Effect of Shale Ash-Based Catalyst on the Pyrolysis of Fushun Oil Shale
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. OS Pyrolysis
2.3. Analytical Methods
3. Results and Discussion
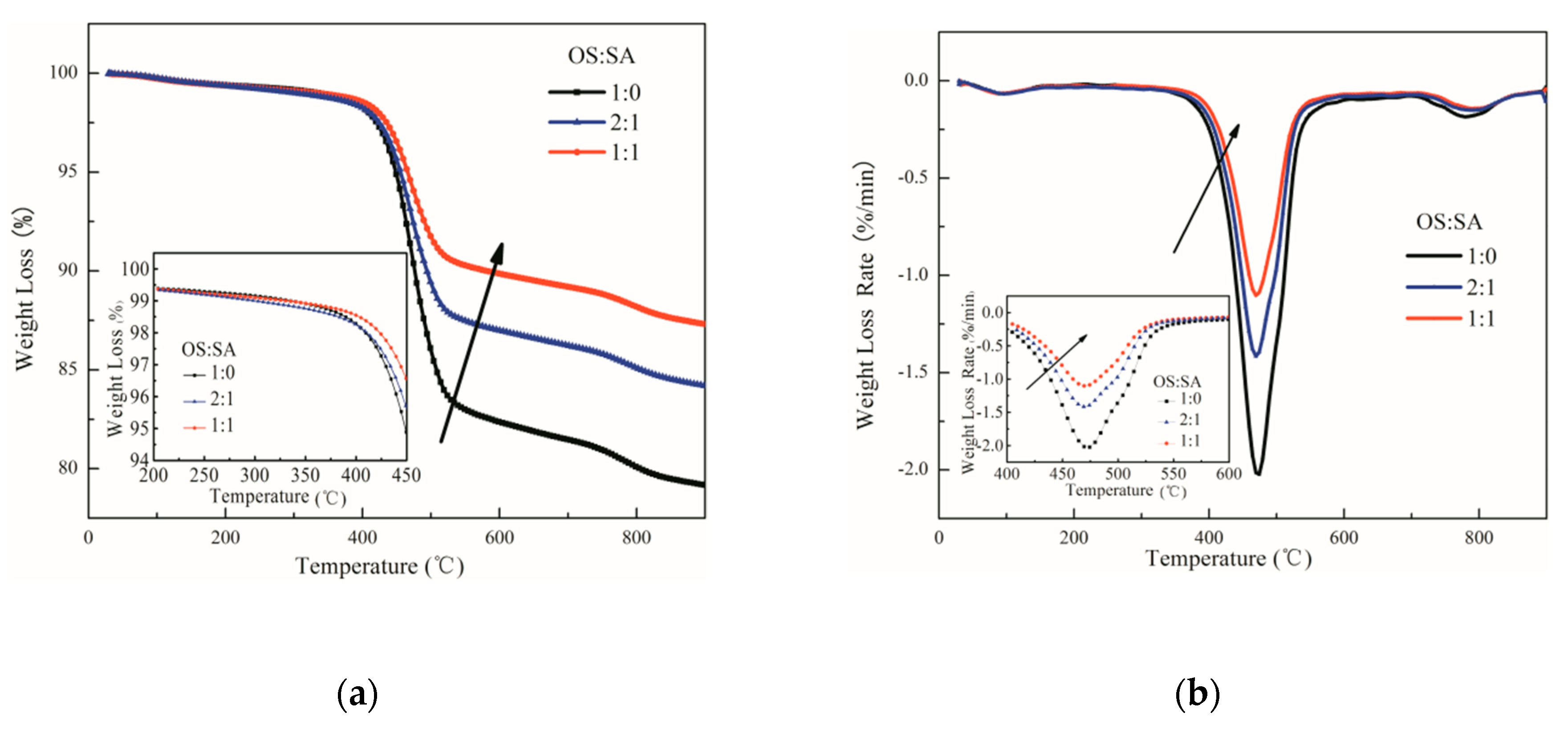
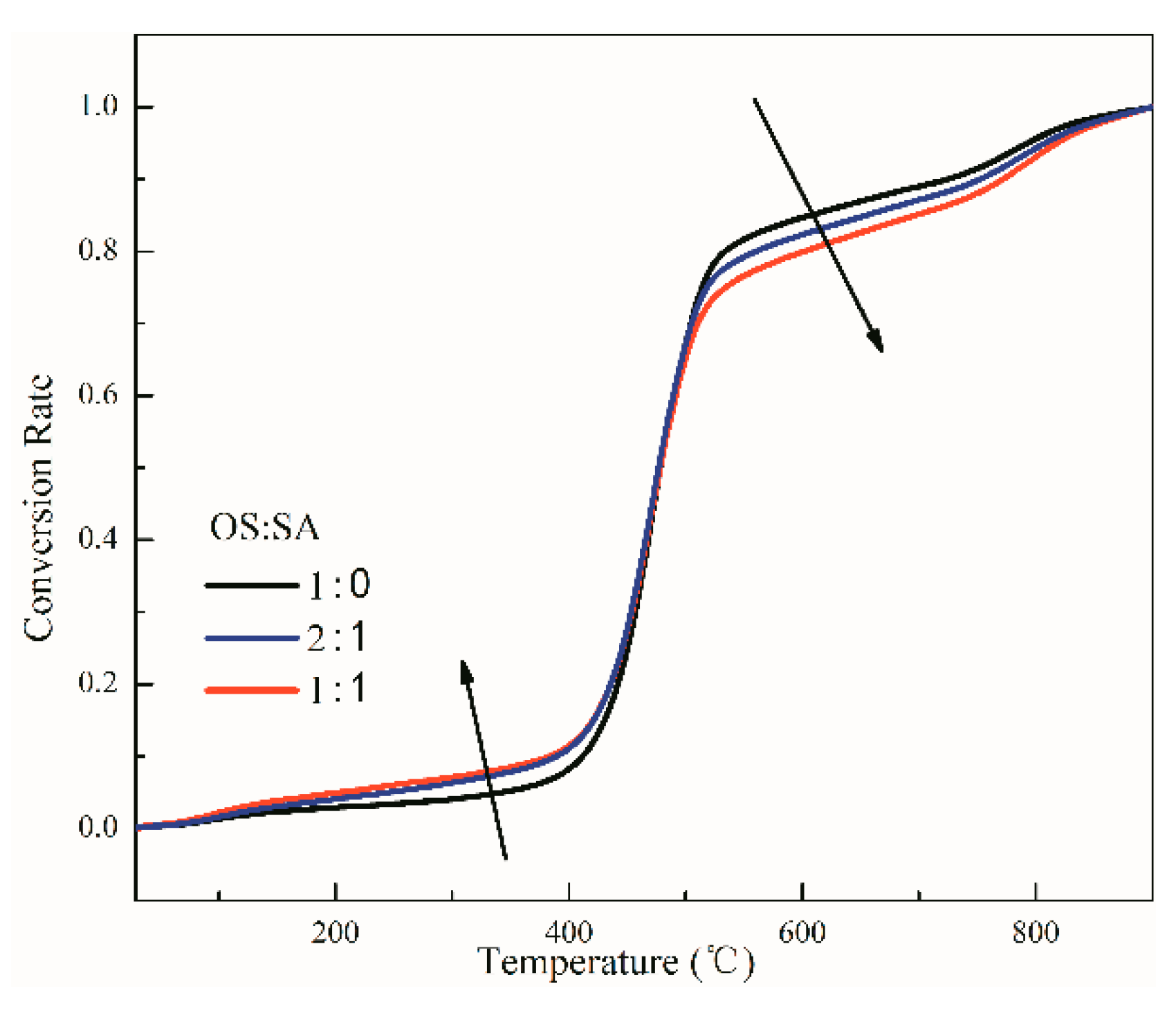
3.1. Influence of SA Content on OS Pyrolysis
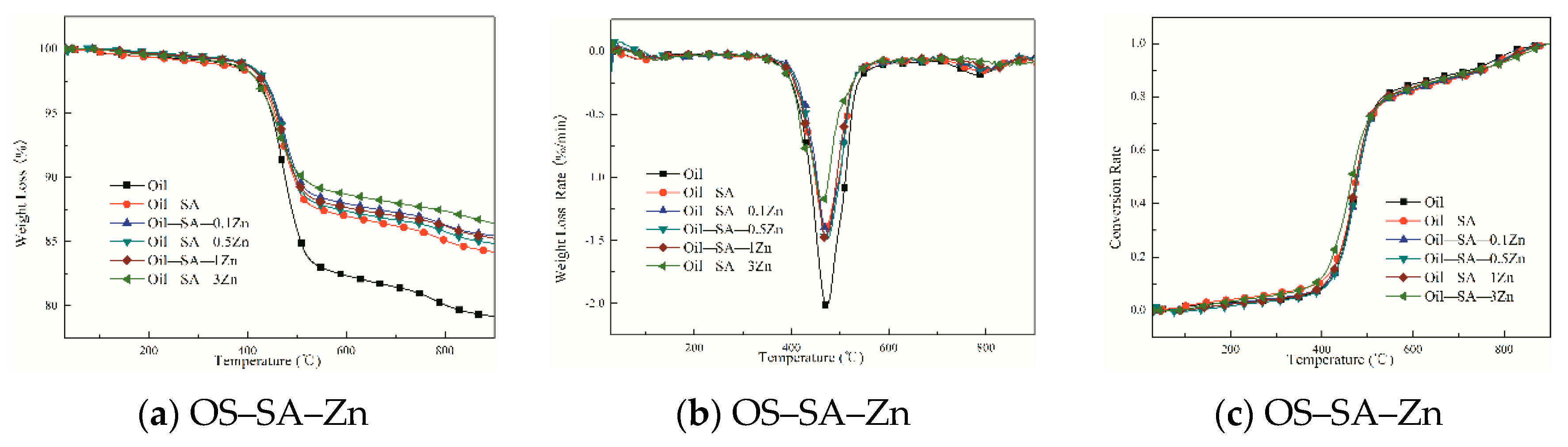
3.2. Effects of Different Loading of Transition Metal Salts on SA on OS Pyrolysis
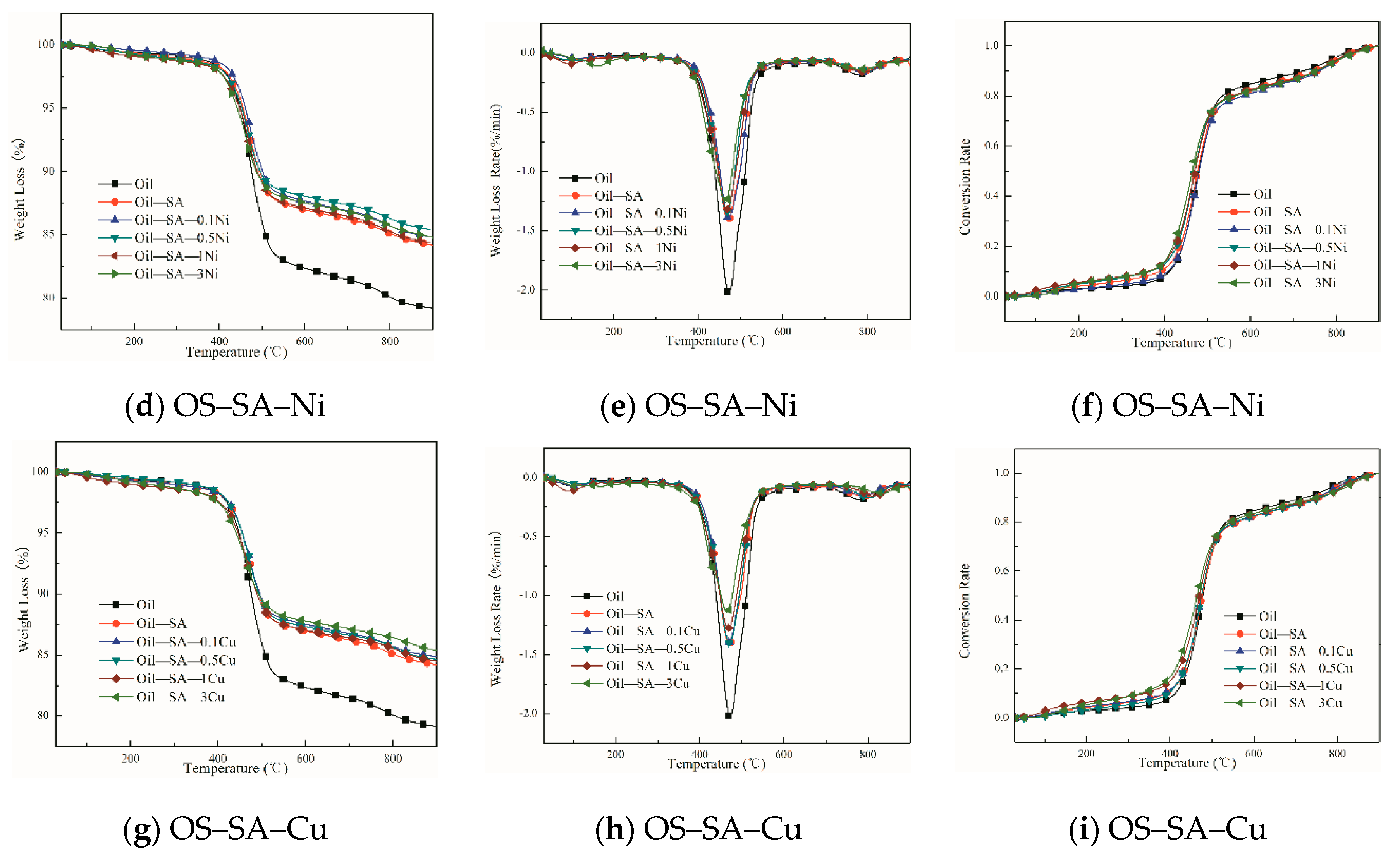
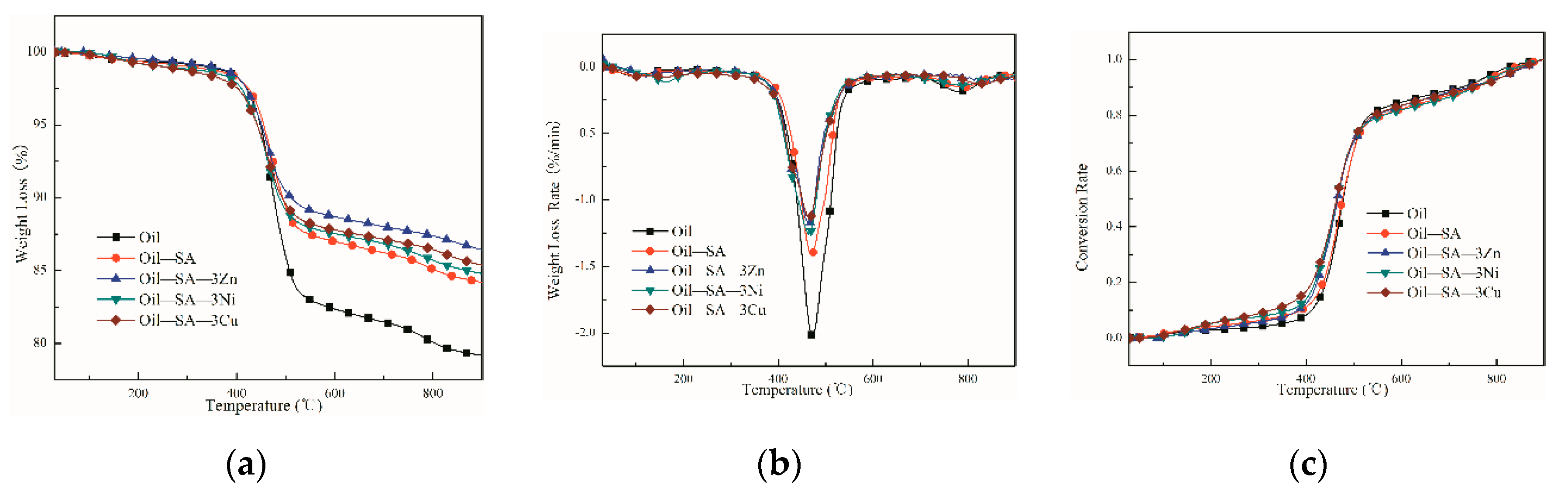
3.3. Effects of Different Transition Metal Salts of SA Loading on OS Pyrolysis
3.3.1. Analysis of Catalytic Pyrolysis Behavior
3.3.2. Kinetics Analysis
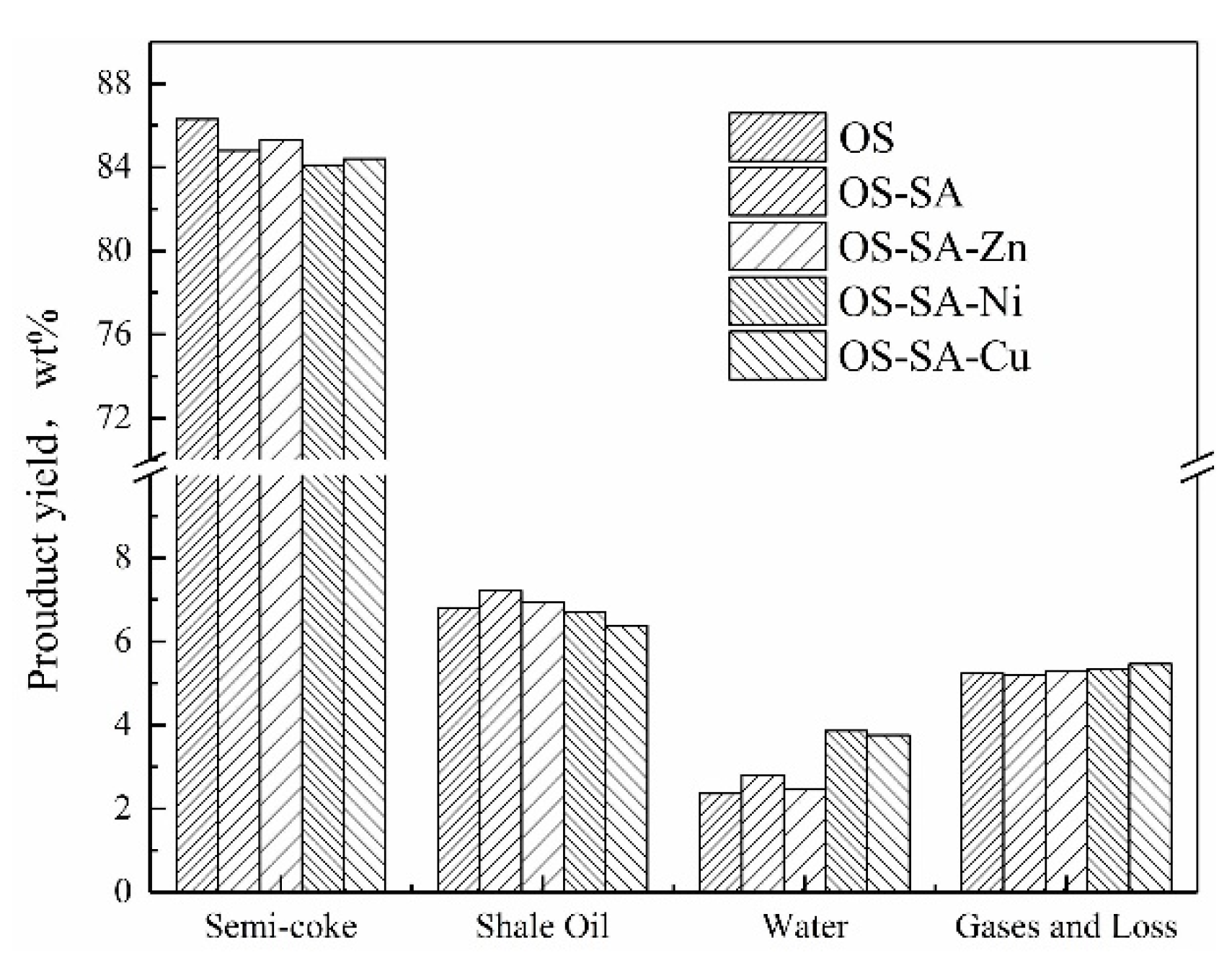
3.4. Product Yield in Fixed Bed Pyrolysis Experiments
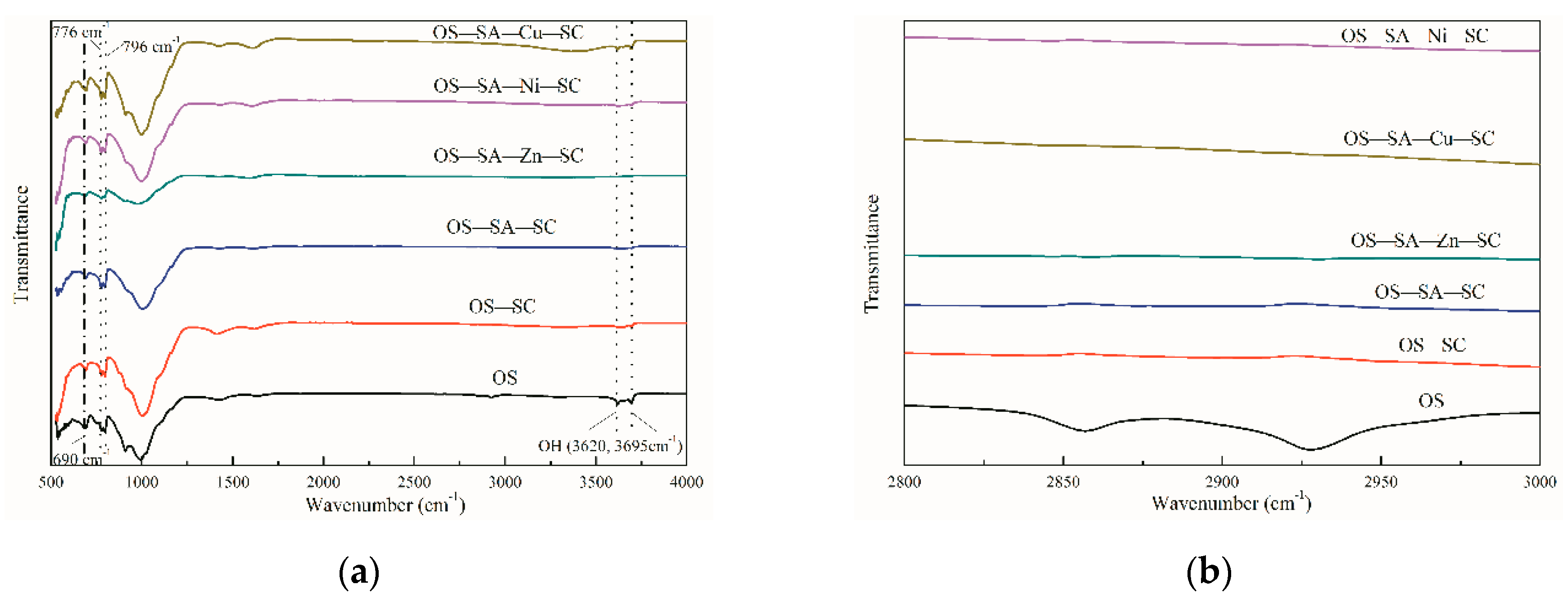
3.5. Characteristics of Semi-Coke after Pyrolysis
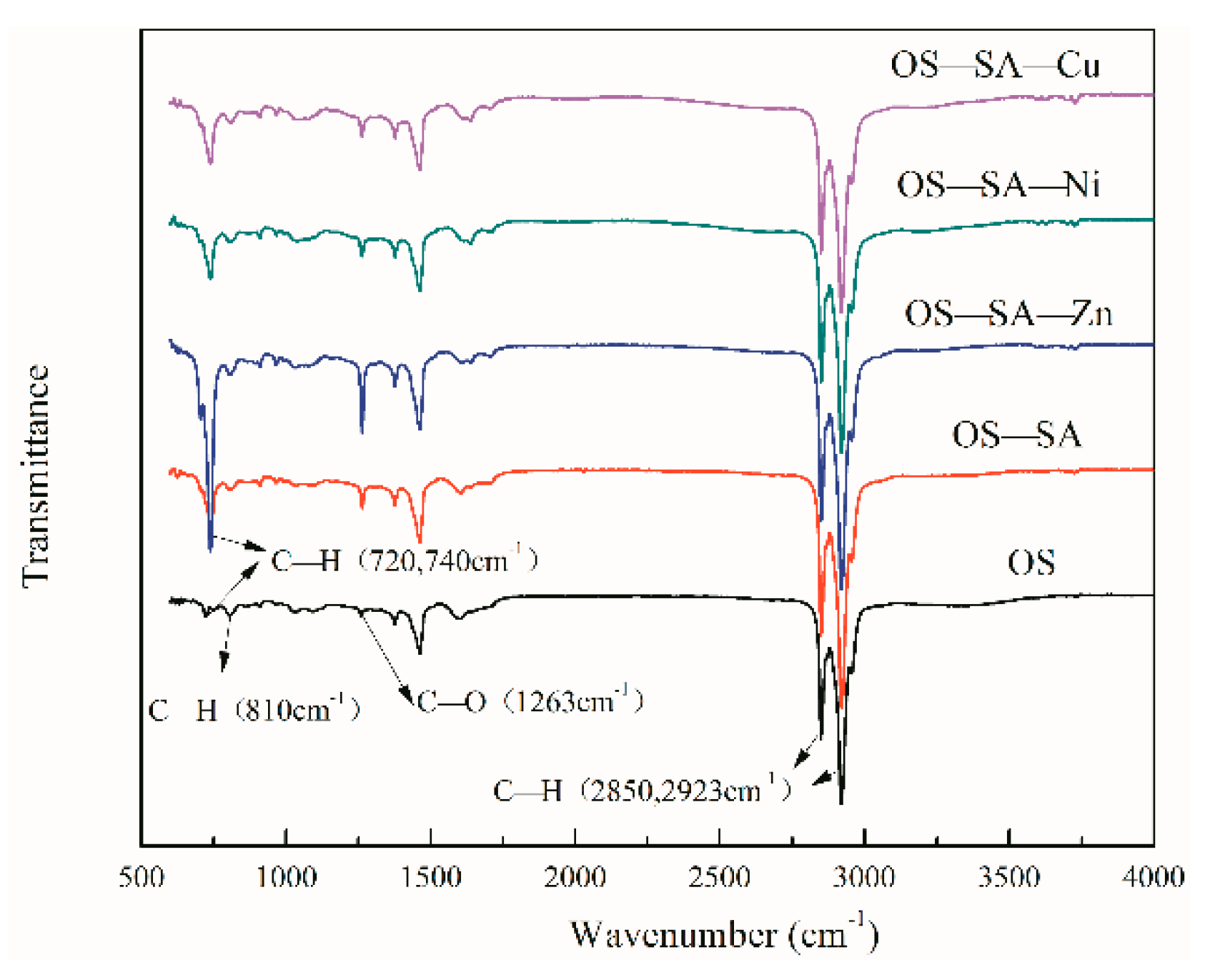
3.6. Characteristics of Shale Oil
3.6.1. Characterization of Shale Oil Produced from Catalytic OS Pyrolysis
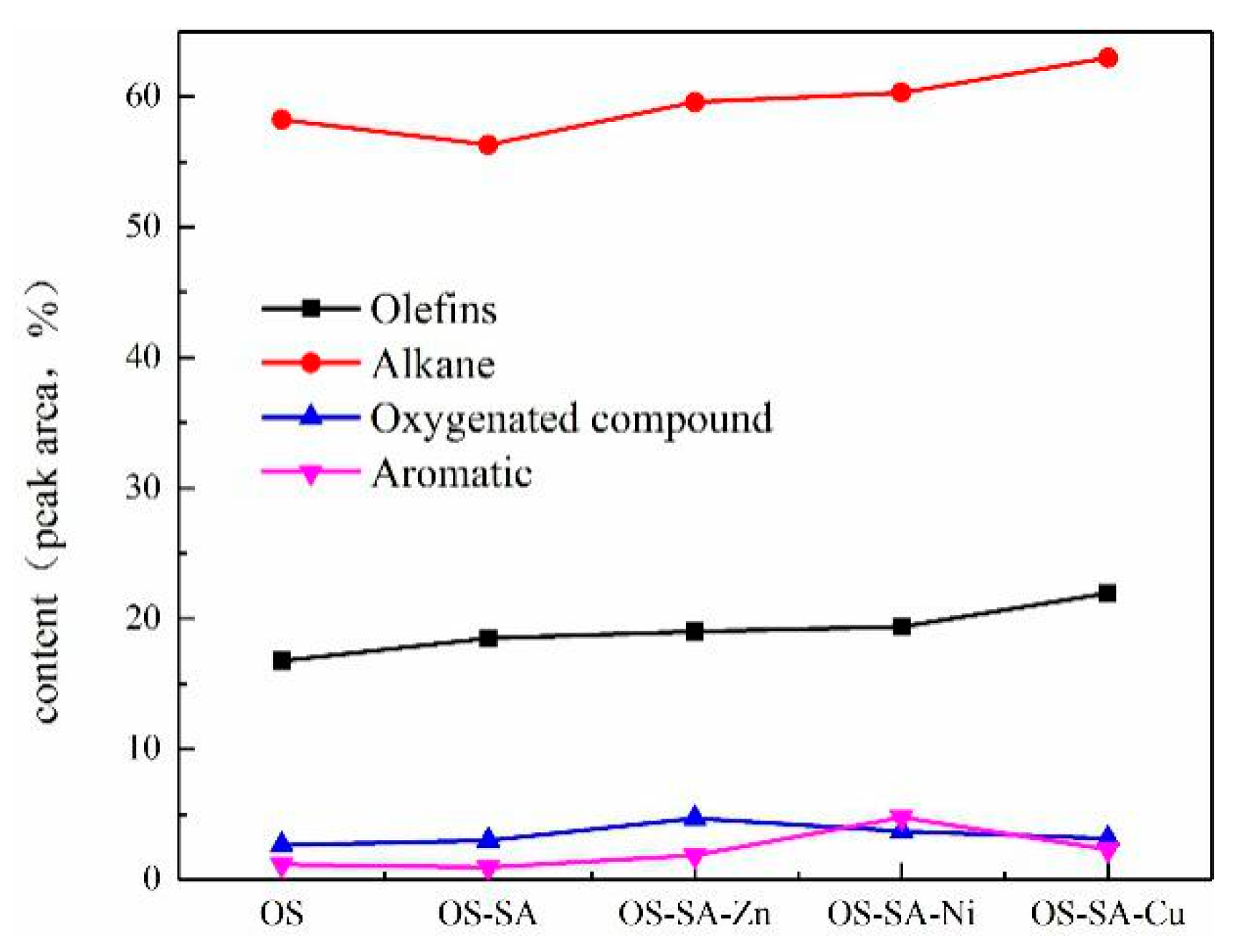
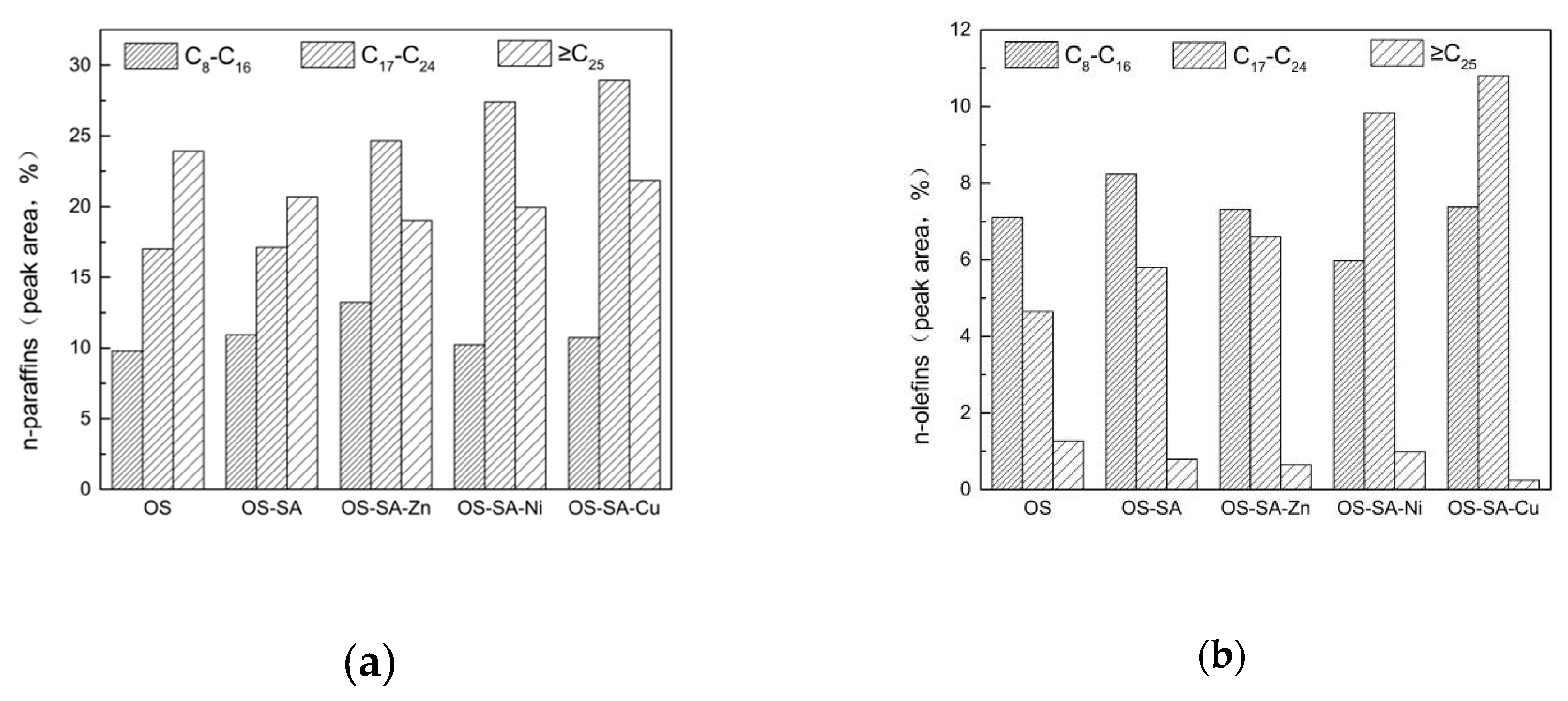
3.6.2. Shale Oil Composition Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.J.; Meng, Q.; Dong, Q.; Zhu, J.; Guo, W.; Ye, S.; Liu, R.; Jia, J.L. Characteristics and resource potential of oil shale in China. Oil Shale 2017, 34, 15–41. [Google Scholar] [CrossRef]
- Liu, Z.J.; Yang, D.; Hu, Y.Q.; Zhang, J.W.; Shao, J.X.; Song, S.; Kang, Z.Q. Influence of In Situ Pyrolysis on the Evolution of Pore Structure of Oil Shale. Energies 2018, 11, 755. [Google Scholar] [CrossRef]
- Fuke, D.; Zijun, F.; Yang, D.; Zhao, Y.S.; Derek, E. Permeability Evolution of Pyrolytically-Fractured Oil Shale under In Situ Conditions. Energies 2018, 11, 3033–3041. [Google Scholar]
- Hu, S.Y.; Xiao, C.L.; Jiang, X.; Liang, X.J. Potential Impact of In-Situ Oil Shale Exploitation on Aquifer System. Water 2018, 10, 649. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.X.; Jiang, X.M.; Han, X.X.; Tong, J.H. Effect of heating rate on products yield and characteristics of non-condensable gases and shale oil obtained by retorting Dachengzi oil shale. Oil Shale 2013, 30, 27–47. [Google Scholar] [CrossRef]
- Araújo, A.M.M.; Queiroz, G.S.M.; Maia, D.O.; Gondim, A.; Souza, L.D.; Fernandes, V.J.; Araujo, A.S. Fast Pyrolysis of Sunflower Oil in the Presence of Microporous and Mesoporous Materials for Production of Bio-Oil. Catalysts 2018, 8, 261. [Google Scholar] [CrossRef]
- Du, W.Z.; Wang, Y.; Liu, X.L.; Sun, L.L. Study on Low Temperature Oxidation Characteristics of Oil Shale Based on Temperature Programmed System. Energies 2018, 11, 2594–2605. [Google Scholar] [CrossRef]
- Williams, P.T.; Chishti, H.M. Two stage pyrolysis of oil shale using a zeolite catalyst. J. Anal. Appl. Pyrolysis 2000, 55, 217–234. [Google Scholar] [CrossRef]
- Williams, P.T.; Chishti, H.M. Influence of residence time and catalyst regeneration on the pyrolysis–zeolite catalysis of oil shale. J. Anal. Appl. Pyrolysis 2001, 60, 187–203. [Google Scholar] [CrossRef]
- Gai, R.H.; Jin, L.J.; Zhang, J.B.; Wang, J.Y.; Hu, H.Q. Effect of inherent and additional pyrite on the pyrolysis behavior of oil shale. J. Anal. Appl. Pyrolysis 2014, 105, 342–347. [Google Scholar] [CrossRef]
- Hu, M.J.; Cheng, Z.Q.; Zhang, M.Y.; Liu, M.Z.; Song, L.H.; Zhang, Y.Q.; Li, J.F. Effect of Calcite, Kaolinite, Gypsum, and Montmorillonite on Huadian Oil Shale Kerogen Pyrolysis. Energy Fuels 2014, 28, 1860–1867. [Google Scholar] [CrossRef]
- Jiang, H.F.; Song, L.H.; Cheng, Z.Q.; Chen, J.; Zhang, L.; Zhang, M.Y.; Hu, M.J.; Li, J.N.; Li, J.F. Influence of pyrolysis condition and transition metal salt on the product yield and characterization via Huadian oil shale pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 230–236. [Google Scholar] [CrossRef]
- Chang, Z.B.; Chu, M.; Zhang, C.; Bai, S.X.; Lin, H.; Ma, L.B. Investigation of the effect of selected transition metal salts on the pyrolysis of huadian oil shale, china. Oil Shale 2017, 34, 354–367. [Google Scholar] [CrossRef]
- Öztaş, N.A.; Yürüm, Y. Effect of Catalysts on the Pyrolysis of Turkish Zonguldak Bituminous Coal. Fuel Energy Abstr. 2000, 14, 820–827. [Google Scholar] [CrossRef]
- Zou, X.W.; Yao, J.Z.; Yang, X.M.; Song, W.L.; Lin, W.G. Catalytic Effects of Metal Chlorides on the Pyrolysis of Lignite. Energy Fuels 2007, 21, 619–624. [Google Scholar] [CrossRef]
- Shi, W.J.; Wang, Z.; Song, W.L.; Li, S.G.; Li, X.Y. Pyrolysis of Huadian oil shale under catalysis of shale ash. J. Anal. Appl. Pyrolysis 2016, 123, 160–164. [Google Scholar] [CrossRef]
- Lai, D.G.; Chen, Z.H.; Chen, Z.H.; Lin, L.X.; Zhang, Y.M.; Gao, S.Q.; Xu, G.W. Secondary Cracking and Upgrading of Shale Oil from Pyrolyzing Oil Shale over Shale Ash. Energy Fuels 2015, 29, 2219–2226. [Google Scholar] [CrossRef]
- Jaber, J.; Abu-Rahmeh, T.; Al-Alawin, A.; Kloub, N.M. Surface Retorting of Jordanian Oil Shale and Associated CO2 Emissions. Jordan J. Mech. Ind. Eng. 2010, 4, 591–596. [Google Scholar]
- Yang, Y.C.; Jia, F.R.; Jian, W.W.; Zhang, S.P.; Ma, D.Z.; Liu, G.X. Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-Walled Carbon Nanotubes. China Pet. Process. Petrochem. Technol. 2019, 1, 23–35. [Google Scholar]
- Jia, F.R.; Li, Z.; Wang, E.G.; He, J.C.; Dong, H.; Liu, G.X.; Jian, W.W. Preparation and SO2 adsorption behavior of coconut shell-based activated carbon via microwave-assisted oxidant activation. China Pet. Process. Petrochem. Technol. 2018, 20, 67–74. [Google Scholar]
- Wang, Z.J.; Deng, S.H.; Gu, Q.; Zhang, Y.M.; Cui, X.J.; Wang, H.Y. Pyrolysis kinetic study of Huadian oil shale, spent oil shale and their mixtures by thermogravimetric analysis. Fuel Process. Technol. 2013, 110, 103–108. [Google Scholar] [CrossRef]
- Pan, L.W.; Dai, F.Q.; Huang, J.J.; Liu, S.; Li, G.Q. Study of the effect of mineral matters on the thermal decomposition of Jimsar oil shale using TG-MS. Thermochim. Acta 2016, 627, 31–38. [Google Scholar] [CrossRef]
- Lai, D.G.; Shi, Y.; Geng, S.L.; Chen, Z.H.; Gao, S.Q.; Zhan, J.H.; Xu, G.W. Secondary reactions in oil shale pyrolysis by solid heat carrier in a moving bed with internals. Fuel 2016, 173, 138–145. [Google Scholar] [CrossRef]
- Burnham, A.K. Chemistry and Kinetics of Oil Shale Retorting. In Oil Shale: A Solution to the Liquid Fuel Dilemma; ASC: Washington, DC, USA, 2010; Volume 1032, pp. 115–134. [Google Scholar]
- Dung, N.V.; Yip, V. Basis of reactor design for retorting Australian oil shales. Fuel 1991, 70, 1336–1341. [Google Scholar] [CrossRef]
- Xiong, L.P.; Hu, S.B.; Wang, J. Analysis on the thermal conductivity of rocks from se china. Acta Petrol. Sin. 1994, 10, 323–329. [Google Scholar]
- Wu, Y.Y.; Qin, Y.; Liu, J.Z.; Wang, A.K. Catalysis Action of Mineral/Metal Elements during Coalderived Hydrocarbons Process, An Example of the Late Permian Coal from Dahebian Coal Mine in Eastern Yunnan and Western Guizhou. J. Nat. Gas Geosci. 2012, 23, 141–152. [Google Scholar]
- Li, S.Y.; Lin, S.J.; Guo, S.H.; Liu, L.F. Effects of inorganic salts on the hydrocarbon generation from kerogens. Geochimica 2002, 31, 15–20. [Google Scholar]
- Han, J.Z.; Wang, X.D.; Yue, J.R.; Gao, S.Q.; Xu, G.W. Catalytic upgrading of coal pyrolysis tar over char-based catalysts. Fuel Process. Technol. 2014, 122, 98–106. [Google Scholar] [CrossRef]
- Bai, F.T.; Guo, W.; Lü, X.S.; Liu, Y.M.; Guo, M.Y.; Li, Q.; Sun, Y.H. Kinetic study on the pyrolysis behavior of Huadian oil shale via non-isothermal thermogravimetric data. Fuel 2015, 146, 111–118. [Google Scholar] [CrossRef]
- Bai, F.T.; Sun, Y.H.; Liu, Y.M.; Liu, B.C.; Guo, M.Y.; Lü, X.S.; Guo, W.; Li, Q.; Hou, C.B.; Wang, Q.W. Kinetic Investigation on Partially Oxidized Huadian Oil Shale by Thermogravimetric Analysis. Oil Shale 2014, 31, 377–393. [Google Scholar] [CrossRef]
- Niu, M.T.; Wang, S.; Han, X.X.; Jiang, X.M. Yield and characteristics of shale oil from the retorting of oil shale and fine oil-shale ash mixtures. Appl. Energy 2013, 111, 234–239. [Google Scholar] [CrossRef]
- Yürüm, Y.; Dror, Y.; Levy, M. Effect of acid dissolution on the mineral matrix and organic matter of Zefa EFE oil shale. Fuel Process. Technol. 1985, 11, 71–86. [Google Scholar] [CrossRef]
- Yariv, S. Infrared Evidence for the Occurrence of SiO Groups with Double-Bond Character in Antigorite, Sepiolite and Palygorskite. Clay Miner. 1986, 21, 925–935. [Google Scholar] [CrossRef]
- Wu, L.M.; Tong, D.S.; Zhao, L.Z.; Yu, W.H.; Zhou, C.H.; Wang, H. Fourier transform infrared spectroscopy analysis for hydrothermal transformation of microcrystalline cellulose on montmorillonite. Appl. Clay Sci. 2014, 95, 74–82. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Farmer, V.C. Transverse and longitudinal crystal modes associated with OH stretching vibrations in single crystals of kaolinite and dickite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 927–930. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, Y.H.; Jia, C.X.; Zhu, Y.C. FTIR analysis and pyrolysis characteristics of oil shale from Gansu province. Chem. Ind. Eng. Prog. 2014, 33, 1730–1734. [Google Scholar]
- Wang, Z.J.; Deng, S.H.; Gu, Q.; Cui, X.J.; Zhang, Y.M.; Wang, H.Y. Subcritical Water Extraction of Huadian Oil Shale under Isothermal Condition and Pyrolysate Analysis. Energy Fuels 2014, 28, 2305–2313. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, Y.C.; Yu, W.Z.; Ren, Z.; Liu, S.H.; Liu, Q.Y.; Liu, Z.Y. A study on the structure of Yilan oil shale kerogen based on its alkali-oxygen oxidation yields of benzene carboxylic acids, 13 C NMR and XPS. Fuel Process. Technol. 2017, 166, 30–40. [Google Scholar] [CrossRef]
- Palayangoda, S.S.; Nguyen, Q.P. An ATR-FTIR procedure for quantitative analysis of mineral constituents and kerogen in oil shale. Oil Shale 2012, 29, 344–356. [Google Scholar] [CrossRef]
- Xing, Q.Y.; Yang, M.; Yang, H.X.; Zu, E.D. Study on the Gemological Characteristics of Amber from Myanmar and Chinese Fushun. Key Eng. Mater. 2013, 544, 172–177. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, J.B.; Yang, H.Y.; Liu, Q. Chemical composition and structural characteristics of oil shales and their kerogens using FTIR and solid-state 13C NMR. Energy Fuels 2016, 30, 6271–6280. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, X.C.; Chi, M.S.; Zhang, H.X.; Cui, D.; Bai, J.R. FT-IR study on composition of oil shale kerogen and its pyrolysis oil generation characteristics. J. Fuel Chem. Technol. 2015, 43, 1158–1166. [Google Scholar]
- Painter, P.C.; Snyder, R.W.; Starsinic, M.; Coleman, M.M.; Kuehn, D.W.; Davis, A. Concerning the Application of FTIR to the Study of Coal: A Critical Assessment of Band Assignments and the Application of Spectral Analysis Programs. Appl. Spectrosc. 1981, 35, 475–485. [Google Scholar] [CrossRef]
- Lin, L.X.; Zhang, C.; Li, H.J.; Lai, D.G.; Xu, G.W. Pyrolysis in indirectly heated fixed bed with internals: The first application to oil shale. Fuel Process. Technol. 2015, 138, 147–155. [Google Scholar] [CrossRef]
- Tiwari, P.K.; Deo, M. Compositional and kinetic analysis of oil shale pyrolysis using TGA–MS. Fuel 2012, 94, 333–341. [Google Scholar] [CrossRef]
- Harfi, K.E.; Mokhlisse, A.; Chanâa, M.B. Yields and composition of oil obtained by isothermal pyrolysis of the Moroccan (Tarfaya) oil shales with steam or nitrogen as carrier gas. J. Anal. Appl. Pyrolysis 2000, 56, 207–218. [Google Scholar] [CrossRef]
- Al-Harahsheh, A.; Al-Ayed, O.; Al-Harahsheh, M.; Abu-El-Halawah, R. Heating rate effect on fractional yield and composition of oil retorted from El-lajjun oil shale. J. Anal. Appl. Pyrolysis 2010, 89, 239–243. [Google Scholar] [CrossRef]
| Industrial Analysis, % | Elemental Analysis, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Mad | Aad | Vad | FCad * | Cad | Had | Nad | Oad * | Sad |
| OS | 2.88 | 77.19 | 17.76 | 2.17 | 10.86 | 1.89 | 0.74 | 6.16 | 0.28 |
| SA | 0.55 | 90.69 | 5.97 | 2.79 | 4.98 | 0.56 | 1.16 | 1.31 | 0.75 |
| Constituent | SiO2 | Al2O3 | Fe2O3 | SO3 | K2O | MgO | TiO2 | P2O5 | CaO | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Content, % | 58.14 | 23.19 | 10.27 | 1.51 | 1.44 | 1.42 | 1.36 | 1.27 | 1.07 | 0.33 |
| Sample | T (K) | E (kJ/mol) | A (min−1) | R2 |
|---|---|---|---|---|
| OS | 697–778 | 116.04 | 1.9 × 107 | 0.9962 |
| OS–SA | 696–768 | 104.96 | 3.1 × 106 | 0.9957 |
| OS–SA–Zn | 681–761 | 93.34 | 4.8 × 105 | 0.9986 |
| OS–SA–Ni | 682–758 | 91.61 | 4.2 × 105 | 0.9985 |
| OS–SA–Cu | 688–753 | 83.20 | 9.9 × 104 | 0.9987 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Jia, F.; Guo, C.; Pan, H.; Long, X.; Liu, G. Effect of Shale Ash-Based Catalyst on the Pyrolysis of Fushun Oil Shale. Catalysts 2019, 9, 900. https://doi.org/10.3390/catal9110900
Lu H, Jia F, Guo C, Pan H, Long X, Liu G. Effect of Shale Ash-Based Catalyst on the Pyrolysis of Fushun Oil Shale. Catalysts. 2019; 9(11):900. https://doi.org/10.3390/catal9110900
Chicago/Turabian StyleLu, Hao, Fengrui Jia, Chuang Guo, Haodan Pan, Xu Long, and Guangxin Liu. 2019. "Effect of Shale Ash-Based Catalyst on the Pyrolysis of Fushun Oil Shale" Catalysts 9, no. 11: 900. https://doi.org/10.3390/catal9110900
APA StyleLu, H., Jia, F., Guo, C., Pan, H., Long, X., & Liu, G. (2019). Effect of Shale Ash-Based Catalyst on the Pyrolysis of Fushun Oil Shale. Catalysts, 9(11), 900. https://doi.org/10.3390/catal9110900






