A Hybrid Reactor System Comprised of Non-Thermal Plasma and Mn/Natural Zeolite for the Removal of Acetaldehyde from Food Waste
Abstract
:1. Introduction
2. Results and Discussion
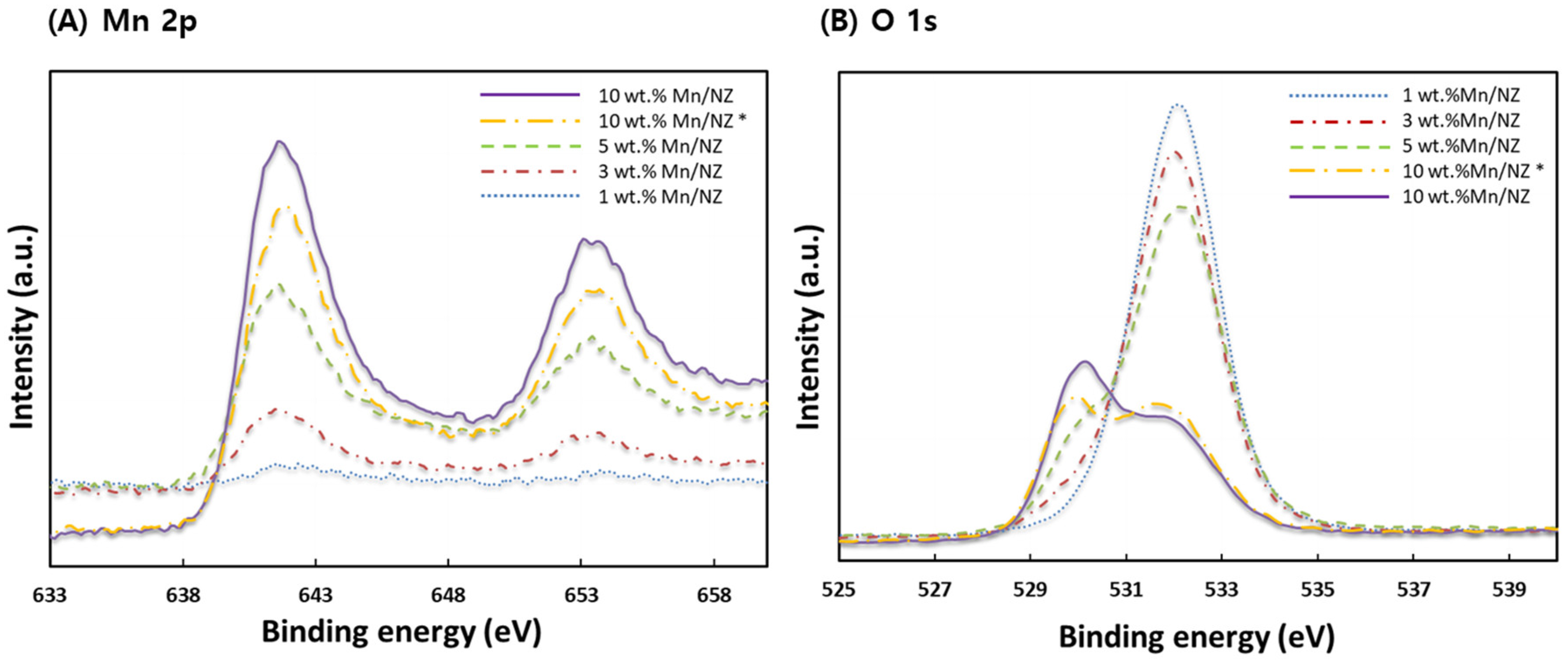
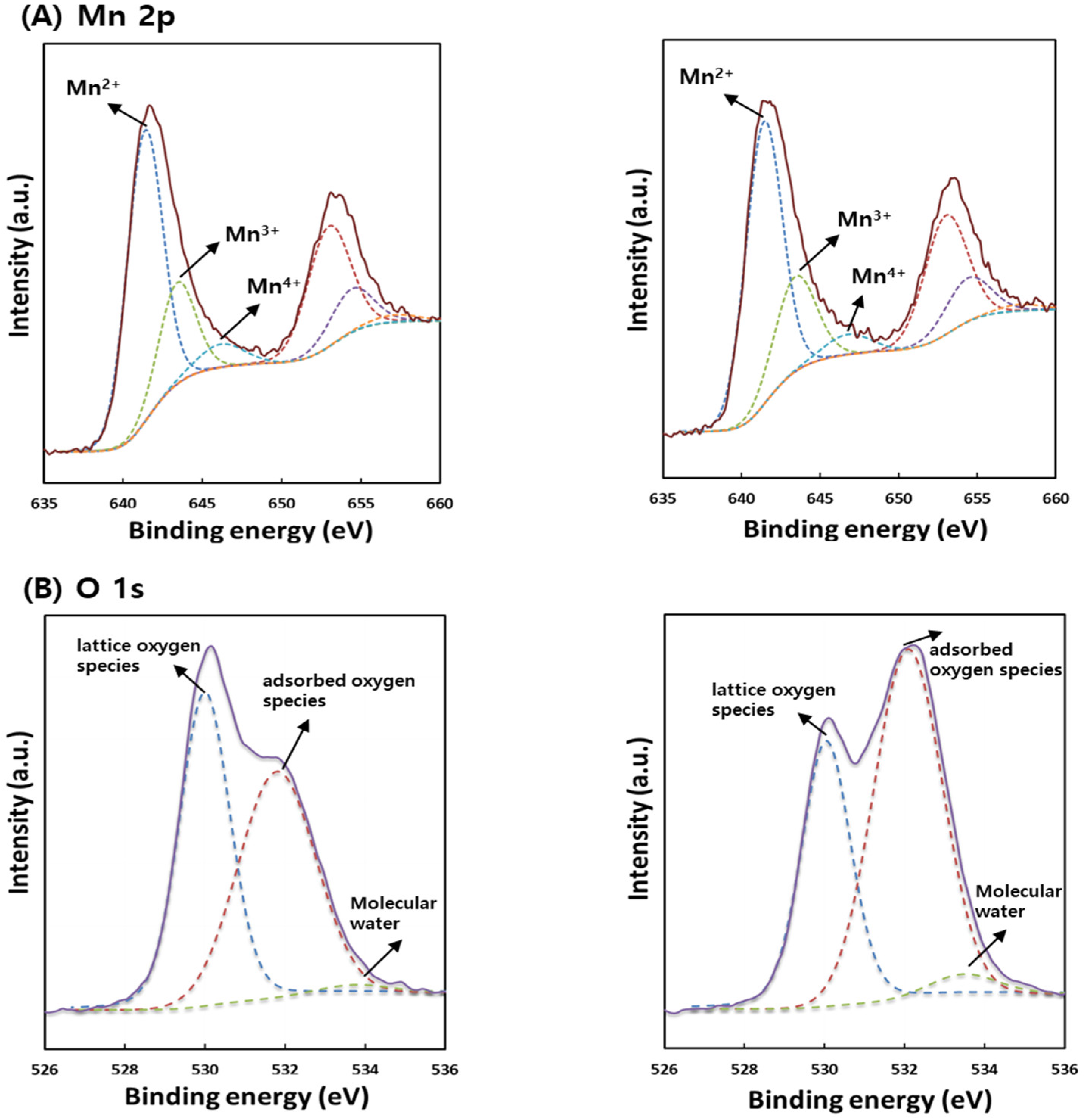
2.1. Characterization of the Catalyst
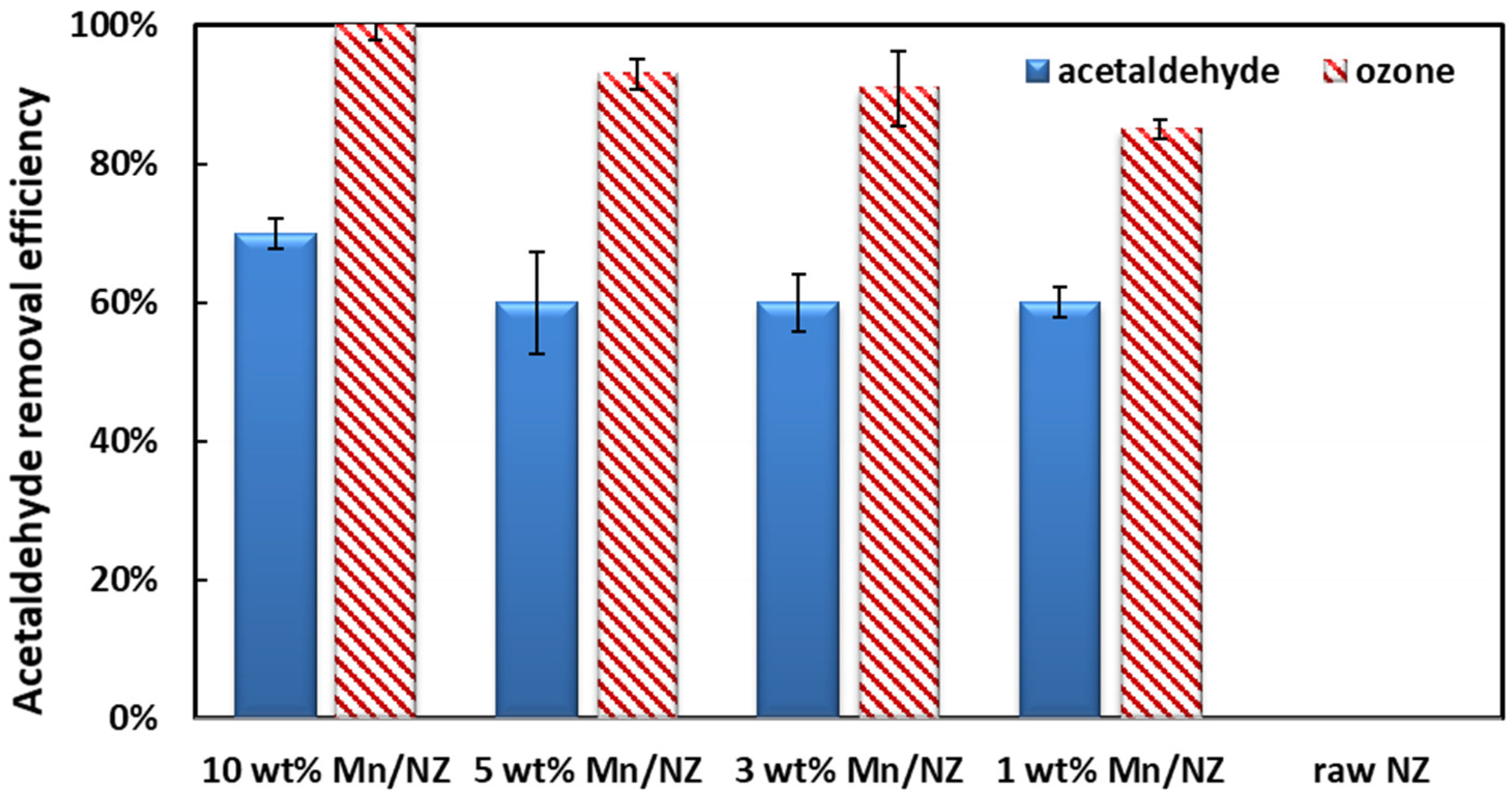
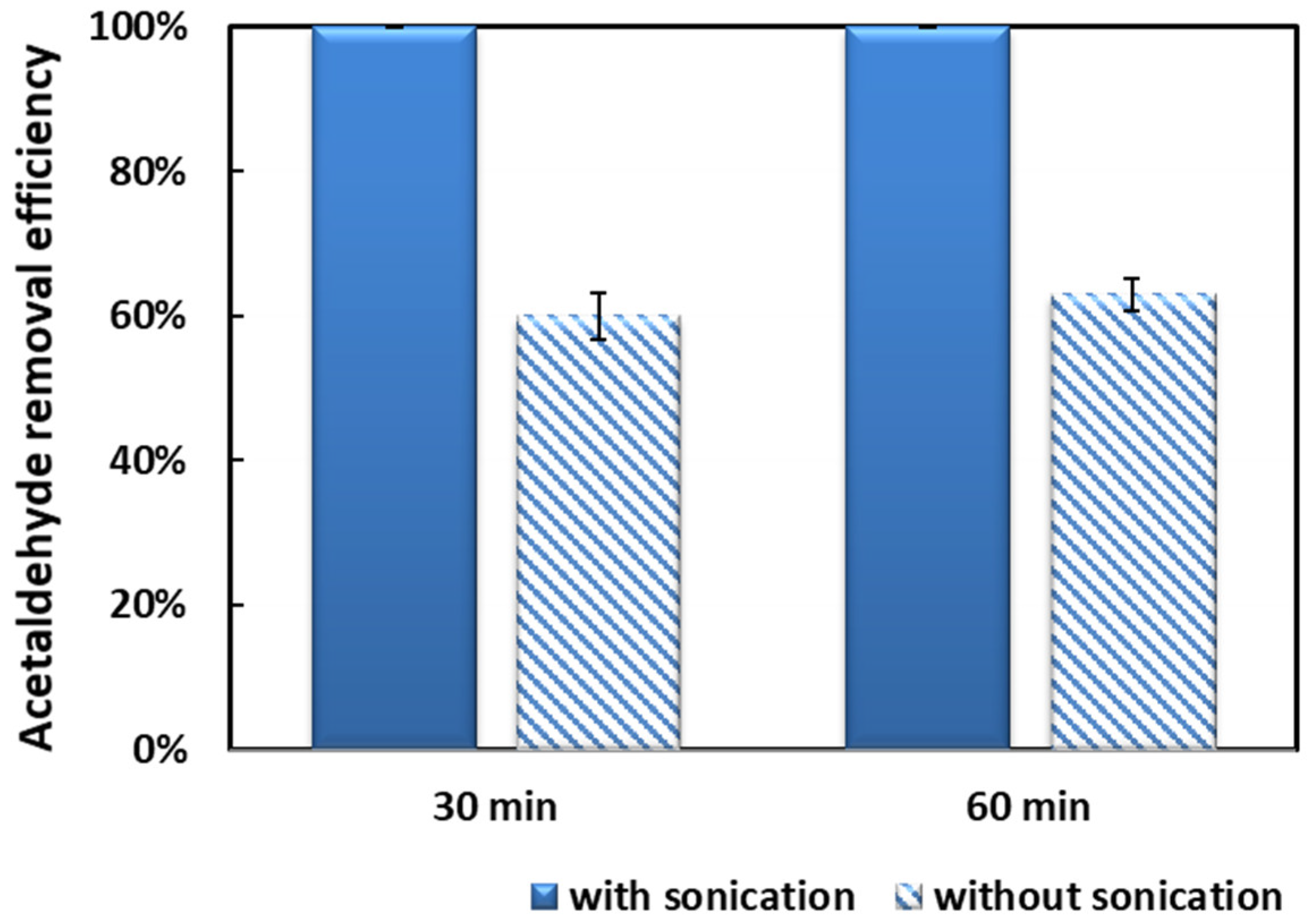
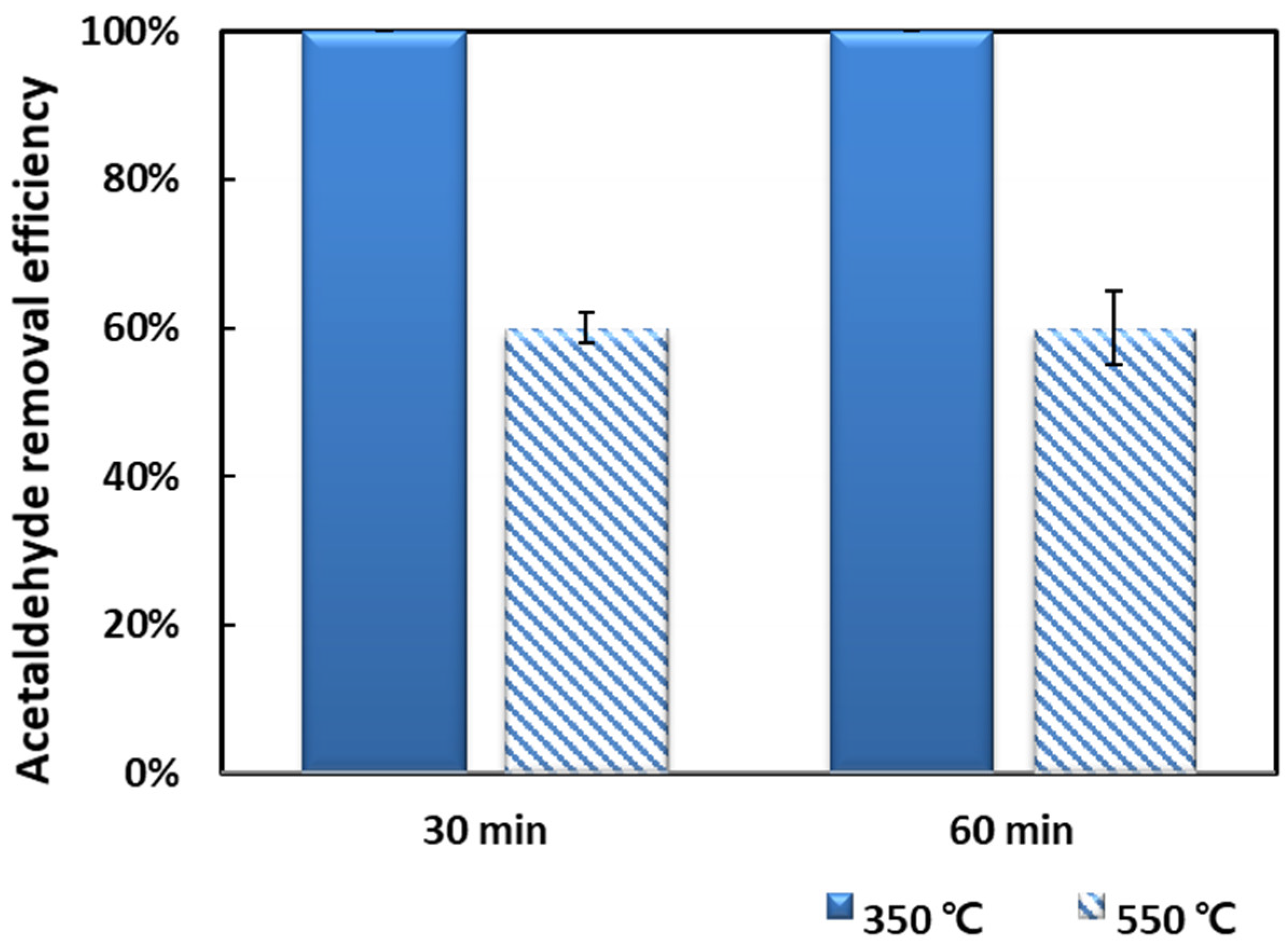
2.2. Acetaldehyde Removal Activity
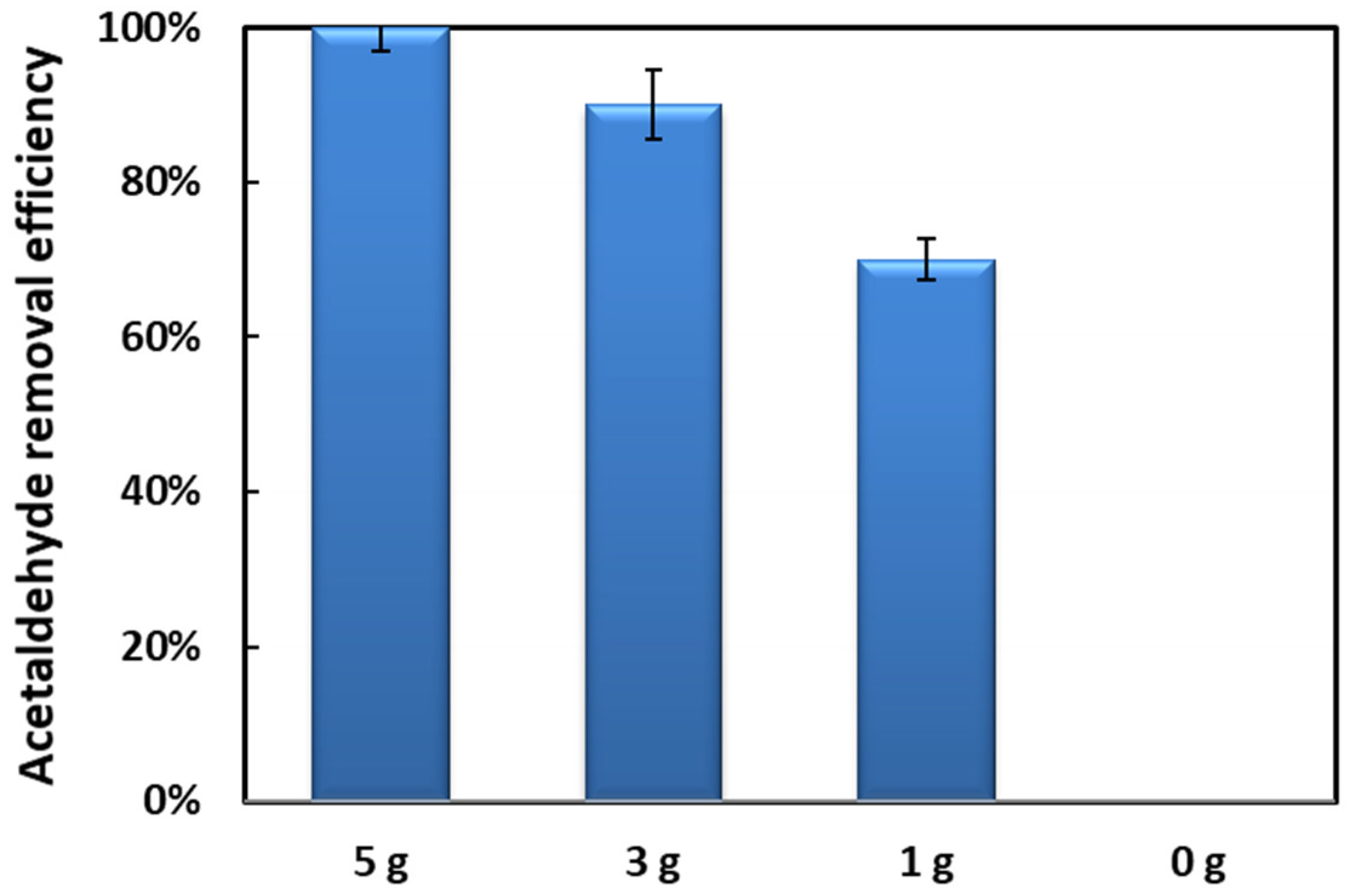
2.3. Long Term Reaction Stability Test
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
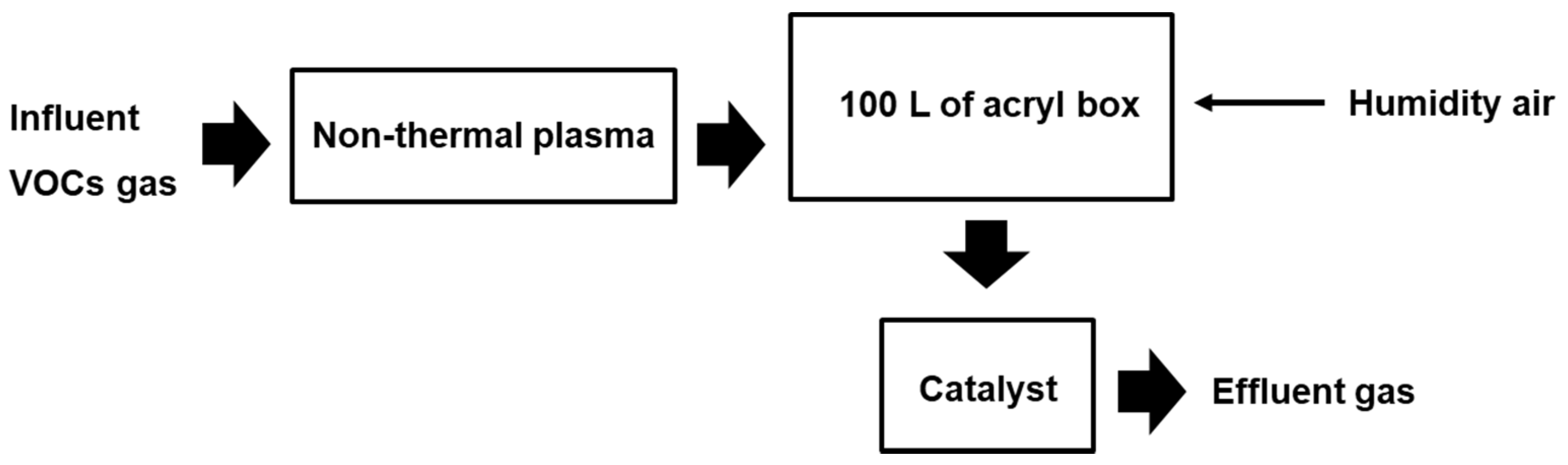
3.3. NTP/Catalyst System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Trinh, Q.H.; Mok, Y.S. Environmental plasma-catalysis for the energy-efficient treatment of volatile organic compounds. Korean J. Chem. Eng. 2016, 33, 735–748. [Google Scholar] [CrossRef]
- Kim, M.; Lalnmunsiama; Lee, S.-M.; Jin, K.-J. Preparation of natural wall paint by using sericite clay. Appl. Chem. Eng. 2017, 28, 501–505. [Google Scholar]
- Kim, M.I.; Kim, S.; Lim, C.; Seo, B. Adsorption of acetaldehyde at room temperature in a continuous system using silica synthesized by the sol-gel method. Korean J. Chem. Eng. 2017, 34, 2773–2779. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Oh, G.-Y. Behavior of toluene adsorption on activated carbon nanofibers prepared by electospinning of a polyacrylonitrile-cellulose acetate blending solution. Korean J. Chem. Eng. 2017, 34, 2731–2737. [Google Scholar] [CrossRef]
- Jeon, M.J.; Park, S.-H.; Lee, H.-D.; Jeon, Y.-W. Practical study of low temperature vacuum swing adsorption process for VOCs removal. Appl. Chem. Eng. 2017, 28, 332–338. [Google Scholar]
- Lee, H.; Park, R.; Lee, H.W.; Hong, Y.; Lee, Y.; Park, S.H.; Jung, S.-C.; Yoo, K.-S.; Jeon, J.-K.; Park, Y.-K. Adsorptive removal of atmospheric pollutants over Pyropia tenera chars. Carbon Lett. 2016, 19, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Balahanigaimani, M.S.; Shim, W.G.; Kim, S.C. Biomass-based carbon materials for energy storage and environmental applications. Appl. Chem. Eng. 2017, 28, 8–16. [Google Scholar]
- Zhu, X.; Yu, J.; Jiang, C.; Chen, B. Enhanced room temperature HCHO decomposition activity of highly-dispersed Pt/Al2O3 hierarchical microsphere with exposed facets. J. Ind. Eng. Chem. 2017, 45, 197–205. [Google Scholar] [CrossRef]
- Kuo, H.-P.; Yao, S.-W.; Huang, A.-N.; Hsu, W.-Y. Photocatalytic degradation of toluene in a staged fluidized bed reactor using TiO2/silica gel. Korean J. Chem. Eng. 2017, 34, 73–80. [Google Scholar] [CrossRef]
- Rangkooy, H.A.; Pour, M.N.; Dehagih, B.F. Efficiency evaluation of the photocatalytic degradation of zinc oxide nanoparicles immobilized on modified zeolites in the removal of styrene vapor from air. Korean J. Chem. Eng. 2017, 34, 3142–3149. [Google Scholar] [CrossRef]
- Li, Y.Z.; Fan, Z.Y.; Shi, J.W.; Liu, Z.Y.; Shangguan, W.F. Post plasma-catalysis for VOCs degradation over different phase structure MnO2 catalysts. Chem. Eng. J. 2014, 241, 251–258. [Google Scholar] [CrossRef]
- Dang, X.; Qin, C.; Huang, J.; Teng, J.; Huang, X. Adsorbed benzen/toluene oxidation using plasm driven catalysis with gas circulation: Elimination of the byproducts. J. Ind. Eng. Chem. 2017, 37, 366–371. [Google Scholar] [CrossRef]
- Pan, K.L.; Chen, D.I.; Pan, G.T.; Chong, S.; Chang, M.B. Removal of phenol from gas streams via combined plasma catalysis. J. Ind. Eng. Chem. 2017, 52, 108–120. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Renken, A.; Kiwi-Minsker, L. Novel catalytic non-thermal plasma reactor for the abatement of VOCs. Chem. Eng. J. 2007, 134, 78–83. [Google Scholar] [CrossRef]
- Marotta, E.; Callea, A.; Rea, M.; Paradisi, C. DC corona electric discharges for air pollution control. Part 1. Efficiency and products of hydrocarbon processing. Environ. Sci. Technol. 2007, 41, 5862–5868. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, Y.; Kim, H.H.; Nobuaki, N.; Atsushi, O. The Role of Ozone in the Reaction Mechanism of a Bare Zeolite-Plasma Hybrid System. Catalysts 2015, 5, 838–850. [Google Scholar] [CrossRef] [Green Version]
- An, H.T.Q.; Huu, T.P.; Le Van, T.; Cormier, J.M.; Khacef, A. Application of atmospheric non thermal plasma-catalysis hybrid system for air pollution control: Toluene removal. Catal. Today 2011, 176, 474–477. [Google Scholar]
- Tang, X.J.; Feng, F.D.; Ye, L.L.; Zhang, X.M.; Huang, Y.F.; Liu, Z.; Yan, K.P. Removal of dilute VOCs in air by post-plasma catalysis over Ag-based composite oxide catalysts. Catal. Today 2013, 211, 39–43. [Google Scholar] [CrossRef]
- Delagrange, S.; Pinard, L.; Tatibouet, J.M. Combination of a non-thermal plasma and a catalyst for toluene removal from air: Manganese based oxide catalysts. Appl. Catal. B Environ. 2006, 68, 92–98. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S. Oxidation behavior of cyclohexane on alumina-supported manganese oxides with ozone. Appl. Catal. B Environ. 2005, 60, 49–55. [Google Scholar] [CrossRef]
- Yosefi, L.; Haghighi, M.; Allahyaria, S.; Ashkriz, S. The beneficial use of HCl-activated naturalzeolite in ultrasound assisted synthesis of Cu/clinoptilolite–CeO2 nanocatalyst used forcatalytic oxidation of diluted toluene in air atlow temperature. J. Chem. Technol. Biotechnol. 2015, 90, 765–774. [Google Scholar] [CrossRef]
- Soylu, G.S.P.; Özçelik, Z.; Boz, İ. Total oxidation of toluene over metal oxides supported on a natural clinoptilolite-type zeolite. Chem. Eng. J. 2010, 162, 380–387. [Google Scholar] [CrossRef]
- Park, E.; Kim, M.; Jung, H.; Chin, S.; Jurng, J. Effect of Sulfur on Mn/Ti Catalysts Prepared Using Chemical Vapor Condensation (CVC) for Low-Temperature NO Reduction. ACS Catal. 2013, 3, 1518–1525. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Tang, W.X.; Wu, X.F.; Li, D.Y.; Wang, Z.; Liu, G.; Liu, H.D.; Chen, Y.F. Oxalate route for promoting activity of manganese oxide catalysts in total VOCs’ oxidation: Effect of calcination temperature and preparation method. J. Mater. Chem. A 2014, 2, 2544–2554. [Google Scholar] [CrossRef]
- Pena, D.A.; Uphade, B.S.; Smirniotis, P.G. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3I. Evaluation and characterization of first row transition metals. J. Catal. 2004, 221, 421–431. [Google Scholar] [CrossRef]
- Xu, X.X.; Wu, J.L.; Xu, W.C.; He, M.L.; Fu, M.L.; Chen, L.M.; Zhu, A.M.; Ye, D.Q. High-efficiency non-thermal plasma-catalysis of cobalt incorporated mesoporous MCM-41 for toluene removal. Catal. Today 2017, 281, 527–533. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of Carbon Nanotubes: Mixing, Sonication, Stabilization, and Composite Properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Jacobs, O.; Wu, W.; Rudiger, G.; Schadel, B. Effect of dispersion method on tribological properties of carbon nanotube reinforced epoxy resin composites. Polym. Test 2007, 26, 351–360. [Google Scholar] [CrossRef]
- Jung, S.C.; Park, Y.K.; Jung, H.Y.; Kang, U.I.; Nah, J.W.; Kim, S.C. Effects of calcination and support on supported manganese catalysts for the catalytic oxidation of toluene as a model of VOCs. Res. Chem. Intermed. 2016, 42, 185–199. [Google Scholar] [CrossRef]
- Tian, W.; Yang, H.; Fan, X.; Zhan, X. Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature. J. Hazard. Mater. 2011, 188, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Kim, J.W.; Kim, J.N.; Jurng, J.; Bae, G.N.; Jeon, J.K.; Park, Y.K. Effect of calcination temperature on the oxidation of benzene with ozone at low temperature over mesoporous α-Mn2O3. Powder Technol. 2011, 214, 458–462. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Demeestere, K.; Leys, C.; Van Langenhove, H. Post-plasma catalytic technology for the removal of toluene from indoor air: Effect of humidity. Appl. Catal. B Environ. 2009, 87, 78–83. [Google Scholar] [CrossRef]
- Vu, V.H.; Belkouch, J.; Ould-Dris, A.; Taouk, B. Removal of hazardous chlorinated VOCs over Mn-Cu mixed oxide based catalyst. J. Hazard. Mater. 2009, 169, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, M.F.; Wei, Z.B.; Xin, Q.; Ying, P.L.; Li, C. Catalytic oxidation of chlorobenzene on supported manganese oxide catalysts. Appl. Catal. B Environ. 2001, 29, 61–67. [Google Scholar] [CrossRef] [Green Version]

| Catalysts | BET Surface (m2·g−1) | Surface Atomic Concentration (%) | ||||
|---|---|---|---|---|---|---|
| O | Al | Si | Ca | Mn | ||
| raw NZ | 134.8 | 65.3 | 4.54 | 22.32 | 0.65 | 0 |
| 1 wt.% Mn/NZ | 29.3 | 65.6 | 4.80 | 21.96 | 0.67 | 1.04 |
| 3 wt.% Mn/NZ | 25.2 | 64.22 | 4.23 | 19.86 | 0.75 | 3.10 |
| 5 wt.% Mn/NZ | 21.4 | 62.15 | 3.49 | 17.76 | 0.84 | 7.37 |
| 10 wt.% Mn/NZ | 20.9 | 55.73 | 1.47 | 7.72 | 1.05 | 20.14 |
| 10 wt.% Mn/NZ (550 °C) | - | 59.71 | 2.77 | 13.95 | 0.85 | 14.61 |
| 10 wt.% Mn/NZ without sonication | 19.1 | 54.65 | 1.49 | 7.06 | 2.87 | 17.09 |
| Catalyst | NZ (g) | Mn(CH3COOH)2·4H2O (g) |
|---|---|---|
| 1 wt.% Mn/NZ | 5 | 0.228 |
| 3 wt.% Mn/NZ | 5 | 0.691 |
| 5 wt.% Mn/NZ | 5 | 1.173 |
| 10 wt.% Mn/NZ | 5 | 2.480 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.Y.; Ryu, H.W.; Jung, S.-C.; Song, J.; Kim, B.-J.; Park, Y.-K. A Hybrid Reactor System Comprised of Non-Thermal Plasma and Mn/Natural Zeolite for the Removal of Acetaldehyde from Food Waste. Catalysts 2018, 8, 389. https://doi.org/10.3390/catal8090389
Song MY, Ryu HW, Jung S-C, Song J, Kim B-J, Park Y-K. A Hybrid Reactor System Comprised of Non-Thermal Plasma and Mn/Natural Zeolite for the Removal of Acetaldehyde from Food Waste. Catalysts. 2018; 8(9):389. https://doi.org/10.3390/catal8090389
Chicago/Turabian StyleSong, Min Young, Hae Won Ryu, Sang-Chul Jung, JiHyeon Song, Byung-Joo Kim, and Young-Kwon Park. 2018. "A Hybrid Reactor System Comprised of Non-Thermal Plasma and Mn/Natural Zeolite for the Removal of Acetaldehyde from Food Waste" Catalysts 8, no. 9: 389. https://doi.org/10.3390/catal8090389
APA StyleSong, M. Y., Ryu, H. W., Jung, S.-C., Song, J., Kim, B.-J., & Park, Y.-K. (2018). A Hybrid Reactor System Comprised of Non-Thermal Plasma and Mn/Natural Zeolite for the Removal of Acetaldehyde from Food Waste. Catalysts, 8(9), 389. https://doi.org/10.3390/catal8090389







