Development of TiO2-Carbon Composite Acid Catalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural
Abstract
1. Introduction
2. Experimental and Methods
2.1. Materials
2.2. Preparation of TiO2 Nanoparticles Using the Sol-Gel Method
2.3. Preparation of Carbon Spheres Using the Microwave-Assisted Method
2.4. Preparation of TiO2C Composites Using the Microwave-Assisted Method
2.5. Preparation of the Sulfonated Carbon and TiO2C Acid Catalysts
2.6. Surface Acid Measurement Tests
2.7. Characterisation
2.8. Catalytic Testing
2.9. Reusability Studies
2.10. Hot Filtration Tests
3. Results and Discussion
3.1. BET Surface Area and Pore Volume of the Catalysts
3.2. Surface Morphology
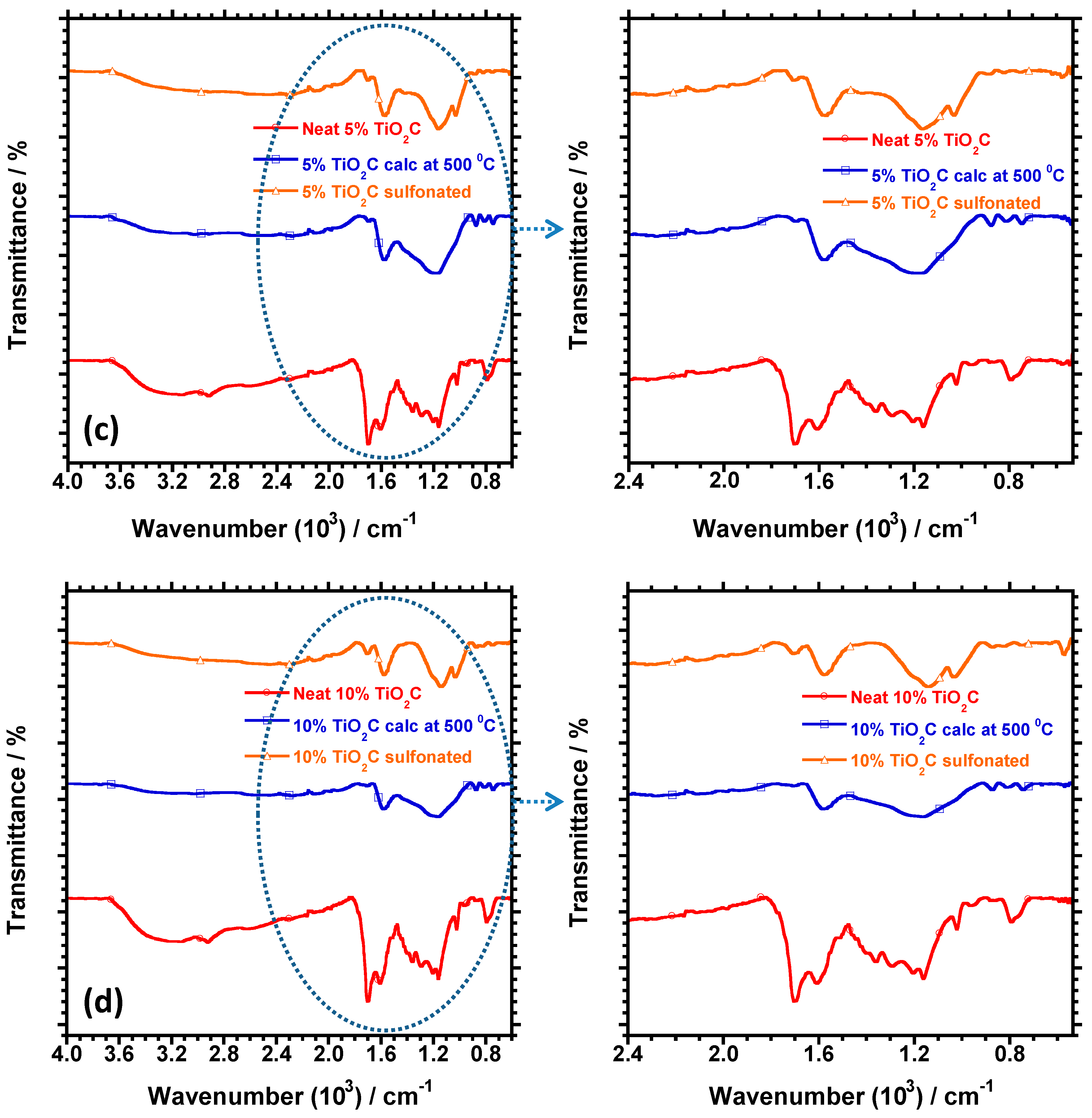
3.3. Chemical Analysis Using FTIR
3.4. Structural Characterisation Using XRD
3.5. Catalytic Testing
3.5.1. Effect of Different Solvents on the Dehydration of Fructose into 5-HMF
3.5.2. Effect of Reaction Temperature on HMF Dehydration
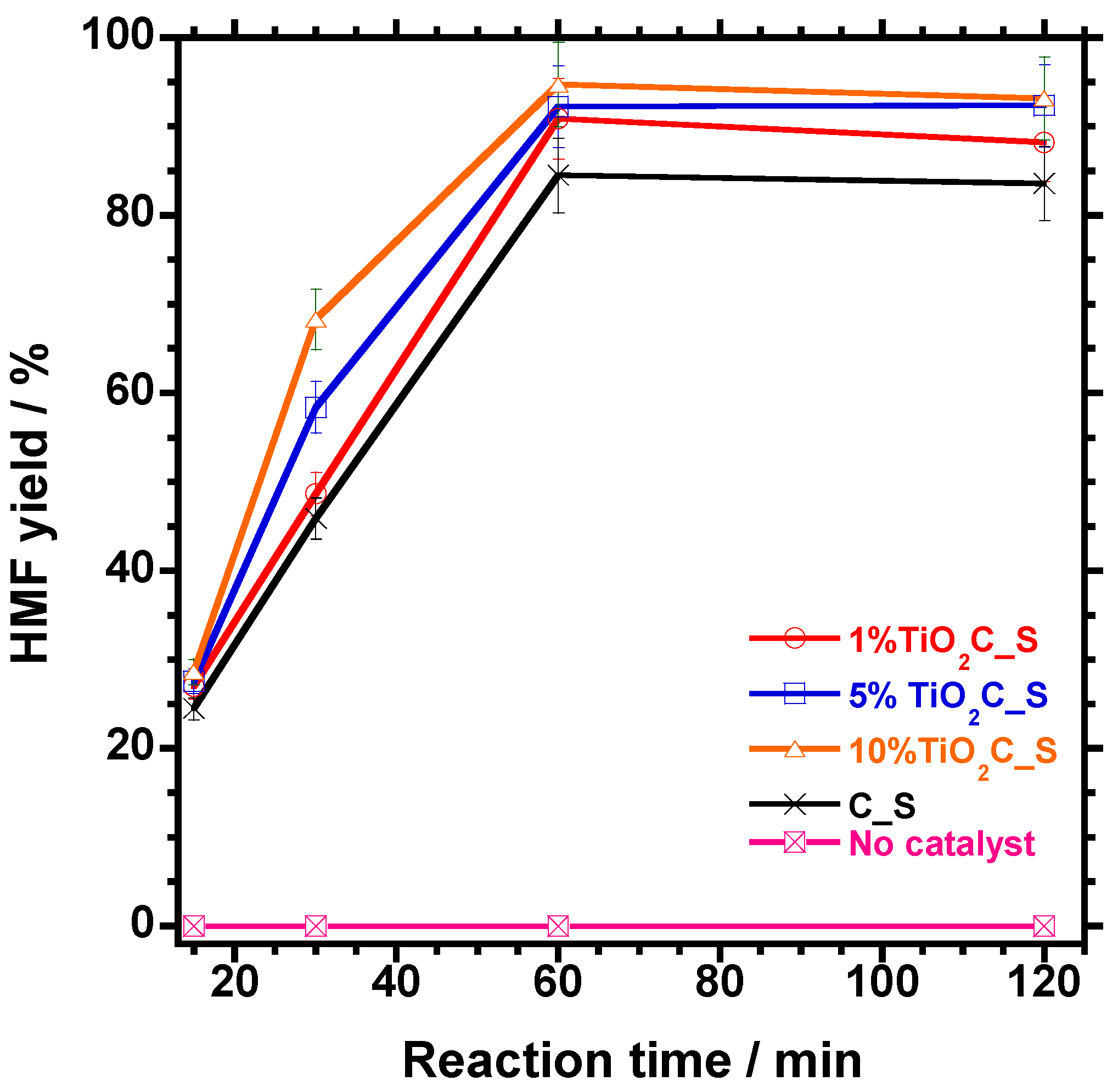
3.5.3. Effect of Reaction Time on 5-HMF Dehydration
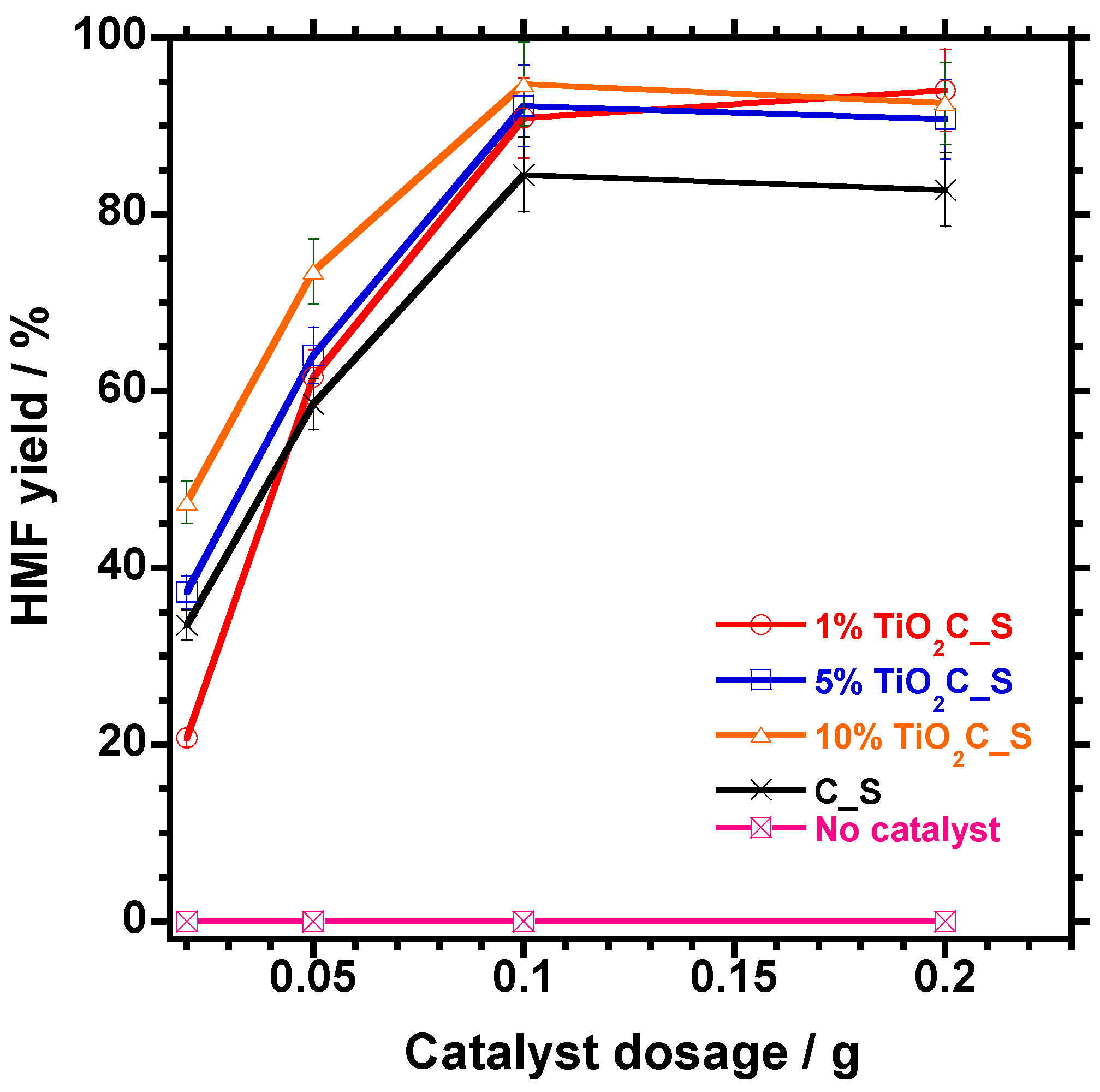
3.5.4. Effect of Catalyst Amount on HMF Dehydration
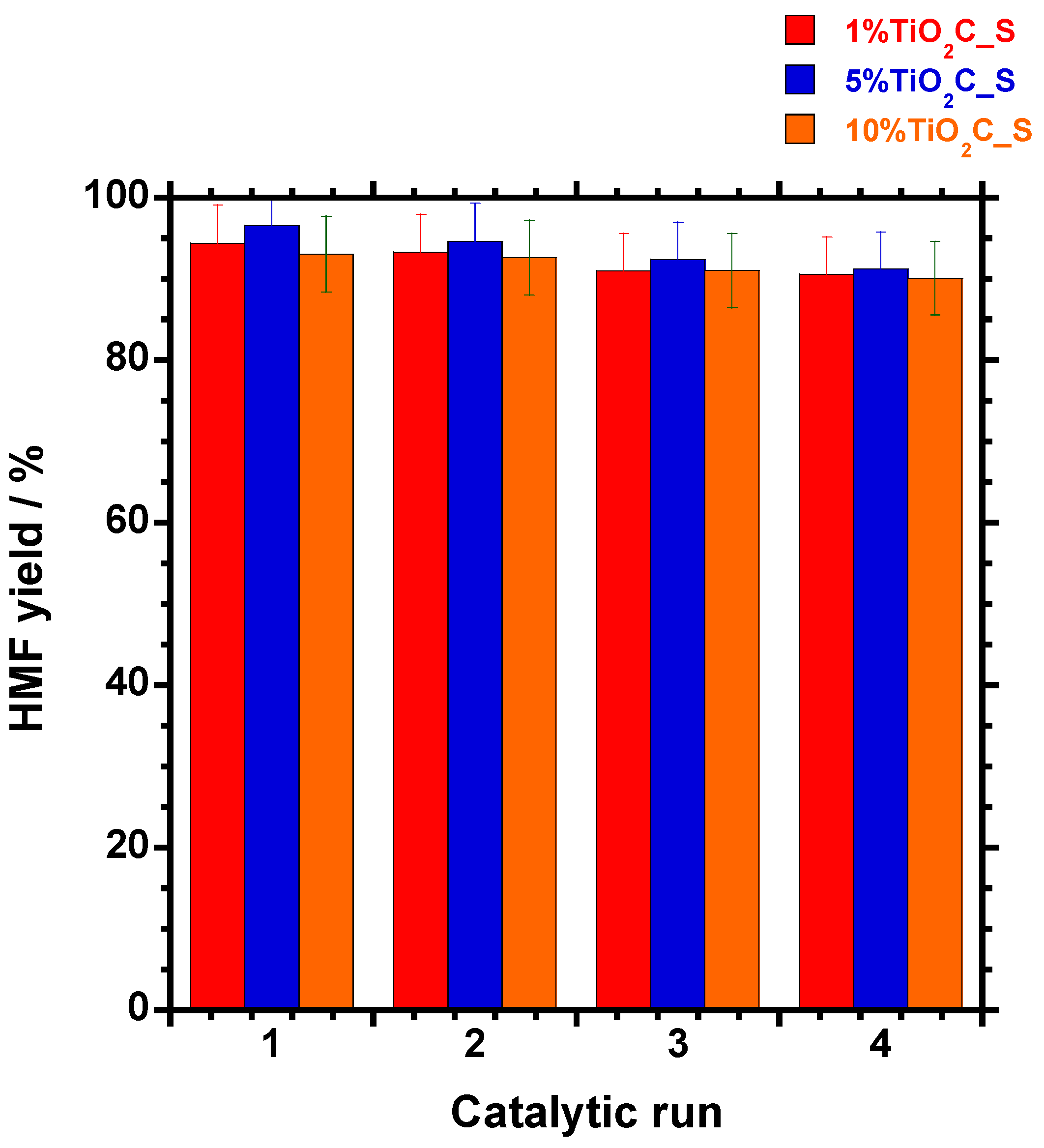
3.5.5. Reusability of the Catalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and applications of alkyl levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- McNeff, C.V.; Nowlan, D.T.; McNeff, L.C.; Yan, B.; Fedie, R.L. Continuous production of 5-hydroxymethylfurfural from simple and complex carbohydrates. Appl. Catal. A Gen. 2010, 384, 65–69. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Catalytical conversion of fructose and glucose into 5-hydroxymethylfurfural in hot compressed water by microwave heating. Catal. Commun. 2008, 9, 2244–2249. [Google Scholar] [CrossRef]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Moreau, C.; Finiels, A.; Vanoye, L. Dehydration of fructose and sucrose into 5 hydroxymethylfurfural in the presence of 1-H-3-methyl imidazolium chloride acting both as solvent and catalyst. J. Mol. Catal. A Chem. 2006, 253, 165–169. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Acid catalysed production of 5-hydroxymethyl furfural from d-Fructose in Subcritical Water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Xu, H.; Miao, Z.; Zhao, H.; Yang, J.; Zhao, J.; Song, H.; Liang, N.; Chou, L. Dehydration of fructose into 5-hydroxymethylfurfural by high stable ordered mesoporous zirconium phosphate. Fuel 2015, 145, 234–240. [Google Scholar] [CrossRef]
- Jain, A.; Shore, A.M.; Jonnalagadda, S.C.; Ramanujachary, K.V.; Mugweru, A. Conversion Of fructose, glucose and sucrose to 5-hydroxymethyl-2-furfural over mesoporous zirconium phosphate catalyst. Appl. Catal. A Gen. 2015, 489, 72–76. [Google Scholar] [CrossRef]
- Kılıc, E.; Yılmaz, S. Fructose dehydration to 5-hydroxymethylfurfural over sulfated TiO2–SiO2, Ti-SBA-15, ZrO2, SiO2, and activated carbon catalysts. Eng. Chem. Res. 2015, 54, 5220–5225. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Liu, B. Catalytic conversion of fructose and 5-hydroxymethylfurfural into 2,5-Furandicarboxylic acid over a recyclable Fe3O4−CoOx magnetite nanocatalyst. ACS Sustain. Chem. Eng. 2015, 3, 406–412. [Google Scholar] [CrossRef]
- Yang, Z.; Qi, W.; Huang, R.; Fang, J.; Su, R.; He, Z. Functionalized silica nanoparticles for conversion of fructose to 5-hydroxymethylfurfural. Chem. Eng. J. 2016, 296, 209–216. [Google Scholar] [CrossRef]
- Morales, G.; Paniagua, M.; Melero, J.A.; Iglesias, J. Efficient production of 5-ethoxymethylfurfural from fructose by sulfonic mesostructured silica using DMSO as co-solvent. Catal. Today 2017, 279, 305–316. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Wang, Y.; Zhu, L.; Cui, H.; Yi, W. Catalytic fructose dehydration to 5-hydroxymethylfurfural over sulfonated carbons with hierarchically ordered pores. J. Fuel Chem. Technol. 2016, 44, 1341–1348. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, C.; He, C.; Dai, Y.; Jia, X.; Yang, Y. Efficient dehydration of fructose to 5 hydroxymethylfurfural over sulfonated carbon sphere solid acid catalysts. Catal. Today 2016, 264, 123–130. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Ren, J.; Liu, X.; Lu, G.; Wang, Y. Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem. 2011, 13, 2678–2681. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Zhou, T.J. Conversion of fructose and glucose into 5-hydroxymethylfurfural with lignin-derived carbonaceous catalyst under microwave irradiation in dimethyl sulfoxide–ionic liquid mixtures. Bioresour. Technol. 2012, 112, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tang, X.; Wu, Z.; Linc, L.; Xu, J.; Xu, N.; Dai, B. Magnetic lignin-derived carbonaceous catalyst for the dehydration of fructose into 5-hydroxymethylfurfural in dimethyl sulfoxide. Chem. Eng. J. 2015, 263, 299–308. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Patra, A.K.; Bhaumik, A.; Saha, B. Self-assembly of mesoporous TiO2 nanospheres via aspartic acid templating pathway and its catalytic application for 5-hydroxymethyl-furfural synthesis. J. Mater. Chem. 2011, 21, 17505–17510. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Patra, A.K.; Sasidharan, M.; Bhaumik, A.; Saha, B. Microwave assisted rapid conversion of carbohydrates into 5-hydroxymethylfurfural catalysed by mesoporous TiO2 nanoparticles. Appl. Catal. A Gen. 2011, 409–410, 133–139. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Casco, M.E.; Militello, M.P.; Silvestre-Albero, J.; Barbero, C.A.; Acevedo, D.F. Sulfonated porous carbon catalysts for biodiesel production: Clear effect of the carbon particle size on the catalyst synthesis and properties. Fuel Process. Technol. 2016, 149, 209–217. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, M.; Ma, H.L.; Zhang, Z.Q.; Gao, J.M.; Zhu, Y.L.; Han, X.J.; Guo, X.Y. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Li, W.; Ju, M.; Liu, L.; Chen, Y.; Yang, Q. One-pot synthesis of sulfonated graphene oxide for efficient conversion of fructose into HMF. RCS Adv. 2016, 16, 104016–104024. [Google Scholar] [CrossRef]
- Qi, X.; Guo, H.; Li, L.; Smith, R.L., Jr. Acid-catalyzed dehydration of fructose into 5-hydroxymethylfurfural by cellulose derived amorphous carbon. ChemSusChem 2012, 5, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Wang, J.; Tang, N.; Zhao, R.; Zhang, D.; Guan, G.; Li, K. Sulfur doped millimetre sized microporous activated carbon spheres derived from sulfonated poly(styrene-devinylbenzene) for CO2 capture. J. Phys. Chem. C 2017, 121, 10000–10009. [Google Scholar] [CrossRef]
- Rao, C.N.R. Contribution to the infrared spectra of organosulphur compounds. Can. J. Chem. 1964, 42, 36–42. [Google Scholar] [CrossRef]
- Yu, D.; Bo, B.; Yunhua, H. Fabrication of TiO2@yeast-carbon hybrid composites with the raspberry like structure and their synergistic adsorption photocatalysis performance. J. Nanomater. 2013, 2013, 851417. [Google Scholar] [CrossRef]
- Kansal, S.K.; Sood, S.; Umar, A.; Mehta, S.K. Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J. Alloys Compd. 2013, 581, 392–397. [Google Scholar] [CrossRef]


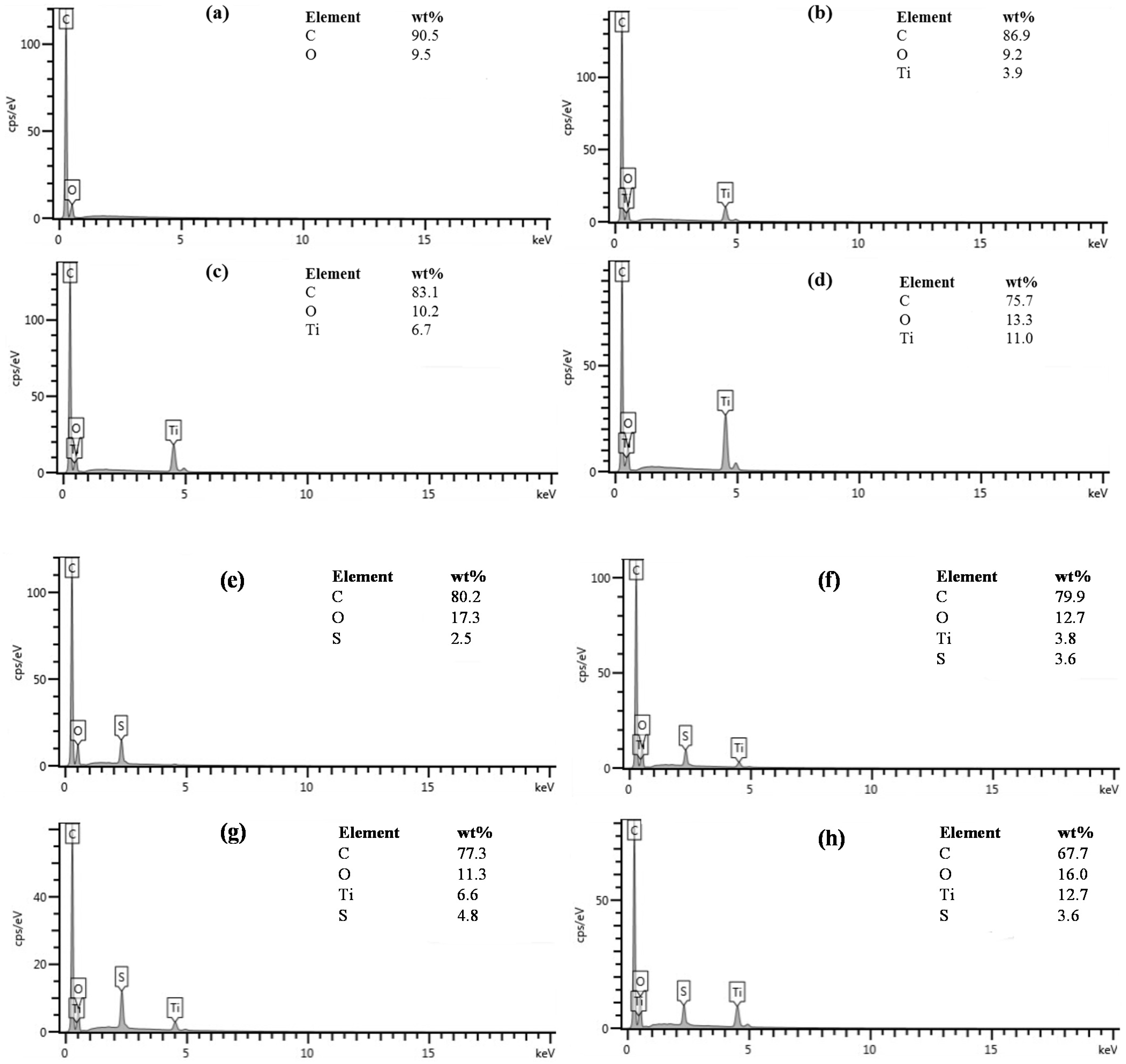



| Catalyst before Sulfonation | Catalyst after Sulfonation | |||||
|---|---|---|---|---|---|---|
| SBET (m2/g) | Vp (cm3/g) | SBET (m2/g) | Vp (cm3/g) | Sulfonic Groups (m·molg−1) a | ||
| C | 517 | - | C_S | 167 | - | 1.34 |
| 1%TiO2C | 413 | 0.26 | 1%TiO2C_S | 83 | 0.07 | 1.46 |
| 5%TiO2C | 273 | 0.13 | 5%TiO2C_S | 61 | 0.04 | 1.55 |
| 10%TiO2C | 202 | 0.10 | 10%TiO2C_S | 59 | 0.03 | 1.49 |
| Catalyst | HMF Dehydration (%) [UV-Vis at the Measured Absorbance of 284 nm] | ||||
|---|---|---|---|---|---|
| Methanol | DI Water | Ethanol | Isopropanol | DMSO | |
| C | 3 | 5 | 11 | 1 | 84 |
| 1%TiO2C | 3 | 6 | 4 | 9 | 91 |
| 5%TiO2C | 3 | 1 | 6 | 0 | 92 |
| 10%TiO2C | 0 | 4 | 12 | 11 | 95 |
| Catalyst | Catalyst Mass (g) | Substrate Mass (g) | T (°C) | Time (min) | 5-HMF Yield (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Uv-Vis a | HPLC b | ||||||
| C_S | 0.1 | 0.5 | 120 | 60 | 84 | - | This work |
| 1%TiO2C_S | 0.1 | 0.5 | 120 | 60 | 91 | - | This work |
| 5%TiO2C-S | 0.1 | 0.5 | 120 | 60 | 92 | - | This work |
| 10%TiO2C_S | 0.1 | 0.5 | 120 | 60 | 95 | - | This work |
| TiO2 | 0.05 | 0.1 | 130 | 2 | 49.5 | 47.8 | [19] |
| TiO2 | 0.1 | 0.05 | 140 | 5 | 53.4 | - | [20] |
| CS | 0.1 | 0.5 | 160 | 90 | - | 90 | [15] |
| C | 0.4 | 0.5 | 130 | 90 | - | 91.2 | [16] |
| Magnetic lignin-derived carbon (MLC)-SO3H | 0.05 | 0.1 | 130 | 40 | - | 81.1 | [18] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songo, M.M.; Moutloali, R.; Ray, S.S. Development of TiO2-Carbon Composite Acid Catalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural. Catalysts 2019, 9, 126. https://doi.org/10.3390/catal9020126
Songo MM, Moutloali R, Ray SS. Development of TiO2-Carbon Composite Acid Catalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural. Catalysts. 2019; 9(2):126. https://doi.org/10.3390/catal9020126
Chicago/Turabian StyleSongo, Morongwa Martha, Richard Moutloali, and Suprakas Sinha Ray. 2019. "Development of TiO2-Carbon Composite Acid Catalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural" Catalysts 9, no. 2: 126. https://doi.org/10.3390/catal9020126
APA StyleSongo, M. M., Moutloali, R., & Ray, S. S. (2019). Development of TiO2-Carbon Composite Acid Catalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural. Catalysts, 9(2), 126. https://doi.org/10.3390/catal9020126







