High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects
Abstract
1. Introduction
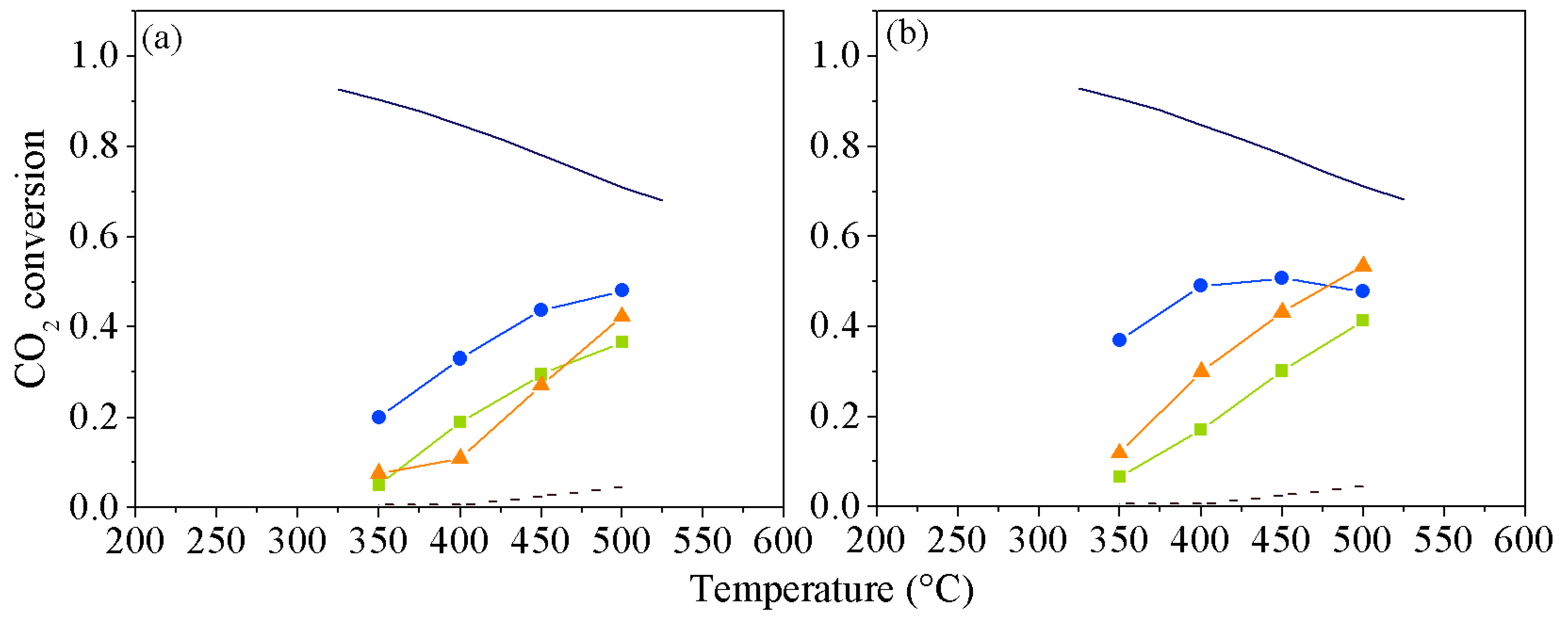
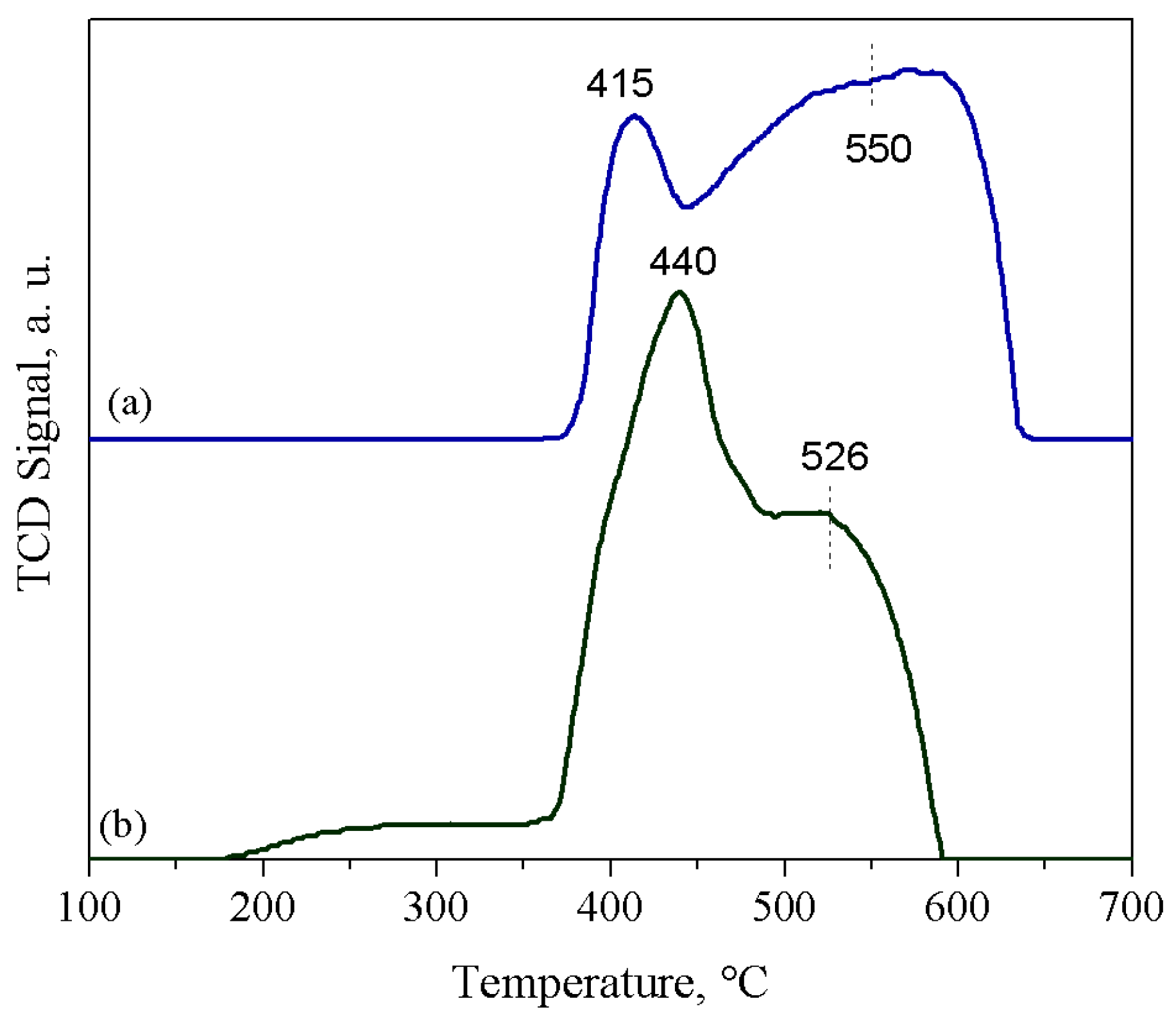
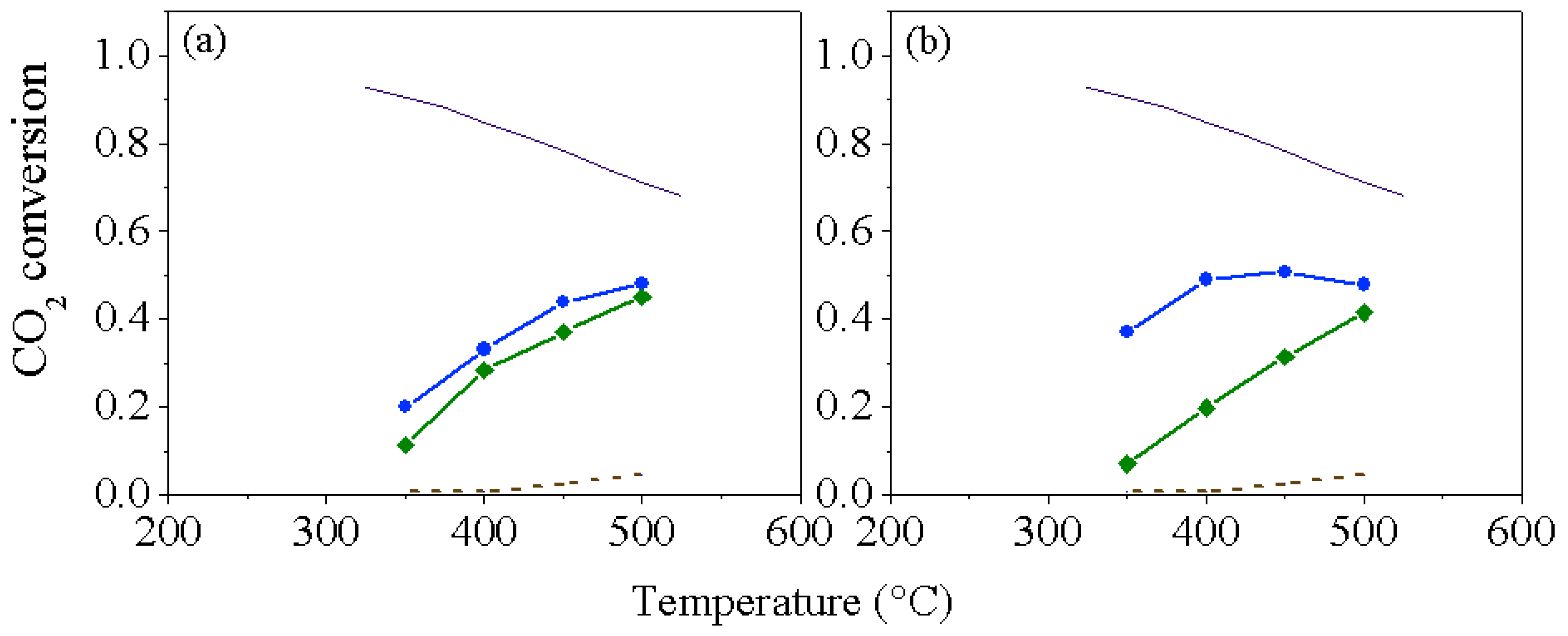
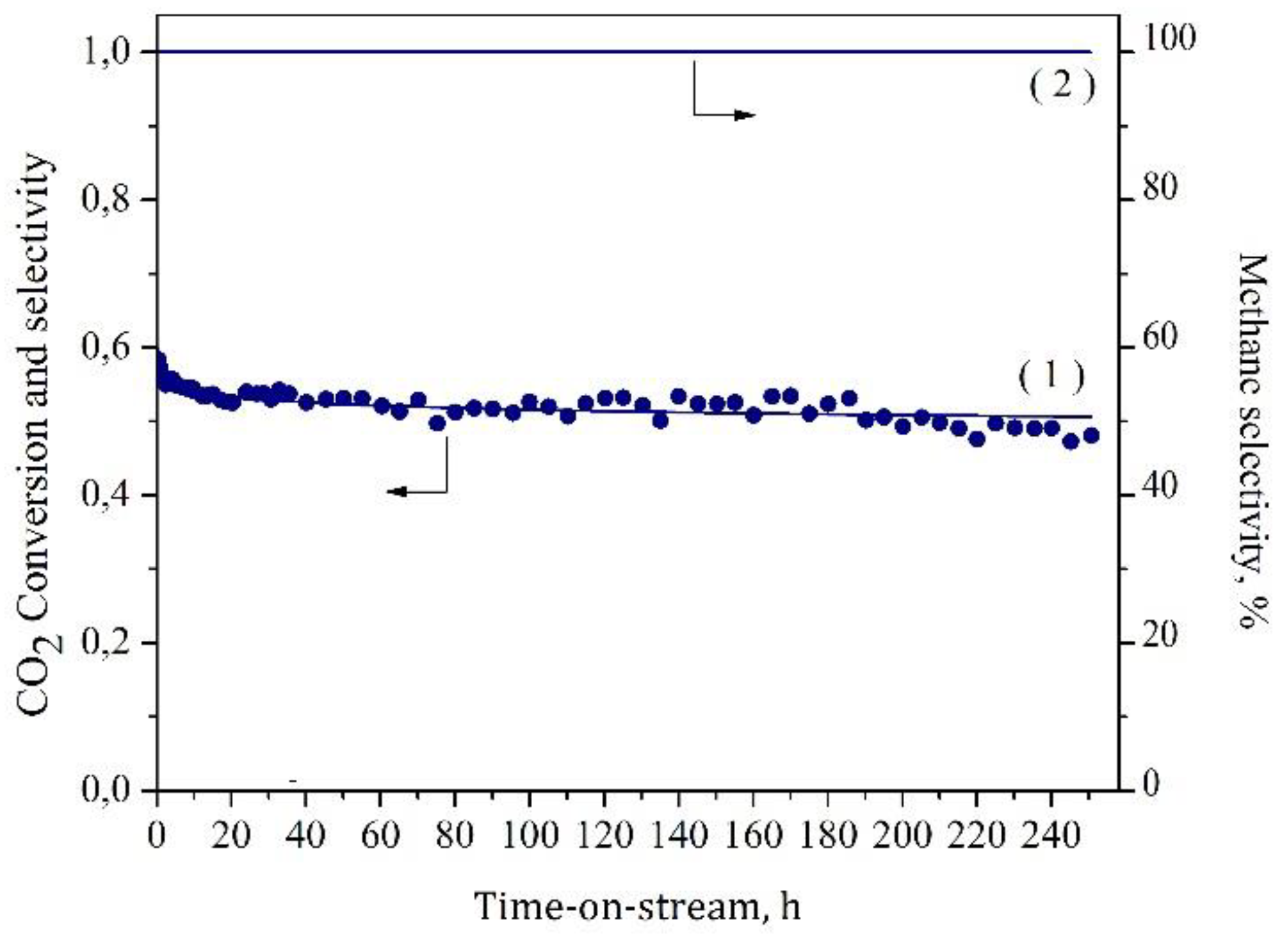
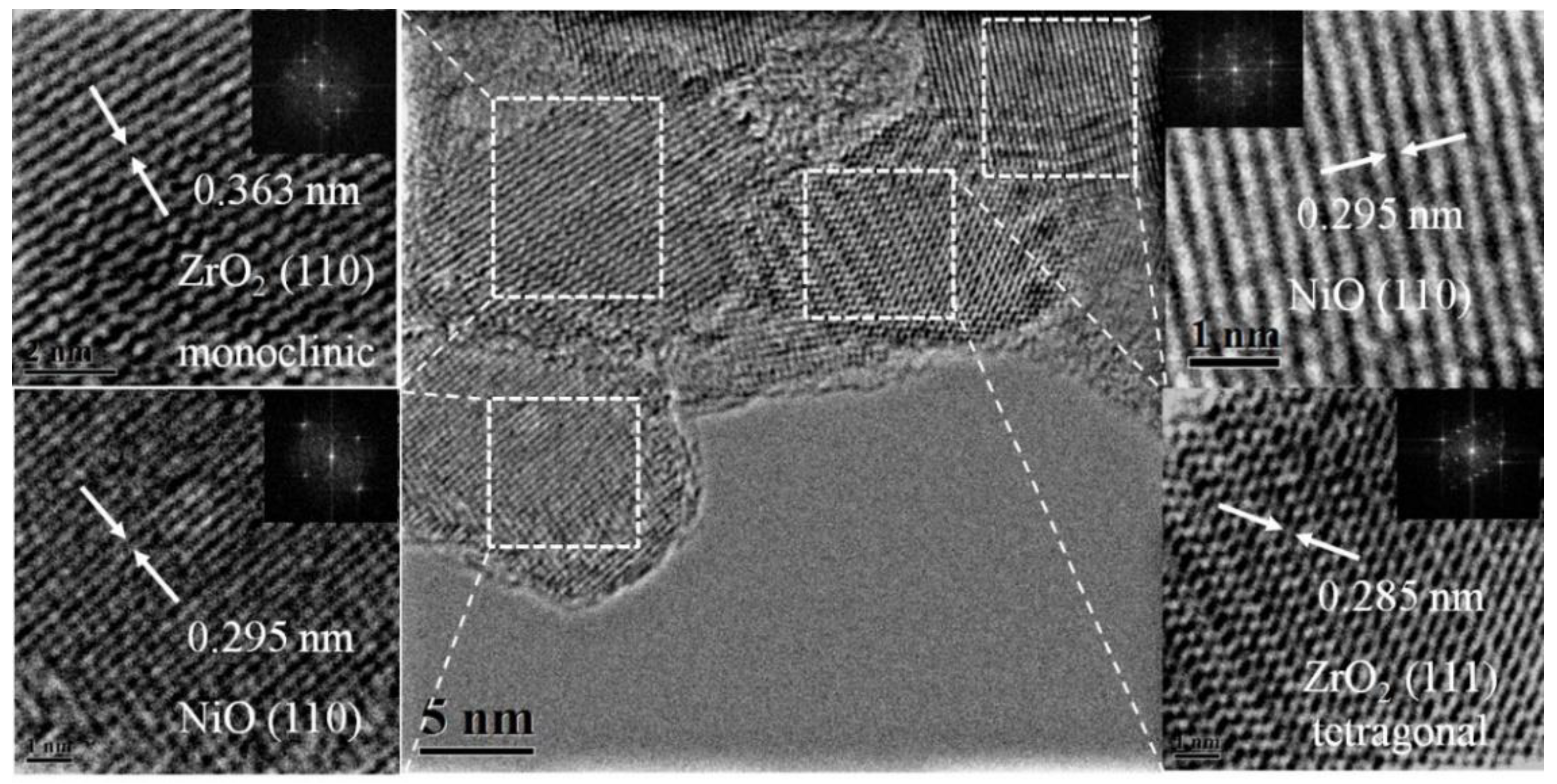
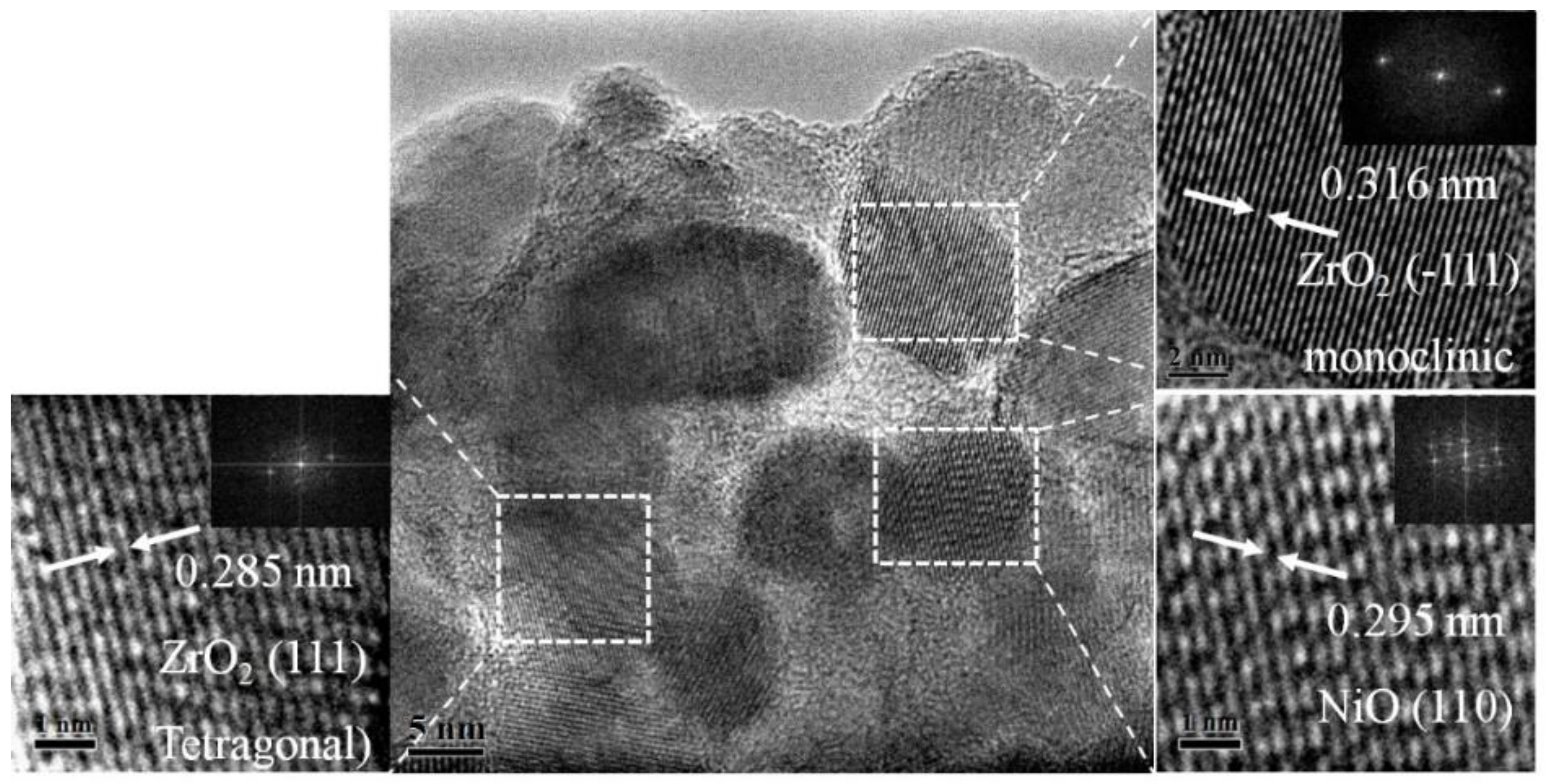
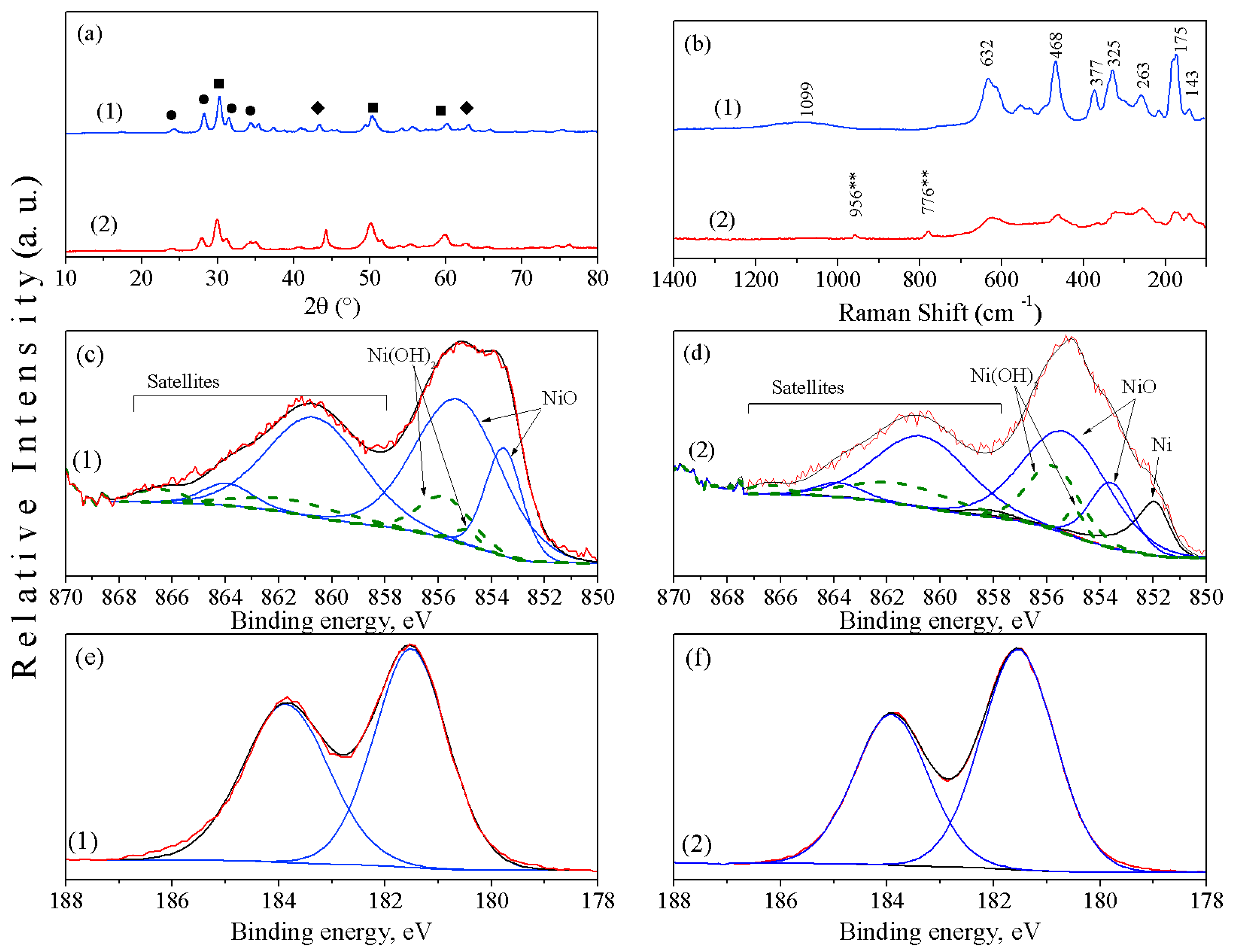
2. Results and Discussion
3. Experimental
3.1. Support Synthesis
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
3.4. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Aeronautics and Space Administration (NASA) USA, ClimateChange: Vital Signs of the Planet: Carbon Dioxide. 2017. Available online: http://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed on 9 June 2018).
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y.J. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Ronsch, S.; Schneider, J.; Matthischke, S.; Schluter, M.; Gotz, M.; Lefebre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Wang, W.; Gong, J. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Younas, M.; Kong, L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P.L. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.Z.; Qin, Z.F.; Guo, H.L.; Lin, J.Y.; Li, Z. Methanation of carbon dioxide over Ni–M/ZrO2 (M = Fe, Co, Cu) catalysts: Effect of addition of a second meta. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]
- Ando, H.; Pujiwara, M.; Matsumura, Y.; Miyamura, H.; Souma, Y. Methanation of carbon dioxide over LaNi4X type catalysts. Energy Convers. Manag. 1995, 36, 653–656. [Google Scholar] [CrossRef]
- Abelló, S.; Berrueco, C.; Montané, D. High-loaded nickel–alumina catalyst for direct CO2 hydrogenation into synthetic natural gas (SNG). Fuel. 2013, 113, 598–609. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Zhao, J.; Chou, L. Methanation of Carbon Dioxide over a Highly Dispersed Ni/La2O3 Catalyst. Chin. J. Catal. 2010, 31, 21–23. [Google Scholar] [CrossRef]
- Ussa, P.A.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyka, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Graca, I.; González, L.V.; Bacariza, M.C.; Fernandes, A.; Henriques, C.; Lopes, J.M.; Ribeiro, M.F. CO2 hydrogenation into CH4 on NiHNaUSY zeolites. Appl. Catal. B Environ. 2014, 147, 101–110. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Su, X.; Duan, H.; Geng, H.; Huang, Y. CO2 methanation over TiO2–Al2O3 binary oxides supported Ru catalysts. Chin. J. Chem. Eng. 2016, 24, 140–145. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Effect of CeO2 addition on Ni/Al2O3 catalysts for methanation of carbon dioxide with hydrogen. J. Nat. Gas Chem. 2012, 21, 703–707. [Google Scholar] [CrossRef]
- Zhi, G.; Guo, X.; Wang, Y.; Jin, G.; Guo, X. Effect of La2O3 modification on the catalytic performance of Ni/SiC for methanation of carbon dioxide. Catal. Commun. 2011, 16, 56–59. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO2 methanation on Ni@MOF-5 via control of active species dispersion. Chem. Commun. 2015, 51, 1728–1731. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Roger, A.C. Methanation of carbon dioxide over nickel-based Ce0.72Zr0.28O2 mixed oxide catalysts prepared by sol–gel method. Appl. Catal. A Gen. 2009, 369, 90–96. [Google Scholar] [CrossRef]
- Gao, J.; Jia, L.S.; Fang, W.P.; Li, Q.B.; Song, H. Methanation of carbon dioxide over the LaNiO3 perovskite catalysts activated under the reactant stream. J. Fuel Chem. Technol. 2009, 37, 573–577. [Google Scholar] [CrossRef]
- Mutz, B.; Carvalho, H.W.P.; Mangold, S.; Kleist, W.; Grunwaldt, J.D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Frey, M.; Édouard, D.; Roger, A.C. Optimization of structured cellular foam-based catalysts for low-temperature carbon dioxide methanation in a platelet milli-reactor. CR Chim. 2015, 18, 283–292. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int. J. Hydrogen Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Bakar, W.A.W.A.; Ali, R.; Mohammad, N.S. The effect of noble metals on catalytic methanation reaction over supported Mn/Ni oxide based catalysts. Arab. J. Chem. 2015, 8, 632–643. [Google Scholar] [CrossRef]
- Chen, K.; Xie, S.; Iglesia, E.; Bell, A.T. Structure and Properties of Zirconia-Supported Molybdenum Oxide Catalysts for Oxidative Dehydrogenation of Propane. J. Catal. 2000, 189, 421–430. [Google Scholar] [CrossRef]
- Chary, J.K.V.R.; Ramesh, K.; Naresh, D.; Rao, P.V.R.; Rao, V.V. The effect of zirconia polymorphs on the structure and catalytic properties of V2O5/ZrO2 catalysts. Catal. Today 2009, 141, 187–194. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Asami, K.; Izumiya, K.; Hashimoto, K. Effect of tetragonal ZrO2 on the catalytic activity of Ni/ZrO2 catalyst prepared from amorphous Ni–Zr alloys. Catal. Commun. 2006, 7, 24–28. [Google Scholar] [CrossRef]
- Solis-Garcia, A.; Louvier-Hernandez, J.F.; Almendarez-Camarillo, A.; Fierro-Gonzalez, J.C. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni. Appl. Catal. B 2017, 218, 611–620. [Google Scholar] [CrossRef]
- Bokhimj, X.; Morales, A.; Novaro, O.; Portilla, M.; López, T.; Tzompantzi, F.; Gómez, R. Tetragonal Nanophase Stabilization in Nondoped Sol–Gel Zirconia Prepared with Different Hydrolysis Catalysts. J. Solid State Chem. 1998, 135, 28–35. [Google Scholar] [CrossRef]
- Knoblauch, N.; Simon, H.; Dörrer, L.; Uxa, D.; Beschnitt, S.; Fielitz, P.; Wendelstorf, J.; Spitzer, K.H.; Schmücker, M.; Borchardt, G. Ceria: Recent Results on Dopant-Induced Surface Phenomena. Inorganics 2017, 5, 76. [Google Scholar] [CrossRef]
- Kaminski, P.; Zioleka, M.; van Bokhovenbc, J.A. Mesoporous cerium–zirconium oxides modified with gold and copper–synthesis, characterization and performance in selective oxidation of glycerol. RSC Adv. 2017, 7, 7801–7819. [Google Scholar] [CrossRef]
- Rahman, A.; Jayaganthan, R. Study of photocatalyst magnesium aluminate spinel nanoparticles. J. Nanostruct. Chem. 2015, 5, 147–151. [Google Scholar] [CrossRef]
- Morris, M.C.; McMurdie, H.F.; Evans, E.H.; Paretzkin, B.; Parker, H.S.; Panagiotopoulos, N.C. Standard X-ray Diffraction Powder Patterns. National Bureau of Standards Monograph, October 1981. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/MONO/nbsmonograph25-5.pdf (accessed on 9 June 2018).
- Thao, N.T.; Nhu, N.T. Evaluation of catalytic activity of MeOx/sepiolite in benzyl alcohol oxidation. JS: AMD 2018, 3, 289–295. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, J.A.P.; Remediakis, I.N.; Bengaard, H.; Nørskov, J.K. Sintering of nickel steam-reforming catalysts: Effects of temperature and steam and hydrogen pressures. J. Catal. 2004, 223, 432–443. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Ji, F.; Li, M. Effect of ZrO2 on catalyst structure and catalytic methanation performance over Ni-based catalyst in slurry-bed reactor. Int. J. Hydrogen Energy 2015, 8833–8843. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Particle size and dispersion measurements. Handb. Heterog. Catal. 2008, 1, 738–765. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin, Germany, 2006; pp. 212–232. ISBN 978-1-4020-2303-3. [Google Scholar]
- Pomfret, M.B.; Owrutsky, J.C.; Walker, R.A. High-Temperature Raman Spectroscopy of Solid Oxide Fuel Cell Materials and Processes. J. Phys. Chem. B 2006, 110, 17305–17308. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Agarkov, D.A.; Burmistrov, I.N.; Tsybrov, F.M.; Tartakovskii, I.I.; Kharton, V.V.; Bredikhin, S.I. In-situ Raman spectroscopy analysis of the interfaces between Ni-based SOFC anodes and stabilized zirconia electrolyte. Solid State Ion. 2017, 302, 133–137. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; MacDougall, B.R. Raman and infrared spectroscopy of α and β phases of thin nickel nydroxide films electrochemically formed on nickel. J. Phys. Chem. A 2012, 116, 6771–6784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xu, X.; Gao, J.; Fu, Q.; Tang, Y. Structure Characterization of WO3/ZrO2 Catalysts by Raman Spectroscopy. J. Raman Spectrosc. 1996, 27, 549–554. [Google Scholar] [CrossRef]
- Baklanova, N.I.; Kolesov, B.A.; Zima, T.M. Raman study of yttria-stabilized zirconia interfacial coatings on Nicalon™ fiber. J. Eur. Ceram. Soc. 2007, 27, 165–171. [Google Scholar] [CrossRef]
- Li, M.; Feng, Z.; Xiong, G.; Ying, P.; Xin, Q.; Li, C. Phase Transformation in the Surface Region of Zirconia Detected by UV Raman Spectroscopy. J. Phys. Chem. B 2001, 105, 8107–8111. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Cao, W.; Kang, J.; Fan, G.; Yang, L.; Li, F. Fabrication of Porous ZrO2 Nanostructures with Controlled Crystalline Phases and Structures via a Facile and Cost-Effective Hydrothermal Approach. Ind. Eng. Chem. Res. 2015, 54, 12795–12804. [Google Scholar] [CrossRef]
- Xie, S.; Iglesia, E.; Bell, A.T. Water-Assisted Tetragonal-to-Monoclinic Phase Transformation of ZrO2 at Low Temperatures. Chem. Mater. 2000, 12, 2442–2447. [Google Scholar] [CrossRef]
- Stocker, C.; Schneider, M.; Baiker, A. Zirconia aerogels and xerogels: Influence of solvent and acid on structural properties. J. Porous Mater. 1995, 2, 171–183. [Google Scholar] [CrossRef]
- Wajler, A.; Tomaszewski, H.; Weglarz, H.; Diduszko, R. Influence of processing on magnesium aluminate precursors morphology prepared by co-precipitation. Ceram. Mater. 2011, 63, 104–108. [Google Scholar]
- Li, J.G.; Ikegami, T.; Lee, J.H.; Mori, T.; Yajima, Y. Synthesis of Mg–Al spinel powder via precipitation using ammonium bicarbonate as the precipitant. J. Eur. Ceram. Soc. 2001, 21, 139–148. [Google Scholar] [CrossRef]
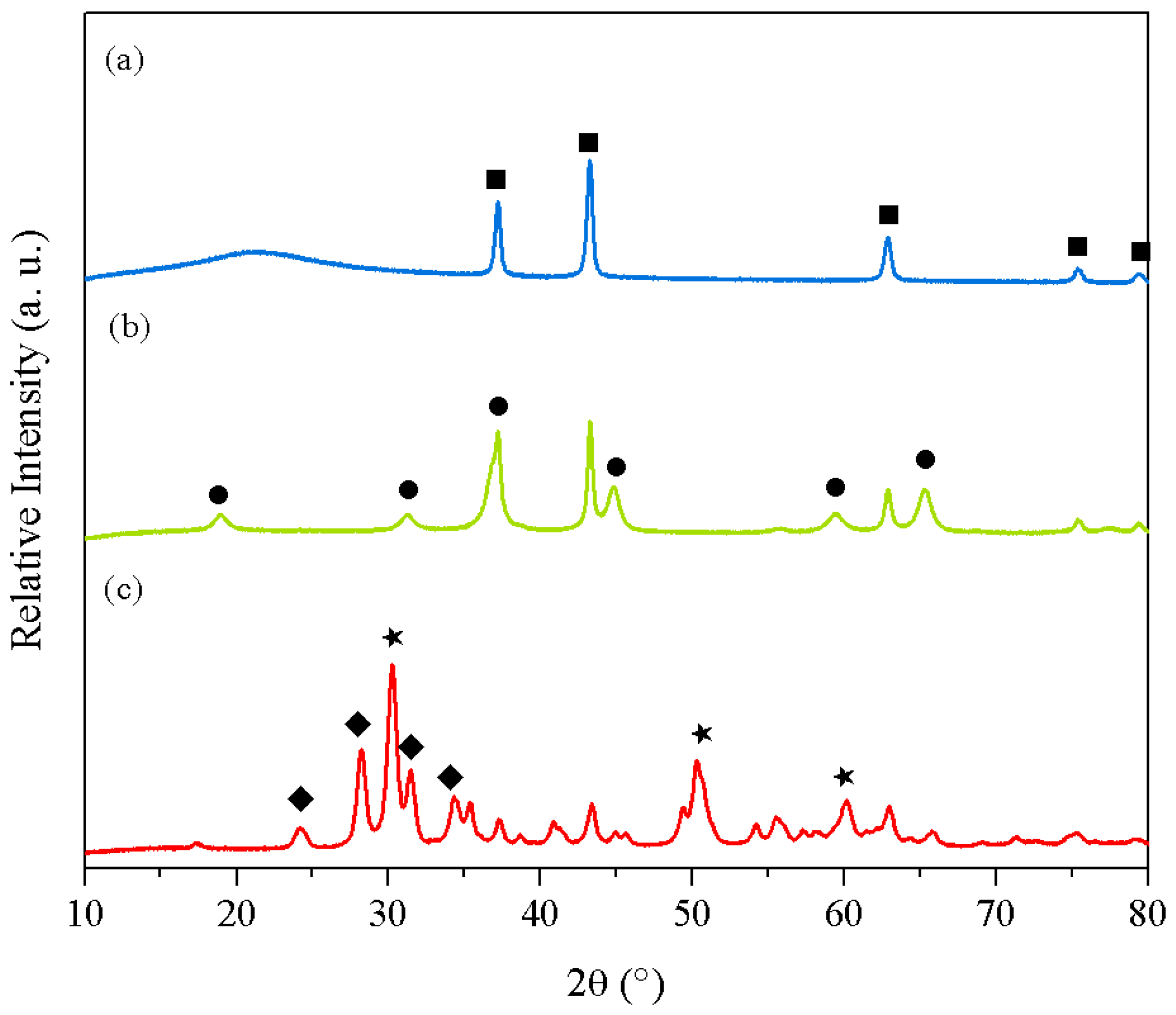
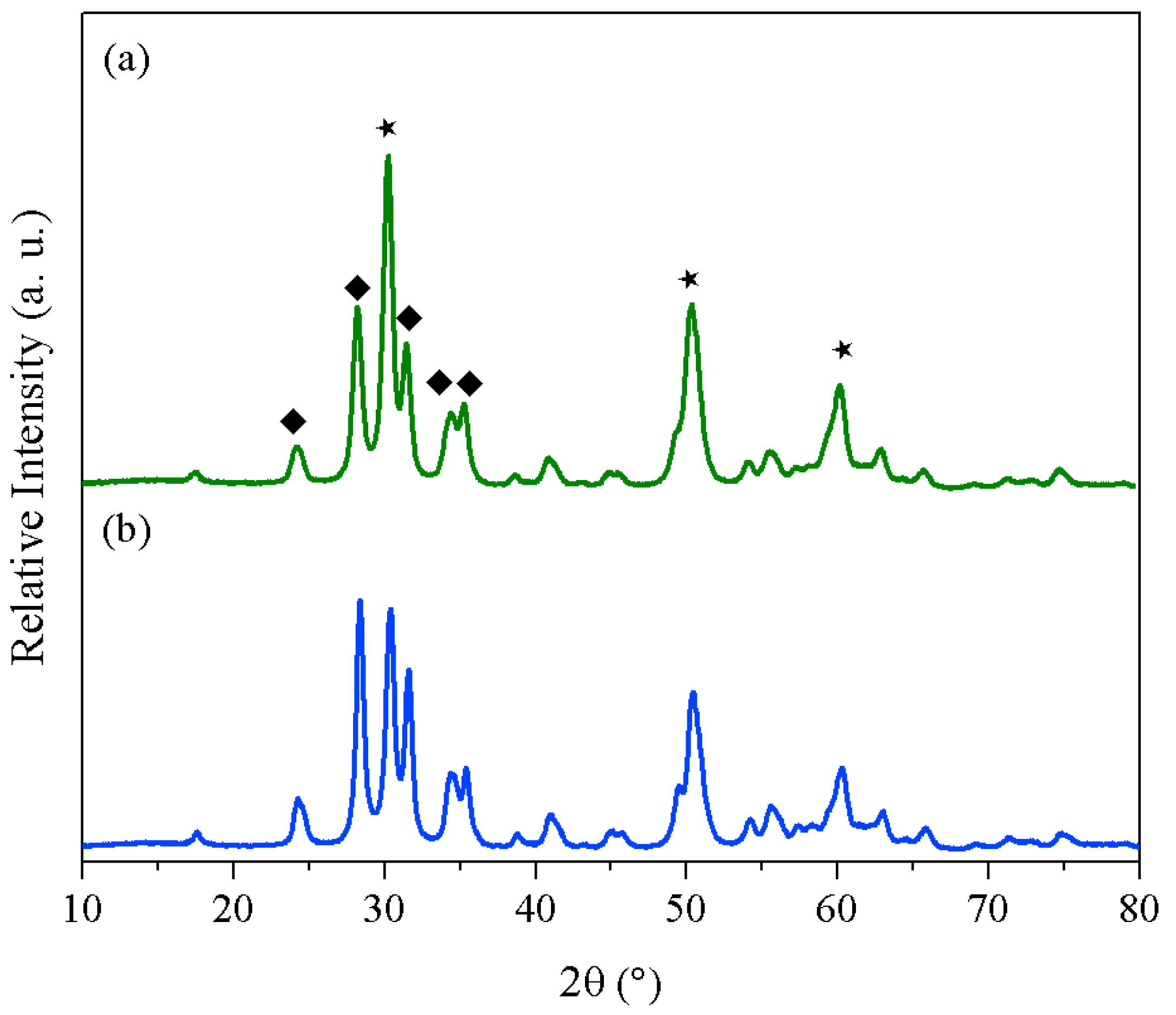
| Support | Co-precipitation | Sol-Gel | ||
|---|---|---|---|---|
| ZrO2 Phase | Monoclinic | Tetragonal | Monoclinic | Tetragonal |
| Phase composition, % | 47 | 53 | 64 | 36 |
| a RSP: | - | - | - | - |
| A | 5.14 | 3.6 | 5.15 | 3.59 |
| B | 5.2 | 3.6 | 5.2 | 3.59 |
| C | 5.32 | 5.18 | 5.32 | 5.19 |
| Crystallite size, nm | 22 | 18 | 25 | 19 |
| Microdeformations | 3.4 × 10−3 | 2.4 × 10−3 | 3.0 × 10−3 | 1.3 × 10−3 |
| b Rwp (%) | 3.56 | 3.52 | ||
| c Rexp (%) | 2.24 | 2.28 | ||
| Support | Preparation Method | Specific Surface Area (BET), m2/g | Average Pore Diameter, nm | |
|---|---|---|---|---|
| ZrO2 | 20% Ni/ZrO2 | |||
| ZrO2-COP | Co-precipitation | 75 | 53 | 8.0 |
| ZrO2-SG | Sol-Gel | 22 | 19 | 19.3 |
| Material | Crystalline Phases Composition, % | Monoclinic/Tetragonal Ratio | |
|---|---|---|---|
| Monoclinic | Tetragonal | ||
| ZrO2-COP (Support) | 47 | 53 | 0.88 |
| 20% Ni/ZrO2-COP-S | 40 | 60 | 0.66 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, J.; Hernández, E.; Alfaro, S.; López Medina, R.; Valverde Aguilar, G.; Albiter, E.; Valenzuela, M.A. High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts 2019, 9, 24. https://doi.org/10.3390/catal9010024
Martínez J, Hernández E, Alfaro S, López Medina R, Valverde Aguilar G, Albiter E, Valenzuela MA. High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts. 2019; 9(1):24. https://doi.org/10.3390/catal9010024
Chicago/Turabian StyleMartínez, Jeremías, Edgar Hernández, Salvador Alfaro, Ricardo López Medina, Guadalupe Valverde Aguilar, Elim Albiter, and Miguel A. Valenzuela. 2019. "High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects" Catalysts 9, no. 1: 24. https://doi.org/10.3390/catal9010024
APA StyleMartínez, J., Hernández, E., Alfaro, S., López Medina, R., Valverde Aguilar, G., Albiter, E., & Valenzuela, M. A. (2019). High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts, 9(1), 24. https://doi.org/10.3390/catal9010024





