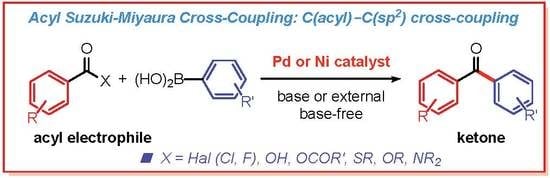
Recent Advances in Acyl Suzuki Cross-Coupling
Abstract
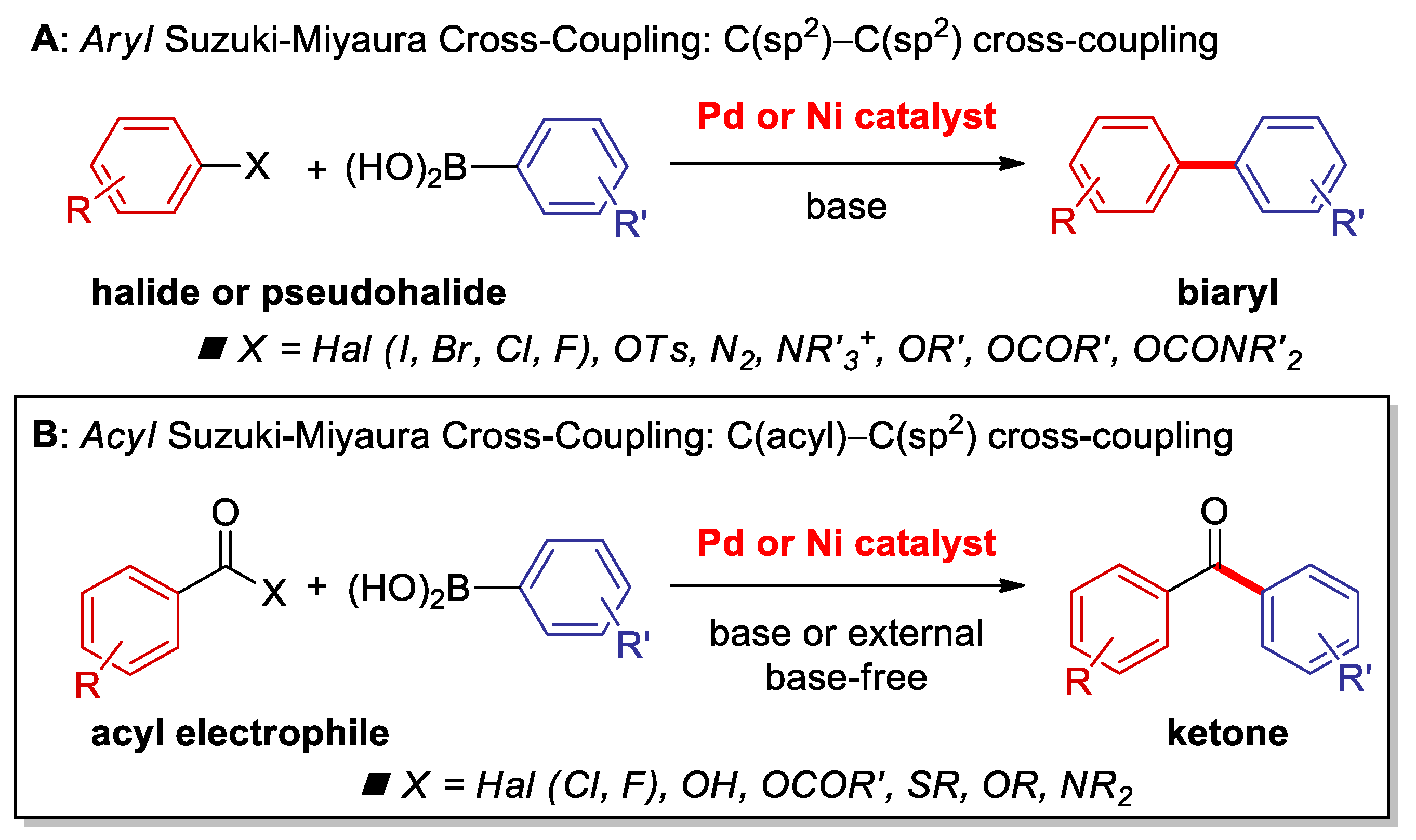
1. Introduction
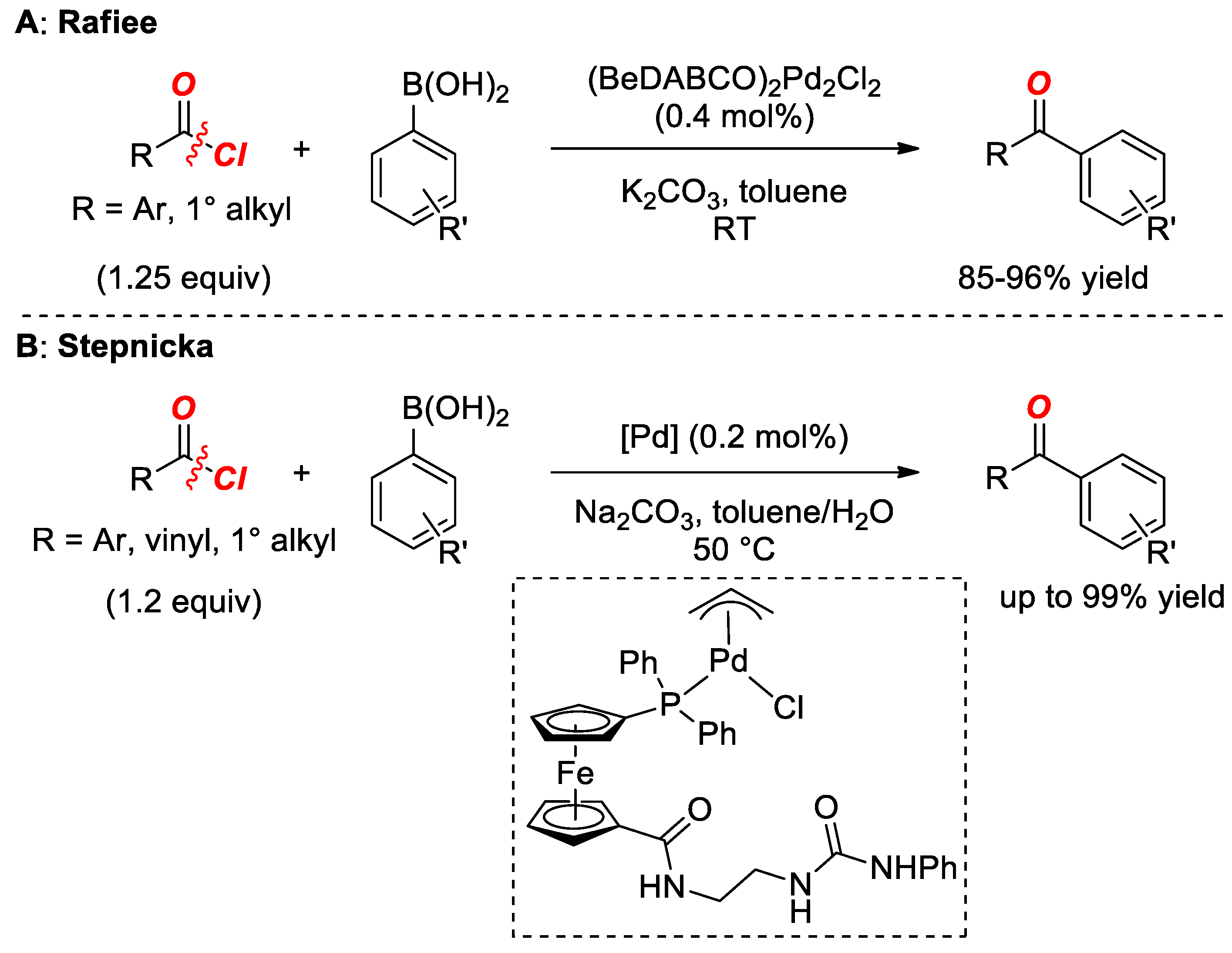
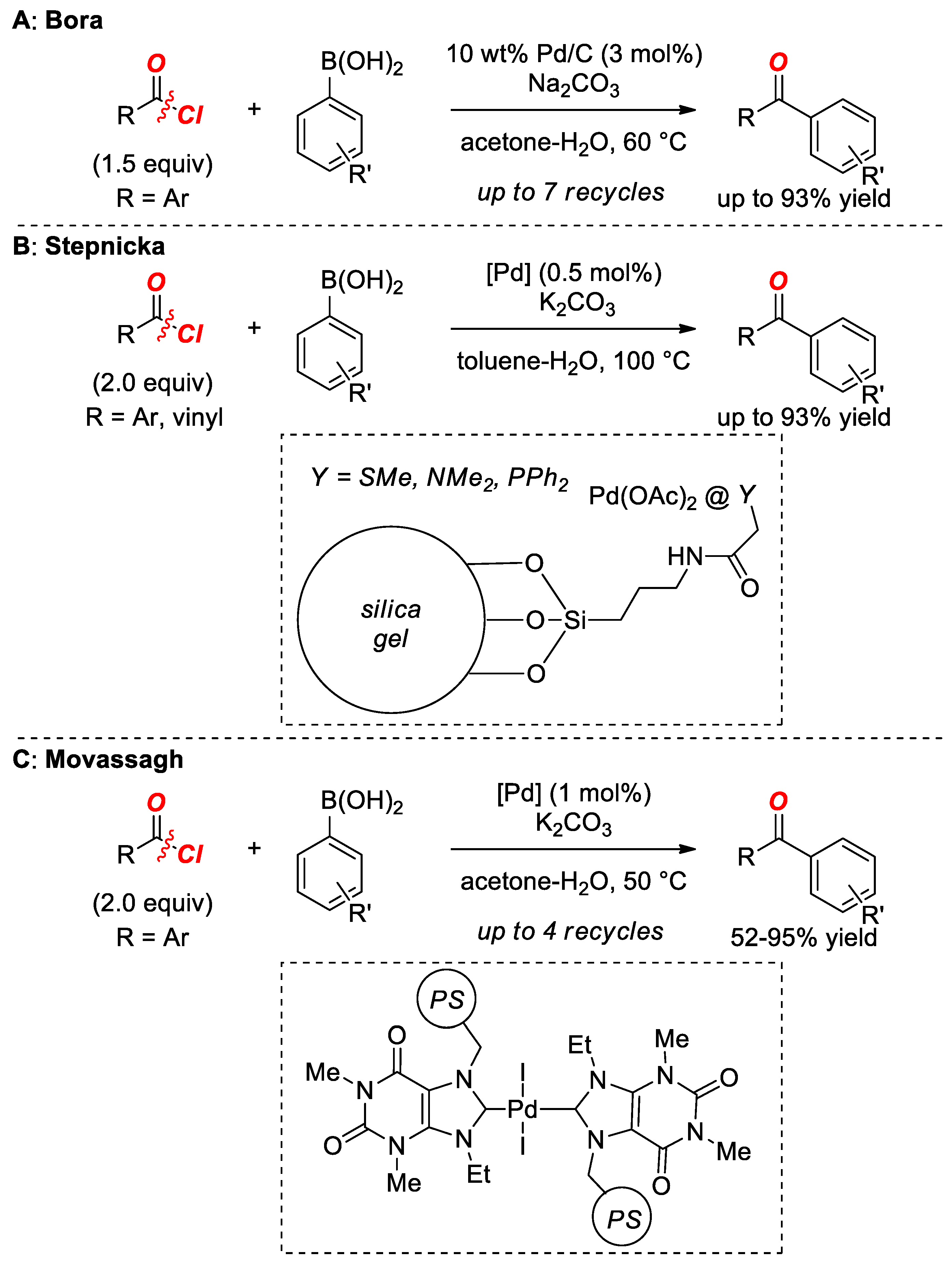
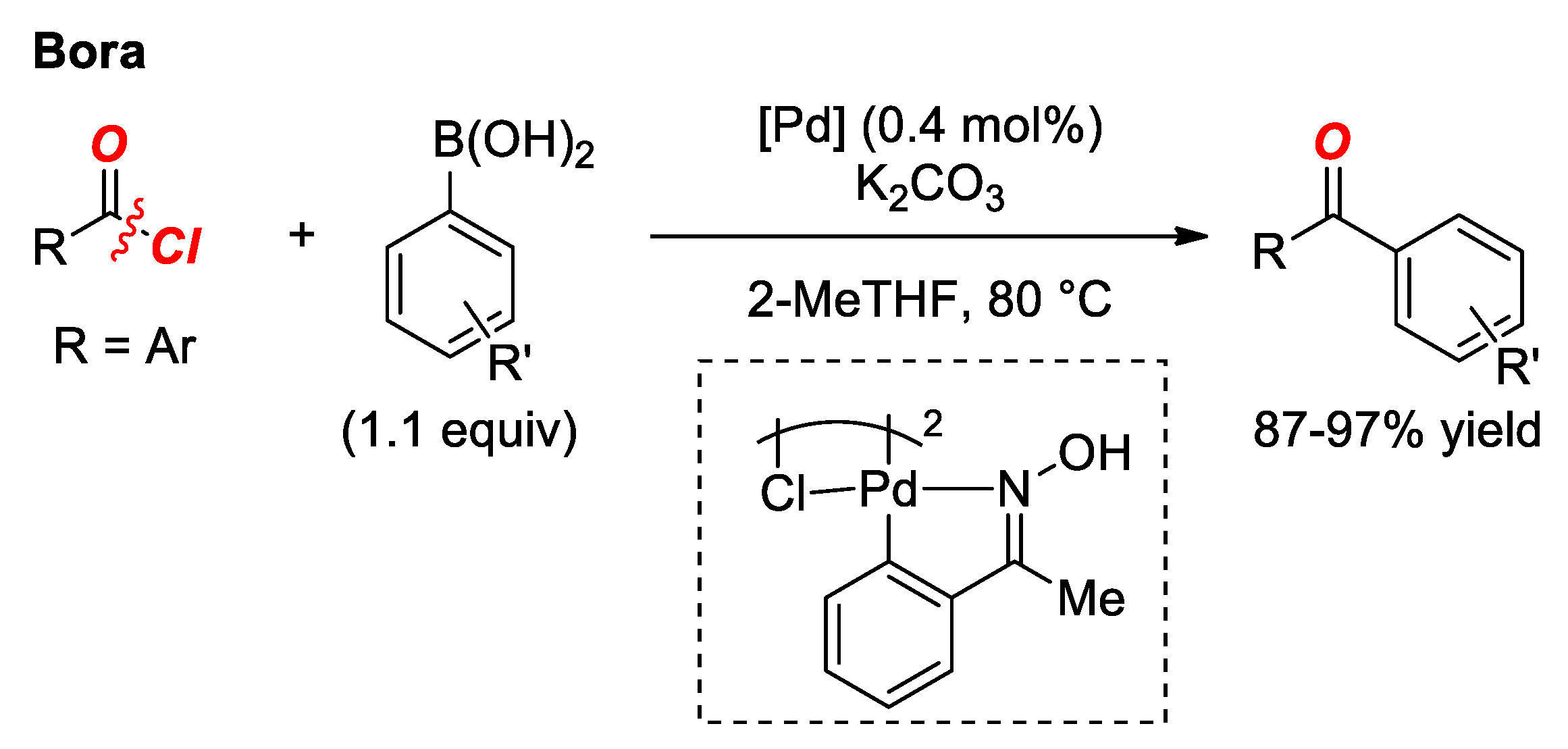
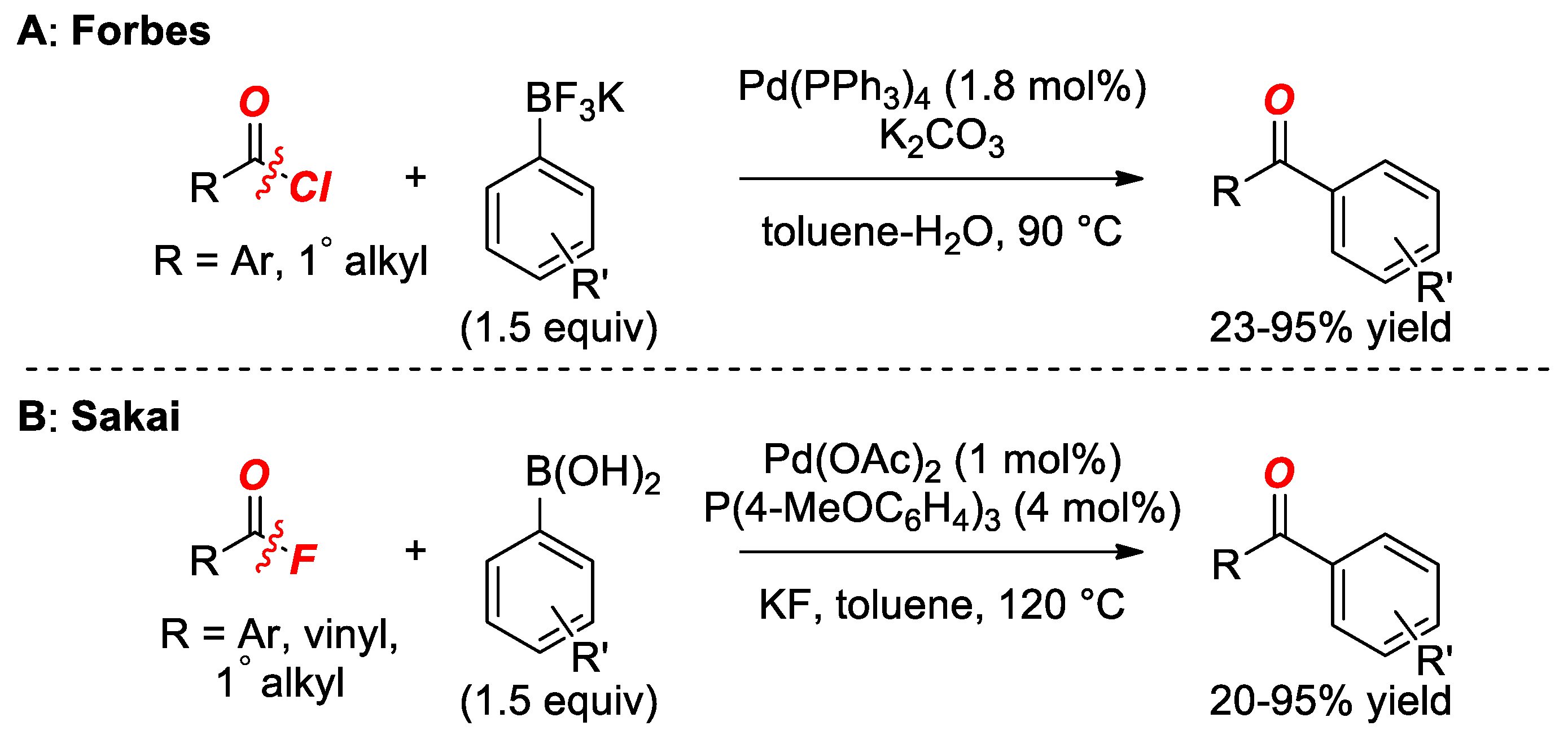
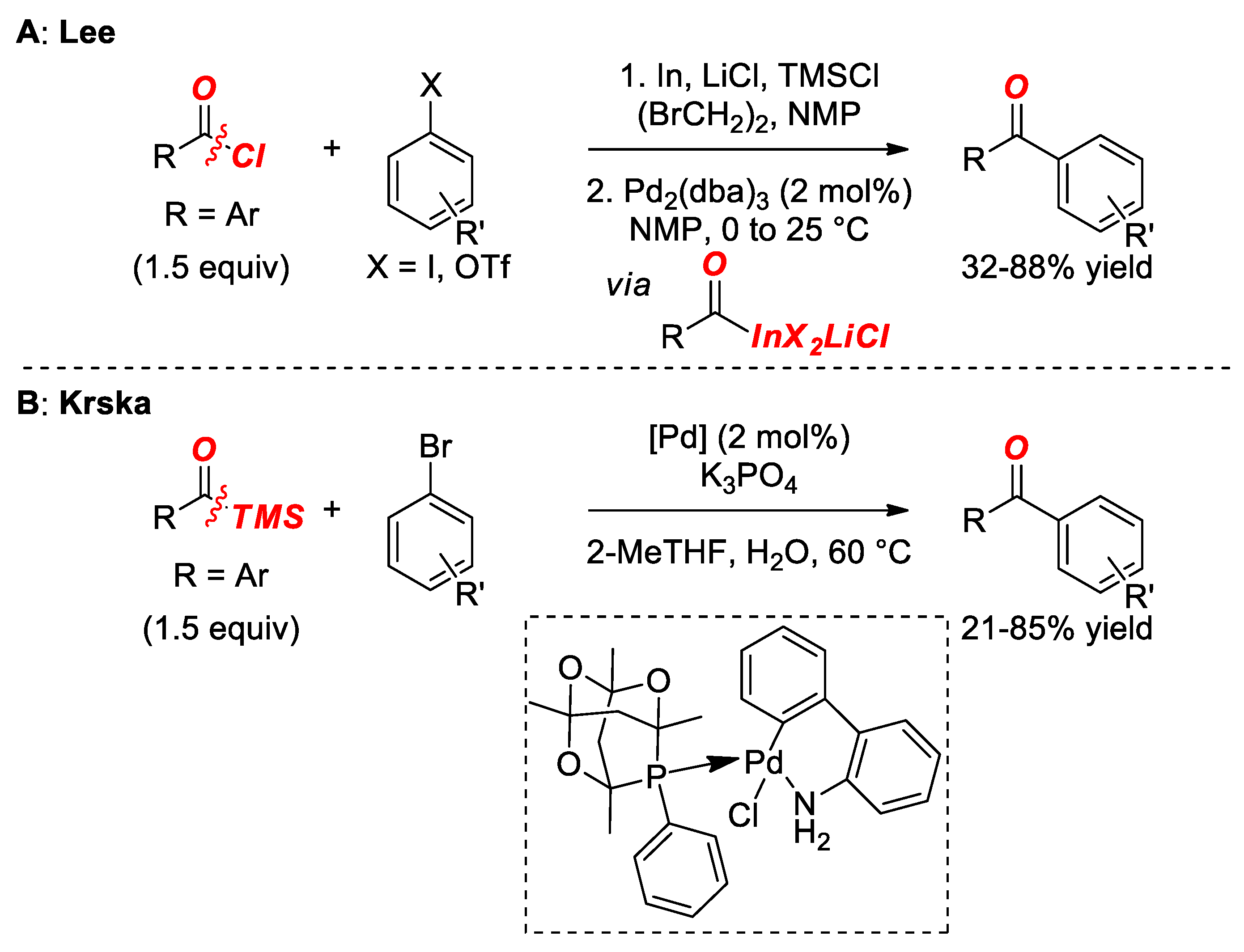
2. Suzuki Cross-Coupling of Acyl Halides
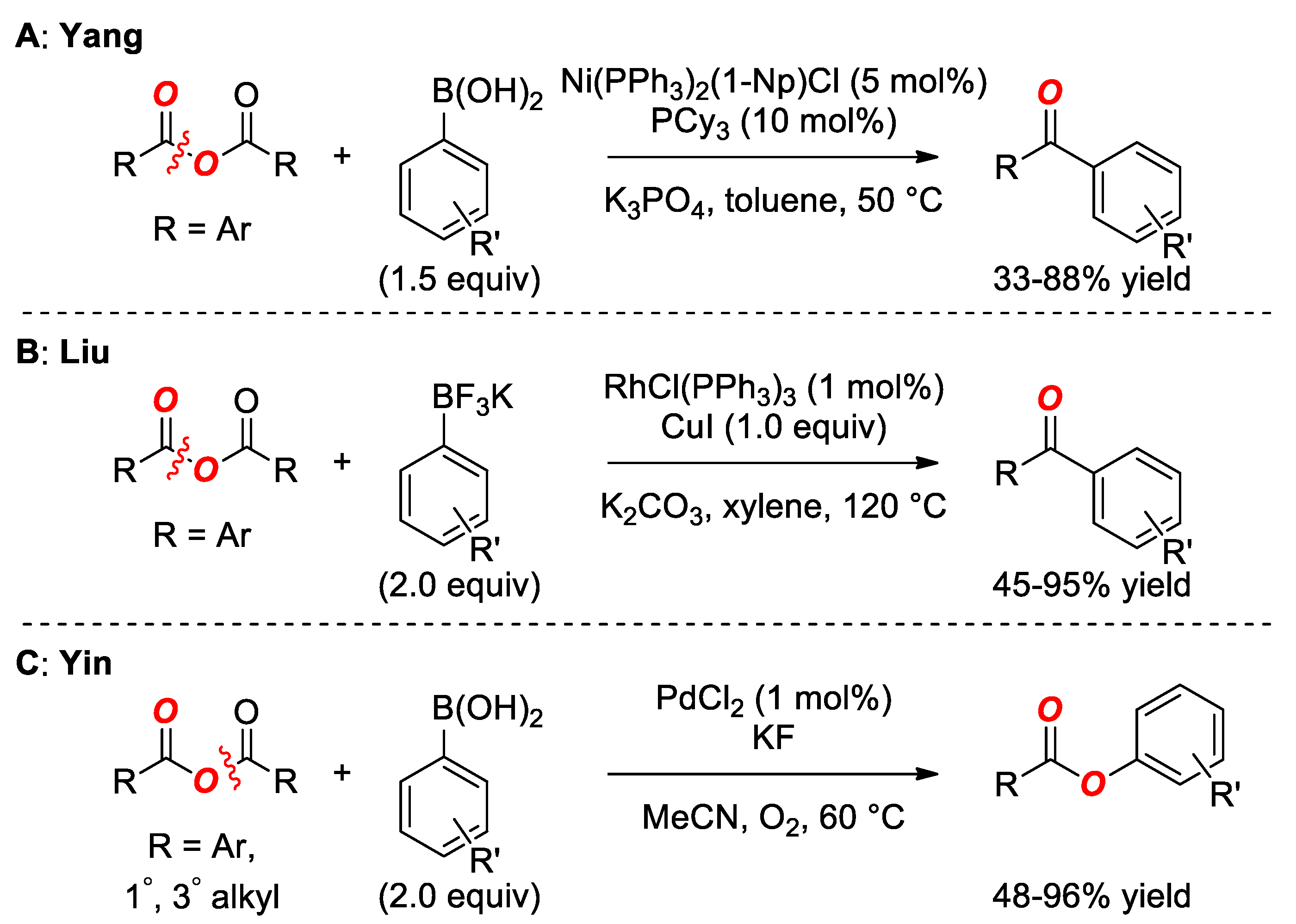
3. Suzuki Cross-Coupling of Anhydrides
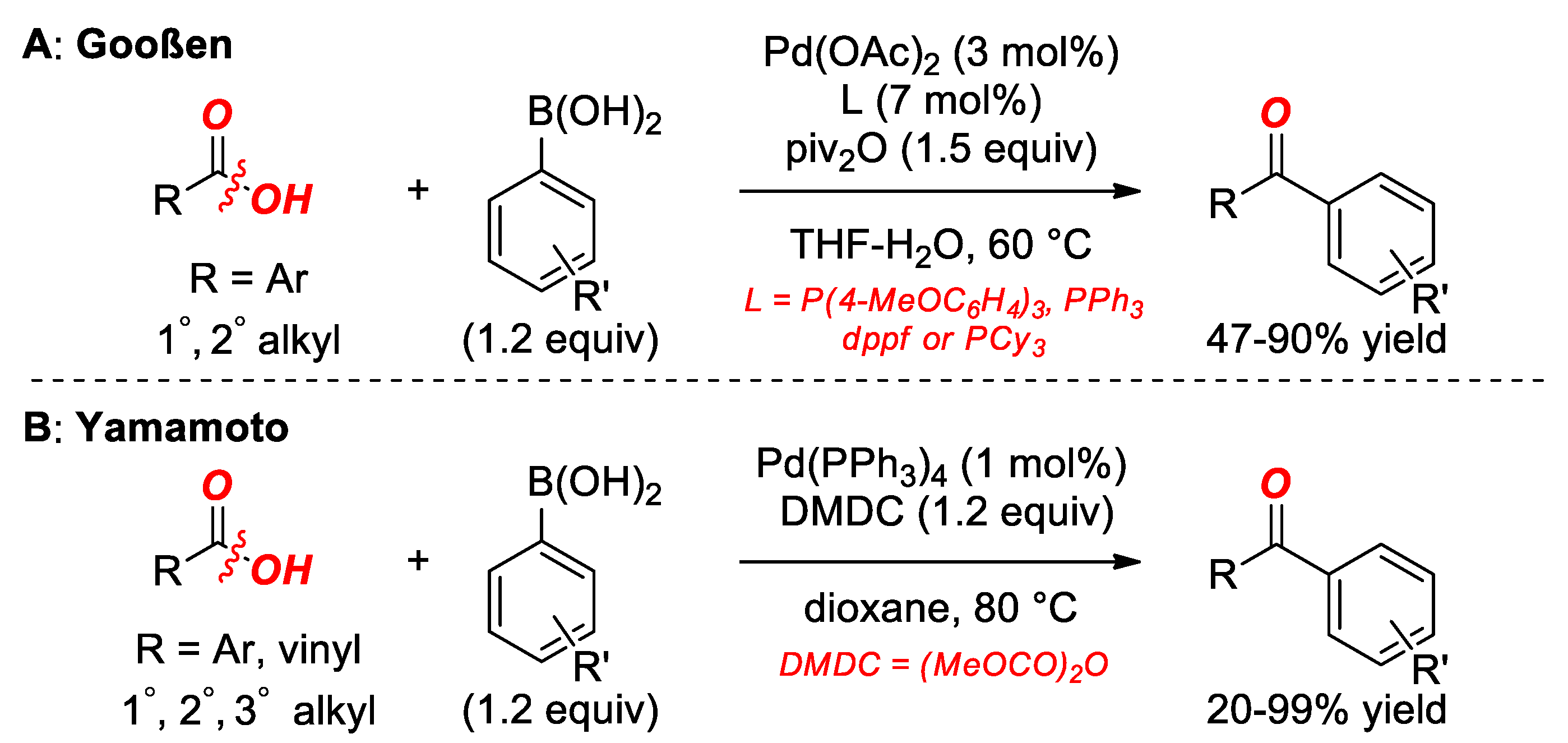
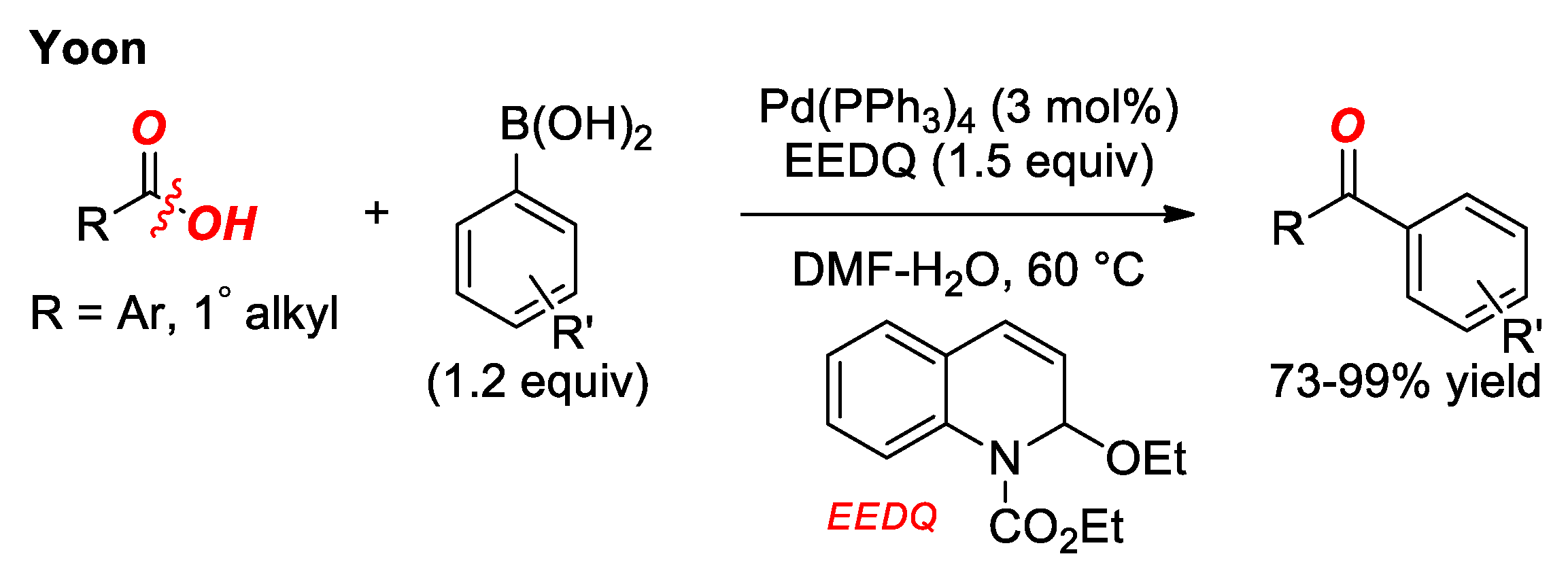
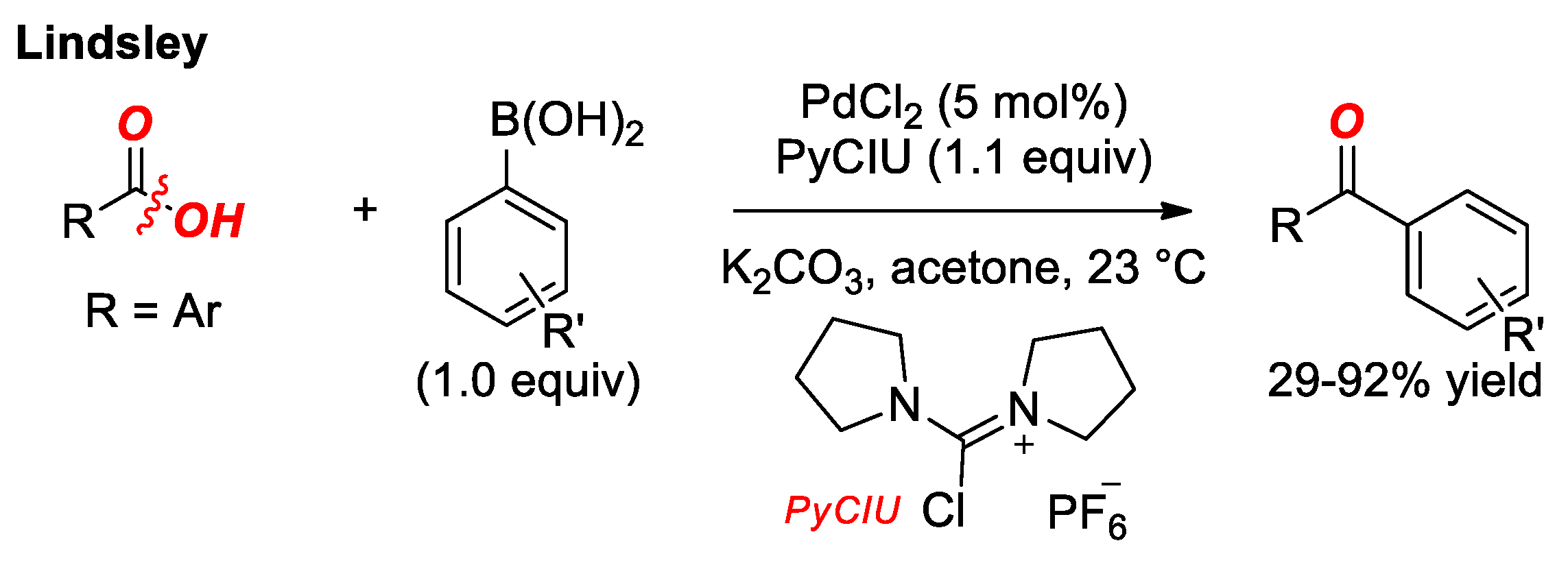
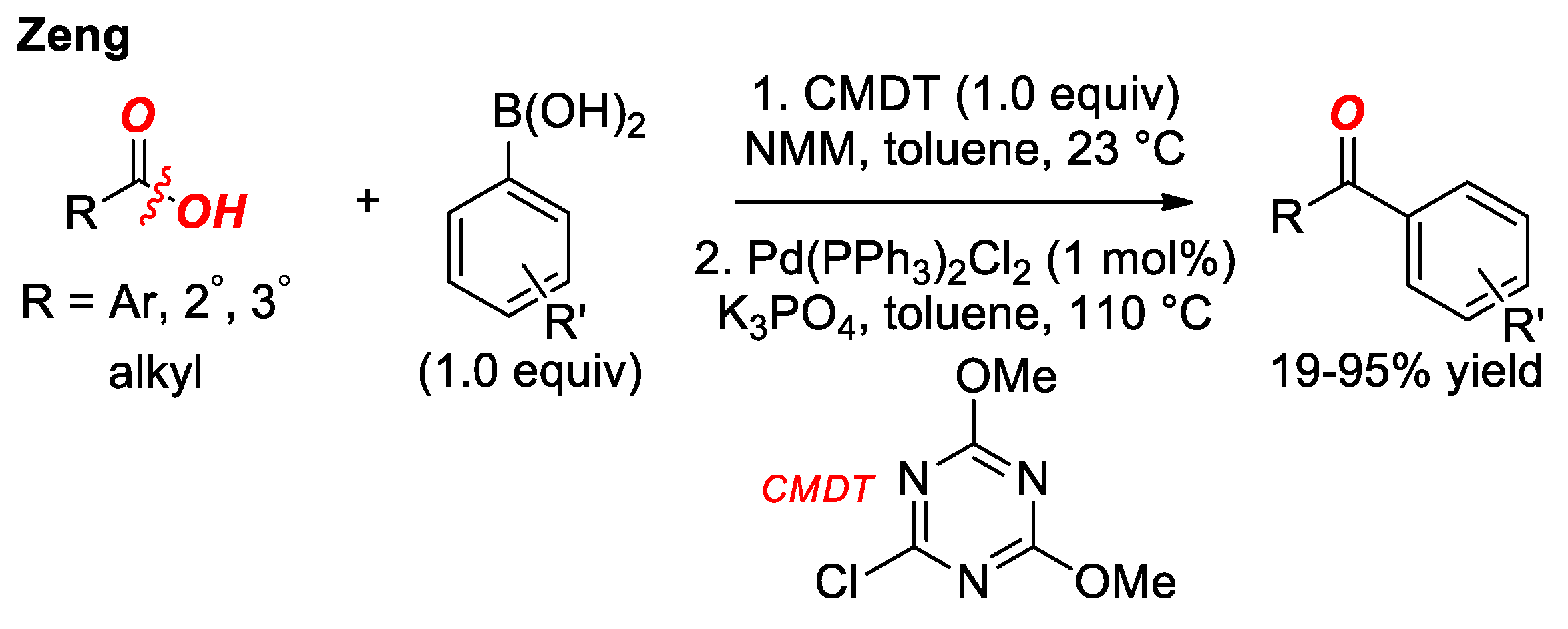
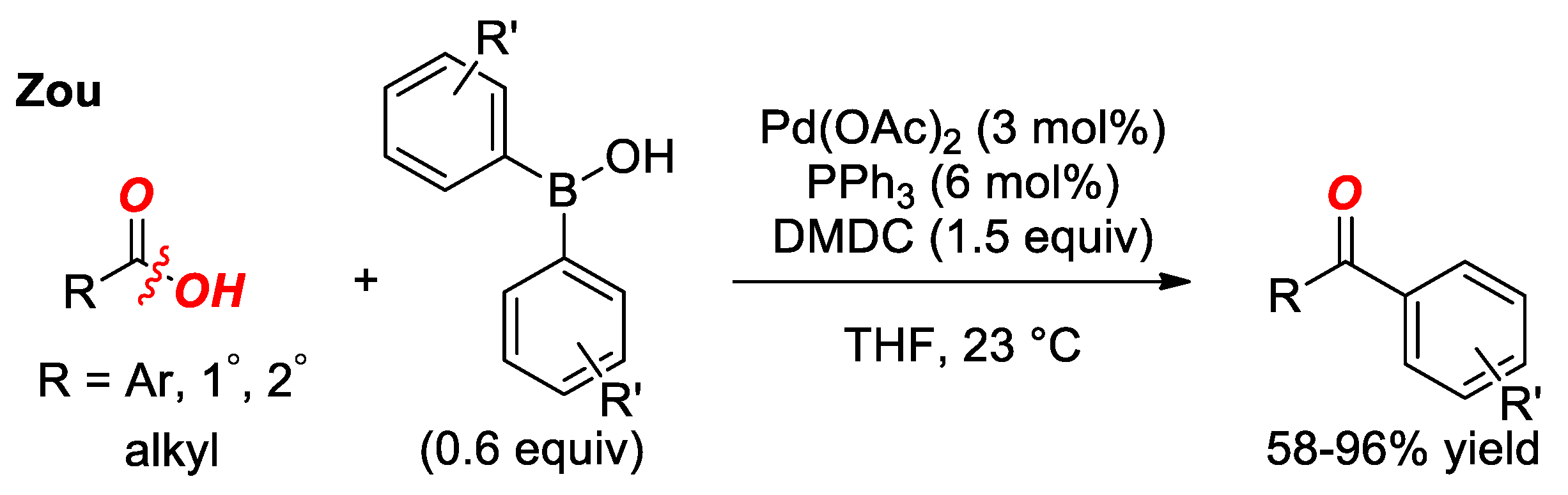
4. Suzuki Cross-Coupling of Carboxylic Acids
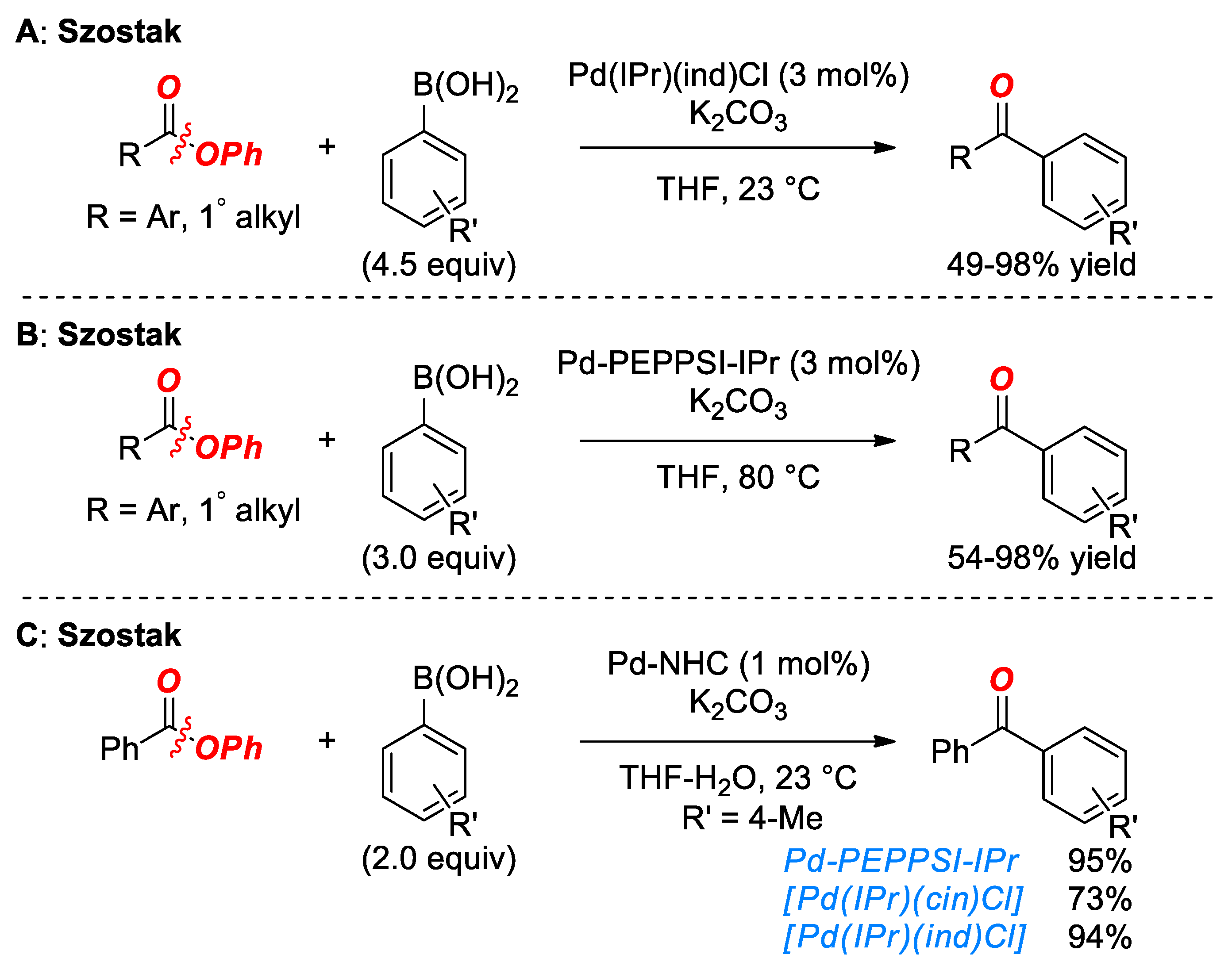
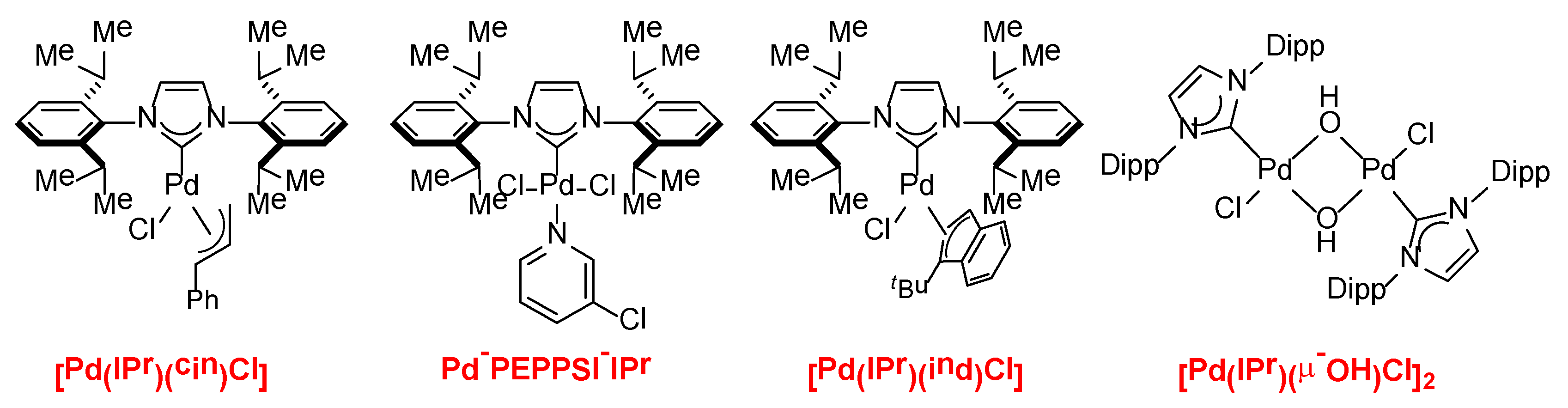
5. Suzuki Cross-Coupling of Esters
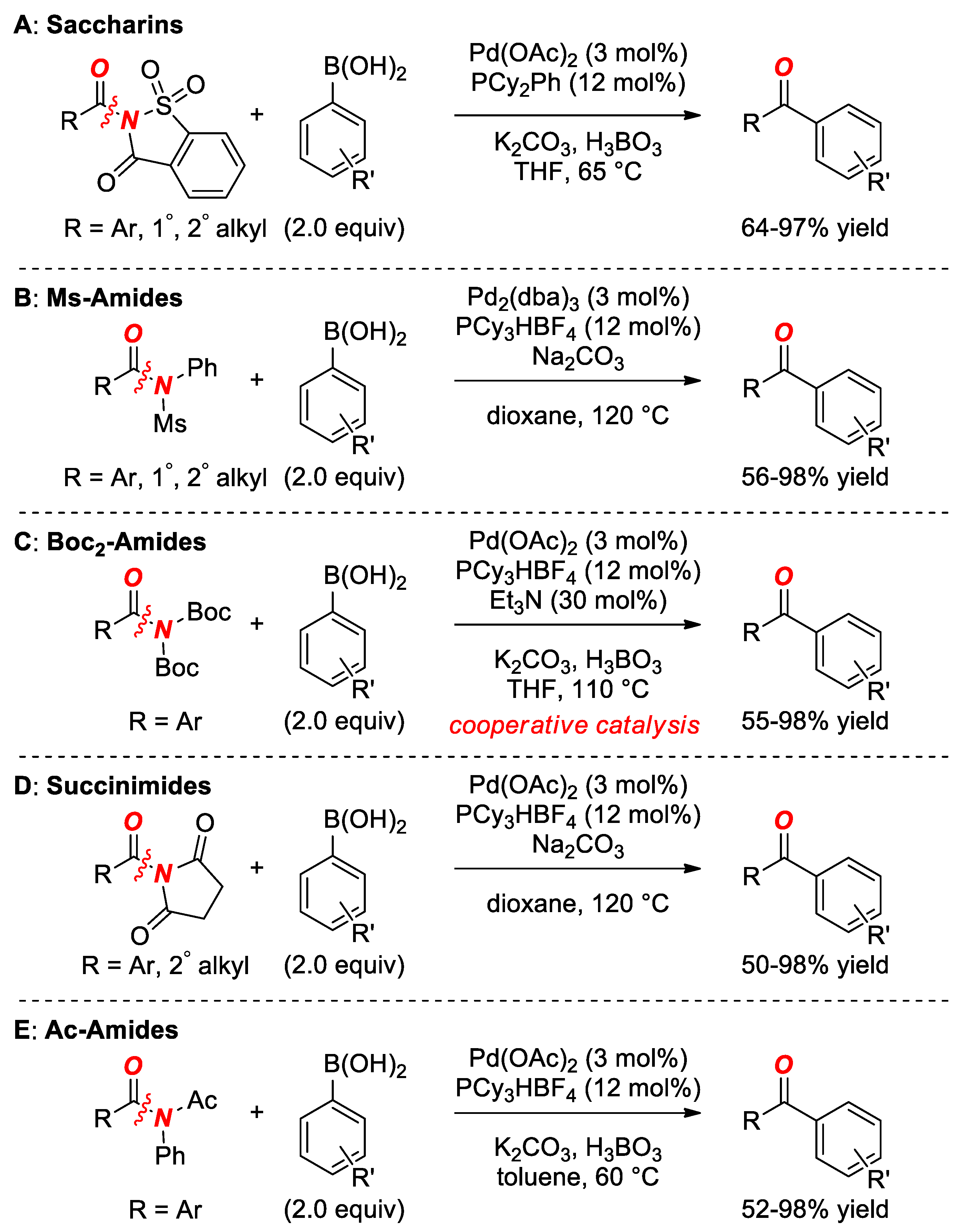
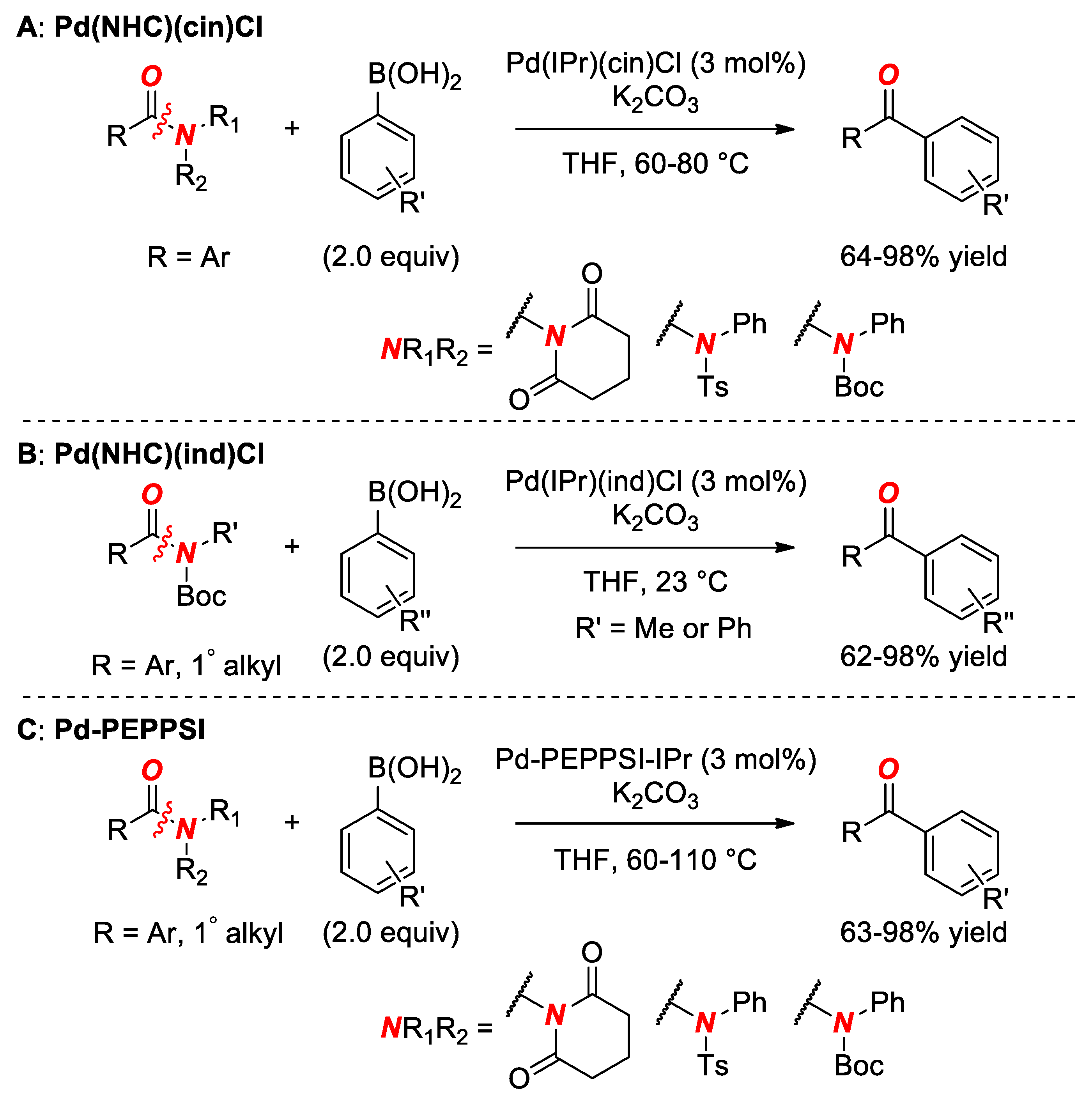
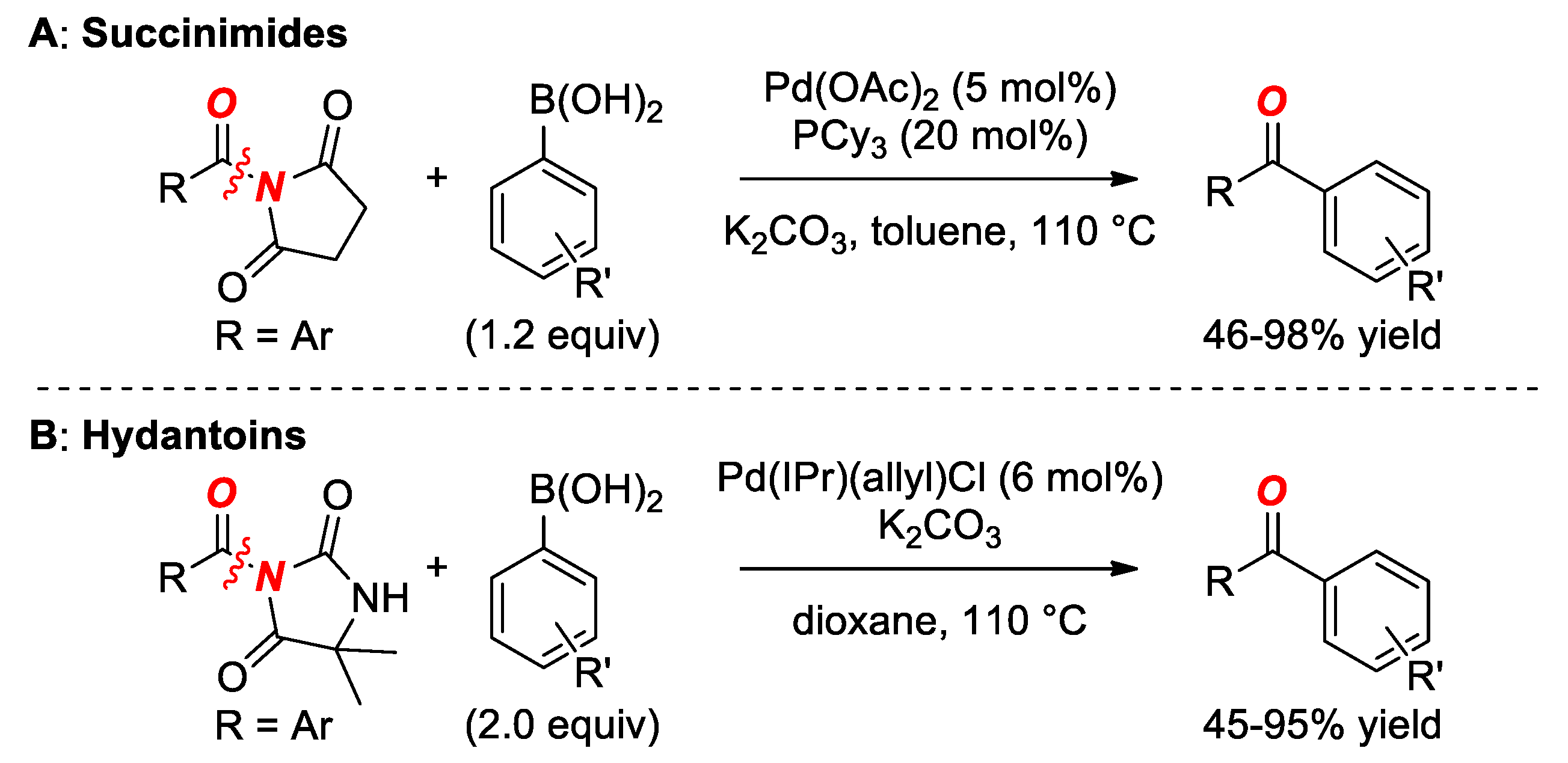
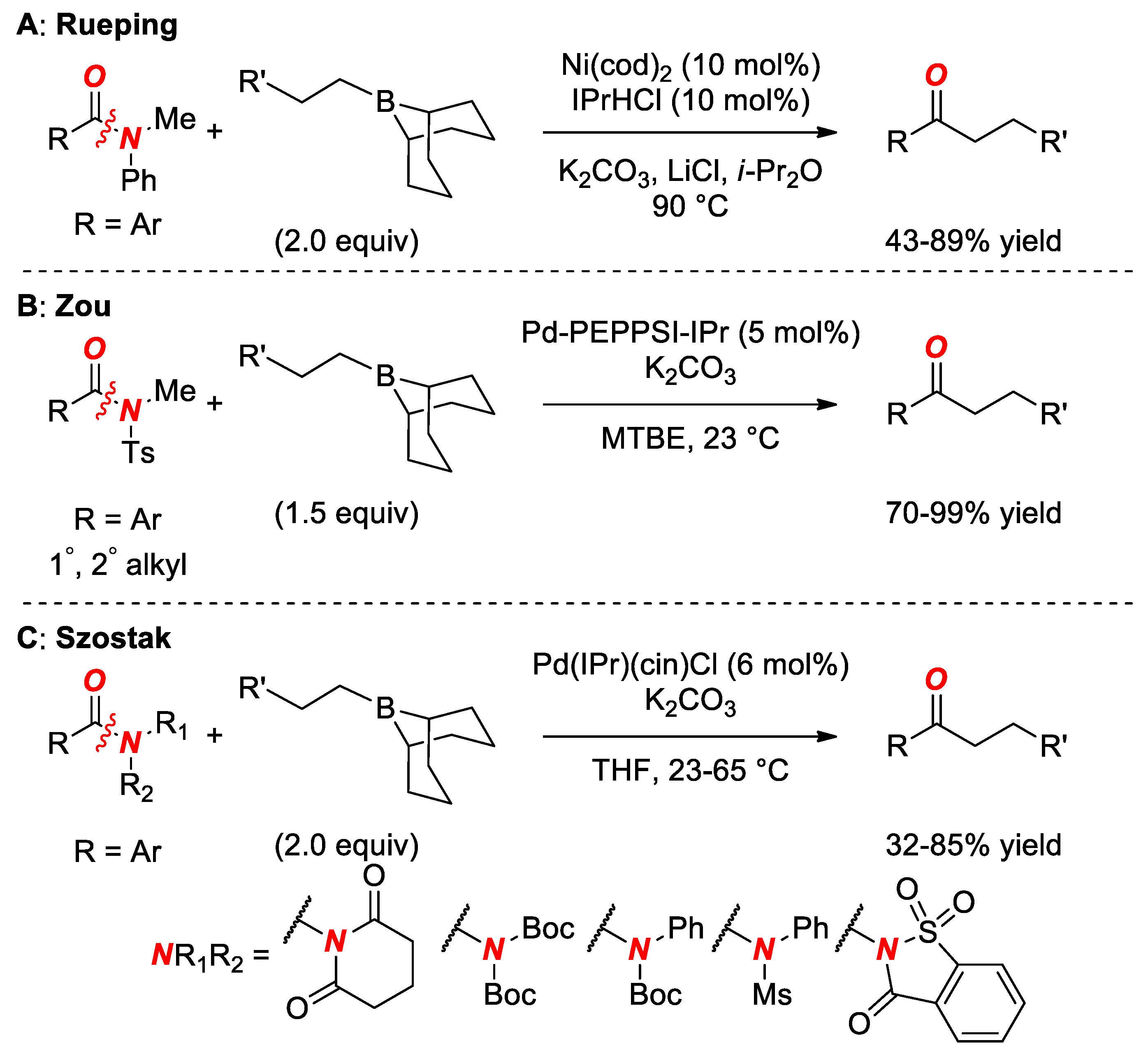
6. Suzuki Cross-Coupling of Amides
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Ackermann, L. (Ed.) Modern Arylation Methods; Wiley: Weinheim, Germany, 2009. [Google Scholar]
- de Meijere, A.; Bräse, S.; Oestreich, M. (Eds.) Metal-Catalyzed Cross-Coupling Reactions and More; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Johansson-Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Zapf, A. Novel Substartes for palladium-catalyzed coupling reactions of Arenes. Angew. Chem. Int. Ed. 2003, 42, 5394–5399. [Google Scholar] [CrossRef] [PubMed]
- Gooßen, L.J.; Rodriguez, N.; Gooßen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 2008, 47, 3100–3120. [Google Scholar] [CrossRef]
- Dieter, R.K. Reaction of acyl chlorides with organometallic reagents: A banquet table of metals for ketone synthesis. Tetrahedron 1999, 55, 4177–4236. [Google Scholar] [CrossRef]
- Negishi, E.; Bagheri, V.; Chatterjee, S.; Luo, F.T.; Miller, J.A.; Stoll, A.T. Palladium-catalyzed acylation of organozincs and other organometallics as a convenient route to ketones. Tetrahedron Lett. 1983, 24, 5181–5184. [Google Scholar] [CrossRef]
- Milstein, D.; Stille, J.K. Mild, selective, general method of ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium. J. Org. Chem. 1979, 44, 1613–1618. [Google Scholar] [CrossRef]
- Mortier, J. Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lenox, A.J.J.; Lloyd-Jones, G.C. Transmetalation in the Suzuki-Miyaura cross-coupling: The fork in the trail. Angew. Chem. Int. Ed. 2013, 52, 7362–7370. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. Advanced Organic Chemistry; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hirschbeck, V.; Gehrtz, P.H.; Fleischer, I. Metal-catalyzed synthesis and use of thioesters: Recent developments. Chem. Eur. J. 2018, 24, 7092–7107. [Google Scholar] [CrossRef]
- Prokopcova, H.; Kappe, C.O. The Liebeskind-Srogl C–C cross-coupling reaction. Angew. Chem. Int. Ed. 2009, 48, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Bumagin, N.A.; Korolev, D.N. Synthesis of Unsymmetric ketones via Ligandless Pd-catalyzed reaction of Acyl chlorides with organoboranes. Tetrahedron Lett. 1999, 40, 3057–3060. [Google Scholar] [CrossRef]
- Haddach, M.; McCarthy, J.R. A new method for the synthesis of ketones: The palladium-catalyzed cross-coupling of acyl chlorides with arylboronic acids. Tetrahedron Lett. 1999, 40, 3109–3112. [Google Scholar] [CrossRef]
- Cho, C.S.; Itotani, K.; Uemura, S. Sodium tetraphenylborate as a phenylating reagent in the palladium-catalyzed phenylation of alkenes and acid chlorides. J. Organomet. Chem. 1993, 443, 253–259. [Google Scholar] [CrossRef]
- Blangetti, M.; Rosso, H.; Prandi, C.; Deagostino, A.; Venturello, P. Suzuki-Miyaura cross-coupling in acylation reactions, scope and recent developments. Molecules 2013, 18, 1188–1213. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, F.; Hajipour, A. A versatile method for the synthesis of diaryl and alkyl aryl ketones via palladium-catalysed cross-coupling reaction of arylboronic acids with acyl chlorides. Appl. Organomet. Chem. 2015, 29, 181–184. [Google Scholar] [CrossRef]
- Solarova, H.; Cisarova, I.; Stepnicka, P. Synthesis, structural characterization, and catalytic evaluation of phosphinoferrocene ligands bearing extended urea-amide. Organometallics 2014, 33, 4131–4147. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. Ligandless heterogeneous palladium: An efficient and recyclable catalyst for Suzuki-type cross-coupling reaction. Appl. Organomet. Chem. 2014, 28, 354–358. [Google Scholar] [CrossRef]
- Semler, M.; Stepnicka, P. Synthesis of aromatic ketones by Suzuki-Miyaura cross-coupling of acyl chlorides with boronic acids mediated by palladium catalysts deposited over donor-functionalized silica gel. Catal. Today 2015, 243, 128–133. [Google Scholar] [CrossRef]
- Movassagh, B.; Hajizadeh, F.; Mohammadi, E. Polystyrene-supported Pd(II)–N-heterocyclic carbenecomplex as a heterogeneous and recyclable precatalyst for cross-coupling of acyl chlorides with arylboronic acids. Appl. Organomet. Chem. 2018, 32, 3982. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. Eco-friendly Suzuki–Miyaura coupling of arylboronic acids to aromatic ketones catalyzed by the oxime-palladacycle in biosolvent 2-MeTHF. New J. Chem. 2016, 40, 3119–3123. [Google Scholar] [CrossRef]
- Forbes, A.; Meier, G.; Jones-Mensah, E.; Magolan, J. Biaryl ketones by suzuki–miyaura cross-coupling of Organotrifluoroborates and acyl chlorides. Eur. J. Org. Chem. 2016, 2983–2987. [Google Scholar] [CrossRef]
- Molander, G.A.; Ellis, N. Organotrifluoroborates: Protected Boronic Acids That Expand the Versatility of the Suzuki Coupling Reaction. Acc. Chem. Res. 2007, 40, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, Y.; Sakino, D.; Sakurai, Y.; Sakai, N. Acid Fluorides as Acyl Electrophiles in Suzuki–Miyaura Coupling. Eur. J. Org. Chem. 2017, 4324–4327. [Google Scholar] [CrossRef]
- Lee, D.; Ryu, T.; Park, Y.; Lee, P. Unmasked acyl anion equivalent from acid chloride with indium: Reversed-Polarity synthesis of unsymmetric aryl-aryl and alkenyl-aryl ketone through palladium-catalyzed cross-coupling reaction. Org. Lett. 2014, 16, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Schmink, J.; Krska, S. Reversed-Polarity Synthesis of Diaryl Ketones via Palladium-Catalyzed Cross-Coupling of Acylsilanes. J. Am. Chem. Soc. 2011, 133, 19574–19577. [Google Scholar] [CrossRef] [PubMed]
- Gooßen, L.; Ghosh, K. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids or Anhydrides. Angew. Chem. Int. Ed. 2001, 40, 3458–3460. [Google Scholar] [CrossRef]
- Kakino, R.; Yasumi, S.; Shimizu, I.; Yamamoto, A. Synthesis of unsymmetrical ketones by palladium-catalyzed cross-coupling reaction of carboxylic anhydrides with organoboron compounds. Bull. Chem. Soc. Jpn. 2002, 75, 137–148. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, X.; Zhang, L.; Yang, L. Nickel-catalyzed cross-coupling of carboxylic anhydrides with arylboronic acids. RSC Adv. 2014, 4, 53885–53890. [Google Scholar] [CrossRef]
- Liu, X.; Yi, M.; Wang, J.; Liu, G. Rhodium-catalyzed synthesis of aryl ketones from carboxylic acid anhydrides and potassium aryltrifluoroborates in the presence of CuI. Tetrahedron Lett. 2015, 71, 4635–4639. [Google Scholar] [CrossRef]
- Yin, W.; He, H.; Zhang, Y.; Long, T. Ligand-free palladium-catalyzed aerobic oxidative coupling of carboxylic anhydrides with arylboronic acids. Chem. Asian J. 2014, 9, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Gooßen, L.; Ghosh, K. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids Activated in situ by Pivalic Anhydride. Eur. J. Org. Chem. 2002, 3254–3267. [Google Scholar] [CrossRef]
- Kakino, R.; Narahashi, H.; Shimizu, I.; Yamamoto, A. General and greener route to ketones by palladium-catalyzed direct conversion of carboxylic acids with organoboronic acids. Bull. Chem. Soc. Jpn. 2001, 30, 1242–1243. [Google Scholar] [CrossRef]
- Gooßen, L.; Ghosh, K. A new practical ketone synthesis directly from carboxylic acids: First application of coupling reagents in palladium catalysis. Chem. Commun. 2001, 2084–2085. [Google Scholar]
- Gooßen, L.; Winkel, L.; Döhring, A.; Ghosh, K.; Paetzold, J. Pd-Catalyzed Synthesis of Functionalized Arylketones from Boronic Acids and Carboxylic Acids Activated in situ with Dimethyl Dicarbonate. Synlett 2002, 1237–1240. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Choi, B.R.; Lee, S.H.; Seo, J.S.; Yoon, C.M. Palladium-Catalyzed Synthesis of Aryl Ketones from Carboxylic Acids and Arylboronic Acids Using EEDQ. Bull. Korean Chem. Soc. 2010, 31, 2672–2674. [Google Scholar] [CrossRef]
- Pathak, A.; Rajput, C.; Bora, P.; Sharma, S. DMC-mediated one pot synthesis of biaryl ketones from aryl carboxylic and boronic acids. Tetrahedron Lett. 2013, 54, 2149–2150. [Google Scholar] [CrossRef]
- Garcia-Barrantes, P.; McGowan, K.; Ingram, S.; Lindsley, C. One pot synthesis of unsymmetrical ketones from carboxylic and boronic acids via PyClU-mediated acylative Suzuki coupling. Tetrahedron Lett. 2017, 58, 898–901. [Google Scholar] [CrossRef]
- Wu, H.; Xu, B.; Li, Y.; Hong, F.; Zhu, D.; Jian, J.; Pu, X.; Zeng, Z. One-Pot Synthesis of Arylketones from Aromatic Acids via Palladium-Catalyzed Suzuki Coupling. J. Org. Chem. 2016, 81, 2987–2992. [Google Scholar] [CrossRef]
- Si, S.; Wang, C.; Zhang, N.; Zou, G. Palladium-Catalyzed Room-Temperature Acylative Suzuki Coupling of High-Order Aryl Borons with Carboxylic Acids. J. Org. Chem. 2016, 81, 4364–4370. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, C.; You, H.; Lin, K.; Gong, H. Mild ketone formation via Ni-catalyzed reductive coupling of unactivated alkyl halides with acid anhydrides. Chem. Commun. 2012, 48, 7034–7036. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, X.; Qian, Q.; Gong, H. Alkyl–aryl ketone synthesis via nickel-catalyzed reductive coupling of alkyl halides with aryl acids and anhydrides. Chem. Commun. 2015, 51, 10302–10305. [Google Scholar] [CrossRef]
- Halima, T.; Zhang, W.; Yalaoui, I.; Hong, X.; Yang, Y.; Houk, K.; Newman, S. Palladium-Catalyzed Suzuki−Miyaura Coupling of Aryl Esters. J. Am. Chem. Soc. 2017, 139, 1311–1318. [Google Scholar] [PubMed]
- Lei, P.; Meng, G.; Shi, S.; Ling, Y.; An, J.; Szostak, R.; Szostak, M. Suzuki–Miyaura cross-coupling of amides and esters at room temperature: Correlation with barriers to rotation around C–N and C–O bonds. Chem. Sci. 2017, 8, 6525–6530. [Google Scholar]
- Shi, S.; Lei, P.; Szostak, M. Pd-PEPPSI: A General Pd-NHC Precatalyst for Suzuki−Miyaura Cross-Coupling of Esters by C–O Cleavage. Organometallics 2017, 36, 3784–3789. [Google Scholar] [CrossRef]
- Li, G.; Shi, S.; Szostak, M. Pd-PEPPSI: Water-Assisted Suzuki-Miyaura Cross-Coupling of Aryl Esters at Room Temperature using a Practical Palladium-NHC (NHC = N-Heterocyclic Carbene) Precatalyst. Adv. Synth. Catal. 2018, 360, 1538–1543. [Google Scholar] [CrossRef]
- Dardir, A.; Melvin, P.; Davis, R.; Hazari, N.; Beromi, M. Rapidly Activating Pd-Precatalyst for Suzuki−Miyaura and Buchwald−Hartwig Couplings of Aryl Esters. J. Org. Chem. 2018, 83, 469–477. [Google Scholar] [CrossRef]
- Buchspies, J.; Pyle, D.J.; He, H.; Szostak, M. Pd-Catalyzed Suzuki-Miyaura Cross-Coupling of Pentafluorophenyl Esters. Molecules 2018, 23, 3134. [Google Scholar] [CrossRef]
- Chatupheeraphat, A.; Liao, H.H.; Srimontree, W.; Guo, L.; Minenkov, Y.; Poater, A.; Cavallo, L.; Rueping, M. Ligand-Controlled Chemoselective C(acyl)–O Bond vs C(aryl)–C Bond Activation of Aromatic Esters in Nickel Catalyzed C(sp2)–C(sp3) Cross-Couplings. J. Am. Chem. Soc. 2018, 140, 3724–3735. [Google Scholar] [CrossRef]
- Masson-Makdissi, J.; Vandavasi, J.; Newman, S. Switchable Selectivity in the Pd-Catalyzed Alkylative Cross-Coupling of Esters. Org. Lett. 2018, 20, 4094–4098. [Google Scholar] [CrossRef]
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Meng, G.; Szostak, M. Sterically Controlled Pd-Catalyzed Chemoselective Ketone Synthesis via N-C Cleavage in Twisted Amides. Org. Lett. 2015, 17, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Szostak, M. Palladium-Catalyzed Suzuki-Miyaura Coupling of Amides by Carbon-Nitrogen Cleavage: General Strategy for Amide N-C Bond Activation. Org. Biomol. Chem. 2016, 14, 5690–5707. [Google Scholar] [CrossRef] [PubMed]
- Pace, V.; Holzer, W.; Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Structures of Highly Twisted Amides Relevant to Amide N–C Cross-Coupling: Evidence for Ground-State Amide Destabilization. Chem. Eur. J. 2016, 22, 14494–14498. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, M. Ground-State Distortion in N-Acyl-tert-butyl-carbamates (Boc) and N-Acyl-tosylamides (Ts): Twisted Amides of Relevance to Amide N–C Cross-Coupling. J. Org. Chem. 2016, 81, 8091–8094. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.; Meng, G.; Szostak, M. Resonance Destabilization in N-Acylanilines (Anilides): Electronically-Activated Planar Amides of Relevance in N–C(O) Cross-Coupling. J. Org. Chem. 2017, 82, 6373–6378. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Reversible Twisting of Primary Amides via Ground State N–C(O) Destabilization: Highly Twisted Rotationally Inverted Acyclic Amides. J. Am. Chem. Soc. 2018, 140, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.; Szostak, M. N-Acyl-Glutarimides: Resonance and Proton Affinities of Rotationally-Inverted Twisted Amides Relevant to N−C(O) Cross-Coupling. Org. Lett. 2018, 20, 1342–1345. [Google Scholar] [CrossRef]
- Li, X.; Zou, G. Acylative Suzuki coupling of amides: Acyl-nitrogen activation via synergy of independently modifiable activating groups. Chem. Commun. 2015, 51, 5089–5092. [Google Scholar] [CrossRef]
- Weires, N.A.; Baker, E.L.; Garg, N.K. Nickel-catalysed Suzuki−Miyaura coupling of Amides. Nat. Chem. 2016, 8, 76–80. [Google Scholar] [CrossRef]
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef]
- Kaiser, D.; Bauer, A.; Lemmerer, M.; Maulide, N. Amide Activation: An Emerging Tool for Chemoselective Synthesis. Chem. Soc. Rev. 2018, 47, 7899–7925. [Google Scholar] [CrossRef]
- Meng, G.; Szostak, M. N-Acyl-Glutarimides: Privileged Scaffolds in Amide N–C Bond Cross-Coupling. Eur. J. Org. Chem. 2018, 20–21, 2352–2365. [Google Scholar] [CrossRef]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem. Eur. J. 2017, 23, 7157–7173. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Szostak, M. Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar]
- Liu, C.; Szostak, M. Decarbonylative Cross-Coupling of Amides. Org. Biomol. Chem. 2018, 16, 7998–8010. [Google Scholar] [CrossRef]
- Dander, J.E.; Garg, N.K. Breaking Amides using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. [Google Scholar] [CrossRef]
- Liu, C.; Meng, G.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R.; Szostak, M. N-Acylsaccharins: Stable Electrophilic Amide-Based Acyl Transfer Reagents in Pd-Catalyzed Suzuki−Miyaura Coupling via N−C Cleavage. Org. Lett. 2016, 18, 4194–4197. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Cui, M.; Jian, J.; Zeng, Z. Suzuki Coupling of Amides via Palladium-Catalyzed C-N Cleavage of N-Acylsaccharins. Adv. Synth. Catal. 2016, 358, 3876–3880. [Google Scholar] [CrossRef]
- Szostak, M.; Liu, C.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling of N-Mesylamides by N−C Cleavage: Electronic Effect of the Mesyl Group. Org. Lett. 2017, 19, 1434–1437. [Google Scholar]
- Szostak, M.; Meng, G.; Shi, S. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling of Amides via Site-Selective N−C Bond Cleavage by Cooperative Catalysis. ACS Catal. 2016, 6, 7335–7339. [Google Scholar]
- Shi, S.; Szostak, M. Nickel-Catalyzed Diaryl Ketone Synthesis by N–C Cleavage: Direct Negishi Cross-Coupling of Primary Amides by Site- Selective N,N-Di-Boc Activation. Org. Lett. 2016, 18, 5872–5875. [Google Scholar] [CrossRef]
- Osumi, Y.; Liu, C.; Szostak, M. N-Acylsuccinimides: Twist-controlled, acyl-transfer reagents in Suzuki–Miyaura cross-coupling by N–C amide bond activation. Org. Biomol. Chem. 2017, 15, 8867–8871. [Google Scholar] [CrossRef]
- Cui, M.; Chen, Z.; Liu, T.; Wang, H.; Zeng, Z. N-Acylsuccinimides: Efficient acylative coupling reagents in palladium-catalyzed Suzuki coupling via C-N cleavage. Tetrahedron Lett. 2017, 58, 3819–3822. [Google Scholar] [CrossRef]
- Shi, S.; Szostak, M. Nickel-Catalyzed Negishi Cross-Coupling of N-Acylsuccinimides: Stable, Amide-Based, Twist-Controlled Acyl-Transfer Reagents via N–C Activation. Synthesis 2017, 49, 3602–3608. [Google Scholar]
- Liu, C.; Li, G.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. Acyl and Decarbonylative Suzuki Coupling of N-Acetyl Amides: Electronic Tuning of Twisted, Acyclic Amides in Catalytic Carbon−Nitrogen Bond Cleavage. ACS Catal. 2018, 8, 9131–9139. [Google Scholar] [CrossRef]
- Liu, C.; Shi, S.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R.; Szostak, M. The Most Twisted Acyclic Amides: Structures and Reactivity. Org. Lett. 2018, 20, 7771–7774. [Google Scholar] [CrossRef]
- Szostak, M.; Aubé, J. Chemistry of Bridged Lactams and Related Heterocycles. Chem. Rev. 2013, 113, 5701–5765. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Szostak, M. General Method for the Suzuki-Miyaura Cross-Coupling of Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (NHC = N-Heterocyclic Carbene) Complexes. ACS Catal. 2017, 7, 1960–1965. [Google Scholar] [CrossRef]
- Li, G.; Lei, P.; Szostak, M.; Casals, E.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanistic Study of Suzuki-Miyaura Cross-Coupling Reactions of Amides Mediated by [Pd(NHC)(allyl)Cl] Precatalysts. ChemCatChem 2018, 10, 3096–3106. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Szostak, M. Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki-Miyaura Cross-Coupling Reactions of Amides. J. Org. Chem. 2017, 82, 6638–6646. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-defined N-heterocyclic carbenes-palladium(II) precatalysts for cross-coupling reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Melvin, P.R.; Nova, A.; Balcells, D.; Dai, W.; Hazari, N.; Hruszkewycz, D.P.; Shah, H.P.; Tudge, M.T. Design of a Versatile and Improved Precatalyst Scaffold for Palladium-Catalyzed Cross-Coupling: (η3-1-t-Bu-indenyl)2(μ-Cl)2Pd2. ACS Catal. 2015, 5, 5596–5606. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd-N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef]
- Meng, G.; Szostak, R.; Szostak, M. Suzuki−Miyaura Cross-Coupling of N-Acylpyrroles and Pyrazoles: Planar, Electronically Activated Amides in Catalytic N-C Cleavage. Org. Lett. 2017, 19, 3596–3599. [Google Scholar] [CrossRef]
- Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. N-Methylamino Pyrimidyl Amides (MAPA): Highly Reactive, Electronically-Activated Amides in Catalytic N-C(O) Cleavage. Org. Lett. 2017, 19, 4656–4659. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Nolan, S.P.; Szostak, M. General Method for the Suzuki-Miyaura Cross-Coupling of Primary Amide-Derived Electrophiles Enabled by [Pd(NHC)(cin)Cl] at Room Temperature. Org. Lett. 2017, 19, 6510–6513. [Google Scholar] [CrossRef]
- Li, X.; Zou, G. Palladium-catalyzed acylative cross-coupling of amides with diarylborinic acids and sodium tetraarylborates. J. Organomet. Chem. 2015, 794, 136–145. [Google Scholar] [CrossRef]
- Wang, C.; Huang, L.; Wang, F.; Zou, G. Highly efficient synthesis of aryl ketones by PEPPSI-palladium catalyzed acylative Suzuki coupling of amides with diarylborinic acids. Tetrahedron Lett. 2018, 59, 2299–2301. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, T.; Guo, W.; Wang, Z.; Huang, J.; Zhu, Y.; Zeng, Z. N-Acyl-5,5-dimethylhydantoin, a New Mild Acyl-Transfer Reagent in Pd Catalysis: Highly Efficient Synthesis of Functionalized Ketones. Org. Process Res. Dev. 2018, 22, 1188–1199. [Google Scholar] [CrossRef]
- Szostak, R.; Liu, C.; Lalancette, R.; Szostak, M. Twisted N-Acyl-hydantoins: Rotationally Inverted Urea-Imides of Relevance in N–C(O) Cross-Coupling. J. Org. Chem. 2018, 83, 14676–14682. [Google Scholar] [CrossRef]
- Wang, T.; Guo, J.; Wang, H.; Guo, H.; Jia, D.; Zhang, W.; Liu, L. N-heterocyclic carbene palladium(II)-catalyzed Suzuki-Miyaura cross coupling of N-acylsuccinimides by C-N cleavage. J. Organomet. Chem. 2018, 877, 80–84. [Google Scholar] [CrossRef]
- Wang, T.; Xie, H.; Liu, L.; Zho, W.X. N-Heterocyclic carbene-palladium(II) complexes with benzoxazole or benzothiazole ligands: Synthesis, characterization, and application to Suzuki-Miyaura cross-coupling reaction. J. Organomet. Chem. 2016, 804, 73–79. [Google Scholar] [CrossRef]
- Lim, M.; Kim, H.; Ban, J.; Son, J.; Lee, J.; MIn, S.; Lee, S.; Rhee, H. Palladium-Catalyzed Carbonylative Coupling Reactions of N,N-Bis(methanesulfonyl)amides through C–N Bond Cleavage. Eur. J. Org. Chem. 2018, 2018, 5717–5724. [Google Scholar] [CrossRef]
- Liu, X.; Hsiao, C.; Guo, L.; Rueping, M. Cross-Coupling of Amides with Alkylboranes via Nickel-Catalyzed C−N Bond Cleavage. Org. Lett. 2018, 20, 2976–2979. [Google Scholar] [CrossRef]
- Shi, W.; Zou, G. Palladium-Catalyzed Room Temperature Acylative Cross-Coupling of Activated Amides with Trialkylboranes. Molecules 2018, 23, 2412. [Google Scholar] [CrossRef]
- Meng, G.; Szostak, M. Palladium/NHC (NHC = N-Heterocyclic Carbene)-Catalyzed B-Alkyl Suzuki Cross-Coupling of Amides by Selective N−C Bond Cleavage. Org. Lett. 2018, 20, 6789–6793. [Google Scholar] [CrossRef]
- Boit, T.; Weires, N.; Kim, J.; Garg, N. Nickel-Catalyzed Suzuki−Miyaura Coupling of Aliphatic Amides. ACS Catal. 2018, 8, 1003–1008. [Google Scholar] [CrossRef]
- Amani, J.; Alam, R.; Badir, S.; Molander, G. Synergistic Visible-Light Photoredox/Nickel-Catalyzed Synthesis of Aliphatic Ketones via N−C Cleavage of Imides. Org. Lett. 2017, 19, 2426–2429. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, W.; Mei, H.; Han, J.; Pan, Y. Ni-Catalyzed Reductive Cross-Coupling of Amides with Aryl Iodide Electrophiles via C−N Bond Activation. Org. Lett. 2017, 19, 2536–2539. [Google Scholar] [CrossRef]
- Shi, S.; Szostak, M. Efficient Synthesis of Diaryl Ketones by Nickel-Catalyzed Negishi Cross-Coupling of Amides via Carbon–Nitrogen Bond Cleavage at Room Temperature Accelerated by Solvent Effect. Chem. Eur. J. 2016, 22, 10420–10424. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Luo, M.; Zeng, X. Kumada Arylation of Secondary Amides Enabled by Chromium Catalysis for Unsymmetric Ketone Synthesis under Mild Conditions. ACS Catal. 2018, 8, 5864–5868. [Google Scholar] [CrossRef]





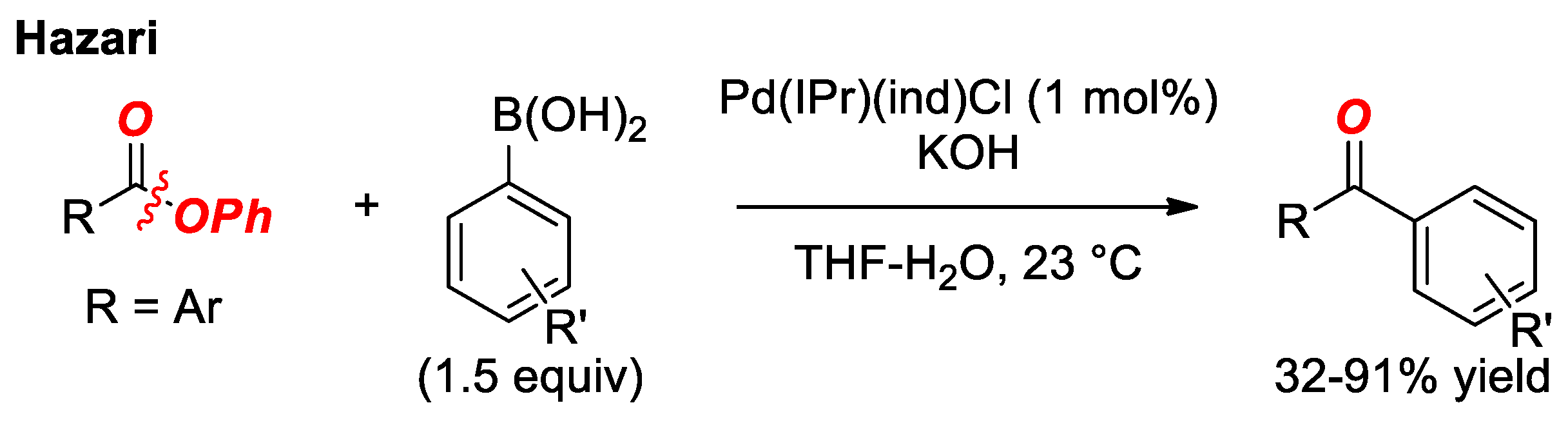
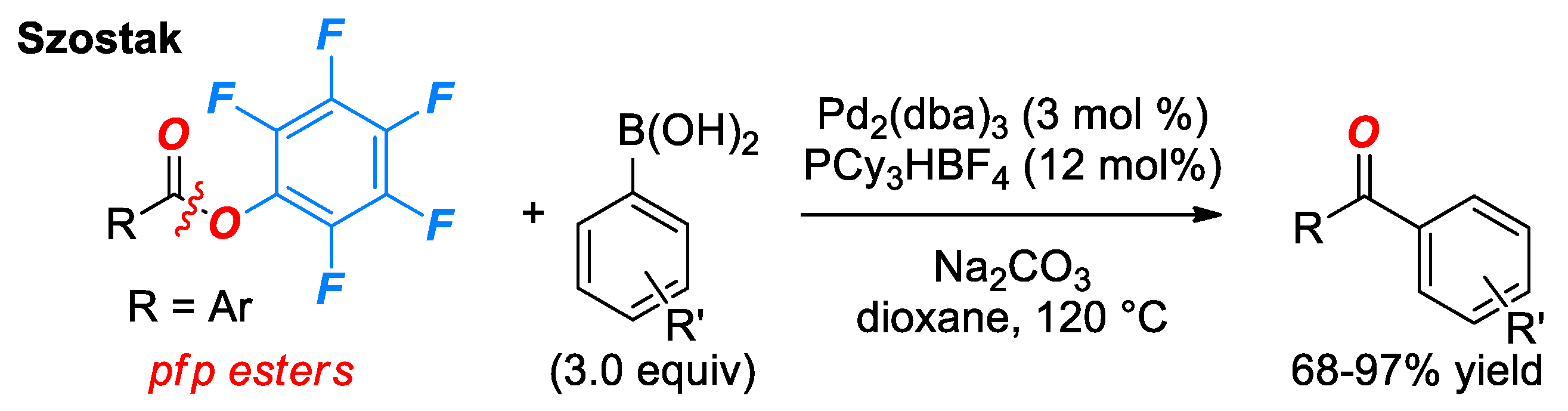
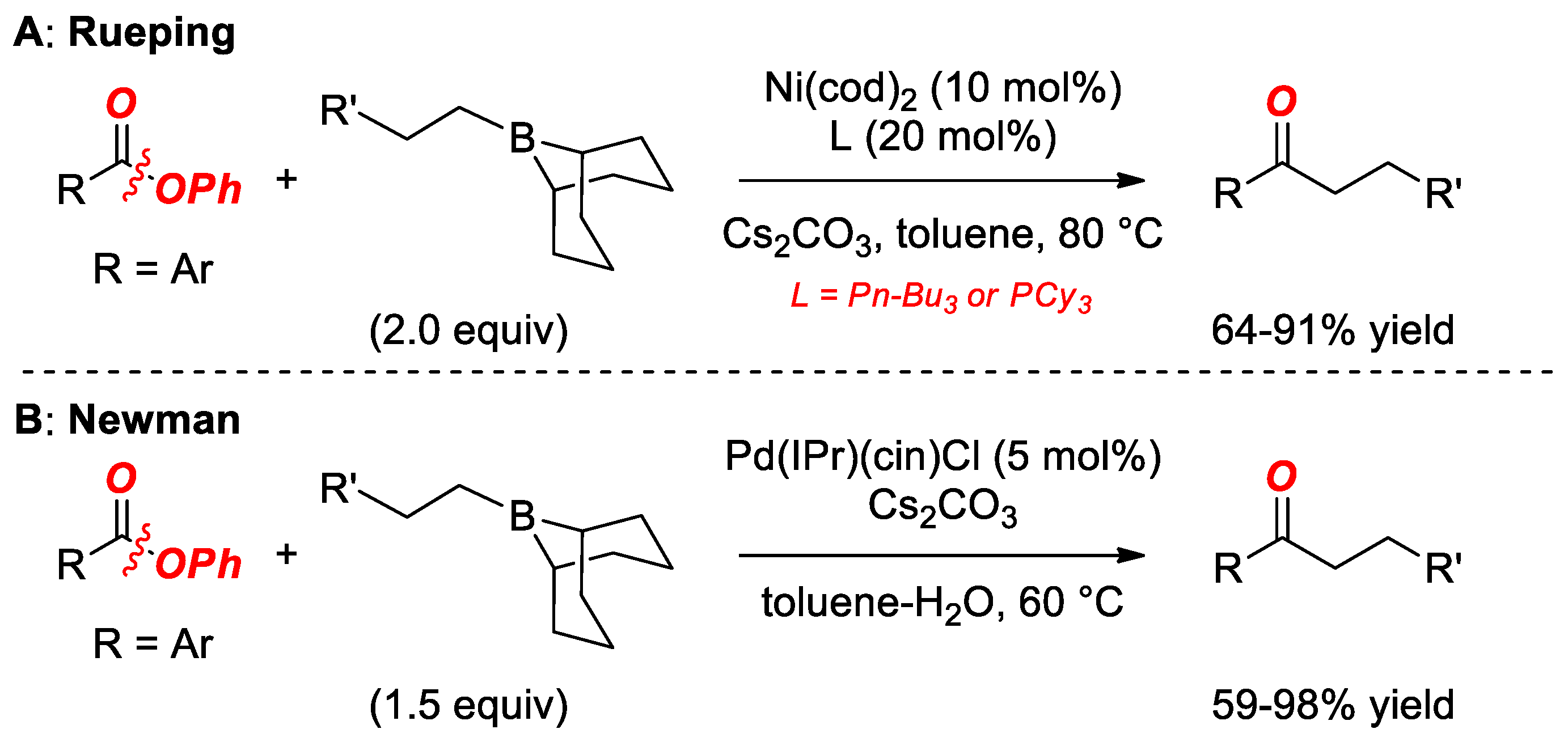





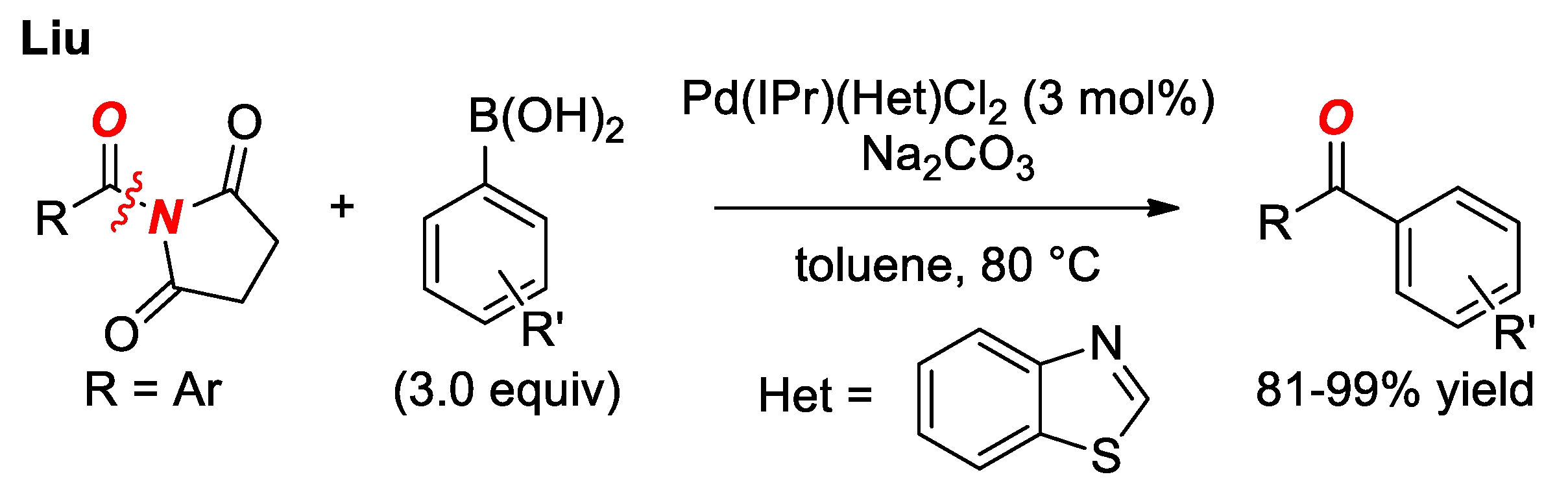
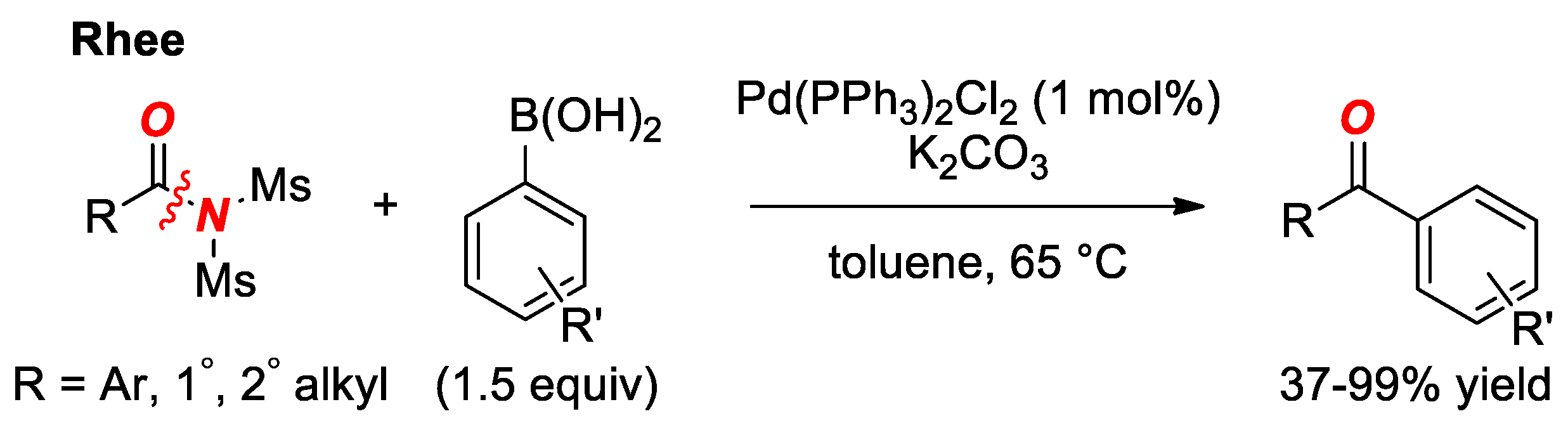
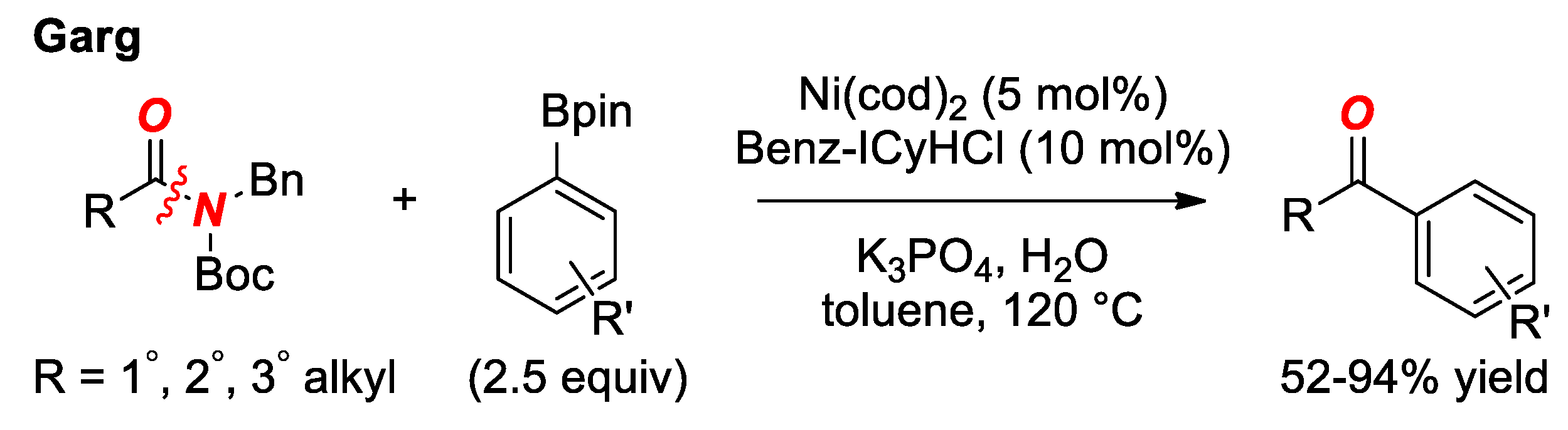
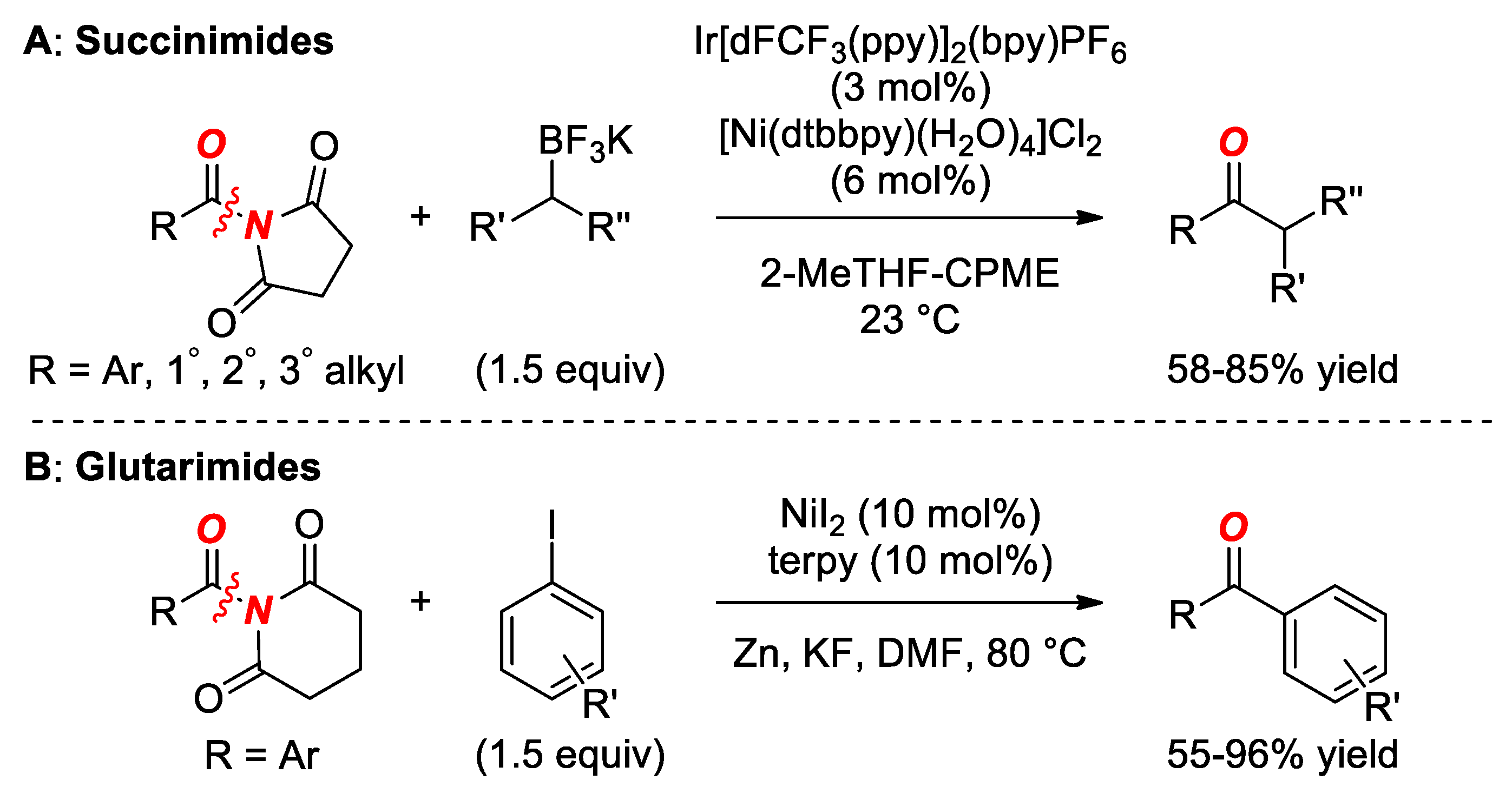
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchspies, J.; Szostak, M. Recent Advances in Acyl Suzuki Cross-Coupling. Catalysts 2019, 9, 53. https://doi.org/10.3390/catal9010053
Buchspies J, Szostak M. Recent Advances in Acyl Suzuki Cross-Coupling. Catalysts. 2019; 9(1):53. https://doi.org/10.3390/catal9010053
Chicago/Turabian StyleBuchspies, Jonathan, and Michal Szostak. 2019. "Recent Advances in Acyl Suzuki Cross-Coupling" Catalysts 9, no. 1: 53. https://doi.org/10.3390/catal9010053
APA StyleBuchspies, J., & Szostak, M. (2019). Recent Advances in Acyl Suzuki Cross-Coupling. Catalysts, 9(1), 53. https://doi.org/10.3390/catal9010053