Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction
Abstract
1. Introduction
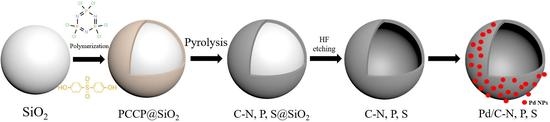
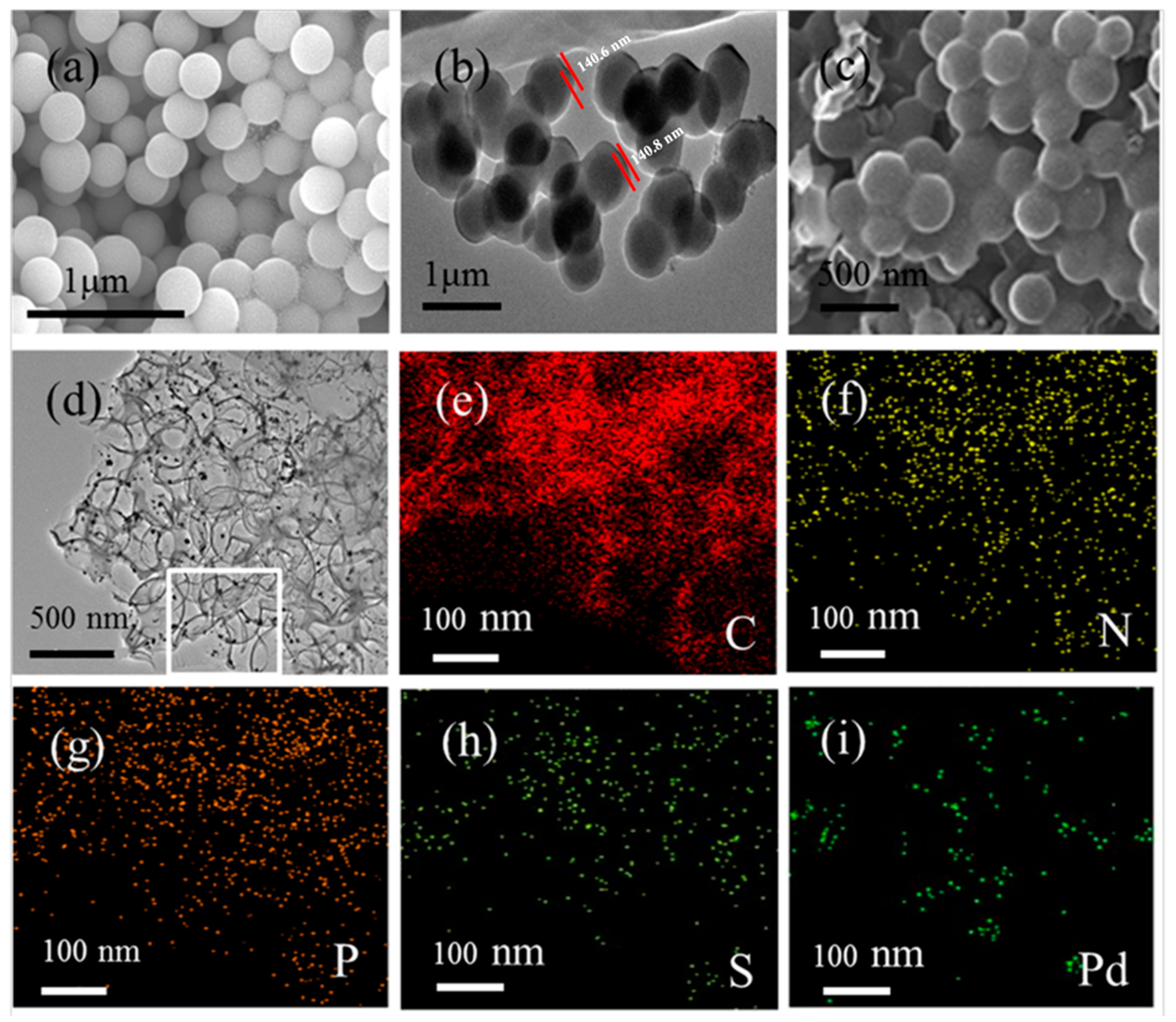
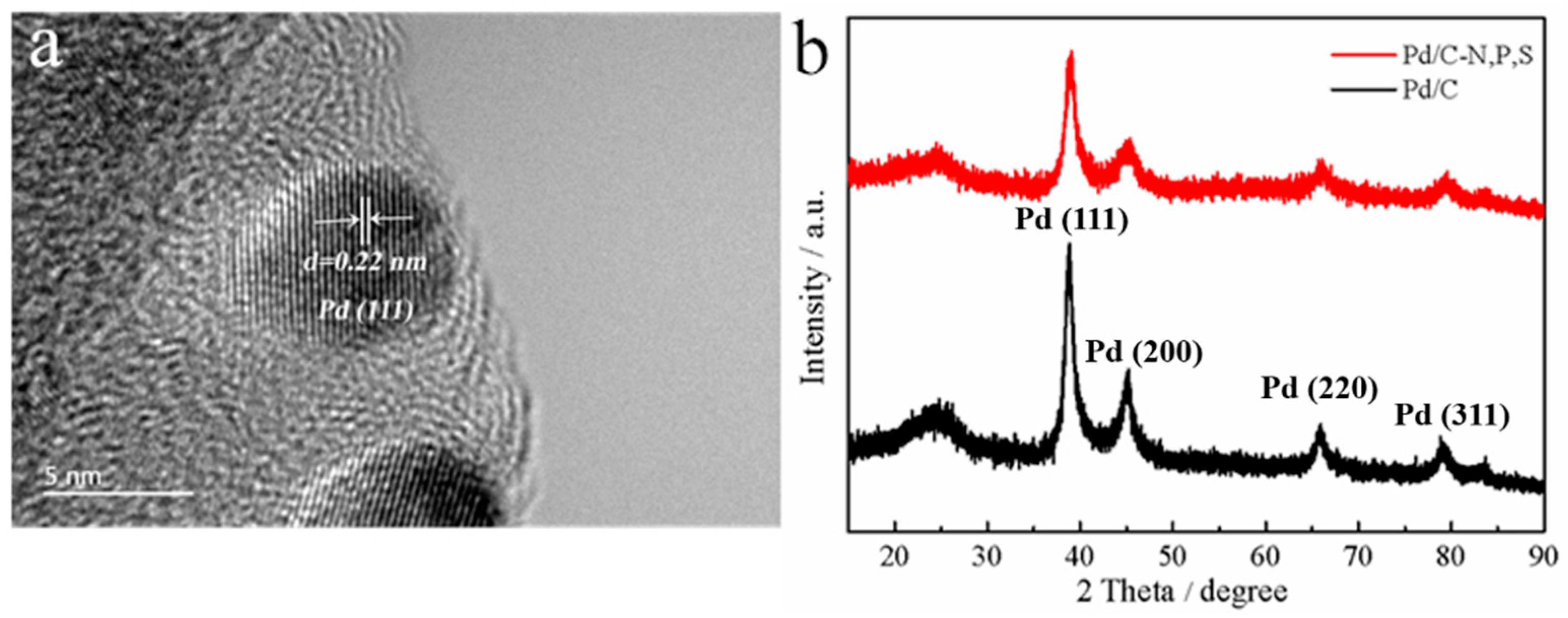
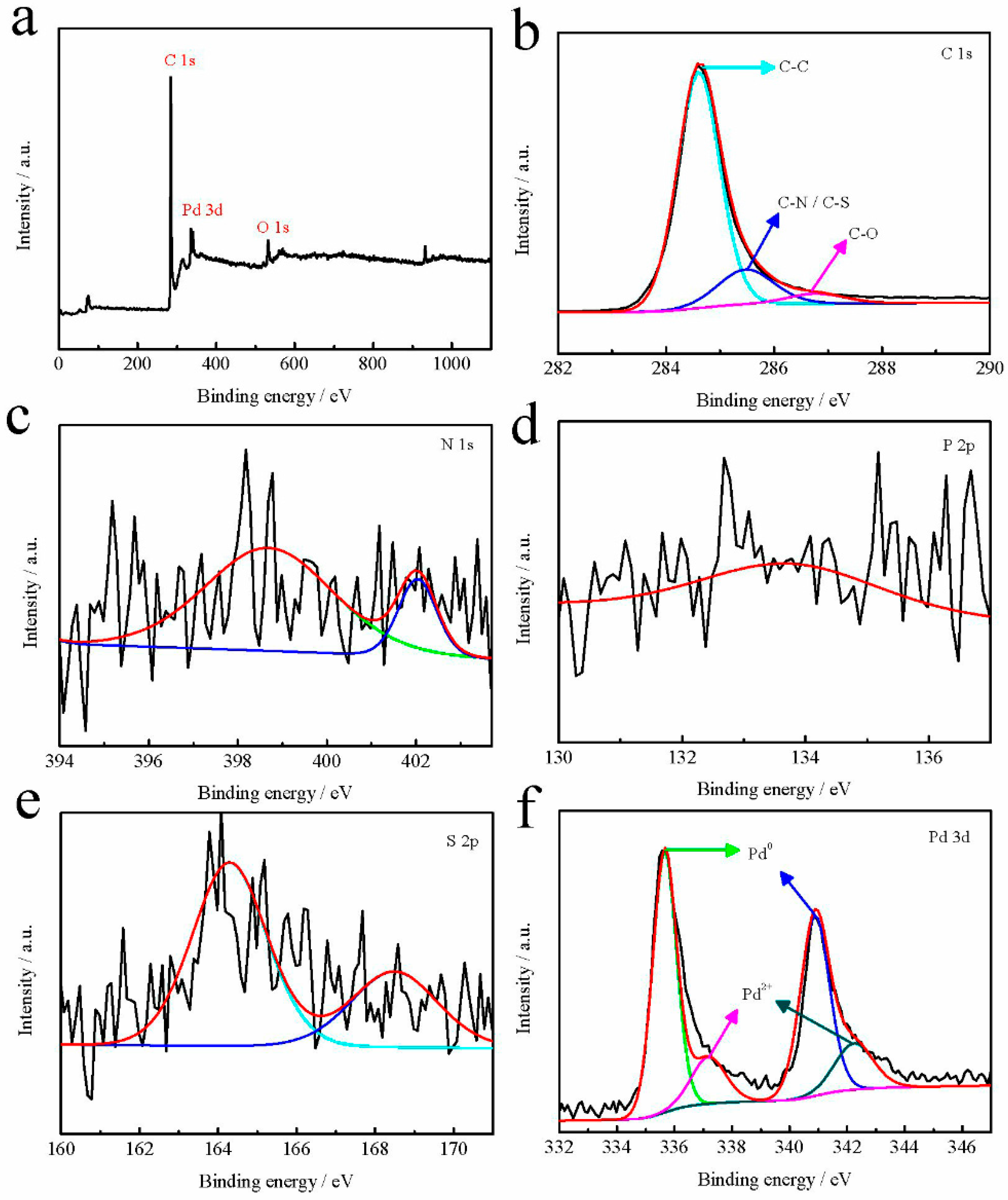
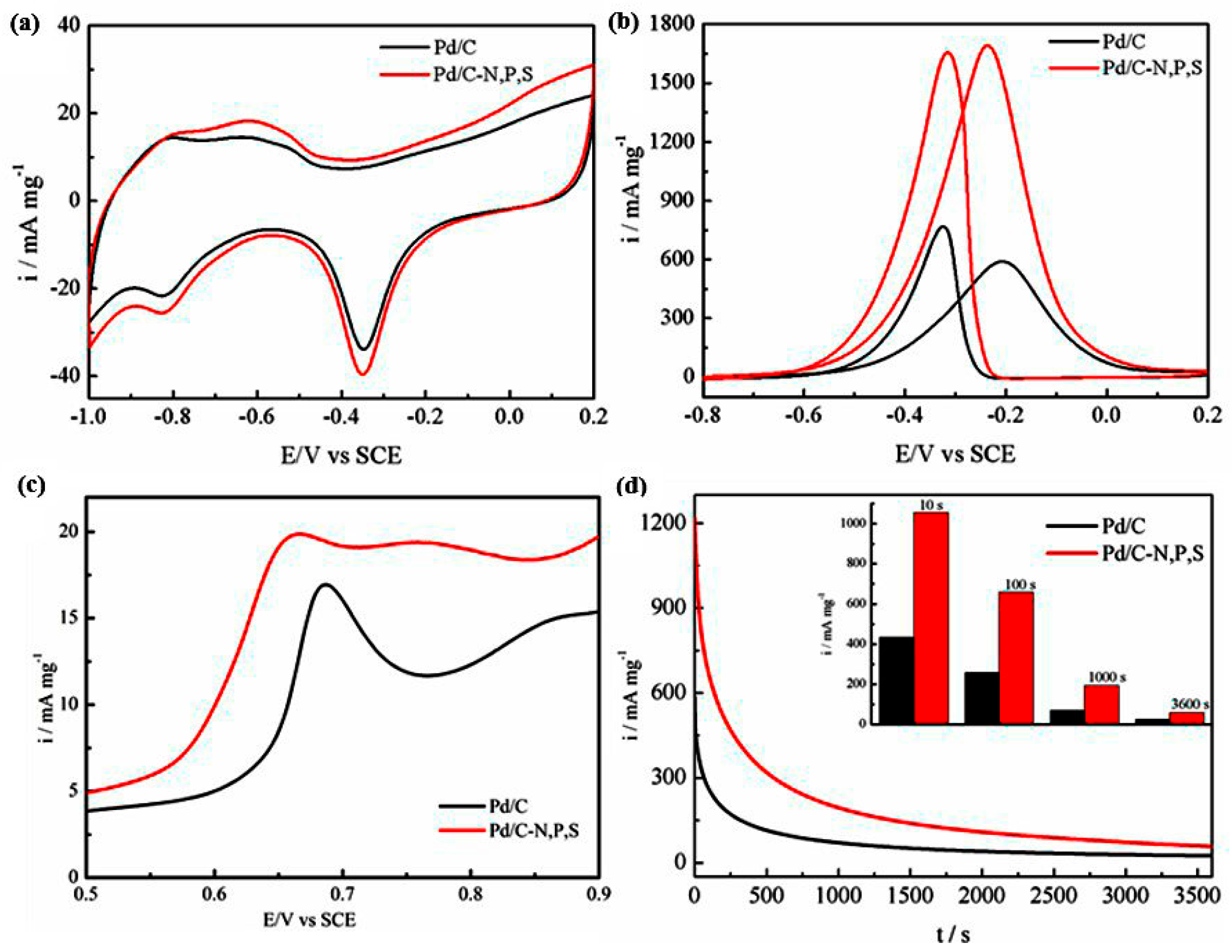
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of C–N,P,S
3.3. Preparation of Pd/C–N,P,S Catalysts
3.4. Electrocatalytic Activity Test
3.5. Catalysts Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Fernandez, P.S.; Lima, F.D.; Troiani, H.E.; Martins, M.E.; Arenillas, A.; Maia, G.; Camara, G.A. Remarkable electrochemical stability of one-step synthesized Pd nanoparticles supported on grapheme and multi-walled carbon nanotubes. Nano Energy 2014, 9, 142–151. [Google Scholar] [CrossRef]
- Dutta, A.; Ouyang, J.Y. Ternary NiAuPt nanoparticles on reduced graphene oxide as catalysts toward the electrochemical oxidation reaction of ethanol. ACS Catal. 2015, 5, 1371–1380. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Sergent, N.; Berthome, G.; Chatenet, M. Huge instability of Pt/C catalysts in alkaline medium. ACS Catal. 2015, 5, 4819–4824. [Google Scholar] [CrossRef]
- Sneed, B.T.; Young, A.P.; Jalalpoor, D.; Golden, M.C.; Mao, S.; Jiang, Y.; Wang, Y.; Tsung, C.K. Shaped Pd-Ni-Pt Core-Sandwich-Shell Nanoparticles: Influence of Ni Sandwich Layers on Catalytic Electrooxidation. ACS Nano 2014, 7, 7239–7250. [Google Scholar] [CrossRef]
- Wang, E.D.; Xu, J.B.; Zhao, T.S. Density Functional Theory Studies of the Structure Sensitivity of Ethanol Oxidation on Palladium Surfaces. J. Phys. Chem. C 2010, 114, 10489–10497. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, F.; Yang, Y.; Cai, W. Carbon supported Pd-Ni-P nanoalloy as an efficient catalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2013, 243, 369–373. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S.W. Highly Active Graphene-Supported NixPd100–x Binary Alloyed Catalysts for Electro-Oxidation of Ethanol in an Alkaline Media. ACS Catal. 2014, 4, 1830–1837. [Google Scholar] [CrossRef]
- Wang, L.; Lavacchi, A.; Bevilacqua, M.; Bellini, M.; Fornasiero, P.; Filippi, J.; Innocenti, M.; Marchionni, A.; Miller, H.A.; Vizza, F. Energy efficiency of alkaline direct ethanol fuel cells employing nanostructured palladium electrocatalysts. ChemCatChem 2015, 7, 2214–2221. [Google Scholar] [CrossRef]
- Singh, R.N.; Awasthi, R. Graphene support for enhanced electrocatalytic activity of Pd for alcohol oxidation. Catal. Sci. Technol. 2011, 1, 778–783. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Q.; Fan, J.C.; Liao, K.X.; Xie, J.W.; Liu, P.; Chen, Y.H.; Min, Y.L.; Xu, Q.J. Highly dispersed palladium nanoparticles on poly (N1, N3-dimethylbenzimidazolium) iodide-functionalized multiwalled carbon nanotubes for ethanol oxidation in alkaline solution. RSC Adv. 2016, 6, 102582–102594. [Google Scholar] [CrossRef]
- Chang, J.F.; Feng, L.G.; Liu, C.P.; Xing, W.; Hu, X.L. An effective Pd–Ni2P/C anode catalyst for direct formic acid fuel cells. Angew. Chem. Int. Ed. 2014, 53, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ostrom, C. Palladium-based nanomaterials: Synthesis and electrochemical applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Meng, H.; Shi, L. Synthesis of mesoporous hollow carbon hemispheres as highly efficient Pd electrocatalyst support for ethanol oxidation. Electrochem. Commun. 2010, 12, 689–692. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, L.; Wang, H. Hollow graphitized carbon nanocage supported Pd catalyst with excellent electrocatalytic activity for ethanol oxidation. ACS Sustain. Chem. Eng. 2018, 6, 7507–7514. [Google Scholar] [CrossRef]
- Kim, A.; Bae, H.S.; Park, J.C.; Song, H. Surfactant-free pd@pSiO2 yolk-shell nanocatalyst for selective oxidation of primary alcohols to aldehydes. New J. Chem. 2015, 39, 8153–8157. [Google Scholar] [CrossRef]
- Jinwoo, K.; Aram, K.; Nallal, M.; Kang, P. PdO/ZnO@mSiO2 hybrid nanocatalyst for reduction of nitroarenes. Catalysts 2018, 8, 280. [Google Scholar]
- Aram, K.; Nallal, M.; Chohye, Y.; Sang, J.; Kang, P. MOF-derived Cu@Cu2O nanocatalyst for oxygen reduction reaction and cycloaddition reaction. Nanomaterials 2018, 8, 138. [Google Scholar]
- Choi, C.H.; Park, S.H.; Woo, S.I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 2012, 8, 7084–7091. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jiang, Z.J.; Maiyalagan, T.; Manthiram, A. Cobalt oxide-coated N- and B-doped graphene hollow spheres as a bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 5877–5889. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; He, S.; Tjiu, W.W.; Pan, J.; Xia, Y.; Liu, T. Nitrogen-doped graphene nanoribbons as efficient metal-free electrocatalysts for oxygen reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Zhang, T.; Silva, T.L.; Almeida, V.C.; Asefa, T. Bone char-derived metal-free N- and S-co-doped nanoporous carbon and its efficient electrocatalytic activity for hydrazine oxidation. Appl. Catal. B 2018, 225, 30–39. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Park, S.H.; Woo, S.I. Additional doping of phosphorus and or sulfur into nitrogen-doped carbon for efficient oxygen reduction reaction in acidic media. Phys. Chem. Chem. Phys. 2013, 15, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fan, J.C.; Min, Y.L.; Wu, T.; Lin, Y.; Xu, Q.J. B, N-codoped graphene nanoribbons supported Pd nanoparticles for ethanol electrooxidation enhancement. J. Mater. Chem. A 2016, 4, 4929–4933. [Google Scholar] [CrossRef]
- Sanchez, M.L.; Primo, A.; Garcia, H. P-doped graphene obtained by pyrolysis of modified alginate as a photocatalyst for hydrogen generation from water–methanol mixtures. Angew. Chem. 2013, 52, 11813–11816. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Park, S.H.; Woo, S.I. N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694–3699. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.; Zhang, L.; Liang, H.W.; Chen, P.; Yu, S.H. N-, P- and Fe-tridoped nanoporous carbon derived from plant biomass: An excellent oxygen reduction electrocatalyst for zinc-air battery. J. Mater. Chem. A 2016, 4, 8602–8609. [Google Scholar]
- Zhang, G.G.; Zang, S.H.; Wang, X.C. Layered Co(OH)2 deposited polymeric carbon nitrides for photocatalytic water oxidation. ACS Catal. 2015, 5, 941–947. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Nasir, M.; Yin, H.; Liu, F.; Hou, Y.L. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Preparation of carbon supported Pd–P catalyst with high content of element phosphorus and its electrocatalytic performance for formic acid oxidation. Electrochem. Commun. 2010, 12, 492–495. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.X.; Chandra, S.T.; Ma, Z.Y.; Huang, H.J.; Zhang, J.F.; Lu, Z.Y.; Huang, W.; Wu, Y.P. Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Interfaces 2016, 8, 10858–10865. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.W.; Xu, Q.; Chen, J.F.; Chen, Z.M.; Huang, X.B.; Tang, X.Z. Controlled fabrication of uniform hollow core porous shell carbon spheres by the pyrolysis of core/shell polystyrene/cross-linked polyphosphazene composites. Chem. Commun. 2010, 46, 6563–6565. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Kim, Y.; Wi, D.H.; Lee, S.; Lee, S.-U.; Lee, Y.W.; Choi, S.-I.; Han, S.W. Ultrathin Free-Standing Ternary-Alloy Nanosheets. Angew. Chem. Int. Ed. 2016, 55, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, P.; Li, Y.; Xia, H.; Wang, D.; Tao, X. Synthesis of open-mouthed, yolk–shell Au@AgPd nanoparticles with access to interior surfaces for enhanced electrocatalysis. Chem. Sci. 2015, 6, 4350–4357. [Google Scholar] [CrossRef]
- Ye, S.-H.; Feng, J.-X.; Li, G.-R. Pd Nanoparticle/CoP Nanosheet Hybrids: Highly Electroactive and Durable Catalysts for Ethanol Electrooxidation. ACS Catal. 2016, 6, 7962–7969. [Google Scholar] [CrossRef]
- Zhang, K.; Bin, D.; Yang, B.; Wang, C.; Ren, F.; Du, Y. Ru-assisted synthesis of Pd/Ru nanodendrites with high activity for ethanol electrooxidation. Nanoscale 2015, 7, 12445–12451. [Google Scholar] [CrossRef]
- Wang, A.-L.; He, X.-J.; Lu, X.-F.; Xu, H.; Tong, Y.-X.; Li, G.-R. Palladium–Cobalt Nanotube Arrays Supported on Carbon Fiber Cloth as High-Performance Flexible Electrocatalysts for Ethanol Oxidation. Angew. Chem. Int. Ed. 2015, 54, 3669–3673. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.; Tang, D.; Zhang, T.; Wang, Y. Ethanol oxidation on Pd/C promoted with CaSiO3 in alkaline medium. Electrochim. Acta 2015, 158, 18–23. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.; Huang, G.; Yang, N.; Zhang, L.; Wang, H.; Liu, Y.; Wang, W.; Gao, J. A green method to prepare Pd–Ag nanoparticles supported on reduced graphene oxide and their electrochemical catalysis of methanol and ethanol oxidation. J. Power Sources 2014, 263, 13–21. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Lu, G.Q.; Kenis, P.J.A.; Wieckowski, A. Electrooxidation of adsorbed CO on Pt(111) and Pt(111)/Ru in alkaline media and comparison with results from acidic media. J. Electroanal. Chem. 2004, 568, 215–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Tian, J.; Wang, F.; Zhan, L. A facile and novel approach toward synthetic polypyrrole oligomers functionalization of multi-walled carbon nanotubes as PtRu catalyst support for methanol electro-oxidation. J. Power Sources 2010, 195, 4634–4640. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Lin, Y.; Fan, J.; Li, Q.; Shi, P.; Xu, Q.; Min, Y. Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts 2019, 9, 114. https://doi.org/10.3390/catal9020114
Yu K, Lin Y, Fan J, Li Q, Shi P, Xu Q, Min Y. Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts. 2019; 9(2):114. https://doi.org/10.3390/catal9020114
Chicago/Turabian StyleYu, Ke, Yan Lin, Jinchen Fan, Qiaoxia Li, Penghui Shi, Qunjie Xu, and Yulin Min. 2019. "Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction" Catalysts 9, no. 2: 114. https://doi.org/10.3390/catal9020114
APA StyleYu, K., Lin, Y., Fan, J., Li, Q., Shi, P., Xu, Q., & Min, Y. (2019). Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts, 9(2), 114. https://doi.org/10.3390/catal9020114