Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Prepared Photocatalysts
2.1.1. XRD Patterns and FT–IR Spectra
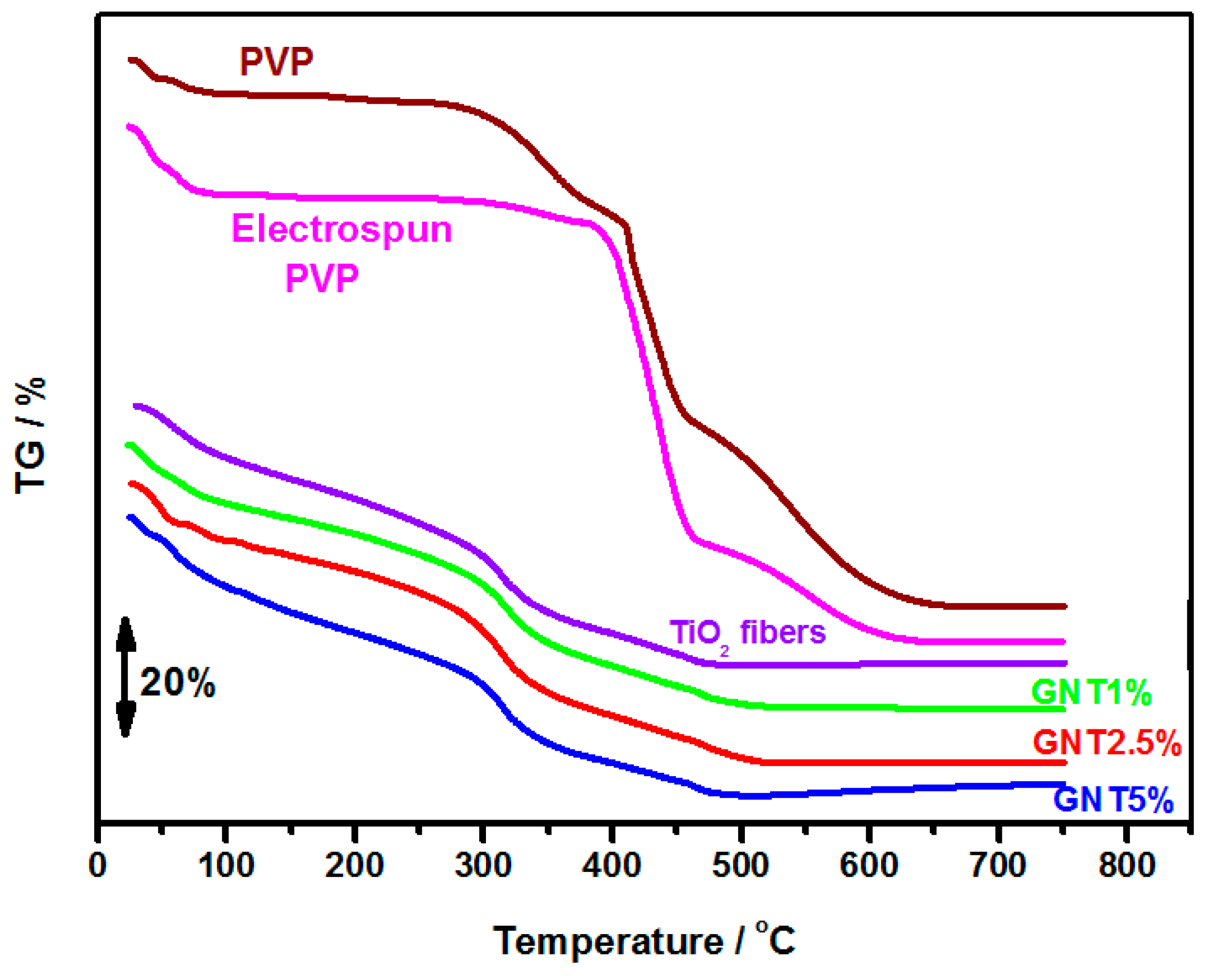
2.1.2. Morphology and Thermal Analysis
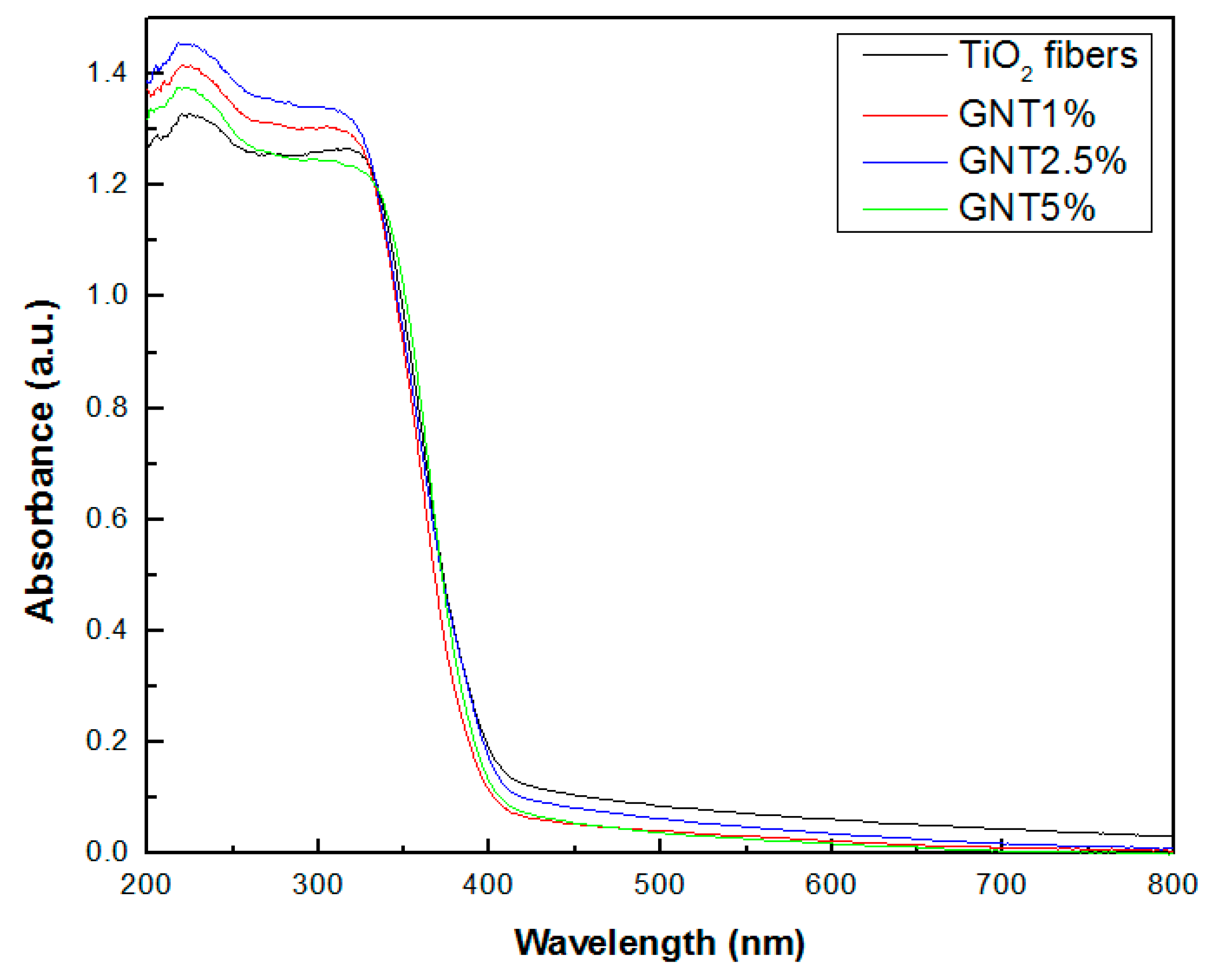
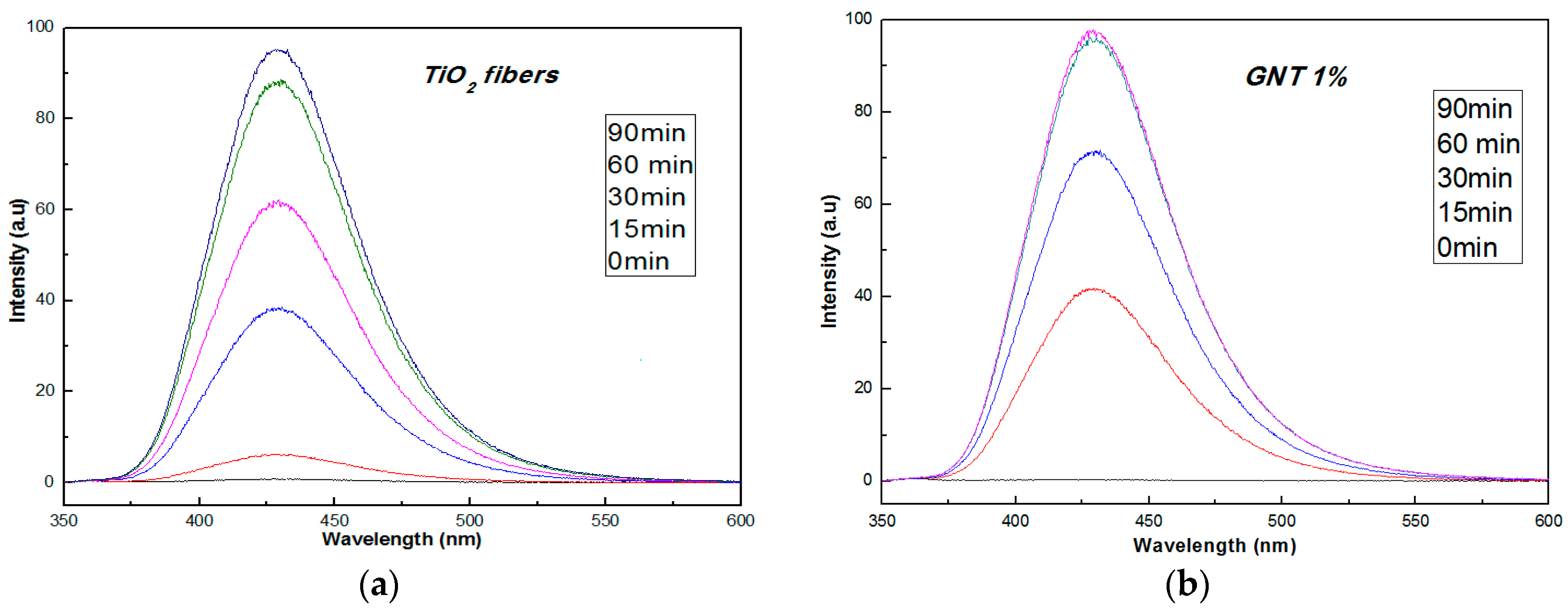
2.1.3. UV-Vis Diffuse Reflectance and Fluorescence Spectra
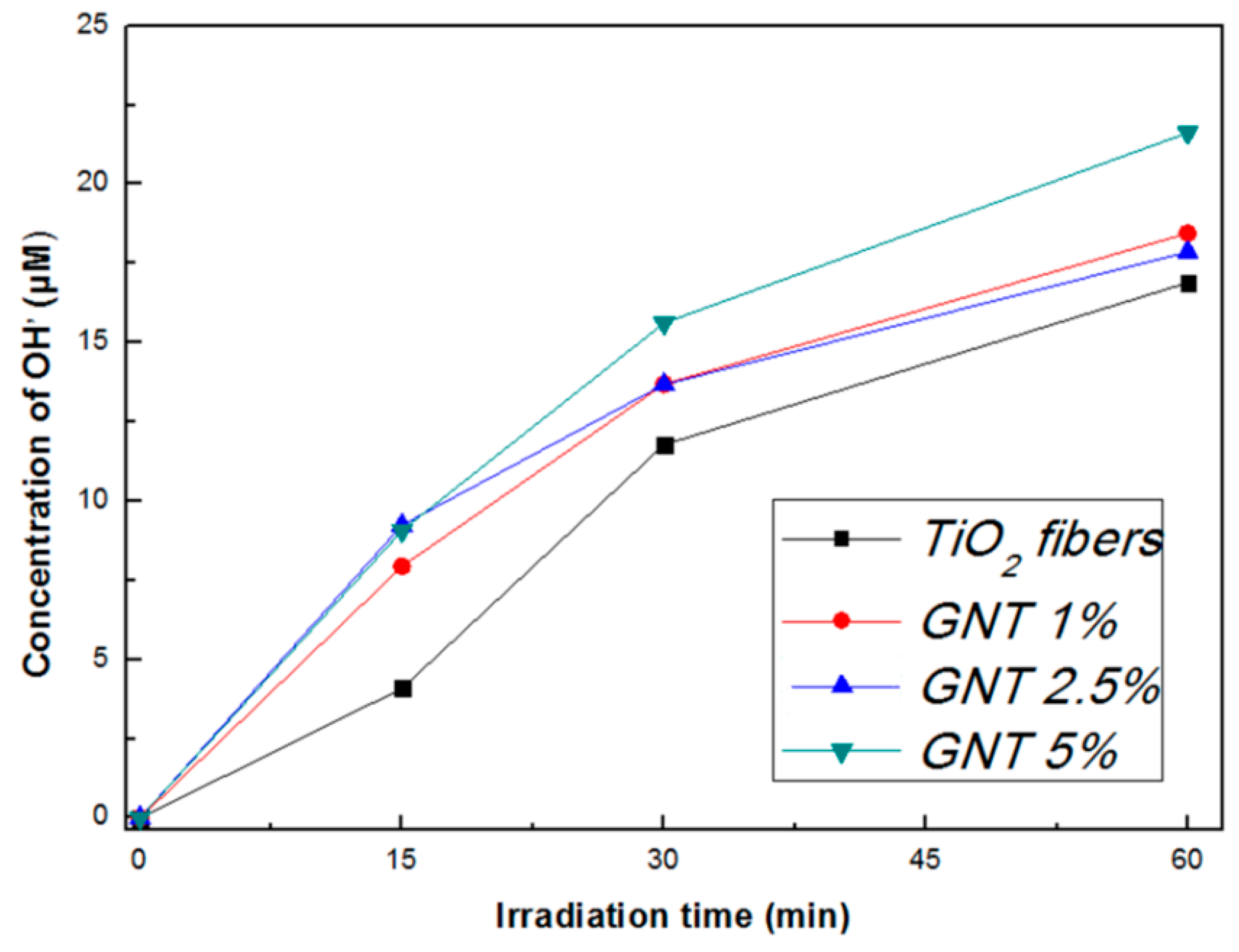
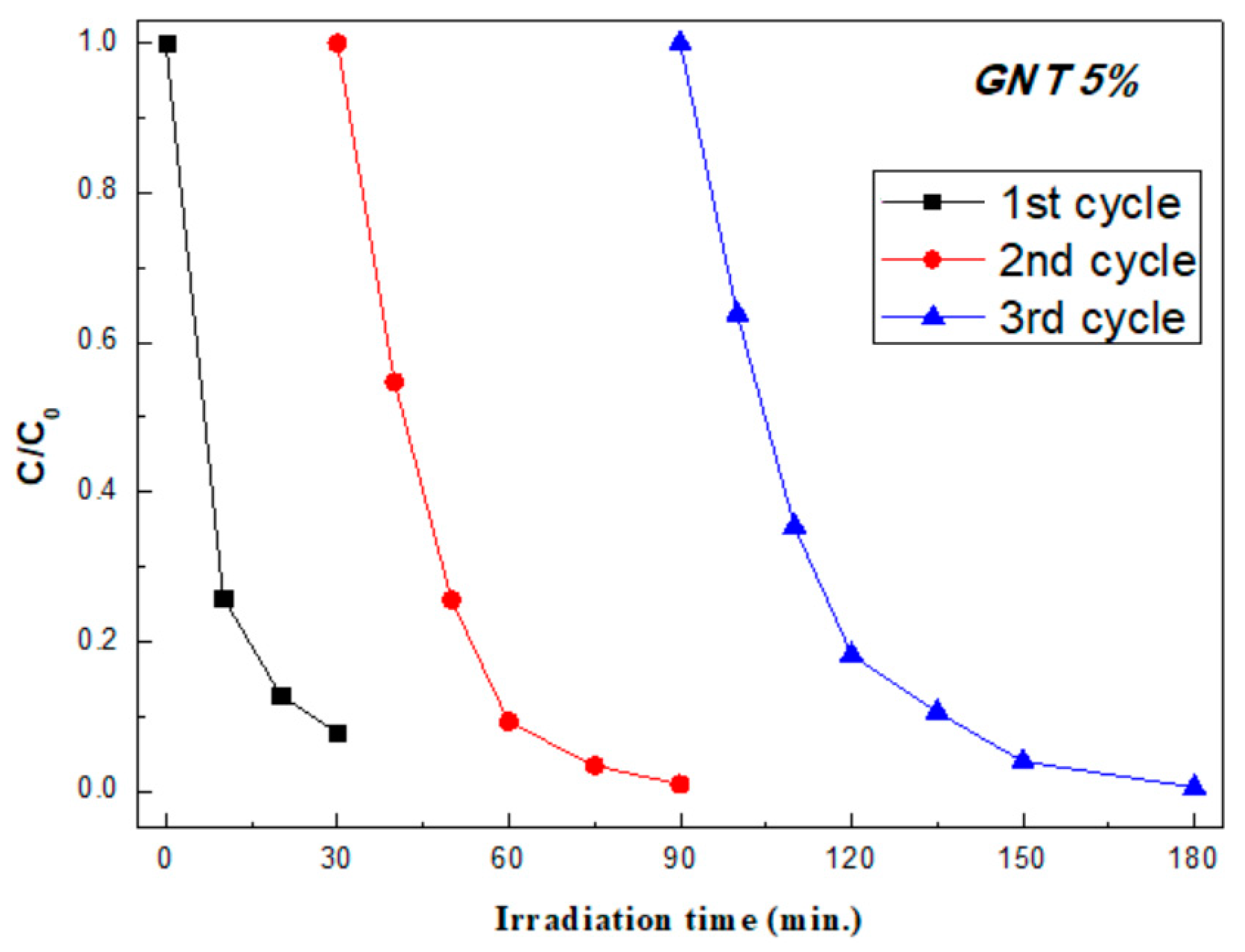
2.1.4. Photocatalytic Activity and Recyclability
2.1.5. Featured Photocatalytic Mechanism for the TiO2/g-C3N4 Composite Fibers
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Electrospun Fibrous TiO2/g-C3N4 Heterojunction Photocatalysts
3.3. Characterization
3.4. Fourier Transform Infrared Spectroscopic (FT–IR) Analysis
3.5. UV–Vis–DRS Measurements
3.6. Determination of •OH Radicals by Fluorescence Measurements
3.7. Evaluation of Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Konstantinou, I.K.; Albanis, T. Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: Intermediates and degradation pathways. Appl. Catal. B Environ. 2003, 42, 319–335. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonato, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, T.N.; Pillai, C.S.; Seery, K.M.; Falaras, P.; Kontos, G.A.; Dunlop, S.M.P.; Hamilton, W.J.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Mamba, G.M.; Mishra, A.K.M. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wang, X.W.; Antonietti, M.A. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Sun, Y.S.; Dong, F.D. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Zhang, M.Z.; Zhu, Y.Z. Photocatalytic enhancement of hybrid C3N4/TiO2 prepared via ball milling method. Phys. Chem. Chem. Phys. 2015, 17, 3647–3652. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Zhang, T.Z.; Li, B.L.; Jiang, S.J.; Zhang, X.Z.; Hai, L.H.; Chen, X.C.; Wu, W.W. An ingenious strategy of preparing TiO2/g-C3N4 heterojunction photocatalyst: In situ growth of TiO2 nanocrystals on g-C3N4 nanosheets via impregnation-calcination method. Appl. Surf. Sci. 2018, 433, 963–974. [Google Scholar] [CrossRef]
- Muňoz-Batista, M.J.M.B.; Kubacka, A.K.; Fernández-Garcia, M.F.G. Effect of g-C3N4 loading on TiO2—Based photocatalysts: UV and visible degradation of toluene. Catal. Sci. Technol. 2014, 4, 2006–2015. [Google Scholar] [CrossRef]
- Yu, J.Y.; Wang, S.W.; Low, J.L.; Xiao, W.X. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Wang, C.W.; Hu, L.H.; Chai, B.C.; Yan, J.Y.; Li, J.L. Enhanced photocatalytic activity of electrospun nanofibrous TiO2/g-C3N4 heterojunction photocatalyst under simulated solar light. Appl. Surf. Sci. 2018, 430, 243–252. [Google Scholar] [CrossRef]
- Tong, Z.W.T.; Yang, D.Y.; Xian, T.X.X.; Tian, Y.T.; Jiang, Z.Y.J. Biomimetic fabrication of g-C3N4/TiO2nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Hao, R.H.; Wang, G.W.; Jiang, C.J.; Tang, H.T.; Xu, Q.X. In situ hydrothermal synthesis of g-C3N4/TiO2 heterojunction photocatalysts with high specific surface area for Rhodamine B degradation. Appl. Surf. Sci. 2017, 411, 400–410. [Google Scholar] [CrossRef]
- Bhardwaj, N.B.; Kundu, S.C.K. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.W.; Liu, Z.L.; Fang, M.F.; Tang, C.T.; Huang, Z.H.; Liu, Y.L.; Wu, X.W.; Mao, Y.M. Enhancement in the photocatalytic activity of TiO2 nanofibers hybridized with g-C3N4 via electrospinning. Sol. Stat. Sci. 2016, 55, 1–7. [Google Scholar] [CrossRef]
- Liu, B.L.; Nakata, K.N.; Sakai, M.S.; Saito, H.S.; Ochiai, T.O.; Murakami, T.M.; Takagi, K.T.; Fujishima, A.F. Mesoporous TiO2 Core-Shell Spheres Composed of Nanocrystals with Exposed High-Energy Facets: Facile Synthesis and Formation Mechanism. Langmuir 2011, 27, 8500–8508. [Google Scholar] [CrossRef]
- Tang, Q.T.; Meng, X.M.; Wang, Z.W.; Zhou, J.Z.; Tang, H.T. One-step electrospinning synthesis of TiO2/g-C3N4 nanofibers with enhanced photocatalytic properties. Appl. Surf. Sci. 2018, 430, 253–262. [Google Scholar] [CrossRef]
- Adhikari, S.P.A.; Awasthi, G.P.A.; Kim, H.J.K.; Park, C.H.P.; Kim, C.S.K. Electrospinning Directly Synthesized Porous TiO2 Nanofibers Modified by Graphitic Carbon Nitride Sheets for Enhanced Photocatalytic Degradation Activity under Solar Light Irradiation. Langmuir 2016, 32, 6163–6175. [Google Scholar] [CrossRef]
- Wei, X.W.; Shao, C.S.; Li, X.L.; Lu, N.L.; Wang, K.W.; Zhang, Z.Z.; Liu, Y.L. Facile in situ synthesis of plasmonic nanoparticles-decorated g-C3N4/TiO2 heterojunction nanofibers and comparison study of their photosynergistic effects for efficient photocatalytic H2 evolution. Nanoscale 2016, 8, 11034–11043. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Chen, X.C.; Li, H.L.; Wu, Y.W.; Wu, H.W.; Wu, L.W.; Tan, P.T.; Pan, J.P.; Xiong, X.X. Facile fabrication of novel porous graphitic carbon nitride/copper sulfide nanocomposites with enhanced visible light driven photocatalytic performance. J. Colloid Interface Sci. 2016, 476, 132–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, D.T.T.; Schwartz, A.S. The glass transition temperature of poly (N-vinyl pyrrolidone) by differential scanning calorimetry. Polymer 1985, 26, 757–762. [Google Scholar] [CrossRef]
- Elishav, O.E.; Beilin, V.B.; Rozent, O.R.; Shter, G.E.S.; Grader, G.S.G. Thermal shrinkage of electrospun PVP nanofibers. J. Polym. Sci. B Polym. Phys. 2018, 56, 248–254. [Google Scholar] [CrossRef]
- Wen, J.W.; Xie, J.X.; Chen, X.C.; Li, X.L. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–133. [Google Scholar] [CrossRef]
- Shen, L.S.; Xing, Z.X.; Zou, J.Z.; Li, Z.L.; Wu, X.W.; Zhang, Y.Z.; Zhu, Q.Z.; Yang, S.Y.; Zhou, W.Z. Black TiO2 nanoblets/g-C3N4 nanosheets Laminated Heterojunctions with Efficient Visible Light-Driven Photocatalytic Performance. Sci. Rep. 2017, 7, 41978. [Google Scholar] [CrossRef]
- Low, J.L.; Jiang, C.J.; Cheng, B.C.; Wageh, S.W.; Al-Ghamdi, A.A.A.G.; Yu, J.Y. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 170080, 1–21. [Google Scholar] [CrossRef]
- Tao, B.T.; Yan, Z.Y. In-situ synthesis of highly efficient visible light driven stannic oxide/graphitic carbon nitride heterostructured photocatalysts. J. Colloid Interface Sci. 2016, 480, 118–125. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhang, T.Z.; Le, L.L.; Ruan, X.R.; Fang, P.F.; Pan, C.P.; Xiong, R.X.; Shi, J.S.; Wei, L.W. Quick and Facile Preparation of Visible light-Driven TiO2 Photocatalyst with High Photocatalytic Activity. Sci. Rep. 2014, 4, 7045. [Google Scholar] [CrossRef]
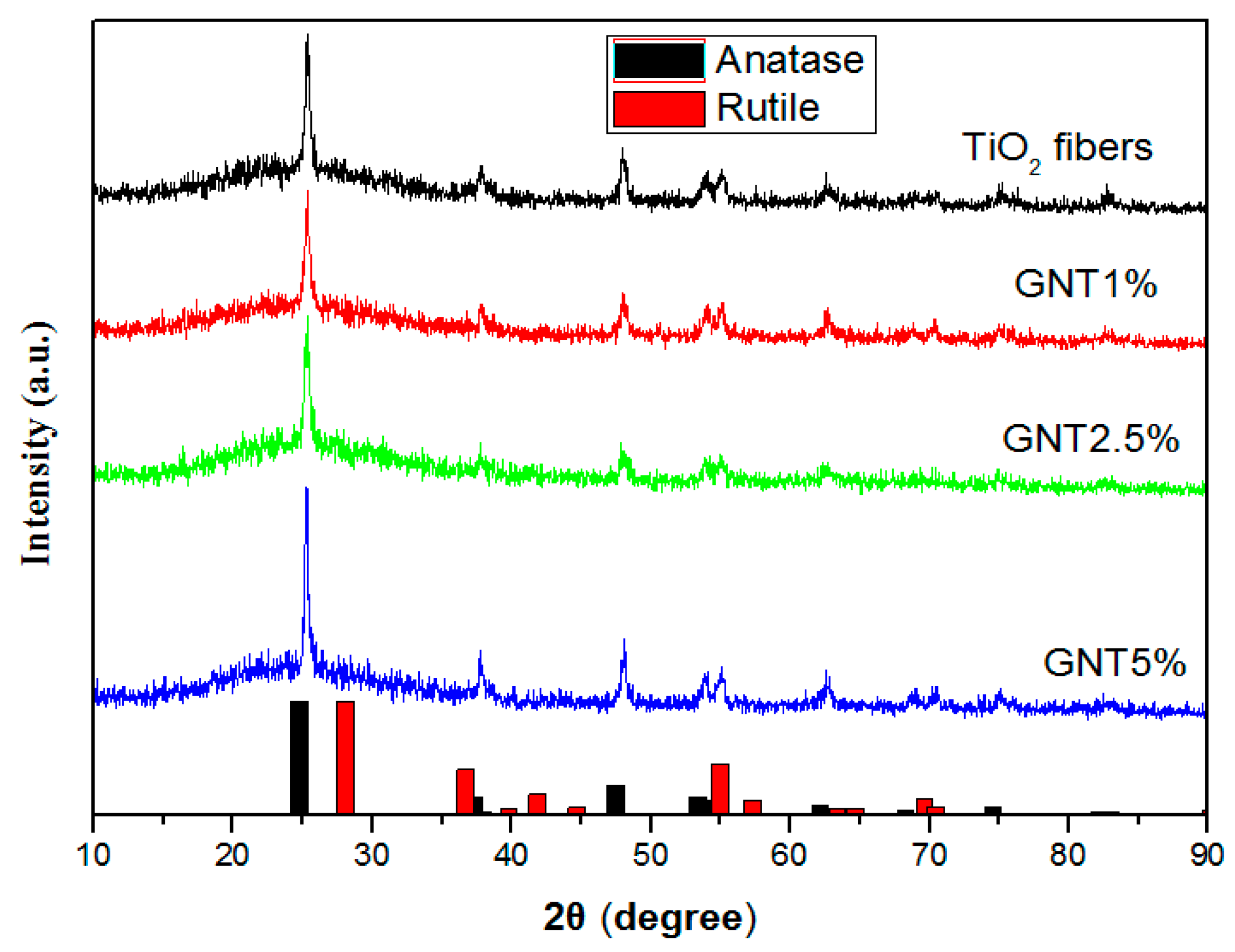
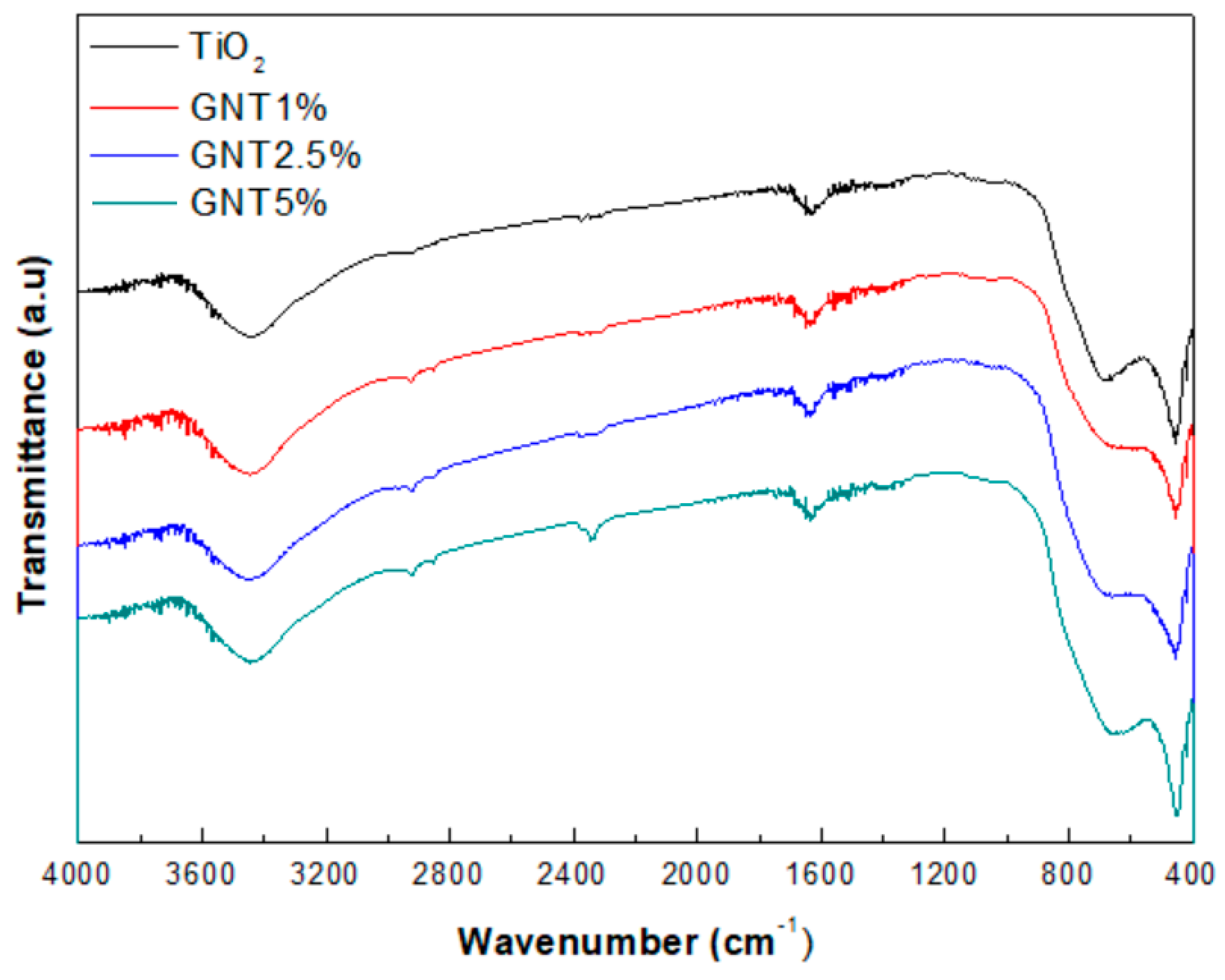
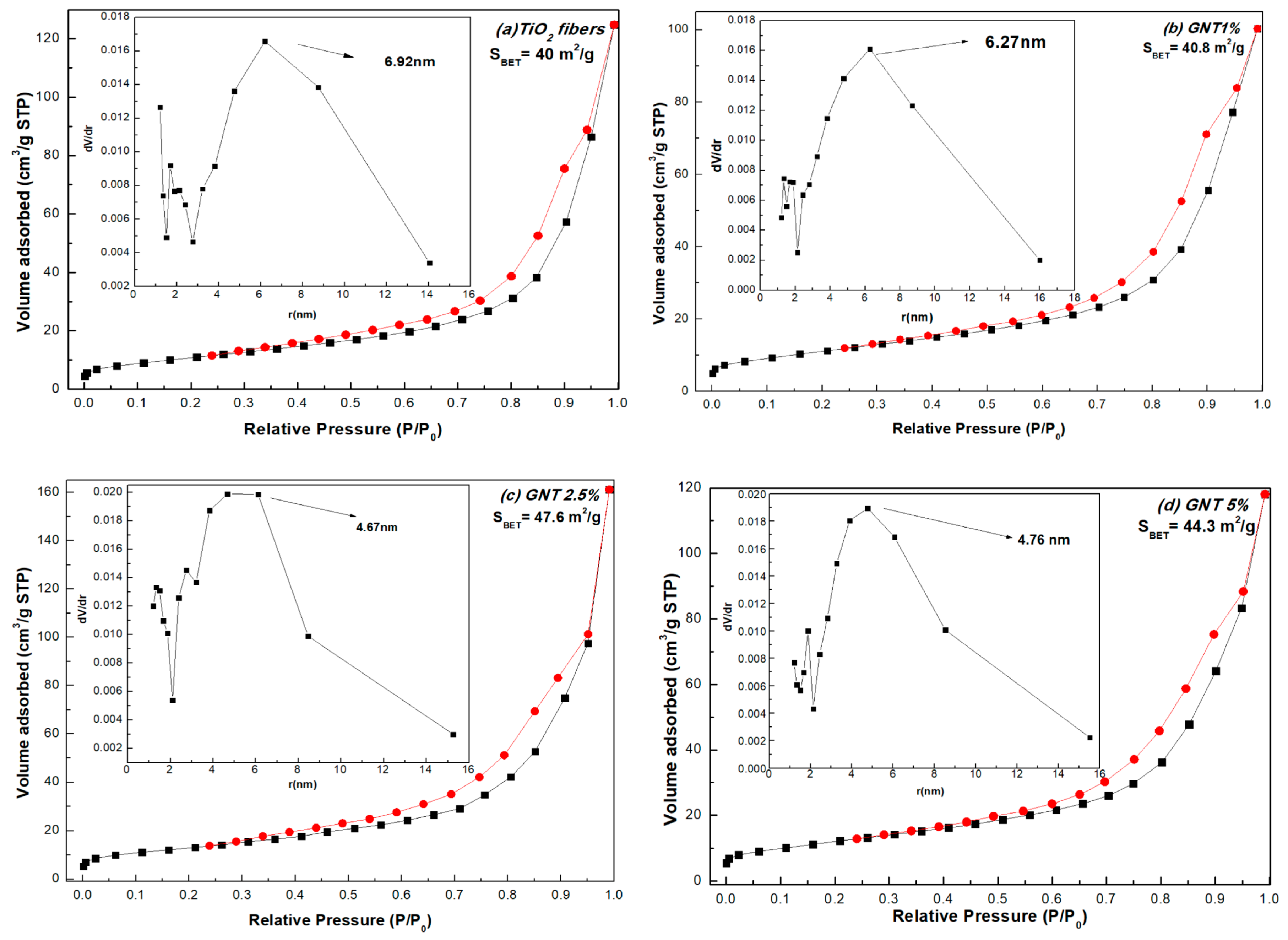
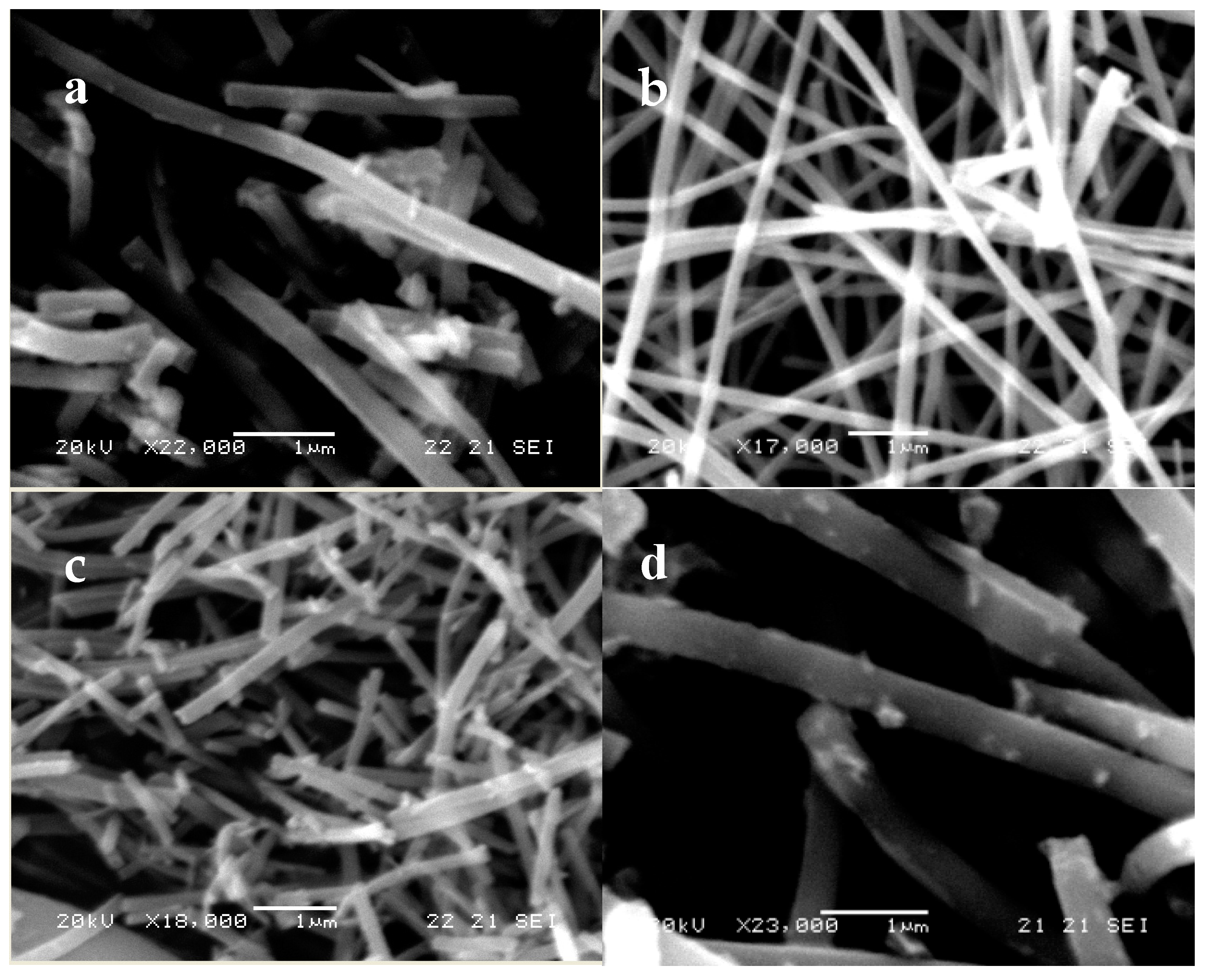
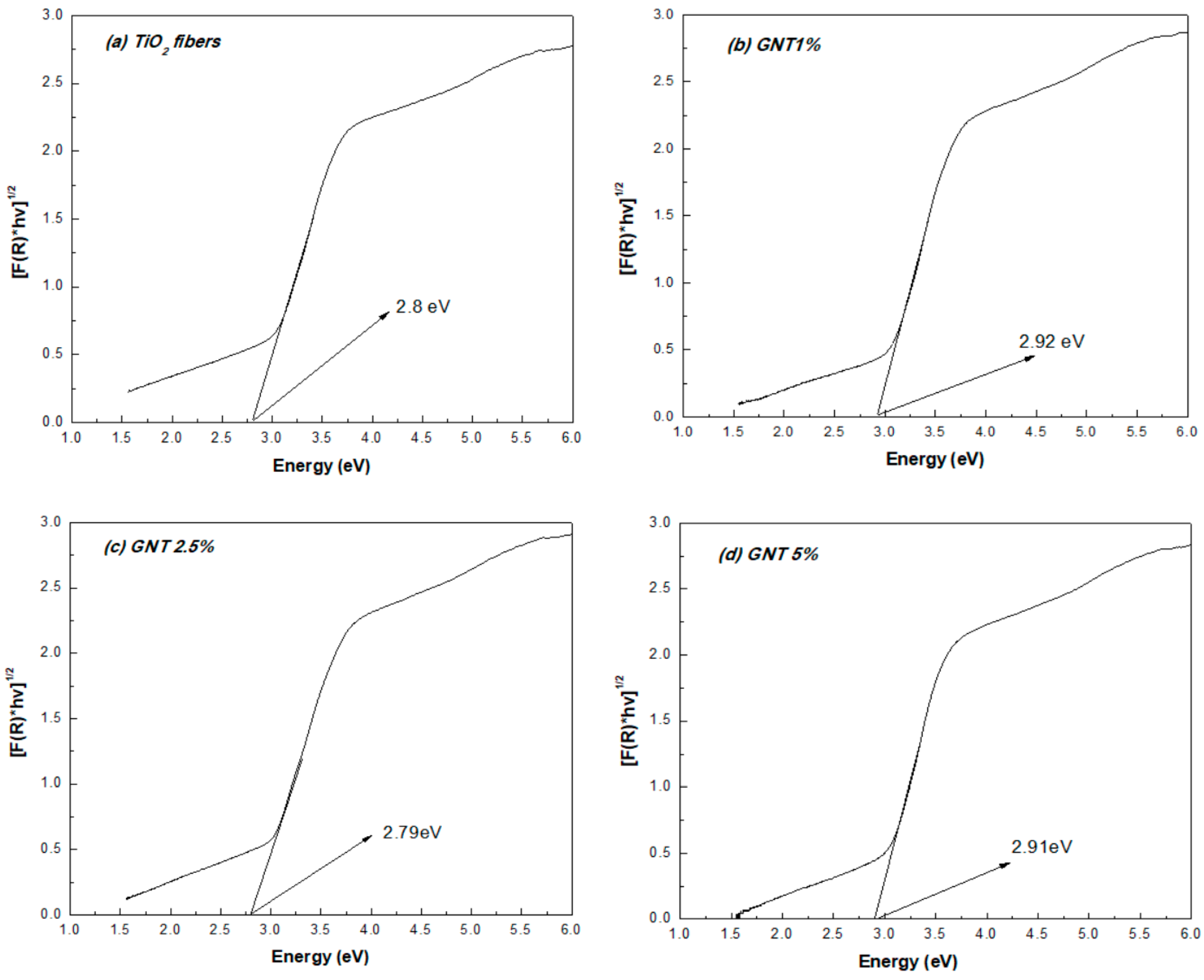



| Sample | % Anatase | % Rutile | Crystal Size (nm) | SBET (m2/g) | Average Pore Diameter (nm) | VTOT (cm3/g) | Energy Band Gap (eV) |
|---|---|---|---|---|---|---|---|
| TiO2 | 84.1 | 15.9 | 15.2 | 40.0 | 13.84 | 0.1589 | 2.80 |
| GNT 1% | 80.0 | 20.0 | 15.2 | 40.8 | 12.54 | 0.1277 | 2.92 |
| GNT 2.5% | 85.1 | 14.9 | 15.2 | 47.6 | 9.34 | 0.2499 | 2.79 |
| GNT 5% | 84.3 | 15.7 | 15.1 | 44.3 | 9.52 | 0.1831 | 2.91 |
| g-C3N4 | - | - | 27.0 | 35.2 | - | - | 2.82 |
| Sample (Fibers) | Formation Rate of •OH Radicals (μΜ/min) | R2 | K (min−1) | t1/2 (min) | R2 |
|---|---|---|---|---|---|
| TiO2 | 0.288 | 0.951 | 0.0628 | 11.1 | 0.9712 |
| GNT 1% | 0.297 | 0.917 | 0.0752 | 9.2 | 0.9869 |
| GNT 2.5% | 0.298 | 0.904 | 0.0781 | 8.87 | 0.9837 |
| GNT 5% | 0.348 | 0.929 | 0.1113 | 6.22 | 0.9789 |
| Sample GNT 5% | k (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| 1st cycle | 0.0836 | 8.3 | 0.9484 |
| 2nd cycle | 0.0797 | 8.7 | 0.9965 |
| 3rd cycle | 0.0736 | 9.4 | 0.9829 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bairamis, F.; Konstantinou, I.; Petrakis, D.; Vaimakis, T. Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue. Catalysts 2019, 9, 880. https://doi.org/10.3390/catal9110880
Bairamis F, Konstantinou I, Petrakis D, Vaimakis T. Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue. Catalysts. 2019; 9(11):880. https://doi.org/10.3390/catal9110880
Chicago/Turabian StyleBairamis, Feidias, Ioannis Konstantinou, Dimitrios Petrakis, and Tiverios Vaimakis. 2019. "Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue" Catalysts 9, no. 11: 880. https://doi.org/10.3390/catal9110880
APA StyleBairamis, F., Konstantinou, I., Petrakis, D., & Vaimakis, T. (2019). Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue. Catalysts, 9(11), 880. https://doi.org/10.3390/catal9110880





