Performance of Catalysts of Different Nature in Model Tar Component Decomposition
Abstract
:1. Introduction
2. Results and Discussion
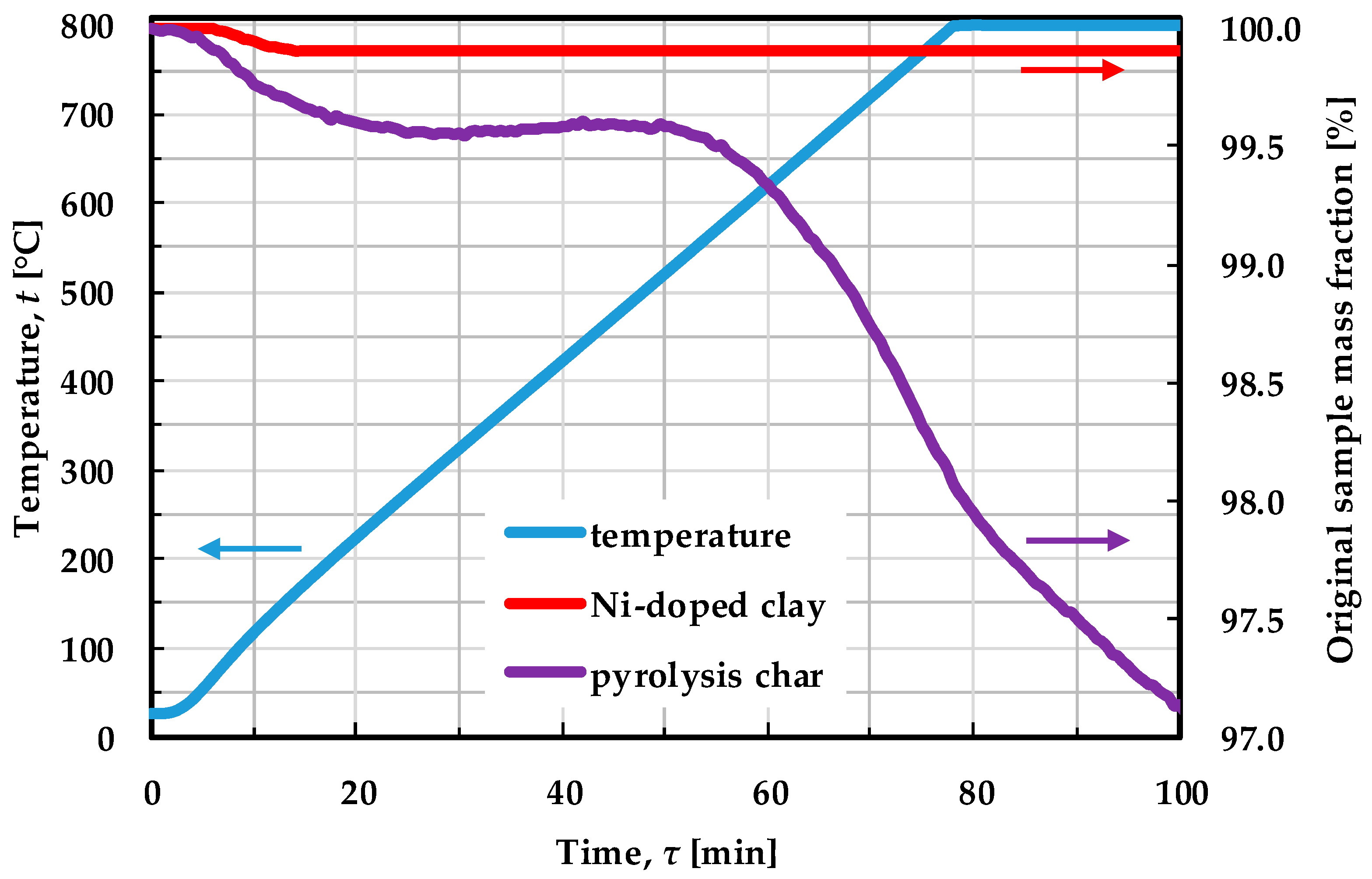
2.1. Catalysts Characterization
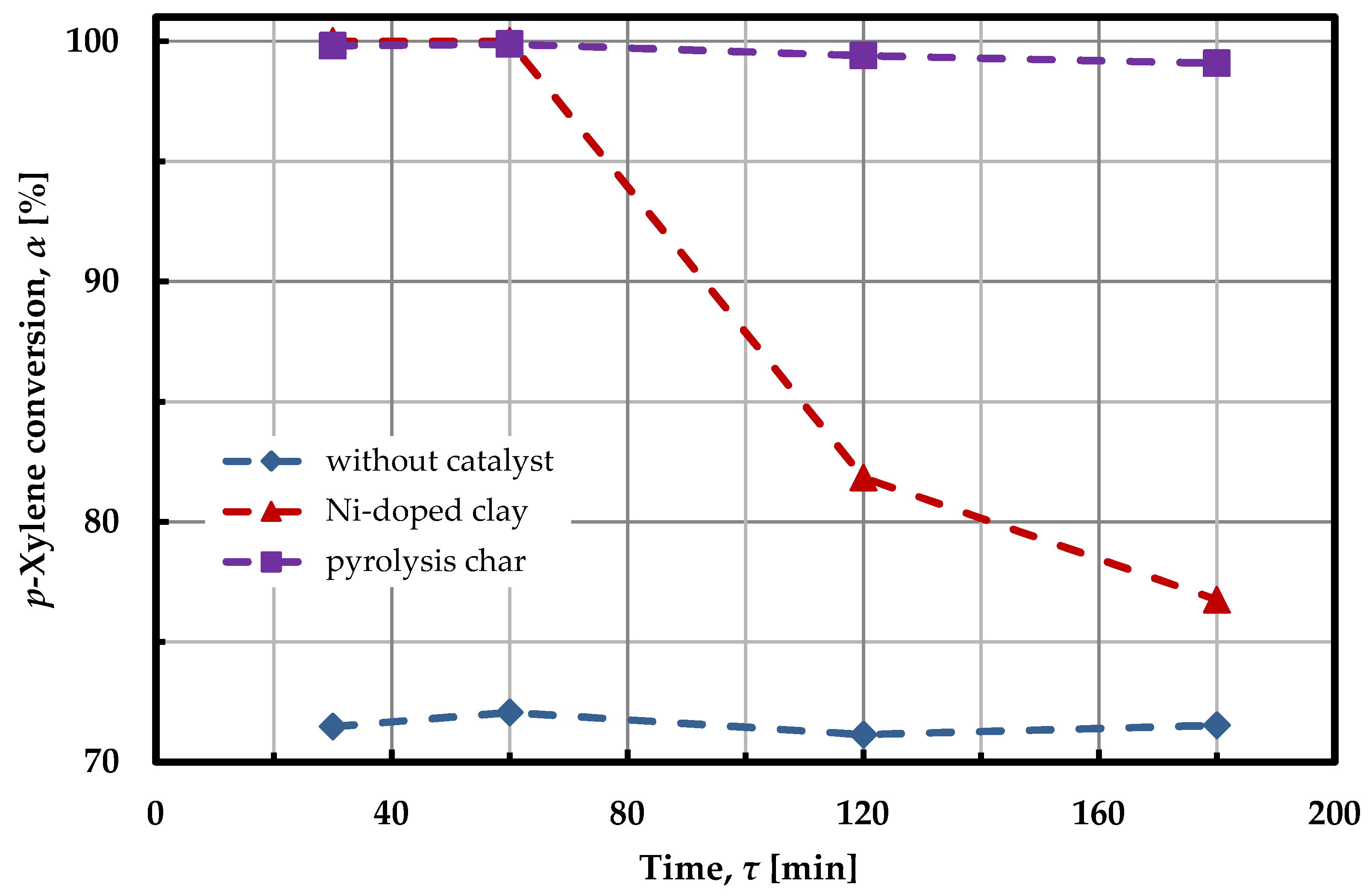
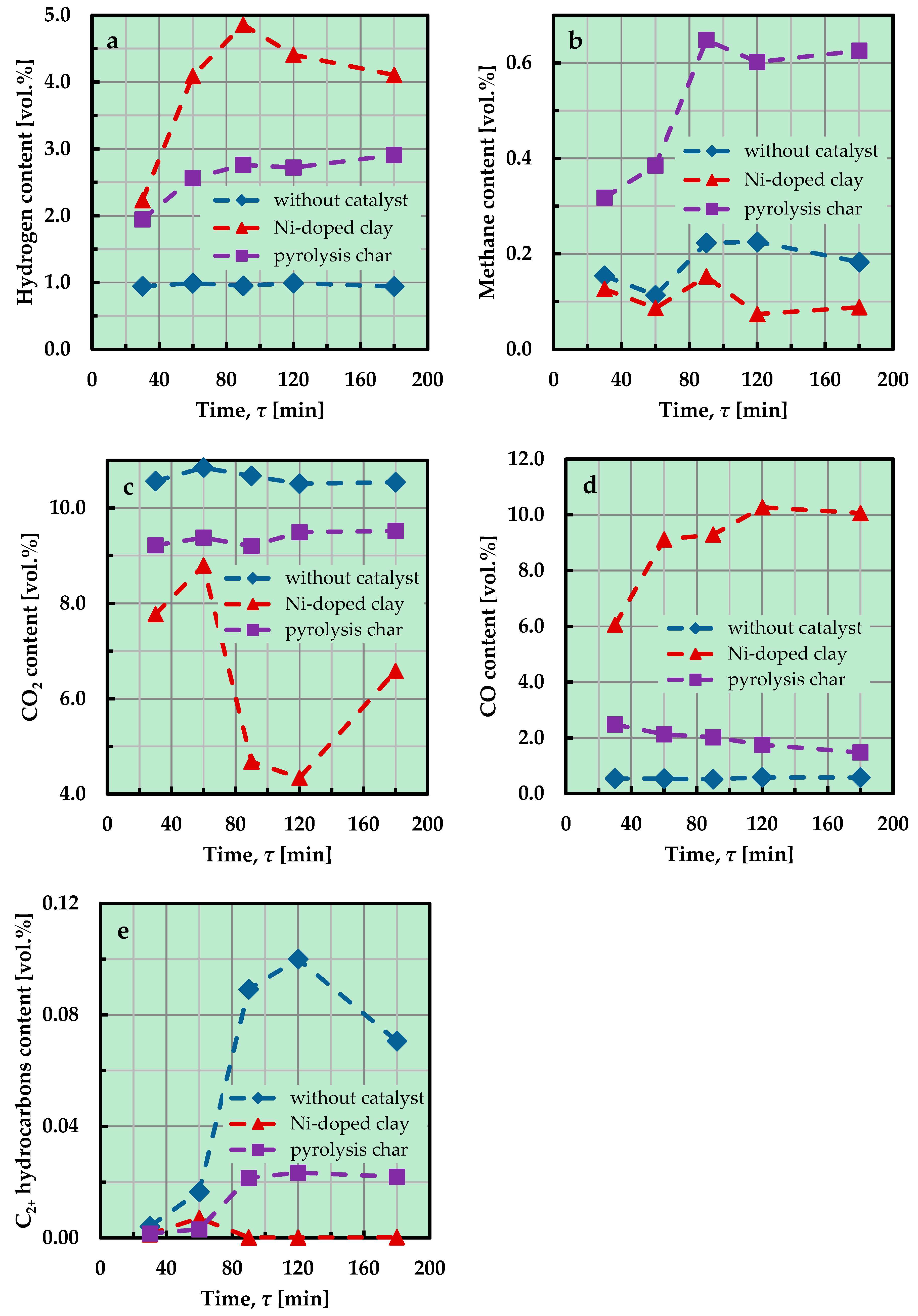
2.2. Model Tar Component Decomposition Experiments
3. Materials and Methods
3.1. Catalysts Preparation and Characterization
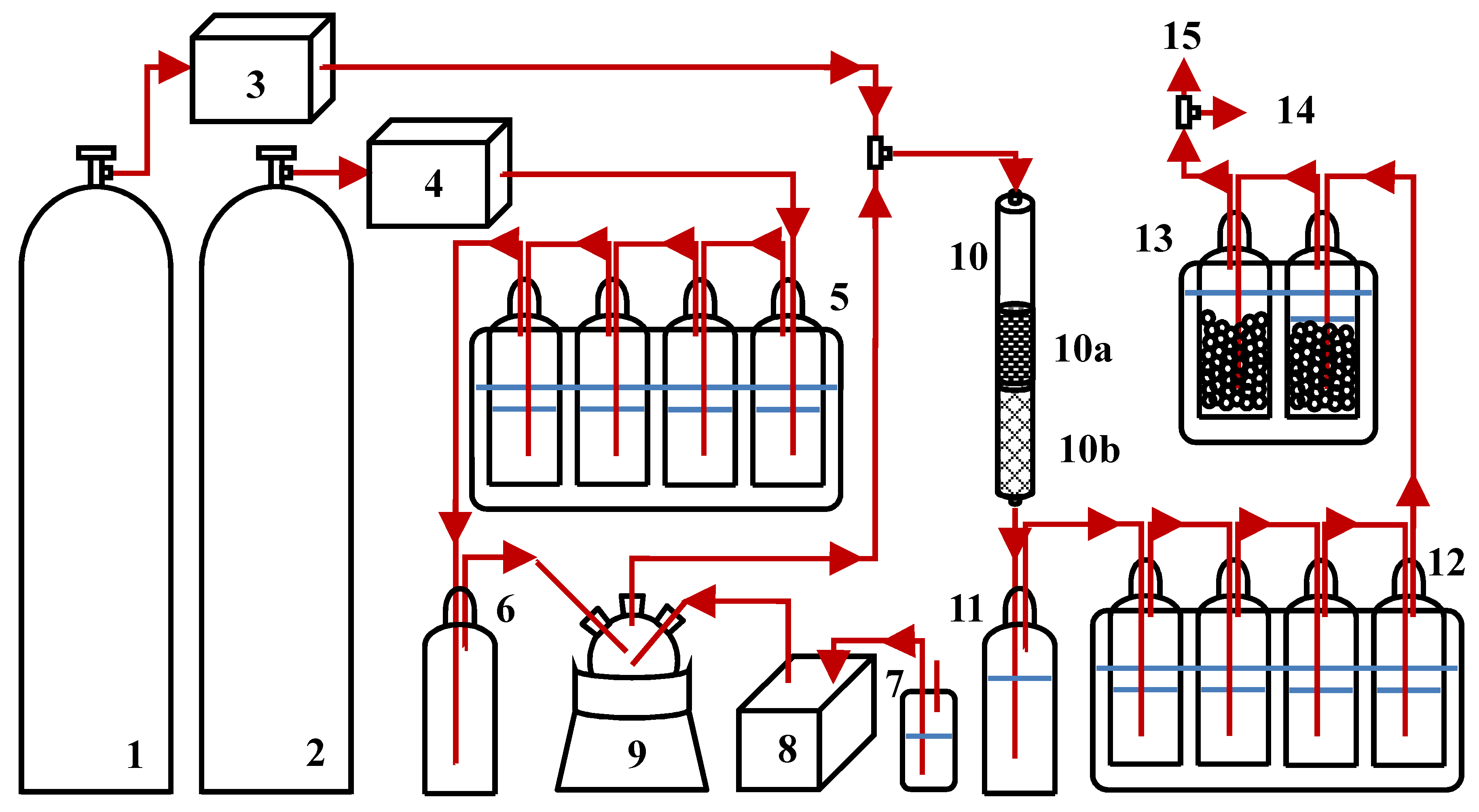
3.2. Experimental Set-Up of Model Tar Component Decomposition Unit
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0; World Bank: Washington, DC, USA, 2018; pp. 17–38. [Google Scholar]
- Fei, F.; Wen, Z.G.; Huang, S.B.; Clercq, D.D. Mechanical biological treatment of municipal solid waste: Energy efficiency, environmental impact and economic feasibility analysis. J. Clean. Prod. 2018, 178, 731–739. [Google Scholar] [CrossRef]
- Gallardo, A.; Carlos, M.; Bovea, M.D.; Colomer, F.J.; Albarrán, F. Analysis of refuse-derived fuel from the municipal solid waste reject fraction and its compliance with quality standards. J. Clean. Prod. 2014, 83, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Šuhaj, P.; Husár, J.; Haydary, J. Modelling of syngas production from municipal solid waste (MSW) for methanol synthesis. Acta Chim. Slovaca 2017, 10, 107–114. [Google Scholar] [CrossRef]
- Kalita, P.; Baruah, D. Investigation of biomass gasifier product. Gas composition and its characterization. In Coal and Biomass Gasification; De, S., Agarwal, A.K., Moholkar, V.S., Thallada, B., Eds.; Springer: Singapore, 2018; pp. 115–150. [Google Scholar]
- Bridgwater, A.V. The technical and economic feasibility of biomass gasification for power generation. Fuel 1995, 74, 631–653. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Abatzoglou, N. Biomass Gasifier “Tars”: Their Nature, Formation, and Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 1998. Available online: https://www.nrel.gov/docs/fy99osti/25357.pdf (accessed on 26 October 2019).
- Maniatis, K.; Beenackers, A.A.C. Tar protocols. IEA bioenergy gasification task. Biomass Bioenergy 2000, 18, 1–4. [Google Scholar] [CrossRef]
- Awais, M.; Li, W.; Arshad, A.; Haydar, Z.; Yaqoob, N.; Hussain, S. Evaluating removal of tar contents in syngas produced from downdraft biomass gasification system. Int. J. Green Energ. 2018, 15, 724–731. [Google Scholar] [CrossRef]
- Hai, I.U.; Sher, F.; Zarrena, G.; Liu, H. Experimental investigation of tar arresting techniques and their evaluation for product syngas cleaning from bubbling fluidized bed gasifier. J. Clean. Prod. 2019, 240, 118239. [Google Scholar] [CrossRef]
- Elliott, D.C. Relation of reaction time and temperature to chemical composition of pyrolysis oils. In Pyrolysis Oils from Biomass: Producing, Analyzing, and Upgrading; ACS Symposium Series; Soltes, E., Milne, T.A., Eds.; American Chemical Society: Washington, DC, USA, 1988; Volume 376, pp. 55–65. [Google Scholar]
- Schildhauer, T.J.; Biollaz, S.M.A. Synthetic Natural Gas from Coal, Dry Biomass, and Power-to-Gas Applications; Wiley: Hoboken, NJ, USA, 2016; pp. 47–76. [Google Scholar]
- Zhang, B.; Zhang, L.; Yang, Z.Q.; He, Z.X. An experiment study of biomass steam gasification over NiO/dolomite for hydrogen-rich gas production. Int. J. Hydrogen Energ. 2017, 42, 76–85. [Google Scholar] [CrossRef]
- Nam, H.S.; Wang, Z.H.; Shanmugam, S.R.; Adhikari, S.; Abdoulmoumine, N. Chemical looping dry reforming of benzene as a gasification tar model compound with Ni- and Fe-based oxygen carriers in a fluidized bed reactor. Int. J. Hydrogen Energ. 2018, 43, 18790–18800. [Google Scholar] [CrossRef]
- Zou, X.H.; Chen, T.H.; Liu, H.B.; Zhang, P.; Ma, Z.Y.; Xie, J.J.; Chen, D. An insight into the effect of calcination conditions on catalytic cracking of toluene over 3Fe8Ni/palygorskite: Catalysts characterization and performance. Fuel 2017, 190, 47–57. [Google Scholar] [CrossRef]
- Šuhaj, P.; Haydary, J.; Husár, J.; Steltenpohl, P.; Šupa, I. Catalytic gasification of refuse-derived fuel in a two-stage laboratory scale pyrolysis/gasification unit with catalyst based on clay minerals. Waste Manag. 2019, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Husár, J.; Haydary, J.; Šuhaj, P.; Steltenpohl, P. Potential of tire pyrolysis char as tar-cracking catalyst in solid waste and biomass gasification. Chem. Pap. 2019, 73, 2091–2101. [Google Scholar] [CrossRef]
- Artetxe, M.; Alvarez, J.; Nahil, M.A.; Olazar, M.; Williams, P.T. Steam reforming of different biomass tar model compounds over Ni/Al2O3 catalysts. Energ. Convers. Manage. 2017, 136, 119–126. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Wang, Y.C.; Brown, R.C. Steam reforming of tar compounds over Ni/olivine catalysts doped with CeO2. Energ. Convers. Manage. 2007, 48, 68–77. [Google Scholar] [CrossRef]
- El-Rub, Z.A.; Bramer, E.; Al-Gharabli, S.; Brem, G. Impact of char properties and reaction parameters on naphthalene conversion in a macro-TGA fixed char bed reactor. Catalysts 2019, 9, 307. [Google Scholar] [CrossRef]
- Guo, F.Q.; Peng, K.Y.; Liang, S.A.; Jia, X.P.; Jiang, X.C.; Qian, L. Evaluation of the catalytic performance of different activated biochar catalysts for removal of tar from biomass pyrolysis. Fuel 2019, 258, 116204. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Mochizuki, Y.; Byambajav, E.; Takahashi, S.; Hanaoka, Y.; Ohtsuka, Y. Catalytic performance of limonite ores in the decomposition of model compounds of biomass-derived tar. Energ. Fuel. 2017, 31, 3898–3904. [Google Scholar] [CrossRef]
- Lestinsky, P.; Grycova, B.; Pryszcz, A.; Martaus, A.; Matejova, L. Hydrogen production from microwave catalytic pyrolysis of spruce sawdust. J. Anal. Appl. Pyrol. 2017, 124, 175–179. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Hydrogen-rich syngas production and tar removal from biomass gasification using sacrificial tyre pyrolysis char. Appl. Energ. 2017, 190, 501–509. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Ko, D.C.K.; McKay, G. Production of active carbons from waste tyres—A review. Carbon 2004, 42, 2789–2805. [Google Scholar] [CrossRef]

| Clay Mineral | ||||||
| Component | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | Other |
| w [mass%] | 63.16 ± 0.14 | 8.89 ± 0.02 | 7.28 ± 0.04 | 6.15 ± 0.04 | 2.87 ± 0.03 | 11.65 |
| Pyrolysis Char | ||||||
| Element | C | S | H | N | O | Ash |
| w [mass%] | 72.91 ± 0.64 | 2.47 ± 0.03 | 0.47 ± 0.01 | 0.45 ± 0.01 | 1.16 | 22.54 |
| Catalyst | SBET [m2 g−1] | vp [cm3 g−1] | dp [nm] |
|---|---|---|---|
| Ni-doped clay | 28.6 | 0.17 | 7.8 |
| Pyrolysis char | 67.5 | 0.32 | 17.4 |
| Catalyst | Temperature, t [°C] | p-Xylene Conversion, α [%] | |
|---|---|---|---|
| τ = 30 min | τ = 180 min | ||
| None | 750 | 70.3 | 71.3 |
| 800 | 71.5 | 71.5 | |
| 850 | 71.1 | 73.0 | |
| Ni-doped clay (10 g) GHSV = 2400 h−1 | 750 | 100 | 76.4 |
| 800 | 100 | 76.8 | |
| 850 | n.a. | n.a. | |
| Pyrolysis char (10 g) GHSV = 1000 h−1 | 750 | 99.8 | 94.4 |
| 800 | 99.8 | 99.1 | |
| 850 | 99.9 | 99.9 | |
| Catalyst | None | Ni-Doped Clay | Pyrolysis Char | |
|---|---|---|---|---|
| Product Distribution | ||||
| Solid phase/% | 1.63 | 29.13 | 27.17 | |
| Liquid phase/% | 29.55 | 1.80 | 1.58 | |
| Gas phase/% | 68.82 | 69.08 | 71.26 | |
| Analyzed Components Content | ||||
| Mass, m [g] | Char | 0.0208 | 0.3495 | 0.3450 |
| p-Xylene | 0.3481 | 0.0027 | 0.0025 | |
| Toluene | 0.0342 | 0.0189 | 0.0175 | |
| Volume fraction, φ [vol.%] | N2 | 87.79 | 83.81 | 86,04 |
| CO2 | 10.56 | 7.77 | 9.22 | |
| CO | 0.54 | 6.06 | 2.48 | |
| H2 | 0.94 | 2.23 | 1.94 | |
| CH4 | 0.15 | 0.13 | 0.32 | |
| Other, C2+ | 0.00 | 0.00 | 0.00 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steltenpohl, P.; Husár, J.; Šuhaj, P.; Haydary, J. Performance of Catalysts of Different Nature in Model Tar Component Decomposition. Catalysts 2019, 9, 894. https://doi.org/10.3390/catal9110894
Steltenpohl P, Husár J, Šuhaj P, Haydary J. Performance of Catalysts of Different Nature in Model Tar Component Decomposition. Catalysts. 2019; 9(11):894. https://doi.org/10.3390/catal9110894
Chicago/Turabian StyleSteltenpohl, Pavol, Jakub Husár, Patrik Šuhaj, and Juma Haydary. 2019. "Performance of Catalysts of Different Nature in Model Tar Component Decomposition" Catalysts 9, no. 11: 894. https://doi.org/10.3390/catal9110894
APA StyleSteltenpohl, P., Husár, J., Šuhaj, P., & Haydary, J. (2019). Performance of Catalysts of Different Nature in Model Tar Component Decomposition. Catalysts, 9(11), 894. https://doi.org/10.3390/catal9110894





