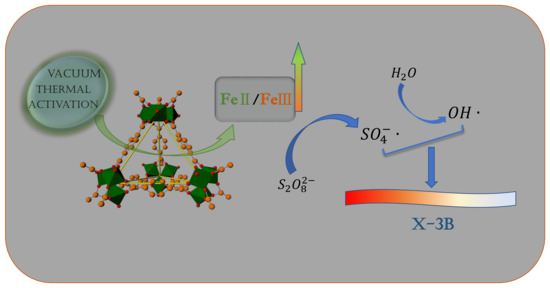
Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center
Abstract
:1. Introduction
2. Results and Discussion
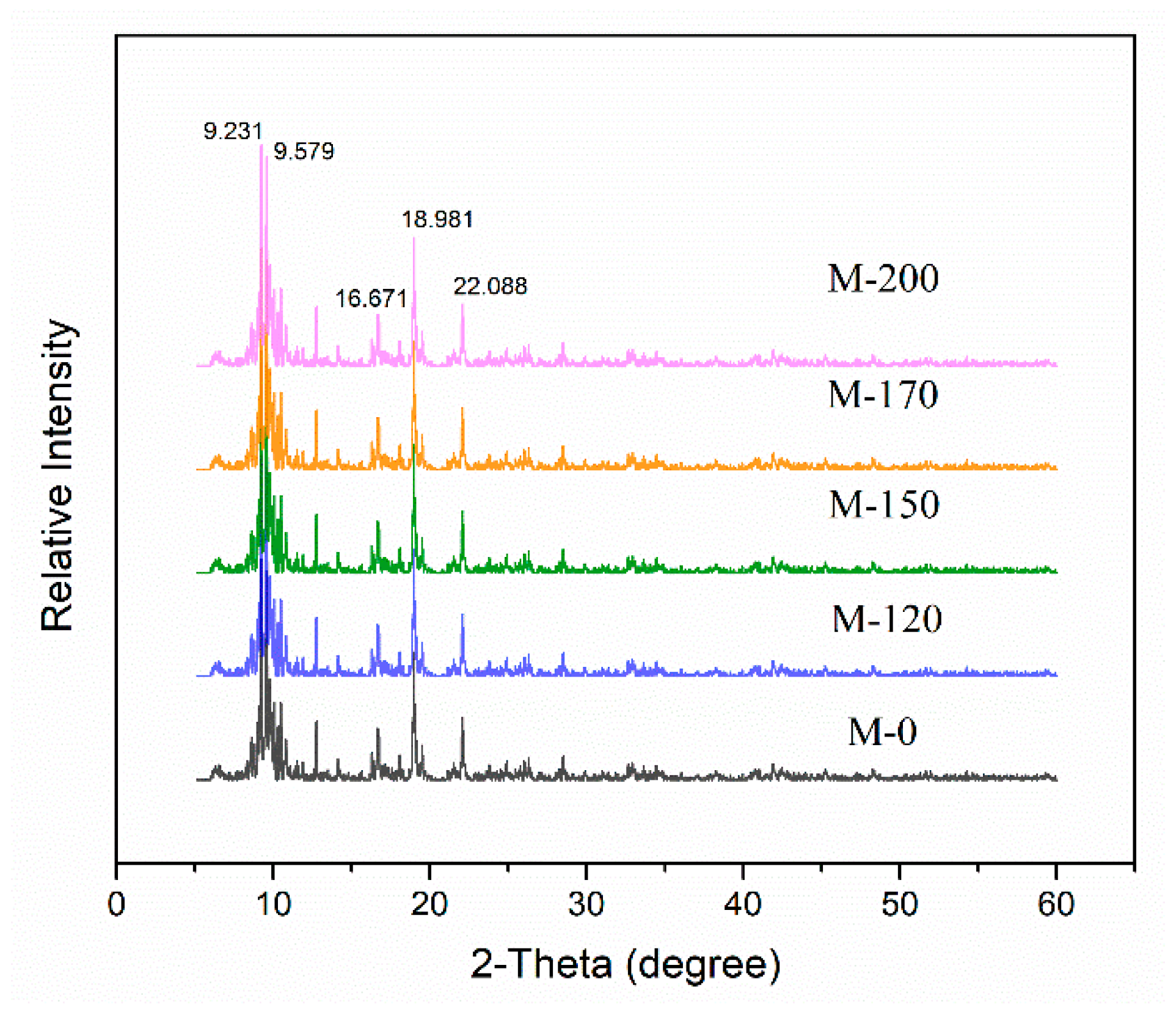
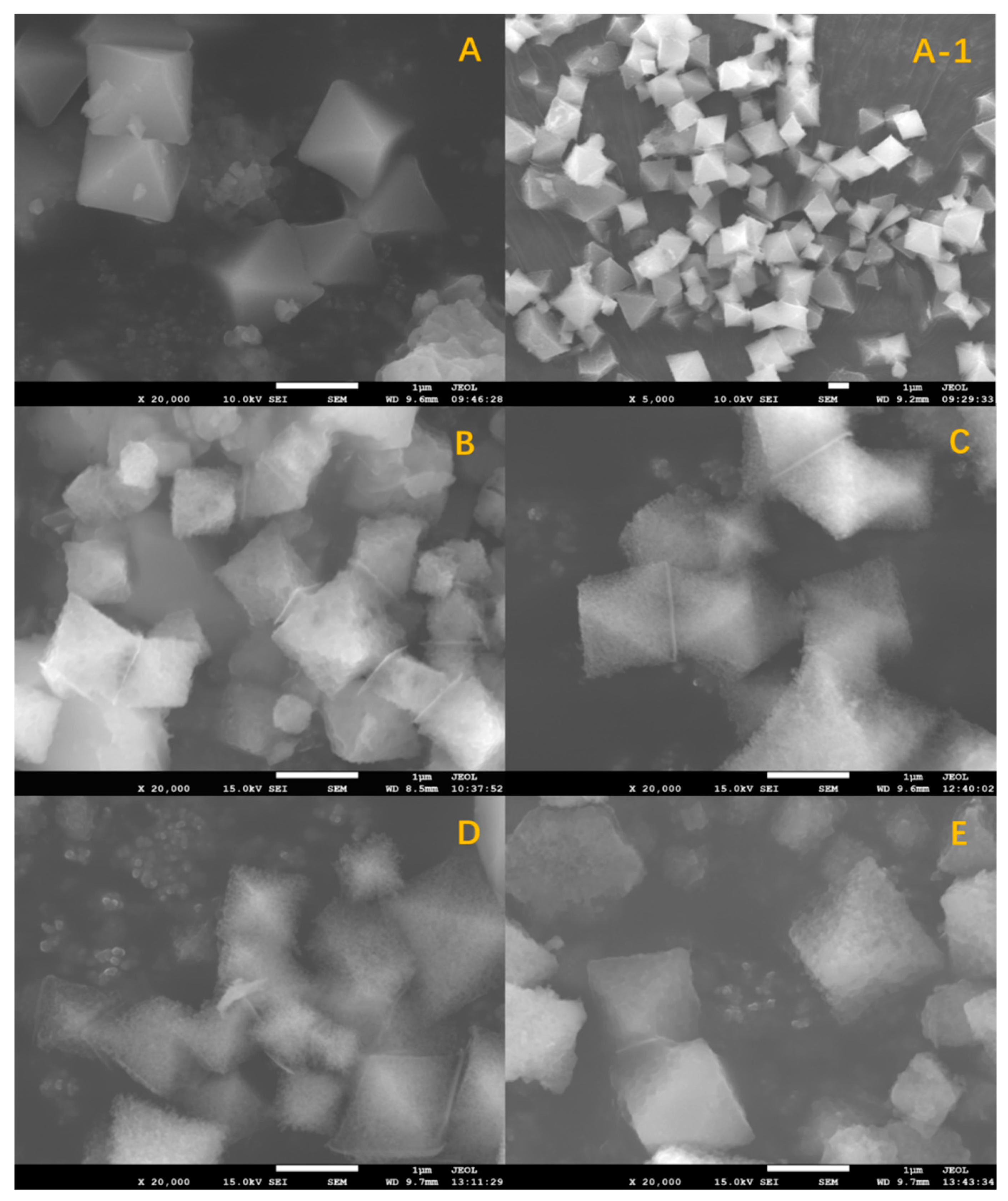
2.1. Characterization
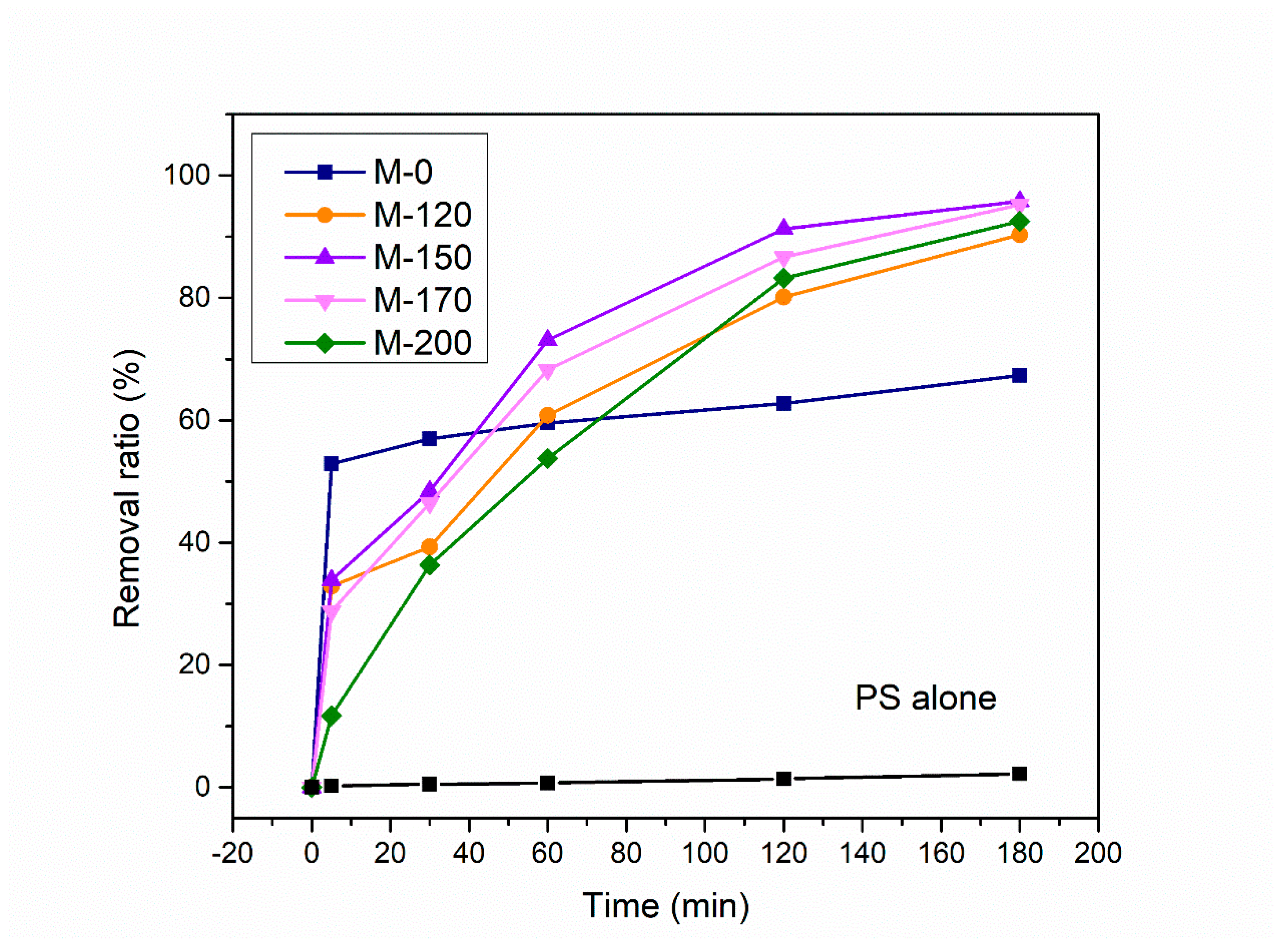
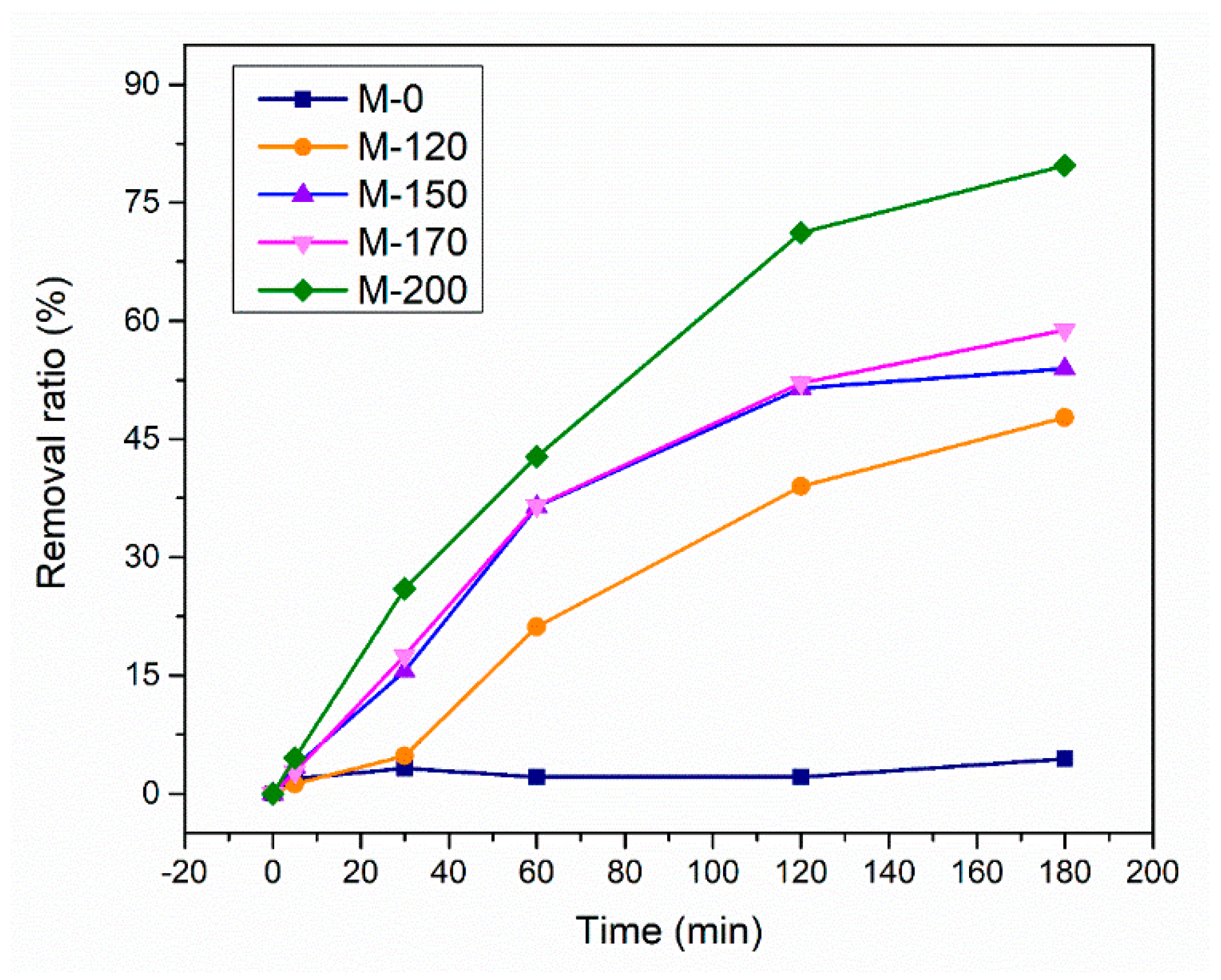
2.2. Catalytic Performance
2.3. Mechanism
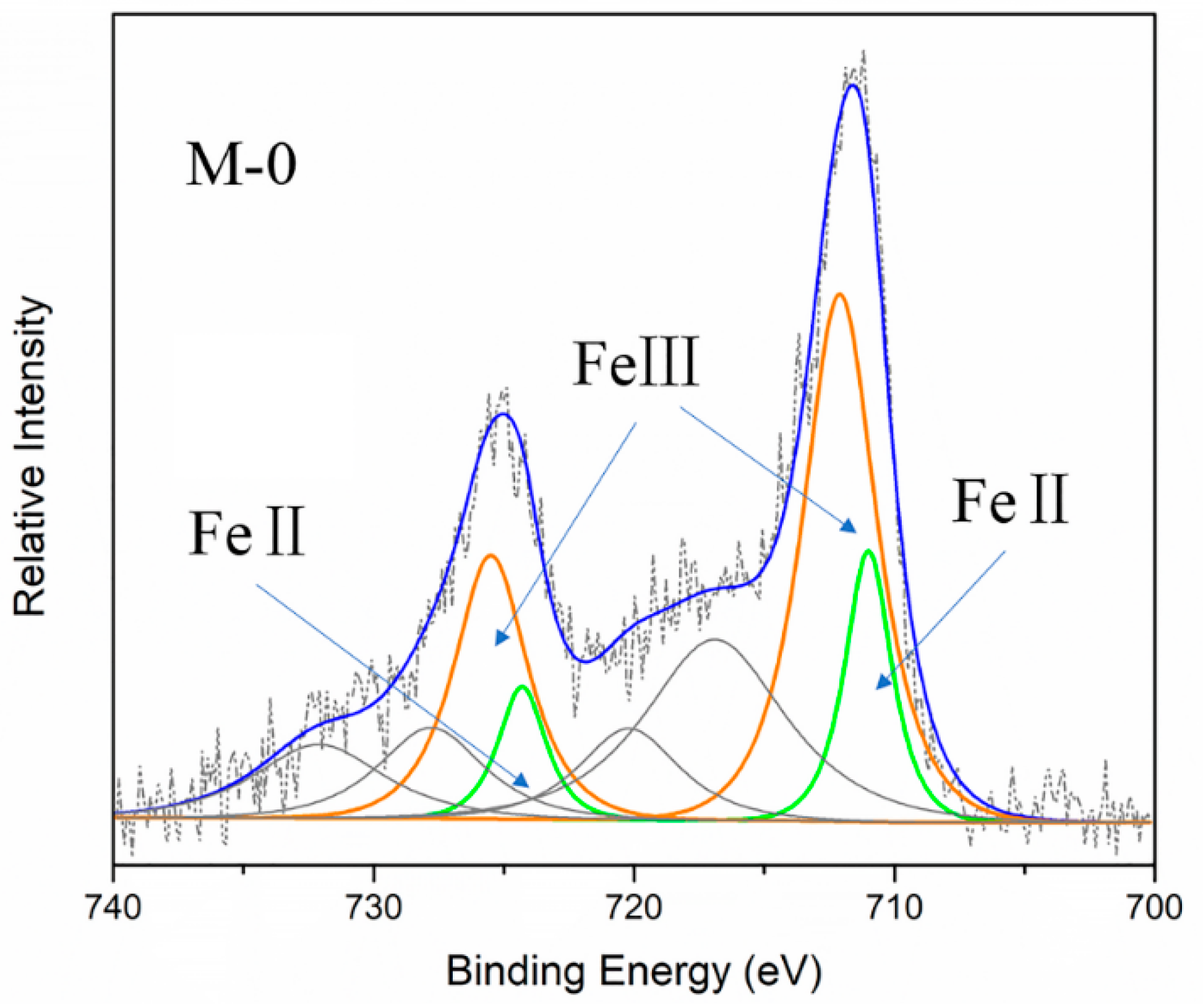
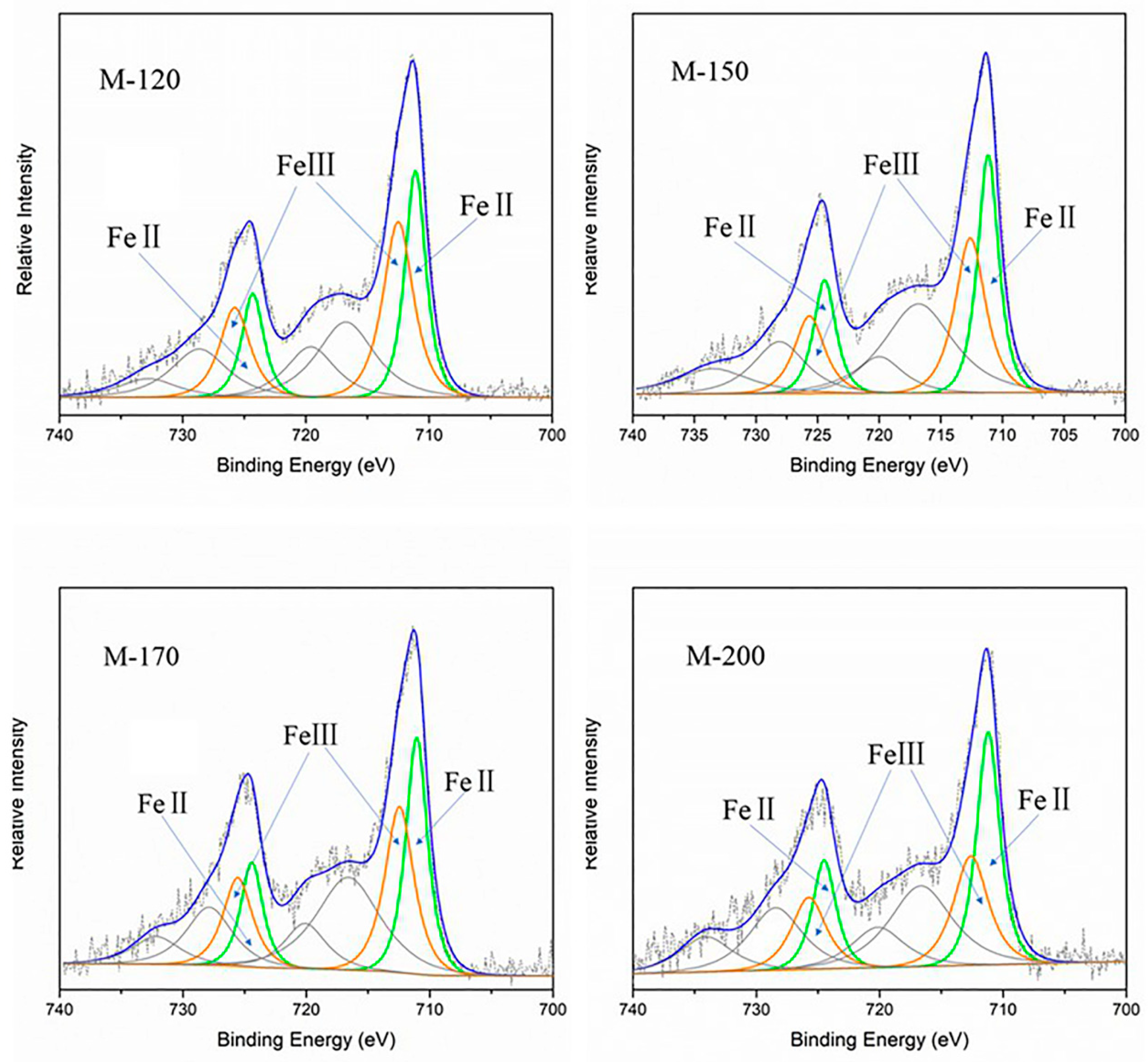
2.3.1. Mixed-Valence State of Iron in MIL-101(Fe)
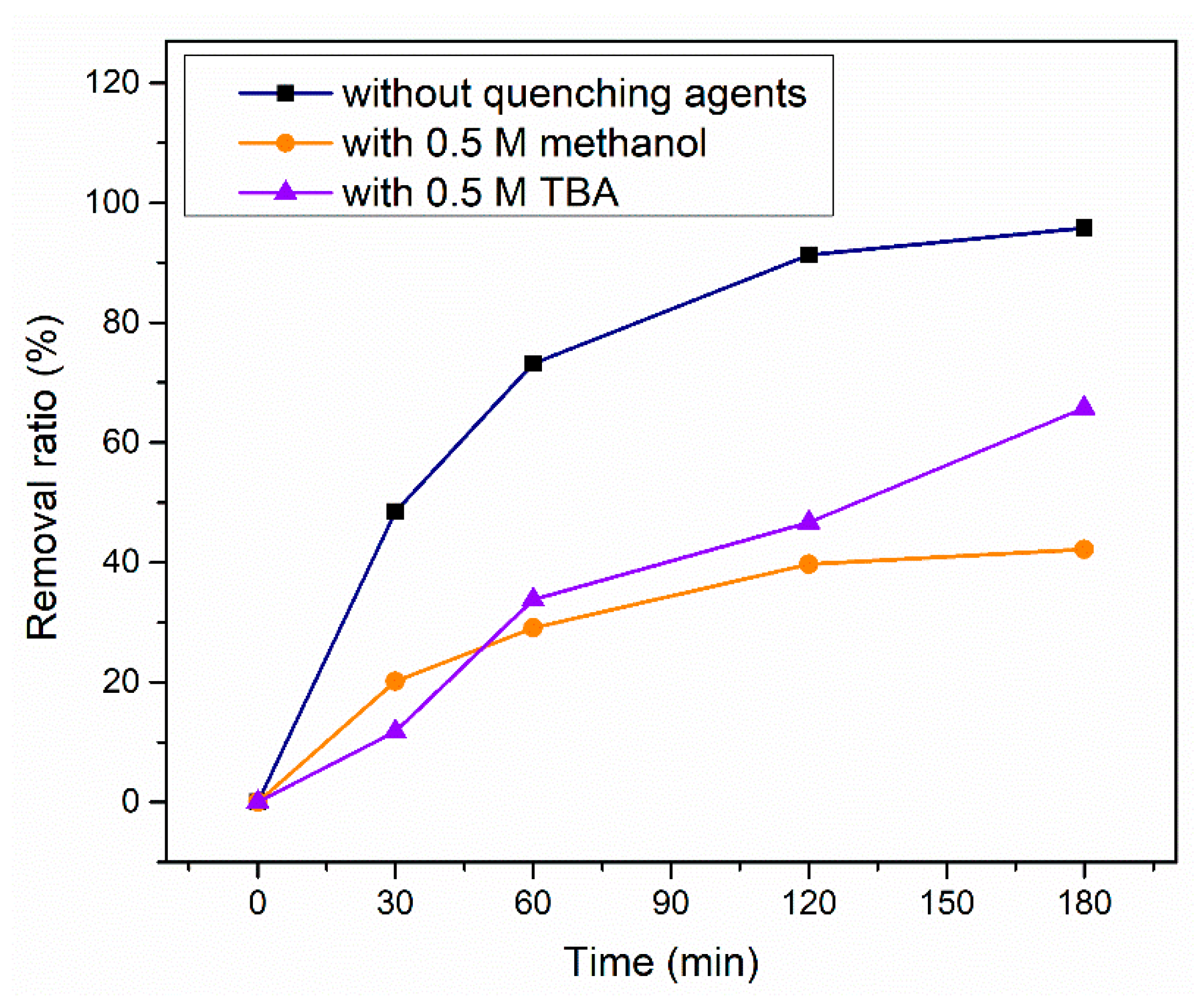
2.3.2. Quenching Experiments and EPR (electron paramagnetic resonance) tests
2.3.3. Mechanism of Activation of PS by MIL-101(Fe)
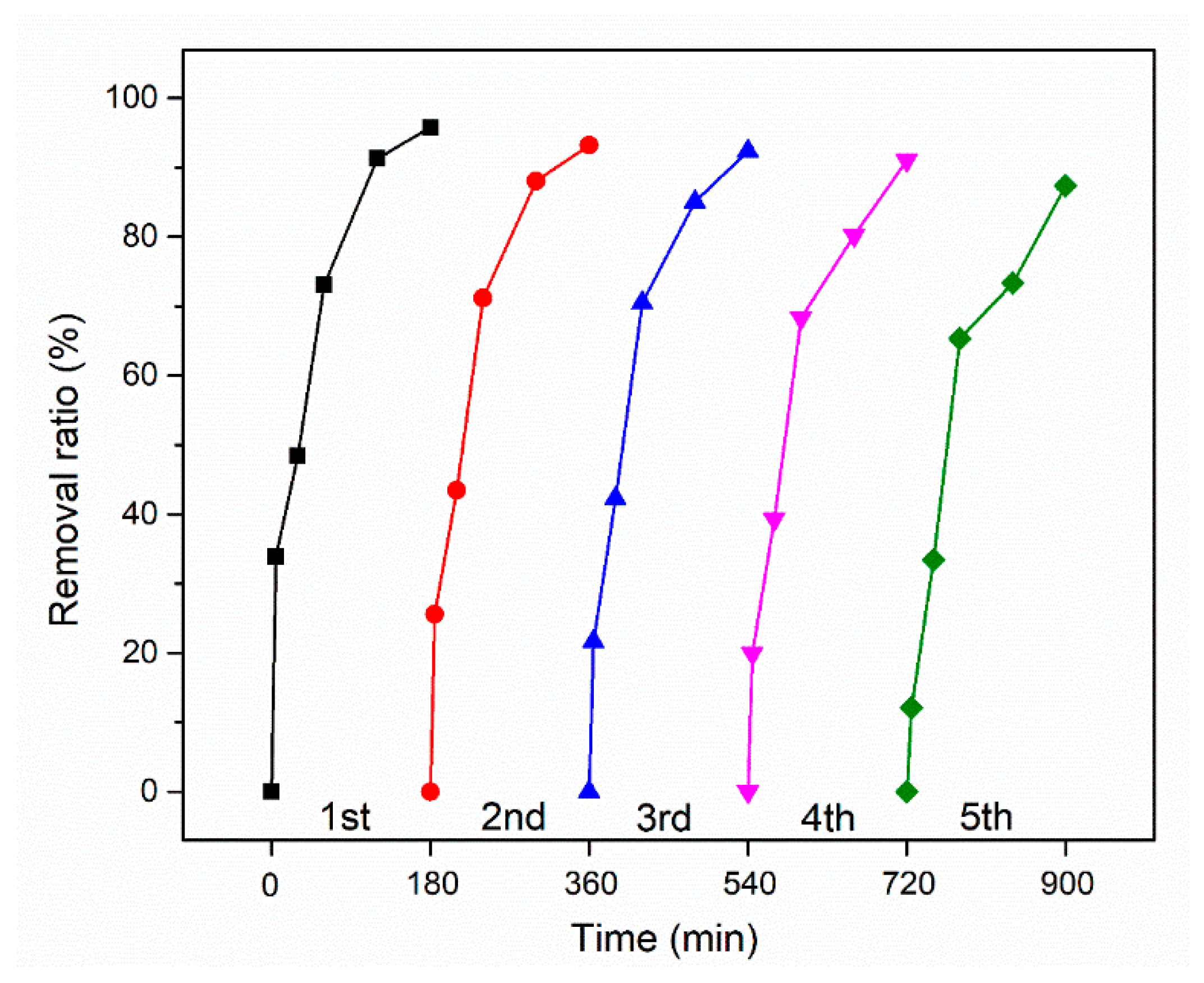
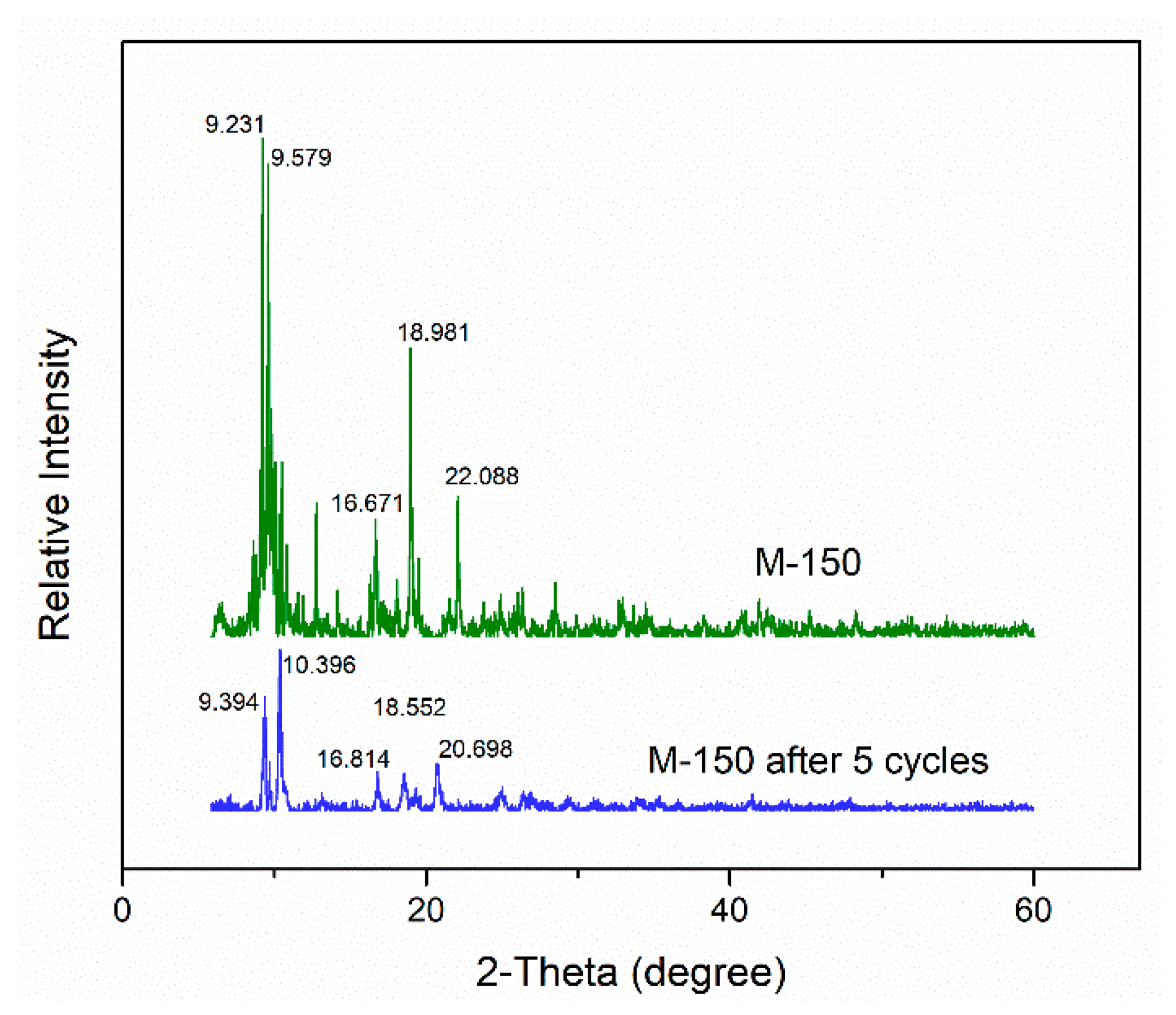
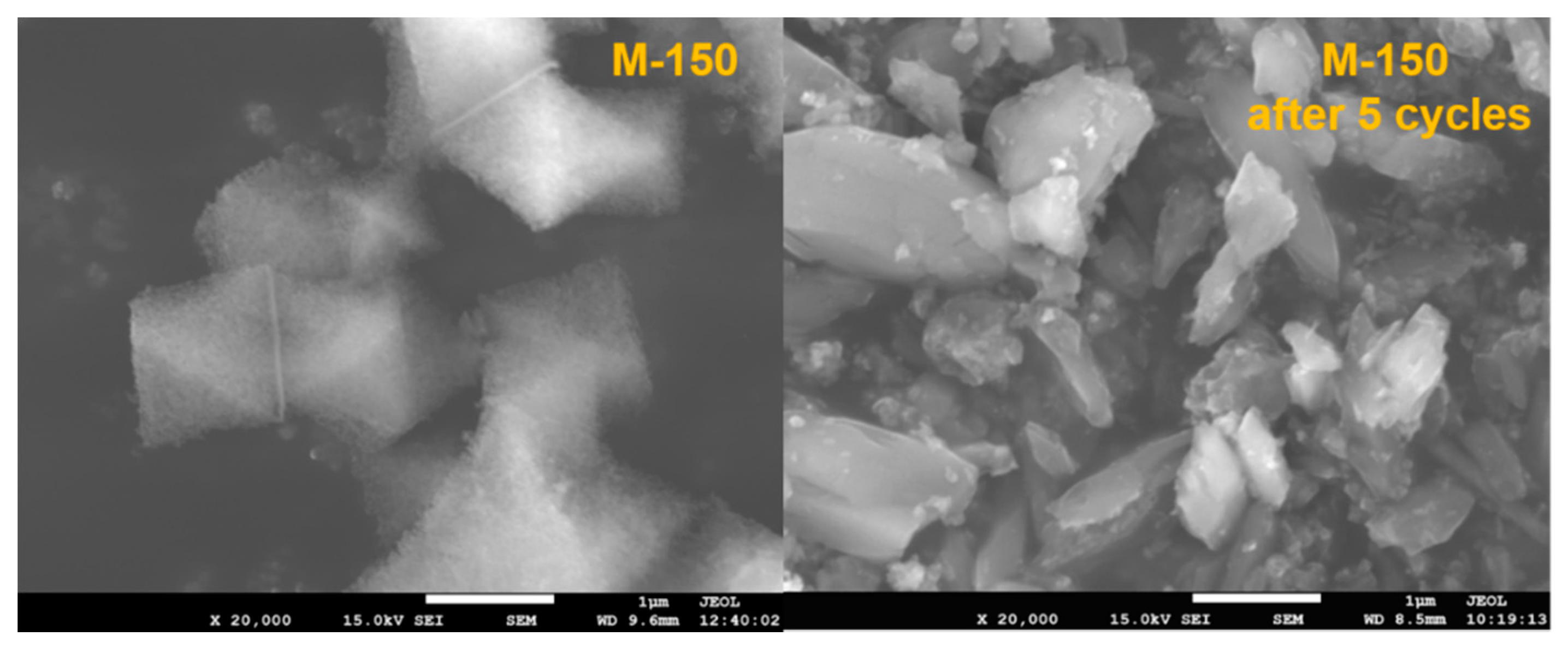
2.4. Stability and Reusability
3. Materials and Methods
3.1. Chemical Reagents
3.2. Preparation and Characterization of Catalysts
3.3. Catalytic and Adsorption Experiments
3.4. EPR Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Neamtu, M.; Siminiceanu, I.; Yediler, A.; Kettrup, A. Kinetics of decolorization and mineralization of reactive azo dyes in aqueous solution by the UV/H2O2 oxidation. Dye. Pigment. 2002, 53, 93–99. [Google Scholar] [CrossRef]
- Hu, C.; Hu, X.; Wang, L.; Qu, J.; Wang, A. Visible-light-induced photocatalytic degradation of azo dyes in aqueous AgI/TiO2 dispersion. Environ. Sci. Technol. 2006, 40, 7903–7907. [Google Scholar] [CrossRef] [PubMed]
- Vinodgopal, K.; Wynkoop, D.E.; Kamat, P.V. Environmental Photochemistry on Semiconductor Surfaces: Photosensitized Degradation of a Textile Azo Dye, Acid Orange 7, on TiO2 Particles Using Visible Light. Environ. Sci. Technol. 1996, 30, 1660–1666. [Google Scholar] [CrossRef]
- de Lima, R.O.A.; Bazo, A.P.; Salvadori, D.M.F.; Rech, C.M.; de Palma Oliveira, D.; de Aragão Umbuzeiro, G. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 626, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 2005, 58, 1409–1414. [Google Scholar] [CrossRef]
- Peternel, I.; Kusic, H.; Marin, V.; Koprivanac, N. UV-assisted persulfate oxidation: The influence of cation type in the persulfate salt on the degradation kinetics of an azo dye pollutant. React. Kinet. Mech. Catal. 2013, 108, 17–39. [Google Scholar] [CrossRef]
- Li, J.J.; Wu, L.; Liu, P.F.; Liu, L.J.; Zhang, B. Effect of Annealing Temperature of Fe78Si9B13 Amorphous Ribbons for Activation of Persulfate on Azo Dye Degradation. Adv. Funct. Mater. 2018, 285–292. [Google Scholar]
- Ma, Y.H.; Chen, F.; Yang, Q.; Zhong, Y.; Shu, X.Y.; Yao, F.B.; Xie, T.; Li, X.M.; Wang, D.B.; Zeng, G.M. Sulfate radical induced degradation of Methyl Violet azo dye with CuFe layered doubled hydroxide as heterogeneous photoactivator of persulfate. J. Environ. Manag. 2018, 227, 406–414. [Google Scholar] [CrossRef]
- Chen, J.B.; Hong, W.; Huang, T.Y.; Zhang, L.M.; Li, W.W.; Wang, Y. Activated carbon fiber for heterogeneous activation of persulfate: Implication for the decolorization of azo dye. Environ. Sci. Pollut. Res. 2016, 23, 18564–18574. [Google Scholar] [CrossRef]
- Zhu, J.P.; Lin, Y.L.; Zhang, T.Y.; Cao, T.C.; Xu, B.; Pan, Y.; Zhang, X.T.; Gao, N.Y. Modelling of iohexol degradation in a Fe(II)-activated persulfate system. Chem. Eng. J. 2019, 367, 86–93. [Google Scholar] [CrossRef]
- Ling, L.; Li, Z.B.; Fang, J.Y.; Shang, C. Control of bromate formation in UV/peroxymonosulfate, UV/persulfate and Co/peroxymonosulfate processes by ammonia, chlorine-ammonia, and ammonia-chlorine processes. Abstr. Papers Am. Chem. Soc. 2015, 250, 26. [Google Scholar]
- Felix-Navarro, R.M.; Lin, S.W.; Zizumbo-Lopez, A.; Perez-Sicairos, S.; Reynoso-Soto, E.A.; Espinoza-Gomez, J.H. 1,4-Dioxane Degradation Using Persulfate Ion and Ag(I) Ion. J. Mex. Chem. Soc. 2013, 57, 127–132. [Google Scholar] [CrossRef]
- Wang, J.-L.; Wang, C.; Lin, W. Metal–Organic Frameworks for Light Harvesting and Photocatalysis. ACS Catal. 2012, 2, 2630–2640. [Google Scholar] [CrossRef]
- Li, D.; Chen, D.; Yao, Y.; Lin, J.; Gong, F.; Wang, L.; Luo, L.; Huang, Z.; Zhang, L. Strong enhancement of dye removal through addition of sulfite to persulfate activated by a supported ferric citrate catalyst. Chem. Eng. J. 2016, 288, 806–812. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Chen, L.; Li, X.; Guan, Y.; Xie, P.; Pan, C. Rapid Acceleration of Ferrous Iron/Peroxymonosulfate Oxidation of Organic Pollutants by Promoting Fe(III)/Fe(II) Cycle with Hydroxylamine. Environ. Sci. Technol. 2013, 47, 11685–11691. [Google Scholar] [CrossRef]
- Sun, L.; Yao, Y.; Wang, L.; Mao, Y.; Huang, Z.; Yao, D.; Lu, W.; Chen, W. Efficient removal of dyes using activated carbon fibers coupled with 8-hydroxyquinoline ferric as a reusable Fenton-like catalyst. Chem. Eng. J. 2014, 240, 413–419. [Google Scholar] [CrossRef]
- Weng, C.-H.; Tao, H. Highly efficient persulfate oxidation process activated with Fe0 aggregate for decolorization of reactive azo dye Remazol Golden Yellow. Arab. J. Chem. 2018, 11, 1292–1300. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Ahn, Y.-Y.; Bae, H.; Kim, H.-I.; Kim, S.-H.; Kim, J.-H.; Lee, S.-G.; Lee, J. Surface-loaded metal nanoparticles for peroxymonosulfate activation: Efficiency and mechanism reconnaissance. Appl. Catal. B Environ. 2019, 241, 561–569. [Google Scholar] [CrossRef]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Li, A.; Qing, Z.; Li, Y. Oxidative degradation of Orange G by persulfate activated with iron-immobilized resin chars. J. Ind. Eng. Chem. 2015, 25, 308–313. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Zalomaeva, O.V.; Skobelev, I.Y.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Metal-organic frameworks of the MIL-101 family as heterogeneous single-site catalysts. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2017–2034. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-Based MOFs for Photocatalytic CO2 Reduction: Role of Coordination Unsaturated Sites and Dual Excitation Pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F. Crystallized Frameworks with Giant Pores: Are There Limits to the Possible? Acc. Chem. Res. 2005, 38, 217–225. [Google Scholar] [CrossRef]
- Gao, Y.W.; Li, S.M.; Li, Y.X.; Yao, L.Y.; Zhang, H. Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate. Appl. Catal. B Environ. 2017, 202, 165–174. [Google Scholar] [CrossRef]
- Wang, J.M.; Wan, J.Q.; Ma, Y.W.; Wang, Y.; Pu, M.J.; Guan, Z.Y. Metal-organic frameworks MIL-88A with suitable synthesis conditions and optimal dosage for effective catalytic degradation of Orange G through persulfate activation. RSC Adv. 2016, 6, 112502–112511. [Google Scholar] [CrossRef]
- Li, X.H.; Guo, W.L.; Liu, Z.H.; Wang, R.Q.; Liu, H. Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation. Appl. Surf. Sci. 2016, 369, 130–136. [Google Scholar] [CrossRef]
- Yue, X.X.; Guo, W.L.; Li, X.H.; Zhou, H.H.; Wang, R.Q. Core-shell Fe3O4@MIL-101(Fe) composites as heterogeneous catalysts of persulfate activation for the removal of Acid Orange 7. Environ. Sci. Pollut. Res. 2016, 23, 15218–15226. [Google Scholar] [CrossRef]
- Zhang, M.W.; Yang, M.T.; Tong, S.P.; Lin, K.Y.A. Ferrocene-modified iron-based metal-organic frameworks as an enhanced catalyst for activating oxone to degrade pollutants in water. Chemosphere 2018, 213, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, W.L.; Li, X.H. Activation of persulfates by ferrocene-MIL-101(Fe) heterogeneous catalyst for degradation of bisphenol A. RSC Adv. 2018, 8, 36477–36483. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, B.; Zhang, H.; Zhao, X. A g-C3N4/MIL-101(Fe) heterostructure composite for highly efficient BPA degradation with persulfate under visible light irradiation. J. Mater. Chem. A 2018, 6, 23703–23711. [Google Scholar] [CrossRef]
- Wuttke, S.; Bazin, P.; Vimont, A.; Serre, C.; Seo, Y.-K.; Hwang, Y.K.; Chang, J.-S.; Férey, G.; Daturi, M. Discovering the Active Sites for C3 Separation in MIL-100(Fe) by Using Operando IR Spectroscopy. Chem. Eur. J. 2012, 18, 11959–11967. [Google Scholar] [CrossRef]
- Aggarwal, H.; Bhatt, P.M.; Bezuidenhout, C.X.; Barbour, L.J. Direct Evidence for Single-Crystal to Single-Crystal Switching of Degree of Interpenetration in a Metal–Organic Framework. J. Am. Chem. Soc. 2014, 136, 3776–3779. [Google Scholar] [CrossRef]
- Yoon, J.W.; Seo, Y.-K.; Hwang, Y.K.; Chang, J.-S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled Reducibility of a Metal–Organic Framework with Coordinatively Unsaturated Sites for Preferential Gas Sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Skobelev, I.Y.; Ivanchikova, I.D.; Kovalenko, K.A.; Fedin, V.P.; Sorokin, A.B. Hydrocarbon oxidation over Fe- and Cr-containing metal-organic frameworks MIL-100 and MIL-101-a comparative study. Catal. Today 2014, 238, 54–61. [Google Scholar] [CrossRef]
- Xie, Q.; Li, Y.; Lv, Z.; Zhou, H.; Yang, X.; Chen, J.; Guo, H. Effective Adsorption and Removal of Phosphate from Aqueous Solutions and Eutrophic Water by Fe-based MOFs of MIL-101. Sci. Rep. 2017, 7, 3316. [Google Scholar] [CrossRef]
- Skobelev, I.Y.; Sorokin, A.B.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Solvent-free allylic oxidation of alkenes with O2 mediated by Fe- and Cr-MIL-101. J. Catal. 2013, 298, 61–69. [Google Scholar] [CrossRef]
- Lebedev, O.I.; Millange, F.; Serre, C.; van Tendeloo, G.; Férey, G. First Direct Imaging of Giant Pores of the Metal−Organic Framework MIL-101. Chem. Mater. 2005, 17, 6525–6527. [Google Scholar] [CrossRef]
- Pu, M.J.; Ma, Y.W.; Wan, J.Q.; Wang, Y.; Wang, J.M.; Brusseau, M.L. Activation performance and mechanism of a novel heterogeneous persulfate catalyst: Metal-organic frameworkMIL-53(Fe) with Fe-II/Fe-III mixed-valence coordinatively unsaturated iron center. Catal. Sci. Technol. 2017, 7, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.P.; Zhang, Y.T.; Li, R.Y.; Meng, W.D.; Song, Z.L.; Qi, F.; Xu, B.B.; Chu, W.; Yuan, D.H.; et al. Enhancement of Fe@porous carbon to be an efficient mediator for peroxymonosulfate activation for oxidation of organic contaminants: Incorporation NH2-group into structure of its MOF precursor. Chem. Eng. J. 2018, 354, 835–848. [Google Scholar] [CrossRef]
- Joo, S.H. Advanced Treatment of Reverse Osmosis Concentrate by Integrated Activated Carbon and Iron-Activated Persulfate Oxidation. Water Air Soil Pollut. 2014, 225, 2076. [Google Scholar] [CrossRef]
- Ortiz-Gomez, I.; Salinas-Castillo, A.; Garcia, A.G.; Alvarez-Bermejo, J.A.; de Orbe-Paya, I.; Rodriguez-Dieguez, A.; Capitan-Vallvey, L.F. Microfluidic paper-based device for colorimetric determination of glucose based on a metal-organic framework acting as peroxidase mimetic. Microchim. Acta 2018, 185, 47. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Pashow, K.M.L.; della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal−Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed]

 DMPO-OH· adduct;
DMPO-OH· adduct;  DMPO-SO4−· adduct; Experimental conditions: (PS) = 15 mmol L−1, M-150 dosage = 0.1 g L−1, (DMPO) = 0.9 mol L−1, (X-3B) = 100 mg L−1, T = 25 °C.
DMPO-SO4−· adduct; Experimental conditions: (PS) = 15 mmol L−1, M-150 dosage = 0.1 g L−1, (DMPO) = 0.9 mol L−1, (X-3B) = 100 mg L−1, T = 25 °C.
 DMPO-OH· adduct;
DMPO-OH· adduct;  DMPO-SO4−· adduct; Experimental conditions: (PS) = 15 mmol L−1, M-150 dosage = 0.1 g L−1, (DMPO) = 0.9 mol L−1, (X-3B) = 100 mg L−1, T = 25 °C.
DMPO-SO4−· adduct; Experimental conditions: (PS) = 15 mmol L−1, M-150 dosage = 0.1 g L−1, (DMPO) = 0.9 mol L−1, (X-3B) = 100 mg L−1, T = 25 °C.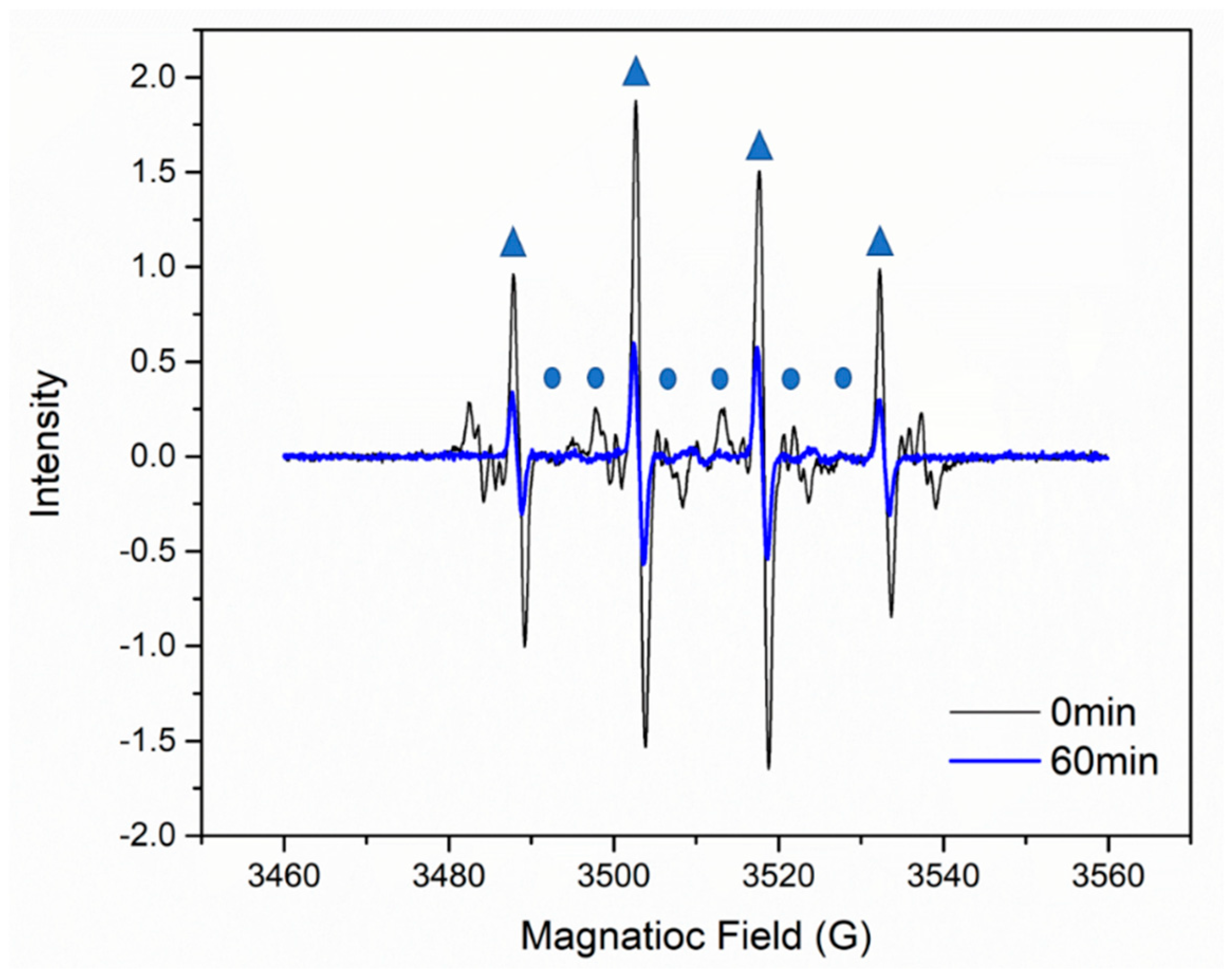
| Samples | Assignment | Peak position | FWHM | FeII/FeIII | |
|---|---|---|---|---|---|
| M-0 | Fe 2p3/2 | FeII | 710.99 | 2.1 | 0.327 |
| FeIII | 712.09 | 3.3 | |||
| Fe 2p1/2 | FeII | 724.29 | 2.15 | ||
| FeIII | 725.49 | 3.3 | |||
| M-120 | Fe 2p3/2 | FeII | 711.09 | 2.0 | 0.890 |
| FeIII | 712.49 | 2.9 | |||
| Fe 2p1/2 | FeII | 724.29 | 2.1 | ||
| FeIII | 725.75 | 2.9 | |||
| M-150 | Fe 2p3/2 | FeII | 711.15 | 2.0 | 1.064 |
| FeIII | 712.60 | 2.89 | |||
| Fe 2p1/2 | FeII | 724.34 | 2.15 | ||
| FeIII | 725.66 | 2.89 | |||
| M-170 | Fe 2p3/2 | FeII | 711.05 | 2.08 | 1.070 |
| FeIII | 712.45 | 2.78 | |||
| Fe 2p1/2 | FeII | 724.42 | 2.1 | ||
| FeIII | 725.55 | 2.8 | |||
| M-200 | Fe 2p3/2 | FeII | 711.19 | 2.2 | 1.417 |
| FeIII | 712.59 | 3.3 | |||
| Fe 2p1/2 | FeII | 724.49 | 2.25 | ||
| FeIII | 725.73 | 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zeng, Z.; Huang, Z.; Cui, Y. Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center. Catalysts 2019, 9, 906. https://doi.org/10.3390/catal9110906
Yang J, Zeng Z, Huang Z, Cui Y. Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center. Catalysts. 2019; 9(11):906. https://doi.org/10.3390/catal9110906
Chicago/Turabian StyleYang, Jieyang, Zequan Zeng, Zhanggen Huang, and Yan Cui. 2019. "Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center" Catalysts 9, no. 11: 906. https://doi.org/10.3390/catal9110906
APA StyleYang, J., Zeng, Z., Huang, Z., & Cui, Y. (2019). Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center. Catalysts, 9(11), 906. https://doi.org/10.3390/catal9110906