Abstract
Fulfilling the direct inert C–H bond functionalization of raw materials that are earth-abundant and commercially available for the synthesis of diverse targeted organic compounds is very desirable and its implementation would mean a great reduction of the synthetic steps required for substrate prefunctionalization such as halogenation, borylation, and metalation. Successful C–H bond functionalization mainly resorts to homogeneous transition-metal catalysis, albeit sometimes suffering from poor catalyst reusability, nontrivial separation, and severe biotoxicity. TiO2 photocatalysis displays multifaceted advantages, such as strong oxidizing ability, high chemical stability and photostability, excellent reusability, and low biotoxicity. The chemical reactions started and delivered by TiO2 photocatalysts are well known to be widely used in photocatalytic water-splitting, organic pollutant degradation, and dye-sensitized solar cells. Recently, TiO2 photocatalysis has been demonstrated to possess the unanticipated ability to trigger the transformation of inert C–H bonds for C–C, C–N, C–O, and C–X bond formation under ultraviolet light, sunlight, and even visible-light irradiation at room temperature. A few important organic products, traditionally synthesized in harsh reaction conditions and with specially functionalized group substrates, are continuously reported to be realized by TiO2 photocatalysis with simple starting materials under very mild conditions. This prominent advantage—the capability of utilizing cheap and readily available compounds for highly selective synthesis without prefunctionalized reactants such as organic halides, boronates, silanes, etc.—is attributed to the overwhelmingly powerful photo-induced hole reactivity of TiO2 photocatalysis, which does not require an elevated reaction temperature as in conventional transition-metal catalysis. Such a reaction mechanism, under typically mild conditions, is apparently different from traditional transition-metal catalysis and beyond our insights into the driving forces that transform the C–H bond for C–C bond coupling reactions. This review gives a summary of the recent progress of TiO2 photocatalytic C–H bond activation for C–C coupling reactions and discusses some model examples, especially under visible-light irradiation.
1. Introduction
In the last decade, we have witnessed a surge of catalytic methodologies for direct functionalization of the inactive C–H bonds of organic molecules to serve different purposes [1,2,3,4,5,6,7,8,9,10,11]. Compared with traditional transition-metal catalyzed cross-coupling reactions commonly start with substrates with indispensable leaving groups such as Cl, Br, I, alkylsilyl, organometallic, and carboxylates [12,13,14], this approach provides multiple advantages for synthetic chemistry. The biggest advantage is that direct C–H bond functionalization can bypass the nontrivial prefunctionalization of substrates such as the preparation of toxic halogenated and hazardous organometallic compounds, greatly simplifying the workup procedure and expanding the range of available substrates. However, because of the well-known large bond dissociation energy and poor selectivity, currently most C–H bond direct functionalization strategies mainly rely on homogeneous transition-metal catalysis with the assistance of complex ligands or auxiliaries, and these strategies are often conducted in the presence of a stoichiometric amount of oxidants such as benzoquinone and the Ag (I) and Cu (II) salts [2,4,11]. Despite this, homogeneous transition-metal catalysis has garnered considerable success in C–H functionalization, though the nontrivial catalyst separation and difficultly in recycling are still inherent drawbacks. Moreover, trace amounts of transition-metal residues in purified products pose a challenge to the wide application of said products in the pharmaceutical industry [15]. From this perspective, heterogeneous catalytic C–H bond transformation reactions are more desirable [16]. Among these reactions, TiO2 photocatalysis, which has been thoroughly explored since the 1970s for its use in water-splitting, dye-sensitized solar cells (DSSCs), and air and water decontamination [17,18,19,20,21], was considered to be a promising choice for use in inert C–H bond functionalization. On the one hand, due to its extremely high stability to light, oxidants, reductants, and strong acidic and basic conditions, TiO2 photocatalysts can be reused and recycled many times using only a simple centrifugation procedure without any apparent loss of catalytic activity [22]. On the other hand, and maybe more importantly, the photogenerated holes on TiO2 nanoparticles show such remarkably high activity in reaction to organic molecules that they can nearly activate any inert C–H, C–C, and C–X bond in term of its oxidizing potential. Figure 1 shows, in principle, the activation of organic substrates by TiO2 photocatalysis. Under ultraviolet (UV) (wavelength λ < 387 nm, or ultraviolet component of sunlight) irradiation (Figure 1a), charge separation occurs on the TiO2 nanoparticles, generating holes in the valence band and electrons in the conduction band. The TiO2 photo-induced valence band hole (h+vb) possesses strong oxidativity (E1/2 = 2.7 V vs. normal hydrogen electrode), which drives the interfacial oxidative cleavage of the inert C–H bonds of organic molecules through the single electron transfer (SET), proton transfer (PT), or proton-coupled electron transfer (PCET) pathway [23,24,25,26,27]. In this case, unlike the pattern of homogenous transition-metal catalysis (TMC), only the substrates that are readily adsorbed while on the surface of TiO2 nanocrystal catalyst can be functionalized, since photo-induced holes cannot diffuse into bulk solutions.
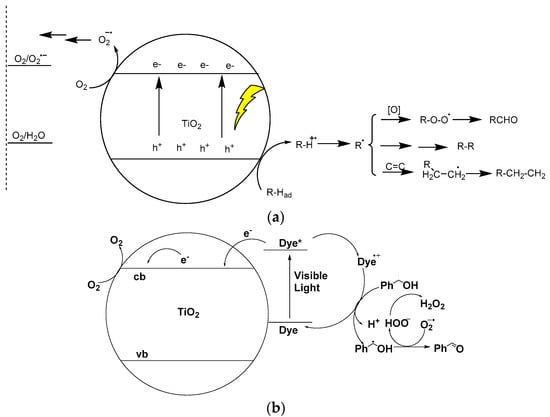
Figure 1.
Activation of organic compound C–H bonds through TiO2 photocatalysis under (a) ultraviolet (UV) and (b) visible-light irradiation. (a) C–H functionalization reactions of generic hydrocarbons started by TiO2 photocatalyst under UV light excitation; (b) C–H functionalization reactions of generic hydrocarbons started by TiO2/dye-sensitization mechanism under visible-light irradiation. Adapted with permission from [47]. Copyright 2014, ACS.
On the other hand, TiO2 photo-induced conductive-band electrons (e−cb) have moderate reductivity (E1/2 = −0.5 V vs. normal hydrogen electrode), which is just suitable enough to utilize molecular dioxygen as an electron acceptor, yielding superoxide radical anion, a hydroperoxide radical, hydrogen peroxide, and H2O successively to fulfill the catalytic cycle [28,29,30,31,32,33,34,35,36]. In this case, some of these reactive oxygen species (ROS) can directly react with organic substrates or indirectly react with the intermediates of the catalytic cycle. Direct reactions of ROS with substrates are generally not selective and render organic substrates as partial or complete mineralization. Therefore, for the purpose of organic synthesis, these side reactions originating from ROS should be avoided by controlling the reaction conditions. For example, in order to eliminate any side reactions of ROS, the reaction must require anaerobic conditions. In this case, conduction band electrons could transfer to some other electron acceptor, such as a proton, benzoquinone, Ag+, or an organic sacrificial agent with concomitant formation of an H2, hydroquinone, or Ag precipitation, to finish the catalytic cycle. In some cases, the C–H bond cleavages are assisted by the direct oxygen atom transfer that is driven synergistically by the TiO2 photogenerated h+vb and e−cb, which yields a stable target product [31,33]. Due to the very high redox potential of TiO2 photo-induced h+vb and ROS, it still remains a challenge to tame these reactive oxidants for highly selective organic transformations [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Regarding this purpose, visible light is used as an irradiation source that significantly increases the selectivity of the desired reactions via a sensitization mechanism different from the case in which the TiO2 catalyst itself is directly excited as UV light (see Figure 1b). In this process, the primary nonselective oxidations of the photo-induced h+vb are totally excluded, and instead, the adsorbed or self-formed sensitizers (common organic dyes) absorb the visible light and become excited, injecting the electron into the TiO2 conduction band and generating a dye radical cation that is left behind. The radical cations perform the C–H bond functionalization task. Recently, by consistently fine-tuning the TiO2 photocatalysts, especially for visible-light excitation and reaction parameter optimization, the following delicate examples of challenging organic synthesis photocatalyzed by TiO2 have appeared: aerobic oxidation of alcohol to aldehydes [31,35,37,51,59,60,61,62,63,64,65,66,67,68,69], aerobic oxidative condensation of benzylamines to imines [30,36,49,70], olefin epoxidation [71,72], C–H arylation with aryl diazonium compounds [73,74], desilylative Michael addition between benzyltrimethylsilane and electron-poor olefins [75,76,77,78], pyridine anti-Markovnikoff addition to diphenylethlyene [38], 4-aryltetralone synthesis by styrene [2 + 2 + 2] cyclo-addition [79,80], and amine alkylation with alcohol as an alkylating reagent [81,82,83,84,85]. These reports successfully fulfilled the organic transformations with high yield, excellent selectivity, mild conditions, and wide substrate scope. Corresponding to this new field, a number of excellent reviews have focused on TiO2 photocatalysis in organic synthesis [39,40,42,43,47,50,52,56,57,63,86,87,88]. However, the incredible potential of C–H bond functionalization by TiO2 photocatalysis for C–C coupling reactions has not been fully tapped yet. In this review, we concentrate on discussing and analyzing the state-of-the-art examples of TiO2 photocatalysis with a focus on C–H bond functionalization for highly selective C–C bond formations.
2. C–H Activation Available to Convert Simple Molecules into Desired Complex C–C Coupling Products
The ultimate aim of organic synthetic chemistry has always been to develop appropriate methods with the least synthetic steps, mildest conditions, most easily available substrates, and excellent yields. A promising strategy to realize this aim is to selectively functionalize, aiming C–H bonds in the substrate chain moiety or cyclic skeleton and placing a desired functional group or branch chain in it. For this purpose, many famous catalytic reactions were developed, such as Friedel–Crafts [89,90,91] and Heck [92,93] reactions. In the conventional Friedel–Crafts reaction, AlCl3 Lewis acid catalyst was applied to catalyze electrophilic C–H alkylation or acylation between alkyl halides, acyl halides, and arenes. This transformation realized the aromatic ring sp2 C–H activation and further set the required alkyl or acyl functional group on the desired position [94,95,96,97]. In the Heck reaction, aryl halides or pseudo-halides and alkenes are efficiently coupled by Pd (0) catalysts. Pd (0) catalyst was suitable for oxidative addition of aryl halides and coordination with alkenyl C=C bonds. The pivotal carbopalladation step realized C–H transformation, while the final reductive elimination released the C–C coupling product and regenerated the Pd (0) catalyst [98] (see Figure 2). The key to this successful transformation was that the ligands around the Pd center accurately tuned its electronic and steric environment during the catalytic cycle. The electron-rich and sterically congested phosphine or N-heterocyclic carbene (NHC) ligands facilitated both the oxidative addition and reductive elimination steps. These transition-metal catalyzed C–H functionalization reactions greatly enlarged the scope of organic synthesis, simplifying the redundant prefunctionalization procedures. However, these important catalytic reactions based on AlCl3 and noble-metal catalysts are either environmentally unfriendly or economically costly. Therefore, to develop more green and sustainable methods, non–noble metal catalyzed C–H functionalization has always been a meaningful subject in the advanced organic synthesis field.
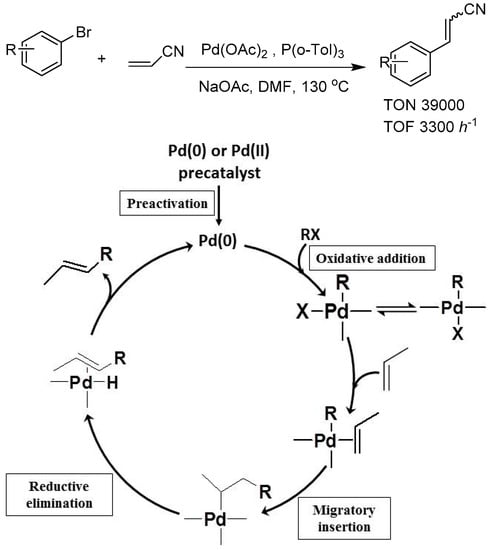
Figure 2.
Scheme of Pd-catalyzed Heck reaction and its catalytic cycle. Adapted with permission from [98]. Copyright 2000, ACS.
TiO2 photocatalysis is receiving attention in the field of C–H bond functionalization not only because of its intrinsic robust redox catalytic activity, but also its unconventional C–H transformation modes. Although some unsystematized and discrete examples could not demonstrate similarly wide substrate scope with Friedel–Crafts and Heck reactions, as they lacked the general strategy and approach for chiral asymmetric C–H functionalization, two points deserve to be highlighted: (i) the cross-dehydrogenative coupling (CDC) reaction could be realized between two parent C–H bonds without any halogenated one, and (ii) some inert C–H bonds that were difficult to be activated in noble-metal catalysis could be efficiently functionalized. These two points demonstrate that TiO2 photocatalytic C–H functionalization does not proceed via a similar pathway as traditional homogeneous noble-metal catalysis or zeolite solid catalysis in high temperature and pressure with acid-base catalysis sites. From the view of TiO2 photocatalysis, the reaction pathways of organic molecules are not limited to the well-known localized valence band h+vb oxidation and conduction band e−cb reduction pathways [36]. In some examples it must couple multiple intermediates generated in the TiO2 interface to realize very inert C–H bond functionalization. Obviously, homogeneous noble-metal catalysis sometimes does not possess this property. Instead, noble-metal catalysis generally has to resort to strong acids, bases, oxidative or reductive co-catalysts, and additives to realize such inert C–H bond activation and functionalization [99,100]. In comparison with AlCl3-based Friedel–Crafts alkylation or acylation, TiO2 photocatalysis has more promise in arene C–H functionalization, since AlCl3 is a too-strong Lewis acid that has narrower functional group tolerability toward acid-sensitive group on aromatic ring than TiO2 photocatalysis. Moreover, since the much stronger coordinating ability and the higher electrophilic property of AlCl3, in AlCl3-catalyzed Friedel–Crafts alkylation, it is very difficult to control the regioselectivity over multisite alkylation. Because of this uniqueness, TiO2 photocatalysis has received considerable attention in initiating C–H transformation for constructing C–C bonds in different target organic compounds. It can be anticipated that TiO2 photocatalysis will realize more challenging C–H activation for C–C bond or other C–X bond formation reactions if its generality is established. With deeper insight into the functionalization mechanisms, one can design and optimize TiO2 photocatalysts to initiate reactions in the most economic and suitable fashion only by irradiating to activate them, unlike conventional transition-metal catalysis with heating of the solvent, substrates, and products.
This review centers on the theme of C–H functionalization and comments on the current accomplishment of TiO2 photocatalytic C–C bond coupling examples. Although there is currently no unified agreement about the reaction mechanism of every example, the facts of every model example are still very concrete and the corresponding mechanism is still very important. Thus, our review is different from most published critical and tutorial reviews in this field. First, we introduce the reaction and its main unique properties. Then the mechanism provided by the original authors is presented. More importantly, we comment on the intrinsic property of the TiO2 photocatalyzed reaction based on the original text’s evidence. The mechanisms and reaction pathways are emphasized and discussed in comparison with traditional homogenous catalysis as much as possible. These discussions and comparisons may contribute to the success of other C–H bond functionalization for new C–C target compound synthesis via mild TiO2 photocatalysis even directly using visible light or sunlight.
2.1. Activating C–H Bond to React with C=C or C–X Bond for C–C Coupling Reactions
The most common pathways of C–H bond functionalization for C–C coupling reactions are through photo-induced h+vb oxidizing the adsorbed organic substrates R–H along the single-electron transfer (SET) scheme, yielding intermediate high-energy R–H+• radical cation in an energetically uphill pathway. The unstable R–H+• experiences a spontaneous deprotonation event, generating carbon-centered radical R•. The reactive radical attacks the C=C bond of another substrate in either an electrophilic or nucleophilic manner, bringing out a new adduct radical species, R–C–C•. Then this new radical accepts conduction band e−cb, yielding carbanion species R–C–C−, and the proton transfer generates the final functionalized C–C coupling R–C–CH compound (see Figure 3). Gaining satisfactory selectivity from this kind of reaction initiated through h+vb oxidation is very challenging, especially in aqueous solution in which OH• free radical formation from solvent H2O oxidation often results in nonselective attacking of substrate [101]. In fact, the organic pollutant decomposition reactions in water usually proceed through this mechanism to deep mineralization [102]. To obtain synthetically useful C–H functionalization, the designed TiO2 photocatalytic process must avoid this kind of aqueous decomposition pathway.
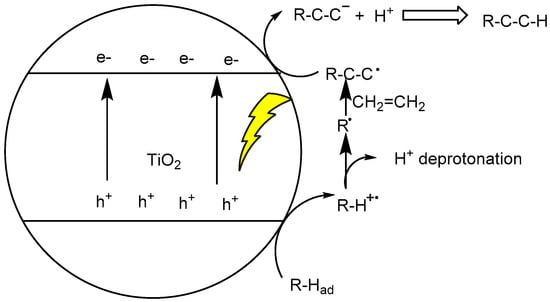
Figure 3.
TiO2 photocatalytic activation of C–H bond for addition reaction with C=C olefinic bond.
Rueping et al. reported that heterogeneous TiO2 photocatalyzed C–H arylation of heteroarenes with aryldiazonium salts [73]. In their investigations, they discovered that this transformation could be successfully accomplished even under visible-light irradiation. By further mechanistic study with UV-Vis spectra, they illustrated that the red shift of the TiO2 catalyst absorption spectrum to the visible-light region was mainly due to the formation of TiO2-diazoether surface species. This surface species was proposed to absorb visible light and initiate the C–H bond transformation for subsequent C–C bond coupling with heteroarene substrates (see Figure 4). The use of visible-light irradiation was crucial to ensure high selectivity and yield, which avoided h+vb random oxidation. This method demonstrated a wide substrate scope in 19 successful examples (as shown in Table 1). Furthermore, an important muscle relaxant drug, dantrolene, was synthesized using this method in two steps with very good yield. This is a very important TiO2 photocatalytic C–H bond activation following the C–C coupling example. The significance is that along normal photochemical reactions under UV irradiation, heteroarenes such as furans and thiophenes are commonly inclined to [2 + 2] cycloaddition or [4 + 2] Diels–Alder reaction products when aromatic diazo compounds are present [103,104]. Although the mechanism the authors provided was not soundly supported by experiment evidence, the proposed first step of formation and transformation of aryldiazoether to aryl radical was possible (see Figure 4). However, the event that photogenerated h+vb and e−cb was attributed to direct excitation of the TiO2 semiconductor was not likely. Herein, >420 nm irradiation did not excite normal TiO2 nanocrystal catalyst, because the energy of incident light was below the gap energy (<387 nm). Thus, the most probable reaction pathway was that the surface complex formed between the diazoether and TiO2 nanoparticle surface hydroxyl would be activated by visible light, and the excited surface complex may sensitize ethanol oxidation to provide electrons injecting into the conduction band. Subsequently, diazo compounds accept the electron with the extrusion of N2, yielding aryl radical. Heteroarene substrates trapped in situ form aryl radical, forming the radical intermediate. Since the h+vb was not formed, the TiO2-diazoether complex continued to accept the electron from the radical intermediate, yielding a carbocation intermediate, which upon spontaneous deprotonation rearomatized to the final product. The net reaction should be ethanol oxidation to acetaldehyde, while diazo was reduced to N2 and proton left behind was combined with BF4− to form HBF4. This example of TiO2 photocatalyzed C–H arylation indicates a direction for designing a feasible protocol of C–H functionalization for C–C coupling reaction by a SET pathway, even mediated by visible light or sunlight. The key to this successful C–H functionalization is based on the use of easily fragmenting aryldiazonium salts, which facilitated aryl radical generation upon TiO2 conduction band e−cb SET reduction.
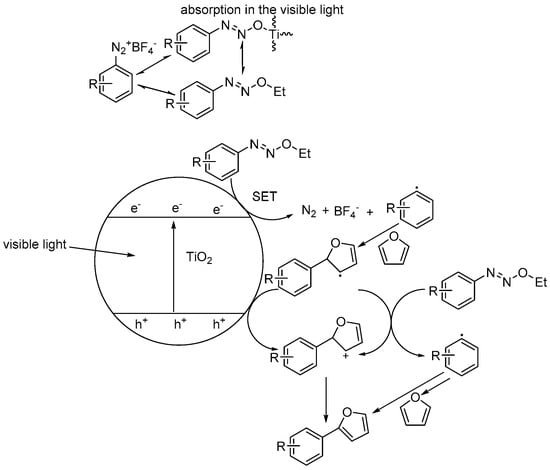
Figure 4.
Proposed mechanism of TiO2 photocatalytic heteroarene C–H arylation by aryl diazonium salts. Adapted with permission from [73]. Copyright 2015, ACS.

Table 1.
Substrate scope of heteroarene C–H arylation with aryldiazonium salts. Adapted with permission from [73]. Copyright 2015, ACS.
Sometime later, the same group reported the continuous-flow reactor version of this photocatalytic C–H bond arylation [74]. They innovated a falling-film membrane reactor (FFMR) (shown in Figure 5) to adapt to the heterogeneous photocatalyst TiO2 nanocrystal film. By elaborate synergy of this FFMR with TiO2 nanoparticles, TiO2 photocatalyst was calcined and bound to the stainless layer of reactor by a polyvinyl alcohol binder. XRD (X-ray diffraction), TEM (Transmission electron microscope), SEM (Scanning Electron Microscope), and EDX characterization displayed unchanged crystallinity, morphology, and size after calcination and immobilization. A comparison of the photocatalytic C–H bond arylation results demonstrated that the FFMR continuous-flow design provided approximately 60,000-fold enhancement of specific reactor compared with conventional batch reactor, along with excellent yields up to 99%. Moreover, this immobilized photocatalyst bed also generated enhanced stability and reusability against TiO2 photocatalysis in batch reactor.
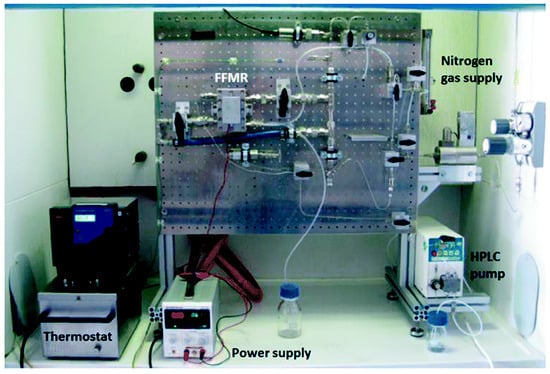
Figure 5.
Falling-film membrane reactor (FFMR) lab plant with an HPLC pump, thermostat, nitrogen gas supply, and power supply with light-emitting diode (LED) array. Reprinted from [74]. Copyright 2018, RSC.
Wang et al. developed a simpler and more user-friendly version of this heterogeneous photocatalytic transformation by a PANI-g-C3N4-TiO2 composite catalyst [105]. They expedited the protocol by in situ generation of aryldiazonium salts through the reaction of aniline with MeSO3H and tBuONO. Sequentially, the diazonium salts participated in the following aryl radical generation by electron reduction, radical addition with heteroarene furan, thiophene and pyridines, and the deprotonation step furnishing the final C–C coupled products (see Figure 6). Apart from the initiation step of evolution of aryldiazonium salts, this ternary composite photocatalyst system proceeded along a similar reaction route as the previous example by Rueping [73]. However, this example differed considerably from Rueping’s work on the composition of photocatalyst. In Rueping’s system, a commercial photocatalyst, P25 TiO2, was applied, whereas in Wang’s system, a hybrid PANI-g-C3N4-TiO2 photocatalyst was utilized. The difference in photocatalyst composition determined the intrinsic difference in the origin of visible-light response. For Rueping’s system, the surface complex of diazoether–TiO2 accounted for the absorption of incident visible-light photons, while in Wang’s system, despite the uncertainty of confirming the catalytic sites, the visible-light absorption was mainly attributed to the narrow-gap semiconductor PANI. Thus, PANI-g-C3N4-TiO2 composite catalyst could respond to visible-light irradiation, thereby initiating a variety of photocatalytic organic transformations, including C–H transformation and C–C formation, only by absorbing visible-light irradiation. TiO2 alone could initiate a few photocatalytic C–H transformations for C–C and C–hetero bond formation under visible-light irradiation, but only to the extent that the substrates themselves must coordinate or complex onto the TiO2 surface to form colored surface complexes for adsorption of visible-light driving ET-reaction. Apparently, available substrates are very limited; only a few polar aromatic compounds containing heteroatoms such as oxygen, nitrogen, or sulfur substituent function groups may be applicable. On the contrary, PANI-g-C3N4-TiO2 absorbs visible light by PANI itself and does not require the specific substrate’s heteroatom. Therefore, this hybrid photocatalysis system has wider substrate scope and reaction types for visible light–mediated transformations than TiO2 alone. In combination with PANI charge separation initiated by incident visible-light photons and consecutive surface reaction of in situ generated aryldiazonium salt with photo-induced h+vb/e−cb pair in an acetone/water or pure aqueous environment, this catalytic reaction yielded the final arylated products with good to excellent yield. Apart from heteroarenes, other kinds of substrates, such as enol ether and benzoquinone, were also easily arylated predominantly in their α-position. Good functional group tolerability and high chemo- and regioselectivity were achieved apart from the excellent yields in the present photocatalysis. The same group also showcased another protocol for easy synthesis of 2,5-diaryl 1,3,4-oxadiazoles by the same synergistic ternary composite photocatalyst (PANI-g-C3N4-TiO2) under visible-light illumination [106]. A broad scope of benzohydrazides and phenylglyoxylic acids were explored, and moderate to good isolated yields were obtained. No reaction occurred when 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) was added or nitrogen was used instead of an air atmosphere. Based on these mechanistic studies and control experiments, the authors proposed a plausible reaction mechanism. Initially, benzohydrazide and phenylglyoxylic acid were condensed in the presence of K2CO3, providing a 2-(2-benzoylhydrazono)-2-phenylacetate intermediate. This intermediate was then converted to its corresponding radical by h+vb SET oxidation. After decarboxylation and intramolecular radical addition to carbonyl oxygen, the new 2,5-diaryl-1,3,4-oxadiazole radical was formed. The final electron transfer from superoxide radical anion closed the total catalytic cycle. The photocatalyst displayed very good recyclability and reusability. After simple centrifugation and washing with ethyl acetate and water, the photocatalyst could be reused for six consecutive runs without apparent loss of catalytic reactivity. This example indicates that under visible-light illumination, modified TiO2 photocatalyst can photocatalyze the formation of five-membered heterocyclic compounds in an environmentally friendly and user-friendly manner.
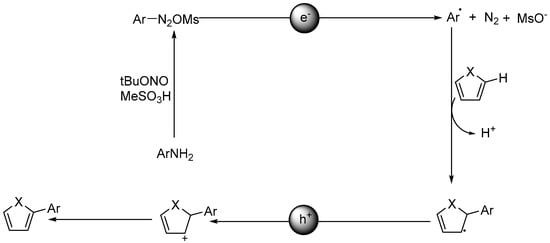
Figure 6.
Proposed mechanism for C–H arylation reactions through aniline activation catalyzed by PANI-g-C3N4-TiO2. Adapted and reprinted from [105]. Copyright 2018, RSC.
Apart from the C–H bond arylation reaction, TiO2 photocatalyst was also applied in the C–H bond alkylation transformations. Koenig et al. reported that upon visible-light irradiation, organic dye–anchored TiO2 photocatalyst could be combined with chiral secondary amine organocatalyst for highly chemoselective and stereoselective aldehyde α-C–H alkylation with electron-deficient alkyl bromide (see Figure 7) [107]. After loading with Texas Red derivative dye, TiO2 nanoparticles could even photosensitize this α-C–H alkylation reaction under 530 nm green-light irradiation and provide good ee value when a chiral secondary amine organocatalyst was added to the suspending solution. However, when chiral organocatalyst was covalently bound to TiO2 photocatalyst, no product formation was observed and the catalytic system was totally ineffective. This was mainly due to the oxidative decomposition of the organocatalyst on the TiO2 surface upon illumination. Much more efficient electron transfer occurred between the anchored chiral organocatalyst and the TiO2 oxidizing surface compared with the free chiral organocatalyst in solution. High chemo- and stereoselectivity achieved in the TiO2-photosensitized C–H alkylation reactions originated from the strong activation capacity of dye-modified TiO2 catalyst even under very low temperature (~−20 °C), since photo-induced ET thermodynamic behavior is generally less influenced by reaction temperature than homogeneous chiral organocatalysts and existing transition-metal catalyst. For the latter, the reaction never took place under low temperature, or lost chemo- and stereoselectivity while the temperature was raised. The findings on surface modification and immobilization for efficient asymmetric C–H functionalization by TiO2 photocatalysis under visible-light irradiation provided many tips and insights into the design of TiO2 photocatalytic organic transformations with more chemo-, regio-, and stereoselectivity. Many asymmetric reactions in which substrates are difficult to activate by the prevailing chiral catalysts under obligatory low temperature for high ee values may go smoothly with the modified TiO2 photocatalysts under low temperature.
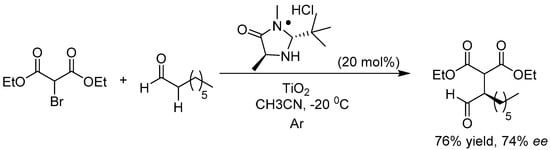
Figure 7.
TiO2 photocatalytic enantioselective alkylations using MacMillan’s chiral secondary amine organocatalyst.
Yoshida et al. reported that TiO2 photocatalyzed perfluoroalkylation via indirect C–H bond activation of electron-rich arenes and styrene derivatives [108,109]. Upon UV light irradiation, the TiO2 conduction band e−cb could reduce perfluoroalkyl iodide to generate perfluoroalkyl radical and iodide anion through the SET process (see Figure 8). The sp2 C–H bond functionalization of arene or styrene was indirectly performed by perfluoroalkyl radical attack to the arene or styrene π system and yielded the radical adduct. Then the radical adduct experienced SET oxidation by TiO2 h+vb on the catalyst surface, yielding carbocation species. The final energetically downhill deprotonation step finished the whole catalytic cycle. In this process, methanol co-solvent and NaBF4 additive acted as hole and electron modulator to accelerate this reaction. Using this method, several arenes and α-methyl styrene could be successfully perfluorohexylated and perfluorobutylated in moderate yields, providing formal C–H functionalized products. This discovery demonstrated the potential of TiO2 photocatalysis to set a perfluoroalkyl group on certain C–H bonds. The selectivity of this transformation was moderate, since the iodide anion, which was generated from photo-induced conduction band e−cb reduction of perflulroiodide, would possibly combine in the last step with arene cation, generating perfluoroaryl iodide byproducts.
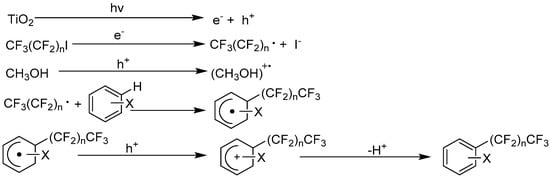
Figure 8.
TiO2 photocatalyzed perfluoroalkylation of arenes.
Not only organic dye efficiently sensitized wide band-gap semiconductor TiO2; transition-metal oxides were also decorated on the P25 TiO2 surface, serving as an effective sensitizer for some important organic synthesis. Shen et al. reported that NiO/TiO2 nanocomposite could photocatalyze the functionalization of N,N-dimethylaniline’s N-methyl C–H bond to generate the C–C coupling cyclization product tetrahydroquinoline by a further addition reaction with electron-poor maleic imides (see Figure 9) [110]. By a simple chemisorption-calcination procedure from Ni(acac)2·2H2O precursor and P25 TiO2, modified NiO/TiO2 nanocomposite was formed. The NiO modification extended the TiO2 absorption edge to the visible-light region by elevating the TiO2 valence band position. This modification greatly decreased the TiO2 band gap and enough oxidation ability remained for N-methyl C–H functionalization. After screening the optimum condition for this C–H transformation, the authors explored a wide variety of substrate scope (see Table 2). Moreover, the authors demonstrated that even with the TiO2 photocatalyst loading only 1% mol, it still provided excellent yield of C–C coupling cyclized tetrahydroquinoline target product. In the proposed mechanism, two C–H bond transformation events were involved: one was a sp3 C–H bond of N-methyl in N,N-dimethylaniline, and the other was the aromatic ring sp2 C–H bond. Besides being a well-known deprotonation route for C–H bond transformation by the photo-induced hole along a SET process, ROS was proposed to play a key role in abstracting hydrogen from N,N-dimethylaniline [111,112], generating α-aminoalkyl radical species. The successful C–H bond transformation and subsequent ring-closing C–C coupling mostly rely on electron-poor Michael-acceptor maleic imides as substrates. This catalytic reaction displayed high selectivity. The origin of this high reactivity was mainly attributed to NiO’s ability to attenuate photo-induced h+vb oxidability. NiO loading levelled up the h+vb redox potential, thus reducing the possibility of other undesired transformations. This example demonstrates that very efficient transformation and high turn-over number (TON) and turn-over frequency (TOF) can be achieved by the transition-metal oxide sensitized TiO2 photocatalysis system for useful C–H functionalization events rivaling or even surpassing its counterparts such as Ru, Ir complexes and organic dye photocatalysts.
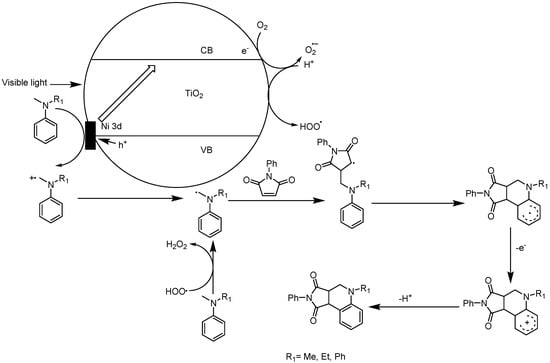
Figure 9.
Surface-modified NiO/TiO2 photocatalyzed reaction mechanism of tertiary anilines with maleic imides. Adapted and reproduced from [110]. Copyright 2015, ACS.

Table 2.
Visible light–mediated cyclization of tertiary anilines and maleimides with P25/NiO as photocatalyst. Adapted and reproduced from [110]. Copyright 2015, ACS.
Almost in the same period of this report, Kappe et al. reported the same transformation by a monodispersed TiO2 nanocrystal (see Figure 10) [113]. In a continuous-flow reactor setup, monodispersed TiO2 nanocrystals in the shape of spheres or rods were synthesized by heating the mixture of titanium isopropoxide and capping reagent oleic acid in a CH2Cl2/toluene mixed solvent at high temperature for a certain period. The as-synthesized nanocrystal sample was characterized by HRTEM and XRD to indicate its monodispersed shape and size. The small nanocrystals displayed nanorod or spherical shape. The authors applied the monodispersed TiO2 nanocrystals to the C–H functionalization of N-methylaniline and N,N-dimethylaniline N-methyl C–H bond. The C–H bond was selectively cleaved and linked with N-methyl maleic imide olefinic carbon by irradiating with a 365 nm UV LED torch. The monodispersed TiO2 nanocrystals demonstrated parallel activity toward efficient C–H transformation compared with commercial P25 nanomaterials.
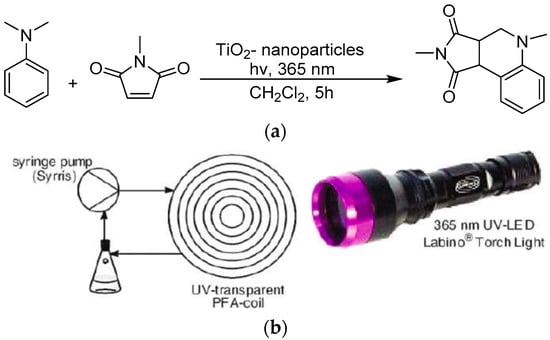
Figure 10.
(a) TiO2 photocatalyzed tandem addition–cyclization reaction of N-methylmaleimide and N,N-dimethylaniline and (b) the continuous-flow photoreactor setup. Adapted and reproduced from [113]. Copyright 2013, Springer.
To widen the scope of TiO2 photocatalysis for inert C–H functionalization, we reported that a more challenging pyridine ortho-C–H bond could be selectively cleaved and connected with an unactivated olefin 1,1-diphenylethlyene along an anti-Markovnikoff mode (Figure 11) [38]. Although the heteroarene scope was limited to pyridine and picolines, the realization of pyridine C–H bond cleavage was not a trivial matter, since the dissociation energy of pyridine sp2 C–H bond was very large. The SET redox potential of pyridine and picolines is high, and it is extremely difficult for the TiO2 photo-induced valence band h+vb to oxidize. However, we discovered that when an excessive amount of pyridine was applied, this transformation proceeded easily by a possible concerted two-electron transfer process. We demonstrated this reaction pathway by Fe(III)-ortho-1,10-phenanthroline titration of photo-induced e−cb and electron-paramagnetic-resonance (EPR) characterization of conduction band e−cb experiments. From the experimental results, no conduction band electron accumulation was detected when 4-picoline was subjected to TiO2 photocatalytic conditions under UV illumination, while other compounds with known SET reaction mechanism in TiO2 photocatalytic conditions all demonstrated notable electron accumulation signals. This result indicated that TiO2 photocatalytic functionalization of pyridine ortho-C–H bond into C–C bond with 1,1-diphenylethlene did not proceed along the same SET pathway as benzyltrimethylsilane, N,N-dimethylaniline, and N-methylpyrrolidine. We excluded the stepwise SET process and suggested that the most probable pathway was the concerted two-electron transfer process. This example demonstrates that by an unconventional concerted two-electron transfer process, TiO2 photocatalysis likely was able to fulfill very challenging inert electron-poor heteroarene sp2 C–H functionalization to construct useful C–C bonds. The good selectivity was mainly rooted in the highly specific concerted two-electron transfer rather than the common free-radical mechanism. Significantly differing from alternative transition-metal catalysis in a kind of anti-Markovnikoff reaction of pyridines with obligatory electron-rich olefins [114,115], the main advantage of the TiO2 photocatalytic method is that between two reactants, olefin and pyridine, only pyridine molecules could be adsorbed on to the polar TiO2 surface and activated into carbocation via this unconventional activation mode, which diffused into the solution phase to react with olefin. This protocol can maximally prevent preferentially activating olefin along the SET route into an undesired polymerization side reaction by the free-radical mechanism.
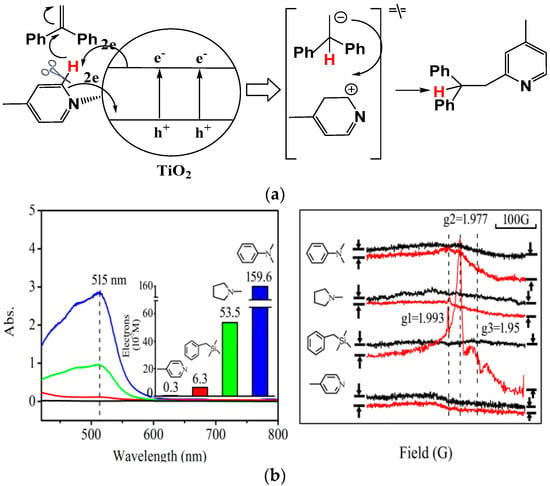
Figure 11.
(a) TiO2 photocatalytic anti-Markovnikoff addition reaction. (b) Accumulated electron evidenced by Fe(III)-o-phenanthroline titration and low-temperature electron-paramagnetic-resonance (EPR) experiments in TiO2 photocatalytic reactions between 1,1-diphenylethylene and different substrates. Reproduced with permission from [38]. Copyright 2015, RSC.
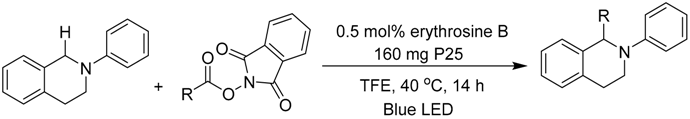
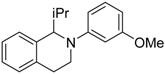
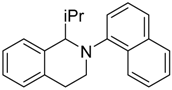
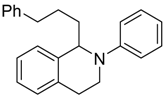
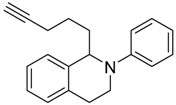
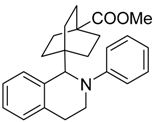
In a very recent report, the C–H bond functionalization of tetrahydroisoquinolines was used to construct C–C bonds with the carbon-centered radical generated from decarboxylation of active esters (N-hydroxyphthalimide esters) by a dye-sensitized TiO2 photocatalyst system (see Table 3) [116]. In this example, commonly used eosin-Y dye was replaced with a similar erythrosine-B dye, which showed much higher reactivity and selectivity toward C–C coupling products. This method was applicable to a wide variety of both tetrahydroisoquinolines and active ester substrates. Electron-rich methyl and methoxy, electron-poor fluorophenyl and chlorophenyl, and fused-ring naphthalene substituents on tetrahydroisoquinoline did not greatly reduce the isolated yield. Moreover, a variety of alkyl radical precursor N-hydroxyphthalimide esters were chosen as the substrates to test the universality of this method. Primary, secondary, and tertiary alkyls were introduced into the target molecule, with moderate to good yield. Alkene, alkyne, thienyl, cyclobutyl, tetrahydropyran, cyclohexanone, and Boc-protected amino functional groups were well tolerated. Moreover, the most sterically congested tertiary alkyls, or even extremely sterically crowded bridge-head carbon, were all effectively coupled with tetrahydroisoquinoline. The cyclopropyl radical clock experiments were undertaken in the present system. The ring-opening olefinic product demonstrated that the photocatalytic C–H alkylation probably proceeded via a radical mechanism (see Figure 12).

Table 3.
Substrate scope of dye-sensitized TiO2 photocatalyzed C–H decarboxylative alkylation. Adapted and reproduced from [116]. Copyright 2018, ACS.
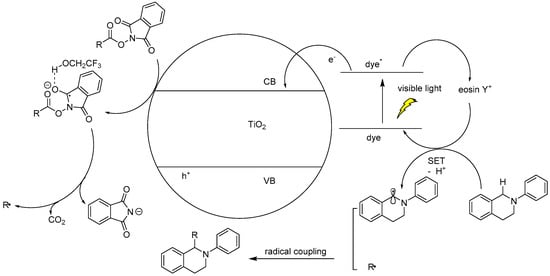
Figure 12.
Proposed mechanisms of dye-sensitized TiO2 photocatalyzed C–H decarboxylative alkylation. Adapted and reproduced from [116]. Copyright 2018, ACS.
Although initial free-radical formation routes such as the extrusion of CO2 from active esters generating stable N-hydroxyphthalimide anion and alkyl radical seemed reasonable, the possibility of a biradical coupling in bulk solution was too low for efficient transformation. Thus, it was more likely that surface binding of the tetrahydroisoquinoline radical extended its life and favored coupling with alkyl radical.
2.2. C–H Bond Functionalization for C–C Bond Coupling Reactions
In the above-mentioned bimolecular coupling transformations, C–X or C=C of one reagent couples with the C–H bond of another reagent, in which the C–H bond cleavage and C–C coupling reaction are usually discrete and passive. Generally, the initiation step is C–X cleavage and activation to generate the carbon-centered radical to involve in the sequential C–C coupling reaction (see Figure 3). Thus, the question has been posed whether TiO2 photocatalysis could realize the cleavage of both C–H bonds in two different molecules to fulfill the most challenging cross-dehydrogenative coupling (CDC) reaction. Recently, Rueping et al. reported that aza-Henry, Mannich-type, and cyanation reactions could be facilitated by TiO2 photocatalyst under visible-light irradiation (Figure 13) [22]. The authors showed that Aeroxide P25 or pure anatase TiO2 particles could mediate the C–H bond functionalization of tetrahydroisoquinoline and initiate the C–C cross-coupling reaction with nitromethane, acetone, and cyanide. In this manner, useful products such as β-nitro amines, β-amino ketones, and α-amino nitriles could be constructed with excellent yields. This work paved the way for further application of TiO2 photocatalysis in visible light–induced C–C (or C–P) bond formation via cleavage of two C–H bonds (or one C–H bond and one P–H bond). The authors did not provide the underlying mechanism of this C–H functionalization event, but this is a milestone discovery to independently realize the transformation and coupling of two C–H bonds in two substrate molecules, especially the functionalization of two saturated sp3 C–H bonds. For the traditional transition-metal catalysts, such an activation of two inert sp3 C–H bonds is almost impossible, unless one substrate is prefunctionalized or other auxiliary reagents are added to the system. The TiO2 photocatalytic mechanism behind this successful transformation remains an interesting mystery for further exploration.
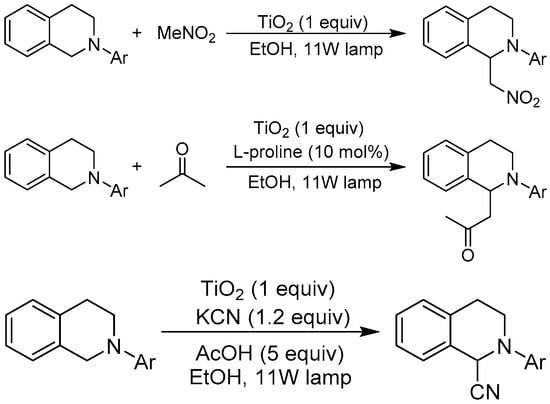
Figure 13.
TiO2 photocatalyzed oxidative Mannich, aza-Henry, and cyanation reactions.
In another example of activating two C–H bonds, N-heterocyclic arene C–H bond was also cleaved by sequential SET and radical attack event via TiO2 photocatalysis (see Figure 14). Sunlight induced the functionalization of some heterocyclic bases in the presence of polycrystalline TiO2 [43,117]. In this work, polycrystalline TiO2 mediated two C–H bond cleavage events to facilitate C–C formation between sp2 C–H bond in heterocyclic bases such as quinoline, quinaldine, lepidine, and quinoxaline and sp3 C–H bond in formamide, dimethylformamide, and dimethylacetamide. The use of H2O2 greatly assisted the sp3 C–H bond activation. TiO2 photo-induced conduction band e−cb reduced H2O2, yielding hydroxyl free radicals and hydroxide ions via the SET process. The hydroxyl free radical initiated a hydrogen-atom transfer (HAT) process to spontaneously abstract hydrogen from aldehyde-H or methyl-H. The as-formed nucleophilic carbon-centered radical attacked heteroarene via a Minisci-type mechanism, and the final deprotonation and electron transfer occurred spontaneously by the driving force of rearomatization and acid-base neutralization to form the final product.
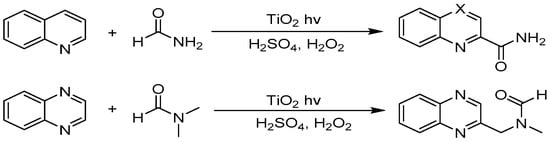
Figure 14.
TiO2 photocatalyzed heterocyclic bases of C–H activation with amides.
The same group later reported that not only formamide aldehyde C–H and N-methyl C–H bonds could be cleaved under TiO2 photocatalysis condition, but more inert ether α-methylene C–H was also functionalized and bridged with electron-poor N-heterocyclic bases such as quinoline, quinaldine, lepidine, quinoxaline, isoquinoline, and 4-cyanopyridine [118]. In the presence of TfOH and H2O2, using H2O or H2O/acetonitrile mixed solvent, tetrahydrofuran, tetrahydropyran, dioxane, diethylether, and dioxolane ether α-methylene, C–H was linked to quinoline via a similar radical Minisci-type pathway. The yields were not satisfactory, ranging from 25% to 75% for four different ethers. The main reason for the moderate yields was the necessary use of protic solvent and an aqueous environment, which on the one hand was helpful in facilitating ET from the polar TiO2 surface to the apolar or weak polar substrate, but on the other hand increased the undesired hydroxyl free-radical production. The more robust hydroxyl free radicals and other possible ROS led to more uncontrollable side reactions such as overoxidation, undesired HAT, and oxygen-atom transfer processes, thereby greatly decreasing the yield of desired main products. Moreover, the authors also explored the substrate scope of heterocyclic bases to couple quinoline, quinaldine, lepidine, quinoxaline, isoquinoline, and 4-cyanopyridine with trioxane. In these transformations, only very moderate yields ranging from 7% to 40% were obtained. Neither anatase nor rutile TiO2 nanoparticles could provide satisfactory yield. Although the yield was moderate in this reaction, the results proved that very challenging and inert C–H bond could be selectively cleaved and functionalized by TiO2 photocatalysis in the SET process.
Apart from the ether α-C–H bond, a similar alcohol α-C–H bond was also utilized for C–C coupling reaction. Zhu et al. reported that when metallic platinum was loaded to TiO2 nanoparticles, the composite photocatalyst could photocatalyze the α-C–H bond functionalization of ethanol, generating hydroxyethyl radicals [119]. The as-formed hydroxyethyl was further dehydrogenated via a “current doubling” effect on the TiO2 surface under UV illumination. This process was detrimental for the desired C–C coupling reaction due to the formation of aldehyde byproducts. However, the selectivity of the C–C coupling main reaction could be significantly enhanced by tuning the TiO2 surface hydroxyl group. The surface hydroxyl group reacted with H2O, generating hydroxyl free radical. If hydroxyethyl radical remained on the TiO2 surface, it suffered from reacting with hydroxyl free radical, yielding acetaldehyde byproducts. To avoid this side reaction, the authors engineered the TiO2 surface by a fluorination modification. The fluorinated P25 TiO2 provided the highest selectivity (64%) of 2,3-butanediol from ethanol compared with untreated anatase, rutile, brookite, and P25 TiO2.
For the same C–C coupling reaction, Zhu et al. also reported that upon phase and pore engineering of TiO2 photocatalyst, the ethanol α-C–H bond could also be efficiently transformed into C–C bond in 2,3-butanediol [120]. P25 TiO2 crystalline phase composition was tuned by sintering at different temperatures. Polycrystalline TiO2 with different anatase-to-rutile ratios was formed by this method. The higher the sintering temperature, the more rutile the phase profile. The authors discovered that the rutile phase was beneficial for the ethanol C–C coupling reaction rather than oxidation and overoxidation to aldehyde, acids, and CO2. Such intrinsic selectivity was due to rutile TiO2 possessing fewer surface hydroxyl groups. The surface hydroxyl group was detrimental for useful C–C coupling reaction as mentioned above. Moreover, the authors demonstrated that a more determining factor for the success of the useful C–H alkylation was the pore effect. They showed that the more developed the pore system, the greater the Brunauer-Emmett-Teller (BET) surface area and the less selectivity for C–H alkylation reaction. The reason was similar to the crystalline phase effect. Dense hydroxyl groups inside pores would interact with hydroxyethyl radical in the internal space of pores, generating many overoxidation byproducts. In order to prove the pore influence on C–H alkylation selectivity, the authors deliberately designed a paraffin-blocked porous rutile Pt–TiO2 photocatalyst system (see Figure 15). The inner space was totally crammed with nonpolar paraffin. The paraffin molecule exhibited very weak polarity and very few hydroxyl groups. In this way, hydroxyethyl radical inside the pore did not effectively interact with catalyst. This facilitated its desorption for efficient coupling reaction in bulk solution, yielding useful C–H alkylation products.
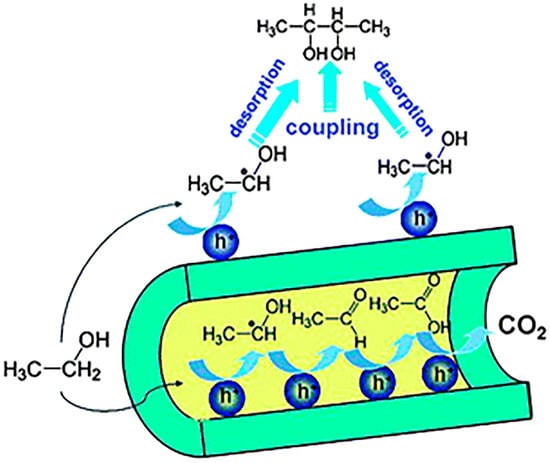
Figure 15.
Schematic of the process of passivating the internal channels of rutile-TiO2 nanotubes (rutile-TNTs) by filling paraffin into the tubes, and an illustration of the pore effect on selectivity control of ethanol oxidation. Reproduced with permission from [120]. Copyright 2011, RSC.
In fact, successful C–H bond functionalization for the purpose of C–C bond coupling based on TiO2 photocatalysis is very limited in both reaction type and substrate scope compared to conventional homogenous catalysis. However, these beneficial attempts provided a sound basis of theory and practice for future designs and modifications of TiO2 photocatalysts as well as corresponding reaction conditions.
3. Conclusions
We have provided an overview of examples of TiO2 photocatalytic C–H transformation for C–C coupling reaction. Although in its infancy compared with its application in water-splitting, DSSC, and water and air pollutant decomposition, TiO2 photocatalysis has already demonstrated potential in C–H bond functionalization for organic synthesis purposes. Various C–H bonds, including amino and ether N- and O-ortho aliphatic α-C–H, inert arene sp2 C–H such as benzene and pyridines, benzylic C–H, C–H adjacent to benzylic and the N-ortho position in tetrahydroisoquinoline, can be efficiently and selectively functionalized via either SET, HAT, or the two-electron transfer mechanism. By means of such a strategy, the inert C–H bonds in nonprefunctionalized organic substrates could be transformed to useful C–C bonds in diverse complex organic functional molecules. This has developed a somewhat robust method to prepare complex and challenging target organic compounds by using simple, ample, and commercially available raw materials. Nevertheless, compared with its homogeneous counterparts Ru, Ir complex and organic dye photocatalysts, current TiO2 photocatalysis demonstrates very narrow substrate scope and limited reaction types and still has plenty of room for improvement. More challenging and unconventional C–H bond cleavage and functionalization transformations are waiting to be realized by more elaborate design of either the TiO2 photocatalyst itself or skillful choice of co-catalyst, additive, and reaction parameters. If research focus is concentrated on TiO2 photocatalytic C–H transformation for useful C–C coupling reactions, this research field will provide us with milder, greener, safer, more significantly sustainable and efficient strategies to supplement the disadvantages of other transition-metal catalysts and organocatalysts in the C–H activation task.
Funding
This work was supported by the National Natural Science Foundation of China (21703005), the Research Foundation for Youth Scholars of Beijing Technology and Business University (Grant No. QNJJ2017-02), and the Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (CIT&TCD 201804025).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tian, C.; Massignan, L.; Meyer, T.H.; Ackermann, L. Electrochemical C–H/N–H Activation by Water-Tolerant Cobalt Catalysis at Room Temperature. Angew. Chem. Int. Ed. 2018, 57, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition-Metal-Catalyzed Direct Arylation of (Hetero)Arenes by C-H Bond Cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9826. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Wang, L.; Ding, Q.; Peng, Y. Nitrile as a Versatile Directing Group for C(sp2)–H Functionalizations. Adv. Synth. Catal. 2017, 359, 3274–3291. [Google Scholar] [CrossRef]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, O.; Yu, J.-Q. Palladium-Catalyzed Transformations of Alkyl C-H Bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.J.; Lin, D.W.; Miura, M.; Zhu, R.Y.; Gong, W.; Wasa, M.; Yu, J.Q. Palladium(II)-catalyzed enantioselective C(sp3) –H activation using a chiral hydroxamic acid ligand. J. Am. Chem. Soc. 2014, 136, 8138–8142. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.F.; Maugel, N.; Zhang, Y.H.; Yu, J.Q. Pd(II)-catalyzed enantioselective activation of C(sp2)–H and C(sp3) –H bonds using monoprotected amino acids as chiral ligands. Angew. Chem. Int. Ed. 2008, 47, 4882–4886. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Engle, K.M.; Wang, D.-H.; Yu, J.-Q. Palladium(II)-Catalyzed C–H Activation/C–C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ren, Z.; Thompson, S.J.; Xu, Y.; Dong, G. Transition-Metal-Catalyzed C-H Alkylation Using Alkenes. Chem. Rev. 2017, 117, 9333–9403. [Google Scholar] [CrossRef] [PubMed]
- Daugulis, O.; Do, H.-Q.; Shabashov, D. Palladium- and Copper-Catalyzed Arylation of Carbon-Hydrogen Bonds. Acc. Chem. Res. 2009, 42, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.G. Organometallic chemistry—C–H activation. Nature 2007, 446, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Hickman, A.J.; Sanford, M.S. High-valent organometallic copper and palladium in catalysis. Nature 2012, 484, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Cherney, A.H.; Kadunce, N.T.; Reisman, S.E. Enantioselective and Enantiospecific Transition-Metal-Catalyzed Cross-Coupling Reactions of Organometallic Reagents To Construct C–C Bonds. Chem. Rev. 2015, 115, 9587–9652. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Melchor, M.; Braga, A.A.C.; Lledos, A.; Ujaque, G.; Maseras, F. Computational Perspective on Pd-Catalyzed C–C Cross-Coupling Reaction Mechanisms. Acc. Chem. Res. 2013, 46, 2626–2634. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jin, H.; Hashmi, A.S.K. The recent achievements of redox-neutral radical C–C cross-coupling enabled by visible-light. Chem. Soc. Rev. 2017, 46, 5193–5203. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.C.; Vila, I.C.; Neild, E. Ecotoxicity assessment of metals and wastewater using multitrophic assays. Environ. Toxicol. 2000, 15, 370–375. [Google Scholar] [CrossRef]
- Santoro, S.; Kozhushkov, S.I.; Ackermann, L.; Vaccaro, L. Heterogeneous catalytic approaches in C–H activation reactions. Green Chem. 2016, 18, 3471–3493. [Google Scholar] [CrossRef]
- Takanabe, K.; Domen, K. Preparation of Inorganic Photocatalytic Materials for Overall Water Splitting. ChemCatChem 2012, 4, 1485–1497. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.T.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Rueping, M.; Zoller, J.; Fabry, D.C.; Poscharny, K.; Koenigs, R.M.; Weirich, T.E.; Mayer, J. Light-Mediated Heterogeneous Cross Dehydrogenative Coupling Reactions: Metal Oxides as Efficient, Recyclable, Photoredox Catalysts in C-C Bond-Forming Reactions. Chem. Eur. J. 2012, 18, 3478–3481. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient Visible Light Nitrogen Fixation with BiOBr Nanosheets of Oxygen Vacancies on the Exposed {001} Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, L.; Wang, J.; Li, Q.; He, W.; Yin, J.J. Surface Structure-Dependent Molecular Oxygen Activation of BiOCl Single-Crystalline Nanosheets. J. Am. Chem. Soc. 2013, 135, 15750–15753. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and Facet-Dependent Photoreactivity of BiOCl Single-Crystalline Nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, W.; Ai, Z.; Song, X.; Zhang, L.; Lee, S. Aerosol-assisted flow synthesis of B-doped, Ni-doped and B-Ni-codoped TiO2 solid and hollow microspheres for photocatalytic removal of NO. Appl. Catal. B Environ. 2009, 89, 398–405. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, W.; Lee, S.; Zhang, L.; Li, G.; Yu, J.C. Effect of carbon doping on the mesoporous structure of nanocrystalline titanium dioxide and its solar-light-driven photocatalytic degradation of NOx. Langmuir 2008, 24, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, Y.; Zhang, H.; Duan, R.; Chen, C.; Song, W.; Zhao, J. Localized TiIII mediated dissociative electron transfer for carbon halogen bond activation on TiO2. Appl. Catal. B Environ. 2017, 219, 322–328. [Google Scholar] [CrossRef]
- Lang, X.J.; Leow, W.R.; Zhao, J.C.; Chen, X.D. Synergistic photocatalytic aerobic oxidation of sulfides and amines on TiO2 under visible-light irradiation. Chem. Sci. 2015, 6, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lang, X.J.; Ma, W.H.; Ji, H.W.; Chen, C.C.; Zhao, J.C. Selective aerobic oxidation of amines to imines by TiO2 photocatalysis in water. Chem. Commun. 2013, 49, 5034–5036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.A.; Chen, C.C.; Ma, W.H.; Zhao, J.C. Visible-Light-Induced Aerobic Oxidation of Alcohols in a Coupled Photocatalytic System of Dye-Sensitized TiO2 and TEMPO. Angew. Chem. Int. Ed. 2008, 47, 9730–9733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, C.; Ma, W.; Zhu, H.; Zhao, J. Pivotal Role of Fluorine in Tuning Band Structure and Visible-Light Photocatalytic Activity of Nitrogen-Doped TiO2. Chem. Eur. J. 2009, 15, 4765–4769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Q.; Chen, C.C.; Zang, L.; Ma, W.H.; Zhao, J.C. Oxygen Atom Transfer in the Photocatalytic Oxidation of Alcohols by TiO2: Oxygen Isotope Studies. Angew. Chem. Int. Ed. 2009, 48, 6081–6084. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Ma, W.H.; Zhao, J.C. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.A.; Chen, C.C.; Ma, W.H.; Zhao, J.C. Photocatalytic Aerobic Oxidation of Alcohols on TiO2: The Acceleration Effect of a Brønsted Acid. Angew. Chem. Int. Ed. 2010, 49, 7976–7979. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.J.; Ji, H.W.; Chen, C.C.; Ma, W.H.; Zhao, J.C. Selective Formation of Imines by Aerobic Photocatalytic Oxidation of Amines on TiO2. Angew. Chem. Int. Ed. 2011, 50, 3934–3937. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, A.; Lu, C.; Chen, C. Photocatalytic Dehydrogenation of Primary Alcohols: Selectivity Goes against Adsorptivity. ACS Omega 2017, 2, 4161–4172. [Google Scholar] [CrossRef]
- Ma, D.; Yan, Y.; Ji, H.W.; Chen, C.C.; Zhao, J.C. Photocatalytic activation of pyridine for addition reactions: An unconventional reaction feature between a photo-induced hole and electron on TiO2. Chem. Commun. 2015, 51, 17451–17454. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, A.; Li, S.; Lu, C.; Chen, C. TiO2 photocatalysis for C-C bond formation. Catal. Sci. Technol. 2018, 8, 2030–2045. [Google Scholar] [CrossRef]
- Augugliaro, V.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Palmisano, L.; Soria, J.; Yurdakal, S. Heterogeneous Photocatalysis and Photoelectrocatalysis: From Unselective Abatement of Noxious Species to Selective Production of High-Value Chemicals. J. Phys. Chem. Lett. 2015, 6, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef]
- Palmisano, G.; Garcia-Lopez, E.; Marci, G.; Loddo, V.; Yurdakal, S.; Augugliaro, V.; Palmisano, L. Advances in selective conversions by heterogeneous photocatalysis. Chem. Commun. 2010, 46, 7074–7089. [Google Scholar] [CrossRef] [PubMed]
- Gambarotti, C.; Punta, C.; Recupero, F.; Caronna, T.; Palmisano, L. TiO2 in Organic Photosynthesis: Sunlight Induced Functionalization of Heterocyclic Bases. Curr. Org. Chem. 2010, 14, 1153–1169. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor Photocatalysis for Chemoselective Radical Coupling Reactions. Acc. Chem. Res. 2017, 50, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor Photocatalysis—Mechanistic and Synthetic Aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.J.; Zhao, J.C.; Chen, X.D. Cooperative photoredox catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.J.; Ma, W.H.; Chen, C.C.; Ji, H.W.; Zhao, J.C. Selective Aerobic Oxidation Mediated by TiO2 Photocatalysis. Acc. Chem. Res. 2014, 47, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.J.; Chen, X.D.; Zhao, J.C. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lang, X. Visible light photocatalysis of dye-sensitized TiO2: The selective aerobic oxidation of amines to imines. Appl. Catal. B Environ. 2018, 224, 404–409. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, J. Integrating TEMPO and Its Analogues with Visible-Light Photocatalysis. Chem. Asian J. 2018, 13, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Lang, X. Merging visible light photocatalysis of dye-sensitized TiO2 with TEMPO: The selective aerobic oxidation of alcohols. Catal. Sci. Technol. 2017, 7, 4955–4963. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M.; Dondi, D.; Albini, A. Significance of TiO2 Photocatalysis for Green Chemistry. J. Adv. Oxid. Technol. 2011, 14, 40–46. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Manley, D.W.; McBurney, R.T.; Miller, P.; Howe, R.F.; Rhydderch, S.; Walton, J.C. Unconventional Titania Photocatalysis: Direct Deployment of Carboxylic Acids in Alkylations and Annulations. J. Am. Chem. Soc. 2012, 134, 13580–13583. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N. Combining Photoredox and Metal Catalysis. Chemcatchem 2015, 7, 393–394. [Google Scholar] [CrossRef]
- Hoffmann, N. Photocatalysis with TiO2 Applied to Organic Synthesis. Aust. J. Chem. 2015, 68, 1621–1639. [Google Scholar] [CrossRef]
- Cherevatskaya, M.; Koenig, B. Heterogeneous photocatalysts in organic synthesis. Russ. Chem. Rev. 2014, 83, 183–195. [Google Scholar] [CrossRef]
- Vila, C.; Rueping, M. Visible-light mediated heterogeneous C-H functionalization: Oxidative multi-component reactions using a recyclable titanium dioxide (TiO2) catalyst. Green Chem. 2013, 15, 2056–2059. [Google Scholar] [CrossRef]
- Scandura, G.; Palmisano, G.; Yurdakal, S.; Tek, B.S.; Ozcan, L.; Loddo, V.; Augugliaro, V. Selective photooxidation of ortho-substituted benzyl alcohols and the catalytic role of ortho-methoxybenzaldehyde. J. Photochem. Photobiol. A Chem. 2016, 328, 122–128. [Google Scholar] [CrossRef]
- Palmisano, G.; Scandura, G.; Augugliaro, V.; Loddo, V.; Pace, A.; Tek, B.S.; Yurdakal, S.; Palmisano, L. Unexpectedly ambivalent O2 role in the autocatalytic photooxidation of 2-methoxybenzyl alcohol in water. J. Mol. Catal. A Chem. 2015, 403, 37–42. [Google Scholar] [CrossRef]
- Ozcan, L.; Yurdakal, S.; Augugliaro, V.; Loddo, V.; Palmas, S.; Palmisano, G.; Palmisano, L. Photoelectrocatalytic selective oxidation of 4-methoxybenzyl alcohol in water by TiO2 supported on titanium anodes. Appl. Catal. B Environ. 2013, 132, 535–542. [Google Scholar] [CrossRef]
- Yurdakal, S.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Enhancing selectivity in photocatalytic formation of p-anisaldehyde in aqueous suspension under solar light irradiation via TiO2 N-doping. New J. Chem. 2012, 36, 1762–1768. [Google Scholar] [CrossRef]
- Palmisano, L.; Augugliaro, V.; Bellardita, M.; Di Paola, A.; Lopez, E.G.; Loddo, V.; Marci, G.; Palmisano, G.; Yurdakal, S. Titania Photocatalysts for Selective Oxidations in Water. Chemsuschem 2011, 4, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; El Nazer, H.A.H.; Loddo, V.; Mele, A.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Partial photocatalytic oxidation of glycerol in TiO2 water suspensions. Catal. Today 2010, 151, 21–28. [Google Scholar] [CrossRef]
- Augugliaro, V.; Kisch, H.; Loddo, V.; Lopez-Munoz, M.J.; Marquez-Alvarez, C.; Palmisano, G.; Palmisano, L.; Parrino, F.; Yurdakal, S. Photocatalytic oxidation of aromatic alcohols to aldehydes in aqueous suspension of home prepared titanium dioxide 2. Intrinsic and surface features of catalysts. Appl. Catal. A Gen. 2008, 349, 189–197. [Google Scholar] [CrossRef]
- Yurdakal, S.; Palmisano, G.; Loddo, V.; Augugliaro, V.; Palmisano, L. Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J. Am. Chem. Soc. 2008, 130, 1568–1569. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; Caronna, T.; Loddo, V.; Marci, G.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Oxidation of aromatic alcohols in irradiated aqueous suspensions of commercial and home-prepared ruffle TiO2: A selectivity study. Chem. Eur. J. 2008, 14, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Yurdakal, S.; Palmisano, G.; Loddo, V.; Alagoz, O.; Augugliaro, V.; Palmisano, L. Selective photocatalytic oxidation of 4-substituted aromatic alcohols in water with rutile TiO2 prepared at room temperature. Green Chem. 2009, 11, 510–516. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhu, Z.P.; Wu, Y.P.; Zhao, T.J.; Li, L. TiO2-photocatalytic acceptorless dehydrogenation coupling of primary alkyl alcohols into acetals. Green Chem. 2014, 16, 4076–4080. [Google Scholar] [CrossRef]
- Lang, X.J.; Ma, W.H.; Zhao, Y.B.; Chen, C.C.; Ji, H.W.; Zhao, J.C. Visible-Light-Induced Selective Photocatalytic Aerobic Oxidation of Amines into Imines on TiO2. Chem. Eur. J. 2012, 18, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Teruhisa, O.; Takayo, K.; Keizo, N.; Michio, M. Stereospecific Epoxidation of 2-Hexene with Molecular Oxygen on Photoirradiated Titanium Dioxide Powder. Chem. Lett. 1998, 27, 877–878. [Google Scholar]
- Ohno, T.; Nakabeya, K.; Matsumura, M. Epoxidation of Olefins on Photoirradiated Titanium Dioxide Powder Using Molecular Oxygen as an Oxidant. J. Catal. 1998, 176, 76–81. [Google Scholar] [CrossRef]
- Zoller, J.; Fabry, D.C.; Rueping, M. Unexpected Dual Role of Titanium Dioxide in the Visible Light Heterogeneous Catalyzed C-H Arylation of Heteroarenes. ACS Catal. 2015, 5, 3900–3904. [Google Scholar] [CrossRef]
- Fabry, D.C.; Ho, Y.A.; Zapf, R.; Tremel, W.; Panthofer, M.; Rueping, M.; Rehm, T.H. Blue light mediated C-H arylation of heteroarenes using TiO2 as an immobilized photocatalyst in a continuous-flow microreactor. Green Chem. 2017, 19, 1911–1918. [Google Scholar] [CrossRef]
- Cermenati, L.; Richter, C.; Albini, A. Solar light induced carbon-carbon bond formation via TiO2 photocatalysis. Chem. Commun. 1998, 805–806. [Google Scholar] [CrossRef]
- Cermenati, L.; Mella, M.; Albini, A. Titanium dioxide photocatalysed alkylation of maleic acid derivatives. Tetrahedron 1998, 54, 2575–2582. [Google Scholar] [CrossRef]
- Cermenati, L.; Albini, A. Titanium dioxide photocatalysis for radical alkylation. J. Adv. Oxid. Technol. 2002, 5, 58–66. [Google Scholar] [CrossRef]
- Cermenati, L.; Fagnoni, M.; Albini, A. TiO2-photocatalyzed reactions of some benzylic donors. Canad. J. Chem. 2003, 81, 560–566. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Tung, C.-H.; Wang, Y. TiO2 Photocatalytic Cyclization Reactions for the Syntheses of Aryltetralones. ACS Catal. 2016, 6, 8389–8394. [Google Scholar] [CrossRef]
- Qiao, X.; Biswas, S.; Wu, W.; Zhu, F.; Tung, C.-H.; Wang, Y. Selective endoperoxide formation by heterogeneous TiO2 photocatalysis with dioxygen. Tetrahedron 2018, 74, 2421–2427. [Google Scholar] [CrossRef]
- Selvam, K.; Sakamoto, H.; Shiraishi, Y.; Hirai, T. Photocatalytic secondary amine synthesis from azobenzenes and alcohols on TiO2 loaded with Pd nanoparticles. New J. Chem. 2015, 39, 2856–2860. [Google Scholar] [CrossRef]
- Hirakawa, H.; Katayama, M.; Shiraishi, Y.; Sakamoto, H.; Wang, K.L.; Ohtani, B.; Ichikawa, S.; Tanaka, S.; Hirai, T. One-Pot Synthesis of Imines from Nitroaromatics and Alcohols by Tandem Photocatalytic and Catalytic Reactions on Degussa (Evonik) P25 Titanium Dioxide. ACS Appl. Mater. Interface 2015, 7, 3797–3806. [Google Scholar] [CrossRef] [PubMed]
- Adolph, C.M.; Werth, J.; Selvaraj, R.; Wegener, E.C.; Uyeda, C. Dehydrogenative Transformations of Imines Using a Heterogeneous Photocatalyst. J. Org. Chem. 2017, 82, 5959–5965. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Fujiwara, K.; Sugano, Y.; Ichikawa, S.; Hirai, T. N-Monoalkylation of Amines with Alcohols by Tandem Photocatalytic and Catalytic Reactions on TiO2 Loaded with Pd Nanoparticles. ACS Catal. 2013, 3, 312–320. [Google Scholar] [CrossRef]
- Tsarev, V.N.; Morioka, Y.; Caner, J.; Wang, Q.; Ushimaru, R.; Kudo, A.; Naka, H.; Saito, S. N-Methylation of Amines with Methanol at Room Temperature. Org. Lett. 2015, 17, 2530–2533. [Google Scholar] [CrossRef] [PubMed]
- Fagnoni, M.; Dondi, D.; Ravelli, D.; Albini, A. Photocatalysis for the Formation of the C−C Bond. Chem. Rev. 2007, 107, 2725–2756. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; Augugliaro, V.; Pagliaro, M.; Palmisano, L. Photocatalysis: A promising route for 21st century organic chemistry. Chem. Commun. 2007, 2007, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity Enhancement in Heterogeneous Photocatalytic Transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.L. Friedel-Crafts Acylations. Ind. Eng. Chem. 1959, 51, 1099–1101. [Google Scholar] [CrossRef]
- You, S.; Cai, Q.; Zeng, M. Chiral Bronsted Acid Catalyzed Friedel-Crafts Alkylation Reactions. Chem. Soc. Rev. 2009, 38, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Lacey, H.T. Friedel-Crafts Reactions. Ind. Eng. Chem. 1954, 46, 1827–1835. [Google Scholar] [CrossRef]
- Watson, W.J. Book Review of The Mizoroki−Heck Reaction. Org. Process Res. Dev. 2010, 14, 748. [Google Scholar] [CrossRef]
- Ozawa, F.; Kubo, A.; Hayashi, T. Catalytic Asymmetric Heck Reaction. In Selectivity in Catalysis; ACS Symposium Series 517; American Chemical Society: Washington, DC, USA, 1993; Volume 517, pp. 75–85. [Google Scholar]
- Calloway, N.O. The Friedel-Crafts Syntheses. Chem. Rev. 1935, 17, 327–392. [Google Scholar] [CrossRef]
- Sartori, G.; Maggi, R. Use of Solid Catalysts in Friedel−Crafts Acylation Reactions. Chem. Rev. 2006, 106, 1077–1104. [Google Scholar] [CrossRef] [PubMed]
- Sartori, G.; Maggi, R. Update 1 of: Use of Solid Catalysts in Friedel−Crafts Acylation Reactions. Chem. Rev. 2011, 111, 181–214. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, T.B.; Jørgensen, K.A. Catalytic Asymmetric Friedel−Crafts Alkylation Reactions—Copper Showed the Way. Chem. Rev. 2008, 108, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, Y.J.; Yuan, W.C.; Cui, X.; Cun, L.F.; Gong, L.Z. Rhodium-catalyzed asymmetric nitroallylation of arylmetallics with cyclic nitroallyl acetates and applications in organic synthesis. Eur. J. Org. Chem. 2006, 18, 4093–4105. [Google Scholar] [CrossRef]
- Wasa, M.; Engle, K.M.; Lin, D.W.; Yoo, E.J.; Yu, J.Q. Pd(II)-catalyzed enantioselective C–H activation of cyclopropanes. J. Am. Chem. Soc. 2011, 133, 19598–19601. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yang, C.; Ji, H.; Chen, C.; Ma, W.; Zhao, J. Mechanistic Studies of TiO2 Photocatalysis and Fenton Degradation of Hydrophobic Aromatic Pollutants in Water. Chem. Asian J. 2016, 11, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Giamalva, D.H. Alkylidenecarbenes from 1-diazo-1-alkenes: Their electrophilicity and stereochemistry of [2 + 2]-cycloaddition. J. Org. Chem. 1992, 57, 4185–4188. [Google Scholar] [CrossRef]
- Qiu, H.; Srinivas, H.D.; Zavalij, P.Y.; Doyle, M.P. Unprecedented Intramolecular [4 + 2]-Cycloaddition between a 1,3-Diene and a Diazo Ester. J. Am. Chem. Soc. 2016, 138, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, J.; Yang, S.; Liu, W.; Chen, Q.; He, M. C-H arylation reactions through aniline activation catalysed by a PANI-g-C3N4-TiO2 composite under visible light in aqueous medium. Green Chem. 2018, 20, 1290–1296. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, Q.; He, M. Photocatalyzed facile synthesis of 2,5-diaryl 1,3,4-oxadiazoles with polyaniline-g-C3N4-TiO2 composite under visible light. Tetrahedron Lett. 2018, 59, 1489–1492. [Google Scholar] [CrossRef]
- Cherevatskaya, M.; Neumann, M.; Fueldner, S.; Harlander, C.; Kuemmel, S.; Dankesreiter, S.; Pfitzner, A.; Zeitler, K.; Koenig, B. Visible-Light-Promoted Stereoselective Alkylation by Combining Heterogeneous Photocatalysis with Organocatalysis. Angew. Chem. Int. Ed. 2012, 51, 4062–4066. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Yoshida, M. Redox system for perfluoroalkylation of arenes and α-methylstyrene derivatives using titanium oxide as photocatalyst. J. Fluorine Chem. 2009, 130, 926–932. [Google Scholar] [CrossRef]
- Iizuka, M.; Fukushima, S.; Yoshida, M. Perfluoroalkylation of alpha-methylstyrene using titanium oxide as a photocatalyst. Chem. Lett. 2007, 36, 1042–1043. [Google Scholar] [CrossRef]
- Tang, J.; Grampp, G.; Liu, Y.; Wang, B.-X.; Tao, F.-F.; Wang, L.-J.; Liang, X.-Z.; Xiao, H.-Q.; Shen, Y.-M. Visible Light Mediated Cyclization of Tertiary Anilines with Maleimides Using Nickel(II) Oxide Surface-Modified Titanium Dioxide Catalyst. J. Org. Chem. 2015, 80, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Wu, T.X.; Zhao, J.C.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. 8. Irreversible degradation of alizarin red under visible light radiation in air-equilibrated aqueous TiO2 dispersions. Environ. Sci. Technol. 1999, 33, 2081–2087. [Google Scholar] [CrossRef]
- Wu, T.X.; Lin, T.; Zhao, J.C.; Hidaka, H.; Serpone, N. TiO2-assisted photodegradation of dyes. 9. Photooxidation of a squarylium cyanine dye in aqueous dispersions under visible light irradiation. Environ. Sci. Technol. 1999, 33, 1379–1387. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Glasnov, T.N.; Kappe, C.O. Continuous-flow production of photocatalytically active titanium dioxide nanocrystals and its application to the photocatalytic addition of n, n-dimethylaniline to n-methylmaleimide. J. Flow Chem. 2013, 3, 109–113. [Google Scholar] [CrossRef]
- Guan, B.-T.; Hou, Z. Rare-earth-catalyzed C-H bond addition of pyridines to olefins. J. Am. Chem. Soc. 2011, 133, 18086–18089. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Wylie, W.N.O.; Hou, Z. Enantioselective C-H bond addition of pyridines to alkenes catalyzed by chiral half-sandwich rare-earth complexes. J. Am. Chem. Soc. 2014, 136, 12209–12212. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cong, H. Visible-light-driven decarboxylative alkylation of C-H bond catalyzed by dye-sensitized semiconductor. Org. Lett. 2018, 20, 3225–3228. [Google Scholar] [CrossRef] [PubMed]
- Caronna, T.; Gambarotti, C.; Palmisano, L.; Punta, C.; Recupero, F. Sunlight induced functionalisation of some heterocyclic bases in the presence of polycrystalline TiO2. Chem. Commun. 2003, 2003, 2350–2351. [Google Scholar] [CrossRef]
- Caronna, T.; Gambarotti, C.; Palmisano, L.; Punta, C.; Recupero, F. Sunlight-induced reactions of some heterocyclic bases with ethers in the presence of TiO2—A green route for the synthesis of heterocyclic aldehydes. J. Photochem. Photobiol A Chem. 2005, 171, 237–242. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, J.; Cao, B.; Li, L.; Wang, Z.; Tian, X.; Jia, S.; Zhu, Z. Selective photocatalytic cc coupling of bioethanol into 2,3-butanediol over Pt-decorated hydroxyl-group-tunable TiO2 photocatalysts. Chemcatchem 2015, 7, 2384–2390. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, J.; Li, L.; Gong, L.; Zheng, J.; Zhang, L.; Wang, Z.; Zhang, J.; Zhu, Z. Selective oxidation of sacrificial ethanol over TiO2-based photocatalysts during water splitting. Energ Environ. Sci. 2011, 4, 3384–3388. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).