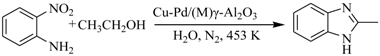Abstract
The direct synthesis of benzimidazoles from 2-nitroaniline and ethanol over Cu-Pd/γ-Al2O3 catalysts has the advantages of requiring easily available starting materials, having high efficiency, and a simple procedure. The modification by Mg of the Cu-Pd/γ-Al2O3 catalyst could improve the catalytic activity significantly. The addition of Mg to the Cu-Pd/γ-Al2O3 catalyst could maintain and promote the formation of CuPd alloy active sites. Meanwhile, the basicity of the support was enhanced appropriately by Mg, which generated more basic sites (Al-Oδ−) to accelerate the dehydrogenation of alcohol and increased the rate of the whole coupled reaction. The 2-nitroaniline was completely converted over Cu-Pd/(Mg)γ-Al2O3 after reacting for six hours, and the yield of 2-methylbenzimidazole was 98.8%. The results of this work provide a simple method to develop a more efficient catalyst for the “alcohol-dehydrogenation, hydrogen transfer and hydrogenation” coupled reaction system.
1. Introduction
Benzimidazoles are important organic intermediates which can be used as the key structural units of many drugs such as antibacterials, antimicrobials, antifungals, antituberculars, and anti-inflammatories [1,2,3,4,5,6]. Thus numerous methods for the synthesis of benzimidazoles from o-phenylenediamine and carbonyl derivatives promoted by inorganic acids and various oxidants have been reported, such as HCl [7], PPA [8], Na2S2O5 [9], molecular iodine [10], oxone [11], SnCl2·H2O [12], In(OTf)3 [13], Yb(OTf)3 [14], BF3·OEt2 [15], (Pd(dppf)Cl2 [16], etc. Although the above reactions were reported to be efficient, there were still many shortcomings in these methods, such as a high demand for equipment and large amounts of waste water due to the use of inorganic acids, difficulty in the separation and recycling of the homogeneous catalysts, many by-products and so on.
In order to find an environmentally friendly, high efficiency, low cost, high atomic and economic method for the synthesis of benzimidazoles, various heterogeneous catalytic systems such as heteropolyacid catalysts [17], modified zeolite catalysts [18], complex metal oxide catalysts [19,20], supported noble metal catalysts [21,22,23], Cu-PMO catalysts [24] and photocatalytic systems over Pt-TiO2 [25] were recently reported. In our recent work, the direct synthesis of benzimidazoles from 2-nitroaniline and alcohol in aqueous media catalyzed by Cu-Pd/γ-Al2O3 solid catalyst was studied [26]. We reported a successfully coupled multi-step reaction including dehydrogenation (Scheme 1. I), transfer hydrogenation (Scheme 1. II) and molecular nucleophilic cyclization, (Scheme 1. III) over a multifunctional heterogeneous catalyst (Scheme 1). The method used a cheaper and more readily available nitroaniline compound as a raw material for the reaction to achieve a simpler process and a substantial enhancement in the yields of desired products [26]. However, the activity of the catalyst was still low and the reaction took at least 12 h to ensure 100% conversion of 2-nitroaniline. It should be the most important factor to limit the reaction rate and the dehydrogenation of alcohol was difficult to achieve, even at 453 K over a Cu-Pd/γ-Al2O3 catalyst.
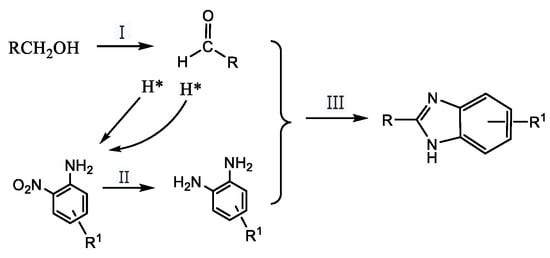
Scheme 1.
The mechanism of one-pot synthesis of benzimidazoles from o-nitrophenol and alcohols.
For the supported Ni catalysts, the nature of support has an important role in dehydrogenation of alcohols [27,28], in which the bifunctional support with both acidity-basicity is superior to the single acid or base support. Shimizu et al. [28] proposed that alcohol was converted to an alkoxide group on Lewis acid sites (Alδ+) and a proton on Lewis basic sites (Al-Oδ−) respectively over alumina. Alkoxide was then reacted with Ni0 to form ketone or aldehyde and Ni-H hydrides, followed by the protolysis of Ni-H hydrides and a neighboring proton to generate H2 and regenerate Ni0 and Al-Oδ− sites. Based on the mechanism presented above, it was suggested that the base sites of support were necessary to abstract the proton from alcohol. Thus, the appropriate amount of acid-base sites on the γ-Al2O3 surface is advantageous to accelerate the dehydrogenation of alcohol, which is the rate-determining step of the direct synthesis process of benzimidazoles from 2-nitroaniline and alcohol.
Therefore, we have reasons to believe that the adjusting of the acidity and basicity of the Cu-Pd/γ-Al2O3 catalyst surface has an important influence on the rate of this complex reaction. Inspired by previous researchers, Mg was selected as the secondary additive to add to the Pd-based catalyst in this paper. The effect of an addition of Mg to the Cu-Pd/γ-Al2O3 bimetallic catalyst in the hydrogenation of 2-Nitroaniline was also investigated. The results of this work can contribute to improving performance of catalysts for coupling of “dehydrogenation-hydrogen transfer-hydrogenation” multi-step reactions.
2. Results and Discussion
2.1. Synthesis of Benzimidazoles Over Pd-Cu/γ-Al2O3 Based Catalysts
The reaction of o-nitroaniline with ethanol over the catalysts was performed, and the results are listed in Table 1. 2-Methylbenzimidazole was the main product while 2-propylbenzimidazole was formed as a byproduct. 2-Propylbenzimidazole might be formed through self-aldolization of acetaldehyde to crotonaldehyde [29]. The Pd/γ-Al2O3 catalyst gave low conversion of o-nitroaniline and a low yield of benzimidazole, whereas the Cu/γ-Al2O3 catalyst showed no activity (entries 1-2). When blending Cu/γ-Al2O3 and Pd/γ-Al2O3 physically as the catalyst, the conversion of o-nitroaniline was only 67.3%, and the yield of 2-methylbenzimidazole was 64.7% within 12 h (entry 3). The yield of 2-methylbenzimidazole reached 89.2% within 6 h using Cu-Pd/γ-Al2O3 as the catalyst (entry 4). From these results, it might be deduced that Cu-Pd bimetallic catalysts could effectively catalyze the formation of benzimidazoles from o-nitroaniline with ethanol. The higher activity of the Cu-Pd catalyst was attributed to the accompanying synergistic interaction between Pd and Cu by forming CuPd alloy compound [26]. Surprisingly the addition of alkali metal and alkaline earth metal could effectively promote the reaction and enhance the selectivity. Also, the modification by different alkali additives can affect the activity of Cu-Pd/γ-Al2O3 catalysts. The conversion of ο-nitroaniline was significantly increased by doping Mg, K and Sr (entries 5, 7, 10), and the highest benzimidazole yield was obtained over the Cu-Pd/(Mg)γ-Al2O3 catalyst. The effect on the catalytic activity of the Ca, Cs, Ba, and La-modified catalysts were relatively weak in comparison with that of the Mg-modified catalyst.

Table 1.
Evaluation of the catalytic activity of various catalysts.
2.2. Effect of Mg Modification on the Formation of CuPd Alloy
According to reported literature, Pd-Cu bimetallic catalysts were used in various reactions to improve the selectivity of target products such as the degradation of nitrates [30] and the steam reforming of methanol [31]. In our previous work [26], the superior catalytic performance of Cu-Pd/γ-Al2O3 catalysts was attributed to the formation of the CuPd compound: The active component Cu promoted the dehydrogenation of alcohol to aldehyde, and at the same time, Pd promoted transfer hydrogenation of o-nitroaniline to o-phenylendiamine, the synergistic interaction between Pd and Cu makes the reaction proceed smoothly. Therefore, the enhancement of catalytic performance of the Mg-doped catalysts in this work might be related to the stimulative formation of the CuPd alloy.
The HRTEM analysis of Cu-Pd/(Mg)γ-Al2O3 catalysts were performed and the results are shown in Figure 1A,B. Lattice fringe images of monometallic Pd particles show the lattice spacings at 2.27 Å, 1.97 Å, and 1.40 Å corresponding to (111), (200), (220) planes of Pd. Fourier transforms analysis (FFT) of high resolution images of Cu-Pd bimetallic catalyst particles show the lattice spacings at 2.18 Å, 1.89 Å, and 1.38 Å corresponding to (111), (200), (220) planes of CuPd, which are lower than the Pd metallic spacing. It could suggest the existence of the CuPd alloy structure in the bimetallic catalysts. The mean size of metal particles for Cu-Pd/(Mg)γ-Al2O3 is about 7.2 nm (Figure 1C). These results suggested that the CuPd alloy was the primary active component in the Cu-Pd/(Mg)γ-Al2O3 catalyst, and that the CuPd alloy was highly dispersed on the surface of the support.
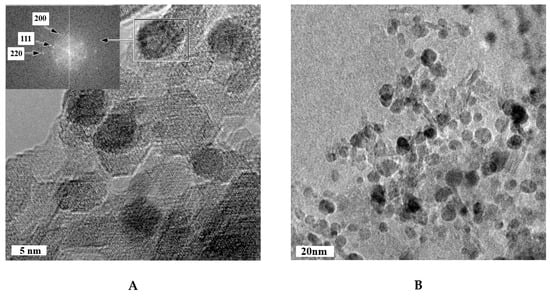
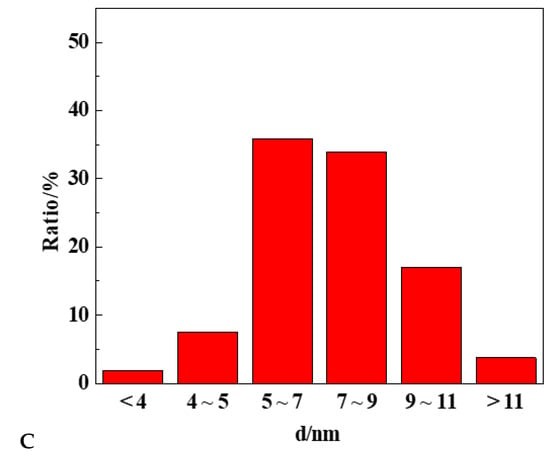
Figure 1.
(A,B) TEM images and (C) particle size distribution profiles of Cu-Pd/(Mg)γ-Al2O3 catalysts.
Figure 2 shows the TPR profiles of the calcined samples. The PdO/γ-Al2O3 sample showed a negative peak at 361 K, corresponding to liberated hydrogen during the decomposition of Pd hydride [32,33,34], which indicated that PdO was already reduced to Pd0 at room temperature. The CuO/γ-Al2O3 sample displayed a reduction peak with two maxima at 477 K and 528 K. The reduction peak at low temperature might be attributed to the reduction of superficial Cu2O to Cu0 and the reduction peak at high temperature might be assigned to the reduction of CuO to Cu0 [35]. However, for the Cu-Pd-based samples, the negative peak at 361 K completely disappeared, and only a hydrogen desorption peak was shown at around 406 K, indicating that the formation of Pd hydride is inhibited with the addition of Cu [33]. The displacement of reduction peak in the TPR profiles of (c) and (d) also indicate that the Cu oxides could be reduced at a much lower temperature in the presence of Pd, resulting from the hydrogen spillover from Pd0 to Cu oxides [34]. Thus, it could be seen that there was an interaction between Pd and Cu species in the Cu-Pd-bimetallic catalysts which generated the CuPd alloy formation. Furthermore, by comparing the TPR profiles of (c) and (d), it indicated that the addition of Mg had negligible influence on the position of reduction peak, but the reduction peak area of CuPd alloy increased obviously.
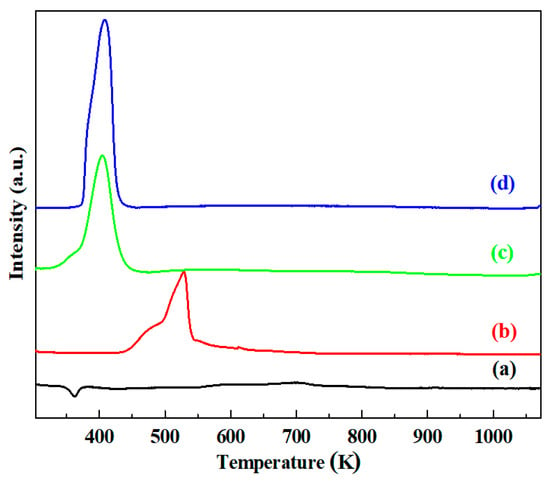
Figure 2.
TPR profiles of catalysts: (a) PdO/γ-Al2O3; (b) CuO/γ-Al2O3; (c) CuO-PdO/γ-Al2O3; (d) CuO-PdO/(Mg)γ-Al2O3.
Figure 3 shows the XRD patterns of the reduced catalyst samples. All catalyst samples exhibited diffraction peaks at 2θ = 66.7°, 45.8° and 37.6° corresponding to a γ-Al2O3 structure. Diffraction peaks of the Pd/γ-Al2O3 catalyst and pure γ-Al2O3 carrier were basically identical. The peaks at 43.3°, 50.4° and 74.1° corresponding to Cu were observed with the Cu/γ-Al2O3 catalyst. For the Cu-Pd/γ-Al2O3 and Cu-Pd/(Mg)γ-Al2O3 catalysts, no Cu diffraction peak was found, but the diffraction peaks at 2θ = 41.6°, 48.3° and 70.8° which are characteristic of (111), (200) and (220) planes for CuPd alloy respectively [31,36], were observed. The intensity of the CuPd alloy diffraction peaks of Cu-Pd/(Mg)γ-Al2O3 was much higher than that of Cu-Pd/γ-Al2O3. Besides, the peak of MgO phase was not found in the XRD patterns of the Cu-Pd/(Mg)γ-Al2O3 catalyst and the characteristic peaks of γ-Al2O3 had no obvious change. The content of Mg was low, and Mg was well dispersed in the γ-Al2O3 rather than assembled in grains. Both of the TPR and XRD results indicated that the addition of Mg might promote the formation of the CuPd alloy.
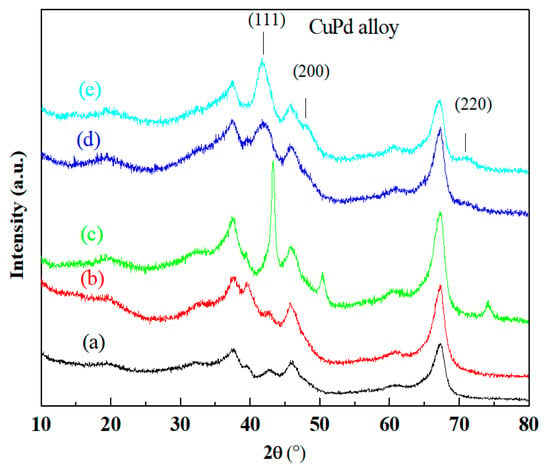
Figure 3.
X-ray diffraction patterns of the reduced catalysts (a) γ-Al2O3; (b) Pd/γ-Al2O3; (c) Cu/γ-Al2O3; (d) Cu-Pd/γ-Al2O3; (e) Cu-Pd/(Mg)γ-Al2O3.
2.3. Effect of Mg Modification on the Surface Acidity and Basicity of Catalysts
Besides the effect on the formation of CuPd alloy, the modification of Mg could influence the surface chemical properties of the Cu-Pd/γ-Al2O3 catalyst significantly. The CO2-TPD analysis was examined to investigate the effect on the surface basicity of the Cu-Pd/γ-Al2O3 catalyst with the addition of Mg, and the results are shown in Figure 4. Both the Cu-Pd/γ-Al2O3 and Cu-Pd/(Mg)γ-Al2O3 catalysts showed the desorption peak of CO2 at 385–395 K, which should be attributed to the low basicity sites on catalyst surface formed by Brønsted OH groups [37,38]. Both of two catalyst samples showed a desorption peak over 573 K, which corresponded to the high basicity sites resulting from low coordination oxygen anions (O2−) [37,38]. However, the concentrations of high and low basicity sites in the Cu-Pd/(Mg)γ-Al2O3 catalyst were higher than that in the Cu-Pd/γ-Al2O3 catalyst, and the peak for high basicity sites shifted to a high temperature range in the Cu-Pd/(Mg)γ-Al2O3 catalyst. Moreover, another desorption peak at 430 K corresponding to medium basicity sites generated from metal-oxygen pairs (M-Oδ− pairs) [37,38] was observed in the Cu-Pd/(Mg)γ-Al2O3 catalyst but not found in the Cu-Pd/γ-Al2O3 catalyst. These CO2-TPD results suggested that the basicity intensity and concentration especially the medium and strong basic sites of the Cu-Pd/γ-Al2O3 catalyst could be promoted by the doping of Mg.
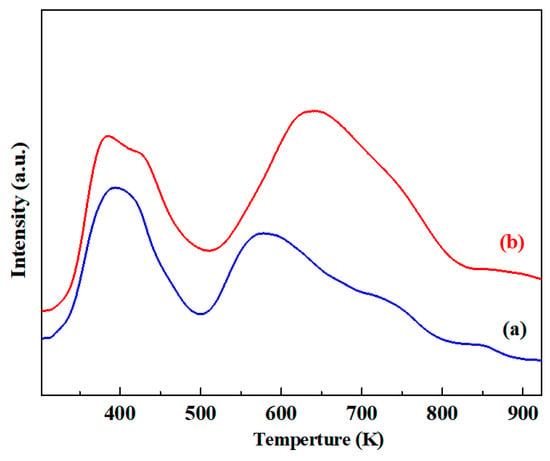
Figure 4.
CO2-TPD profiles for catalyst samples: (a) Cu-Pd/γ-Al2O3; (b) Cu-Pd/(Mg)γ-Al2O3.
Figure 5 shows the FTIR spectra of adsorbed pyridine (Py-FTIR) at different temperatures for various catalysts. The spectrum of the Cu-Pd/γ-Al2O3 catalyst contained three main peaks at 1448, 1488, 1598 cm−1, which are responsible for Lewis acid sites [39], after being evacuated at 303 K. However, the intensity of the three peaks was much higher than that of Cu-Pd/(Mg)γ-Al2O3. The spectrum of the Cu-Pd/γ-Al2O3 catalyst after being evacuated at 473 K contained two peaks at 1449 and 1608 cm−1, which indicated the presence of strong Lewis acid sites. Furthermore, the peak at 1549 cm−1, that is responsible for Brønsted acid sites [40], was detected in the Cu-Pd/γ-Al2O3 catalyst but disappeared with the addition of Mg, indicating a deactivation of acid sites through Mg doping.
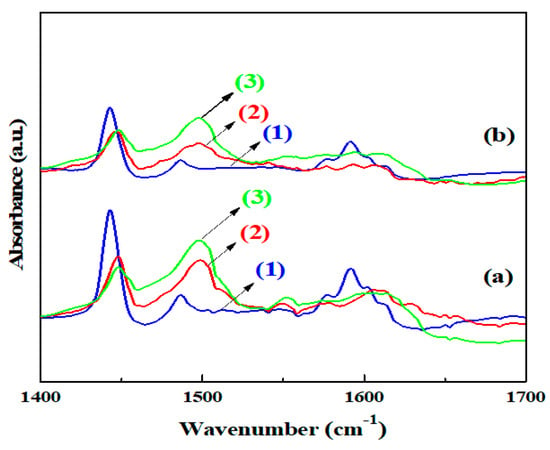
Figure 5.
FTIR spectra of pyridine adsorbed of the samples: (a) Cu-Pd/γ-Al2O3 measured after heating at (1) 303 K, (2) 373 K, (3)473 K; (b) Cu-Pd/(Mg)γ-Al2O3 measured after heating at (1) 303 K, (2) 373 K, (3) 473 K.
The CO2-TPD and Py-FTIR results suggested that the strong Lewis and Brønsted acid sites on the Cu-Pd/γ-Al2O3 catalyst surface were reduced by the doping of Mg. Meanwhile, the basic Al-Oδ− groups of Cu-Pd/(Mg)γ-Al2O3 catalyst were increased, which made Lewis acid (Alδ+)-base (Al-Oδ−) equilibrium of the catalyst. The Cu-Pd/(Mg)γ-Al2O3 catalyst was most likely to follow a multifunctional mechanism in the dehydrogenation of alcohols which was similar to previous reports [28]. Both the Cu0 and the acid-basic sites on the (Mg)γ-Al2O3 support were responsible for promoting dehydrogenation of alcohol to aldehyde, resulting in the acceleration of the whole reaction system.
Finally, the fascinating applicability of the method towards diversified 4,4’-Diamino-3,3’-dinitrobiphenyl and alcohol prompted us to design some more complex benzimidazole derivatives that can serve as lead compounds in pharmaceutical research. To our delight, we were able to synthesize a bisbenzimidazole (3) through a reaction between 4,4’-Diamino-3,3’-dinitrobipheny (1) and alcohol (2) at an excellent yield (Scheme 2). As experimental results showed, 4,4’-Diamino-3,3’-dinitrobiphenyl was almost completely converted to bisbenzimidazole over the Cu-Pd/(Mg)γ-Al2O3 catalyst within 6 h, and bisbenzimidazole was obtained an a 98% yield.
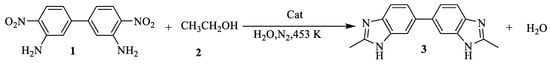
Scheme 2.
Synthesis of bisbenzimidazole from 4,4’-diamino-3,3’-dinitrobipheny and alcohol. Reaction conditions: 3,3'-Dinitrobenzidine (2 g), H2O (20 mL), ethanol (50 mL), Cat (0.2 g), 453 K, 3.5 MPa, N2 atmosphere, Yield: 98% (6 h).
3. Materials and Methods
3.1. Catalyst Preparation
The (M)γ-Al2O3 supports (M = Na, K, Cs, Mg, Ca, Sr, Ba, La) were prepared by an incipient wetness method. All supports were prepared by the impregnation of γ-Al2O3 with an aqueous solution of alkaline or alkaline earth nitrate (5 wt. % content for M). After impregnation, the sample was dried in the oven at 383 K for 12 h and then was calcined at 673 K for 4 h in air. The Cu-Pd/(M)γ-Al2O3 catalysts were prepared by a deposition-precipitation method. Sufficient amounts of H2PdCl4 and Cu (NO3)2·3H2O aqueous solutions were mixed together (to produce a catalyst containing 5 wt. % Pd and 5 wt. % Cu). This mixture was added dropwise to a 10 wt. % slurry of γ-Al2O3 (or (M)γ-Al2O3) and deionized water, with constant stirring by a magnetic stirring apparatus. The temperature was increased to 353 K and kept at this temperature for 5 h. After impregnation, the NaHCO3 solution (1 M) was used to adjust the pH value of the solution to 8–9, and stirring continued for 30 min. Then, the solid was filtered, washed, dried at 383 K for 4 h and calcined in air at 533 K for 4 h. Finally, the samples were reduced in H2 at 533 K for 2 h.
3.2. Catalyst Characterization
The X-ray diffraction (XRD) of catalysts was carried out with an X’ Pert PRO diffractometer (PNAlytical Co., Almelo, Netherlands) at 45 kV and 40 mA using a Cu Kα radiation source with a scanning rate of 2°/min and a step of 0.02°. TEM measurements were performed on a JEOL JEM-200CX instrument (Tokyo, Japan) operating at 160 kV. We randomly selected 300 particles of the catalyst in the TEM images to determine the mean particle size. Temperature programmed reduction under H2 (TPR) was carried out with a BELCAT instrument (BELL Japan Inc., Osaka, Japan). The calcined sample (0.1 g of CuO-PdO/γ-Al2O3 or CuO-PdO/(M)γ-Al2O3) was heated from 303 K to 1073 K at a rate of 10 K/min in a flow of 5% (V/V) H2/Ar (25 mL/min), and the hydrogen consumption was obtained by a thermal conductivity detector. Temperature-programmed desorption (TPD) of CO2 was conducted on a BELCAT (Osaka, Japan). For example, 0.075 g of catalyst was heated to 533 K in a flow of He (30 mL/min) and maintained for 40 min. Then the catalyst was cooled to 303 K under He flow, and followed by exposing it to a flow of 100% CO2 for 30 min. Subsequently, the catalyst was purged in He at 303 K for 60 min. Finally, the catalyst was heated to 923 K at a rate of 10 K/min in a flow of He, and CO2 (m/e = 44), the outlet gas was detected by the mass spectrometer (BELMASS, Osaka, Japan). The Py-FTIR spectra was obtained on a Nicolet 6700 spectrometer (Madison, WI, USA). The sample was heated to 533 K and kept at this temperature for 1 h in a vacuum (1 × 10−2 MPa), and then cooled down to 303 K, followed by exposure to the vapor of pyridine. The Py-IR spectra was recorded at 303 K, 373 K and 473 K respectively, in a vacuum for 30 min.
3.3. Catalytic Tests
In the experiment, the mixture, composed of 8 g of the nitroaromatic compound, 0.8 g of catalyst, 120 mL of alcohol and 80 mL of deionized water, was placed in a 500 mL stainless steel autoclave. The reactor was sealed and purged by high purity N2 five times. Then the pressure in the reactor was raised to 3.5 MPa using N2 after the reactor was heated to the desired temperature, at a stirring rate of 1000 rpm. The temperature, pressure and stirring rate were kept constant during the reaction. After the specified reaction time, the reactor was cooled down to room temperature and the pressure in the reaction system was released. The catalyst was filtered from the mixture and the liquid product was qualitatively analyzed using gas chromatography-mass spectrometry (GC-MS, Agilent 5973N, Palo Alto, CA, USA) and quantitatively analyzed by GC (Agilent 7890A, Shanghai, China) equipped with a flame ionization detector (FID) and a DB-1 capillary column (30 m × 0.32 mm × 3 μm) using the area-normalization method.
4. Conclusions
We efficiently synthesized benzimidazole from low-cost alcohol and o-nitroaniline over a Cu-Pd/(Mg)γ-Al2O3 heterogeneous catalyst. The Cu-Pd bimetallic catalyst had better catalytic activity than that of monometallic Cu or Pd catalyst in the reaction system. It was mainly attributed to the formation of the CuPd alloy and the synergistic effect between Pd and Cu. Moreover, the catalytic activity of the Cu-Pd/(Mg)γ-Al2O3 catalyst with the addition of Mg was superior to Cu-Pd/γ-Al2O3, because of the addition of Mg. The formation of the CuPd alloy was promoted and the surface acid sites of Cu-Pd/γ-Al2O3 catalyst were neutralized, which improved the basicity of the support, as well as enhanced the rate of ethanol dehydrogenation, and finally accelerated the whole reaction system.
Author Contributions
Conceptualization, X.L.; methodology, F.F. and Q.Z; investigation and validation, Y.D. and Z.C.; catalysts characterization, F.F. and C.L.; mechanism analysis, X.X. and L.M.; writing—original draft preparation, F.F.
Funding
Financial support by the National Natural Science Foundation of China (21406199, 21776258 and 21476208) and the Program for Science and Technology Department of Zhejiang Province (LGG18B060004 and LY17B060008) are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, S.; Gangal, S.; Rauf, A. Convinient one-pot synthesis of novel 2-substituted benzimidazoles, tetrahydrobenzimidazoles and imidazoles and evaluation of their in vitro antibacterial and antifungal activities. Eur. J. Med. Chem. 2009, 44, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S. Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles. Eur. J. Med. Chem. 2009, 44, 4244–4248. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.F.; Lal, C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur. J. Med. Chem. 2009, 44, 4028–4033. [Google Scholar] [CrossRef] [PubMed]
- Mungra, D.C.; Patel, M.P.; Patel, R.G. Microwave-assisted synthesis of some new tetrazolo[1,5-a]quinoline-based benzimidazoles catalyzed by p-TsOH and investigation of their antimicrobial activity. Med. Chem. Res. 2011, 20, 782–789. [Google Scholar] [CrossRef]
- Jain, R.; Agarwal, D.D.; Sahu, P.K.; Selvam, D.T.; Sharma, Y.; Gupta, R.; Prakash, A. Mild and highly efficient copper(II) sulfate catalyzed one pot synthesis of 2-aryl benzimidazole using atmospheric air as an oxidant and its antibacterial study. Med. Chem. Res. 2013, 22, 1788–1794. [Google Scholar] [CrossRef]
- Chen, G.Z.; Liu, Z.G.; Zhang, Y.L.; Shan, X.O.; Jiang, L.L.; Zhao, Y.J.; He, W.F.; Feng, Z.G.; Yang, S.L.; Liang, G. Synthesis and anti-inflammatory evaluation of novel benzimidazole and imidazopyridine derivatives. ACS Med. Chem. Lett. 2013, 4, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, X.X.; Yu, X.Q.; Shi, L.; Sun, Y. Acid-catalyzed solvent-free synthesis of 2-arylbenzimidazoles under microwave irradiation. J. Mol. Catal. A Chem. 2013, 22, 1788–1794. [Google Scholar] [CrossRef]
- Lu, J.; Ge, H.G.; Bai, Y.J. Solvent-free synthesis of 2-substituted benzimidazoles under microwave-irradiation using PPA as a catalyst. Chin. J. Org. Chem. 2002, 22, 782–784. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Moreno-Diaz, H.; Aguirre-Crespo, F.; León-Rivera, I.; Villalobos-Molina, R.; Muñoz-Muñiz, O.; Estrada-Soto, S. Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbene bioisosteres. Bioorg. Med. Chem. Lett. 2006, 16, 4169–4173. [Google Scholar] [CrossRef]
- Sun, P.P.; Hu, Z.X. The convenient synthesis of benzimidazole derivatives catalyzed by I2 in aqueous media. Heterocycl. Chem. 2006, 43, 773–775. [Google Scholar] [CrossRef]
- Lin, S.N.; Yang, L.H. A simple and efficient procedure for the synthesis of benzimidazoles using air as the oxidant. Tetrahedron Lett. 2005, 46, 4315–4319. [Google Scholar] [CrossRef]
- Duan, L.P.; Li, Q.; Wu, N.B.; Xu, D.F.; Zhang, H.B. Synthesis of 2,5-disubstitued benzimidazole using SnCl2-catalyzed reduction system at room temperature. Chin. Chem. Lett. 2014, 25, 155–158. [Google Scholar] [CrossRef]
- Trivedi, R.; De, S.K.; Gibbs, R.A. A convenient one-pot synthesis of 2-substituted benzimidazoles. J. Mol. Catal. A Chem. 2006, 245, 8–11. [Google Scholar] [CrossRef]
- Shen, M.G.; Cai, C. Ytterbium perfluorooctanesulfonates catalyzed synthesis of benzimidazole derivatives in fluorous solvents. J. Fluor. Chem. 2007, 128, 232–235. [Google Scholar] [CrossRef]
- Nagawade, R.R.; Shinde, D.B. BF3.OEt2 promoted solvent-free synthesis of benzimidazole derivatives. Chin. Chem. Lett. 2006, 17, 453–456. [Google Scholar]
- Li, X.T.; Hu, R.H.; Tong, Y.; Pan, Q.; Miao, D.Z.; Han, S.Q. An efficient route for the synthesis of benzimidazoles via a hydrogentransfer strategy between o-nitroanilines and alcohols. Tetrahedron Lett. 2016, 57, 4645–4649. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H. A Heterogeneous catalyst for efficient and green synthesis of 2-arylbenzothiazoles and 2-arylbenzimidazoles. Appl. Catal. A 2009, 353, 74–79. [Google Scholar] [CrossRef]
- Gadekar, L.S.; Arbad, B.R.; Lande, M.K. Eco-friendly synthesis of benzimidazole derivatives using solid acid scolecite catalyst. Chin. Chem. Lett. 2010, 21, 1053–1056. [Google Scholar] [CrossRef]
- Shingalapur, R.V.; Hosamani, K.M. An Efficient and eco-friendly tungstate promoted zirconia (WOx/ZrO2) solid acid catalyst for the synthesis of 2-aryl benzimidazoles. Catal. Lett. 2010, 137, 63–68. [Google Scholar] [CrossRef]
- Rathod, S.B.; Lande, M.K.; Arbad, B.R. Synthesis, characterization and catalytic application of MoO3/CeO2-ZrO2 solid heterogeneous catalyst for the synthesis of benzimidazole derivatives. Bull. Korean Chem. Soc. 2010, 31, 2835–2840. [Google Scholar] [CrossRef]
- Tateyama, K.; Wada, K.; Miura, H.; Hosokawa, S.; Abe, R.; Inoue, M. Dehydrogenative synthesis of benzimidazoles under mild conditions with supported iridium catalysts. Catal. Sci. Technol. 2016, 6, 1677–1684. [Google Scholar] [CrossRef]
- Ruiz, V.R.; Corma, A.; Sabater, M.J. New route for the synthesis of benzimidazoles by a one-pot multistep process with mono and bifunctional solid catalysts. Tetrahedron 2010, 66, 730–735. [Google Scholar] [CrossRef]
- Chaudhari, C.; Siddiki, S.; Shimizu, K. Acceptorless dehydrogenative synthesis of benzothiazoles and benzimidazoles from alcohols or aldehydes by heterogeneous Pt catalysts under neutral conditions. Tetrahedron Lett. 2015, 56, 4885–4888. [Google Scholar] [CrossRef]
- Sun, Z.H.; Bottari, B.; Barta, K. Supercritical methanol as solvent and carbon source in the catalytic conversion of 1,2-diaminobenzenes and 2-nitroanilines to benzimidazoles. Green Chem. 2015, 17, 5172–5181. [Google Scholar] [CrossRef]
- Selvam, K.; Swaminathan, M. An easy one-step photocatalytic synthesis of 1-aryl-2-alkylbenzimidazoles by platinum loaded TiO2 nanoparticles under UV and solar light. Tetrahedron Lett. 2011, 52, 3386–3392. [Google Scholar] [CrossRef]
- Feng, F.; Ye, J.; Cheng, Z.; Xu, X.X.; Zhang, Q.F.; Ma, L.; Lu, C.S.; Li, X.N. Cu–Pd/γ-Al2O3 catalyzed the coupling of multistep reactions: Direct synthesis of benzimidazole derivatives. RSC Adv. 2016, 6, 72750–72755. [Google Scholar] [CrossRef]
- Fang, W.H.; Zhang, Q.H.; Chen, J.; Deng, W.P.; Wang, Y. Gold nanoparticles on hydrotalcites as efficient catalysts for oxidant-free dehydrogenation of alcohols. Chem. Commun. 2010, 46, 1547–1549. [Google Scholar] [CrossRef]
- Shimizu, K.; Kon, K.; Shimura, K.; Hakim, S.S. Acceptor-free dehydrogenation of secondary alcohols by heterogeneous cooperative catalysis between Ni nanoparticles and acid–base sites of alumina supports. J. Catal. 2013, 300, 242–250. [Google Scholar] [CrossRef]
- Scalbert, J.; Thibault-Starzyk, F.; Jacquot, R.; Morvan, D.; Meunier, F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization? J. Catal. 2014, 311, 28–32. [Google Scholar] [CrossRef]
- Sá, J.; Gross, S.; Vinek, H. Effect of the reducing step on the properties of Pd-Cu bimetallic catalysts used for denitration. Appl. Catal. A 2005, 294, 226–234. [Google Scholar] [CrossRef]
- Mierczynskia, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T.P. Highly selective Pd–Cu/ZnAl2O4 catalyst for hydrogen production. Appl. Catal. A 2014, 479, 26–34. [Google Scholar] [CrossRef]
- Das, N.N.; Das, R. Synthesis, characterization and activation of quaternary layered double hydroxides for the one-pot synthesis of methyl isobutyl ketone. React. Kinet. Mech. Catal. 2010, 99, 397–408. [Google Scholar] [CrossRef]
- Marínez-Ortiz, M.J.; Tichit, D.; Gonzalez, P.; Coq, B. The “one-pot” synthesis of 4-methyl-2-pentanone (methyl isobutyl ketone) from acetone over PdCu catalysts prepared from layered double hydroxides. J. Mol. Catal. A Chem. 2003, 201, 199–210. [Google Scholar] [CrossRef]
- Batista, J.; Pintar, A.; Mandrino, D.; Jenko, M.; Martin, V. XPS and TPR examinations of γ-alumina-supported Pd-Cu catalysts. Appl. Catal. A 2001, 206, 113–124. [Google Scholar] [CrossRef]
- Sá, J.; Vinek, H. Catalytic hydrogenation of nitrates in water over a bimetallic catalyst. Appl. Catal. B 2005, 57, 247–256. [Google Scholar] [CrossRef]
- Guy, K.A.; Xu, H.P.; Yang, J.C.; Werth, C.J.; Shapley, J.R. Catalytic nitrate and nitrite reduction with Pd-Cu/PVP colloids in water: composition, structure, and reactivity correlations. J. Phys. Chem. C 2009, 113, 8177–8185. [Google Scholar] [CrossRef]
- Cosimo, J.I.D.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Díez, V.K.; Apesteguía, C.R.; Cosimo, J.I.D. Effect of the chemical composition on the catalytic performance of MgyAlOx catalysts for alcohol elimination reactions. J. Catal. 2003, 215, 220–233. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Devassy, B.M.; Lefebvre, F.; Halligudi, S.B. Zirconia-supported 12-tungstophosphoric acid as a solid catalyst for the synthesis of linear alkyl benzenes. J. Catal. 2005, 231, 1–10. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).