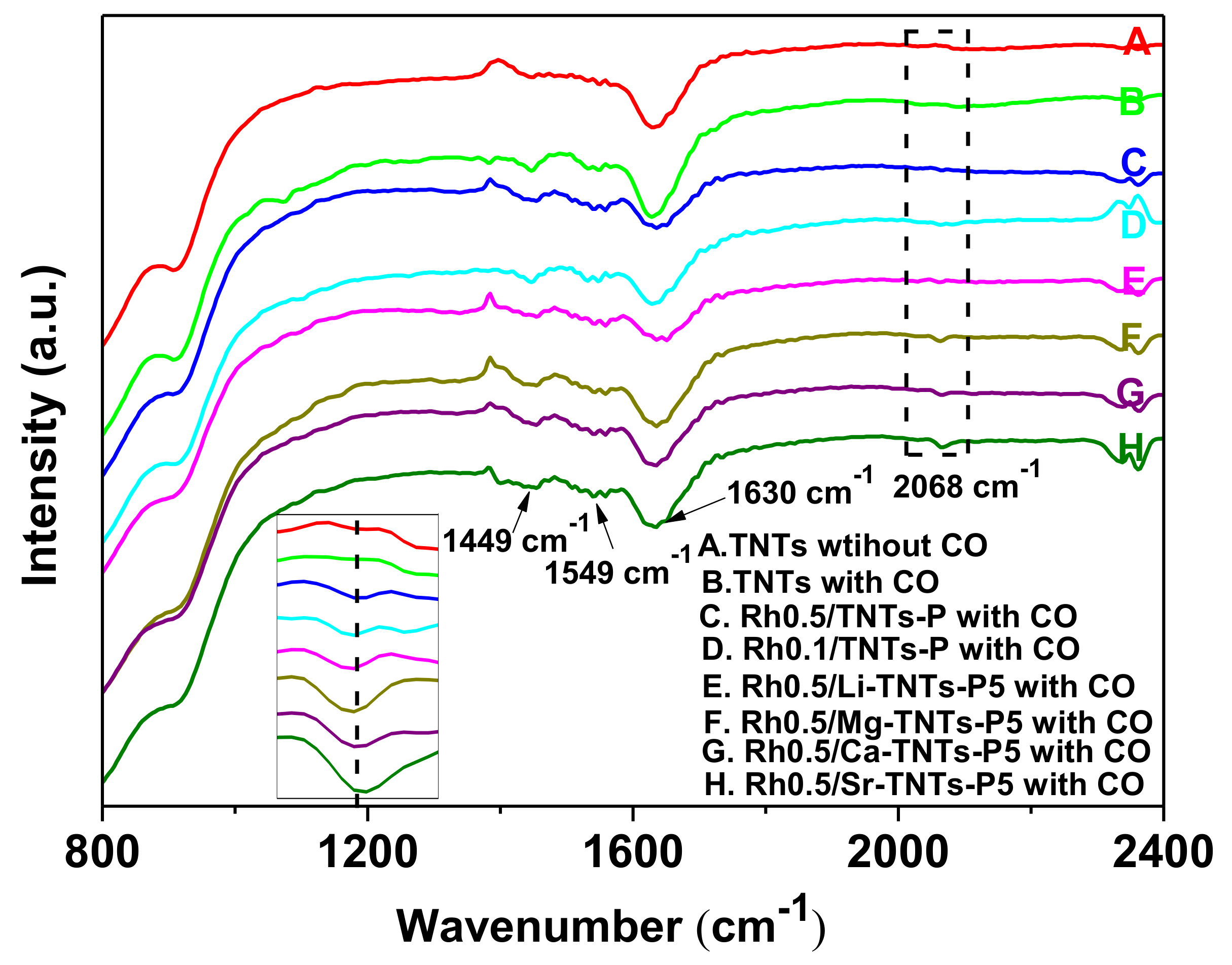
2.1. Characterization of the Catalysts
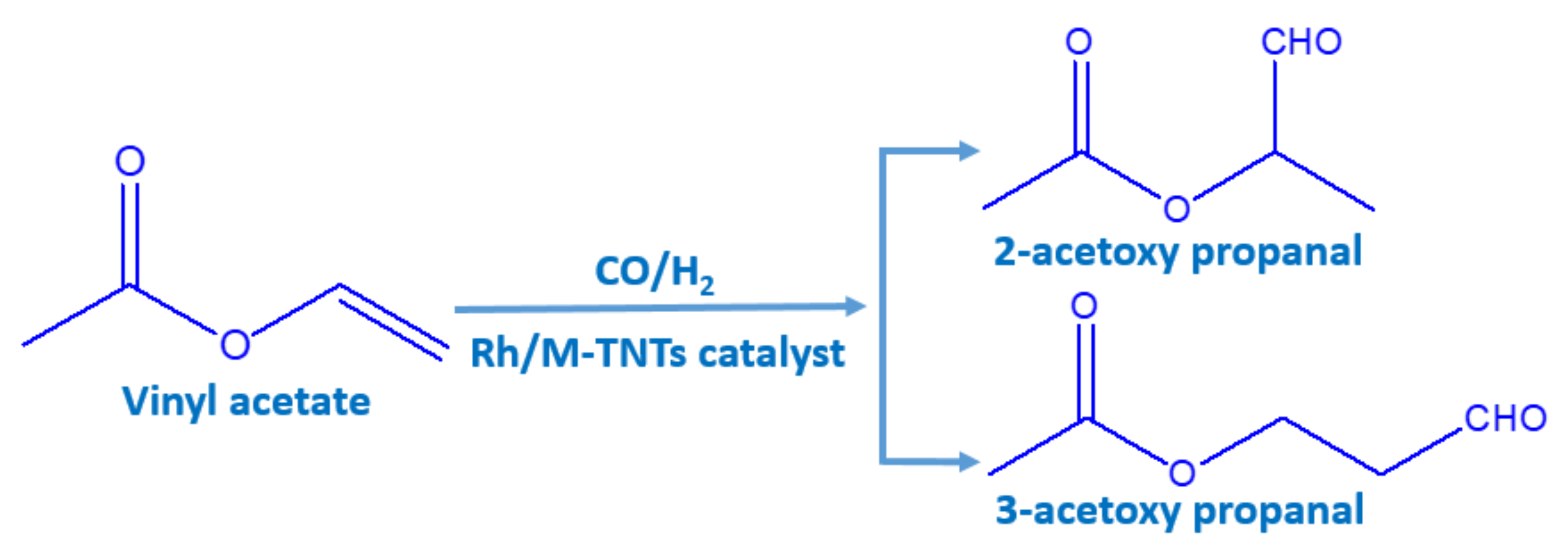
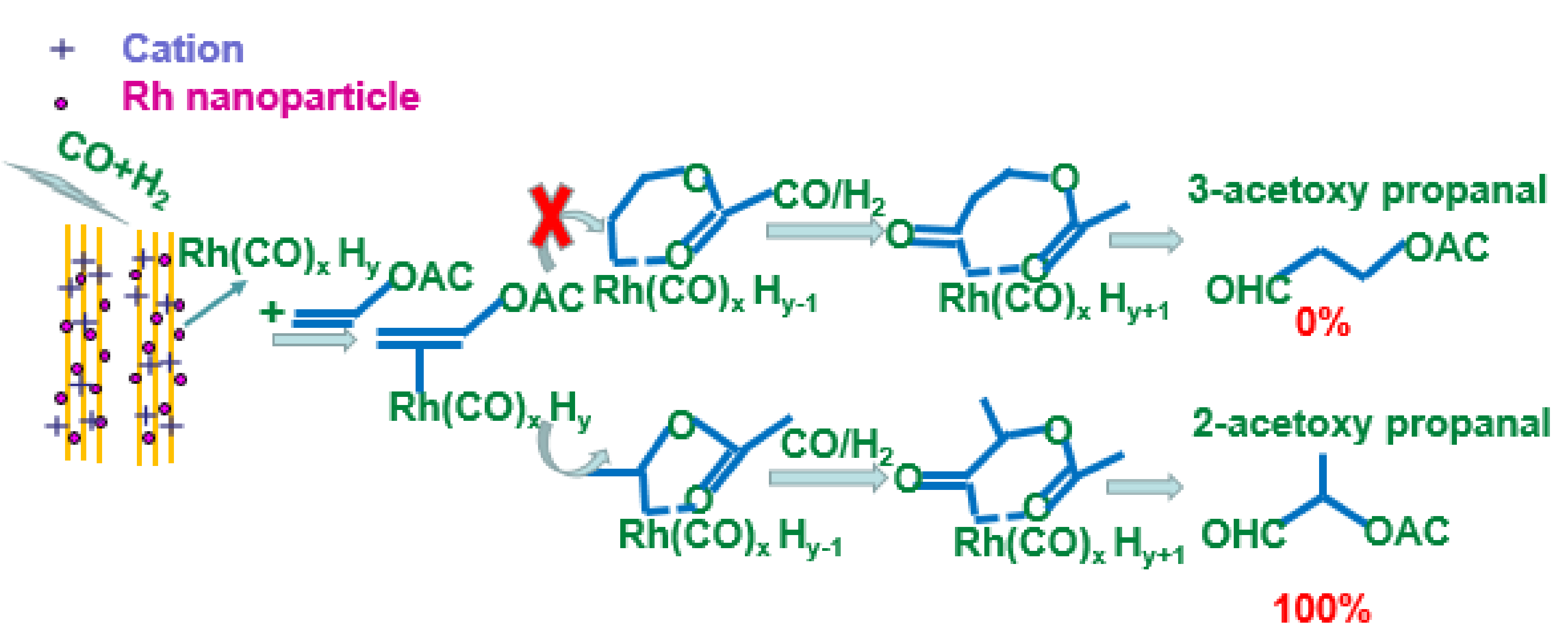
2.2. Catalytic Performances of the Catalysts
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goanvic, L.L.; Ternel, J.; Couturier, J.L.; Dubois, J.L.; Carpentier, J.F. Rhodium-biphephos-catalyzed tandem isomerization–hydroformylation of oleonitrile. Catalysts 2018, 8, 21. [Google Scholar] [CrossRef]
- Cousin, K.; Menuel, S.; Monflier, E.; Hapiot, F. Hydroformylation of alkenes in a planetary ball mill: From additive-controlled reactivity to supramolecular control of regioselectivity. Angew. Chem. Int. Ed. 2017, 56, 10564–10568. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.B.; Fu, J.; Gao, Z.X.; Zhang, L.B.; Li, C.Y.; Wang, T.F. Dicyclopentadiene hydroformylation to value-added fine chemicals over magnetically separable Fe3O4-supported Co-Rh bimetallic catalysts: Effects of cobalt loading. Catalysts 2017, 7, 103. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yan, L.; Li, C.Y.; Jiang, M.; Wang, W.L.; Ding, Y.J. Highly efficient porous organic copolymer supported Rh catalysts for heterogeneous hydroformylation of butenes. Appl. Catal. A Gen. 2018, 551, 98–105. [Google Scholar] [CrossRef]
- Kontkanen, M.J.; Tuikka, M.; Kinnunen, N.M.; Suvanto, S.; Haukka, M. Hydroformylation of 1-hexene over Rh/nano-oxide catalysts. Catalysts 2013, 3, 324–337. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Zhang, X.M.; Sanjeevi, J.; Yang, Q.H. Hydroformylation of 1-octene in Pickering emulsion constructed by amphiphilic mesoporous silica nanoparticles. J. Catal. 2016, 334, 52–59. [Google Scholar] [CrossRef]
- Gual, A.; Godard, C.; Castillon, S.; Claver, C. Highly efficient Rhodium catalysts for the asymmetric hydroformylation of vinyl and allyl ethers using C1-symmetrical diphosphite ligands. Adv. Synth. Catal. 2010, 352, 463–477. [Google Scholar] [CrossRef]
- Khan, S.R.; Bhanage, B.M. Regioselective hydroformylation of vinyl esters catalyzed by Rh(acac)(CO)2 with simple and efficient diphosphinite ligands. Catal. Commun. 2014, 46, 109–112. [Google Scholar] [CrossRef]
- Chuai, H.Y.; Liu, X.T.; Chen, Y.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Hydroformylation of vinyl acetate and cyclohexene over TiO2 nanotube supported Rh and Ru nanoparticle catalysts. RSC. Adv. 2018, 8, 12053–12059. [Google Scholar] [CrossRef]
- Chen, Y.; Su, P.H.; Liu, T.; Liu, H.C.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Titanate nanotube-supported Au–Rh bimetallic catalysts: Characterization and their catalytic performances in hydroformylation of vinyl acetate. Catalysts 2018, 8, 420. [Google Scholar] [CrossRef]
- Li, J.F.; Cheng, X.F.; Zhang, C.H.; Chang, Q.; Wang, J.; Wang, X.P.; Lv, Z.G.; Dong, W.S.; Yang, Y.; Li, Y.W. Effect of alkalis on iron-based Fischer-Tropsch synthesis catalysts: Alkali-FeOx interaction, reduction, and catalytic performance. Appl. Catal. A Gen. 2016, 528, 131–141. [Google Scholar] [CrossRef]
- Tao, Z.C.; Yang, Y.; Zhang, C.H.; Li, T.Z.; Wang, J.H.; Wan, H.J.; Xiang, H.W.; Li, Y.W. Effect of calcium promoter on a precipitated iron–manganese catalyst for Fischer–Tropsch synthesis. Catal. Commun. 2006, 7, 1061–1066. [Google Scholar] [CrossRef]
- Pour, A.N.; Shahri, S.M.K.; Bozorgzadeh, H.R.; Tavasoli, Y.Z.A.; Marvast, M.A. Effect of Mg, La and Ca promoters on the structure and catalytic behavior of iron-based catalysts in Fischer—Tropsch synthesis. Appl. Catal. A Gen. 2008, 348, 201–208. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, C.H.; Cheng, X.F.; Qing, M.; Xu, J.; Wu, B.S.; Yang, Y.; Li, Y.W. Effects of alkaline-earth metals on the structure, adsorption and catalytic behavior of iron-based Fischer–Tropsch synthesis catalysts. Appl. Catal. A Gen. 2013, 464–465, 10–19. [Google Scholar] [CrossRef]
- Farias, F.E.M.; Sales, F.G.; Fernandes, F.A.N. Effect of operating conditions and potassium content on Fischer-Tropsch liquid products produced by potassium-promoted iron catalysts. J. Nat. Gas Chem. 2008, 17, 175–178. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Zhang, C.H.; Qin, S.D.; Xiang, H.W.; Li, Y.W. Effect of interaction between potassium and structural promoters on Fischer–Tropsch performance in iron-based catalysts. J. Mol. Catal. A Chem. 2008, 285, 137–142. [Google Scholar] [CrossRef]
- Hussain, S.T.; Nadeem, M.A.; Mazhar, M. Effect of potassium addition on the product selectivity of Ru:Mn/alumina supported catalyst system for Fischer–Tropsch reaction. Catal. Commun. 2008, 9, 2048–2052. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, H.W.; Xu, Y.Y.; Bai, L.; Li, Y.W. Effect of potassium promoter on precipitated iron-manganese catalyst for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2004, 266, 181–194. [Google Scholar] [CrossRef]
- Fusi, A.; Psaro, R.; Dossi, C.; Garlaschelli, L.; Cozzi, F. A molecular approach to heterogeneous catalysis. Ethylene hydroformylation catalysed by silica-supported [Rh12(CO)30]2− cluster anion: Influence of the countercations Li+, Na+, K+, Zn2+. J. Mol. Catal. A. 1996, 107, 255–261. [Google Scholar] [CrossRef]
- Sordelli, L.; Guidotti, M.; Andreatta, D.; Vlaic, G.; Psaro, R. Characterization and catalytic performances of alkali-metal promoted Rh/SiO2 catalysts for propene hydroformylation. J. Mol. Catal. A Chem. 2003, 204, 509–518. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.Y.; Zhang, J.L.; Schwanka, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Jiang, Y.J.; Zhu, B.L.; Zhang, S.M.; Hunag, W.P. Ti-O nanotubes supported Rh-catalysts and their catalytic performances for hydroformylation reaction of olefin containing—CN Functional group. J. Mol. Catal. 2016, 30, 505–514. [Google Scholar]
- Shi, Y.K.; Hu, X.J.; Chen, L.; Lu, Y.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Boron modified TiO2 nanotubes supported Rh-nanoparticle catalysts for highly efficient hydroformylation of styrene. New J. Chem. 2017, 14, 6120–6126. [Google Scholar] [CrossRef]
- Gannouna, C.; Turki, A.; Kochkar, H.; Delaigle, R.; Eloy, P.; Ghorbel, A.; Gaigneauxc, E.M. Elaboration and characterization of sulfated and unsulfated V2O5/TiO2 nanotubes catalysts for chlorobenzene total oxidation. Appl. Catal. B Environ. 2014, 147, 58–64. [Google Scholar] [CrossRef]
- Hu, X.J.; Shi, Y.K.; Zhang, Y.J.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Nanotubular TiO2-supported amorphous Co–B catalysts and their catalytic performances for hydroformylation of cyclohexene. Catal. Commun. 2015, 59, 45–49. [Google Scholar] [CrossRef]
- Shi, Y.K.; Hu, X.J.; Zhu, B.L.; Wang, S.R.; Zhang, S.M.; Huang, W.P. Synthesis and characterization of TiO2 nanotube supported Rh-nanoparticle catalysts for regioselective hydroformylation of vinyl acetate. RSC Adv. 2014, 4, 62215–62222. [Google Scholar] [CrossRef]
- Ma, R.; Fukuda, K.; Sasaki, T.; Osada, M.; Bando, Y. Structural features of titanate nanotubes/nanobelts revealed by raman, X-ray absorption fine structure and electron diffraction characterizations. J. Phys. Chem. B 2005, 109, 6210–6214. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wong, S.S. Size- and shape-dependent transformation of nanosized titanate into analogous anatase titania nanostructures. J. Am. Chem. Soc. 2006, 128, 8217–8226. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.R.; Zhang, H.M.; Liu, H.W.; Wang, Y.; Yao, X.D.; Zhu, G.S.; Zhang, S.Q.; Zhao, H.J. A facile vapor-phase hydrothermal method for direct growth of titanate nanotubes on a titanium substrate via a distinctive nanosheet roll-up mechanism. J. Am. Chem. Soc. 2011, 133, 19032–19035. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.M.; Howard, R.A.; Kadish, K.M.; Bean, J.L.; Brace, J.; Winograd, N. X-ray photoelectron spectra of some dirhodium carboxylate complexes. Inorg. Chim. Acta 1980, 44, L139–L141. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M. X-ray photoelectron spectroscopic study of montmorillonite containing exchangeable divalent cations. J. Chem. Soc. Faraday Trans. 1984, 80, 237–248. [Google Scholar] [CrossRef]
- Bandura, A.V.; Evarestov, R.A. From anatase (1 0 1) surface to TiO2 nanotubes: Rolling procedure and first principles LCAO calculations. Surf. Sci. 2009, 603, L117–L120. [Google Scholar] [CrossRef]
- Hossain, F.M.; Evteev, A.V.; Belova, I.V.; Nowotny, J.; Murch, G.E. Electronic and optical properties of anatase TiO2 nanotubes. Comput. Mater. Sci. 2010, 48, 854–858. [Google Scholar] [CrossRef]
- Rasko, J.; Bontovics, J. FTIR study of the rearrangement of adsorbed CO species on Al2O3-supported rhodium catalysts. Catal. Lett. 1999, 58, 27–32. [Google Scholar] [CrossRef]
- Ding, D.; Yu, J.; Guo, Q.S.; Guo, X.M.; Xiao, X.J.; Mao, D.S.; Lu, G.Z. The effects of PVP-modified SiO2 on the catalytic performance of CO hydrogenation over Rh–Mn–Li/SiO2 catalysts. RSC Adv. 2017, 7, 48420–48428. [Google Scholar] [CrossRef]
- Zhang, S.H.; Han, X.X. Study on preparation and morphology of Aluminum-coated titanium dioxide. Semicond. Optoelectronics 2018, 39, 65–76. [Google Scholar]
- Flores-Escamilla, G.A.; Fierro-Gonzalez, J.C. Infrared spectroscopic study of dimethyl ether carbonylation catalysed by TiO2-supported rhodium carbonyls. Catal. Sci. Technol. 2015, 5, 843–850. [Google Scholar] [CrossRef]
- Iqbal, S.; Kondrat, S.A.; Jones, D.R.; Schoenmakers, D.C.; Edwards, J.K.; Lu, L.; Yeo, B.R.; Wells, P.P.; Gibson, E.K.; Morgan, D.J.; et al. Ruthenium nanoparticles supported on carbon: An active catalyst for the hydrogenation of lactic acid to 1,2-propanediol. ACS Catal. 2015, 5, 5047–5059. [Google Scholar] [CrossRef]
- Ichikawa, M.; Lang, A.J.; Shriver, D.F.; Sachtler, W.M.H. Selective hydroformylation of ethylene on rhodium-zinc-silica. An apparent example of site isolation of rhodium and Lewis acid-promoted carbonyl insertion. J. Am. Chem. Soc. 1985, 107, 7216–7218. [Google Scholar] [CrossRef]
- Sachtler, W.M.H.; Ichikawa, M. Catalytic site requirements for elementary steps in syngas conversion to oxygenates over promoted rhodium. J. Phys. Chem. 1986, 90, 4752–4758. [Google Scholar] [CrossRef]
- Borole, Y.L.; Chaudhari, R.V. New Route for the Synthesis of Propylene Glycols via Hydroformylation of Vinyl Acetate. Ind. Eng. Chem. Res. 2005, 44, 9601–9608. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshikawa, S. Synthesis and thermal analyses of TiO2-derived nanotubes prepared by the hydrothermal method. J. Mater. Res. 2004, 19, 982–985. [Google Scholar] [CrossRef]
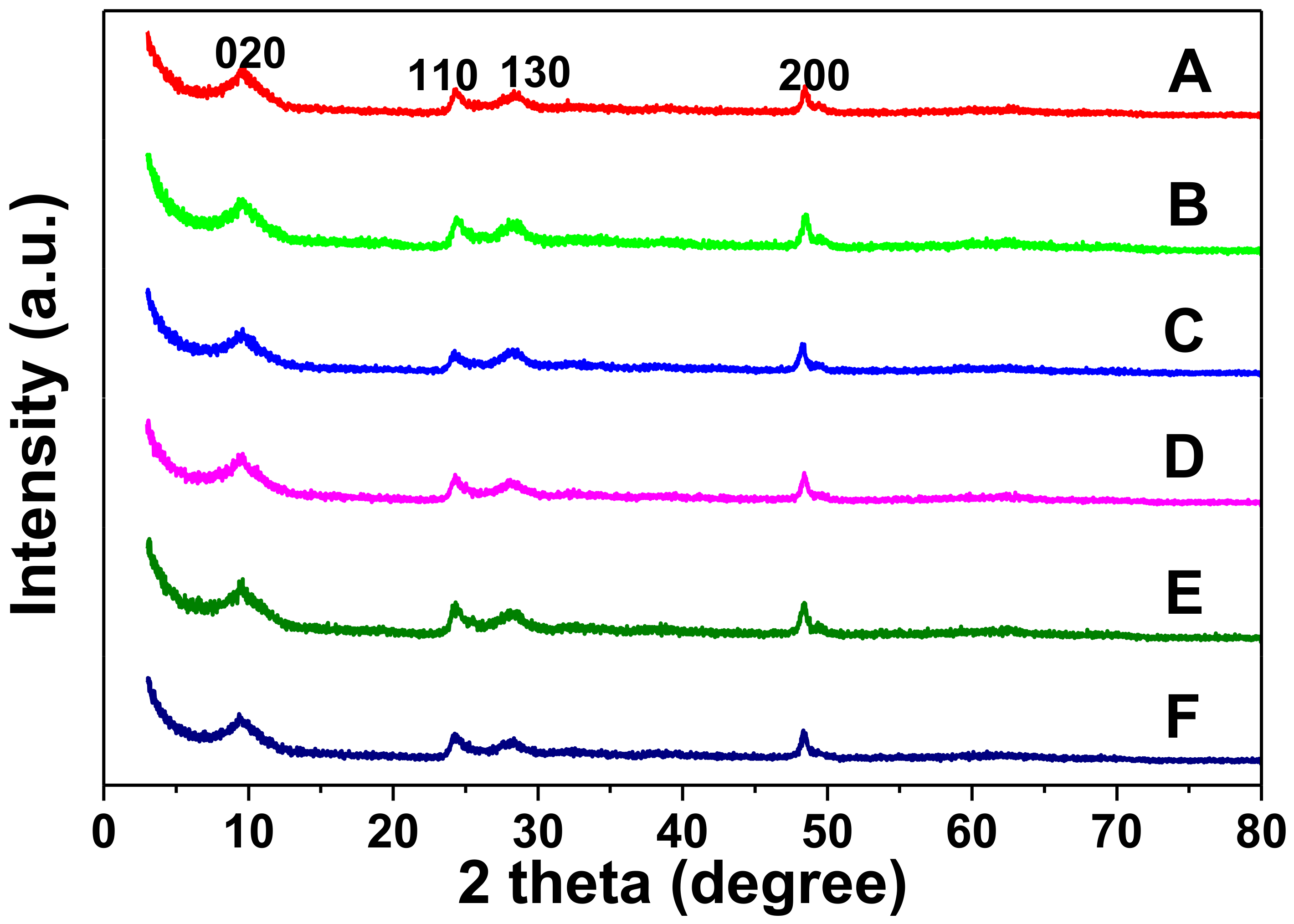
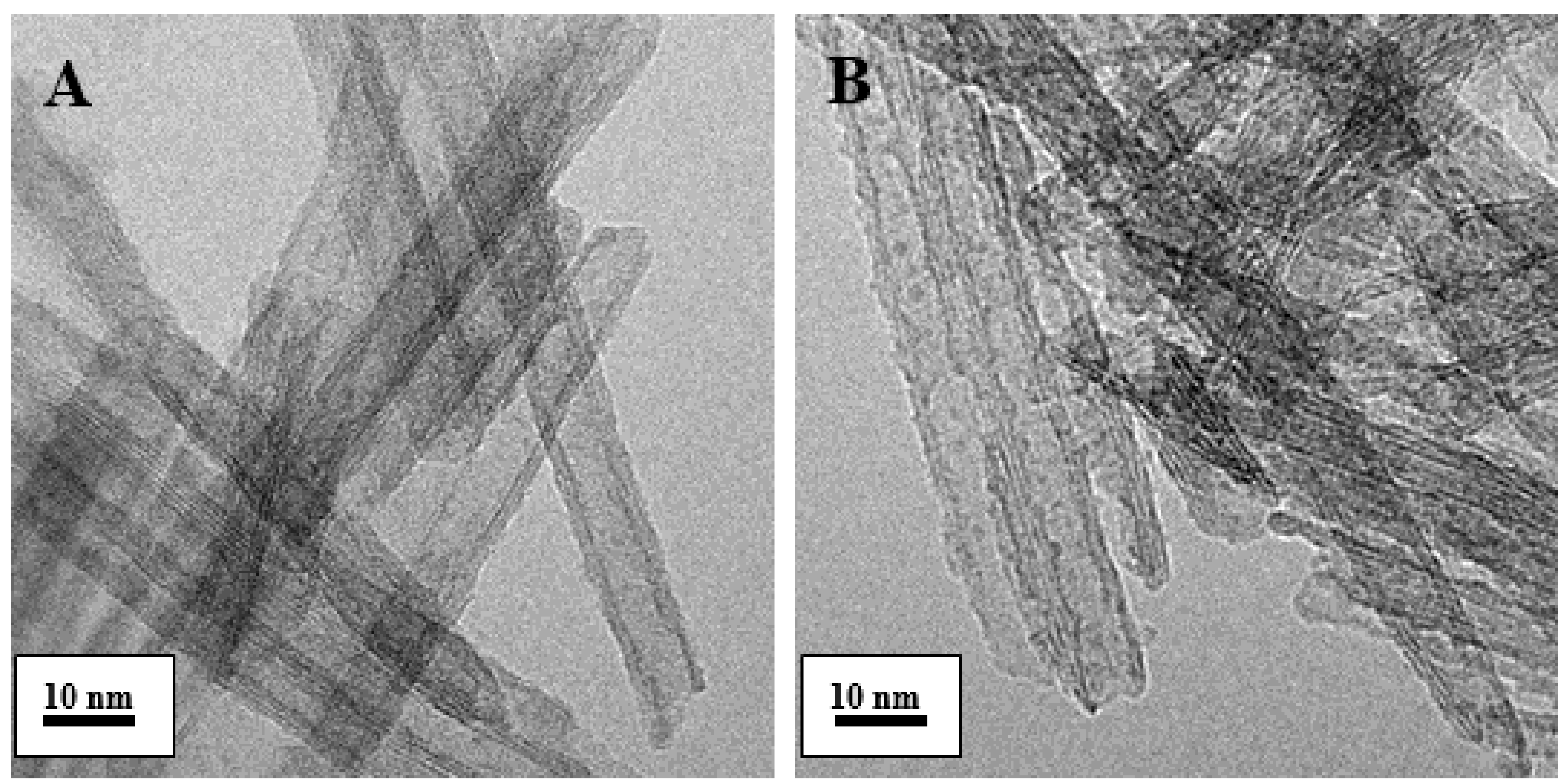
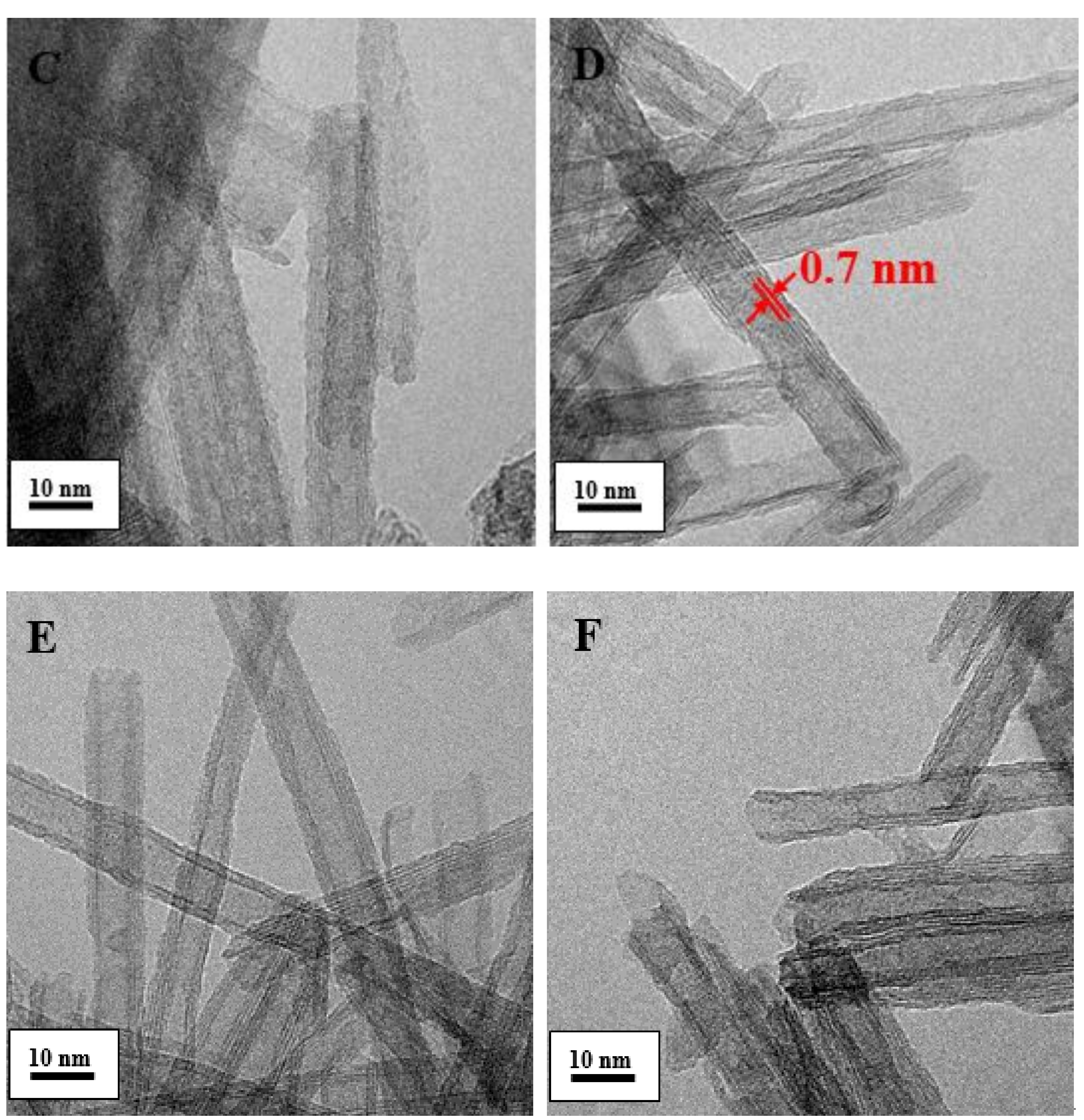
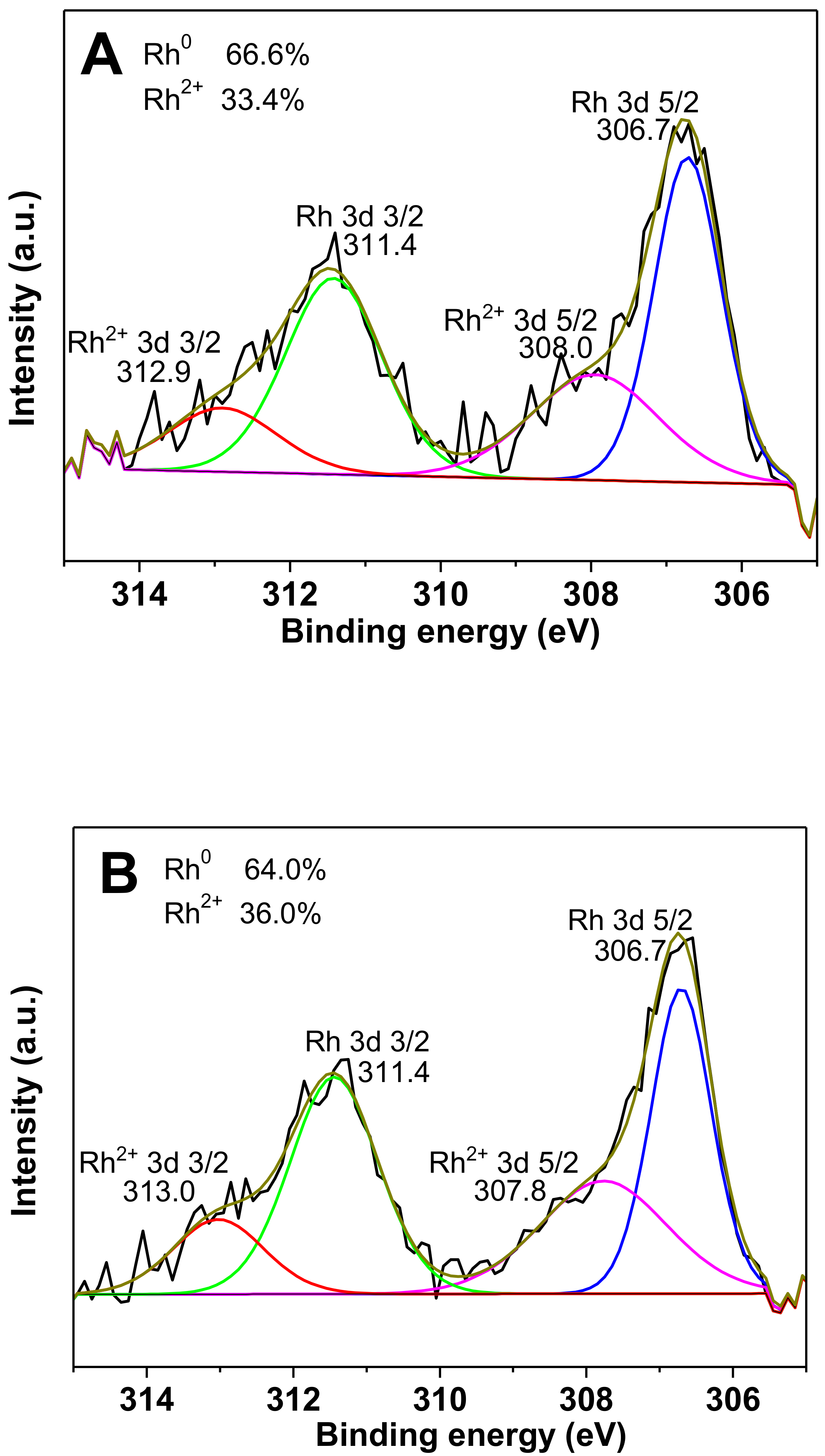
| Entry | Catalyst | Rh (Nominal) (wt.%) | M/Rh (Nominal) (Molar Ratio) | Reduction Method | Rh, M ICP (wt.%) | SSA BET (m2/g) |
|---|---|---|---|---|---|---|
| 1 | Rh0.5/TNTs-P | 0.5 | — | photo-reduction | 0.35, — | 300.08 |
| 2 | Rh0.25/TNTs-P | 0.25 | — | photo-reduction | 0.20, — | — |
| 3 | Rh0.1/TNTs-P | 0.1 | — | photo-reduction | 0.15, — | 302.83 |
| 4 | Rh0.5/Li-TNTs-P1 | 0.5 | 1 | photo-reduction | 0.43, 0.02 | — |
| 5 | Rh0.5/Li-TNTs-P5 | 0.5 | 5 | photo-reduction | 0.38, 0.08 | — |
| 6 | Rh0.5/Li-TNTs-B1 | 0.5 | 1 | borohydride | 0.27, 0.04 | — |
| 7 | Rh0.5/Li-TNTs-B5 | 0.5 | 5 | borohydride | 0.27, 0.10 | — |
| 8 | Rh0.25/Li-TNTs-P5 | 0.25 | 5 | photo-reduction | 0.26, 0.07 | — |
| 9 | Rh0.1/Li-TNTs-P5 | 0.1 | 5 | photo-reduction | 0.15, 0.04 | — |
| 10 | Rh0.5/Na-TNTs-P5 | 0.5 | 5 | photo-reduction | 0.38, 3.45 | — |
| 11 | Rh0.5/K-TNTs-P5 | 0.5 | 5 | photo-reduction | 0.38, 1.01 | — |
| 12 | Rh0.5/Mg-TNTs-P5 | 0.5 | 5 | photo-reduction | 0.37, 0.57 | 301.49 |
| 13 | Rh0.1/Mg-TNTs-P5 | 0.1 | 5 | photo-reduction | 0.17, 0.22 | — |
| 14 | Rh0.5/Ca-TNTs-P5 | 0.5 | 5 | photo-reduction | 0.36, 0.95 | — |
| 15 | Rh0.1/Ca-TNTs-P5 | 0.1 | 5 | photo-reduction | 0.14, 0.30 | — |
| 16 | Rh0.5/Sr-TNTs-P5 | 0.5 | 5 | photo-reduction | 0.40, 1.29 | — |
| 17 | Rh0.1/Sr-TNTs-P5 | 0.1 | 5 | photo-reduction | 0.12, 0.50 | — |
| Entry | Catalyst | Conversion of Vinyl Acetate (%) | Selectivity of Aldehyde (%) | TOF a (h−1) |
|---|---|---|---|---|
| 1 | Rh0.5/TNTs-P | 100 | 60 | 2382 |
| 2 | Rh0.25/TNTs-P | 60 | 60 | 2501 |
| 3 | Rh0.1/TNTs-P | 40 | 78 | 2890 |
| 4 | Rh0.5/Li-TNTs-P1 | 100 | 75 | 2424 |
| 5 | Rh0.5/Li-TNTs-P5 | 100 | 81 | 2962 |
| 6 | Rh0.5/Li-TNTs-B1 | 70 | 92 | 3315 |
| 7 | Rh0.5/Li-TNTs-B5 | 84 | 63 | 2724 |
| 8 | Rh0.25/Li-TNTs-P5 | 100 | 67 | 3581 |
| 9 | Rh0.1/Li-TNTs-P5 | 44 | 74 | 3016 |
| 10 | Rh0.5/Na-TNTs-P5 | 100 | 80 | 2926 |
| 11 | Rh0.5/K-TNTs-P5 | 100 | 79 | 2889 |
| 12 | Rh0.5/Mg-TNTs-P5 | 99 | 76 | 2826 |
| 13 | Rh0.1/Mg-TNTs-P5 | 58 | 64 | 3034 |
| 14 | Rh0.5/Ca-TNTs-P5 | 100 | 60 | 2316 |
| 15 | Rh0.1/Ca-TNTs-P5 | 46 | 72 | 3287 |
| 16 | Rh0.5/Sr-TNTs-P5 | 100 | 77 | 2675 |
| 17 | Rh0.1/Sr-TNTs-P5 | 43 | 68 | 3386 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuai, H.; Su, P.; Liu, H.; Zhu, B.; Zhang, S.; Huang, W. Alkali and Alkaline Earth Cation-Decorated TiO2 Nanotube-Supported Rh Catalysts for Vinyl Acetate Hydroformylation. Catalysts 2019, 9, 194. https://doi.org/10.3390/catal9020194
Chuai H, Su P, Liu H, Zhu B, Zhang S, Huang W. Alkali and Alkaline Earth Cation-Decorated TiO2 Nanotube-Supported Rh Catalysts for Vinyl Acetate Hydroformylation. Catalysts. 2019; 9(2):194. https://doi.org/10.3390/catal9020194
Chicago/Turabian StyleChuai, Hongyuan, Penghe Su, Hongchi Liu, Baolin Zhu, Shoumin Zhang, and Weiping Huang. 2019. "Alkali and Alkaline Earth Cation-Decorated TiO2 Nanotube-Supported Rh Catalysts for Vinyl Acetate Hydroformylation" Catalysts 9, no. 2: 194. https://doi.org/10.3390/catal9020194
APA StyleChuai, H., Su, P., Liu, H., Zhu, B., Zhang, S., & Huang, W. (2019). Alkali and Alkaline Earth Cation-Decorated TiO2 Nanotube-Supported Rh Catalysts for Vinyl Acetate Hydroformylation. Catalysts, 9(2), 194. https://doi.org/10.3390/catal9020194





