Optimization of Biodiesel Production from Waste Cooking Oil Using S–TiO2/SBA-15 Heterogeneous Acid Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Prepared Catalyst
2.1.1. Physical Properties of the Catalyst
2.1.2. Low- and Wide-Angle X-ray Diffraction
2.1.3. Scanning Electron Microscopy
2.1.4. Acid–Base Neutralization Technique
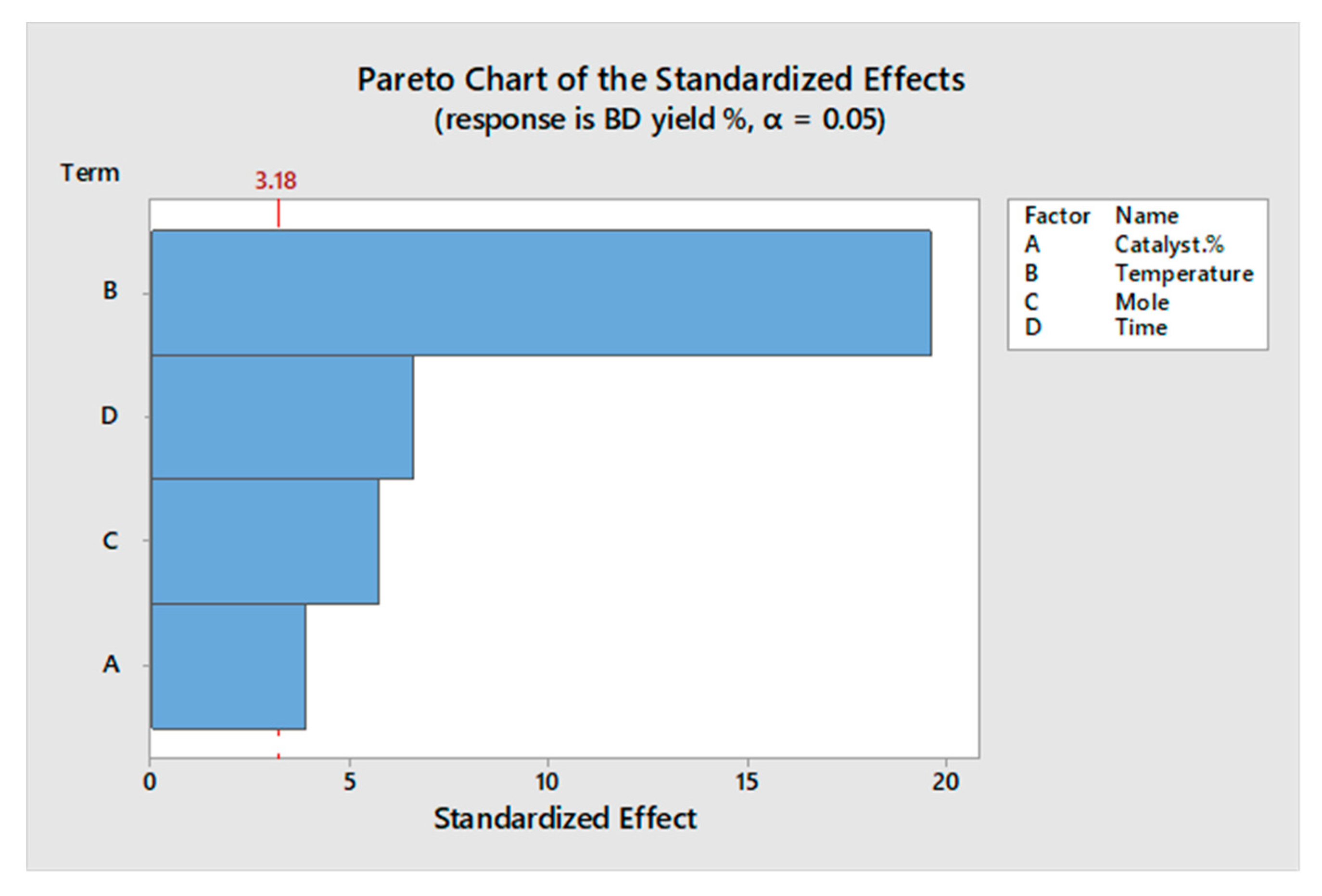
2.2. Statistical Analysis
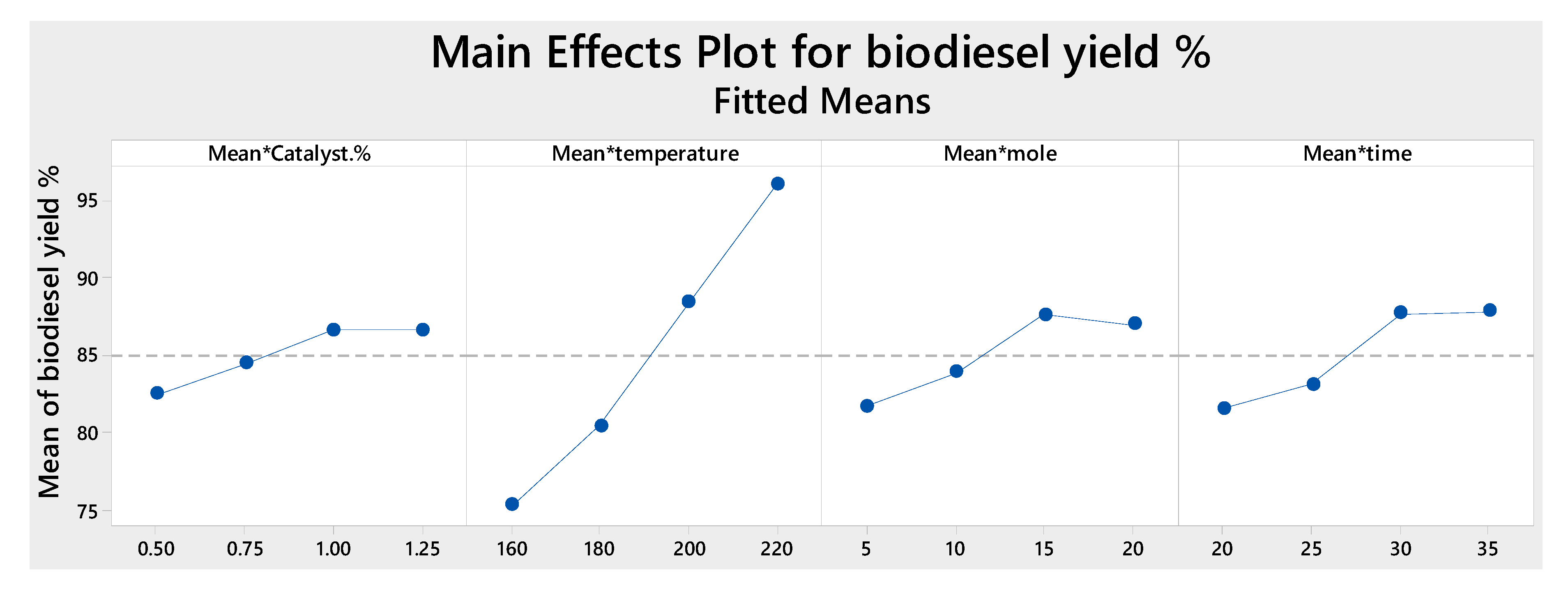
2.3. Effect of Process Parameters and Optimization
2.4. Catalytic Activity of S–TiO2/SBA-15 Solid Acid Catalyst in Esterification and Comparison with Other Solid Acid Catalysts in Transesterification
2.5. Reusability and Stability of the Catalyst
2.6. Properties of Biodiesel
2.7. Biodiesel Production from Various Sources by Heterogeneous Catalysis
3. Materials and Method
3.1. Materials
3.2. Pretreatment of Waste Cooking Oil (WCO)
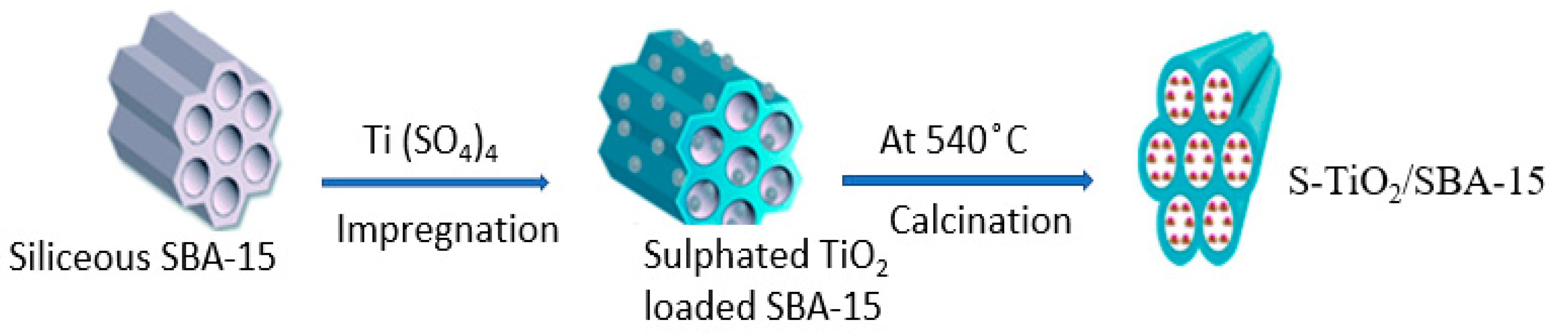
3.3. Catalyst Preparation
3.4. Experimental Procedure
3.4.1. Purification of Biodiesel
3.4.2. Experiment Design for Optimization of the Biodiesel Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandit, P.R.; Fulekar, M.H. Egg shell waste as heterogeneous nanocatalyst for biodiesel production: Optimized by response surface methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef]
- Hossain, M.N.; Bhuyan, M.S.U.S.; Alam, A.H.M.A.; Seo, Y.C. Biodiesel from Hydrolyzed Waste Cooking Oil Using a S-ZrO2/SBA-15 Super Acid Catalyst under Sub-Critical Conditions. Energies 2018, 11, 299. [Google Scholar] [CrossRef]
- Goodrum, J.W.; Geller, D.P.; Adams, T.T. Rheological characterization of animal fats and their mixtures with #2 fuel oil. Biomass Bioenergy 2003, 24, 249–256. [Google Scholar]
- Bhuyan, M.S.U.S.; Alam, A.H.M.A.; Chu, Y.; Seo, Y.C. Biodiesel Production Potential from Littered Edible Oil Fraction Using Directly Synthesized S-TiO2/MCM-41 Catalyst in Esterification Process via Non-Catalytic Subcritical Hydrolysis. Energies 2017, 10, 1290. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Phan, A.N.P.T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, Sh.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2–MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceride as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Negi, N.A.; Sayed, G.H.; Yehia, F.Z.; Habib, O.I.; Mohamed, E.A. Biodiesel production from one-step heterogeneous catalyzed process of Castor oil and Jatropha oil using novel sulphonated phenyl silane montmorillonite catalyst. J. Mol. Liq. 2017, 234, 157–163. [Google Scholar]
- Soriano, N.U., Jr.; Venditti, R.; Argyropoulos, D.S. Biodiesel synthesis via homogeneous Lewis acid-catalyzed transesterification. Fuel 2009, 88, 560–565. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Faruq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Haigh, K.F.; Vladisavljevic, G.T.; Reynolds, J.C.; Nagy, Z.; Saha, B. Kinetics of the pre-treatment of used cooking oil using Novozyme 435 for biodiesel production. Chem. Eng. Res. Des. 2014, 9, 713–719. [Google Scholar] [CrossRef]
- Negm, N.A.; Sayed, G.H.; Habib, O.I.; Yehia, F.Z.; Mohamed, E.A. Heterogeneous catalytic transformation of vegetable oils into biodiesel in one-step reaction using super acidic sulfonated modified mica catalyst. J. Mol. Liq. 2017, 237, 38–45. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Two-step preparation for catalyst-free biodiesel fuel production. Appl. Biochem. Biotechnol. 2004, 115, 781–791. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Synthesis of hybrid SBA-15 functionalized with molybdophosphoric acid as efficient catalyst for glycerol esterification to fuel additives. Appl. Catal. A Gen. 2012, 433–434, 152–161. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: Catalyst characterization and biodiesel yield performance. Appl. Energy 2015, 160, 58–70. [Google Scholar] [CrossRef]
- Veillette, M.; Giroir-Fendler, A.; Faucheux, N.; Heitz, M. Esterification of free fatty acids with methanol to biodiesel using heterogeneous catalysts: From model acid oil to microalgae lipids. Chem. Eng. J. 2017, 308, 101–109. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhang, Y. Mesoporous silica-supported chromium catalyst: Characterization and excellent performance in dehydrogenation of propane to propylene with carbon dioxide. Catal. Commun. 2007, 8, 565–570. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent progress in synthesis and surface functionalization of mesoporous acidic heterogeneous catalysts for esterification of free fatty acid feedstocks: A review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Kılıç, M.; Uzun, B.B.; Pütün, E.; Pütün, A.E. Optimization of biodiesel production from castor oil using factorial design. Fuel Process. Technol. 2013, 111, 105–110. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Aziz, A.R.A. “Activity of solid catalysts for biodiesel production”: A critical review. Appl. Catal. A Gen. 2014, 470, 140–161. [Google Scholar] [CrossRef]
- Wan, L.; Liu, H.; Skala, D. Biodiesel production from soyabin oil in subcritical methanol using MnCO3/ZnO as catalyst. Appl. Catal. B Environ. 2014, 152–153, 352–359. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Accelerating transesterification reaction with biodiesel as co-solvent: A case study for solid acid sulfated tin oxide catalyst. Fuel 2010, 89, 3866–3870. [Google Scholar] [CrossRef]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient production of biodiesel from high free fatty acid containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Jacobson, K.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production from waste cooking oil. Appl. Catal. B 2008, 85, 86–91. [Google Scholar] [CrossRef]
- Kaur, M.; Malhotra, R.; Ali, A. Tungsten supported Ti/SiO2 nanoflowers as reusable heterogeneous catalyst for biodiesel production. Renew. Energy 2018, 116, 109–119. [Google Scholar] [CrossRef]
- Available online: https://www.scribd.com/document/343180251/Viscosity-of-Fluids-Lab-Ball-Drop-Method (accessed on 10 December 2018).
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; Lorza, R.L.; García, R.E.; Gómez, F.S.; González, E.P.V. An Improvement in Biodiesel Production from Waste Cooking Oil by Applying Thought Multi-Response Surface Methodology Using Desirability Functions. Energies 2017, 10, 130. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; Martínez, R.F.; Lorza, R.L.; Gómez, F.S.; González, E.P.V. Optimizing Biodiesel Production from Waste Cooking Oil Using Genetic Algorithm-Based Support Vector Machines. Energies 2018, 11, 2995. [Google Scholar] [CrossRef]
- Istadi, I.; Anggoro, D.D.; Buchori, L.; Rahmawati, D.A.; Intaningrum, D. Active acid catalyst of sulphated zinc oxide for transesterification of soybean oil with methanol to biodiesel. Procedia Environ. Sci. 2015, 23, 385–393. [Google Scholar] [CrossRef]
- Hidayat, A.; Sutrisno, B. Free Fatty Acids Esterification on Palm Oil Sludge using Zirconia-supported Indonesian Natural Zeolite as Heterogeneous Catalyst. OJCHEG 2018, 34, 2464–2470. [Google Scholar]
- Zanette, A.F.; Barella, R.A.; Pergher, S.B.; Treichel, H.; Oliveira, D.; Mazutti, M.A.; Silva, E.A.; Oliveira, J.V. Screening, optimization and kinetics of Jatropha curcas oil transesterification with heterogeneous catalysts. Renew. Energy 2011, 36, 726–731. [Google Scholar] [CrossRef]
- Kansedo, J.; Lee, K.T.; Bhatia, S. Cerbera odollam (sea mango) oil as a promisingnon-edible feedstock for biodiesel production. Fuel 2009, 88, 1148–1150. [Google Scholar] [CrossRef]
- Kafuku, C.; Lam, M.K.; Kansedo, J.; Lee, K.T.; Mbarawa, M. Heterogeneous catalyzed biodiesel production from Moringa oleifera oil. Fuel Process. Technol. 2010, 91, 1525–1529. [Google Scholar] [CrossRef]
- Kirubakaran, M. Eggshell as heterogeneous catalyst for synthesis of biodiesel from high free fatty acid chicken fat and its working characteristics on a CI engine. J. Environ. Chem. Eng. 2018, 6, 4490–4503. [Google Scholar]
- Uddin, M.R.; Ferdous, K.; Uddin, M.R.; Rahman, M.; Khan, R.; Salam, M.A. Synthesis of biodiesel from waste cooking oil. Chem. Eng. Sci. 2013, 1, 22–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Ma, P.; Cai, J.; Zhang, Y. Acid Value Determination and Pre-Esterification of Crude Euphorbialathyris L. Oil. World J. Eng. Technol. 2015, 32, 70–75. [Google Scholar] [CrossRef]
- Corro, G.; Bañuelos, F.; Vidal, E.; Cebada, S. Measurements of surface acidity of solid catalysts for free fatty acids esterification in Jatropha curcas crude oil for biodiesel production. Fuel 2014, 115, 625–628. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Xue, F.; Tang, S. Comparison of two different processes to synthesize biodiesel by waste cooking oil. J. Mol. Catal. A Chem. 2006, 252, 107–112. [Google Scholar] [CrossRef]

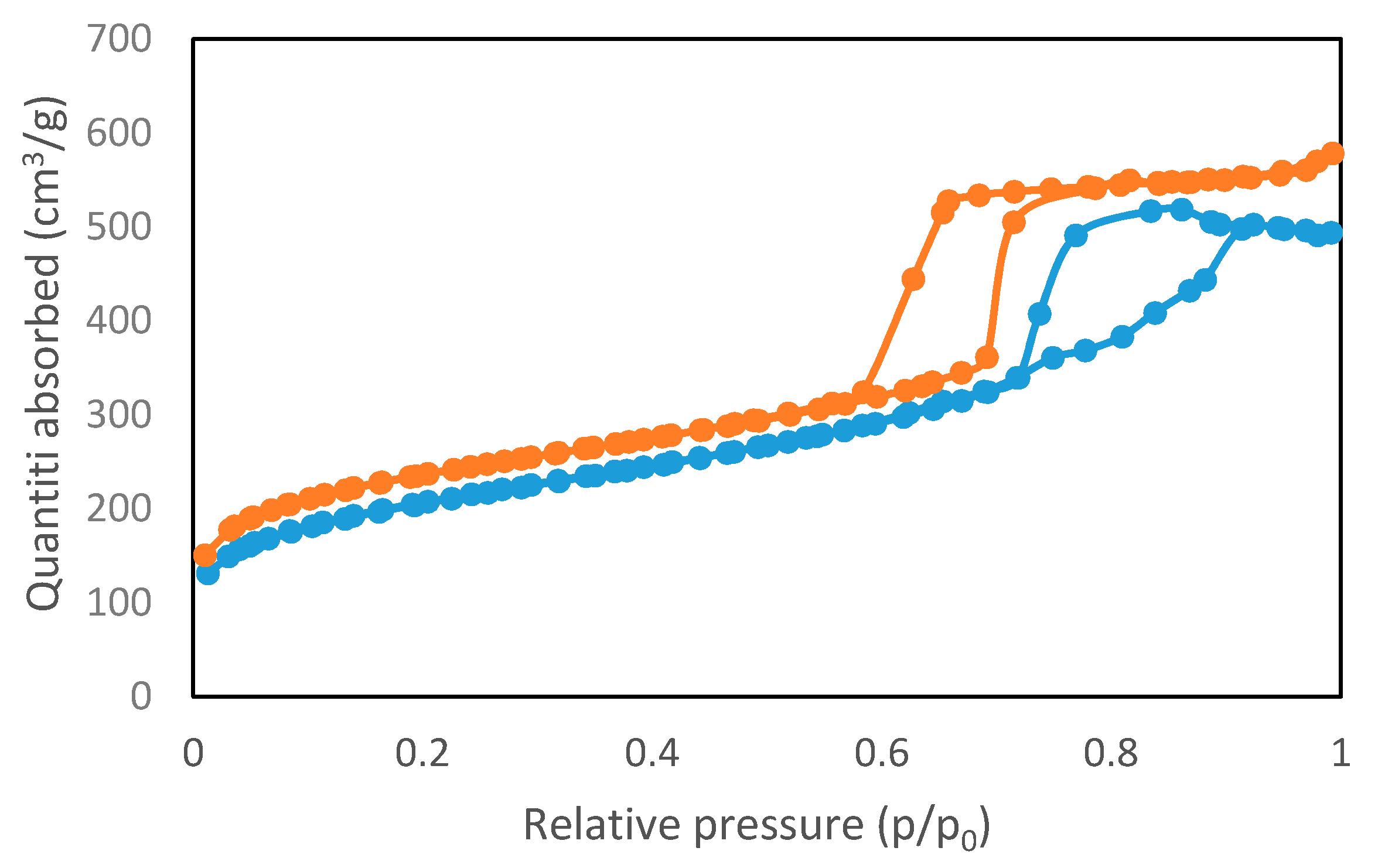
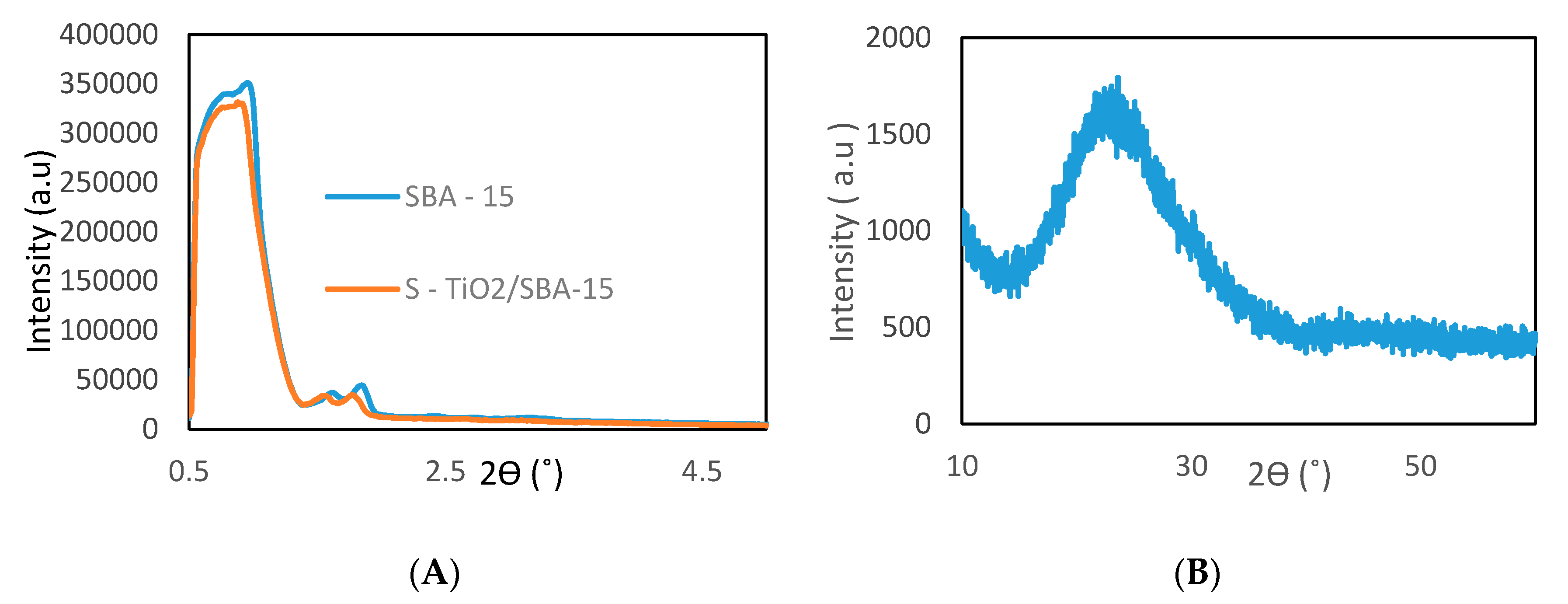


| Sample | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Surface Acidity (mmole/g) |
|---|---|---|---|---|
| SBA-15 [2] | 833.80 | 5.38 | 0.13 | - |
| S–TiO2/SBA-15 | 733.98 | 5.10 | 0.09 | 0.18 |
| Std Order | Run Order | Blocks | Pt Type | Catalyst % | Temperature | Mole | Time | Biodiesel Yield % | Predicted |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1.00 | 160 | 20 | 20 | 75.35 | 78.03 |
| 2 | 2 | 1 | 1 | 1.00 | 180 | 20 | 20 | 80.49 | 83.17 |
| 3 | 3 | 1 | 1 | 1.00 | 200 | 20 | 20 | 89.04 | 90.76 |
| 4 | 4 | 1 | 1 | 1.00 | 220 | 20 | 20 | 96.03 | 98.35 |
| 5 | 5 | 1 | 1 | 0.50 | 220 | 20 | 20 | 91.83 | 94.51 |
| 6 | 6 | 1 | 1 | 0.75 | 220 | 20 | 20 | 93.80 | 96.48 |
| 7 | 7 | 1 | 1 | 1.00 | 220 | 20 | 20 | 96.03 | 98.35 |
| 8 | 8 | 1 | 1 | 1.25 | 220 | 20 | 20 | 95.99 | 98.67 |
| 9 | 9 | 1 | 1 | 1.00 | 200 | 5 | 20 | 83.15 | 85.83 |
| 10 | 10 | 1 | 1 | 1.00 | 200 | 10 | 20 | 85.36 | 88.04 |
| 11 | 11 | 1 | 1 | 1.00 | 200 | 15 | 20 | 88.68 | 91.41 |
| 12 | 12 | 1 | 1 | 1.00 | 200 | 20 | 20 | 87.83 | 90.76 |
| 13 | 13 | 1 | 1 | 1.00 | 200 | 15 | 20 | 89.50 | 91.41 |
| 14 | 14 | 1 | 1 | 1.00 | 200 | 15 | 25 | 90.65 | 93.33 |
| 15 | 15 | 1 | 1 | 1.00 | 200 | 15 | 30 | 95.18 | 97.85 |
| 16 | 16 | 1 | 1 | 1.00 | 200 | 15 | 35 | 95.36 | 98.04 |
| Source | DF | Adj SS | Adj MS | F Value | p Value |
|---|---|---|---|---|---|
| Model | 12 | 560.380 | 46.698 | 131.90 | 0.001 |
| Linear | 12 | 560.380 | 46.698 | 131.90 | 0.001 |
| Catalyst % | 3 | 14.234 | 4.745 | 13.40 | 0.030 |
| Temperature | 3 | 344.069 | 114.690 | 323.93 | 0.000 |
| Mole | 3 | 29.986 | 9.995 | 28.23 | 0.011 |
| Time | 3 | 40.033 | 13.344 | 37.69 | 0.007 |
| Error | 3 | 1.062 | 0.354 | ||
| Total | 15 | 561.443 |
| Catalyst | Reaction Temp. °C | Catalyst Load % | Methanol-to-Oil Mole Ratio | Reaction Time min | Biodiesel Yield % | Reference |
|---|---|---|---|---|---|---|
| Blank | 200 | 15:1 | 30 | 62.55 | This study | |
| SBA-15 | 200 | 1 | 15:1 | 30 | 71.96 | This study |
| SO42−/SnO2–SiO2 | 150 | 6 | 15:1 | 90 | 88.2 | [25] |
| Carbon-based catalyst derived from starch | 80 | 10 | 30:1 | 480 | 92 | [26] |
| ZS/Si | 200 | 3 | 18:1 | 300 | 98% | [27] |
| S–TiO2/MCM-41 | 240 | 1 | 20:1 | 20 | 99.29 | [4] |
| S–TiO2/SBA-15 | 240 | 1 | 20:1 | 20 | 97.50 | This study |
| S–TiO2/SBA-15 | 200 | 1 | 15:1 | 30 | 94.96 | This study |
| Properties | Produced Biodiesel | ASTM Test Values [1,22] | Test Method |
|---|---|---|---|
| Density at 15 °C (gm/cm3) | 0.876 | 0.860–0.894 | measurement |
| Viscosity (mm2/s) at 40 °C | 5.8 | 1.9–6.0 | ball drop method [29] |
| Iodine value (g I2/100 g) | 115.67 | titrimetric | |
| Pour point (°C) | −3.25 | −15–10 | ASTM D2500 |
| Cloud point (°C) | 7.6 | −3–12 | ASTM D97 |
| Feedstocks (Oil) | Catalyst | Optimum Reaction Conditions | % Biodiesel Yield | References |
|---|---|---|---|---|
| Soybean | SO42−/ZnO) | Temperature = 65 °C, methanol-to-oil mole ratio = 6, Catalyst = 4 wt %, Reaction time = 4 h | 80.19 | [33] |
| Palm oil | Zirconia-supported activated natural zeolite | Temperature = 65 °C, methanol-to-oil mole ratio = 10, Catalyst = 10 wt %, Reaction time = 2 h | 84.2 | [34] |
| Jatropha | Montmorillonite KSF/1–20 | Temperature = 160 °C, methanol-to-oil mole ratio = 12, Catalyst = 4.8 wt %, Reaction time = 6 h | 68 | [35] |
| Cerbera odollam | SO42−/ZrO2 | Temperature = 180 °C, methanol-to-oil mole ratio = 8, Catalyst = 6 wt %, Reaction time = 3 h | 84 | [36] |
| Moringa oleifera | SO42−/SnO2−SiO | Temperature = 150 °C, methanol-to-oil mole ratio = 5, Catalyst = 19 wt %, Reaction time = 2.5 h | 84 | [37] |
| Chicken fat | Eggshell | Temperature = 57.5 °C, methanol-to-oil mole ratio = 13, Catalyst = 8.5 wt %, Reaction time = 5 h | 92.29 | [38] |
| Waste cooking oil | S–TiO2/SBA-15 | Temperature = 200 °C, methanol-to-oil mole ratio = 15, Catalyst = 1 wt %, Reaction time = 30 min | 94.96 | This study |
| Chemical | Supplier | Purity (Mass Percentage) |
|---|---|---|
| Anhydrous sodium sulfate | Samchun Pure Chemical Co., Ltd Pyeongtaek Korea | 98.5% |
| Ethyl alcohol | Dae-Jung Chemical & Metals Co., Ltd., Gyeonggi-do Korea | 99.9% |
| Methanol | Dae-Jung Chemical & Metals Co., Ltd., Gyeonggi-do Korea | 99.9% |
| Diethyl ether | Dae-Jung Chemical & Metals Co., Ltd., Gyeonggi-do Korea | >99.0% |
| Hydrochloric acid | Dae-Jung Chemical & Metals Co., Ltd., Gyeonggi-do Korea | 35~37% |
| Potassium hydroxide | Dae-Jung Chemical & Metals Co., Ltd., Gyeonggi-do Korea | >85.0% |
| Pluronics P123 | Sigma-Aldrich. Co St. Louis, USA | >99.5% |
| Titanium sulfate solution Ti(SO4)2 | Fisher Scientific, Loughborough, UK | >24% |
| Tetraethyl orthosilicate (TEOS) | ACROS Organics Co. (Morris, NJ, USA) | ≥98% |
| Properties | Experimental Value | Test Method |
|---|---|---|
| Acid value (mg KOH/g of oil) | 2.92 | titrimetric |
| Iodine value (g I2/100 g) | 107.13 | titrimetric |
| Density (gm/cm3) | 0.891 | measurement |
| Viscosity (mm2/s) at 15 °C | 58.62 | ball drop method [29] |
| Saponification value (mg KOH/g of oil) | 188.39 | titrimetric |
| Factors | Levels | Values |
|---|---|---|
| Catalyst % | 4 | 0.50, 0.75, 1.00, 1.25 |
| Temperature | 4 | 160, 180, 200, 220 |
| Mole ratio | 4 | 5, 10, 15, 20 |
| Reaction time | 5 | 20, 25, 30, 35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.N.; Siddik Bhuyan, M.S.U.; Md Ashraful Alam, A.H.; Seo, Y.C. Optimization of Biodiesel Production from Waste Cooking Oil Using S–TiO2/SBA-15 Heterogeneous Acid Catalyst. Catalysts 2019, 9, 67. https://doi.org/10.3390/catal9010067
Hossain MN, Siddik Bhuyan MSU, Md Ashraful Alam AH, Seo YC. Optimization of Biodiesel Production from Waste Cooking Oil Using S–TiO2/SBA-15 Heterogeneous Acid Catalyst. Catalysts. 2019; 9(1):67. https://doi.org/10.3390/catal9010067
Chicago/Turabian StyleHossain, Muhammad Nobi, Md Sufi Ullah Siddik Bhuyan, Abul Hasnat Md Ashraful Alam, and Yong Chan Seo. 2019. "Optimization of Biodiesel Production from Waste Cooking Oil Using S–TiO2/SBA-15 Heterogeneous Acid Catalyst" Catalysts 9, no. 1: 67. https://doi.org/10.3390/catal9010067
APA StyleHossain, M. N., Siddik Bhuyan, M. S. U., Md Ashraful Alam, A. H., & Seo, Y. C. (2019). Optimization of Biodiesel Production from Waste Cooking Oil Using S–TiO2/SBA-15 Heterogeneous Acid Catalyst. Catalysts, 9(1), 67. https://doi.org/10.3390/catal9010067





