Plasma-Assisted Surface Interactions of Pt/CeO2 Catalyst for Enhanced Toluene Catalytic Oxidation
Abstract
1. Introduction
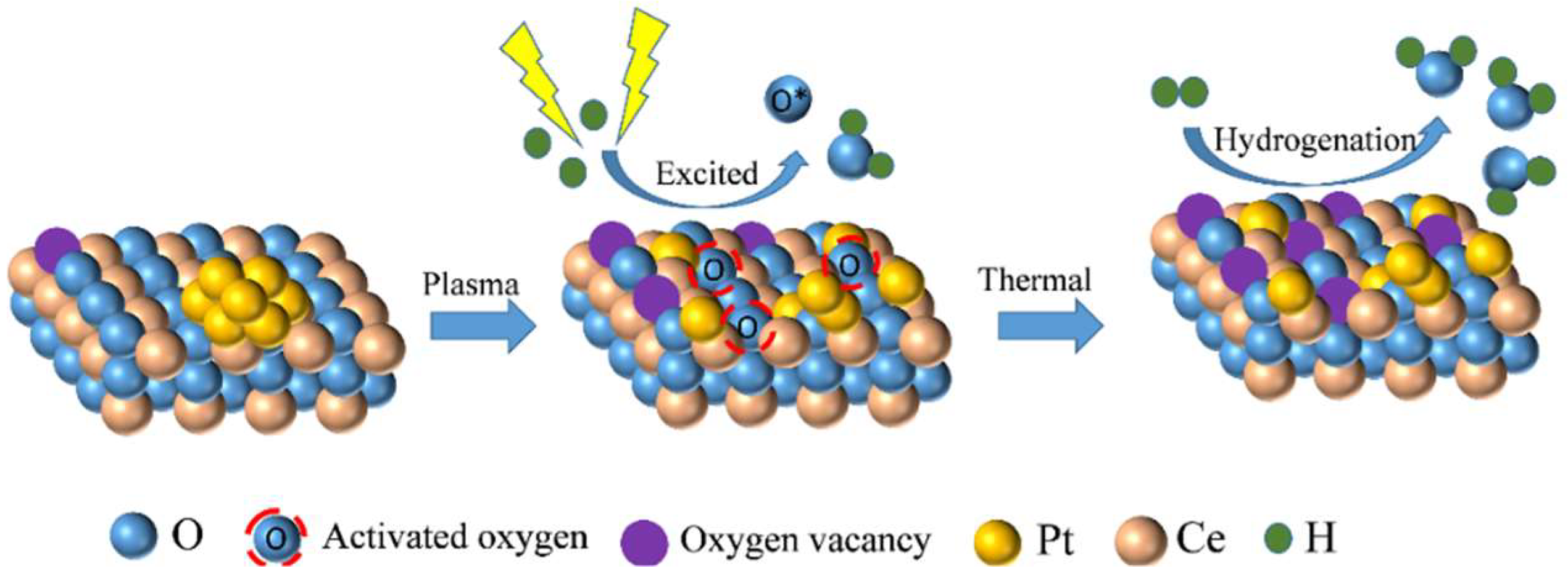
2. Results
3. Discussion
4. Materials and Methods
4.1. Catalysts Preparation
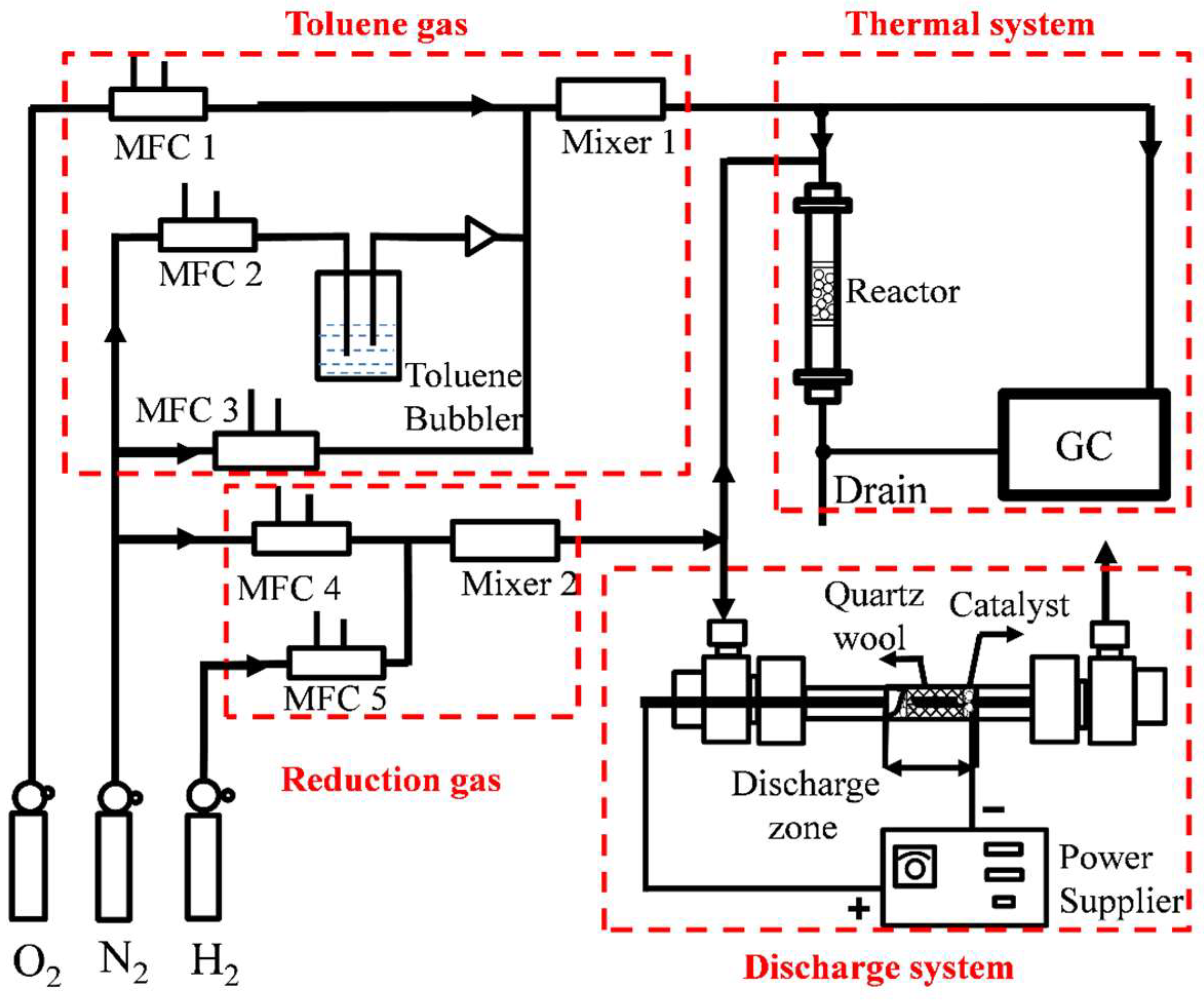
4.2. Experimental Setup
4.3. Catalysts Characterization
4.4. Catalytic Evaluation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Riedel, T.P.; Demarini, D.M.; Zavala, J.; Warren, S.H.; Corse, E.W.; Offenberg, J.H.; Kleindienst, T.E.; Lewandowski, M. Mutagenic atmospheres resulting from the photooxidation of aromatic hydrocarbon and NOx mixtures. Atmos. Environ. 2018, 178, 164–172. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.; Xu, W.; Wang, N.; Xiao, H.; Sun, Y.; Huang, H.; Yu, L.; Fu, M.; Wu, J.; et al. The Mechanism of Non-thermal Plasma Catalysis on Volatile Organic Compounds Removal. Catal. Surv. Asia 2018, 22, 73–94. [Google Scholar] [CrossRef]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Torrentemurciano, L.; Solsona, B.; Agouram, S.; Sanchis, R.; López, J.M.; Garcia, T.; Zanella, R. Low temperature total oxidation of toluene by bimetallic Au–Ir catalysts. Catal. Sci. Technol. 2017, 7, 2886–2896. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Liu, Y.; Xie, S.; Wu, Z.; Dai, H. Preparation and catalytic performance of Ag, Au, Pd or Pt nanoparticles supported on 3DOM CeO2–Al2O3 for toluene oxidation. J. Mol. Catal. A-Chem. 2016, 414, 9–18. [Google Scholar] [CrossRef]
- Zhao, S.; Li, K.; Jiang, S.; Li, J. Pd–Co based spinel oxides derived from pd nanoparticles immobilized on layered double hydroxides for toluene combustion. Appl. Catal. B Environ. 2016, 181, 236–248. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Ju, Y.; Lefkowitz, J.K.; Reuter, C.B.; Sang, H.W.; Yang, X.; Suo, Y.; Sun, W.; Jiang, Z.; Qi, C. Plasma Assisted Low Temperature Combustion. Plasma Chem. Plasma Process. 2016, 36, 85–105. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Estifaee, P. Synthesis and characterization of Pt/Al2O3–CeO2 nanocatalyst used for toluene abatement from waste gas streams at low temperature: Conventional vs. plasma–ultrasound hybrid synthesis methods. Micropor. Mesopor. Mat. 2014, 185, 213–223. [Google Scholar] [CrossRef]
- Di, L.; Li, Z.; Lee, B.; Park, D.-W. An alternative atmospheric-pressure cold plasma method for synthesizing Pd/P25 catalysts with the assistance of ethanol. Int. J. Hydrogen Energy 2017, 42, 11372–11378. [Google Scholar] [CrossRef]
- Gulyaev, R.V.; Slavinskaya, E.M.; Novopashin, S.A.; Smovzh, D.V.; Zaikovskii, A.V.; Osadchii, D.Y.; Bulavchenko, O.A.; Korenev, S.V.; Boronin, A.I. Highly active PdCeOx composite catalysts for low-temperature CO oxidation, prepared by plasma-arc synthesis. Appl. Catal. B Environ. 2014, 147, 132–143. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects Engineered Monolayer MoS2 for Improved Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Di, L.; Xu, W.; Zhang, X. Dry plasma reduction to prepare a high performance Pd/C catalyst at atmospheric pressure for CO oxidation. J. Mater. Chem. A 2014, 2, 11885–11890. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Chan, X.; Kim, T.J.; Kim, D.H. How Pt Interacts with CeO2 under the Reducing and Oxidizing Environments at Elevated Temperature: The Origin of Improved Thermal Stability of Pt/CeO2 Compared to CeO2. J. Phys. Chem. 2016, 120, 25870–25879. [Google Scholar] [CrossRef]
- Pastor-Perez, L.; Belda-Alcazar, V.; Marini, C.; Pastor-Blas, M.M.; Sepulveda-Escribano, A.; Ramos-Fernandez, E.V. Effect of cold Ar plasma treatment on the catalytic performance of Pt/CeO2 in water-gas shift reaction (WGS). Appl. Catal. B Environ. 2018, 225, 121–127. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, J.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; Xiao, F.-S. Enhanced performance in catalytic combustion of toluene over mesoporous Beta zeolite-supported platinum catalyst. Appl. Catal. B Environ. 2013, 140, 199–205. [Google Scholar] [CrossRef]
- Jamalzadeh, S.; Haghighi, M.; Asgari, N. Synthesis and physicochemical characterizations of nanostructured Pd/carbon-clinoptilolite-CeO2 catalyst for abatement of xylene from waste gas streams at low temperature. J. Ind. Eng. Chem. 2014, 20, 2735–2744. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Wang, B.; Chen, B.; Sun, Y.; Xiao, H.; Xu, X.; Fu, M.; Wu, J.; Chen, L.; Ye, D. Effects of dielectric barrier discharge plasma on the catalytic activity of Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 238, 328–338. [Google Scholar] [CrossRef]
- Beck, A.; Horvath, A.; Szucs, A.; Schay, Z.; Horvath, Z.E.; Zsoldos, Z.; Dekany, I.; Guczi, L. Pd nanoparticles prepared by “controlled colloidal synthesis” in solid/liquid interfacial layer on silica. I. Particle size regulation by reduction time. Catal. Lett. 2000, 65, 33–42. [Google Scholar]
- Aslam, M.; Qamar, M.T.; Soomro, M.T.; Ismail, I.M.I.; Salah, N.; Almeelbi, T.; Gondal, M.A.; Hameed, A. The effect of sunlight induced surface defects on the photocatalytic activity of nanosized CeO2 for the degradation of phenol and its derivatives. Appl. Catal. B Environ. 2016, 180, 391–402. [Google Scholar] [CrossRef]
- Meher, S.K.; Cargnello, M.; Troiani, H.; Montini, T.; Rao, G.R.; Fornasiero, P. Alcohol induced ultra-fine dispersion of Pt on tuned morphologies of CeO2 for CO oxidation. Appl. Catal. B Environ. 2013, 130–131, 121–131. [Google Scholar] [CrossRef]
- Castkova, K.; Matousek, A.; Bartonickova, E.; Cihlar Jr., J.; Vanysek, P.; Cihlar, J. Sintering of Ce, Sm, and Pr Oxide Nanorods. J. Am. Ceram. Soc. 2016, 99, 1155–1163. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.-W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef]
- Roldan Cuenya, B. Metal Nanoparticle Catalysts Beginning to Shape-up. Acc. Chem. Res. 2013, 46, 1682–1691. [Google Scholar] [CrossRef]
- Farmer, J.A.; Campbell, C.T. Strong Metal-Support Bonding. Science 2010, 329, 933–936. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.T.; Liu, C.; Wang, J. Hydrogen Storage on Carbon Doped with Platinum Nanoparticles Using Plasma Reduction. Ind. Eng. Chem. Res. 2007, 46, 8277–8281. [Google Scholar] [CrossRef]
- Murugan, R.; Vijayaprasath, G.; Mahalingam, T.; Ravi, G. Enhancement of room temperature ferromagnetic behavior of rf sputtered Ni-CeO2 thin films. Appl. Surf. Sci. 2016, 390, 583–590. [Google Scholar] [CrossRef]
- Si, W.; Wang, Y.; Zhao, S.; Hu, F.; Li, J. A Facile Method for in Situ Preparation of the MnO2/LaMnO3 Catalyst for the Removal of Toluene. Environ. Sci.Technol. 2016, 50, 4572–4578. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Capel Sanchez, M.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B Environ. 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Liotta, L.F.; Longo, A.; Macaluso, A.; Martorana, A.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Influence of the SMSI effect on the catalytic activity of a Pt(1%)/Ce0.6Zr0.4O2 catalyst: SAXS, XRD, XPS and TPR investigations. Appl. Catal. B Environ. 2004, 48, 133–149. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, H.; Li, T.; Wu, Y.; Ni, Y. Study on Pt-structured anodic alumina catalysts for catalytic combustion of toluene: Effects of competitive adsorbents and competitive impregnation methods. Appl. Surf. Sci. 2016, 360, 1066–1074. [Google Scholar] [CrossRef]
- Lopez, J.M.; Gilbank, A.L.; Garcia, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174, 403–412. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Qin, Z.; Wang, G.; Zhang, Y.; Wu, Z.; Li, Z.; Chen, G.; Dong, W.; Wu, Z.; et al. Morphologic effects of nano CeO2–TiO2 on the performance of Au/CeO2–TiO2 catalysts in low-temperature CO oxidation. Appl. Catal. B Environ. 2014, 144, 498–506. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158, 96–105. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.; Delariva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Wang, B.; Chi, C.; Xu, M.; Wang, C.; Meng, D. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge. Chem. Eng. J. 2017, 322, 679–692. [Google Scholar] [CrossRef]
- Zhou, G.; Lan, H.; Gao, T.; Xie, H. Influence of Ce/Cu ratio on the performance of ordered mesoporous CeCu composite oxide catalysts. Chem. Eng. J. 2014, 246, 53–63. [Google Scholar] [CrossRef]
- Chang, S.; Li, M.; Hua, Q.; Zhang, L.; Ma, Y.; Ye, B.; Huang, X. Shape-dependent interplay between oxygen vacancies and Ag–CeO2 interaction in Ag/CeO2 catalysts and their influence on the catalytic activity. J. Catal. 2012, 293, 195–204. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M., III; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Gallon, H.J.; Whitehead, J.C. Plasma-assisted reduction of a NiO/Al2O3 catalyst in atmospheric pressure H2/Ar dielectric barrier discharge. Catal. Today 2013, 211, 120–125. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, X.; Wei, M.; Evans, D.G.; Duan, X. Catalytic behavior of supported Ru nanoparticles on the {1 0 0}, {1 1 0}, and {1 1 1} facet of CeO2. J. Catal. 2015, 329, 177–186. [Google Scholar] [CrossRef]
- Alayoglu, S.; An, K.; Melaet, G.; Chen, S.; Bernardi, F.; Wang, L.W.; Lindeman, A.E.; Musselwhite, N.; Guo, J.; Liu, Z.; et al. Pt-Mediated Reversible Reduction and Expansion of CeO2 in Pt Nanoparticle/Mesoporous CeO2 Catalyst: In Situ X-ray Spectroscopy and Diffraction Studies under Redox (H2 and O2) Atmospheres. J. Phys. Chem. 2013, 117, 26608–26616. [Google Scholar] [CrossRef]
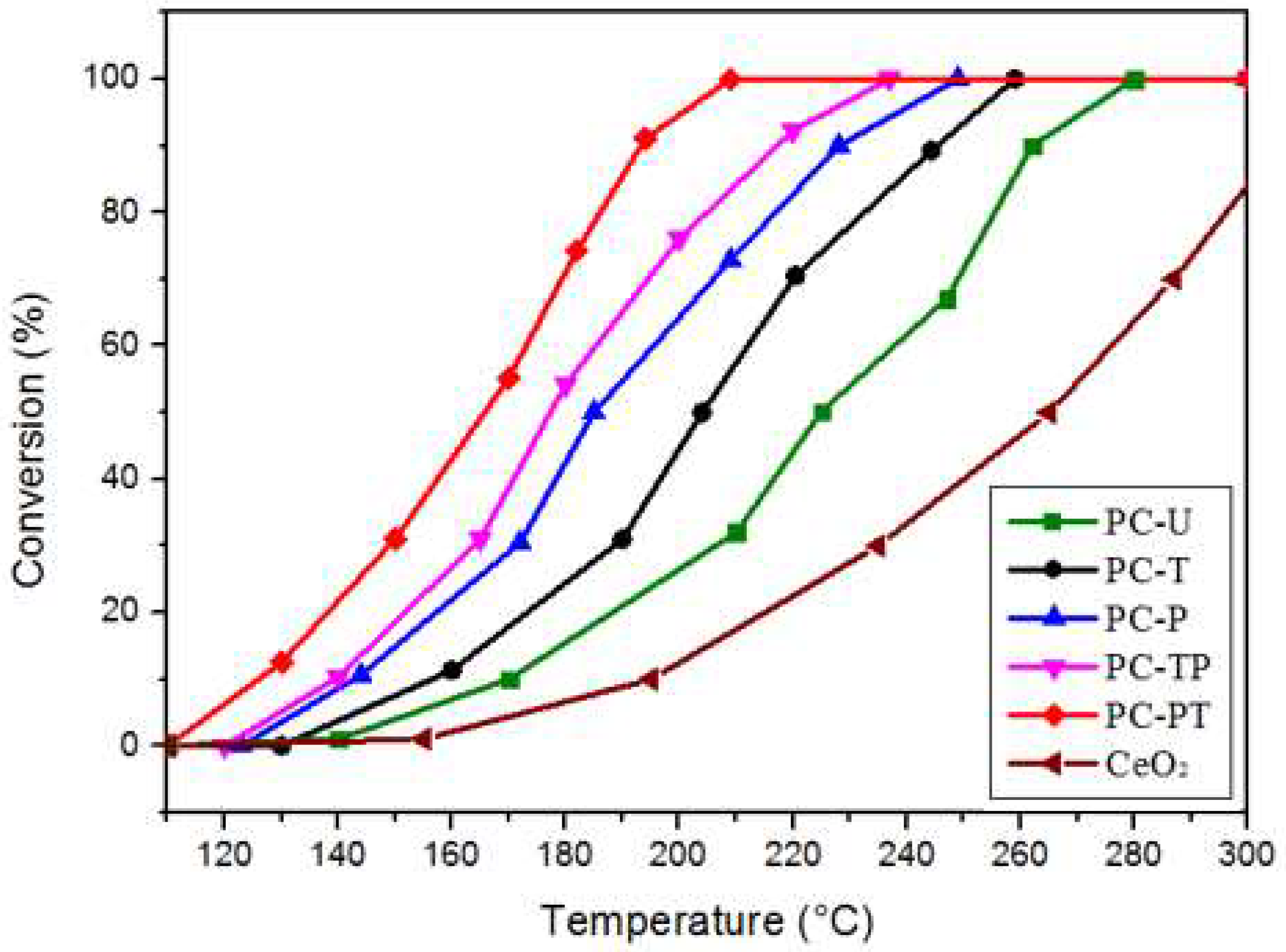
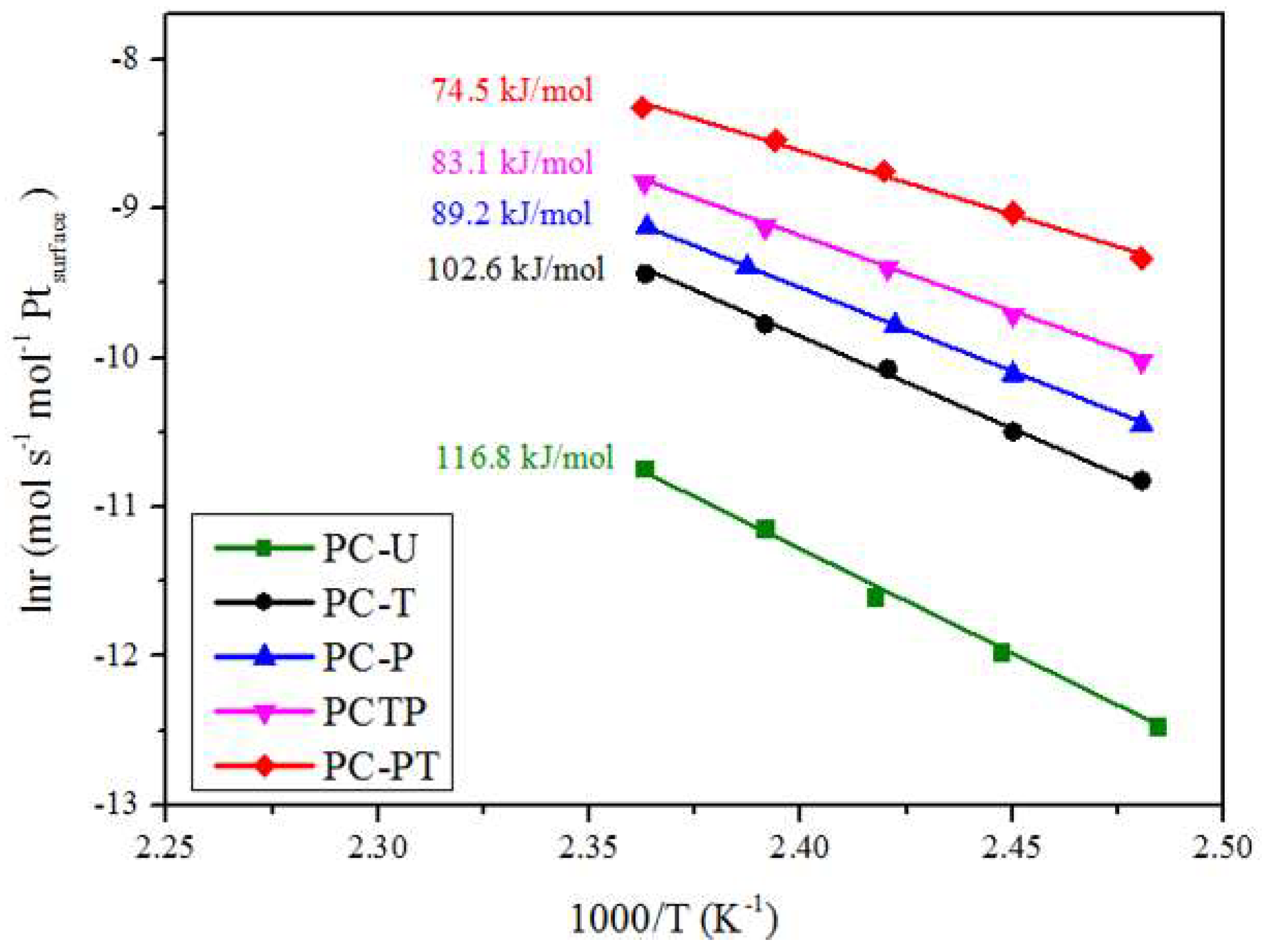
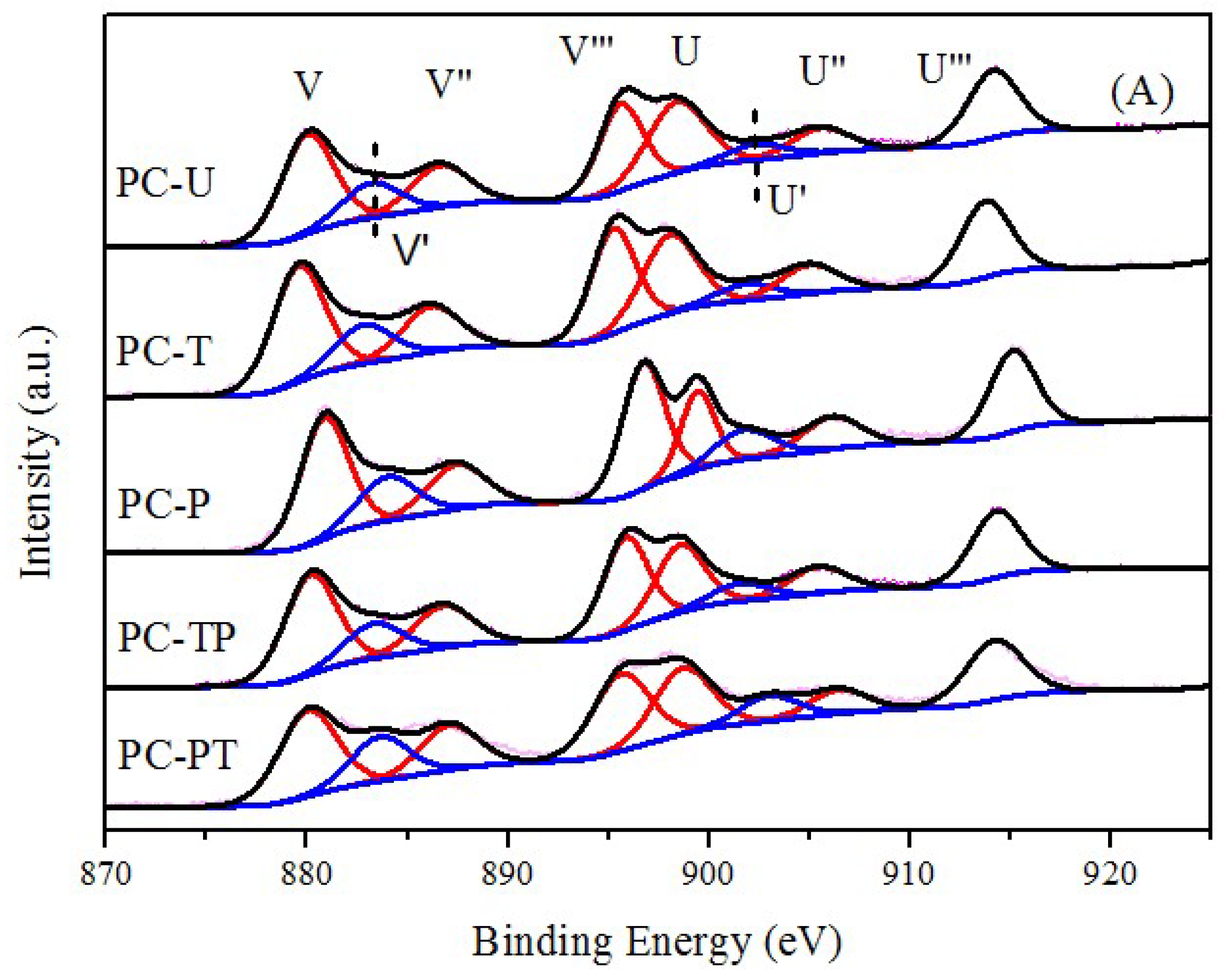
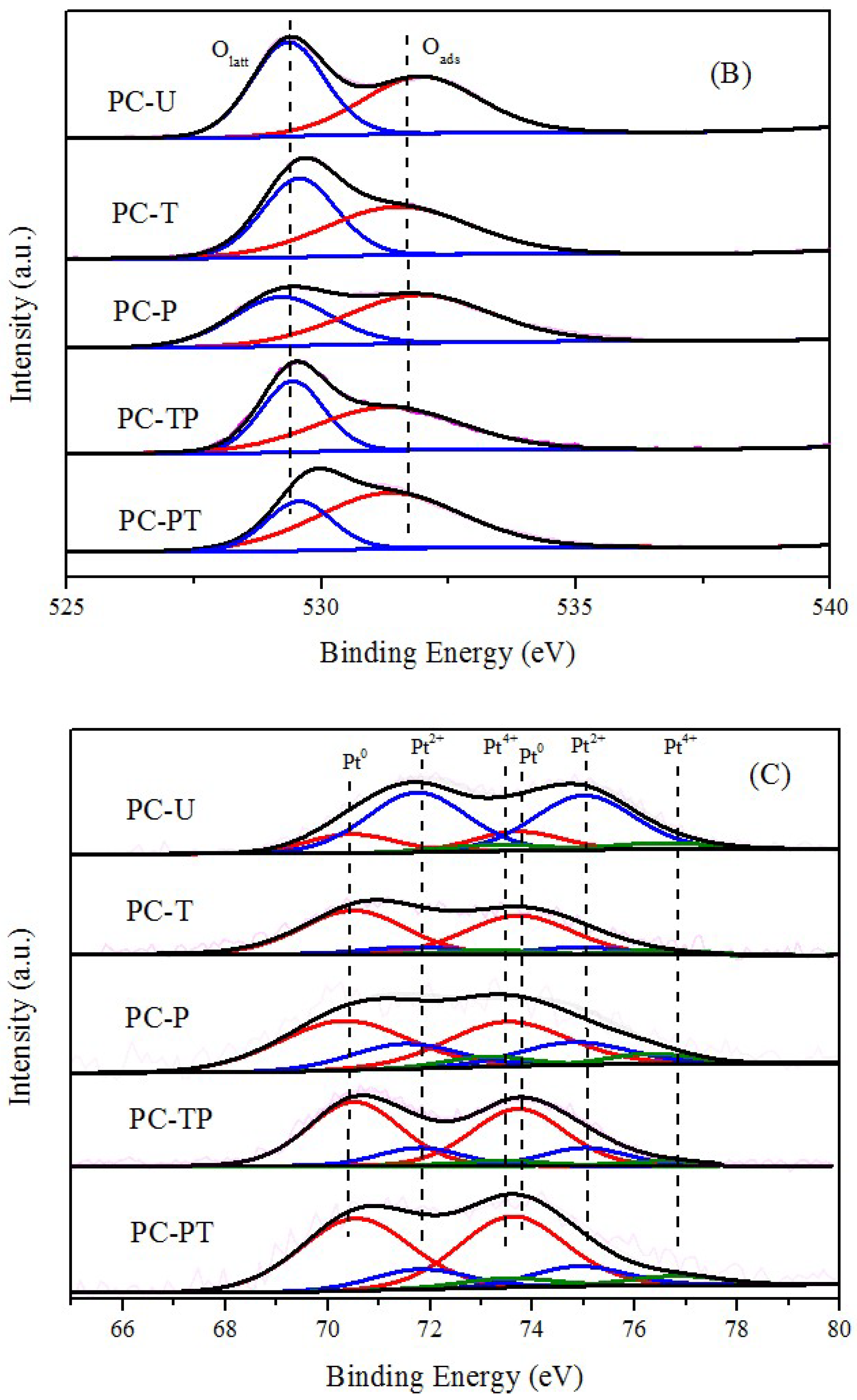
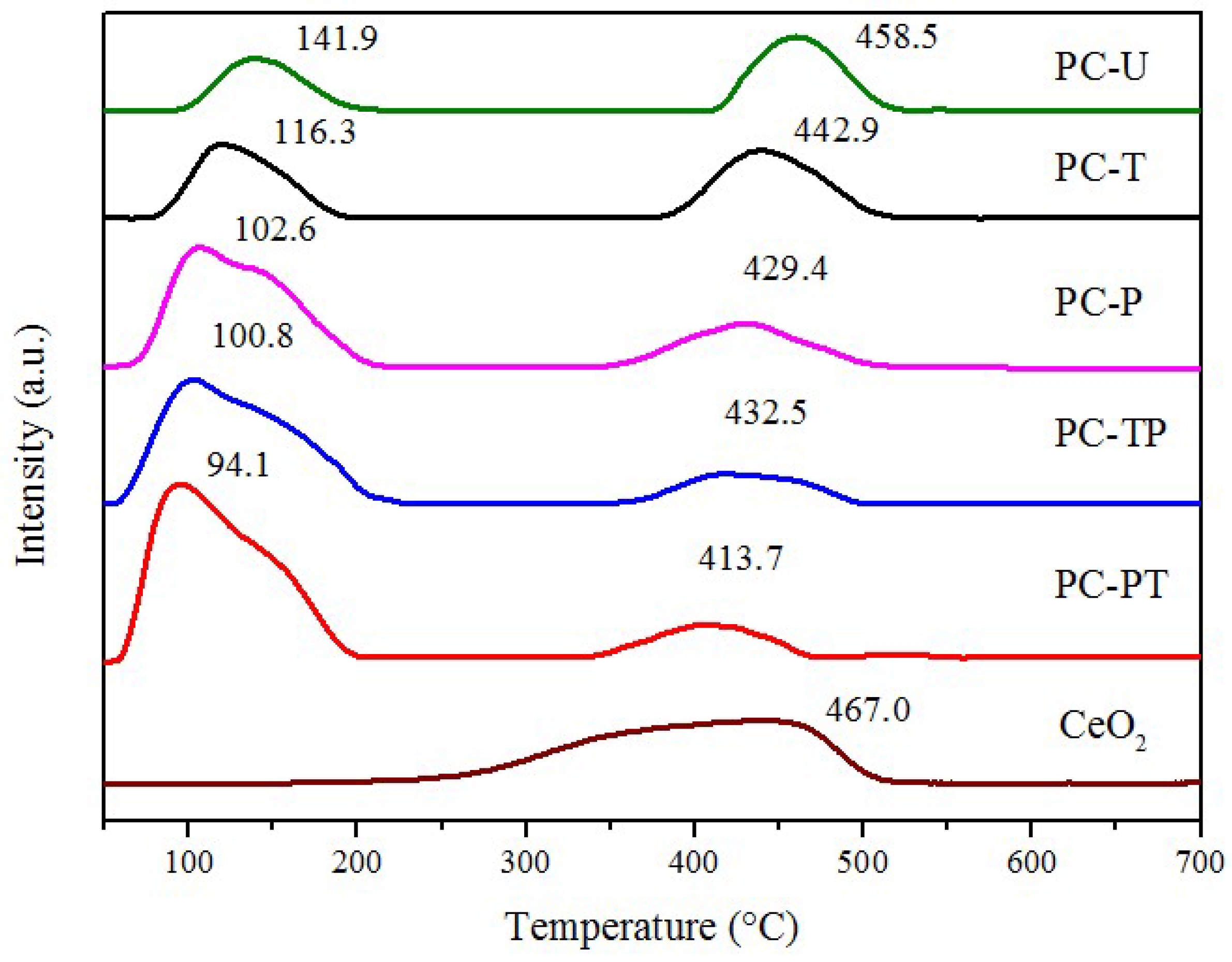
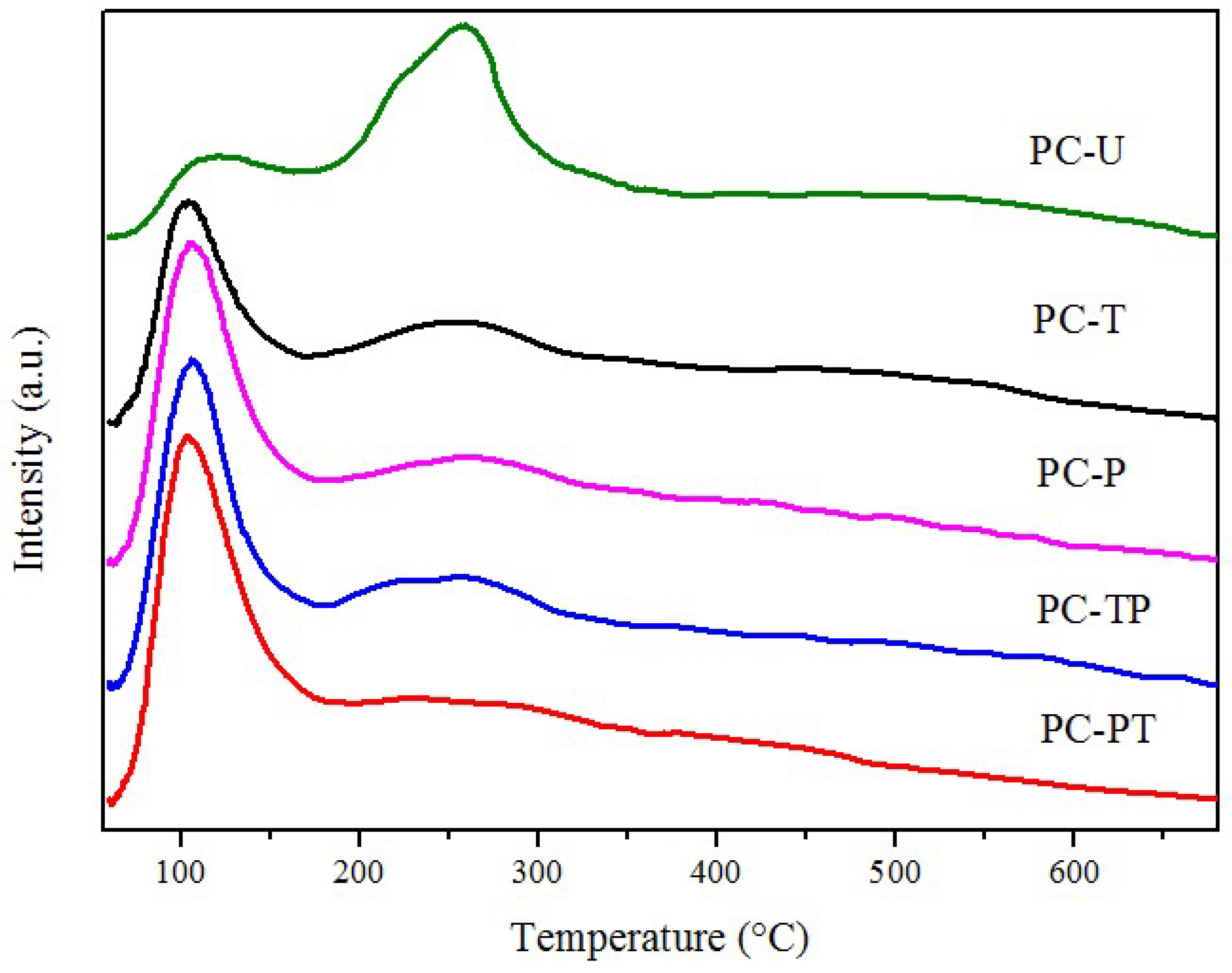

| Sample | Catalytic activity | Ea (kJ mol−1) | TOFPt 1 (×10−3 s−1) | TOFOV 1 (×10−4 s−1) | |
|---|---|---|---|---|---|
| T50 (°C) | T100 (°C) | ||||
| CeO2 | 262 | 328 | - | - | - |
| PC-U | 214 | 279 | 116.8 | 3.1 | 2.0 |
| PC-T | 204 | 256 | 102.6 | 6.1 | 2.6 |
| PC-P | 183 | 247 | 89.2 | 6.2 | 2.8 |
| PC-TP | 178 | 236 | 83.1 | 7.4 | 3.4 |
| PC-PT | 167 | 205 | 74.5 | 7.9 | 3.6 |
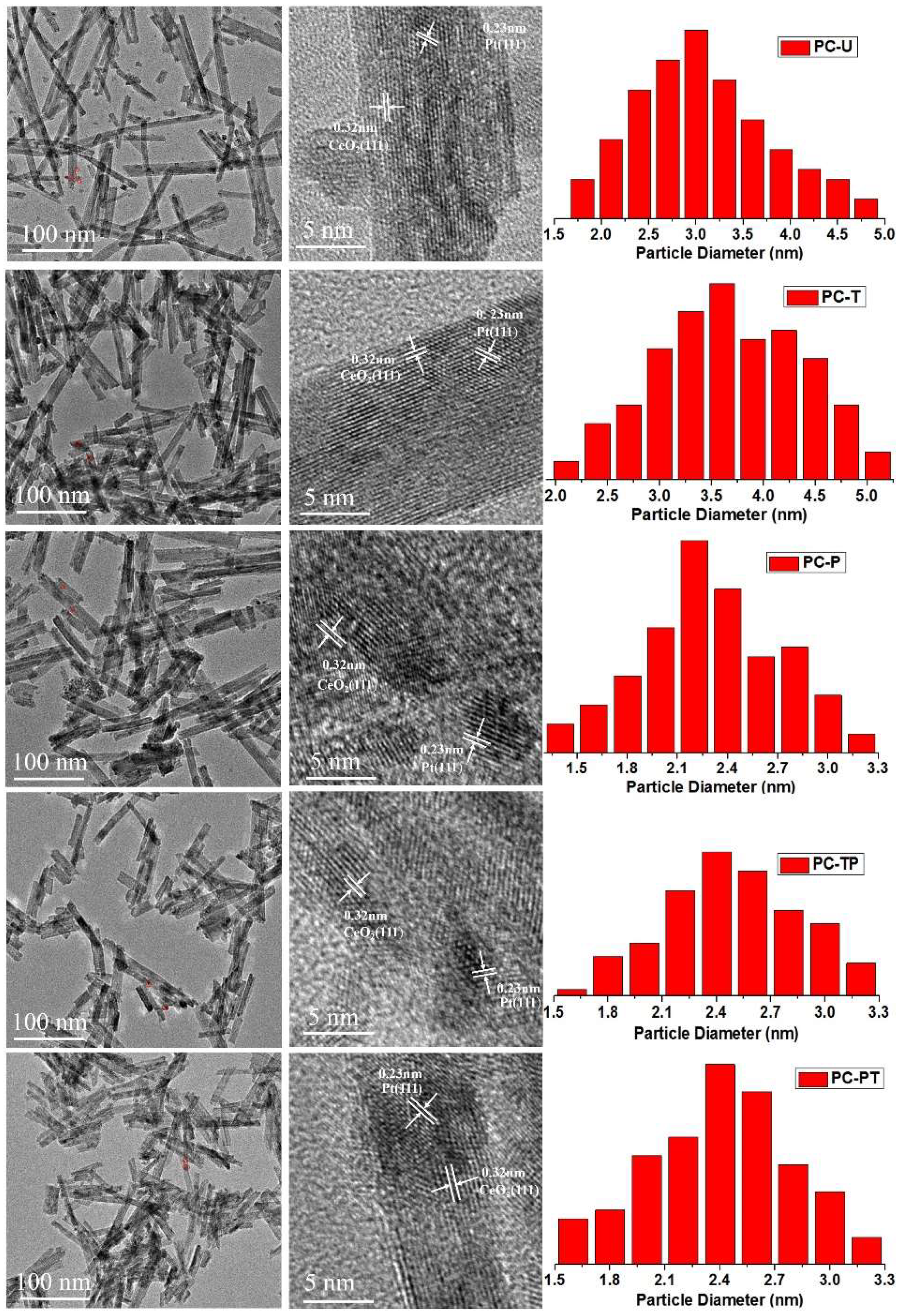
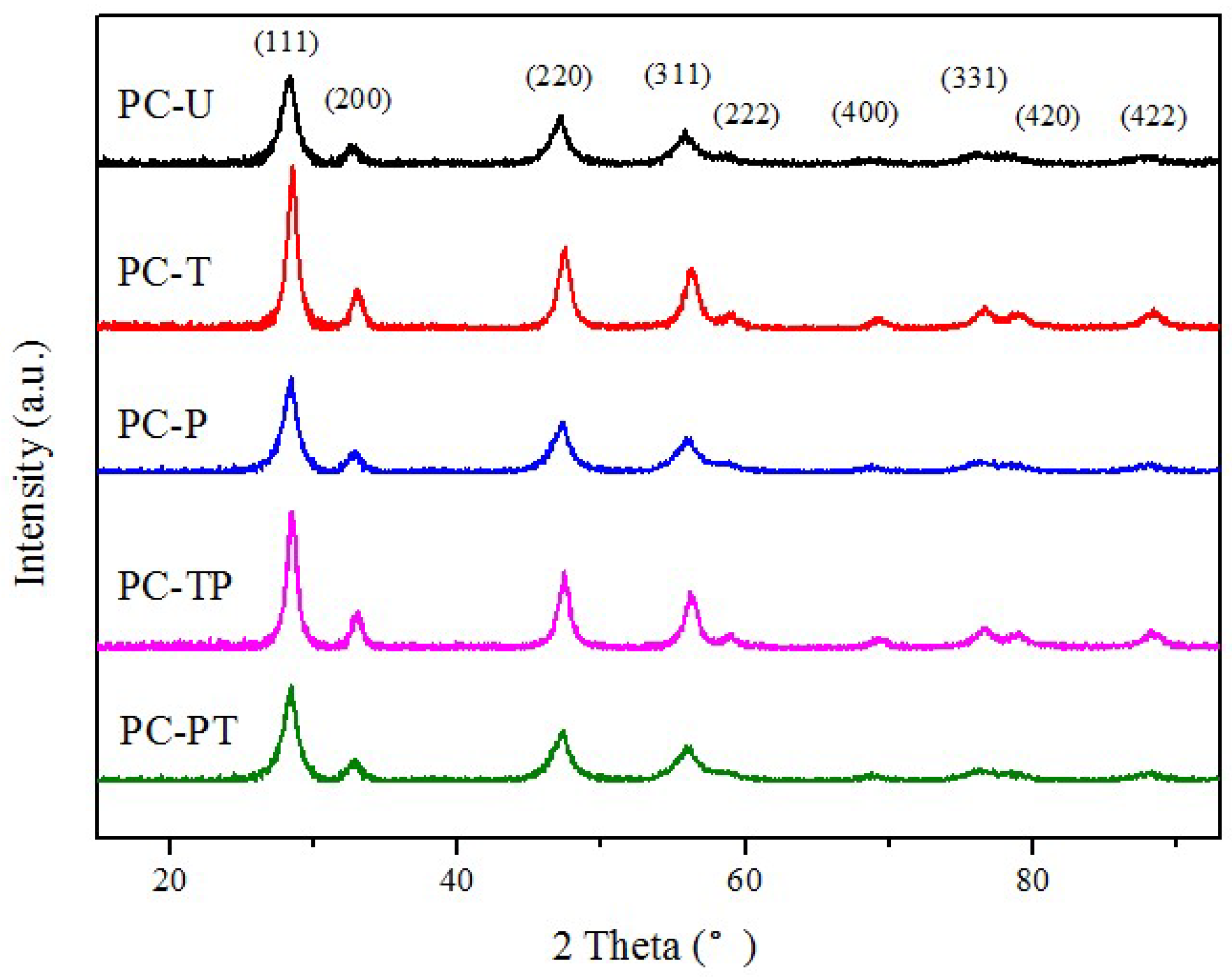
| Sample | Pt loading (%) 1 | Pt mean size(nm) | Pt dispersion (%) | SBET (m2 g−1) 4 | Crystalline size (nm) 5 | ||
|---|---|---|---|---|---|---|---|
| d1Pt 2 | d2Pt 3 | D1Pt 2 | D2Pt 3 | ||||
| PC-U | 0.81 | 2.8 | 3.2 | 40.2 | 35.2 | 90.02 | 7.0 |
| PC-T | 0.80 | 3.2 | 3.6 | 35.1 | 31.2 | 87.88 | 9.0 |
| PC-P | 0.80 | 2.3 | 2.6 | 48.9 | 45.0 | 99.31 | 6.8 |
| PC-TP | 0.80 | 2.5 | 2.8 | 45.0 | 40.2 | 95.55 | 8.0 |
| PC-PT | 0.79 | 2.4 | 2.7 | 46.9 | 41.7 | 96.94 | 7.3 |
| Sample | Pt/Ce | Pt0/(Pt0+Pt2++ Pt4+) % | Ce3+/(Ce3++Ce4+) % | Oads/(Oads+Olat) % |
|---|---|---|---|---|
| PC-U | 0.0196 | 20.13 | 20.44 | 51.07 |
| PC-T | 0.0104 | 76.37 | 22.35 | 53.69 |
| PC-P | 0.0183 | 62.50 | 26.40 | 63.78 |
| PC-TP | 0.0165 | 75.26 | 27.07 | 64.20 |
| PC-PT | 0.0162 | 75.10 | 30.30 | 70.11 |
| Sample | H2-TPR | O2-TPD | Raman spectra | OSC (μmol O g−1) | |||
|---|---|---|---|---|---|---|---|
| peak position (°C) | H2 consumption (μmol H2 g−1) | peak position (°C) | desorption amount (μmol O g−1) | ID/IF2g | OSCPt | OSCsurface | |
| CeO2 | 470 | 128 | - | - | - | - | - |
| PC-U | 138,461 | 46,94 | 119,262 | 39,104 | 1.55 | 32.8 | 40.1 |
| PC-T | 116,443 | 58,91 | 107,265 | 87,59 | 1.73 | 28.7 | 58.2 |
| PC-P | 103,429 | 128,52 | 106,249 | 124,69 | 2.49 | 40.1 | 99.3 |
| PC-TP | 101,432 | 135,48 | 106,258 | 125,72 | 2.52 | 36.9 | 102.9 |
| PC-PT | 94,414 | 182,44 | 104,254 | 161,81 | 2.82 | 38.4 | 125.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Wang, B.; Sun, Y.; Wang, X.; Fu, M.; Wu, J.; Chen, L.; Tan, Y.; Ye, D. Plasma-Assisted Surface Interactions of Pt/CeO2 Catalyst for Enhanced Toluene Catalytic Oxidation. Catalysts 2019, 9, 2. https://doi.org/10.3390/catal9010002
Chen B, Wang B, Sun Y, Wang X, Fu M, Wu J, Chen L, Tan Y, Ye D. Plasma-Assisted Surface Interactions of Pt/CeO2 Catalyst for Enhanced Toluene Catalytic Oxidation. Catalysts. 2019; 9(1):2. https://doi.org/10.3390/catal9010002
Chicago/Turabian StyleChen, Bingxu, Bangfen Wang, Yuhai Sun, Xueqin Wang, Mingli Fu, Junliang Wu, Limin Chen, Yufei Tan, and Daiqi Ye. 2019. "Plasma-Assisted Surface Interactions of Pt/CeO2 Catalyst for Enhanced Toluene Catalytic Oxidation" Catalysts 9, no. 1: 2. https://doi.org/10.3390/catal9010002
APA StyleChen, B., Wang, B., Sun, Y., Wang, X., Fu, M., Wu, J., Chen, L., Tan, Y., & Ye, D. (2019). Plasma-Assisted Surface Interactions of Pt/CeO2 Catalyst for Enhanced Toluene Catalytic Oxidation. Catalysts, 9(1), 2. https://doi.org/10.3390/catal9010002







