Z-Schemed WO3/rGO/SnIn4S8 Sandwich Nanohybrids for Efficient Visible Light Photocatalytic Water Purification
Abstract
1. Introduction
2. Results and Discussion
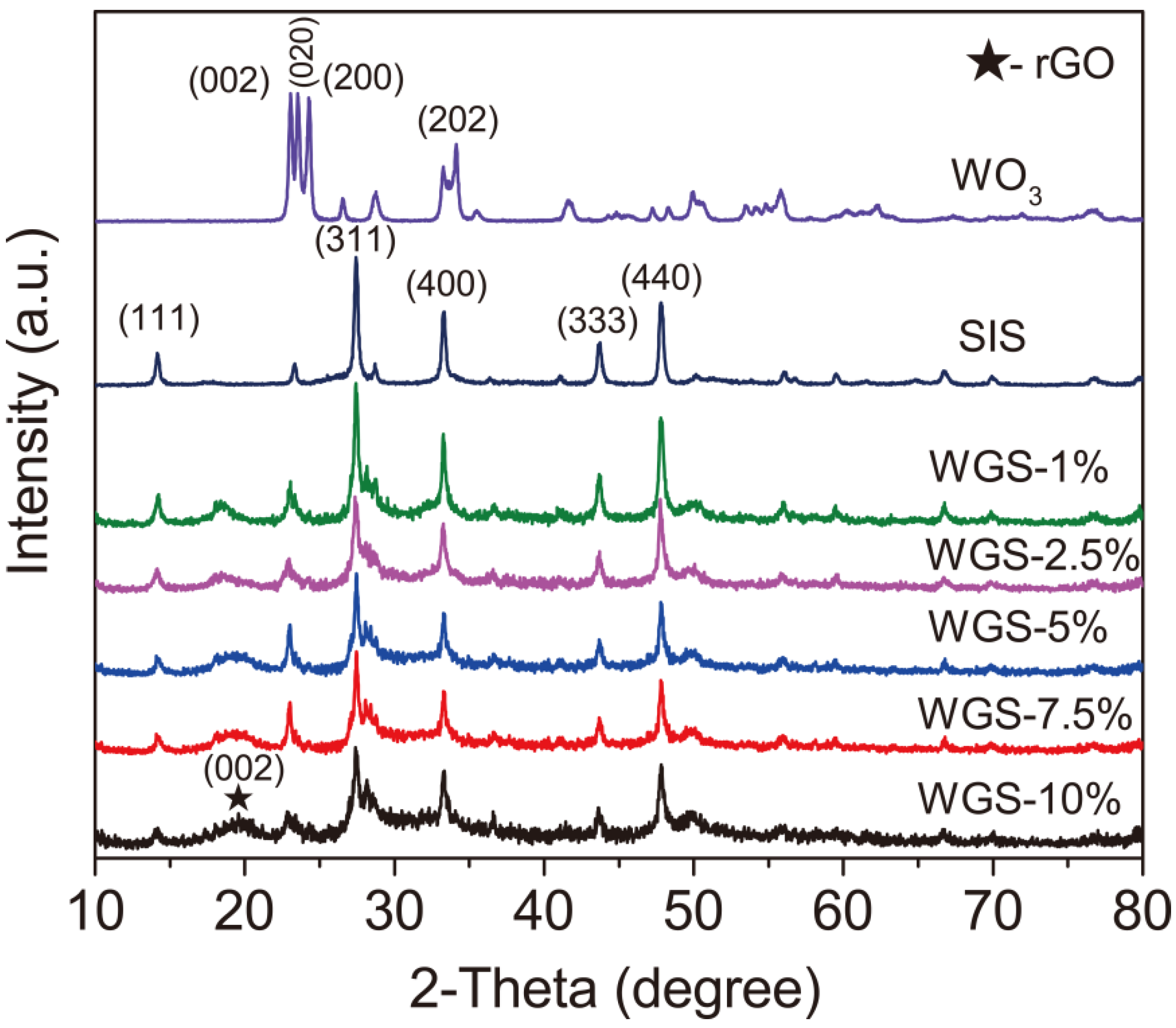
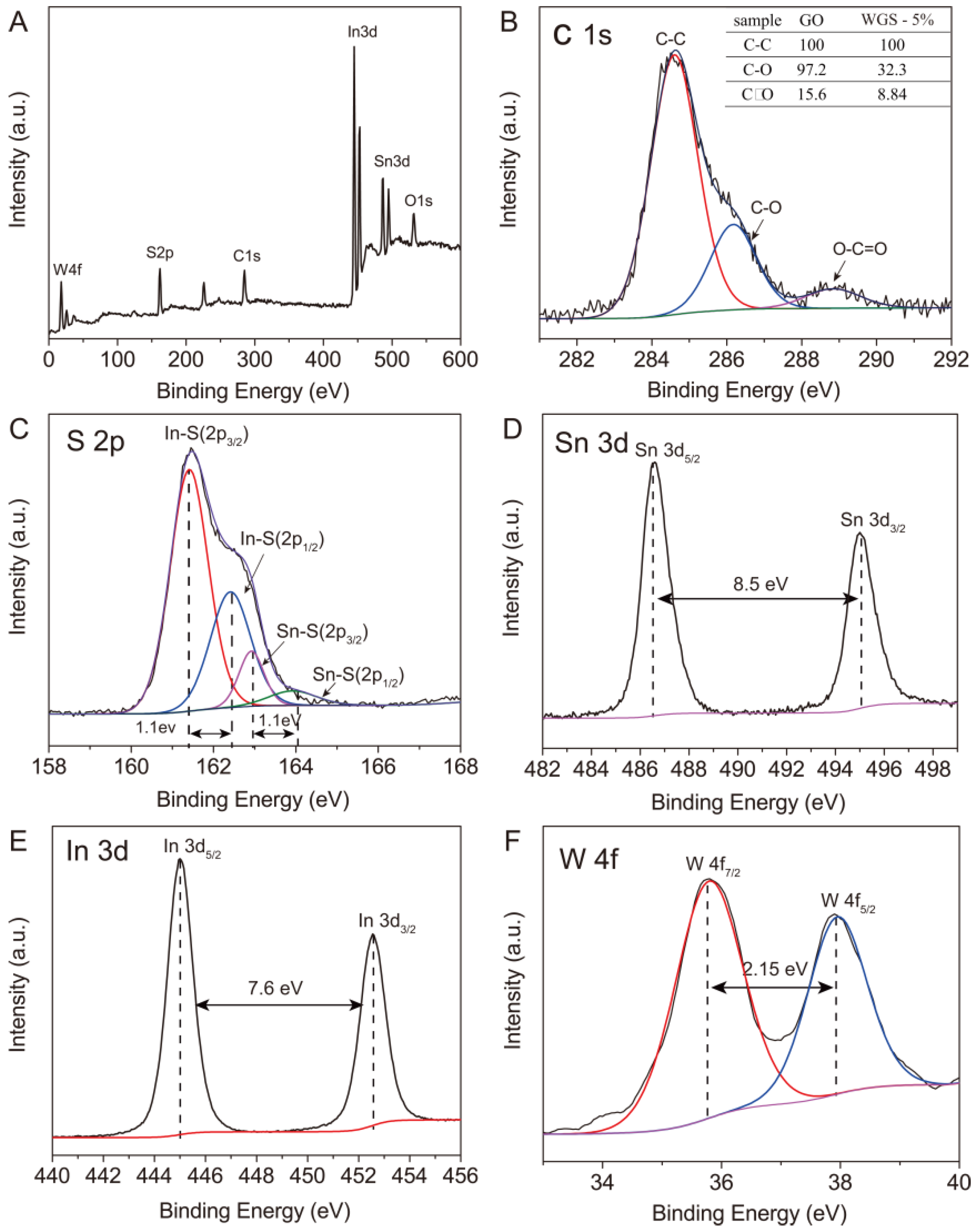
2.1. Structural Characterization of WGS Photocatalysts
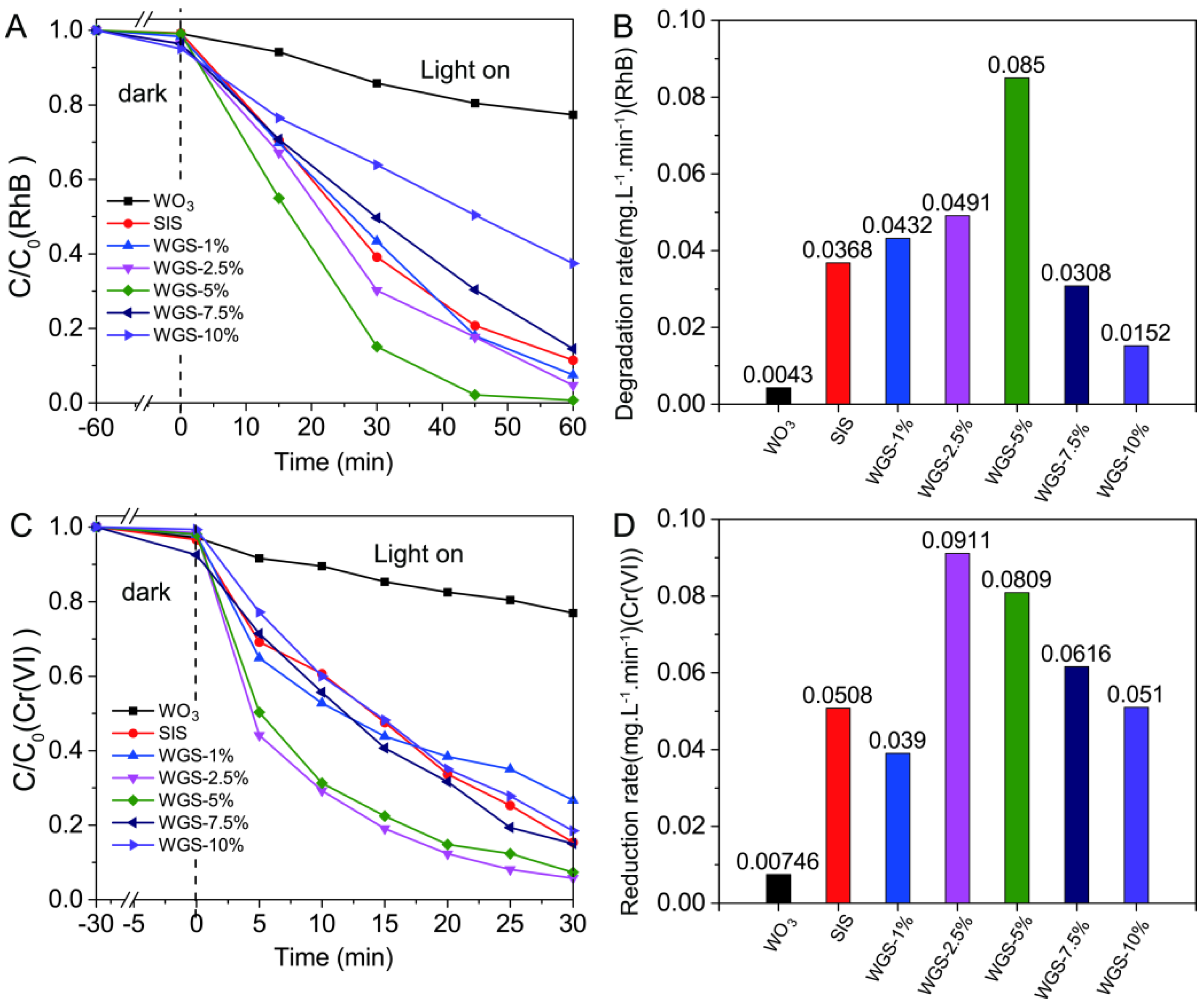
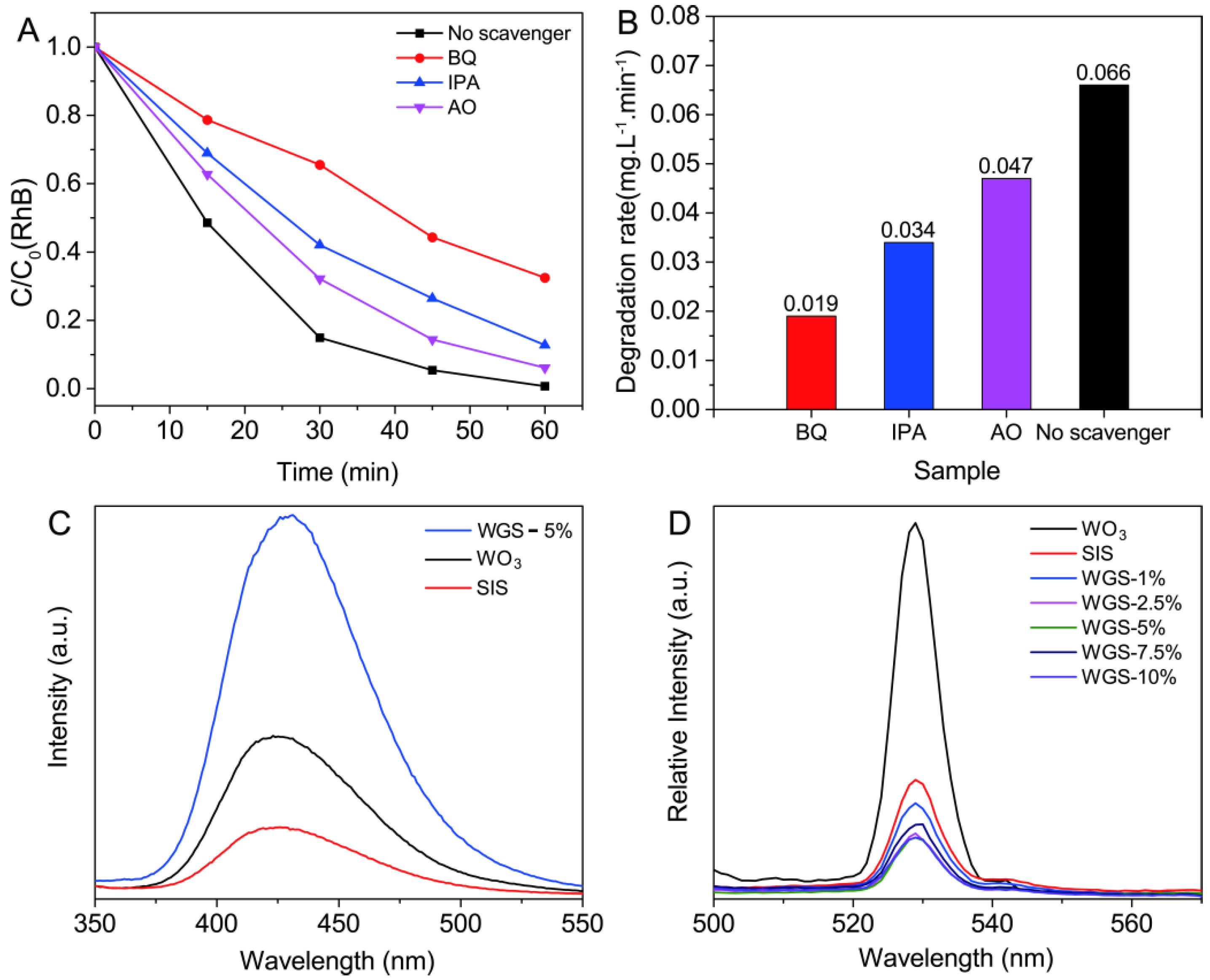
2.2. Photocatalytic Performance
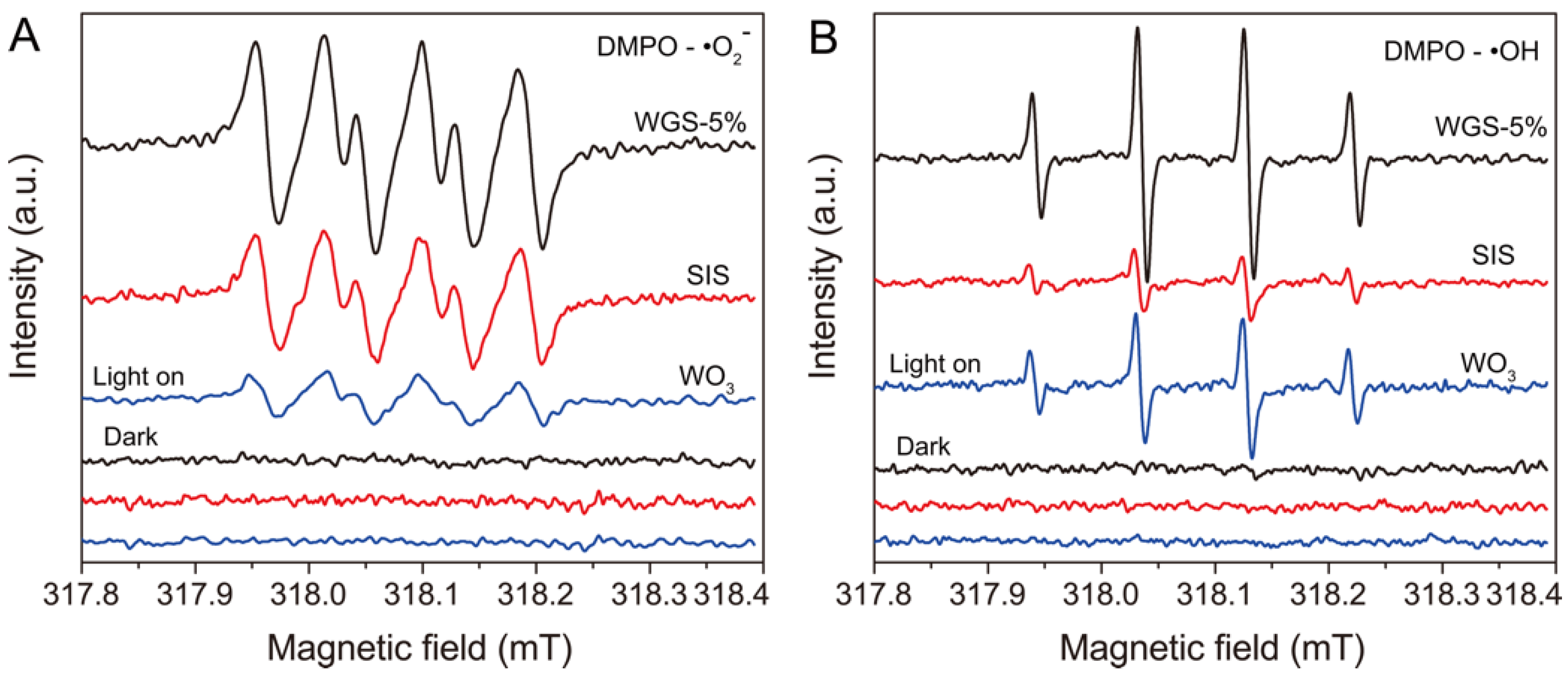
2.3. Mechanism of the Photocatalytic Activity
3. Materials and Methods
3.1. Materials
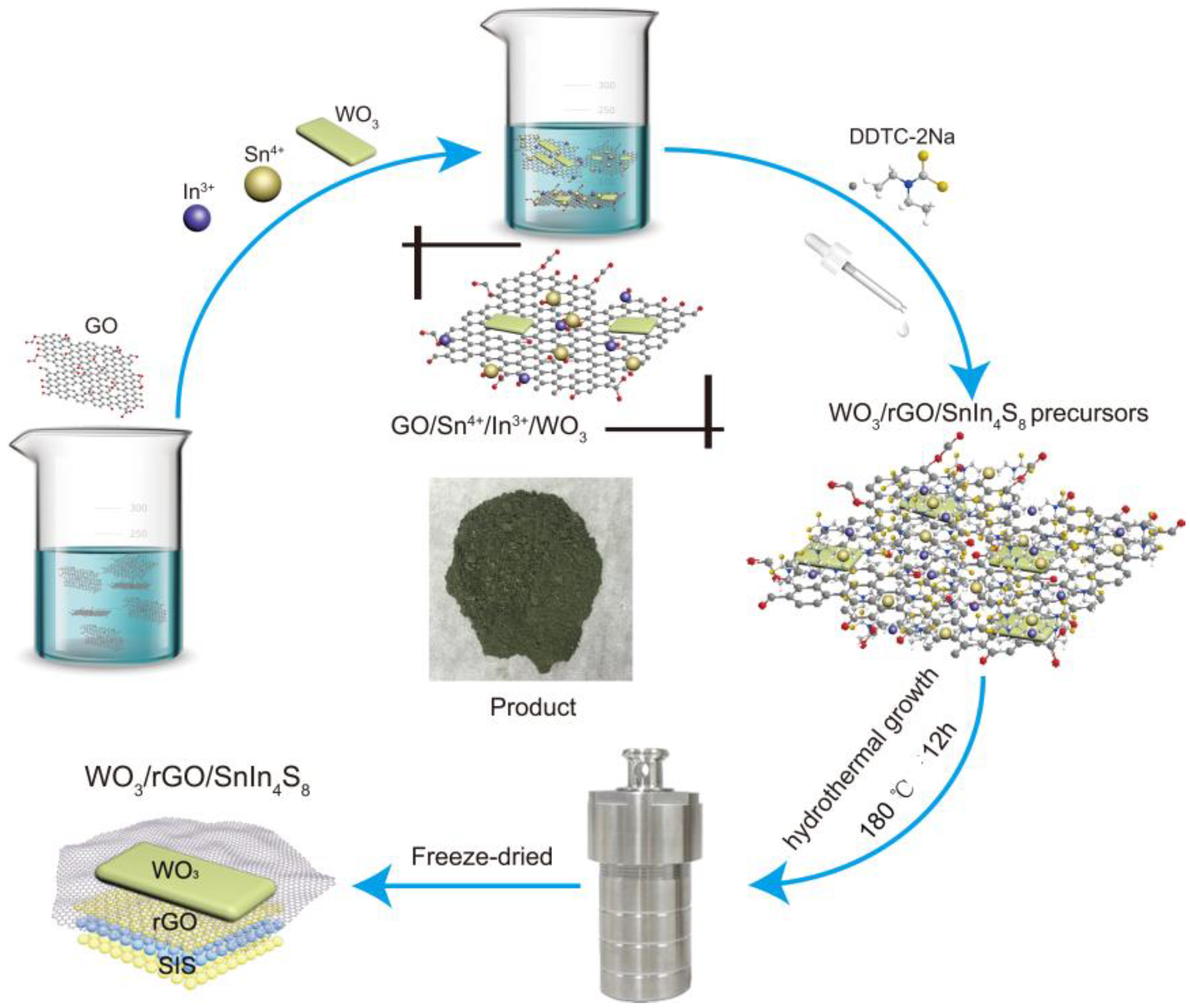
3.2. Synthesis of Photocatalysts
3.3. Characterization
3.4. Photocatalytic Testing
3.5. Radical Species Trapping and ESR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Huang, H.; Lin, J.; Zhu, G.; Weng, Y.; Wang, X.; Fu, X.; Long, J. A Long-Lived Mononuclear Cyclopentadienyl Ruthenium Complex Grafted onto Anatase TiO2 for Efficient CO2 Photoreduction. Angew. Chem. Int. Ed. 2016, 55, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Zhang, Y.; Chu, Z.; Long, J.; An, X.; Zhang, H.; Lin, H.; Zhang, Z.; Wang, X. Synergy of metal and nonmetal dopants for visible light photocatalysis: A case-study of Sn and N co-doped TiO2. Phys. Chem. Chem. Phys. 2016, 18, 9636–9644. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Z.; Liu, G.; Ma, X.; Cheng, H. CdS–mesoporous ZnS core–shell particles for efficient and stable photocatalytic hydrogen evolution under visible light. Energy Environ. Sci. 2014, 7, 1895. [Google Scholar] [CrossRef]
- Meng, L.; Chen, Z.; Ma, Z.; He, S.; Hou, Y.; Li, H.; Yuan, R.; Huang, X.; Wang, X.; Wang, X.; et al. Gold plasmon-induced photocatalytic dehydrogenative coupling of methane to ethane on polar oxide surfaces. Energy Environ. Sci. 2018, 11, 294–298. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, L.; Ming, J.; Liu, B.; Zhao, Y.; Hou, Y.; Ding, Z.; Xu, C.; Zhang, Z.; Long, J. Amorphous Ta2OxNy-enwrapped TiO2 rutile nanorods for enhanced solar photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 243, 481–489. [Google Scholar] [CrossRef]
- Gu, Q.; Long, J.; Fan, L.; Chen, L.; Zhao, L.; Lin, H.; Wang, X. Single-site Sn-grafted Ru/TiO2 photocatalysts for biomass reforming: Synergistic effect of dual co-catalysts and molecular mechanism. J. Catal. 2013, 303, 141–155. [Google Scholar] [CrossRef]
- Fan, L.; Long, J.; Gu, Q.; Huang, H.; Lin, H.; Wang, X. Single-site nickel-grafted anatase TiO2 for hydrogen production: Toward understanding the nature of visible light photocatalysis. J. Catal. 2014, 320, 147–159. [Google Scholar] [CrossRef]
- Gu, Q.; Long, J.; Zhou, Y.; Yuan, R.; Lin, H.; Wang, X. Single-site tin-grafted anatase TiO2 for photocatalytic hydrogen production: Toward understanding the nature of interfacial molecular junctions formed in semiconducting composite photocatalysts. J. Catal. 2012, 289, 88–99. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Domen, K.; Arakawa, H. A new type of water splitting system composed of two different TiO2 photocatalysts (anatase, rutile) and a IO3−/I− shuttle redox mediator. Chem. Phys. Lett. 2001, 344, 339–344. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thampi, K.R.; Tayade, R.J. Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B Environ. 2018, 227, 296–311. [Google Scholar] [CrossRef]
- Jo, W.; Selvam, N.C.S. Z-scheme CdS/g-C3N4 composites with RGO as an electron mediator for efficient photocatalytic H2 production and pollutant degradation. Chem. Eng. J. 2017, 317, 913–924. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Bhattacharyya, D.; Free, M.L.; Misra, M. Augmented Z scheme blueprint for efficient solar water splitting system using quaternary chalcogenide absorber material. J. Phys. Chem. Lett. 2012, 3, 629–639. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Scott, J.A.; Amal, R. Progress in Heterogeneous Photocatalysis: From Classical Radical Chemistry to Engineering Nanomaterials and Solar Reactors. J. Phys. Chem. Lett. 2012, 3, 629–639. [Google Scholar] [CrossRef]
- Xin, G.; Guo, W.; Ma, T. Effect of annealing temperature on the photocatalytic activity of WO3 for O2 evolution. Appl. Surf. Sci. 2009, 256, 165–169. [Google Scholar] [CrossRef]
- Morales, W.; Cason, M.; Aina, O.; Tacconi, N.R.; Rajeshwar, K. Combustion Synthesis and Characterization of Nanocrystalline WO3. J. Am. Chem. Soc. 2008, 130, 6318–6319. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Arakawa, H. The visible light induced photocatalytic activity of tungsten trioxide powders. Appl. Catal. A Gen. 2001, 210, 181–191. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Lv, Y.; Chen, Y.; Liu, M.; Fan, H. Surfactant-assisted hydrothermal synthesis of rGO/SnIn4S8 nanosheets and their application in complete removal of Cr(VI). RSC. Adv. 2018, 8, 5749–5759. [Google Scholar] [CrossRef]
- Zhang, L.; Wong, K.; Chen, Z.; Yu, J.; Zhao, J.; Hu, C.; Chan, C.; Wong, P. AgBr-Ag-Bi2WO6 nanojunction system: A novel and efficient photocatalyst with double visible light active components. Appl. Catal. A Gen. 2009, 363, 221–229. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhang, Y.; Cao, Y.; Luo, M.; Feng, Z.; Yang, W.; Liu, K.; Cao, H.; Yan, H. Pt@Cu2O/WO3 composite photocatalyst for enhanced photocatalytic water oxidation performance. Appl. Catal. B Environ. 2018, 237, 309–317. [Google Scholar] [CrossRef]
- Sayama, K.; Yoshida, R.; Kusama, H.; Okabe, K.; Abe, Y.; Arakawa, H. Photocatalytic decomposition of water into H2 and O2 by a two-step photoexcitation reaction using a WO3 suspension catalyst and an Fe3+/Fe2+ redox system. Chem. Phys. Lett. 1997, 4, 387–391. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Sugihara, H. Development of New Photocatalytic Water Splitting into H2 and O2 using Two Different Semiconductor Photocatalysts and a Shuttle Redox Mediator IO3−/I−. J. Phys. Chem. B. 2005, 109, 16052–16061. [Google Scholar] [CrossRef]
- Sasaki, Y.; Iwase, A.; Kato, H.; Kudo, A. The effect of co-catalyst for Z-scheme photocatalysis systems with an Fe3+/Fe2+ electron mediator on overall water splitting under visible light irradiation. J. Catal. 2008, 259, 133–137. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef]
- Han, C.; Zhang, N.; Xu, Y. Structural diversity of graphene materials and their multifarious roles in heterogeneous photocatalysis. Nano Today 2016, 3, 351–372. [Google Scholar] [CrossRef]
- Xu, J.; He, S.; Zhang, H.; Huang, J.; Lin, H.; Wang, X.; Long, J. Layered metal–organic framework/graphene nanoarchitectures for organic photosynthesis under visible light. J. Mater. Chem. A 2015, 3, 24261–24271. [Google Scholar] [CrossRef]
- Xu, J.; Luo, L.; Xiao, G.; Zhang, Z.; Lin, H.; Wang, X.; Long, J. Layered C3N3S3Polymer/Graphene Hybrids as Metal-Free Catalysts for Selective Photocatalytic Oxidation of Benzylic Alcohols under Visible Light. ACS Catal. 2014, 4, 3302–3306. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Bernardi, M.; Grossman, J.C. The Impact of Functionalization on the Stability, Work Function, and Photoluminescence of Reduced Graphene Oxide. ACS Nano 2013, 7, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Halek, G.; Baikie, I.D.; Teterycz, H.P.; Halek, P.; Suchorska-Woźniak, K.; Wiśniewski, K. Work function analysis of gas sensitive WO3 layers with Pt doping. Sens. Actuators B Chem. 2013, 187, 379–385. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S. Covalently bonded sulfonic acid magnetic graphene oxide: Fe3O4@GO-Pr-SO3H as a powerful hybrid catalyst for synthesis of indazolophthalazinetriones. J. Colloid Int. Sci. 2016, 478, 280–287. [Google Scholar] [CrossRef]
- Heidarizadeh, M.; Doustkhah, E.; Rostamnia, S.; Rezaei, P.F.; Harzevili, F.D.; Zeynizadeh, B. Dithiocarbamate to modify magnetic graphene oxide nanocomposite (Fe3O4-GO): A new strategy for covalent enzyme (lipase) immobilization to fabrication a new nanobiocatalyst for enzymatic hydrolysis of PNPD. Int. J. Biol. Macromol. 2017, 101, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Rostamnia, S.; Doustkhah, E.; Karimi, Z.; Amini, S.; Luque, R. Surfactant-Exfoliated Highly Dispersive Pd-Supported Graphene Oxide Nanocomposite as a Catalyst for Aerobic Aqueous Oxidations of Alcohols. ChemCatChem 2015, 7, 1678–1683. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Zhang, X.; Dong, X.; Ma, C.; Zhang, X.; Ma, H.; Xue, M. Construction of g-C3N4 and FeWO4 Z-scheme photocatalyst: Effect of contact ways on the photocatalytic performance. RSC Adv. 2018, 8, 18419–18426. [Google Scholar] [CrossRef]
- Chen, A.; Bian, Z.; Xu, J.; Xin, X.; Wang, H. Simultaneous removal of Cr(VI) and phenol contaminants using Z-scheme bismuth oxyiodide/reduced graphene oxide/bismuth sulfide system under visible light irradiation. Chemosphere 2017, 188, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Ye, J.; Ma, Q.; Su, N.; Bai, H.; Wang, C. In Situ Growth of Metal Particles on 3D Urchin-like WO3 Nanostructures. J. Am. Chem. Soc. 2012, 134, 6508–6511. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, A.; Mohamed, N.M.; Mazhar, M.; Ehsan, M.A.; Mohamed Saheed, M.S. Core–Shell Vanadium Modified Titania@β-In2S3 Hybrid Nanorod Arrays for Superior Interface Stability and Photochemical Activity. ACS Appl. Mater. Interfaces 2016, 8, 9037–9049. [Google Scholar] [CrossRef] [PubMed]
- Bagus, P.S.; Nelin, C.J.; Al-Salik, Y.; Ilton, E.S.; Idriss, H. Multiplet splitting for the XPS of heavy elements: Dependence on oxidation state. Surf. Sci. 2016, 643, 142–149. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Xu, J.F.; Khalid, N.R.; Elhissi, A.; Ahmed, W. A facile one-step approach to synthesizing ZnO/graphene composites for enhanced degradation of methylene blue under visible light. Appl. Surf. Sci. 2013, 274, 273–281. [Google Scholar] [CrossRef]
- Deng, F.; Pei, X.; Luo, Y.; Luo, X.; Dionysiou, D.D.; Wu, S.; Luo, S. Fabrication of Hierarchically Porous Reduced Graphene Oxide/SnIn4S8 Composites by a Low-Temperature Co-Precipitation Strategy and Their Excellent Visible light Photocatalytic Mineralization Performance. Catalysts 2016, 6, 113. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Zhong, M.; Li, Y.; Wang, Y.; Li, J.; Wei, Z. Direct Vapor Phase Growth and Optoelectronic Application of Large Band Offset SnS2/MoS2 Vertical Bilayer Heterostructures with High Lattice Mismatch. Adv. Electron. Mater. 2016, 2, 1600298. [Google Scholar] [CrossRef]
- Randeniya, L.K.; Bendavid, A.; Martin, P.J.; Preston, E.W. Photoelectrochemical and Structural Properties of TiO2 and N-Doped TiO2 Thin Films Synthesized Using Pulsed Direct Current Plasma-Activated Chemical Vapor Deposition. J. Phys. Chem. C 2007, 111, 18334–18340. [Google Scholar] [CrossRef]
- Aleksandrzak, M.; Adamski, P.; Kukułka, W.; Zielinska, B.; Mijowska, E. Effect of graphene thickness on photocatalytic activity of TiO2-graphene nanocomposites. Appl. Surf. Sci. 2015, 331, 193–199. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Liu, W.; Zhang, S. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4 -RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci. 2017, 405, 60–70. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kansal, S.K. Bi2WO6 nanocuboids: An efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]
- Jin, L.; Qu, Y.; Wang, B.; Li, S.; Jiang, B.; Yang, L.; Fu, W.; Fu, H.; Sun, J. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar]
- Kang, J.; Kim, T.; Choi, J.; Park, J.; Kim, Y.; Chang, M.; Jung, H.; Park, K.; Yang, S.; Park, C. Hidden Second Oxidation Step of Hummers Method. Chem. Mater. 2016, 28, 756–764. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Daikopoulos, C.; Konstantinou, I. Characterization and catalytic performance of B-doped, B-N co-doped and B-N-F tri-doped TiO2 towards simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl. Catal. B Environ. 2016, 184, 44–54. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Huang, S.; Liu, M.; Lv, Y.; Wang, Z.; Long, J.; Zhang, W.; Fan, H. Z-Schemed WO3/rGO/SnIn4S8 Sandwich Nanohybrids for Efficient Visible Light Photocatalytic Water Purification. Catalysts 2019, 9, 187. https://doi.org/10.3390/catal9020187
Xu P, Huang S, Liu M, Lv Y, Wang Z, Long J, Zhang W, Fan H. Z-Schemed WO3/rGO/SnIn4S8 Sandwich Nanohybrids for Efficient Visible Light Photocatalytic Water Purification. Catalysts. 2019; 9(2):187. https://doi.org/10.3390/catal9020187
Chicago/Turabian StyleXu, Pingfan, Siyi Huang, Minghua Liu, Yuancai Lv, Zhonghui Wang, Jinlin Long, Wei Zhang, and Haojun Fan. 2019. "Z-Schemed WO3/rGO/SnIn4S8 Sandwich Nanohybrids for Efficient Visible Light Photocatalytic Water Purification" Catalysts 9, no. 2: 187. https://doi.org/10.3390/catal9020187
APA StyleXu, P., Huang, S., Liu, M., Lv, Y., Wang, Z., Long, J., Zhang, W., & Fan, H. (2019). Z-Schemed WO3/rGO/SnIn4S8 Sandwich Nanohybrids for Efficient Visible Light Photocatalytic Water Purification. Catalysts, 9(2), 187. https://doi.org/10.3390/catal9020187







