Carboxylation of Hydroxyaromatic Compounds with HCO3− by Enzyme Catalysis: Recent Advances Open the Perspective for Valorization of Lignin-Derived Aromatics
Abstract
1. Introduction
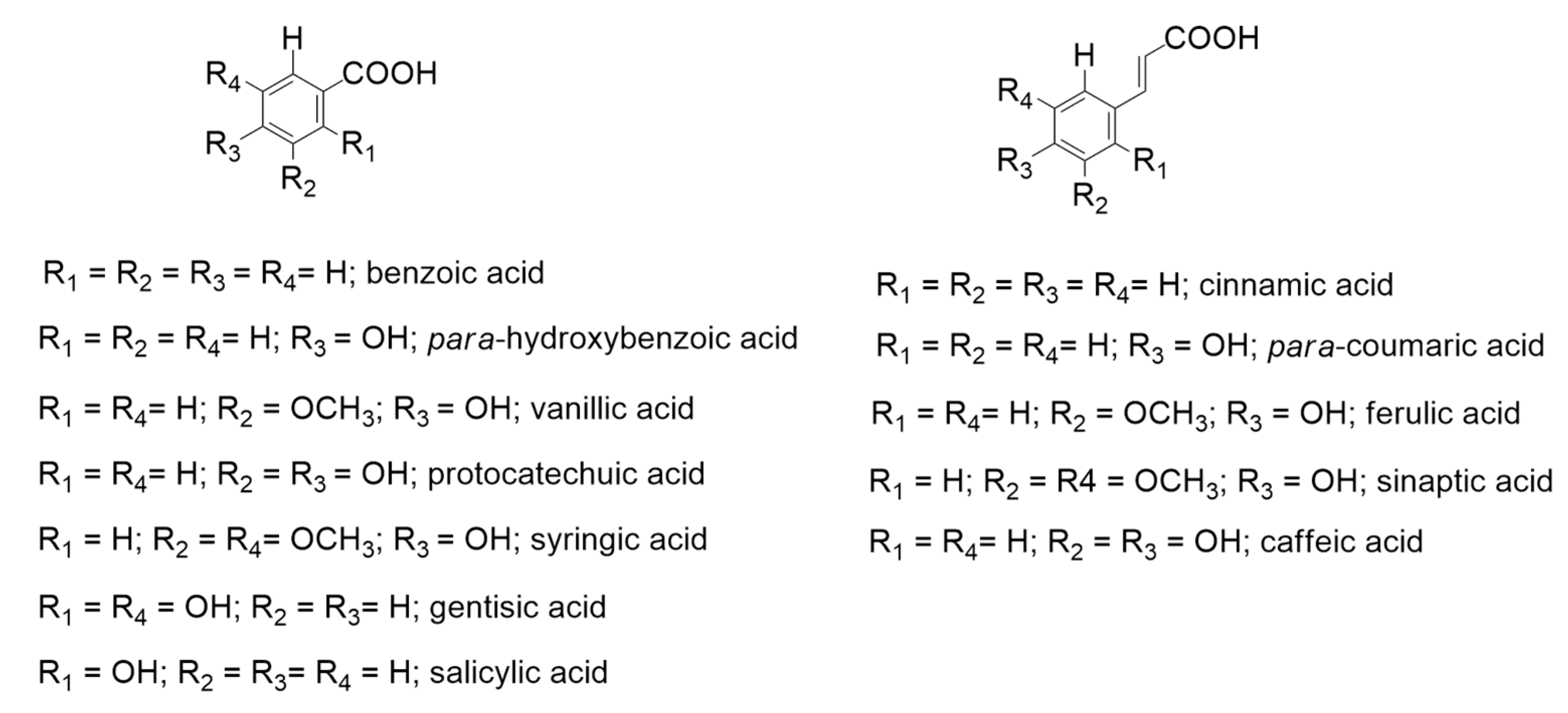
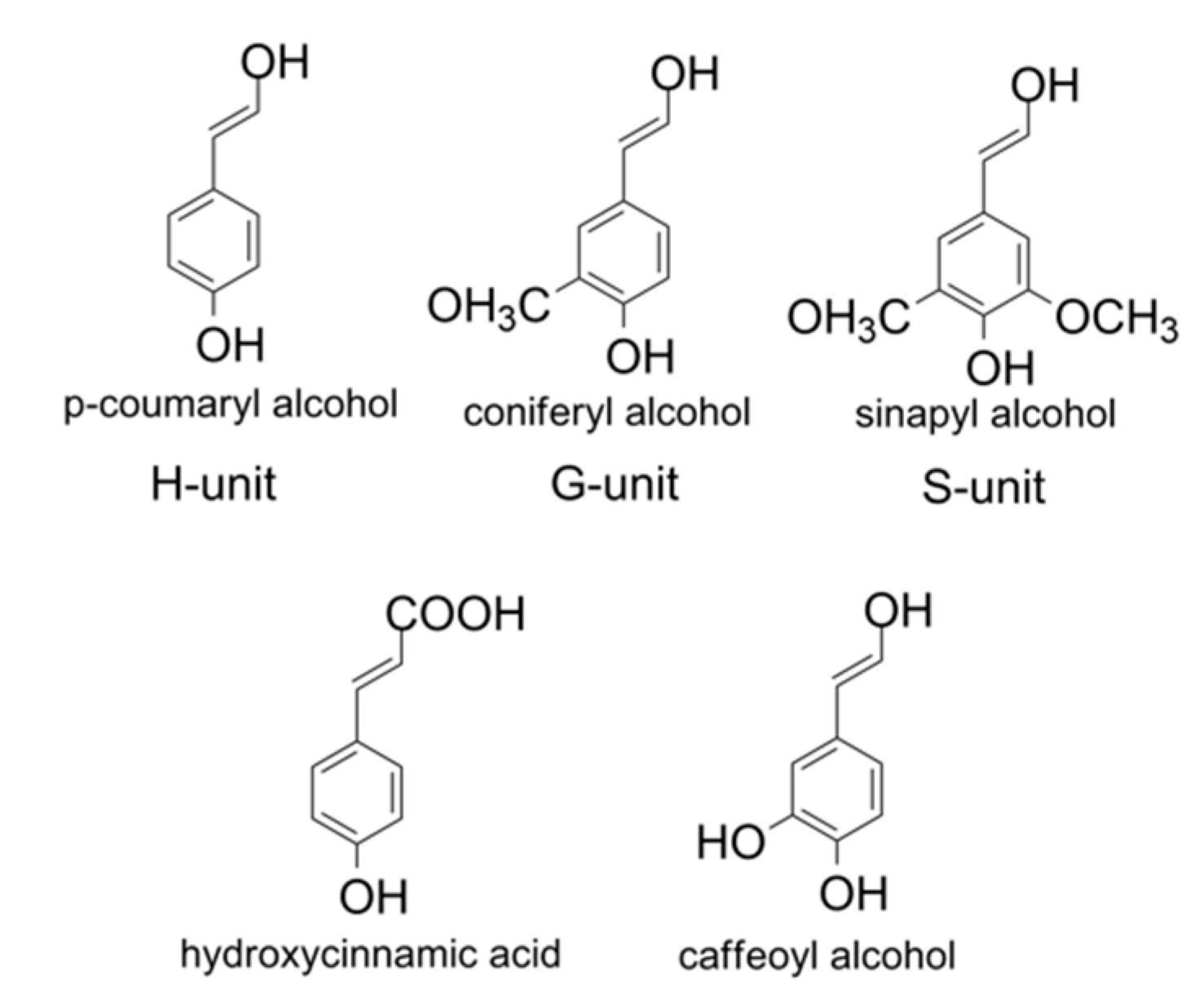
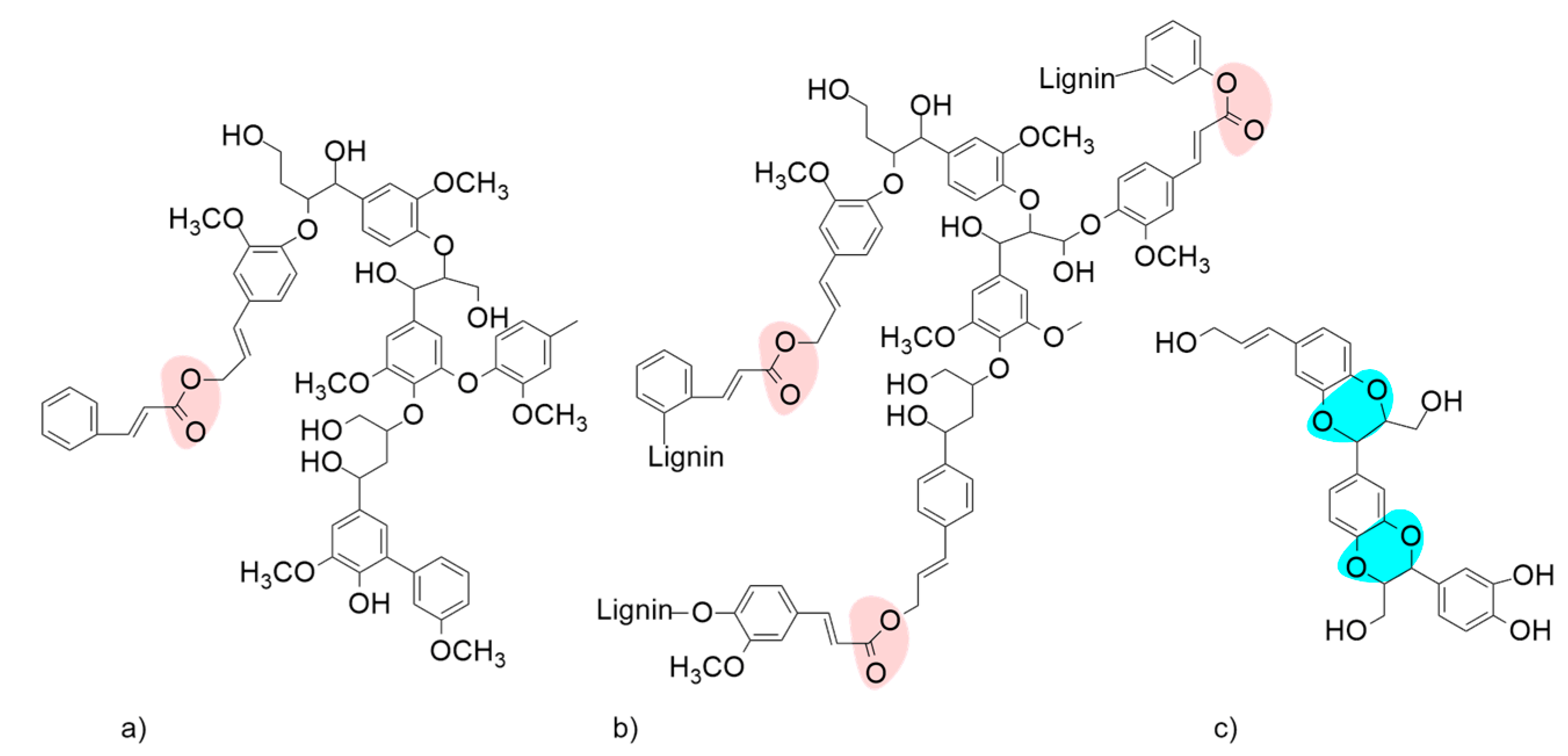
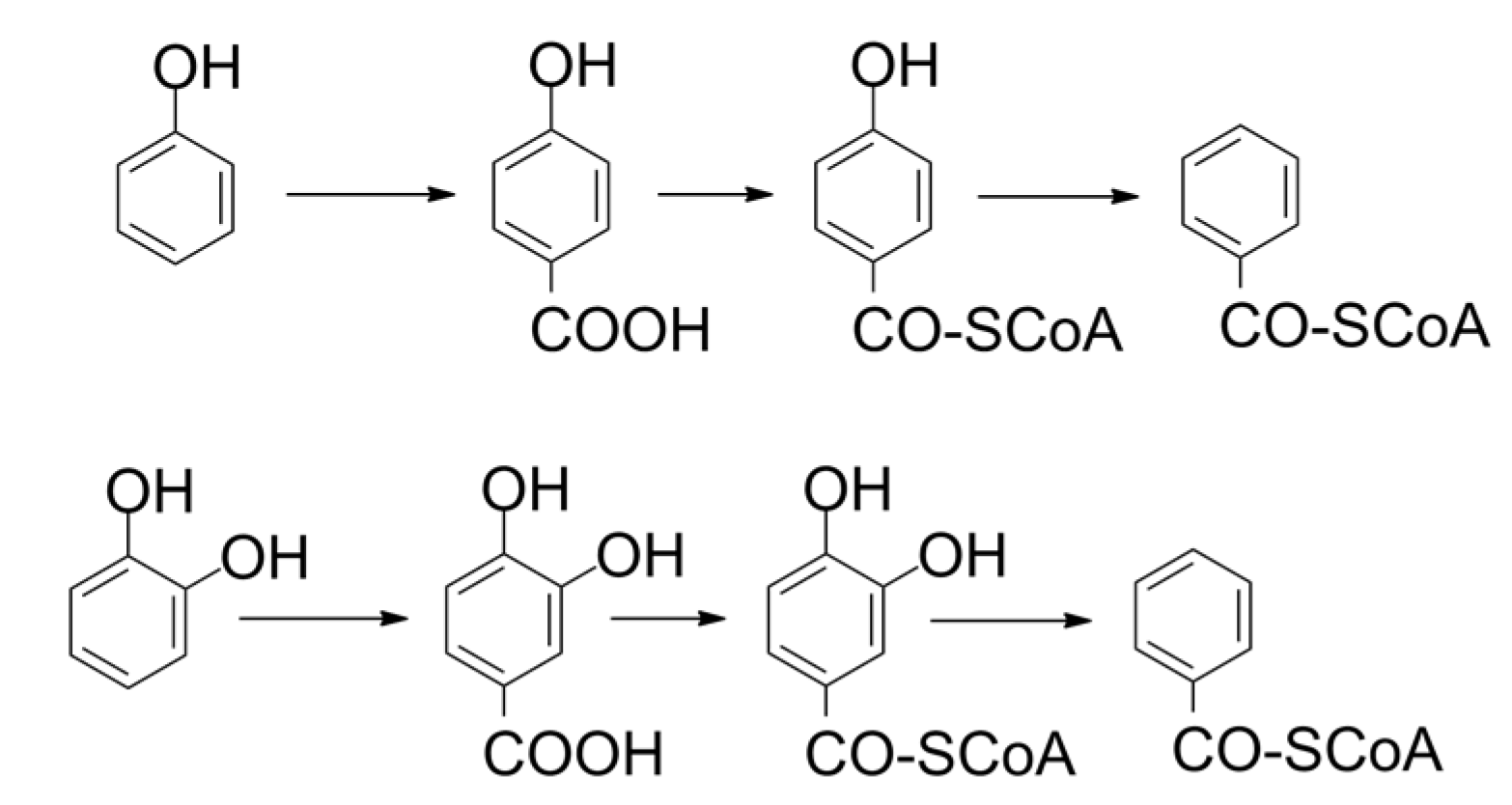
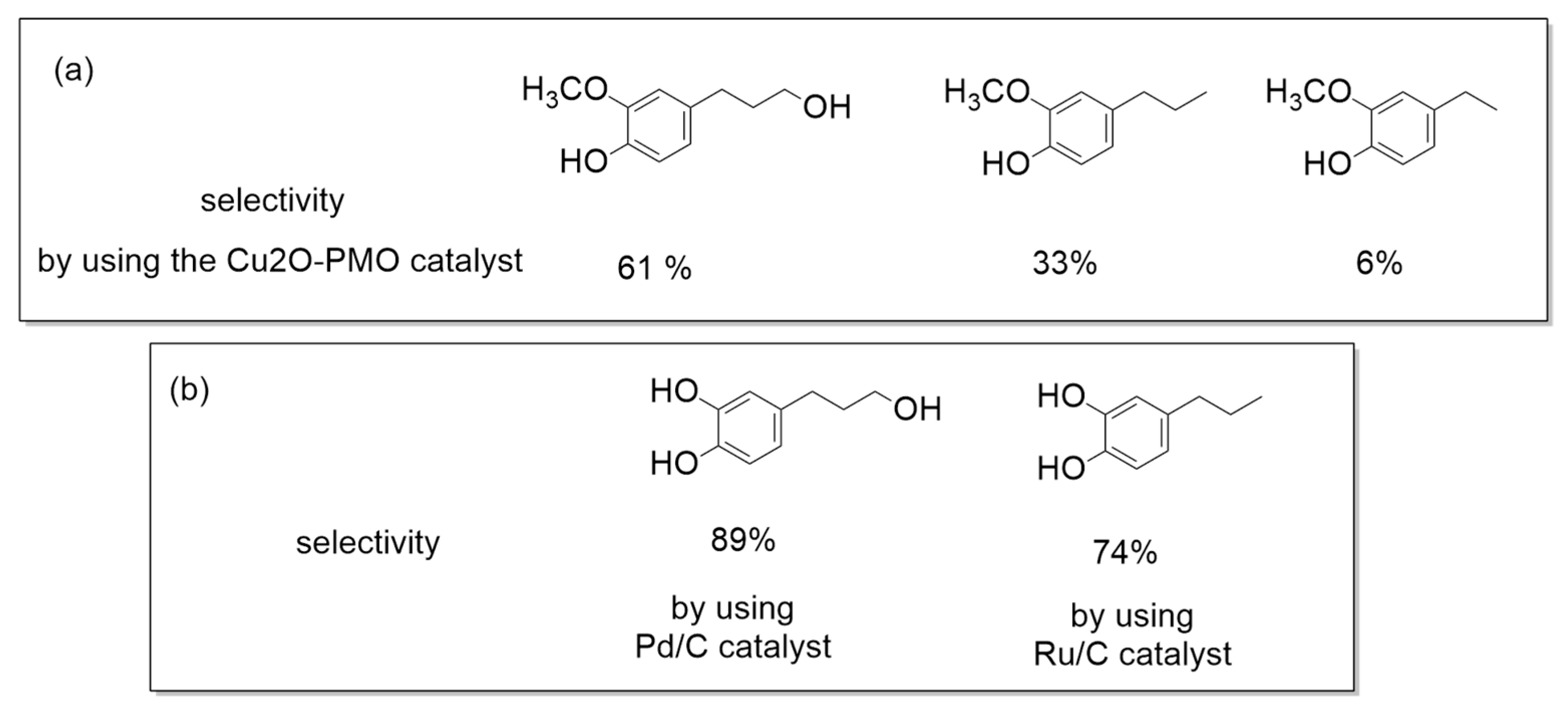
2. Biotechnological Exploitation of Lignin from Biorefineries
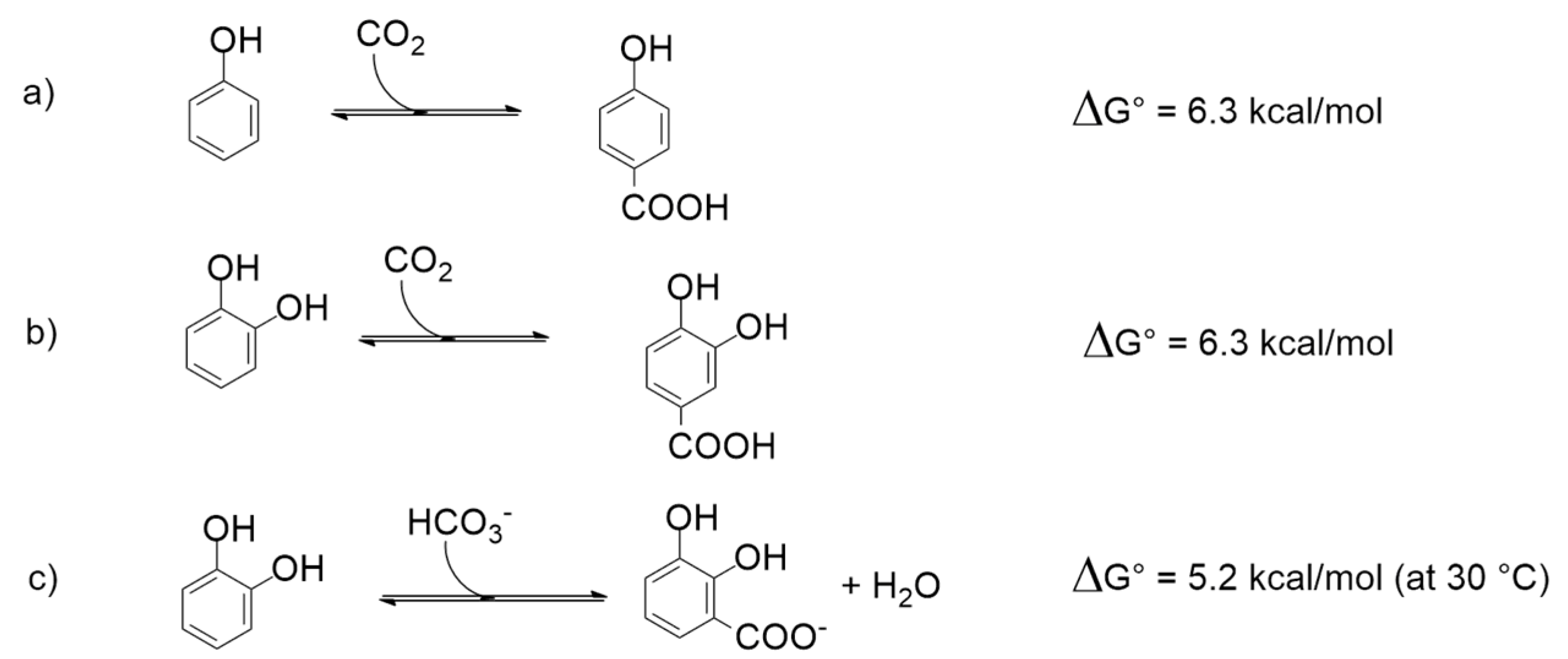
3. Thermodynamic Considerations
4. Electron-Rich Heteroaromatic Biocarboxylations
- 1)
- Decarboxylase enzymes, including salicylic acid decarboxylase, 2,3-dihydroxybenzoate decarboxylase, and 2,6-dihydroxybenzoate decarboxylase, catalysing the regioselective ortho-carboxylation of phenols to the directing phenolic group;
- 2)
- carboxylase enzymes, including 4-hydrobenzoate and 3,4-dihydroxybenzoate decarboxylases, catalysing the regioselective para-carboxylation of phenols to the directing phenolic group. Phenylphosphate carboxylases also belong to this category, but require an adenosine triphosphate (ATP) consuming activation prior to carboxylation;
- 3)
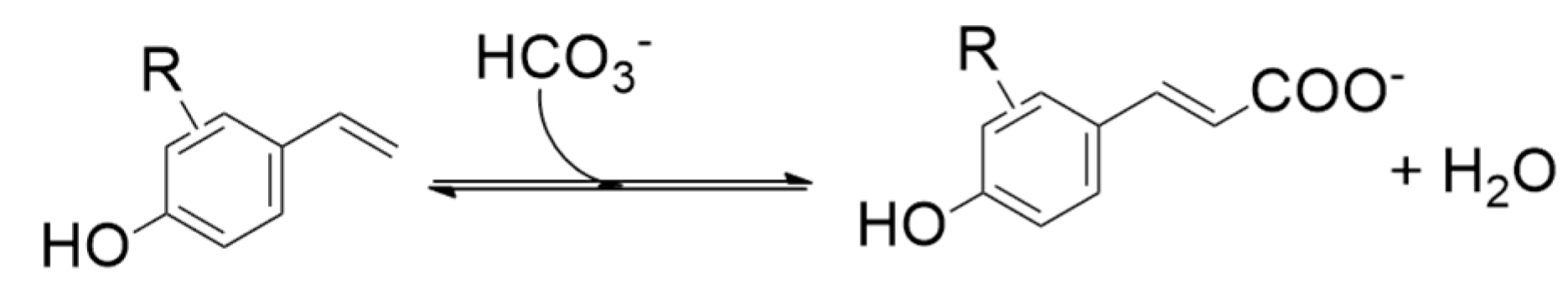
- enzymes catalyzing the carboxylation of para-hydroxystyrenes at the β-carbon of the styrene side chain, yielding (E)-4-hydroxycinnamic acids;
- 4)
- enzymes catalyzing carboxylation of heteroaromatics, such as pyrrole and indole, as pyrrole-2-carboxylate and indole-3-carboxylate decarboxylases.
- 1)
- Do not need cofactors as pyridoxal 5’-phosphate or thiamine pyrophosphate;
- 2)
- substrates are not preactivated by phosphorylation (except for phenylphosphate carboxylase from facultative anaerobes);
- 3)
- use of HCO3− as a CO2 source.
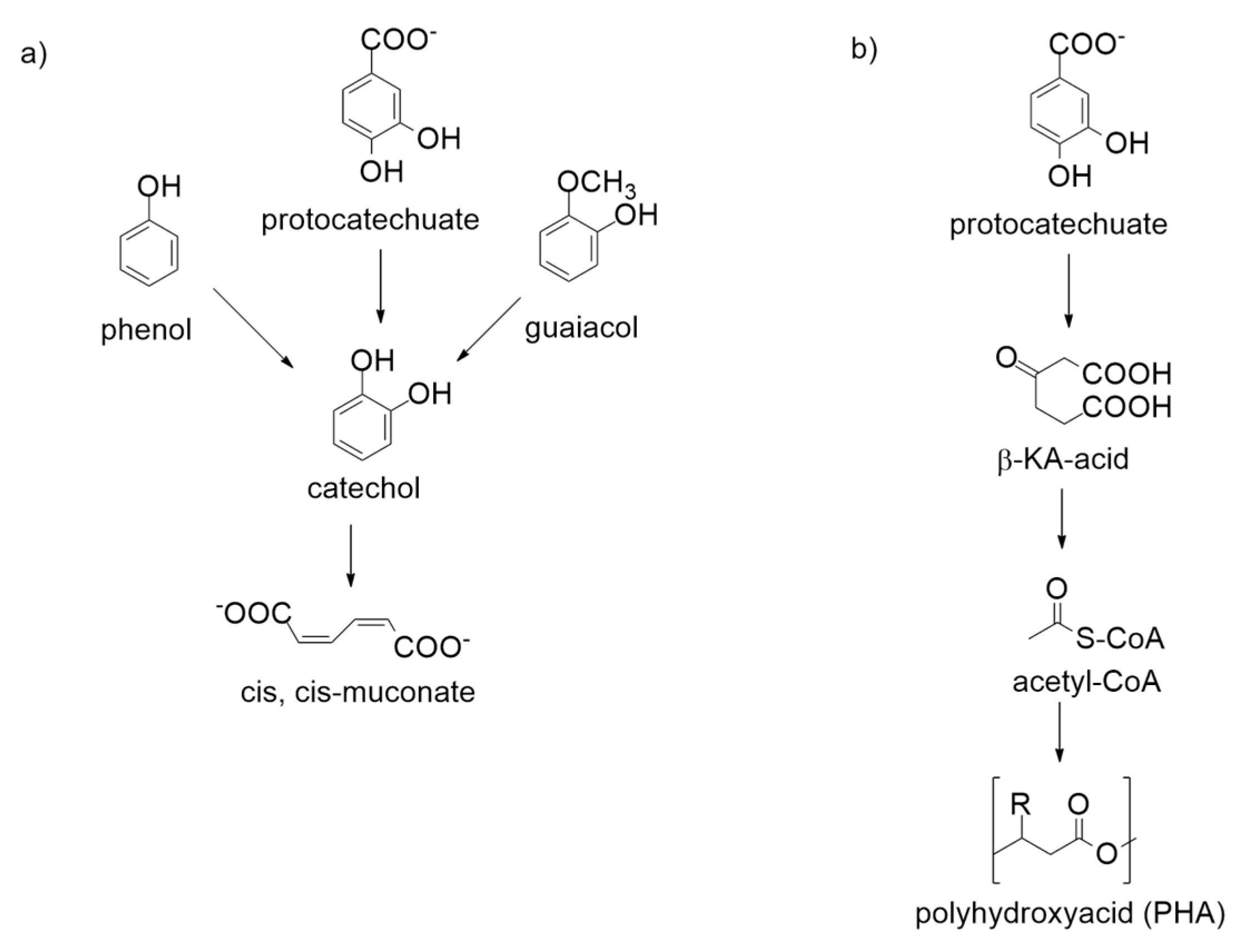
5. Enzymatic Regioselective Ortho-Carboxylation of Phenols: The Enzyme’s Catalytic Reactivity
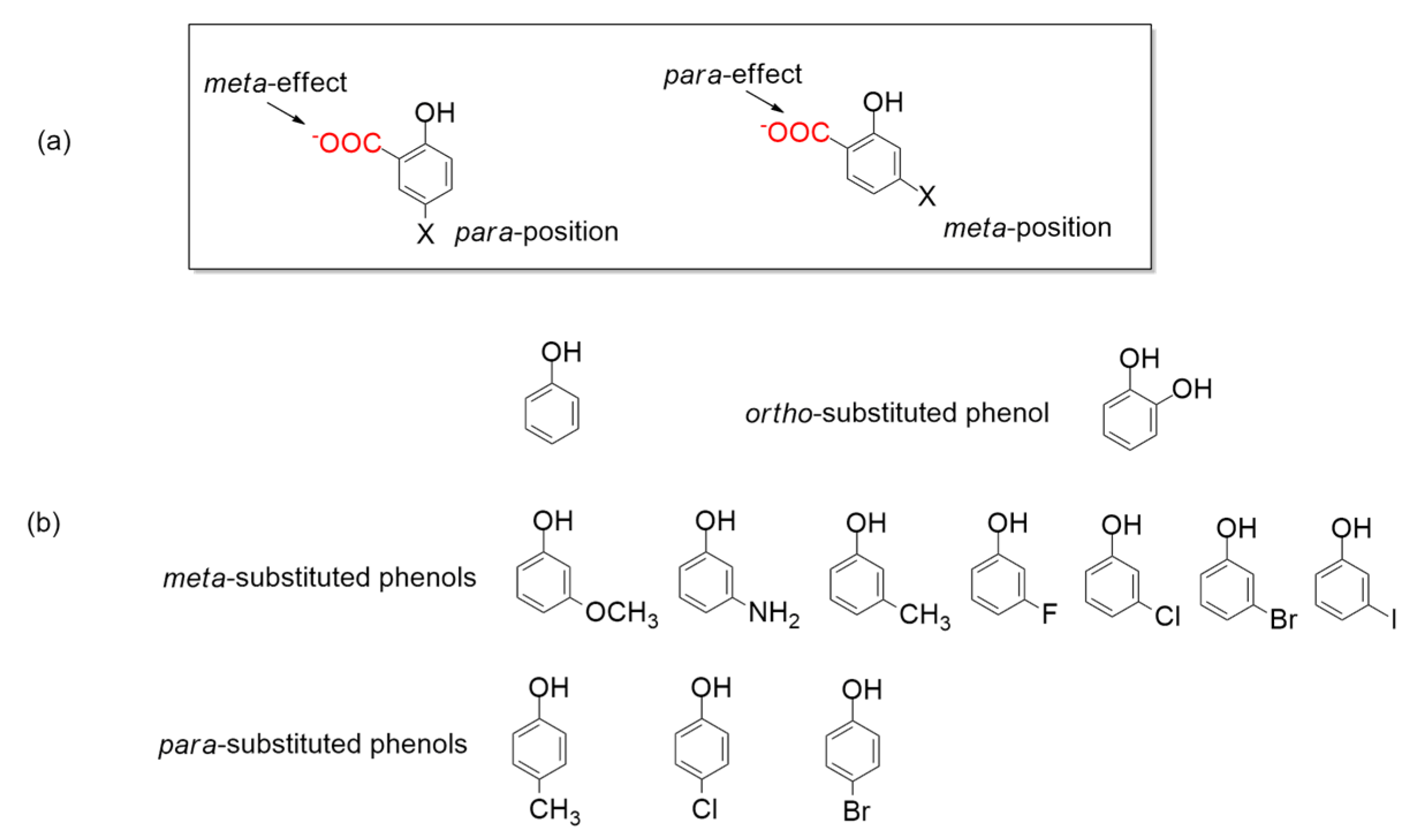
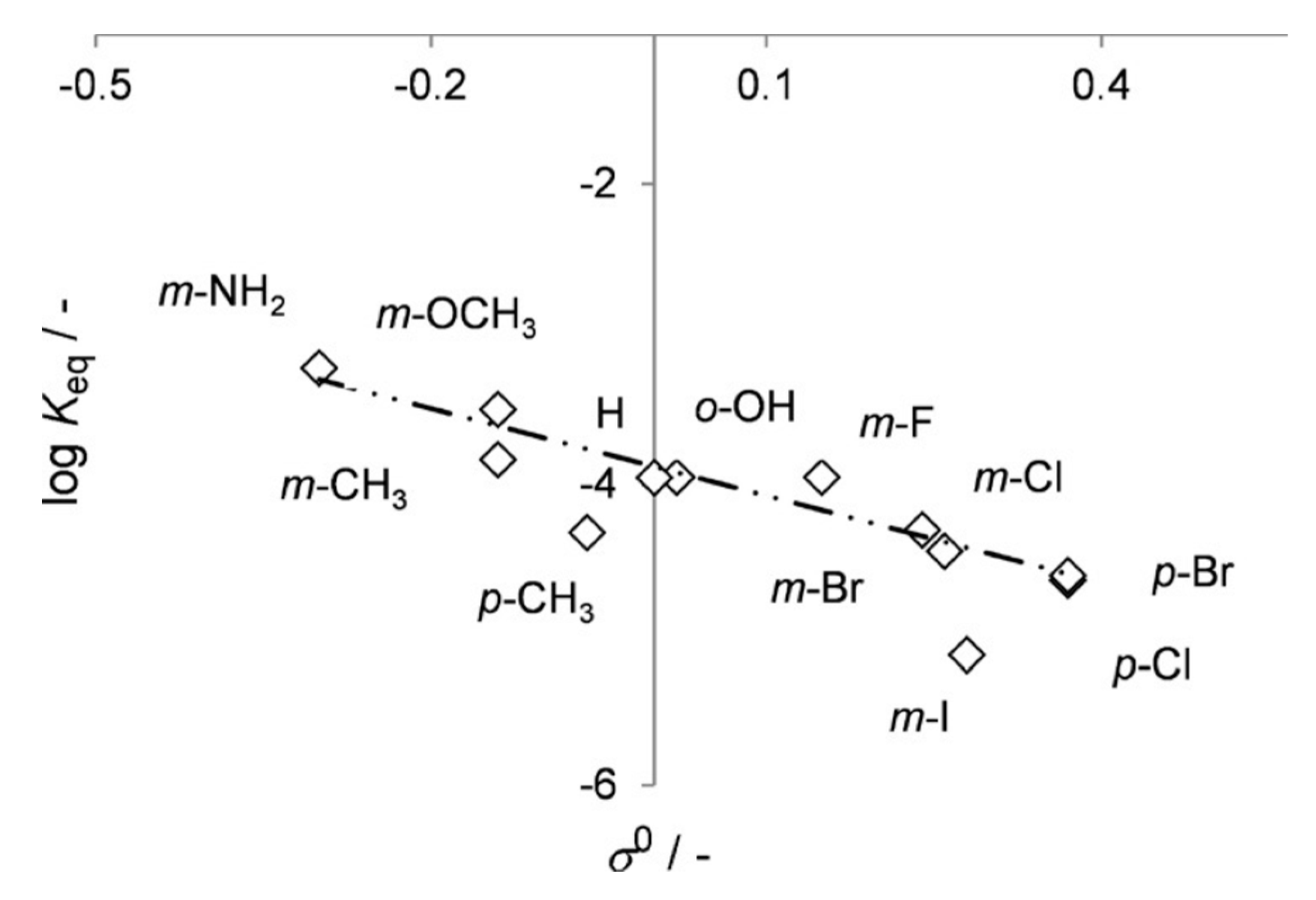
5.1. Catalytic Activity of 2,6-Dihydroxybenzoate Decarboxylase from Rhizobium sporomusa, 2,3-Dihydroxybenzoate Decarboxylase from Aspergillus oryzae and Salicylic Acid Decarboxylase from Trichosporon moniliiforme
5.2. Catalytic Activity of 2,3-Dihydroxybenzoic Acid Decarboxylase from Fusarium oxysporum (2,3-DHBD_Fo)
5.3. Catalytic Activity of Mutated Salicylic Acid Decarboxylase from Trichosporon moniliiforme
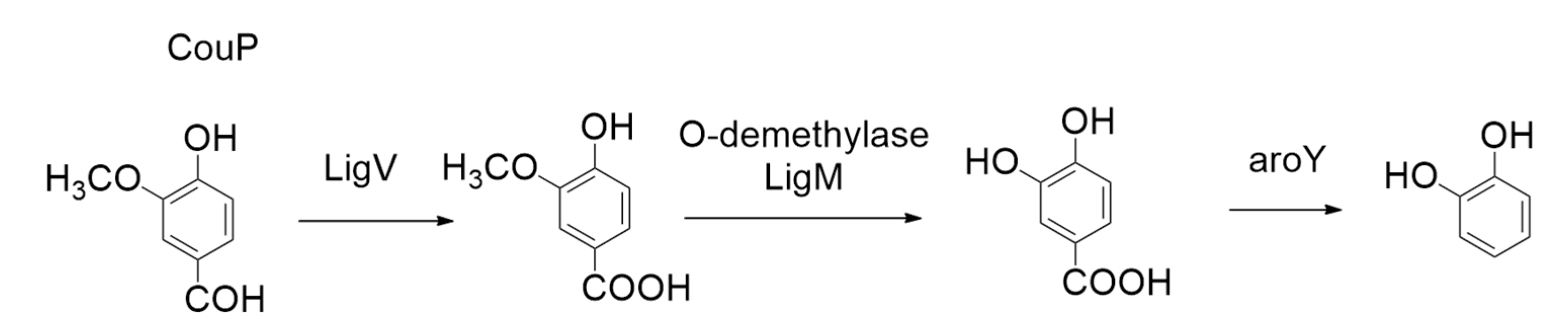
5.4. Bio-Catalysis of 5-Carboxyvanillate Decarboxylase Enzymes from Sphingomonas paucimobilis SYK-6 (LigW_Sp and LigW2_Sp)
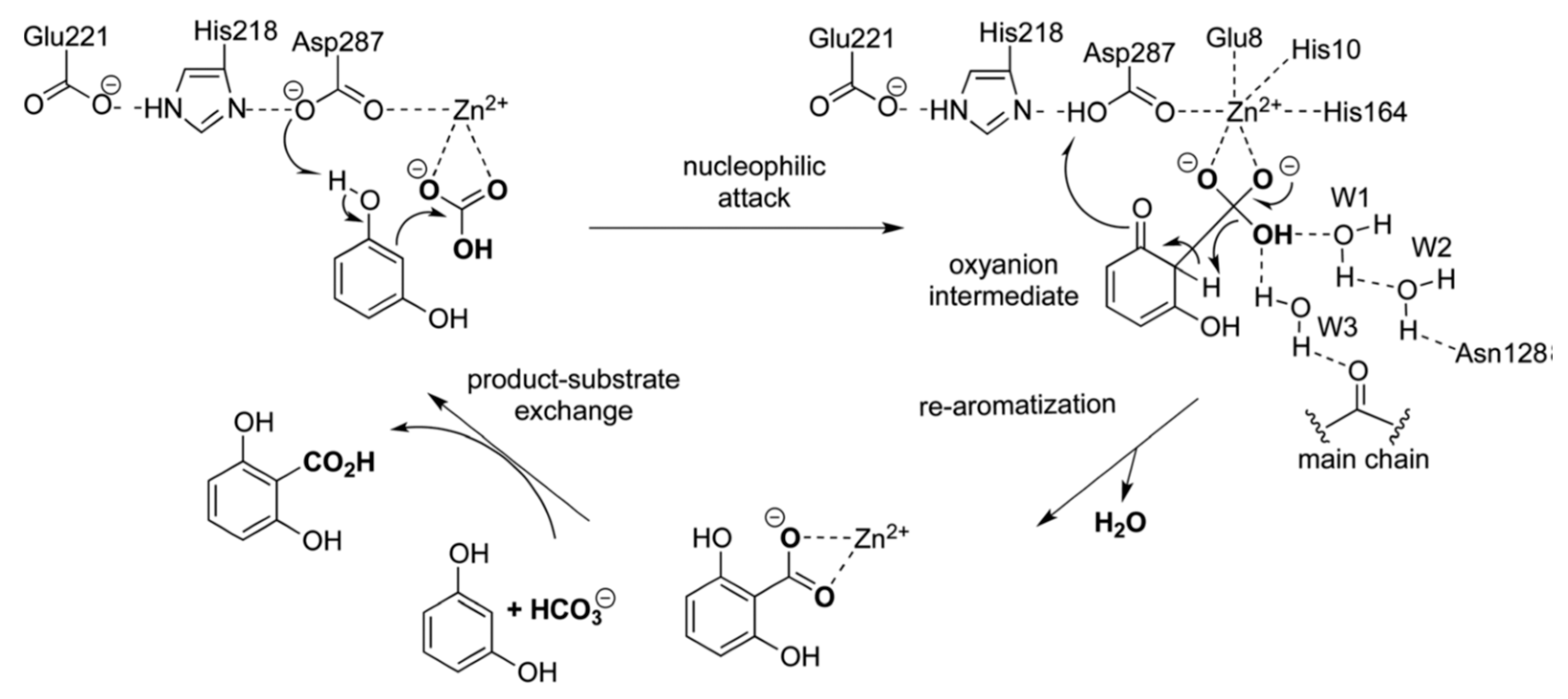
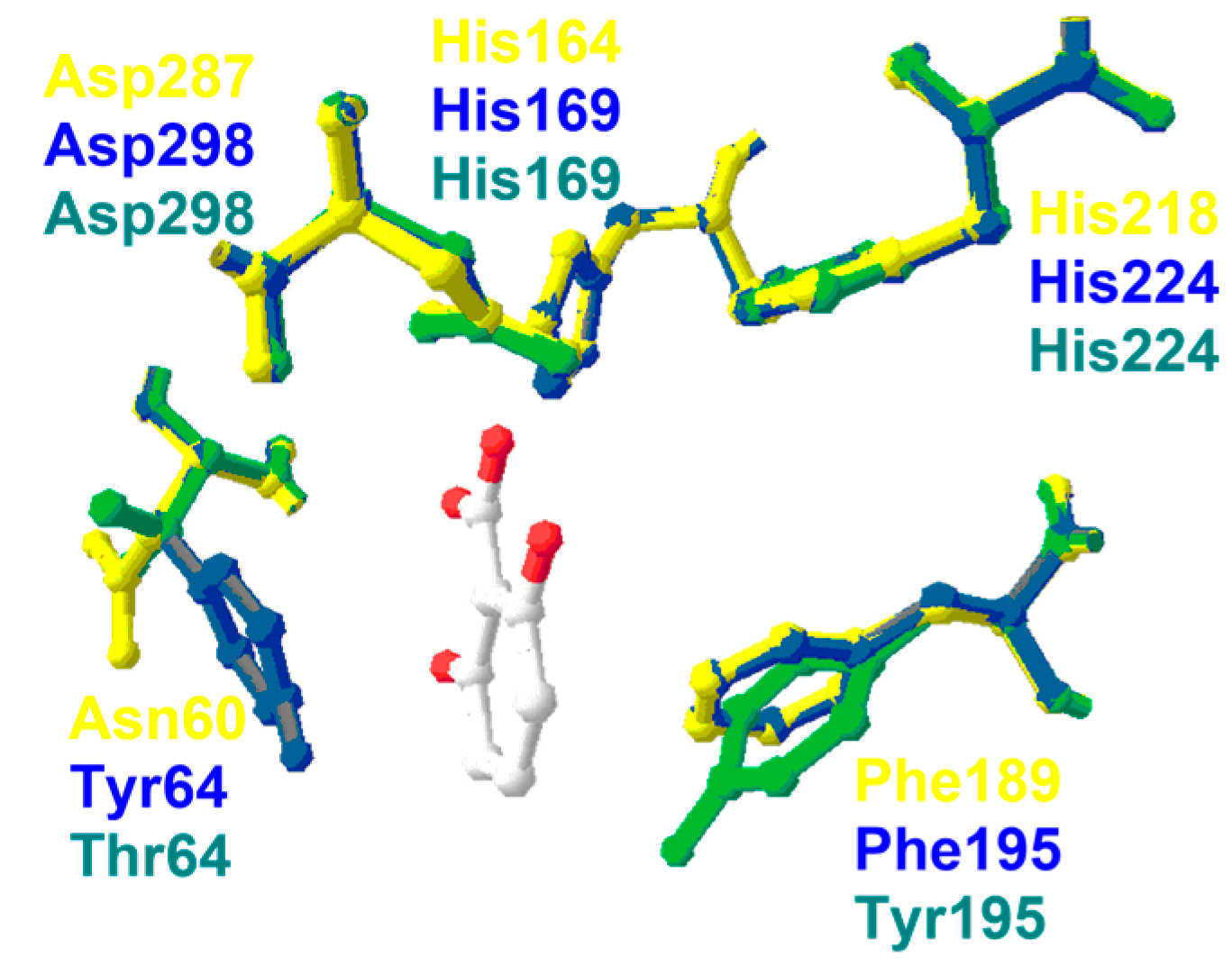
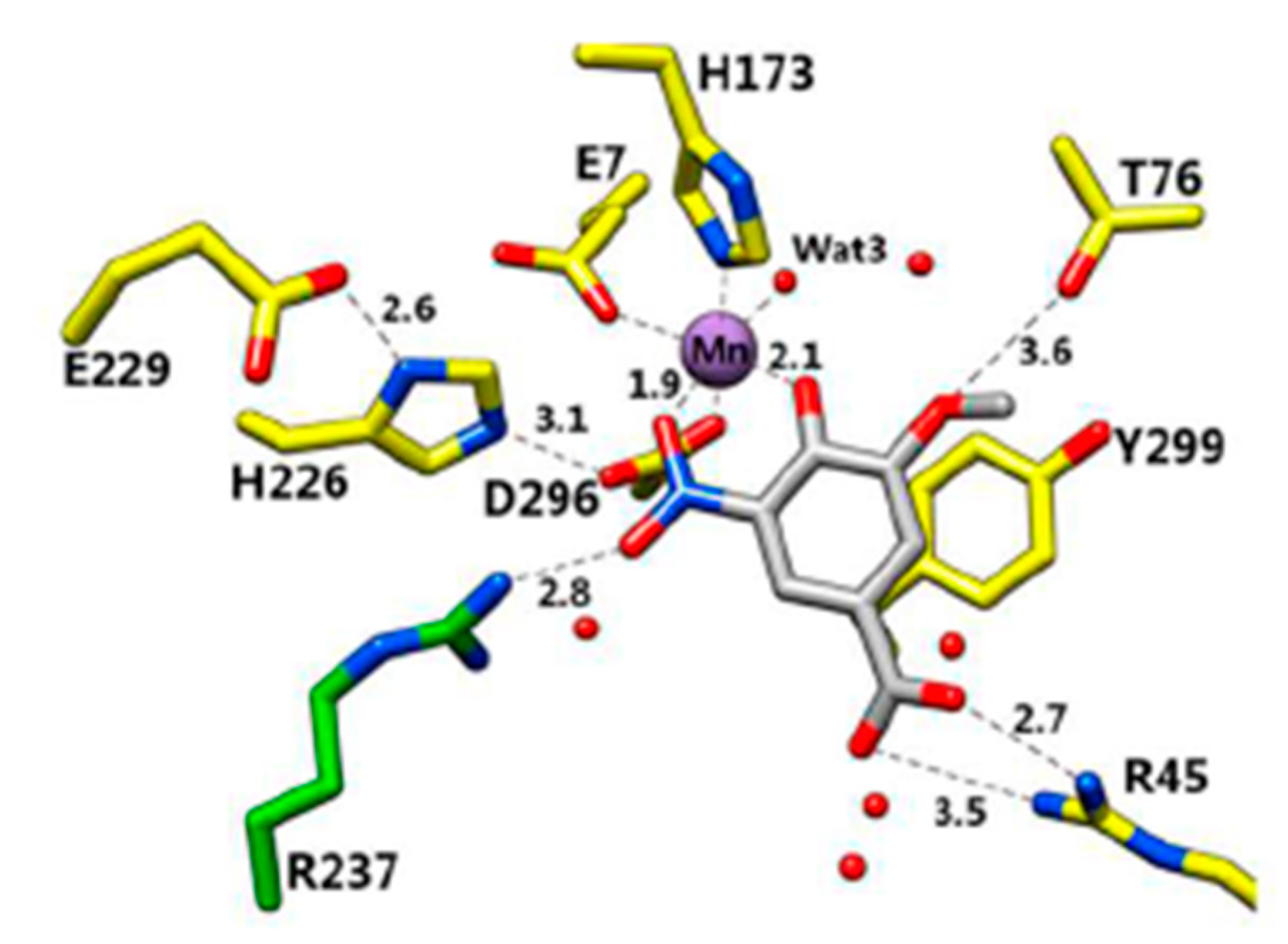
6. Insights into the Reaction Mechanism of Decarboxylase Enzymes: Protein Structure
6.1. 2,6-Dihydroxybenzoate Decarboxylase from Rhizobium sporomusa
6.2. 2,6-Dihydroxybenzoate Decarboxylase from Polaromonas sporomusa JS666
6.3. Salicylic Acid Decarboxylase from Trichosporon moniliiforme
6.4. 5-Carboxyvanillate Decarboxylase from Sphingomonas paucimobilis SYK-6
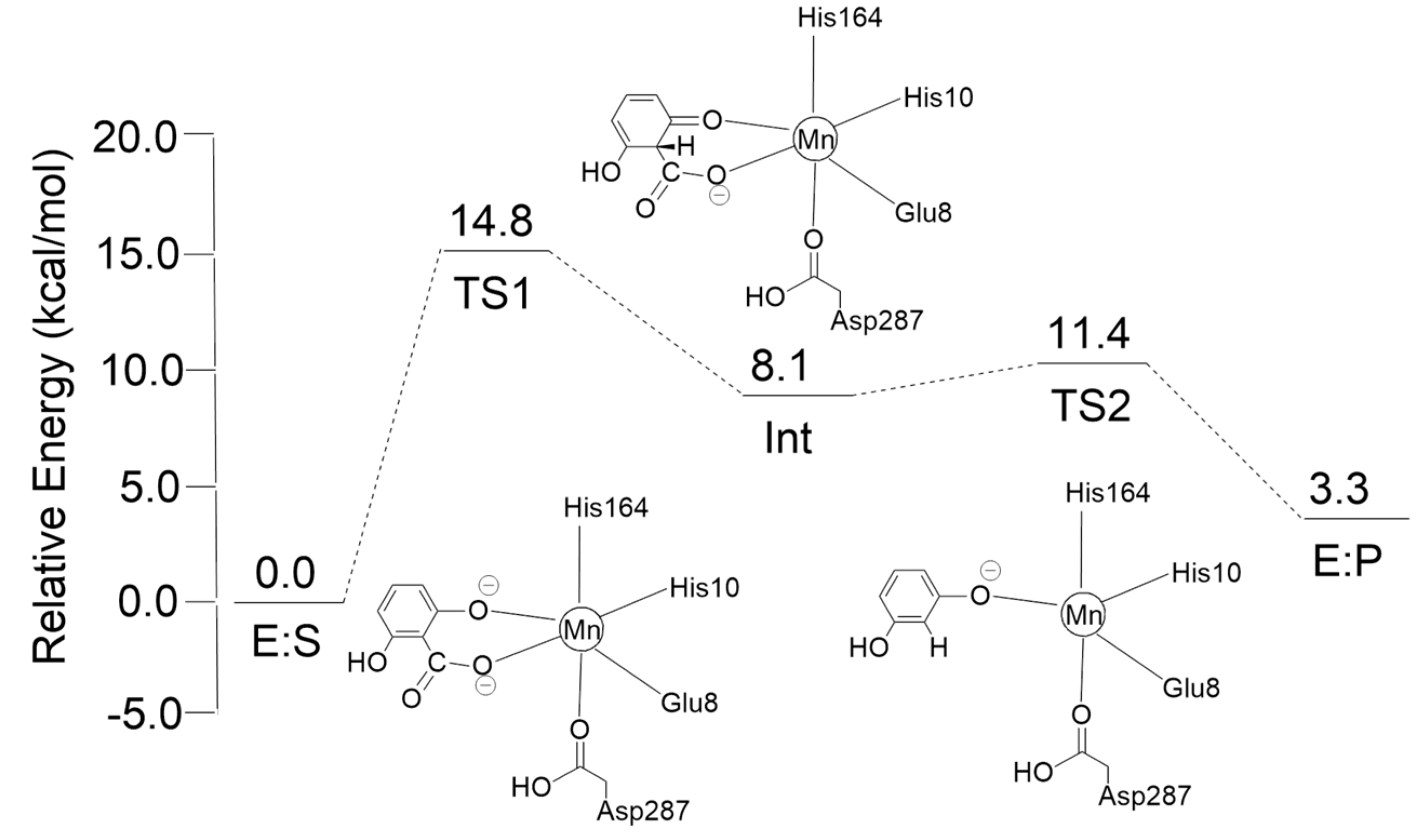
7. Insights into the Reaction Mechanism of 2,6-Dihydroxybenzoate Decarboxylase from Rhizobium sporomusa: Kinetic Studies
8. Regioselective Enzymatic Para-Carboxylation of Phenols: The Enzymes’ Reactivity
8.1. Regioselective Para-Carboxylation of Phenol by 4-Hydroxybenzoate Decarboxylase from Enterobacter cloacae P240
8.2. Regioselective Para-Carboxylation of Phenol by Phenylphosphate Carboxylase from Thauera aromatica K172
8.3. Regioselective Para-Carboxylation of Phenol by 3,4-Dihydroxybenzoate Decarboxylase from Enterobacter cloacae P241
8.4. Regioselective Para-Carboxylation of Guaiacol by Vanillate Decarboxylase from Bacillus subtilis ATCC 6051
9. Para-Hydroxystyrenes β-Carboxylation by Phenolic Acid Decarboxylase (PAD) Enzymes
10. Enzymatic Carboxylation of Aromatic Hydrocarbons
11. Enzymatic Carboxylation Reactions Open the Perspective for Valorization of Lignin-Derived Aromatics.
12. Conclusions
- 1)
- 2,3-DHBD_Ao, 2,3-DHBD_Fo, 2,6-DHDB_Rs, SAD_Tm enzymes analyzed in the above Sections have been shown as being versatile enzymes able to functionalise with high regioselectivity a wide range of phenols to valuable ortho-hydroxybenzoic acids using HCO3− as a CO2 source (see Table 3, Table 4 and Table 6). However, due to thermodynamic constraints, the reaction must be carried out under CO2 pressure or in the presence of quaternary ammonium salts to achieve high conversions (up to 97%).
- 2)
- 2,3-DHBD_Ao and 2,6-DHDB_Rs enzymes show better conversion (up to 97%) in the carboxylation of polyphenolic compounds (Table 4 and Table 5) possessing the resorcinol moiety. Scaling up of the reaction has been shown to be feasible. As noticed by Faber, the carboxylation of polyphenols increases the stability of the molecule toward light-degradation. In addition, γ-resorcylic acids bearing a lipophilic substituent are known as thrombolytic and anti-inflammatory agents.
- 3)
- The newly discovered LigW_Sp and LigW2_Sp decarboxylase enzymes are able to carboxylate hydroxycinnamic acid derivatives as well as guaiacol-derivatives, with excellent selectivity toward ortho-carboxylation with respect to the -OH directing group. At the moment, they are the best candidates for targeting the valorization of lignin-derived aromatics through carboxylation reactions. Also, phenolic acid decarboxylase enzymes (PAD) are good candidates for lignin valorization provided that para-hydroxystyrene derivatives can be efficiently recovered from natural lignin sources.
- 4)
- Enzymes catalyzing selective para-carboxylation of phenol, phenylphosphate, catechol, and guaiacol (with respect to the -OH directing group) summarized in Table 9 present a valuable tool for the synthesis of para-hydroxybenzoic acid derivatives.
- 5)
- Functionalisation of aromatic hydrocarbons as benzene and naphthalene is also a synthetically attractive procedure, although research in this specific field seems at a “nascent stage”.
- 6)
- Advances in the structural and “kinetics” characterization of the enzymes will significantly contribute to elucidate the enzymatic reaction mechanism and to enhance the enzyme’s performance through mutational studies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Soekamto, N.H.; Islam, M.F. Phenetyl ester and amide of Ferulic Acids: Synthesis and bioactivity against P388 Leukemia Murine Cells, IOP Sciences. J. Phys. Conf. Ser. 2018, 979, 012016. [Google Scholar] [CrossRef]
- Dasagrandhi, C.; Park, S.; Jung, W.-K.; Kim, Y.-M. Antibacterial and Biofilm Modulating Potential of Ferulic Acid Grafted Chitosan against Human Pathogenic Bacteria. Int. J. Mol. Sci. 2018, 19, 2157. [Google Scholar] [CrossRef]
- Tomi, M.J.; Sharania, C.S.; Mahapatra, D.K.; Suresh, K.I.; Sabu, A.; Haridas, M. In vitro assessment of selected benzoic acid derivatives as anti-inflammatory compounds. J. Sci. Ind. Res. 2017, 77, 330–336. [Google Scholar]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; Green, K.A.; Mustard, K.J.; et al. The Ancient Drug Salicylate Directly Activates AMP-Activated Protein Kinase. Science 2012, 336, 918–922. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Mukhopadhyay, S.K.; Behara, K.K.; Dey, S.; Singh, N.D.P. 1-Acetylpyrene−Salicylic Acid: Photoresponsive Fluorescent Organic Nanoparticles for the Regulated Release of a Natural Antimicrobial Compound, Salicylic Acid. ACS Appl. Mater. Interfaces 2014, 6, 7045–7054. [Google Scholar] [CrossRef]
- Wang, Z.; Miller, B.; Mabin, M.; Shahni, R.; Wang, Z.D.; Ugrinov, A.; Chu, Q.R. Cyclobutane-1,3-Diacid (CBDA): A Semi-Rigid Building Block Prepared by [2+2] Photocyclization for Polymeric Materials. Sci. Rep. 2017, 7, 13704. [Google Scholar] [CrossRef] [PubMed]
- Wiley-VCH Verlag GmbH & Co. KGaA. Ullmann’s Encyclopedia of Industrial Chemistry, 2nd ed.; Carboxylic Acids, Aromatic; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Lindsey, A.S.; Jeskey, A. The Kolbe-Schmitt reaction. J. Chem. Soc. 1957, 57, 583–620. [Google Scholar] [CrossRef]
- Luo, J.; Preciado, S.; Xie, P.; Larrosa, I. Carboxylation of Phenols with CO2 at Atmospheric Pressure. Chem. Eur. J. 2016, 22, 6798–6802. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.W.; Romesberg, F.E. Expansion of the Genetic Alphabet: A Chemist’s Approach to Synthetic Biology. Acc. Chem. Res. 2018, 51, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, H.U. Systems strategies for developing industrial microbial strains. Nat. Biotechnol. 2015, 33, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Bornscheuer, U.T. Protein Engineering Handbook; Vol. 1 and Vol. 2; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Kahl, L.J.; Endy, D. A survey of enabling technologies in synthetic biology. J. Biol. Eng. 2013, 7, 13. [Google Scholar] [CrossRef]
- Chubukov, V.; Mukhopadhyay, A.; Petzold, C.J.; Keasling, J.D.; Martín, H.G. Synthetic and systems biology for microbial production, of commodity chemicals. Syst. Biol. Appl. 2016, 2, 16009. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J. The frontiers of Energy. Nat. Energy 2016, 1, 15020. [Google Scholar]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Chena, F.; Tobimatsuc, Y.; Havkin-Frenkeld, D.; Dixona, R.A.; Ralph, J. A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. USA 2012, 109, 1772–1777. [Google Scholar] [CrossRef]
- Fitzgerald, N.D. Chemistry challenges to enable a sustainable bioeconomy. Nat. Rev. Chem. 2017, 1, 0080. [Google Scholar] [CrossRef]
- Strk, K.; Taccardi, N.; Bçsmann, A.; Wasserscheid, P. Oxidative Depolymerization of Lignin in Ionic Liquids. ChemSusChem 2010, 3, 719–723. [Google Scholar] [CrossRef]
- de Wild, P.; Reith, H.; Heeres, E. Biomass pyrolysis for chemicals. Biofuels 2011, 2, 185–208. [Google Scholar] [CrossRef]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnosn, D. Top Value-Added Chemicals from Biomass. Volume II, Results of Screening for Potential Candidates from Biorefinery Lignin; Prepared for the DOE, PNNL; Pacific Northwest National Lab: Richland, WA, USA, 2007; p. 16983.
- Gosselink, R.J.A.; Teunissen, W.; van Dam, J.E.G.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P.M. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef]
- Hu, J.; Shen, D.; Wu, S.; Zhang, H.; Xiao, R. Composition Analysis of Organosolv Lignin and Its Catalytic Solvolysis in Supercritical Alcohol. Energy Fuels 2014, 28, 4260–4266. [Google Scholar] [CrossRef]
- Chan, J.M.W.; Bauer, S.; Sorek, H.; Sreekumar, S.; Wang, K.; Toste, F.D. Studies on the Vanadium-Catalyzed Nonoxidative Depolymerization of Miscanthus giganteus-Derived Lignin. ACS Catal. 2013, 3, 1369–1377. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachua, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef]
- Wilkerson, C.G.; Mansfield, S.D.; Lu, F.; Withers, S.; Park, J.-Y.; Karlen, S.D.; Gonzales-Vigil, E.; Padmakshan, D.P.; Unda, F.; Rencoret, J.; et al. Monolignol Ferulate Transferase Introduces Chemically Labile Linkages into the Lignin Backbone. Science 2014, 344, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shuai, L.; Kim, H.; Motagamwala, A.M.; Mobley, J.K.; Yue, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Chen, F.; Dixon, R.A.; et al. An “ideal lignin” facilitates full biomass utilization. Sci. Adv. 2018, 4, eaau2968. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial degradation of aromatic compounds—From one strategy to four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Saa, L.; Jaureguibeitia, A.; Largo, E.; Llama, M.J.; Serra, J.L. Cloning, purification and characterization of two components of phenol hydroxylase from Rhodococcus erythropolis UPV-1. Appl. Microbiol. Biotechnol. 2010, 86, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Lingera, J.G.; Vardona, D.R.; Guarnieria, M.Y.; Karpa, E.M.; Hunsingera, G.B.; Frandena, M.A.; Johnson, C.W.; Chupkad, G.; Strathmannc, T.J.; Pienkosa, P.T.; et al. Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. USA 2014, 111, 12013–12018. [Google Scholar] [CrossRef]
- Duan, J.; Huo, X.; Du, W.J.; Liang, J.D.; Wang, D.Q.; Yang, S.C. Biodegradation of kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett. Appl. Microbiol. 2015, 62, 55–62. [Google Scholar] [CrossRef]
- Schink, B.; Müller, B.P.J. Anaerobic Degradation of Phenolic Compounds. Naturwissenschaften 2000, 87, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Zamarro, M.T.; Blazquez, B.; Durante-Rodríguez, G.; Juarez, J.F.; Valderrama, J.A.; Barragan, M.J.L.; García, J.L.; Eduardo Díaz, E. Anaerobic Catabolism of Aromatic Compounds: A Genetic and Genomic View. Microbiol. Mol. Biol. Rev. 2009, 73, 71–133. [Google Scholar] [CrossRef] [PubMed]
- Tinikul, R.; Chenprakhon, P.; Maenpuen, S.; Chaiyen, P. Biotransformation of Plant-Derived Phenolic Acids. Biotechnol. J. 2018, 13, 1700632. [Google Scholar] [CrossRef] [PubMed]
- Kourist, R.; Guterl, J.-K.; Miyamoto, K.; Sieber, V. Enzymatic Decarboxylation—An Emerging Reaction for Chemicals Production from Renewable Resources. ChemCatChem 2014, 6, 689–701. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Enzymatic Conversion of CO2 (Carboxylation Reactions and Reduction to Energy-Rich C1 Molecules). In Reaction Mechanisms in Carbon Dioxide Conversion; Aresta, M., Dibenedetto, A., Quaranta, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 347–371. [Google Scholar]
- Aresta, M.; Dibenedetto, A. Development of environmentally friendly syntheses: Use of enzymes and biomimetic systems for the direct carboxylation of organic substrates. Rev. Mol. Biotechnol. 2002, 90, 113–128. [Google Scholar] [CrossRef]
- Lewin, R.; Thompson, M.L.; Micklefield, J. Enzyme Carboxylation and Decarboxylation. In Science of Synthesis: Biocatalysis in Organic Synthesis; Faber, K., Fessner, W.-D., Turner, N.J., Eds.; Thieme: Stuttgart, NY, USA, 2015; Volume 2, pp. 133–157. [Google Scholar]
- Yoshida, T.; Nagasawa, T. Chapter 5—Biological Kolbe-Schmitt carboxylation: Possible use of enzymes for the direct carboxylation of organic substrates. In Future Directions in Biocatalysis; Elsevier: Amsterdam, The Netherlands, 2007; pp. 83–105. [Google Scholar]
- Glueck, S.M.; Gümüs, S.; Fabian, W.M.F.; Faber, K. Biocatalytic carboxylation. Chem. Soc. Rev. 2010, 39, 313–328. [Google Scholar] [CrossRef]
- Pesci, L.; Glueck, S.M.; Gurikov, P.; Smirnova, I.; Faber, K.; Liese, A. Biocatalytic carboxylation of phenol derivatives: Kinetics and thermodynamics of the biological Kolbe–Schmitt Synthesis. FEBS J. 2015, 282, 1334–1345. [Google Scholar] [CrossRef]
- Silverman, R.B. The Organic Chemistry of Enzyme-Catalyzed Reactions; Chapter 7, Carboxylations; Academic Press: San Diego, CA, USA, 2002; pp. 289–320. [Google Scholar]
- Wuensch, C.; Gross, J.; Steinkellner, G.; Lyskowski, A.; Gruber, K.; Glueck, S.M.; Faber, K. Regioselective ortho-carboxylation of phenols catalyzed by benzoic acid decarboxylases: A biocatalytic equivalent to the Kolbe–Schmitt reaction. RSC Adv. 2014, 4, 9673–9679. [Google Scholar] [CrossRef]
- Erb, T.J. Carboxylases in natural and synthetic microbial pathways. Appl. Environ. Microbiol. 2011, 77, 8466–8477. [Google Scholar] [CrossRef]
- Schmeling, S.; Fuchs, G. Anaerobic metabolism of phenol in proteobacteria and further studies of phenylphosphate carboxylase. Arch. Microbiol. 2009, 191, 869–878. [Google Scholar] [CrossRef]
- Yoshida, M.; Fukuhara, N.; Oikawa, T. Thermophilic, reversible γ-resorcylate decarboxylase from Rhizobium sp. strain MTP-10005: Purification, molecular characterization, and expression. J. Bacteriol. 2004, 186, 6855–6863. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Oikawa, T.; Obata, H.; Abe, K.; Mihara, H.; Esaki, N. Biochemical and genetic analysis of the γ-resorcylate (2,6-dihydroxybenzoate) catabolic pathway in Rhizobium sp. strain MTP-10005: Identification and functional analysis of its gene cluster. J. Bacteriol. 2007, 189, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Narimatsu, Y.; Iwasaki, Y.; Arai, N.; Kino, K.; Kirimura, K. Reversible and nonoxidative gamma-resorcylic acid decarboxylase: Characterization and gene cloning of a novel enzyme catalyzing carboxylation of resorcinol, 1,3-dihydroxybenzene, from Rhizobium radiobacter. Biochem. Biophys. Res. Commun. 2004, 324, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hayakawa, Y.; Matsui, T.; Nagasawa, T. Purification and characterization of 2,6-dihydroxybenzoate decarboxylase reversibly catalyzing nonoxidative decarboxylation. Arch. Microbiol. 2004, 181, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Yoshida, T.; Yoshimura, T.; Nagasawa, T. Regioselective carboxylation of 1,3-dihydroxybenzene by 2,6-dihydroxybenzoate decarboxylase of Pandoraea sp. 12B-2. Appl. Microbiol. Biotechnol. 2006, 73, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kasai, D.; Araki, N.; Motoi, K.; Yoshikawa, S.; Iino, T.; Imai, S.; Masai, E.; Fukuda, M. γ-Resorcylate catabolic-pathway genes in the soil actinomycete Rhodococcus jostii RHA1. Appl. Environ. Microbiol. 2015, 81, 7656–7665. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Patskovsky, Y.; Vladimirova, A.; Bonanno, J.B.; Almo, S.C.; Himo, F.; Frank, M.; Raushel, F.M. Mechanism and Structure of γ-Resorcylate Decarboxylase. Biochemistry 2018, 57, 3167–3175. [Google Scholar] [CrossRef]
- Santa, R.; Rao, N.A.; Vaidyanathan, C.S. Identification of the active-site peptide of 2,3-dihydroxybenzoic acid decarboxylase from Aspergillus oryzae. Biochim. Biophys. Acta 1996, 1293, 191–200. [Google Scholar] [CrossRef]
- NCBI Database, GemBank: EXM04459.1, 2,3-dihydroxybenzoate decarboxylase [Fusarium oxysporum f. sp. Cubense tropical race 4 54006]. 2015. Available online: https://www.ncbi.nlm.nih.gov/search/all/?term=EXM04459.1 (accessed on 28 December 2018).
- Ywasaki, Y.; Gunji, H.; Kino, K.; Hottori, T.; Ishii, Y.; Kirimura, K. Novel metabolic pathway for salicylate biodegradation via phenol in yeast Trichosporon moniliiforme. Biodegradation 2010, 21, 557–564. [Google Scholar] [CrossRef]
- Patskovsky, V.A.; Fedorov, Y.; Bonanno, A.A.; Fedorov, J.B.; Toro, E.V.; Hillerich, R.; Seidel, B.; Richards, N.R.D.; Almo, S.C.; Raushel, F.M. Substrate distortion and the catalytic reaction mechanism of 5-carboxyvanillate decarboxylase. J. Am. Chem. Soc. 2016, 138, 826–836. [Google Scholar]
- Xiang, S.; Zhu, W.; Huddleston, J.; Xiang, D.F.; Raushel, F.M.; Richards, N.G.J.; Himo, F. A combined experimental-theoretical study of the ligW-catalyzed decarboxylation of 5-carboxyvanillate in the metabolic pathway for lignin degradation. ACS Catal. 2017, 7, 4968–4974. [Google Scholar]
- Enzymatic carboxylation reactions are highly selective. Therefore often Authors report the amount of a specific compound undergoing the reaction as “% conversion”. In this review we will adopt the terms “% conversion” and “% yield” as used by Authors in the original papers.
- Plasch, K.; Resch, V.; Hitce, J.; Popłoński, J.; Faber, K.; Glueck, S.M. Regioselective Enzymatic Carboxylation of Bioactive (Poly) phenols. Adv. Synth. Catal. 2017, 359, 959–965. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysgu, L. Plant Polyphenols: Chemical Properties, Biological, Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Klausing, K.; Smith, V.; Shen, M.-J.R.; Moore, J.; Hall, K. Methods Using Polyphenols for Inhibiting Light-Induced Degradation of Nucleic Acids and for Detecting Fluorescent-Tagged Nucleic Acids. U.S. Patent 20120196758A1, 2 August 2012. [Google Scholar]
- Plasch, K.; Hofer, G.; Keller, W.; Hay, S.; Heyes, D.J.; Dennig, A.; Glueck, S.M.; Faber, K. Pressurized CO2 as a carboxylating agent for the biocatalytic ortho-carboxylation of resorcinol. Green Chem. 2018, 20, 1754–1759. [Google Scholar] [CrossRef]
- Ren, J.; Yao, P.; Yu, S.; Dong, W.; Chen, Q.; Feng, J.; Wu, Q.; Zhu, D. An Unprecedented Effective Enzymatic Carboxylation of Phenols. ACS Catal. 2016, 6, 564–567. [Google Scholar] [CrossRef]
- Zhanga, X.; Renb, J.; Yaob, P.; Gong, R.; Wanga, M.; Wub, Q.; Zhu, D. Biochemical characterization and substrate profiling of a reversible 2,3-dihydroxybenzoic acid decarboxylase for biocatalytic Kolbe-Schmitt reaction. Enzym. Microb. Technol. 2018, 113, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pesci, L.; Gurikov, P.; Liese, A.; Kara, S. Amine-Mediated Enzymatic Carboxylation of Phenols Using CO2 as Substrate Increases Equilibrium Conversions and Reaction Rates. Biotechnol. J. 2017, 12, 1700332. [Google Scholar] [CrossRef]
- Kirimura, K.; Gunji, H.; Wakayama, R.; Hattori, T.; Ishii, Y. Enzymatic Kolbe–Schmitt reaction to form salicylic acid from phenol: Enzymatic characterization and gene identification of a novel enzyme, Trichosporon moniliiforme salicylic acid decarboxylase. Biochem. Biophys. Res. Commun. 2010, 394, 279–284. [Google Scholar] [CrossRef]
- Kirimura, K.; Yanaso, S.; Kosaka, S.; Koyama, K.; Hattori, T.; Ishii, Y. Production of p-Aminosalicylic Acid through Enzymatic Kolbe Schmitt Reaction Catalyzed by Reversible Salicylic Acid Decarboxylase. Chem. Lett. 2011, 40, 206–208. [Google Scholar] [CrossRef]
- Ienaga, S.; Kosaka, S.; Honda, Y.; Ishii, Y.; Kirimura, K. p-Aminosalicylic Acid Production by Enzymatic Kolbe-Schmitt Reaction Using Salicylic Acid Decarboxylases Improved through Site-Directed Mutagenesis. Bull. Chem. Soc. Jpn. 2013, 86, 628–634. [Google Scholar] [CrossRef]
- Peng, X.; Masai, E.; Kitayama, H.; Harada, K.; Katayama, Y.; Fukuda, M. Cloning and Characterization of the Ferulic Acid Catabolic Genes of Sphingomonas paucimobilis SYK-6. Appl. Environ. Microb. 2002, 68, 4416–4424. [Google Scholar]
- Peng, X.; Masai, E.; Kasai, D.; Miyauchi, K.; Katayama, Y.; Fukuda, M. A Second 5-Carboxyvanillate Decarboxylase Gene, ligW2 Is Important for Lignin-Related Biphenyl Catabolism in Sphingomonas paucimobilis SYK-6. Appl. Environ. Microb. 2005, 71, 5014–5021. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liu, Y.; Guo, X.; Liu, W.; Li, Q.; Zhao, Z.K. Selective carboxylation of substituted phenols with engineered Escherichia coli whole-cells. Tetrahedron Lett. 2018, 59, 3810–3815. [Google Scholar] [CrossRef]
- Goto, M.; Hayashi, H.; Miyahara, I.; Hirotsu, K.; Yoshida, M.; Oikawa, T. Crystal Structures of Non-oxidative Zinc-dependent 2,6-Dihydroxybenzoate (γ-Resorcylate) Decarboxylasefrom Rhizobium sp. Strain MTP-10005. J. Biol. Chem. 2006, 281, 34365–34373. [Google Scholar] [CrossRef] [PubMed]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 2009, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pesci, L.; Kara, S.; Liese, A. Evaluation of the Substrate Scope of Benzoic Acid (De)carboxylases According to Chemical and Biochemical Parameters. ChemBioChem 2016, 17, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Yoshida, T.; Hayashi, T.; Nagasawa, T. Purification, characterization, and gene cloning of 4-hydroxybenzoate decarboxylase of Enterobacter cloacae P240. Arch. Microbiol. 2006, 186, 21–29. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zhou, S.; Tao, P.; Liu, J. Purification and characterization of a 4-hydroxybenzoate decarboxylase from Chlamydophila pneumonia AR39. Curr. Microbiol. 2007, 54, 102–107. [Google Scholar] [CrossRef]
- Li, T.; Juteau, P.; Beaudet, R.; Lepine, F.; Villemur, R.; Bisaillon, J-G. Purification and characterization of a 4-hydroxybenzoate decarboxylase from an anaerobic coculture. Can. J. Microbiol. 2000, 46, 856–859. [Google Scholar] [CrossRef]
- Tschech, A.; Fuchs, G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch. Microbiol. 1987, 148, 213–217. [Google Scholar] [CrossRef]
- Lack, A.; Tommasi, I.; Aresta, M.; Fuchs, G. Catalytic properties of phenol carboxylase in vitro study of CO2: 4-hydroxybenzoate isotope exchange reaction. Eur. J. Biochem. 1991, 197, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Muller, N. Enzymes involved in the anaerobic degradation of phenol by the sulfate-reducing bacterium Desulfatiglans anilini. BMC Microbiol. 2018, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Rajkumar, B.; Jothikumar, N. Anoxygenic degradation of aromatic substances by Rhodopseudomonas palustris. Curr. Microbiol. 1992, 25, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Inami, Y.; Matsui, T.; Nagasawa, T. Regioselective carboxylation of catechol by 3,4-dihydroxybenzoate decarboxylase of Enterobacter cloacae P241. Biotechnol. Lett. 2010, 32, 701–705. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wiegel, J. Purification and characterization of an oxygensensitive, reversible 3,4-dihydroxybenzoate decarboxylase from Clostridium hydroxybenzoicum. J Bacteriol. 1996, 178, 3539–3543. [Google Scholar] [CrossRef] [PubMed]
- Lupa, B.; Lyon, D.; Shaw, L.N.; Sieprawska-Lupa, M.; Wiegel, J. Properties of the reversible nonoxidative vanillate/4-hydroxybenzoate decarboxylase from Bacillus subtilis. Can. J. Microbiol. 2008, 54, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, C.; Pavkov-Keller, T.; Steinkellner, G.; Gross, J.; Fuchs, M.; Hromic, A.; Lyskowski, A.; Fauland, K.; Gruber, K.; Glueck, S.M.; et al. Regioselective Enzymatic β-Carboxylation of para-Hydroxystyrene Derivatives Catalyzed by Phenolic Acid Decarboxylases. Adv. Synth. Catal. 2015, 357, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, C.; Glueck, S.M.; Gross, J.; Koszelewski, D.; Schober, M.; Faber, K. Regioselective Enzymatic Carboxylation of Phenols and Hydroxystyrene Derivatives. Org. Lett. 2012, 14, 1974–1977. [Google Scholar] [CrossRef]
- Gu, W.; Yang, J.; Lou, Z.; Liang, L.; Sun, Y.; Huang, J.; Li, X.; Cao, Y.; Meng, Z.; Zhang, K.-Q. Structural Basis of Enzymatic Activity for the Ferulic Acid Decarboxylase (FADase) from Enterobacter sp. Px6-4. PLoS ONE 2011, 6, e16262. [Google Scholar] [CrossRef]
- Sheng, X.; Himo, K. Theoretical Study of Enzyme Promiscuity: Mechanisms of Hydration and Carboxylation Activities of Phenolic Acid Decarboxylase. ACS Catal. 2017, 7, 1733–1741. [Google Scholar] [CrossRef]
- Aleku, G.A.; Prause, C.; Bradshaw-Allen, R.T.; Plasch, K.; Glueck, S.M.; Bailey, S.S.; Payne, K.A.P.; Parker, D.A.; Faber, K.; Leys, D. Terminal Alkenes from Acrylic Acid Derivatives via Non-Oxidative Enzymatic Decarboxylation by Ferulic Acid Decarboxylases. ChemCatChem 2018, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Cunha Tarouco, P.; Weyrauch, P.; Don, X.; Himmelberg, A.M. Anaerobic Degradation of Benzene and Polycyclic Aromatic Hydrocarbons. J. Mol. Microbiol. Biotechnol. 2016, 26, 92–118. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, S.; Hornung, B.; van der Waals, M.J.; Nunes da Rocha, U.; Hugenholtz, F.; Nijsse, B.; Molenaar, D.; van Spanning, R.; Stams, A.J.M.; Gerritse, J.; et al. A benzene-degrading nitrate reducing microbial consortium displays aerobic and anaerobic benzene degradation pathways. Sci. Rep. 2018, 8, 4490. [Google Scholar] [CrossRef] [PubMed]
- Laban, N.A.; Selesi, D.; Rattei, T.; Tischler, P.; Meckenstock, R.U. Identification of enzymes involved in anaerobic benzene degradation by a strictly anaerobic iron-reducing enrichment culture. Environ. Microbiol. 2010, 12, 2783–2796. [Google Scholar] [CrossRef] [PubMed]
- Mouttaki, H.; Johannes, J.; Meckenstock, R.U. Identification of naphthalene carboxylase as a prototype for the anaerobic activation of non-substituted aromatic hydrocarbons. Environ. Microbiol. 2012, 14, 2770–2774. [Google Scholar] [CrossRef] [PubMed]
- Oulame, M.Z.; Pion, F.; Allauddin, S.; Raju, K.V.S.N.; Ducrot, P.-H.; Allais, F. Renewable alternating aliphatic-aromatic poly(ester-urethane)s prepared from ferulic acid and bio-based diols. Eur. Polym. J. 2015, 63, 186–193. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Barta, K.; Ford, P.C. Catalytic Conversion of Nonfood Woody Biomass Solids to Organic Liquids. Acc. Chem. Res. 2014, 47, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Ata, M.S.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. RSC Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Sedó, J.; Saiz-Poseu, J.; Busqué, F.; Ruiz-Molina, D. Catechol-Based Biomimetic Functional Materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef] [PubMed]
- Wua, W.; Liua, F.; Singha, S. Toward engineering E. coli with an autoregulatory system for lignin valorization. Proc. Natl. Acad. Sci. USA 2018, 115, 2970–2975. [Google Scholar] [CrossRef] [PubMed]












| Technology | Products Obtained | Reference |
|---|---|---|
| Thermochemical Process (Fast Pyrolysis) | ||
| The Energy Research Center of the Netherlands (ECN) developed at the laboratory-scale a plant for pyrolysis of technical lignin followed by hydrodeoxygenation using a ruthenium-based catalyst. | Product: Pyrolitic lignin oil (PLO) containing 13–20 wt.% of phenolic fraction consisting of both monomeric and oligomeric phenols. Low molecular weight phenolic compounds was approx. 7–9 wt.%. | [22] |
| Depolymerization of Organosolv Lignin | ||
| Oxidative depolymerization of lignin in ionic liquids (1-ethyl-3-methylimidazolium trifluoromethanesulfonate in the presence of Mn(NO3)2 catalyst. (11.2 g of lignin, 111.1 g of IL, 2.22 g of catalyst, treated at 100 °C with air during 24 h). | Product: 66% of lignin conversion (as % weight of initial lignin); 32.7 wt.% of monomeric products extracted from converted lignin (as % weight of initial lignin). | [21] |
| Organosolv hardwood and wheat straw lignin depolymerized in supercritical carbon dioxide/acetone/water fluid (300-370 °C, 100 bar, during 3.5 h). | Product: Lignin oligomers produced in 18.6% yield (as % weight of dry lignin). | [24] |
| Chinese fir (softwood) treated by catalytic solvolysis in supercritical alcohol (supercritical ethanol/1-butanol at 300 °C with Ru/C). | Product (when treating Chinese fir lignin): 32.4% of guaiacol-type products 2.34% of syringol-type products (as % selectivity of products detected by GC/MS analysis). | [25] |
| Non-oxidative depolymerization of Miscanthus giganteus-derived lignin in the presence of CH3CN/THF (10:1) or EtOAc/THF (8:1), 10 wt.% of Vanadium catalyst, at 80 °C, during 24 h. | Product: Approx. 3% of monophenolic compounds (as % weight of treated lignin). | [26] |
| Entry | Enzyme | Microorganism | Reference |
|---|---|---|---|
| 1 | 2,6-dihydroxybenzoic acid decarboxylase (2,6-DHBD) also named γ-resorcylate decarboxylase (γ-RSD)  | Rhizobium sporomusa MTP-10005 Rhizobium radiobacter WU-0108 Agrobacterium tumefaciens IAM12048 Pandoraea sp. 12B-2 Rhodococcus jostii RHA1 Polaromonas sporomusa JS666 | [48,49] [50] [51] [52] [53] [54] |
| 2 | 2,3-dihydroxybenzoate decarboxylase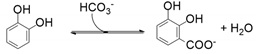 | Aspergillus oryzae Fusarium oxysporum | [55] [56] |
| 3 | salicylic acid decarboxylase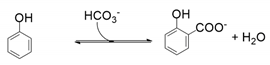 | Trichosporon moniliiforme | [57] |
| 4 | 5-carboxyvanillate decarboxylase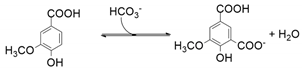 | Sphingomonas paucimobilis SYK-6 (LigW_Sp and LigW2_Sp) | [58,59] |
| Entry | Substrate | Product | 2,3-DHBD_Ao Conversion (%) | SAD_Tm Conversion (%) | 2,6-DHBD_Rs Conversion (%) |
|---|---|---|---|---|---|
| 1 |  |  | 43 | 43 | <1 |
| 2 | 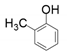 | 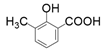 | 16 | 30 | <1 |
| 3 | 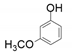 | 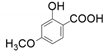 | 50 | 48 | 12 |
| 4 | 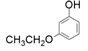 | 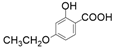 | 48 | 47 | 18 |
| 5 | 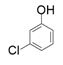 | 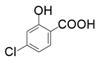 | 25 | 22 | 1 |
| 6 |  | 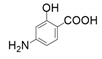 | 74 | 80 | 5 |
| 7 | 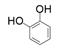 | 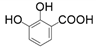 | 30 | 21 | 35 |
| 8 |  | 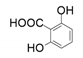 | 22 | 30 | 31 |
| 9 |  |  | 29 | 40 | <1 |
| 10 | 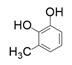 | 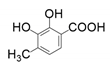 | 15 | 28 | 37 |
| 11 | 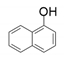 | 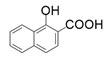 | 54 | 62 | 46 |
| 12 | 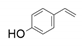 | 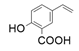 | 58 | 29 | <1 |
| 13 | 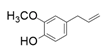 | 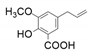 | 14 | 5 | <1 |
| Entry | Substrate | Product | 2,3-DHBD_Ao Conversion (%) | 2,6-DHBD_Rs Conversion (%) | SAD_Tm Conversion (%) |
|---|---|---|---|---|---|
| 1 | 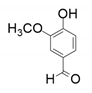 | 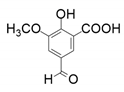 | 40 | <1 | 33 |
| 2 | 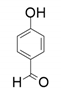 | 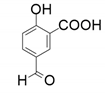 | 62 | <1 | <1 |
| 3 | 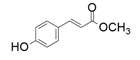 |  | 33 | <1 | 32 |
| 4 | 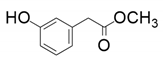 |  | 66 | <1 | <1 |
| 5 | 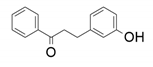 | 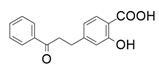 | 68 | 57 | 68 |
| 6 |  | 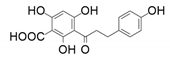 | 97 | 97 | 60 |
| 7 | 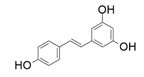 |  | 85 | 97 | 96 |
| 8 | 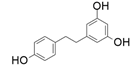 | 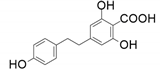 | 65 | 77 | 45 |
| 9 | 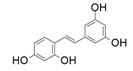 | 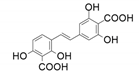 | 83 | 97 | 96 |
| Entry | Substrate | Product | 2,3-DHBD_Ao Yield (%) | 2,6-DHBD_Rs Yield (%) |
|---|---|---|---|---|
| 3 | 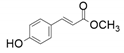 |  | 32 | - |
| 4 | 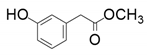 | 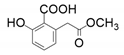 | 43 | - |
| 6 |  |  | 67 | - |
| 7 | 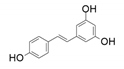 |  | - | 95 |
| 8 | 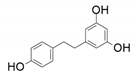 | 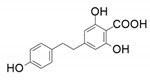 | - | 45 |
| 10 | 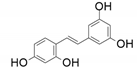 | 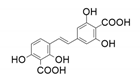 | - | 66 |
| Entry | Substrate | Product | 2,3-DHBD_Fo Conversion (%) |
|---|---|---|---|
| 1 | 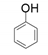 |  | 33 |
| 2 | 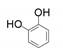 |  | 28 |
| 3 |  | 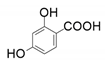 | 9 |
| 4 | 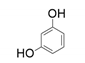 | 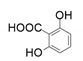 | 35 |
| 5 | 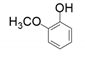 | 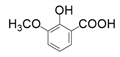 | 15 |
| 6 | 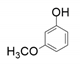 | 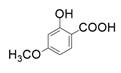 | 46 |
| 7 | 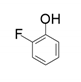 |  | 4.8 |
| 8 | 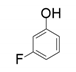 | 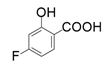 | 7.8 |
| 2,3-DHBD_Ao (a) Conversion % | 2,6-DHBD_Rs (a) Conversion% | 2,3-DHBD_Ao (b) Conversion % | 2,6-DHBD_Rs (c) Conversion % | 2,3-DHBD_Ao (d) Conversion % | 2,3-DHBD_Fo (e) Conversion % | 2,3-DHBD_Fo (f) Conversion % | |
|---|---|---|---|---|---|---|---|
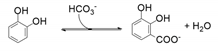 | 30 | 35 | - | 97 | 25 | 28 | 95 |
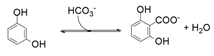 | 22 | 31 | 68 | 97 | - | 35 | - |
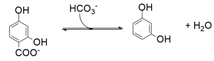 | 29 | <1 | - | 0.1 | - | 9 | - |
| Entry | Substrate | Product | LigW_Sp Conversion (%) | LigW2_Sp Conversion (%) | 2,3-DHBD_Ao Conversion (%) | |
|---|---|---|---|---|---|---|
| 1 | 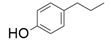 | 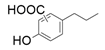 | 6 | 1 | 9.9 | |
| 2 |  |  | 10.2 | 7.3 | 4.2 | |
| 3 | 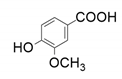 |  | 19.4 | 20.3 | <1 | |
| 4 |  |  | 16.6 | 13.9 | 9.3 | |
| 5 |  |  | 9.0 | 26.6 | 7.2 | |
| 6 | 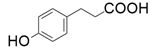 |  | 5.1 | 28.0 | <1 | |
| 7 |  | 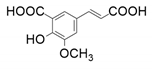 | 31.3 | 18.0 | 25.4 | |
| 8 | 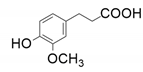 |  | 18.7 | 12.4 | 4.4 | |
| Entry | Enzyme | Microorganism | Reference |
|---|---|---|---|
| 1 | 4-hydroxybenzoate decarboxylase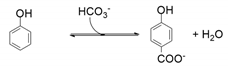 | Enterobacter cloacae P240 Clamidophila pneumonia AR39 Clostridium-like bacterium | [77] [78] [79] |
| 2 | phenylphosphate carboxylase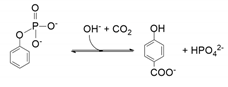 | Thauera aromatica K172 Desulfatiglans anilini Rhodopseudomonas palustris | [80,81] [82] [83] |
| 3 | 3,4-dihydroxybenzoate decarboxylase | Enterobacter cloacae P241 Clostridium hydroxybenzoicum JW/Z-1T | [84] [85] |
| 4 | 4-hydroxy-3-methoxybenzoate decarboxylase (fermenting anaerobes) | Bacillus subtilis ATCC6051 | [86] |
| Entry | Substrate | Product | PAD_Mc Conversion (%) | PAD_Ps Conversion (%) | PAD_LI Conversion (%) |
|---|---|---|---|---|---|
| 1 | 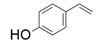 |  | 18 | 17 | 3 |
| 2 |  |  | 28 | 20 | 2 |
| 3 |  |  | 11 | 35 | 35 |
| 4 |  | 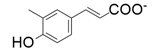 | 28 | 13 | 1 |
| 5 | 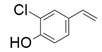 | 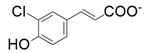 | 30 | 18 | 26 |
| 6 | 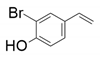 | 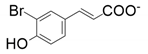 | 18 | <1 | <1 |
| 7 |  | 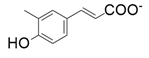 | 26 | 14 | 10 |
| 8 | 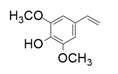 |  | 1 | 5 | 4 |
| Entry | Column A Guaiacol and Guaiacol Derivatives | Column B Carboxylated Products | Column C Syringol and Syringol Derivatives |
|---|---|---|---|
| 1 | 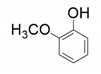 |  | 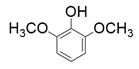 |
| 2 |  | 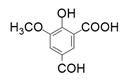 |  |
| 3 |  | 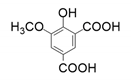 | 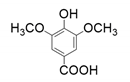 |
| 4 | 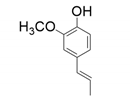 | 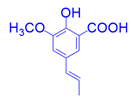 | 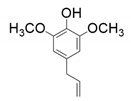 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommasi, I.C. Carboxylation of Hydroxyaromatic Compounds with HCO3− by Enzyme Catalysis: Recent Advances Open the Perspective for Valorization of Lignin-Derived Aromatics. Catalysts 2019, 9, 37. https://doi.org/10.3390/catal9010037
Tommasi IC. Carboxylation of Hydroxyaromatic Compounds with HCO3− by Enzyme Catalysis: Recent Advances Open the Perspective for Valorization of Lignin-Derived Aromatics. Catalysts. 2019; 9(1):37. https://doi.org/10.3390/catal9010037
Chicago/Turabian StyleTommasi, Immacolata C. 2019. "Carboxylation of Hydroxyaromatic Compounds with HCO3− by Enzyme Catalysis: Recent Advances Open the Perspective for Valorization of Lignin-Derived Aromatics" Catalysts 9, no. 1: 37. https://doi.org/10.3390/catal9010037
APA StyleTommasi, I. C. (2019). Carboxylation of Hydroxyaromatic Compounds with HCO3− by Enzyme Catalysis: Recent Advances Open the Perspective for Valorization of Lignin-Derived Aromatics. Catalysts, 9(1), 37. https://doi.org/10.3390/catal9010037




