Metal Chalcogenides on Silicon Photocathodes for Efficient Water Splitting: A Mini Overview
Abstract
1. Introduction
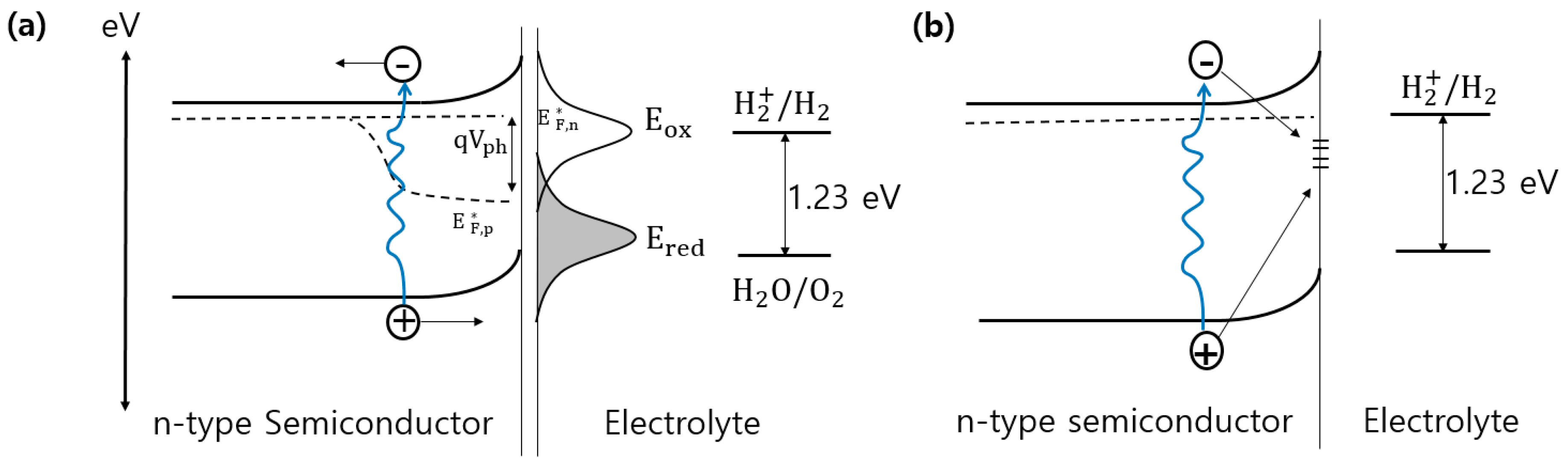
2. Photo-Assisted Electrochemical Water Splitting
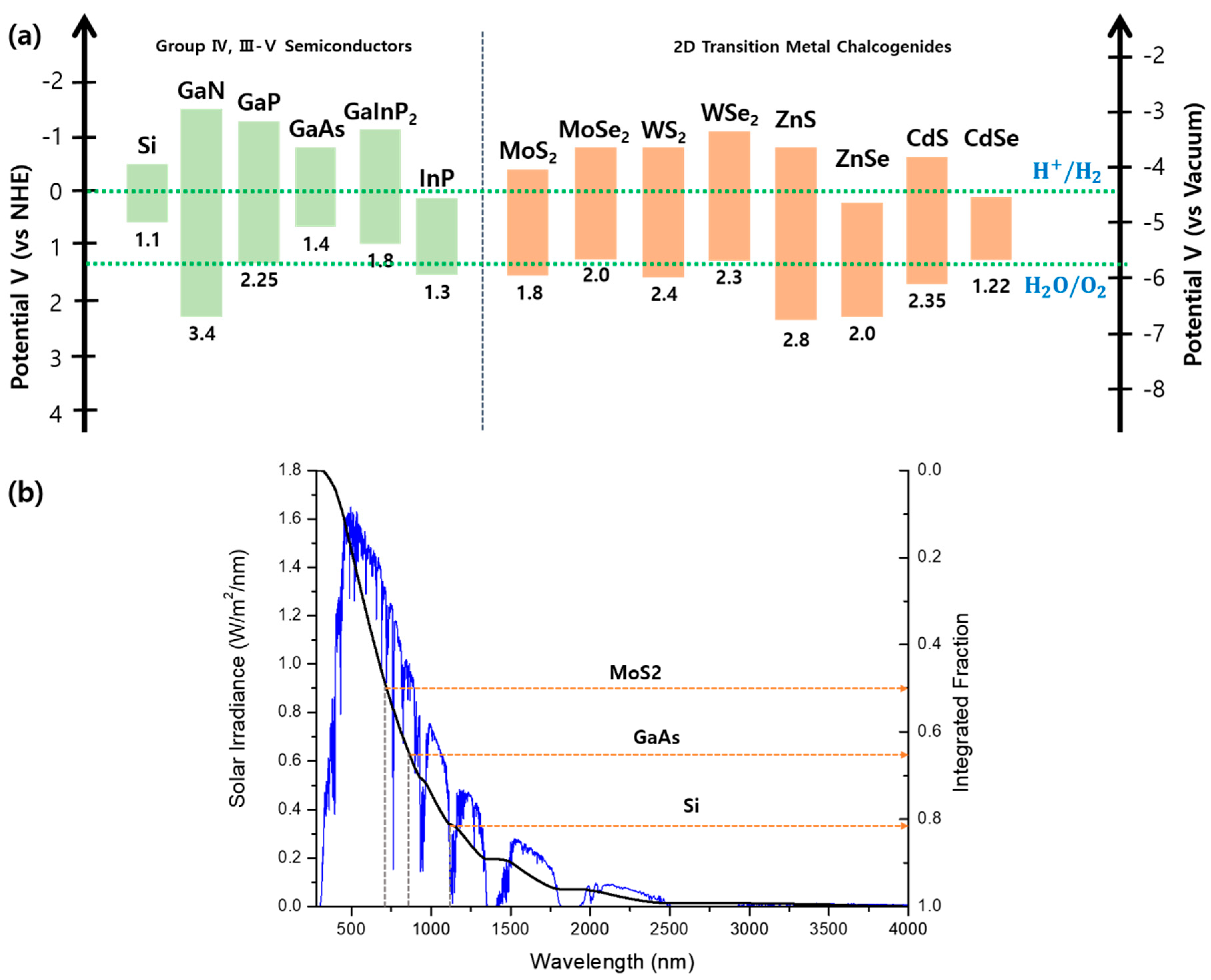
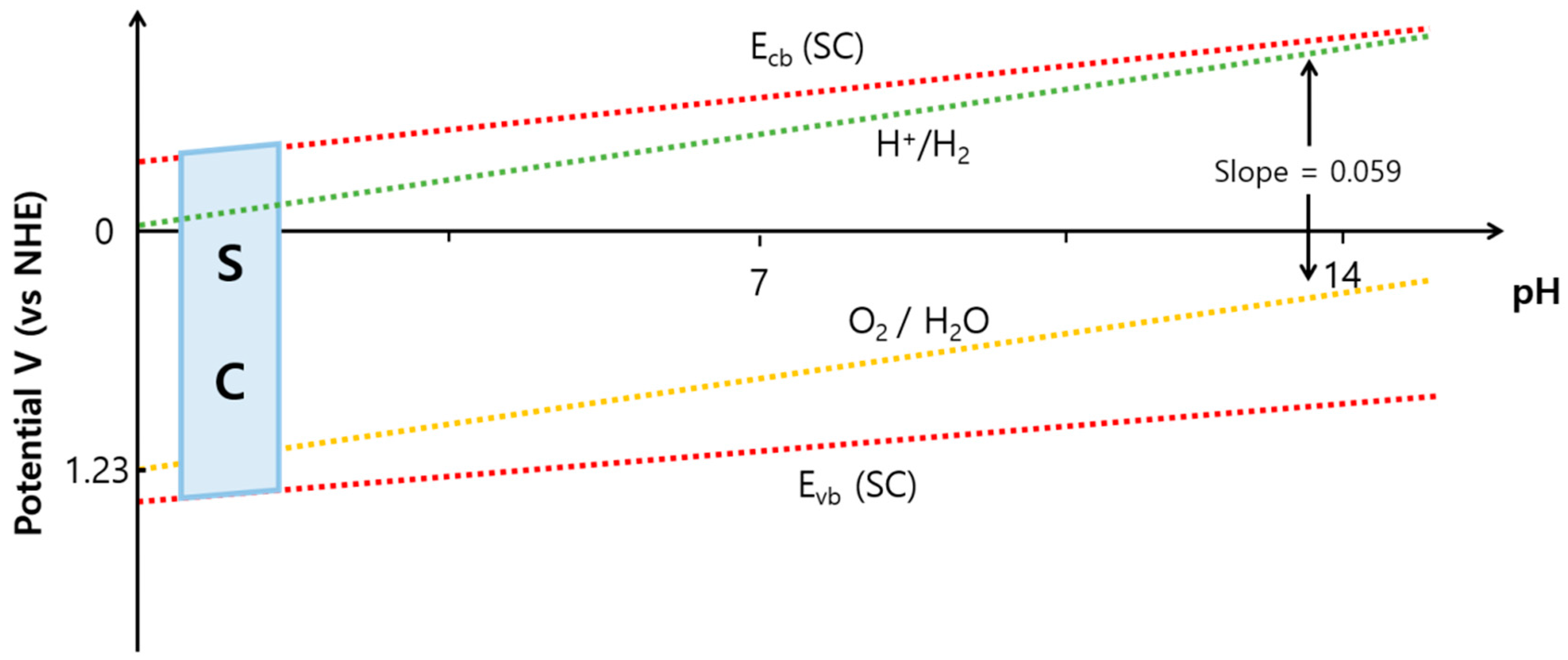
2.1. Requirements for Semiconductor Photoelectrodes
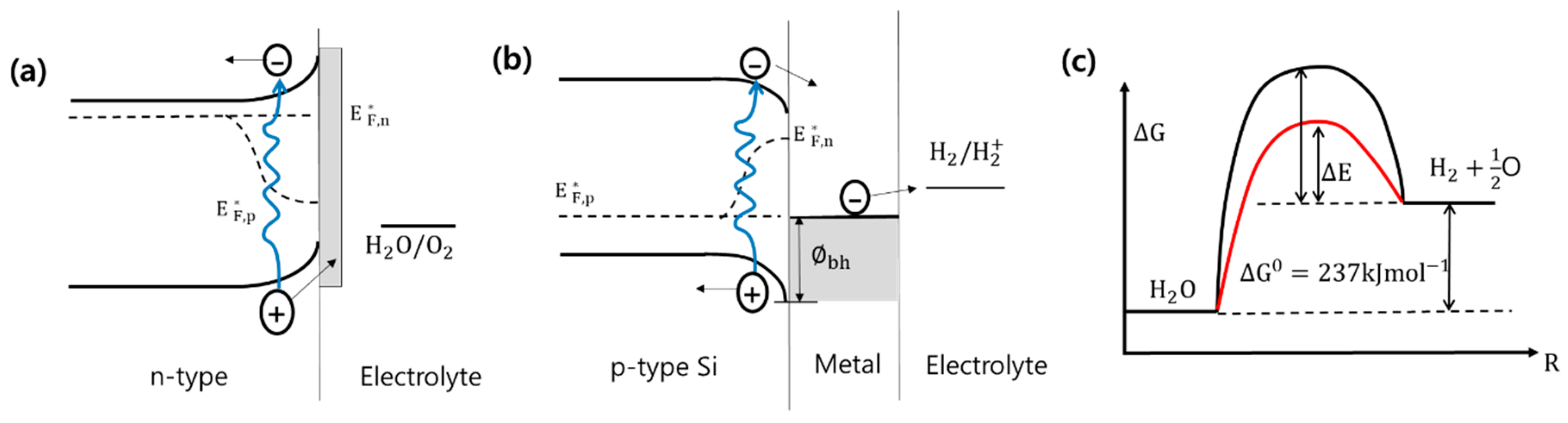
2.2. Properties of Si Photoelectrodes
2.3. Co-Catalyst on Si
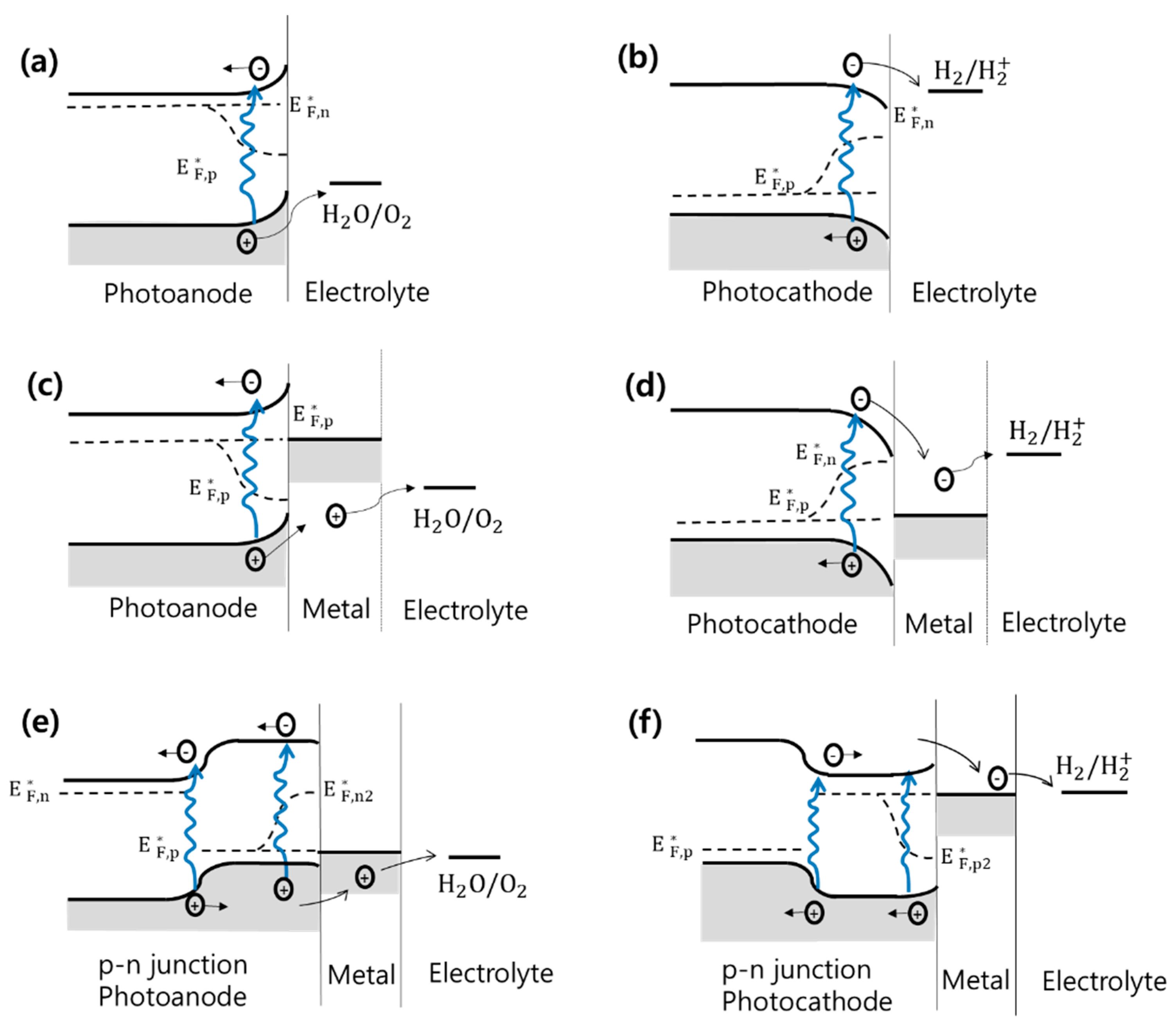
2.4. Photoelectrode Half-Cell Configurations
3. Flat-Band Potential Measurement
3.1. Concepts of Flat-Band Potential
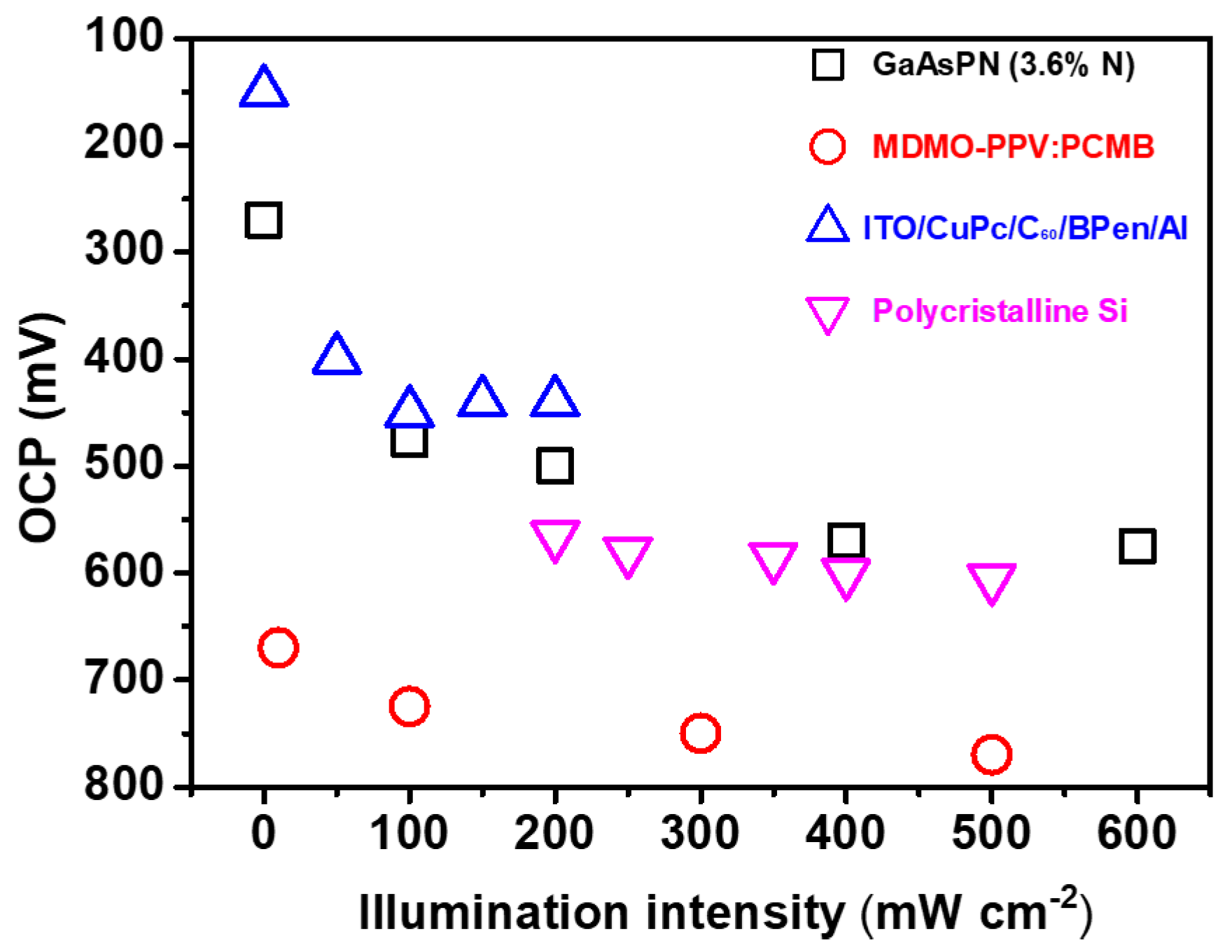
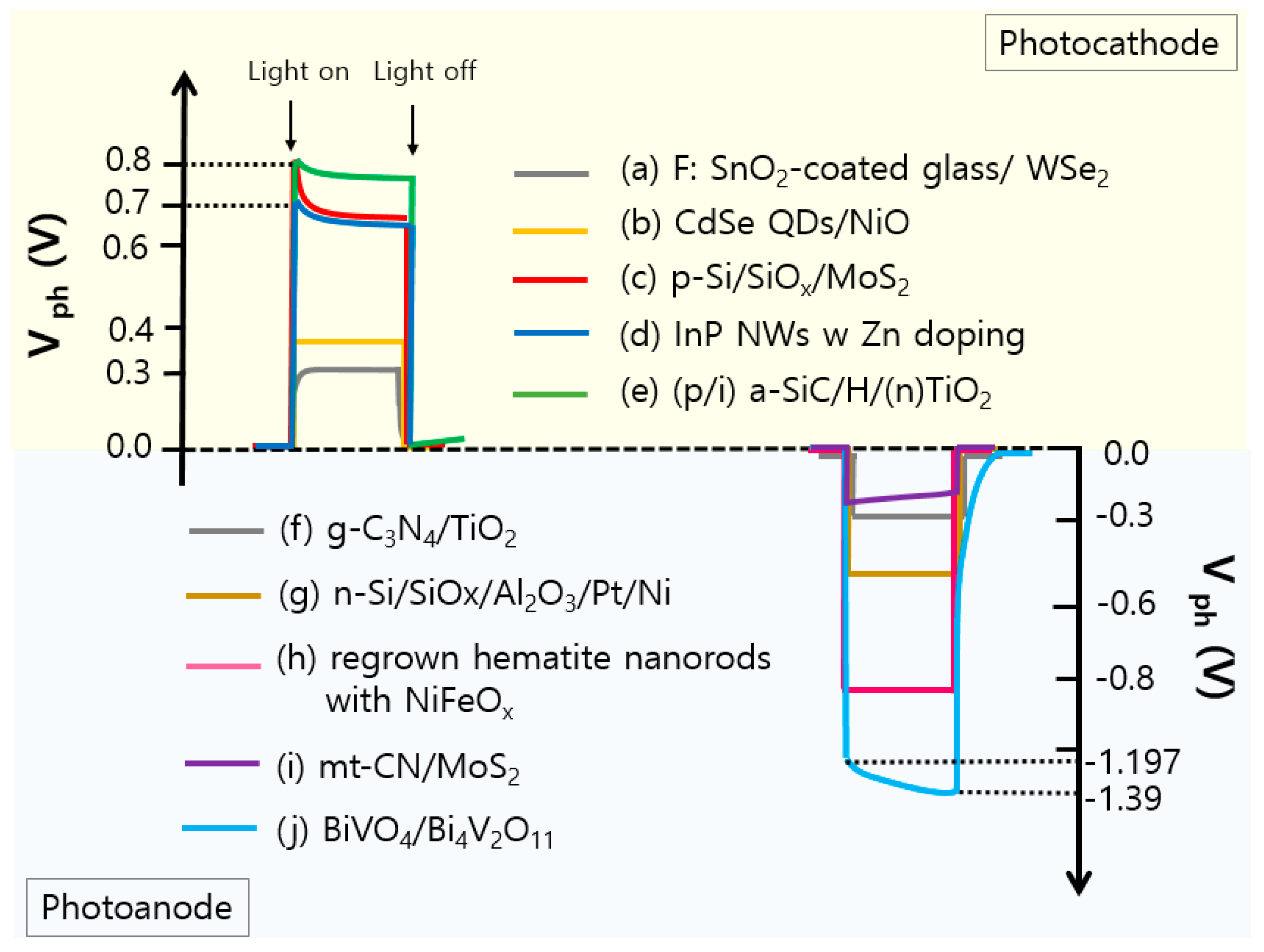
3.2. Illuminated Open-Circuit Potential (OCP)
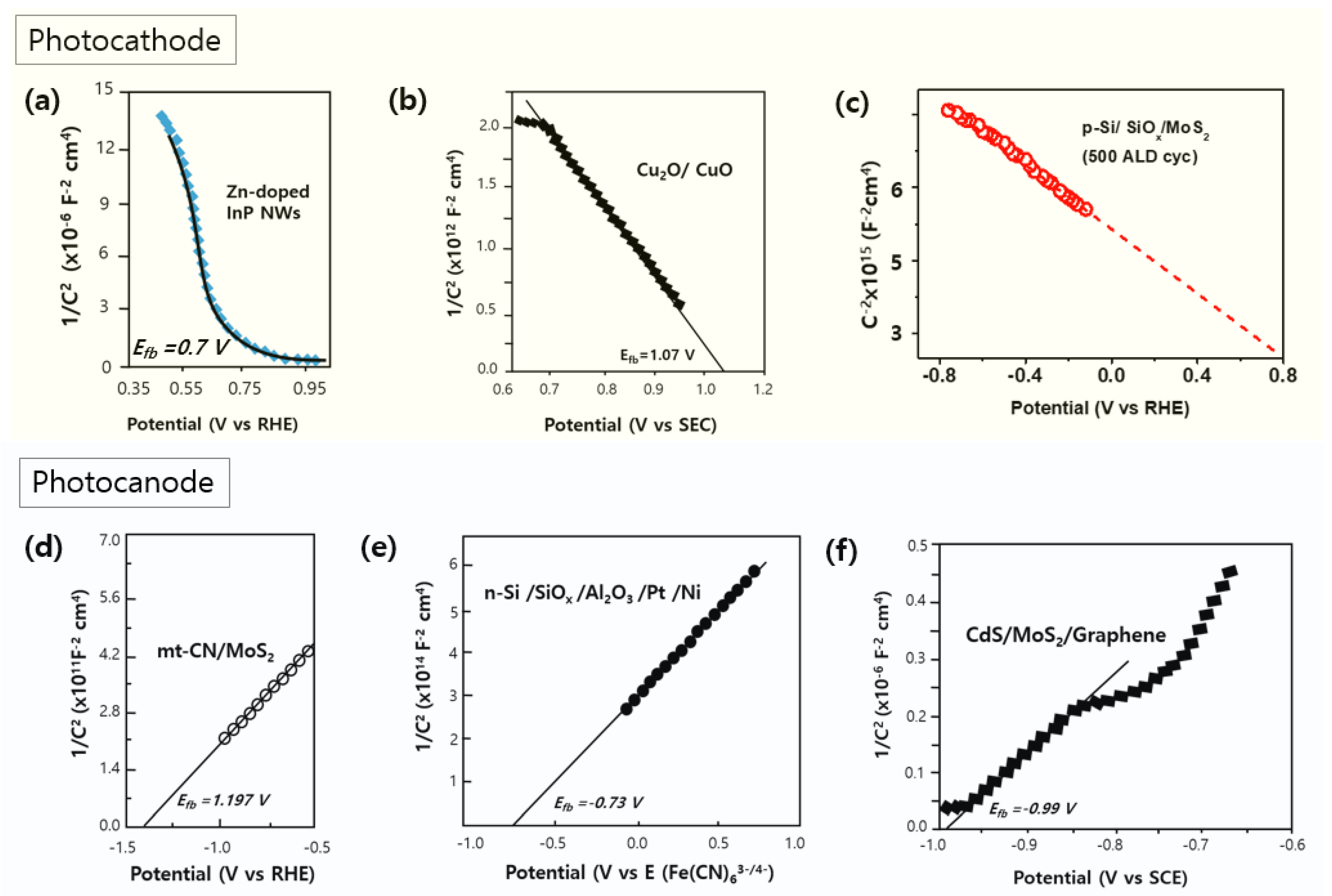
3.3. Mott–Schottky Analysis
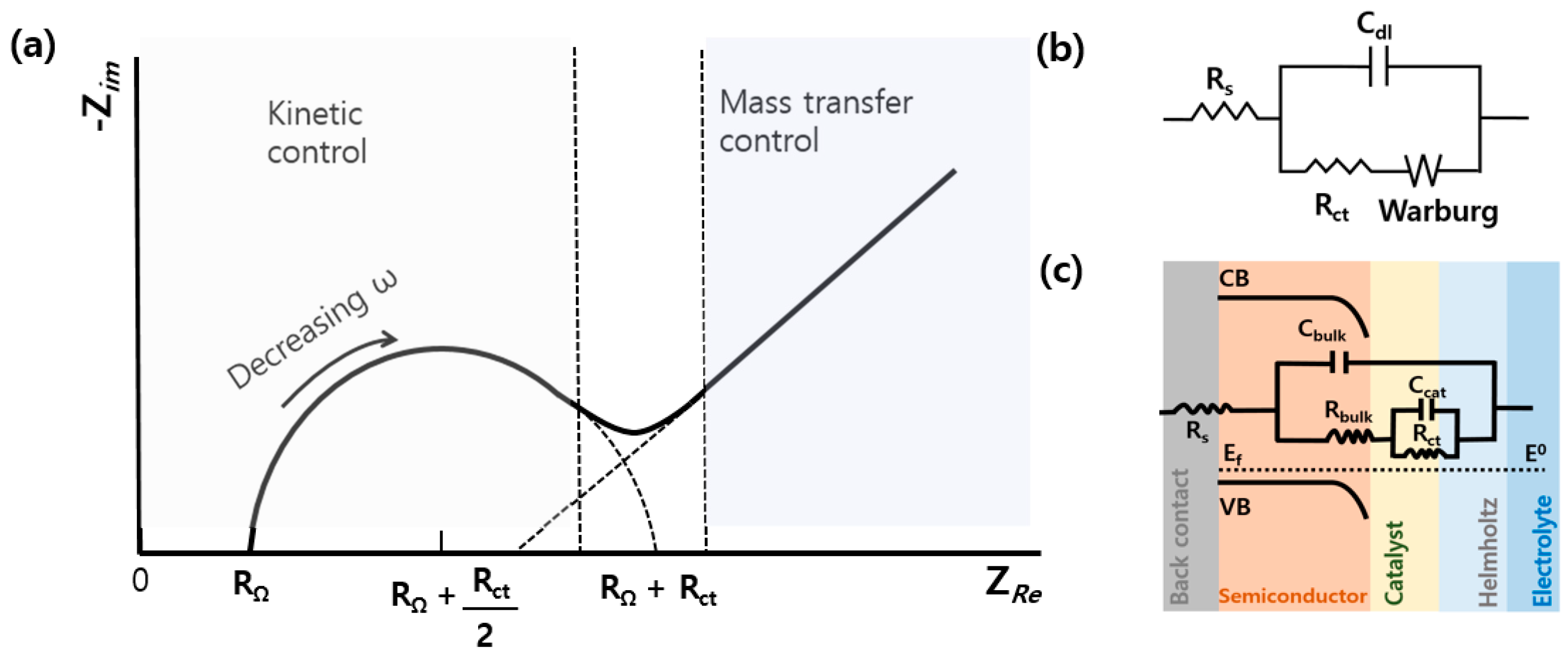
4. Electrochemical Impedance Spectroscopy (EIS) for Photoelectrochemical (PEC) Cells
5. Si/Si/Transition Metal (di)Chalcogenides [TM(D)C] Photocathode
6. Atomic Layer Deposition (ALD) of TM(D)C
6.1. Defect Chemistry of TM(D)C and Its Implication on Low-Temperature ALD
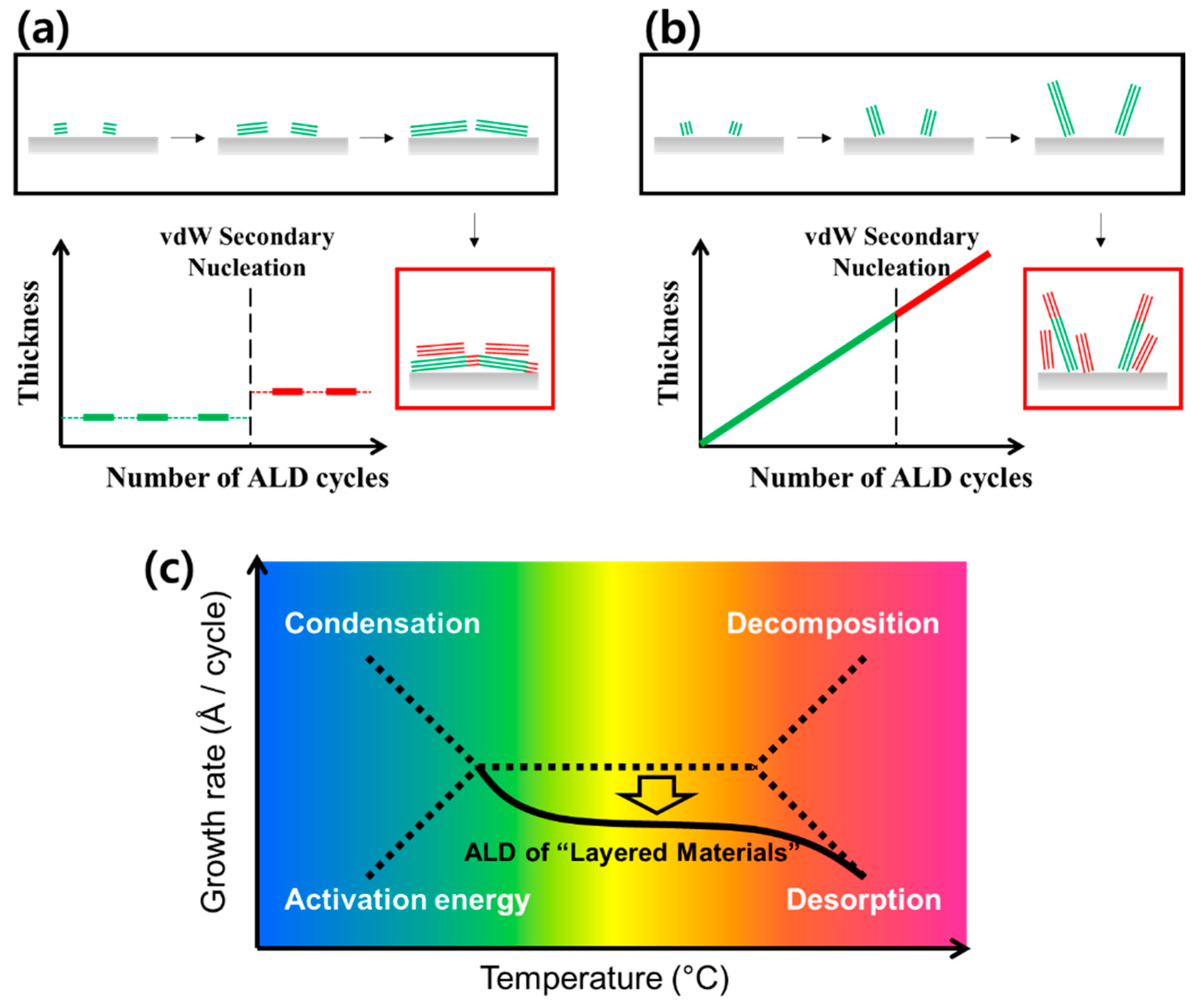
6.2. Growth Mechanisms of TM(D)C in ALD
7. Measurement Tools for Understanding Operation Mechanisms
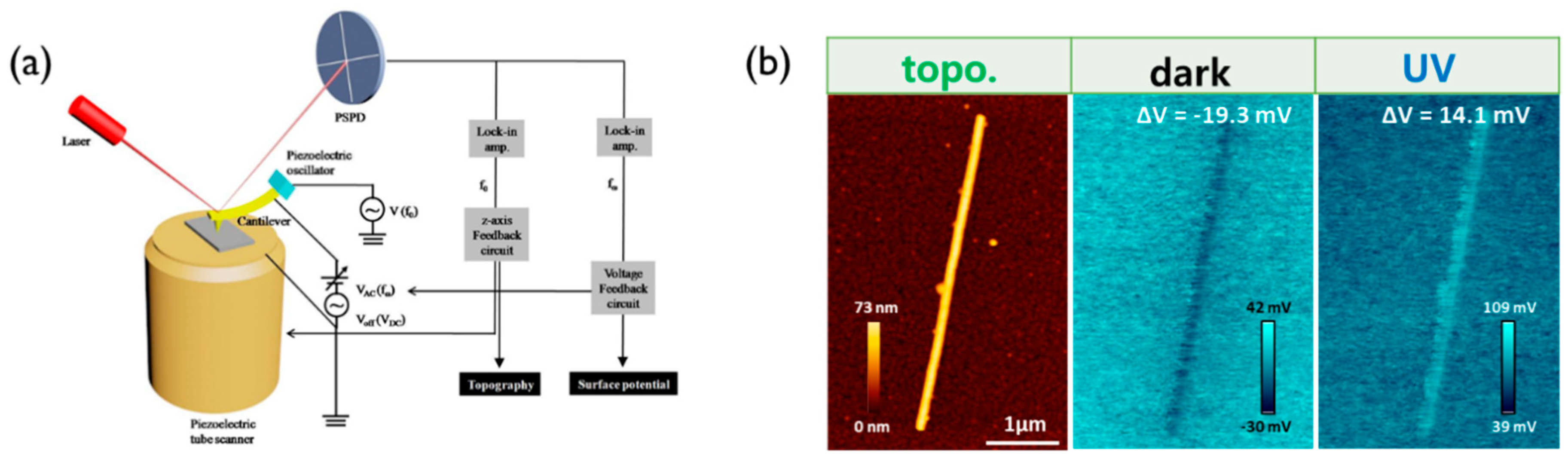
7.1. Kelvin Probe Force Microscopy (KPFM)
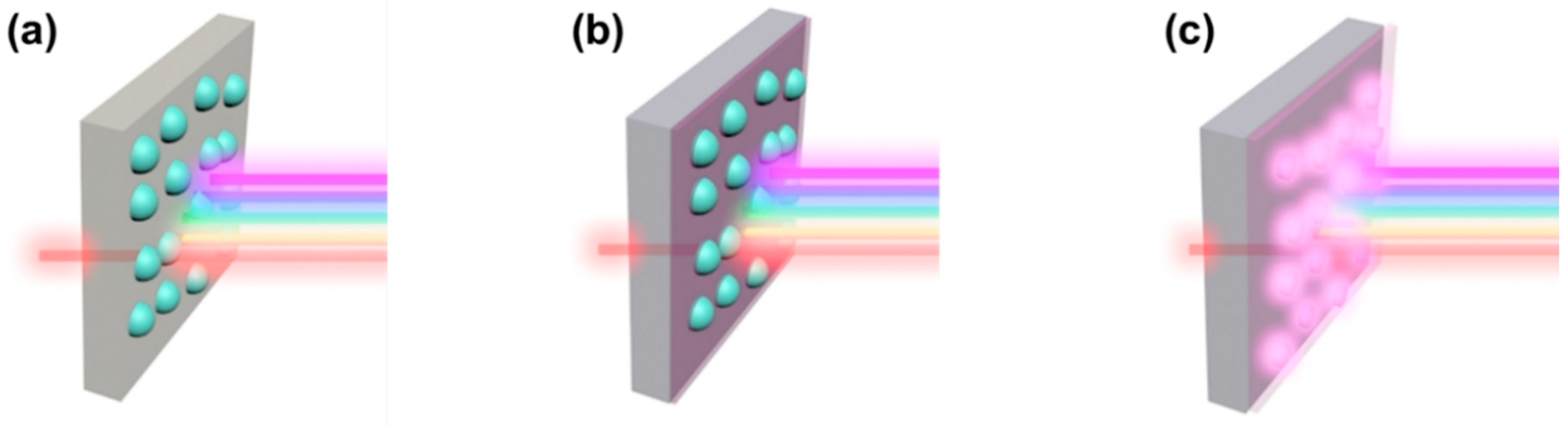
7.2. Optical Spectroscopy
8. Toward Multi-Phase TM(D)C Co-Catalysts on Si
9. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Bocarsly, A.B.; Bookbinder, D.C.; Dominey, R.N.; Lewis, N.S.; Wrighton, M.S. Photoreduction at Illuminated p-Type Semiconducting Silicon Photoelectrodes. Evidence for Fermi Level Pinning. J. Am. Chem. Soc. 1980, 102, 3683–3688. [Google Scholar] [CrossRef]
- Chu, S.; Li, W.; Yan, Y.; Hamann, T.; Shih, I.; Wang, D.; Zetian, M. Roadmap on solar water splitting: Current status and future prospects. Nano Futures 2017, 1, 022001. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Thorne, J.E.; Li, S.; Du, C.; Qin, G.; Wang, D. Energetics at the Surface of Photoelectrodes and Its Influence on the Photoelectrochemical Properties. J. Phys. Chem. Lett. 2015, 6, 4083–4088. [Google Scholar] [CrossRef]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-Complete Suppression of Surface Recombination in Solar Photoelectrolysis by Co-Pi Catalyst-Modified W: BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef]
- Hu, S.; Xiang, C.; Haussener, S.; Berger, D.A.; Lewis, N.S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy. Environ. Sci. 2013, 6, 2984–2993. [Google Scholar] [CrossRef]
- Abdi, F.F.; Han, L.; Smets, A.H.M.; Zeman, M.; Dam, B.; Van De Krol, R. Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode. Nat. Commun. 2013, 4, 2195:1–2195:7. [Google Scholar] [CrossRef]
- Urbain, F.; Wilken, K.; Smirnov, V.; Astakhov, O.; Lambertz, A.; Becker, J.P.; Rau, Y.; Ziegler, J.; Kaiser, B.; Jaegermann, W.; et al. Development of Thin Film Amorphous Silicon Tandem Junction Based Photocathodes Providing High Open-Circuit Voltages for Hydrogen Production. Int. J. Photoenergy 2014, 249317:1–249317:10. [Google Scholar] [CrossRef]
- Benchamekh, R.; Nesoklon, M.; Jancu, J.-M.; Voisin, P. Semiconductor Modeling Techniques, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-43467-9. [Google Scholar]
- Wang, S.; Tian, H.; Ren, C.; Yu, J.; Sun, M. Electronic and optical properties of heterostructures based on transition metal dichalcogenides and graphene-like zinc oxide. Sci. Rep. 2018, 8, 12009. [Google Scholar] [CrossRef]
- Pfluger, E.; Savan, A. Modern Solid Lubricants, Especially Based on MoS2. Vak. Forsch. Prax. 1999, 11, 236–240. [Google Scholar] [CrossRef]
- Bae, C.; Böhnert, T.; Gooth, J.; Lim, S.; Lee, S.; Kim, H.; Heimann, S.; Schulz, S.; Shin, H.; Nielsch, K. Thermopower Engineering of Bi2Te3 without Alloying: The interplay between nanostructuring and defect activation. Semicond. Sci. Technol. 2014, 29, 064003. [Google Scholar] [CrossRef]
- Yang, E.; Ji, H.; Jung, Y. Two-Dimensional Transition Metal Dichalcogenide Monolayers as Promising Sodium Ion Battery Anodes. J. Phys. Chem. C 2015, 119, 26374–26380. [Google Scholar] [CrossRef]
- Gu, X.; Cui, W.; Li, H.; Wu, Z.; Zeng, Z.; Lee, S.-T.; Zhang, H.; Sun, B. A Solution-Processed Hole Extraction Layer Made from Ultrathin MoS2 Nanosheets for Efficient Organic Solar Cells. Adv. Energy. Mater. 2013, 3, 1262–1268. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Guio, C.G.M.; Stern, L.A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef]
- Van de Krol, R. Principles of Photoelectrochemical Cells, 1st ed.; Springer: Boston, MA, USA, 2011; ISSN 978-1-4614-1380-6. [Google Scholar]
- Rasmussen, A.F.; Thygesen, S.K. Computational 2D Materials Database: Electronic Structure of Transition-Metal Dichalcogenides and Oxides. J. Phys. Chem. C 2015, 119, 13169–13183. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Galicia-Hernandez, J.M.; Sanchez-Castillo, A.; Garza, L.M.; Cocoletzi, H.G. Two-dimensional cadmium selenide electronic and optical properties: First principles studies. Bull. Mater. Sci. 2017, 40, 1111–1119. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Sub, W.N.; Hwang, B.J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Mak, F.; Lee, C.; Hone, J.; Shan, J.; Heinz, F. Atomically Thin MoS2: A New Direct Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805:1–136805:4. [Google Scholar] [CrossRef]
- Simon, T.; Bouchonville, N.; Berr, M.; Vaneski, A.; Adrovic, A.; Volbers, D.; Wyrwich, R.; Doblinger, M.; Susha, A.; Rogach, A.; et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014, 13, 1013–1018. [Google Scholar] [CrossRef]
- Wiensch, J.D.; John, J.; Velazquez, J.M.; Torelli, D.A.; Pieterick, A.P.; McDowell, M.T.; Sun, K.; Zhao, X.; Brunschwig, B.S.; Lewis, N.S. Comparative Study in Acidic and Alkaline Media of the Effects of pH and Crystallinity on the Hydrogen-Evolution Reaction on MoS2 and MoSe2. ACS Energy Lett. 2017, 2, 2234–2238. [Google Scholar] [CrossRef]
- Memming, R. Semiconductor Electrochemistry, 2nd ed.; Wiley: New York, NY, USA, 2000; ISBN 978-3-527-31281-8. [Google Scholar]
- Lewis, N.S. A Quantitative Investigation of the Open-Circuit Photovoltage at the Semiconductor/Liquid Interface. J. Electrochem. Soc. 1984, 131, 2496–2503. [Google Scholar] [CrossRef]
- Baglio, J.A.; Calabrese, G.S.; Harrion, D.J.; Kamieniecki, E.; Ricco, A.J.; Wrighton, M.S.; Zoski, G.D. Electrochemical characterization of p-type semiconducting tungsten disulfide photocathodes: Efficient photoreduction processes at semiconductor/liquid electrolyte interfaces. J. Am. Chem. Soc. 1983, 105, 2246–2256. [Google Scholar] [CrossRef]
- Markovic, N.M.; Grgur, B.N.; Ross, R.N. Temperature-Dependent Hydrogen Electrochemistry on Platinum Low-Index Single-Crystal Surfaces in Acid Solutions. J. Phys. Chem. B 1997, 101, 5405–5413. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Reichman, J. The current-voltage characteristic of semiconductor-electrolyte junction photovoltaic cells. J. Appl. Phys. Lett. 1980, 36, 574–576. [Google Scholar] [CrossRef]
- Gartner, W.W. Depletion-Layer photoeffects in Semiconductors. Phys. Rev. 1959, 116, 84–87. [Google Scholar] [CrossRef]
- Peng, K.Q.; Wang, X.; Wu, X.L.; Lee, S.T. Platinum Nanoparticle Decorated Silicon Nanowires for Efficient Solar Energy Conversion. Nano Lett. 2009, 9, 3704–3709. [Google Scholar] [CrossRef]
- Bao, N.Z.; Shen, L.M.; Takata, T.; Domen, K. Self-Templated Synthesis of Nanoporous CdS nanostructures for Highly Efficient Photocatalytic Hydrogen Production under Visible Light. Chem. Mater. 2008, 20, 110–117. [Google Scholar] [CrossRef]
- Thorne, J.E.; Jang, J.W.; Liu, E.Y.; Wang, D. Understanding the origin of photoelectrode performance enhancement by probing surface kinetics. Chem. Sci. 2016, 7, 3347–3354. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soinb, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Li, H.; Hu, H.; Bao, C.; Guo, F.; Zhang, X.; Liu, X.; Hua, J.; Tan, J.; Wang, A.; Zhou, H.; et al. Forming heterojuction: An effective strategy to enhance the photocatalytic efficiency of a new metal-free organic photocatalyst for water splitting. Sci. Rep. 2016, 6, 29327. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- White, J.R.; Fan, F.R.F.; Bard, A. Semiconductor Electrodes LVI. Principles of Multijunction Electrodes and Photoelectrosynthesis at Texas Instruments’p/n-Si Solar Arrays. J. Electrochem. Soc. 1985, 132, 544–550. [Google Scholar] [CrossRef]
- Delahoy, A.E.; Gau, S.C.; Murphy, O.J.; Kapur, M.; JO’M, B. A one-unit photovoltaic electrolysis system based on a triple stack of amorphous silicon (pin) cells. Int. J. Hydrog. Energy 1985, 10, 113–116. [Google Scholar] [CrossRef]
- Kronik, L.; Shapira, Y. Surface photovoltage phenomena: Theory, experiment, and applications. Surf. Sci. Rep. 1999, 37, 1–206. [Google Scholar] [CrossRef]
- Han, L.; Digdaya, I.A.; Buijs, T.W.; Abdi, F.F.; Huang, Z.; Liu, R.; Dam, B.; Zeman, M.; Smith, W.A.; Smets, A.H. Gradient dopant profiling and spectral utilization of monolithic thin-film silicon photoelectrochemical tandem devices for solar water splitting. J. Mater. Chem. A 2015, 3, 4155–4162. [Google Scholar] [CrossRef]
- Sabba, D.; Kumar, M.H.; Wong, L.H.; Barber, J.; Grätzel, M.; Mathews, N. Perovskite–Hematite Tandem Cells for Efficient Overall Solar Driven Water Splitting. Nano Lett. 2015, 15, 3833–3839. [Google Scholar]
- Li, F.; Fan, K.; Xu, B.; Gabrielsson, E.; Daniel, Q.; Li, L.; Sun, L. Organic Dye-Sensitized Tandem Photoelectrochemical Cell for Light Driven Total Water Splitting. J. Am. Chem. Soc. 2015, 137, 9153–9159. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dinh, H.N.; Miller, E. Photoelectrochemical Water Splitting standards, Experimental Methods, and Protocol, 1st ed.; Springer: New York, NY, USA, 2013; pp. 63–103. [Google Scholar]
- Dos Santos, W.S.; Rodriguez, M.; Afonso, A.S.; Mesquita, J.P.; Nascimento, L.L.; Patrocínio, A.O.T.; Silva, A.C.; Oliveira, L.C.A.; Fabris, J.D.; Pereira, M.C. A hole inversion layer at the BiVO4/Bi4V2O11 interface produces a high tunable photovoltage for water splitting. Sci. Rep. 2016, 4, 31406. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, T.G.; Koval, C.A.; Turner, J.A. III-V Nitride Epilayers for Photoelectrochemical Water Splitting: GaPN and GaAs PN. J. Phys. Chem. B 2006, 110, 25297–25307. [Google Scholar] [CrossRef] [PubMed]
- Koster, L.J.A.; Mihailetchi, V.D.; Ramaker, R.; Blom, P.W.M. Light Intensity dependence of open-circuit voltage of polymer-fullerene solar cells. Appl. Phys. Lett. 2005, 86, 123509. [Google Scholar] [CrossRef]
- Kumar, P.; Jain, S.C.; Kumar, H.; Chand, S.; Kumar, V. Effect of illumination intensity and temperature on open circuit voltage in organic solar cells. Appl. Phys. Lett. 2009, 94, 183505. [Google Scholar] [CrossRef]
- Chegaar, M.; Hamzaoui, A.; Namoda, A.; Petit, P.; Aillerie, M.; Herguth, A. Effect of illumination intensity on solar cells parameters. Energy Procedia 2013, 36, 722–729. [Google Scholar] [CrossRef]
- Yu, X.; Sivula, K. Photogenerated Charge Harvesting and Recombination in Photocathodes of Solvent-Exfoliated WSe2. Chem. Mater. 2017, 29, 6863–6875. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.-B.; Gao, Y.-J.; Li, Z.-J.; Meng, Q.-Y.; Tung, C.-H.; Wu, L.Z. A solution-processed, mercaptoacetic acid-engineered CdSe quantum dot photocathode for efficient hydrogen production under visible light irradiation. Energy. Envorn. Sci. 2015, 8, 1443–1449. [Google Scholar] [CrossRef]
- Joe, J.; Bae, C.; Kim, E.; Ho, T.A.; Yang, H.; Park, J.H.; Shin, H. Mixed-Phase (2H and 1T) MoS2 Catalyst for a Highly Efficient and Stable Si Photocathode. Catalysts 2018, 8, 580. [Google Scholar] [CrossRef]
- Kornienko, N.; Gibson, N.A.; Zhang, H.; Eaton, S.W.; Yu, Y.; Aloni, S.; Leone, S.R.; Yang, P. Growth and Photoelectrochemical Energy Conversion of Wurtzite Indium Phosphide nanowire Arrays. ACS Nano 2016, 10, 5525–5535. [Google Scholar] [CrossRef]
- Digdaya, I.A.; Han, L.; Buijs, T.W.F.; Zeman, M.; Dam, B.; Smets, A.H.M.; Smith, W.A. Extracting large photovoltages from a-SiC photocathodes with an amorphous TiO2 front surface field layer for solar hydrogen evolution. Energy Environ. Sci. 2015, 8, 1585–1593. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Zhang, J.; Wang, K.; Qiu, Y.; Liang, Q.; Chen, Z. Efficient Photoelectrochemical Water Splitting by g-C3N4/TiO2 Nanotube Array Heterostructures. Nano-Micro Lett. 2018, 10, 37. [Google Scholar] [CrossRef]
- Digdaya, I.A.; Adhyaksa, G.W.P.; Trzes´niewski, B.J.; Garnett, E.C.; Smith, W.A. Interfacial engineering of metal-insulator-semiconductor junctions for efficient and stable photoelectrochemical water oxidation. Nat. Commun. 2017, 8, 15968. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, D.; Chen, S. Fabrication and Enhanced Photoelectrochemical Performance of MoS2/S-Doped g-C3N4 Heterojunction Film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Tewary, V.k.; Jain, S.C. Open-Circuit Voltage Decay in Solar cells. Adv. Electron. Phys. 1986, 67, 319–414. [Google Scholar]
- Wang, Q. Fast Voltage Decay in Perovskite Solar Cells Caused by Depolarization of Perovskite Layer. J. Phys. Chem. C 2018, 122, 4822–4827. [Google Scholar] [CrossRef]
- Sekizawa, K.; Oh-ishi, K.; Kataoka, K.; Arai, T.; Suzuki, T.M.; Morikawa, T. Stoichiometric water splitting using a p-type Fe2O3 based photocathode with the aid of a multiheterojunction. J. Mater. Chem. A 2017, 5, 6483–6493. [Google Scholar] [CrossRef]
- Bisquert, J.; Zaban, A.; Greenshtein, M.; Mora-Sero, I. Determination of Rate Constants for Charge Transfer and the Distribution of Semiconductor and Electrolyte Electronic Energy Levels in Dye-Sensitized Solar Cells by Open-Circuit Photovoltage Decay Method. J. Am. Chem. Soc. 2004, 126, 13550–13559. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Z.; Guo, L.; Bao, N.Z.; Grimes, C.A. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Du, R.; Li, B.; Zhang, Y.; Liu, H.; Qu, J.; An, X. Biomolecule-assisted self-assembly of CdS/MoS2/graphene hollow spheres as high-efficiency photocatalysts for hydrogen evolution without noble metals. Appl. Catal. B Environ. 2016, 182, 504–512. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.Y.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Landers, A.T.; Switzer, J.A. An electrodeposited inhomogeneous metal-insulator-semiconductor junction for efficient photoelectrochemical water oxidation. Nat. Mater. 2015, 14, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Pawar, G.S.; Tahir, A.A. Unbiased Spontaneous Solar Fuel Production using Stable LaFeO3 Photoelectrode. Sci. Rep. 2018, 8, 3501:1–3501:9. [Google Scholar] [CrossRef]
- Hu, D.; Xiang, J.; Zhou, Q.; Su, S.; Zhang, Z.; Wang, X.; Jin, M.; Nian, L.; Nözel, R.; Zhou, G.; et al. One-step chemical vapor deposition of MoS2 noanosheet on SiNWs as photocathodes for efficint and stable solar-driven hydrogen production. Nanoscale 2018, 10, 3518–3525. [Google Scholar] [CrossRef]
- Zhou, J.; Dai, S.; Dong, W.; Su, X.; Fang, L.; Zheng, F.; Wang, X.; Shen, M. Efficient and stable MoS2 catalyst integrated on Si photocathodes by photoreduction and post-annealing for water splitting. Appl. Phys. Lett. 2016, 108, 213905. [Google Scholar] [CrossRef]
- Devarapalli, R.R.; Kamaja, C.K.; Shelke, M.V. Quantum dot-decorated silicon nanowires as efficient photoelectrodes for photoelectrochemical hydrogen generation. J. Mater. Chem. A 2014, 2, 133352–133358. [Google Scholar] [CrossRef]
- Lopes, T.; Andrade, L.; Formal, F.L.; Gratzel, M.; Sivula, K.; Mendes, A. Hematite photoelectrodes for water splitting: Evaluation of the role of film thickness by impedance spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 16515–16523. [Google Scholar]
- Lopes, T.; Andrade, L.; Ribeiro, H.A.; Medes, A. Characterization of photoelectrochemical cells for water splitting by electrochemical impedance spectroscopy. Int. J. Hydrog. Energy 2010, 23, 11601–11608. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Andoshe, M.D.; Jin, G.; Lee, C.; Kim, C.; Kwon, K.C.; Choi, S.; Sohn, W.; Moon, C.W.; Lee, S.H.; Suh, J.M.; et al. Directly Assembled 3D Molybdenum Disulfide on Silicon Wafer for Efficient Photoelectrochemical Water Reduction. Adv. Sustain. Syst. 2018, 2, 1700142. [Google Scholar] [CrossRef]
- Ding, Q.; Meng, F.; English, C.R.; Acevedo, M.C.; Shearer, M.J.; Liang, D.; Daniel, A.S.; Hamers, R.J.; Jin, S. Efficient photoelectrochemical Hydrogen Generation Using Heterostructures of Si an Chemically Exfoliated Metallic MoS2. J. Am. Chem. Soc. 2014, 136, 8504–8507. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.A.; Bae, C.; Lee, S.; Kim, M.; Montero-Moreno, J.M.; Park, J.H.; Shin, H. Edge-On MoS2 Thin Films by Atomic Layer Deposition for Understanding the Interplay between the Active Area and Hydrogen Evolution Reaction. Chem. Mater. 2017, 29, 7604–7614. [Google Scholar] [CrossRef]
- Fan, R.; Mao, J.; Yin, Z.; Jie, J.; Dong, W.; Fang, L.; Zheng, F.; Shen, M. Efficient and Stable Silicon Photocathodes Coated with Vertically Standing Nano-MoS2 Films for Solar Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 6123–6129. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, J.B.; Song, J.T.; Oh, J.H.; Kim, S.H. Atomic layer deposited molybdenum disulfide on Si photocathodes for highly efficient photoelectrochemical water reduction reaction. J. Mater. Chem. A 2017, 5, 3304–3310. [Google Scholar] [CrossRef]
- Du, P.; Zhu, Y.; Zhang, J.; Xu, D.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Metallic 1T phase MoS2 nanosheets as a highly efficient co-catalyst for the photocatalytic hydrogen evolution of CdS nanorods. RSC Adv. 2016, 6, 74394–74399. [Google Scholar] [CrossRef]
- Kwon, K.C.; Choi, S.; Hong, K.; Moon, C.W.; Shim, Y.-S.; Kim, D.H.; Kim, T.; Sohn, W.; Jeon, J.-M.; Lee, C.H.; et al. Wafer-scale transferable molybdenum disulphide thin-film catalyst for photoelectrochemical hydrogen production. Energy Environ. Sci. 2016, 9, 2240–2248. [Google Scholar] [CrossRef]
- Fujii, K.; Ono, M.; Iwaki, Y.; Sato, K.; Ohkawa, K.; Yao, T. Photoelectrochemical Properties of the p−n Junction in and near the Surface Depletion Region of n-Type GaN. J. Phys. Chem. C 2010, 114, 22727–22735. [Google Scholar] [CrossRef]
- Tran, D.P.; Pramana, S.S.; Kale, S.V.; Nguyen, M.; Chiam, T.S.; Batabyal, K.S.; Wong, H.L.; Barber, J.; Loo, J. Novel Assembly of an MoS2 Electrocatalyst onto a Silicon Nanowire Array Electrode to Construct a Photocathode Composed of Elements Abundant on the Earth for Hydrogen Generation. Chem. Eur. J. 2012, 18, 13994–13999. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, S.; Hu, D.; Lin, L.; Yan, Z.; Gao, X.; Zhang, Z.; Liu, J. Ultrathin MoS2-coated Ag@Si nanosphere arrays as an efficient and stable photocathode for solar-driven hydrogen production. Nanotechnology 2018, 29, 105402–105410. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Lin, S.; Qarony, W.; Tao, L.; Chai, Y.; Zhang, X.; Lau, P.S.; Tsang, H.Y. High photoelectrochemical activity and stability of Au-WS2/silicon heterojunction photocathode. Sol. Energy Mater. Sol. Cells 2018, 174, 300–306. [Google Scholar] [CrossRef]
- Benck, D.J.; Lee, S.; Fong, D.K.; Kibsgaard, J.; Sinclair, R.; Jaramillo, F.T. Designing Active and Stable Silicon Photocathodes for Solar Hydrogen Production Using Molybdenum Sulfide Nanomaterials. Adv. Energy. Mater. 2014, 4, 1400739. [Google Scholar] [CrossRef]
- King, L.A.; Hellstern, T.R.; Park, J.; Sinclair, R.; Jaramillo, T.F. Highly Stable Molybdenum Disulfide Protected Silicon Photocathodes for Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 36792–36798. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Mao, J.; Fan, R.; Yin, Z.; Wu, X.; Jie, J.; Kang, Z.; Shen, M. Integrated MoSe2 with n+p-Si photocathodes for solar water splitting with high efficiency and stability. Appl. Phys. Lett. 2018, 112, 013902. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Wong, B.A.; Resasco, J.; Yang, P. MoS2-wrapped silicon nanowires for photoelectrochemical water reduction. Nano Res. 2015, 8, 281–287. [Google Scholar] [CrossRef]
- Chandra, G.; Roy, M.A.; Bhattacharya, S.S. Effect of n-Si on the Photocatalytic Production of Hydrogen by Pt-loaded CdS and CdS/ZnS Catalyst. Int. J. Hydrog. Energy 1996, 21, 19–23. [Google Scholar] [CrossRef]
- Hou, T.; Abrams, L.B.; Vesborg, C.K.P.; Bjorketun, E.M.; Herbst, K.; Bech, L.; Setti, M.A.; Damsgaard, D.C.; Pedersen, T.; Hansen, O.; et al. Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution. Nat. Mater. 2011, 10, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhu, Z.; Xu, Y.; Guo, F.; Zhang, J.; Wang, X. Efficient photoelectrochemical hydrogen production over p-Si nanowire arrays coupled with molybdenume-sulfur clusters. Int. J. Hydrog. Energy 2017, 42, 2832–2838. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, C.; Pan, L.; Tian, F.; Zhang, X.; Zhang, C. Enhanced photoelectrochemical hydrogen production using silicon nanowires@MoS3. Nano Energy 2013, 2, 1337–1346. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, Q.; He, D.; Liu, H.; Liu, W.; Li, Z.; Yang, B.; Zhang, X.; Lei, L.; Jin, S. A p-Si/NiCoSex core/shell nanopillar array photocathode for enhanced photoelectrochemical hydrogen production. Energy Environ. Sci. 2016, 9, 3113–3119. [Google Scholar] [CrossRef]
- Chen, Y.; Tran, P.; Boix, P.; Ren, Y.; Chiam, Y.S.; Li, Z.; Fu, K.; Wong, H.L.; Barber, J. Silicon Decorated with Amorphous Cobalt Molybdenum Sulfide Catalyst as an Efficient Photocathode for Solar Hydrogen Generation. ACS Nano 2015, 9, 3829–3836. [Google Scholar] [CrossRef]
- Chen, C.; Chen, P.; Basu, M.; Yang, K.; Lu, Y.; Dong, C.; Ma, C.; Shen, C.; Hu, S.; Liu, R. An integrated cobalt disulfide (CoS2) co-catalyst passivation layer on silicon microwires for photoelectrochemical hydrogen evolution. J. Mater. Chem. A 2015, 3, 23466–23476. [Google Scholar] [CrossRef]
- Chen, C.; Yang, K.; Basu, M.; Lu, T.; Lu, Y.; Dong, C.; Hu, S.; Liu, R. Wide Range pH-Tolerable Silicon@Pyrite Cobalt Dichalcogenide Microwire Array Photoelectrodes for Solar Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 5400–5407. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Zhang, Z.; Chen, C.; Chen, P.; Yang, K.; Ma, C.; Lin, C.; Hu, S.; Liu, R. Heterostructure of Si and CoSe2: A Promising Photocathode Based on a Non-noble Metal Catalyst for Photoelectrochemical Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 6211–6216. [Google Scholar] [CrossRef] [PubMed]
- Bazri, B.; Lin, Y.; Lu, T.; Chen, C.; Kowsari, E.; Hu, S.; Liu, R. A heteroelectrode structure for solar water splitting: Integrated cobalt ditelluride across a TiO2-passivated silicon microwire array. Catal. Sci. Technol. 2017, 7, 1488–1496. [Google Scholar] [CrossRef]
- Seger, B.; Laursen, B.A.; Vesborg, C.K.P.; Pedersen, T.; Hansen, O.; Dahl, S.; Chorkendorff, I. Hydrogen Production Using a Molybdenum Sulfide Catalyst on a Titanium-Protected n+p-Silicon Photocathode. Angew. Chem. Int. Ed. 2012, 51, 9128–9131. [Google Scholar] [CrossRef] [PubMed]
- Yadian, B.; Rao, Y.; Zhu, B.; Liu, Z.; Liu, Q.; Gan, L.C.; Chen, X.; Huang, Y. Metal-sulfide-decorated ZnO/Si nano-heterostructure arrays with enhanced photoelectrochemical performance. Mater. Res. Bull. 2017, 96, 503–508. [Google Scholar] [CrossRef]
- Nie, X.; Zhuo, S.; Maeng, G.; Sohlberg, K. Doping of TiO2 Polymorphs for Altered Optical and Photocatalytic Properties. Int. J. Photoenergy 2009, 294042:1–294042:22. [Google Scholar]
- Morgan, B.; Watson, G. Intrinsic n-type Defect Formation in TiO2: A Comparison of Rutile and Anatase from GGA+U Calculations. J. Phys. Chem. C 2010, 114, 2321–2328. [Google Scholar] [CrossRef]
- Fahey, P.; Griffin, P.; Plummer, J. Point defects and dopant diffusion in silicon. Rev. Mod. Phys. 1989, 61, 289–384. [Google Scholar] [CrossRef]
- Scanlon, D.; King, P.; Singh, R.; Torre, A.; Walker, S.; Balakrishnan, G.; Baumberger, F.; Catlow, C. Controlling Bulk Conductivity in Topological Insulators: Key Role of Anti-Site Defects. Adv. Mater. 2012, 24, 2154–2158. [Google Scholar] [CrossRef]
- Fuschillo, N.; Bierly, N.J.; Donahoe, F.J. Transport properties of the pseudo-binary alloy system Bi2Te3−ySey. J. Phys. Chem. Solids 1959, 8, 430–433. [Google Scholar] [CrossRef]
- Miller, G.R.; Li, C. Evidence for the existence of antistructure defects in bismuth telluride by density measurements. J. Phys. Chem. Solids 1965, 26, 173–177. [Google Scholar] [CrossRef]
- Ghoshtagore, R.N. Self-Diffusion in Tellurium. I. Anisotropy and Impurity Effect. Phys. Rev. 1967, 155, 598–602. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.B.R.; Maeng, W.J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Films 2009, 517, 2563–2580. [Google Scholar] [CrossRef]
- Jin, Z.; Shin, S.; Kwon, D.H.; Han, S.J.; Min, Y.S. Novel chemical route for atomic layer deposition of MoS2 thin film on SiO2/Si substrate. Nanoscale 2014, 6, 14453–14458. [Google Scholar] [CrossRef]
- Song, J.G.; Ryu, G.H.; Lee, S.J.; Sim, S.; Lee, C.W.; Choi, T.; Jung, H.; Kim, Y.; Lee, Z.; Myoung, J.M.; et al. Controllable synthesis of molybdenum tungsten disulfide alloy for vertically composition-controlled multilayer. Nat. Commun. 2015, 6, 7817. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Bae, C.; Yang, Y.; Lee, S.; Kim, M.; Kim, H.; Kim, Y.; Shin, H. Spatial Charge Separation in Asymmetric Structure of Au Nanoparticle on TiO2 Nanotube by Light-Induced Surface Potential Imaging. Nano Lett. 2014, 14, 4413–4417. [Google Scholar] [CrossRef] [PubMed]
- Polak, L.; Rector, J.; Slaman, M.; Wijngaarden, R. NaTaO3 Photoanode for Bias-Free Water Splitting: A Photo-Electrochemical and Kelvin Probe Surface Photovoltage Study. J. Phys. Chem. C 2016, 120, 23559–23565. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Feng, J.; Huang, H.; Zhang, C.; Qian, Q.; Fang, T.; Xu, J.; Wang, P.; Li, Z.; et al. Rational design of electrocatalysts for simultaneously promoting bulk charge separation and surface charge transfer in solar water splitting photoelectrodes. J. Mater. Chem. A 2018, 6, 2568–2576. [Google Scholar] [CrossRef]
- Almadori, Y.; Bendiab, N.; Grévin, B. Multimodal Kelvin Prove Force Microscopy Investigations of a Photovoltaic WSe2/MoS2 Type-ll interface. ACS Appl. Mater. Interfaces 2018, 10, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.-Y.; Wang, J.-Y.; Zhen, L. Photodiode-Like Behavior and Excellent Photoresponse of Vertical Si/Monolyer MoS2 Heterostructures. Sci. Rep. 2014, 4, 7186:1–7186:8. [Google Scholar]
- Kim, J.; Kim, G.Y.; Moon, H.; Yoon, S.; Seo, I.W.; Lee, Y.; Moon, D.G.; Ahn, S.J.; Jo, W. Identification of marcasite in pyrite FeS2 fhim films and the films’ carrier transport characeristics. RSC Adv. 2016, 6, 81394–81399. [Google Scholar] [CrossRef]
- Chi, C.F.; Cho, H.W.; Teng, H.; Chuang, C.Y.; Chang, Y.M.; Hsu, Y.J.; Lee, Y.L. Energy level alignment, electron injection, and charge recombination characteristics in CdS/CdSe cosensitized TiO2 photoelectrode. Appl. Phys. Lett. 2011, 98, 012101. [Google Scholar] [CrossRef]
- Fingerle, M.; Tengeler, S.; Calvet, W.; Mayer, T.; Jaegermann, W. Water Interaction with Sputter-Deposited Nickel Oxide on n-Si Photoanode: Cryo Photoelectron Spectroscopy on Adsorbed Water in the Frozen Electrolyte Approach. J. Electrochem. Soc. 2018, 165, H3148–H3153. [Google Scholar] [CrossRef]
- Wan, D.; Zhao, Y.; Cai, Y.; Asmara, T.; Huang, Z.; Chen, J.; Hong, J.; Yin, S.; Nelson, C.; Motapothula, M.; et al. Electron transport and visible light absorption in a plasmonic photocatalyst based on strontium niobite. Nat. Commun. 2017, 8, 15070. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Furube, A.; Shiozawa, T.; Ishikawa, A.; Wada, A.; Domen, K.; Hirose, C. Femtosecond Transient Absorption Spectroscopy on Photocatalysts: K4Nb6O17 and Ru(bpy)3 2+-Intercalated K4Nb6O17 Thin Films. J. Phys. Chem. B 2002, 106, 3065–3072. [Google Scholar] [CrossRef]
- Moss, B.; Lim, K.; Beltram, A.; Moniz, S.; Tang, J.; Fornasiero, P.; Barnes, P.; Durrant, J.; Kafizas, A. Comparing photoelectrochemical water oxidation, recombination kinetics and charge trapping in the three polymorphs of TiO2. Sci. Rep. 2017, 8, 2938:1–2938:7. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dai, M.; Wu, J.; Hu, Y.; Zhang, Q.; Cui, J.; Wang, Y.; Tan, H.H.; Wu, Y. Electrochemical hydrogenation of mixed-phase TiO2 nanotube arrays enables remarkably enhanced photoelectrochemical water splitting performance. Sci. Bull. 2018, 63, 194–202. [Google Scholar] [CrossRef]
- Kappera, R.; Voiry, D.; Yalcin, S.E.; Branch, B.; Gupta, G.; Mohite, A.D.; Chhowalla, M. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nat. Mater. 2014, 13, 1128–1134. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Kim, J.H.; Zhao, J.; Seok, J.; Keum, D.H.; Baik, J.; Choe, D.-H.; Chang, K.J.; Suenaga, K.; et al. Phase patterning for ohmic homojunction contact in MoTe2. Science 2015, 349, 625–628. [Google Scholar] [CrossRef]
- Li, M.Y.; Shi, Y.; Cheng, C.C.; Lu, L.S.; Lin, Y.C.; Tang, H.L.; Tsai, M.L.; Chu, C.W.; Wei, K.H.; He, J.H.; et al. Epitaxial growth of a monolayer WSe2-MoS2 lateral p-n junction with an atomically sharp interface. Science 2015, 349, 524–528. [Google Scholar] [CrossRef]
- Agrawal, A.; Lin, J.; Barth, M.; White, R.; Zheng, B.; Chopra, S.; Gupta, S.; Wang, K.; Gelatos, J.; Mohney, S.E.; et al. Fermi level depinning and contact resistivity reduction using a reduced titania interlayer in n-silicon metal-insulator-semiconductor ohmic contactsn nanowire array electrode to construct a photocathode composed of elements abundant on the earth for hydrogen generation. Appl. Phys. Lett. 2014, 104, 112101–112105. [Google Scholar]
- Smit, G.D.J.; Rogge, S.; Klapwijk, T.M. Scaling of nano-Schottky-diodes. Appl. Phys. Lett. 2002, 81, 3852–3854. [Google Scholar] [CrossRef]
- Rossi, R.C.; Lewis, N.S. Investigation of the Size-Scaling Behavior of Spatially Nonuniform Barrier Height Contacts to Semiconductor Surfaces Using Ordered Nanometer-Scale Nickel Arrays on Silicon Electrodes. J. Phys. Chem. B 2001, 105, 12303–12318. [Google Scholar] [CrossRef]


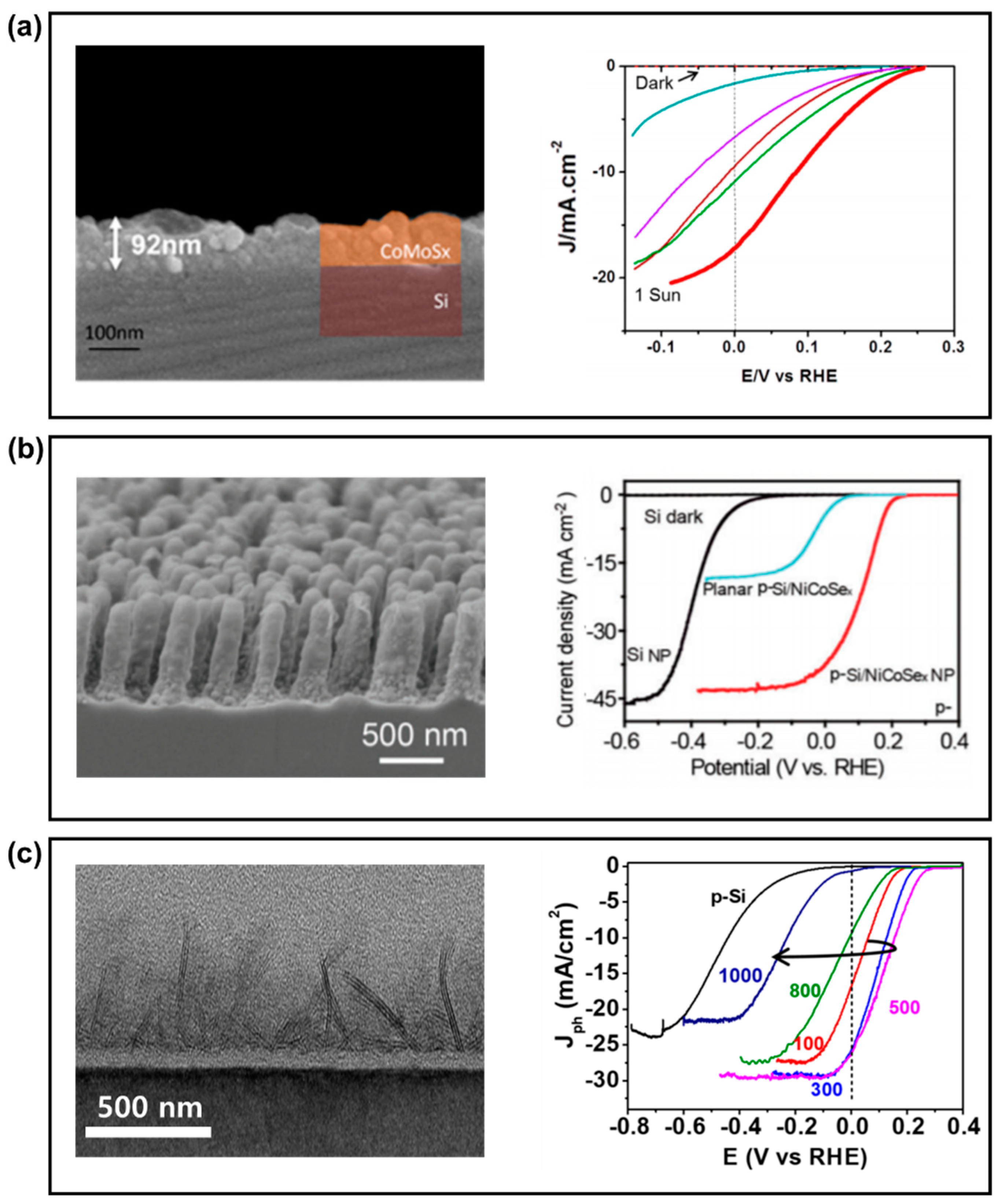
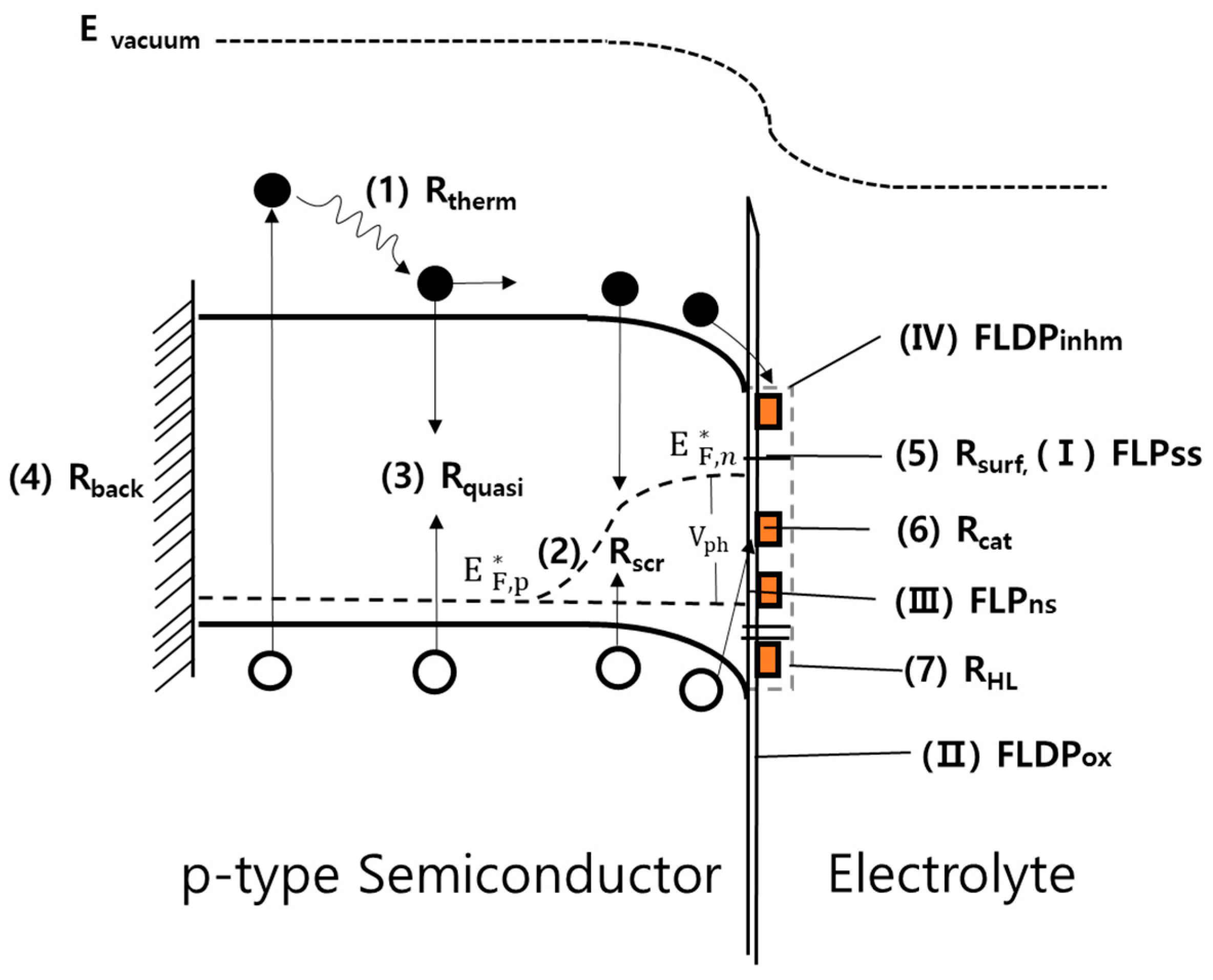
| PEC Process | Cell Type | Configuration | MX2 Compound | Experimental Condition | Current Density (mAcm−2) | Onset Potential (V vs RHE) | Light Source (mWcm−2) | Stability (min) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| TMDC | Photocathode | TMDC/Si | MoS2/p-Si | MoS2 | 0.5 M H2SO4 (pH = 0.3)/SCE Ref./Pt plate Counter | 24.6 (0V vs RHE) | +0.17 | Xe Lamp (100) | 1666.7 (10000s) | [81] |
| MoS2/p-Si | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl/1 M KCl Ref./Graphite rod Counter | 17.6 (0V vs RHE) | +0.23 | 1 sun (100) | 180 | [75] | ||||
| MoS2/p-Si | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl/3M KCl Ref./Pt wire Counter | 21.7 (0V vs RHE) | +0.23 | 1 sun (100) | 1440 | [78] | ||||
| MoS2/p-Si NW | 1.0 M Na2SO4 buffer (pH = 5.0)/Not available | 1 (0V vs RHE) | +0.25 | Xe Lamp (100) | 60 | [82] | ||||
| 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl Ref./Pt wire Counter | 16.5 (0V vs RHE) | +0.26 | Xe Lamp (100) | 2880 | [68] | |||||
| MoS2/Ag@Si NS | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl Ref./Pt wire Counter | 33.3 (Saturated) | +0.11 | 1 sun (100) | 720 | [83] | ||||
| p-Si/SiOx/MoS2 | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl Ref./Pt wire Counter | 30(Saturated) | +0.35 | Xe Lamp (100) | 4320 | [53] | ||||
| Au/WS2/Si | WS2 | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl Ref. Pt Counter | 0.37 (-1.0V vs RHE) | Not Available | Not Available | 50 | [84] | |||
| TMDC/ n+p-Si | MoS2/MoxSi Mo/n+p Si | MoS2 | 0.5 M H2SO4 (pH = 0.3)/Hg/Hg2SO4 in saturated K2SO4 Ref./Ir Counter | 17 (0V vs RHE) | +0.32 | 1 sun (100) | 6000 | [85] | ||
| MoS2/MoxSi Mo/n+pSi | 0.5 M H2SO4 (pH = 0.3)/Hg/Hg2SO4 in saturated K2SO4 Ref./Ir Counter | 19 (0V vs RHE) | +0.28 | 36360 (606h) | [86] | |||||
| MoSe2/n+p-Si | MoSe2 | 1.0 M HClO4 (pH = 1.0)/Ag/AgCl/3M KCl Ref./Pt Counter | 29.3 (Saturated) | +0.40 | Xe Lamp (100) | 7200 | [87] | |||
| TMDC/Interlayer/ Si | MoS2/ALD TiO2/p-Si NW | MoS2 | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl/1M NaCl Ref. | 15 (0V vs RHE) | +0.30 | 1 sun (100) | 75 | [88] | ||
| 3D MoS2/TiO2/p-Si | 0.5 M H2SO4 (pH = 0.3)/SCE Ref./Graphite Counter | 28 (0V vs RHE) | +0.35 | Xe Lamp (100) | 6000 | [74] | ||||
| TMDC/Interlayer/n+p-Si | MoS2/Al2O3 n+p-Si | MoS2 | 1.0 M HClO4 (pH = 1.0)/Ag/AgCl/3M KCl Ref./Pt wire Counter | 35.6 (Saturated) | +0.40 | 1 sun (100) | 7200 | [77] | ||
| TMC | Photocathode | TMC/Si | CdS, ZnS/ n-Si powder | CdS, ZnS | 0.24 M Na2S + 0.35 M Na2SO3 (pH = 11.5)/Not Available | Not Available (7.23mL/gh) | Not Available | (25) | Not Available | [89] |
| Mo3S4 cluster/Si NP | Mo3S4 | 1.0 M HClO4 (pH = 1.0)/Hg/HgSO4 Ref./Pt mesh Counter | 9 (0V vs RHE) | +0.15 | Xe Lamp (28.3) | 60 | [90] | |||
| MoSx/P-Si | MoSx | 0.5 M H2SO4 (pH = 0.3)/SCE Ref./Pt mesh Counter | 14.3 (0V vs RHE) | +0.25 | Xe Lamp (27.3) | 180 | [91] | |||
| a-NiCoSex/ p-Si NP | NiCoSex | 0.5 M H2SO4 (pH = 0.3)/SCE Ref./Pt wire Counter | 37.5 (0V vs RHE) | +0.25 | 1 sun (100) | 120 | [93] | |||
| a-CoMoSx/Si | CoMoSx | Phosphate (pH = 4.25)/Ag/AgCl/3M KCl Ref./Pt wire Counter | 17.5 vs (0.25V vs RHE) | 0.25 | Xe Lamp (100) | 180 | [94] | |||
| CdSe QD/ n-Si NW | CdSe | 0.25 M Na2S + 0.35 M Na2SO3 (pH = 11.5)/Hg/HgO Ref./ Pt foil Counter | 6.1 (1.0V vs RHE) | Not Available | 1 sun (100) | < 1 | [70] | |||
| CoS2/p-Si MW | CoS2 | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl Ref./Pt plate Counter | 3.22 (0V vs RHE) | 0.249 | Xe Lamp (100) | 180 | [94] | |||
| CoS2, CoSe2/p-Si MW | CoS2, CoSe2 | 0.5 M H2SO4 (pH = 0.3)/Ag/AgCl Ref./Pt plate Counter | 3.22 (CoS2), 2.55 (CoSe2) | 0.248 (CoS2) 0.137 (CoSe2) | Xe Lamp (100) | Not Available | [96] | |||
| CoSex/Si MW | CoSex | 0.5 M Na2SO4 (pH =1)/Ag/AgCl Ref./Pt foil Counter | 9 (-0.14V vs RHE) | 0.18 | Xe Lamp (100) | 50 cycles | [97] | |||
| TMC/Interlayer/Si | CoTex/ALD - TiO2/Si MW | CoTex | 0.5 M H2SO4 (pH = 0.3)/0.5M Ag/AgCl Ref./Pt foil Counter | 24.0 (Saturated) | 0.17 | Xe Lamp (100) | 66.7 (4000s) | [98] | ||
| TMC/Interlayer/n+p Si | MoSx/Ti-TiOx/n+p-Si | MoSx | 1.0 M HClO4 (pH = 1.0)/Hg/HgSO4 Ref./Pt mesh Counter | 12 (0.2V vs RHE) | +0.20 | (38.6) | 60 | [99] | ||
| a-MoS2/Al2O3/n+p-Si | MoS2 | 1.0 M HClO4 (pH = 1.0)/Ag/AgCl Ref./Pt wire Counter | 34.5 (Saturated) | +0.35 | Xe Lamp (100) | 2400 | [69] | |||
| Photoanode | TMC/Interlayer/Si | Ag2S NP/ZnS/ZnO/p-Si NC | Ag2S, ZnS | 0.2 M Na2SO4 + PBS (pH = 7.25)/Ag/AgCl Ref./Pt foil Counter | 0.0588 vs (2.1V vs RHE) | Not Available | Not Available | Not Available | [100] |
| Methods | Compounds | Specific Processes | Ref. |
|---|---|---|---|
| CVD | MoS2 | MoS2: (NH4)2MoS4 + H2 at 500 °C ~ 950 °C MoS2: Mo (MoCl5, MoO3) + S (Sulfur powder) from 475 °C to 730 °C | [68,74,75,81,83] |
| Sputtering | MoSe2, WS2 | MoSe2: MoSe2 target Sputtering (400 °C, 50 W) WS2: target Sputtering | [84,87] |
| Photo-electrodeposition | MoS2, NiCoSe | MoS2: (NH4)2[MoS4]/(NH4)2MoS4 + NaClO4 NiCoSex: NiCl + CoCl + SeO2 | [77,82,89,93] |
| ALD (PLD) | MoS2 | MoS2: 200 °C PLD, Mo(CO)6 + H2S, annealing in 500 ~ 700 °C H2S condition | [53,78] |
| Hydrothermal | WS2, MoSx, CoSex, ZnS | MoSx: (NH4)6Mo7O24·4H2O + ammonium polysulfide (96 °C, 120 h) CoSex: EDTA + CoCl2·6H2O + Se Powder + NaOH (180 °C, 18 h) ZnS2: 0.2 M thioacetamide +ZnNO3 (90 °C) | [91,96,97,100] |
| Drop-casting | Mo3S4, CoTe2 | Mo3S4: methylcyclopentadienyl ligands CoTe2: Co(NO3)2·6H2O + Na2TeO4 in methanol | [90,92,98] |
| Electrodeposition | a-MoS2, a-CoMoS | a-MoS2: (NH4)2MoS4 + NaClO4/(NH4)2MoS4 + DMF a-CoMoS: 0.25 mM [Co(MoS4)2]2- | [69,94,99] |
| SILAR | CdSe | CdSe: Cd(NO3)2 +NaHSe + NaBH4 + SeO2 | [70] |
| Thermal Sulfidation | MoS2, CoS2, CoSe2 | MoS2: Mo Sputtering/Sulfidize (250 °C, H2S gas, 1 h) CoS2: Co(OH)2 + Sulfur powder (500 °C, 1 h) CoSe2: Co(NO)3·6(H2O) + S, Se powder | [85,86,95] |
| Thermolysis | MoS2 | MoS2: (NH4)2MoS4 (573–673 K, N2/H2) | [88] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joe, J.; Yang, H.; Bae, C.; Shin, H. Metal Chalcogenides on Silicon Photocathodes for Efficient Water Splitting: A Mini Overview. Catalysts 2019, 9, 149. https://doi.org/10.3390/catal9020149
Joe J, Yang H, Bae C, Shin H. Metal Chalcogenides on Silicon Photocathodes for Efficient Water Splitting: A Mini Overview. Catalysts. 2019; 9(2):149. https://doi.org/10.3390/catal9020149
Chicago/Turabian StyleJoe, Jemee, Hyunwoo Yang, Changdeuck Bae, and Hyunjung Shin. 2019. "Metal Chalcogenides on Silicon Photocathodes for Efficient Water Splitting: A Mini Overview" Catalysts 9, no. 2: 149. https://doi.org/10.3390/catal9020149
APA StyleJoe, J., Yang, H., Bae, C., & Shin, H. (2019). Metal Chalcogenides on Silicon Photocathodes for Efficient Water Splitting: A Mini Overview. Catalysts, 9(2), 149. https://doi.org/10.3390/catal9020149






