Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review
Abstract
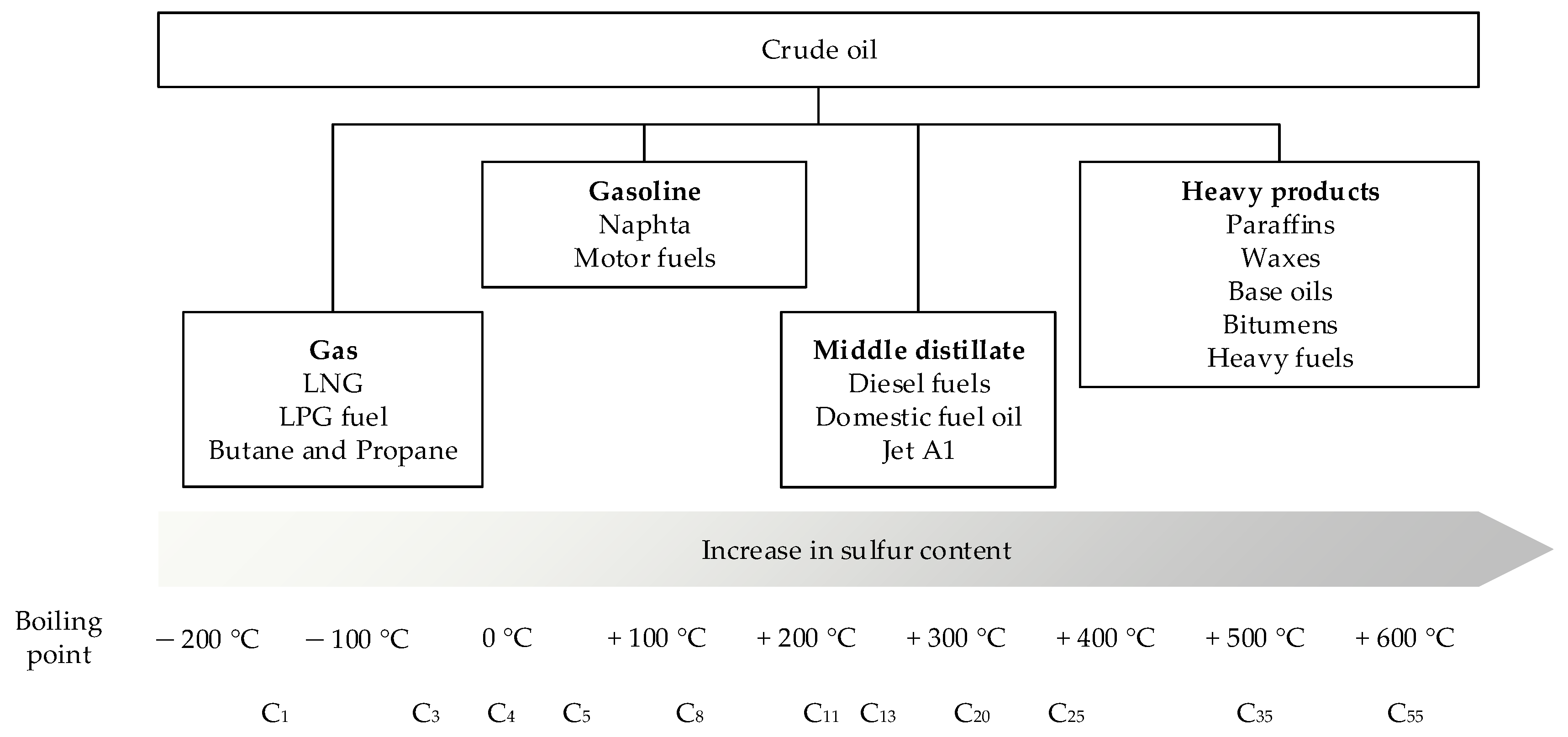
1. Introduction
2. ODS Technology and Efficiency Evaluation
2.1. Sulfones Separation
- Solvent selectivity toward sulfones
- Solvent capacity: solubility of sulfones in the solvent
- Recoverability of solvent for reuse
- Low solubility of hydrocarbon mixture to avoid loss of fraction in the solvent
- Partition ratio and number of extraction stages.
2.2. Efficiency Evaluation
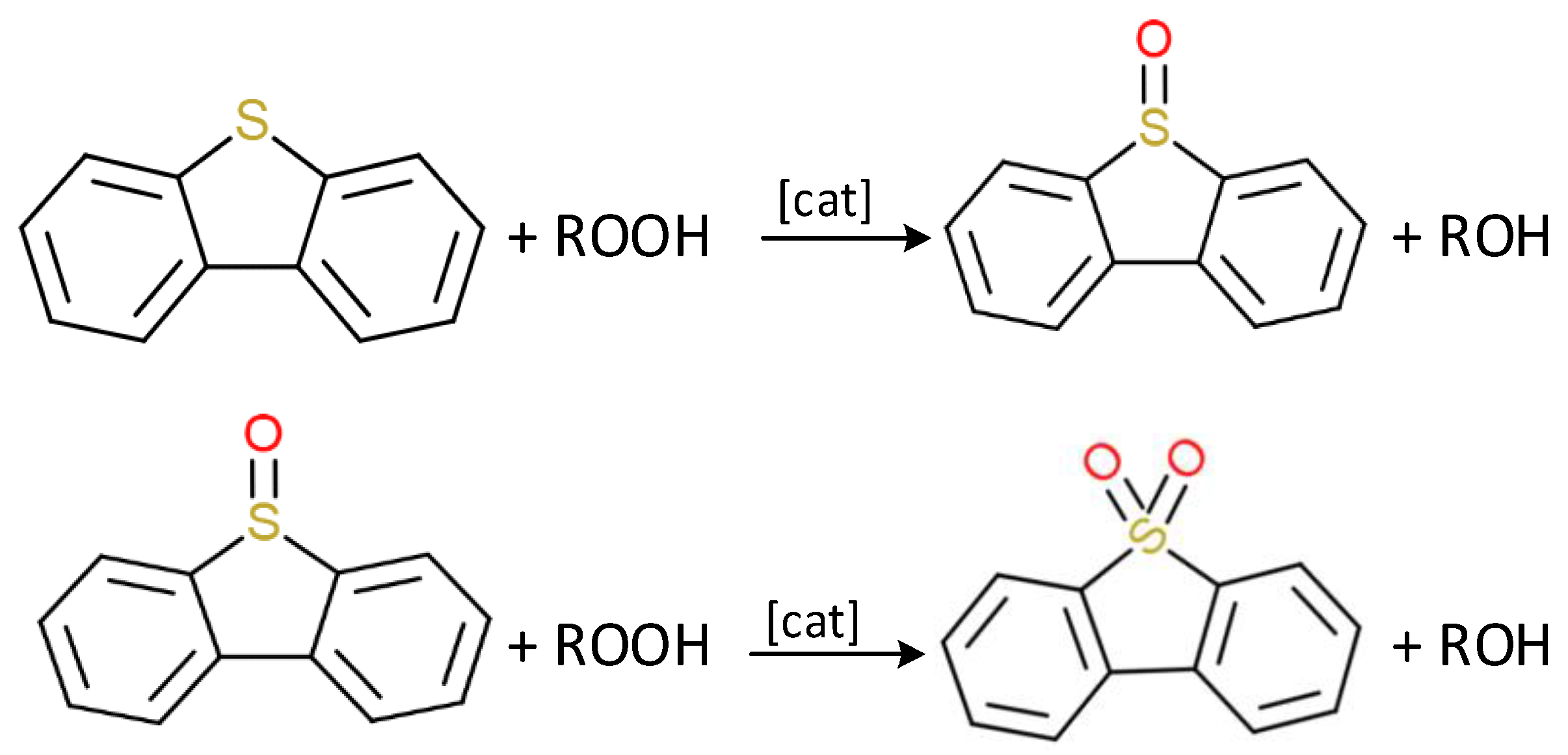
2.3. Sulfur Molecules Reactivity
2.4. Noncatalytic Process
3. Homogeneous Catalytic Systems
3.1. Homogeneous Catalysts
3.2. Effect of Reaction Temperature
3.3. Reaction Time
3.4. Oxidant
3.5. Reactivity of Different Fractions of a Heavy Fuel
3.6. Stirring Conditions
3.7. Additional Reagents
4. Heterogeneous Catalysts
5. Assisted ODS Process
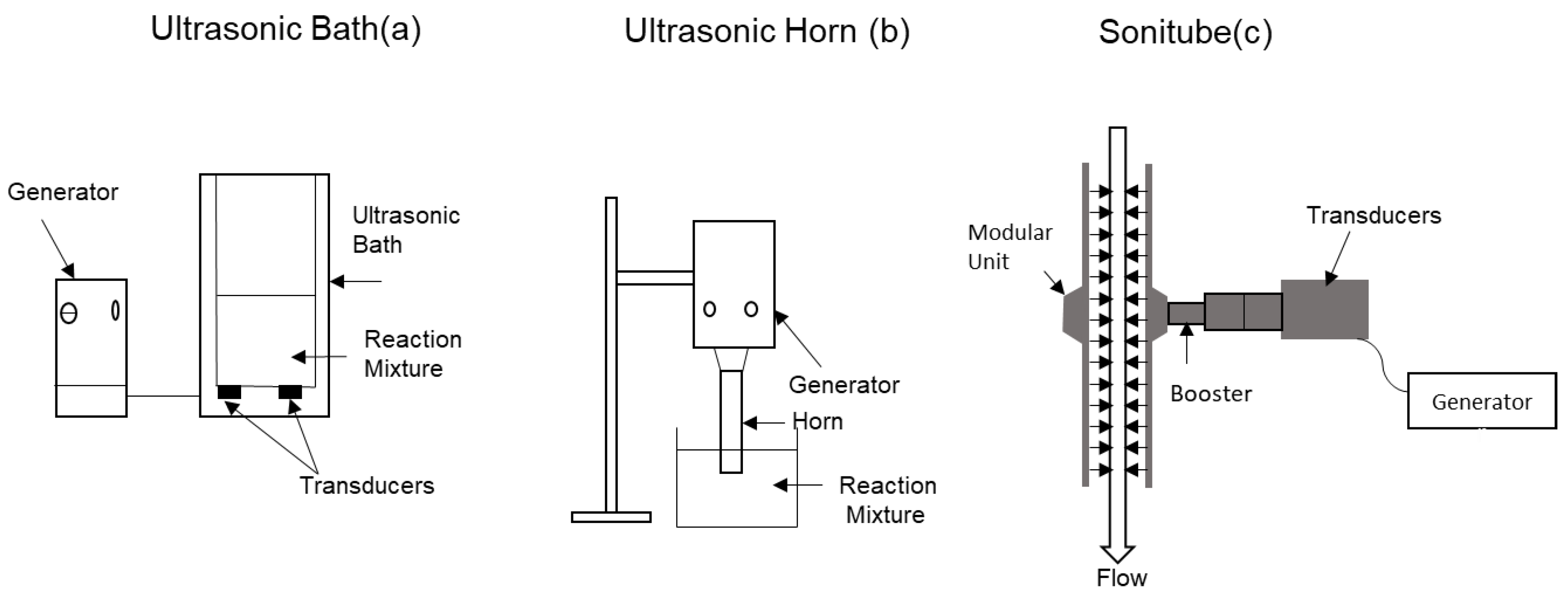
5.1. Ultrasound Technology
5.2. Ionic Liquids
5.3. Ultrasound and Ionic Liquid
6. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| DBT | Dibenzothiophene |
| DMDBT | Dimethyldibenzothiophene |
| DMF | N,N-Dimethylformamide |
| GC | Gas chromatography |
| HFO | Heavy Fuel Oil |
| IL | Ionic Liquid |
| LCO | Light Cycle Oil |
| ODS | Oxidative desulfurization |
| PTA | Phase Transfer Agents |
| PTC | Phase Transfer Catalysts |
| SR-LGO | Straight Run Light Gas Oil |
| SRGO | Straight Run Gas Oil |
| TAOF | Tetraoctyl ammonium fluoride |
| tBHP | tert-butylhydroperoxide |
| TOAB | Tetraoctyl ammonium bromide |
| UAOD | Ultrasound Assisted Oxidative Desulfurization |
| ULSD | Ultra-Low Sulfur Diesel |
| VGO | Vaccum Gas Oil |
References
- WEO 2017. Available online: http://www.iea.org/weo2017/#section-2-1 (accessed on 21 June 2018).
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Estephane, G.; Lancelot, C.; Blanchard, P.; Toufaily, J.; Hamiye, T.; Lamonier, C. Sulfur compounds reactivity in the ODS of model and real feeds on W–SBA based catalysts. RSC Adv. 2018, 8, 13714–13721. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J.M.; Huh, K.-S.; Woo, H.C. Oxidative desulfurization of marine diesel using WOx/SBA-15 catalyst and hydrogen peroxide. Korean Chem. Eng. Res. 2017, 55, 567–573. [Google Scholar] [CrossRef]
- Javadli, R.; de Klerk, A. Desulfurization of Heavy Oil–Oxidative Desulfurization (ODS) As Potential Upgrading Pathway for Oil Sands Derived Bitumen. Energy Fuels 2012, 26, 594–602. [Google Scholar] [CrossRef]
- Aitani, A.M.; Ali, M.F.; Al-Ali, H.H. A Review of Nonconventional Methods for the Desulfurization of Residual Fuel Oil. Pet. Sci. Technol. 2000, 18, 537–553. [Google Scholar] [CrossRef]
- Lin, L.; Hong, L.; Jianhua, Q.; Jinjuan, X. Progress in the Technology for Desulfurization of Crude Oil. China Pet. Process. Petrochem. 2010, 12, 1–6. [Google Scholar]
- Imtiaz, A.; Waqas, A.; Muhammad, I. Desulfurization of liquid fuels using air-assisted performic acid oxidation and emulsion catalyst. Chin. J. Catal. 2013, 34, 1839–1847. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Afonso, C.A.M. Deep desulfurization of diesel fuel using ionic liquids: Current status and future challenges. Green Chem. 2010, 12, 1139–1149. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Prasad, V.V.D.N.; Jeong, K.-E.; Chae, H.-J.; Kim, C.-U.; Jeong, S.-Y. Oxidative desulfurization of 4,6-dimethyl dibenzothiophene and light cycle oil over supported molybdenum oxide catalysts. Catal. Commun. 2008, 9, 1966–1969. [Google Scholar] [CrossRef]
- Anisimov, A.V.; Fedorova, E.V.; Lesnugin, A.Z.; Senyavin, V.M.; Aslanov, L.A.; Rybakov, V.B.; Tarakanova, A.V. Vanadium peroxocomplexes as oxidation catalysts of sulfur organic compounds by hydrogen peroxide in bi-phase systems. Catal. Today 2003, 78, 319–325. [Google Scholar] [CrossRef]
- Toteva, V.; Georgiev, A.; Topalova, L. Oxidative desulphurization of light cycle oil: Monitoring by FTIR spectroscopy. Fuel Process. Technol. 2009, 90, 965–970. [Google Scholar] [CrossRef]
- Krivtsov, E.B.; Golovko, A.K. The kinetics of oxidative desulfurization of diesel fraction with a hydrogen peroxide-formic acid mixture. Pet. Chem. 2014, 54, 51–57. [Google Scholar] [CrossRef]
- Trakarnpruk, W.; Rujiraworawut, K. Oxidative desulfurization of Gas oil by polyoxometalates catalysts. Fuel Process. Technol. 2009, 90, 411–414. [Google Scholar] [CrossRef]
- Zannikos, F.; Lois, E.; Stournas, S. Desulfurization of petroleum fractions by oxidation and solvent extraction. Fuel Process. Technol. 1995, 42, 35–45. [Google Scholar] [CrossRef]
- Hassan, S.I.; Sif El-Din, O.I.; Tawfik, S.M.; Abd El-Aty, D.M. Solvent extraction of oxidized diesel fuel: Phase equilibrium. Fuel Process. Technol. 2013, 106, 127–132. [Google Scholar] [CrossRef]
- Mokhtar, W.N.A.W.; Bakar, W.A.W.A.; Ali, R.; Kadir, A.A.A. Development of bimetallic and trimetallic oxides doped on molybdenum oxide based material on oxidative desulfurization of diesel. Arabian J. Chem. 2016. [Google Scholar] [CrossRef]
- Najafi, I.; Makarem, M.A.; Amani, M. Application of ultrasound waves to increase the efficiency of oxidative desulfurization process. Adv. Pet. Explor. Dev. 2011, 2, 63–69. [Google Scholar] [CrossRef]
- Wan, M.-W.; Yen, T.-F. Enhance efficiency of tetraoctylammonium fluoride applied to ultrasound-assisted oxidative desulfurization (UAOD) process. Appl. Catal. A Gen. 2007, 319, 237–245. [Google Scholar] [CrossRef]
- Mei, H.; Mei, B.W.; Yen, T.F. A new method for obtaining ultra-low sulfur diesel fuel via ultrasound assisted oxidative desulfurization☆. Fuel 2003, 82, 405–414. [Google Scholar] [CrossRef]
- Mello, P.D.A.; Duarte, F.A.; Nunes, M.A.G.; Alencar, M.S.; Moreira, E.M.; Korn, M.; Dressler, V.L.; Flores, É.M.M. Ultrasound-assisted oxidative process for sulfur removal from petroleum product feedstock. Ultrason. Sonochem. 2009, 16, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Rakhmanov, E.V.; Domashkin, A.A.; Myltykbaeva, Z.K.; Kairbekov, Z.; Shigapova, A.A.; Akopyan, A.V.; Anisimov, A.V. Peroxide oxidative desulfurization of a mixture of nonhydrotreated vacuum gas oil and diesel fraction. Pet. Chem. 2016, 56, 742–744. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Hirai, T. Desulfurization of Vacuum Gas Oil Based on Chemical Oxidation Followed by Liquid−Liquid Extraction. Energy Fuels 2004, 18, 37–40. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Alade, O.S.; Tshentu, Z.R. Vanadium(IV) catalysed oxidation of organosulfur compounds in heavy fuel oil. C. R. Chim. 2017, 20, 164–168. [Google Scholar] [CrossRef]
- Farshi, A.; Shiralizadeh, P. Sulfur reduction of heavy fuel oil by oxidative desulfurization (ODS) method. Pet. Coal 2015, 57, 1337–7027. [Google Scholar]
- Flores, R.; Rodas, A.; Chavarria, W. Desulfurization of fuel oils using an advanced oxidation method. Am. Chem. Soc. Div. Fuel Chem. 2004, 9, 341–342. [Google Scholar]
- Tang, Q.; Lin, S.; Cheng, Y.; Liu, S.; Xiong, J.-R. Ultrasound-assisted oxidative desulfurization of bunker-C oil using tert-butyl hydroperoxide. Ultrason. Sonochem. 2013, 20, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, A.A.; Tarakanov, G.V.; Ionov, N.G. Mechanisms of Oxidative Desulfurization of Straight-Run Residual Fuel Oil Using Ozonized Air. Chem. Technol. Fuels Oils 2016, 52, 33–37. [Google Scholar] [CrossRef]
- Tetrisyanda, R.; Wiguno, A.; Ginting, R.R.; Dzikrillah, M.C.; Wibawa, G. Residue Oil Desulfurization Using Oxidation and Extraction Method. Indonesian J. Chem. 2017. [Google Scholar] [CrossRef]
- Mohammed, A.D.; Isah, A.G.; Umaru, M.; Ahmed, S.; Abdullahi, Y.N. Comparative study on sulphur reduction from heavy petroleum-Solvent extraction and microwave irradiation approach. IJEE 2012, 3, 949–960. [Google Scholar]
- Abubakar, A.; Ahmed, I.A.M.-D.; Ahmed, A.S. Reduction of Sulphur Content of Urals Crude Oil Prior To Processing Using Oxidative Desulphurization. NJBAS 2016, 24, 19–24. [Google Scholar] [CrossRef]
- Rafiee, E.; Sahraei, S.; Moradi, G.R. Extractive oxidative desulfurization of model oil/crude oil using KSF montmorillonite-supported 12-tungstophosphoric acid. Pet. Sci. 2016, 13, 760–769. [Google Scholar] [CrossRef]
- Otaibi, R.L.A.; Liu, D.; Hou, X.; Song, L.; Li, Q.; Li, M.; Almigrin, H.O.; Yan, Z. Desulfurization of Saudi Arabian crudes by oxidation–extraction method. Appl Petrochem. Res. 2015, 5, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Hamidi, A. Sulfur Removal of Crude Oil by Ultrasound Assisted Oxidative Method. In Proceedings of the International Conference on Biological, Civil and Environmental Engineering (BCEE-2014), Dubai, United Arab Emirates, 17–18 March 2014; pp. 17–18. [Google Scholar]
- Haruna, S.Y.; Faruq, U.Z.; Zubairu, A.Y.; Liman, M.G.; Riskuwa, M.L. Comparative Studies on Reduction of Sulphur Content of Heavy Crude Oil Using KMnO4 + H2O2/CH3COOH and KMnO4+ H2O2/HCOOH Via Oxidative Desulphurization (ODS). AJAC 2018, 6, 15–24. [Google Scholar]
- García-Gutiérrez, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; Murrieta, F.; Navarrete, J.; Jiménez-Cruz, F. Ultra-deep oxidative desulfurization of diesel fuel with H2O2 catalyzed under mild conditions by polymolybdates supported on Al2O3. Appl. Catal. A Gen. 2006, 305, 15–20. [Google Scholar] [CrossRef]
- García-Gutiérrez, J.L.; Laredo, G.C.; García-Gutiérrez, P.; Jiménez-Cruz, F. Oxidative desulfurization of diesel using promising heterogeneous tungsten catalysts and hydrogen peroxide. Fuel 2014, 138, 118–125. [Google Scholar] [CrossRef]
- Abdullah, W.N.W.; Ali, R.; Bakar, W.A.W.A. In depth investigation of Fe/MoO3–PO4/Al2O3 catalyst in oxidative desulfurization of Malaysian diesel with TBHP-DMF system. J. Taiwan Inst. Chem. Eng. 2016, 58, 344–350. [Google Scholar] [CrossRef]
- Lo, W.-H.; Yang, H.-Y.; Wei, G.-T. One-pot desulfurization of light oils by chemical oxidation and solvent extraction with room temperature ionic liquids. Green Chem. 2003, 5, 639–642. [Google Scholar] [CrossRef]
- Ali, M.F.; Al-Malki, A.; El-Ali, B.; Martinie, G.; Siddiqui, M.N. Deep desulphurization of gasoline and diesel fuels using non-hydrogen consuming techniques. Fuel 2006, 85, 1354–1363. [Google Scholar] [CrossRef]
- Cho, K.-S.; Lee, Y.-K. Effects of nitrogen compounds, aromatics, and aprotic solvents on the oxidative desulfurization (ODS) of light cycle oil over Ti-SBA-15 catalyst. Appl. Catal. B Environ. 2014, 147, 35–42. [Google Scholar] [CrossRef]
- Rivoira, L.P.; Vallés, V.A.; Ledesma, B.C.; Ponte, M.V.; Martínez, M.L.; Anunziata, O.A.; Beltramone, A.R. Sulfur elimination by oxidative desulfurization with titanium-modified SBA-16. Catal. Today 2016, 271, 102–113. [Google Scholar] [CrossRef]
- Yan, X.-M.; Lei, J.-H.; Liu, D.; Wu, Y.-C.; Guo, L.-P. Oxidative Desulfurization of Diesel Oil Using Mesoporous Phosphotungstic Acid/SiO2 as Catalyst. J. Chin. Chem. Soc. 2007, 54. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wang, X.; Jin, C. Oxidative Desulphurization of 4,6-Dimethyldibenzothiophene with Hydrogen Peroxide over Ti-HMS. Energy Fuels 2007, 21, 1415–1419. [Google Scholar] [CrossRef]
- Chen, T.-C.; Shen, Y.-H.; Lee, W.-J.; Lin, C.-C.; Wan, M.-W. The study of ultrasound-assisted oxidative desulfurization process applied to the utilization of pyrolysis oil from waste tires. J. Clean. Prod. 2010, 18, 1850–1858. [Google Scholar] [CrossRef]
- Hamiye, R.; Lancelot, C.; Blanchard, P.; Toufaily, J.; Hamieh, T.; Lamonier, C. Diesel HDS performance of alumina supported CoMoP catalysts modified by sulfone molecules produced by ODS process. Fuel 2017, 210, 666–673. [Google Scholar] [CrossRef]
- Hulea, V.; Fajula, F.; Bousquet, J. Mild Oxidation with H2O2 over Ti-Containing Molecular Sieves—A very Efficient Method for Removing Aromatic Sulfur Compounds from Fuels. J. Catal. 2001, 198, 179–186. [Google Scholar] [CrossRef]
- Safa, M.A.; Al-Majren, R.; Al-Shamary, T.; Park, J.-I.; Ma, X. Removal of sulfone compounds formed in oxidative desulfurization of middle distillate. Fuel 2017, 194, 123–128. [Google Scholar] [CrossRef]
- Wang, D.; Qian, E.W.; Amano, H.; Okata, K.; Ishihara, A.; Kabe, T. Oxidative desulfurization of fuel oil. Appl. Catal. A Gen. 2003, 253, 91–99. [Google Scholar] [CrossRef]
- Zapata, B.; Pedraza, F.; Valenzuela, M.A. Catalyst screening for oxidative desulfurization using hydrogen peroxide. Catal. Today 2005, 106, 219–221. [Google Scholar] [CrossRef]
- Bazyari, A.; Khodadadi, A.A.; Haghighat Mamaghani, A.; Beheshtian, J.; Thompson, L.T.; Mortazavi, Y. Microporous titania–silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 65–77. [Google Scholar] [CrossRef]
- Chica, A.; Gatti, G.; Moden, B.; Marchese, L.; Iglesia, E. Selective catalytic oxidation of organosulfur compounds with tert-butyl hydroperoxide. Chemistry (Weinheim an der Bergstrasse, Germany) 2006, 12, 1960–1967. [Google Scholar] [CrossRef]
- Qian, E. Development of Novel Nonhydrogenation Desulfurization Process-Oxidative Desulfurization of Distillate. J. Jpn. Pet. Inst. 2008, 51, 14–31. [Google Scholar] [CrossRef]
- Safa, M.A.; Ma, X. Oxidation kinetics of dibenzothiophenes using cumene hydroperoxide as an oxidant over MoO3/Al2O3 catalyst. Fuel 2016, 171, 238–246. [Google Scholar] [CrossRef]
- Bukowski, A.; Milczarska, T. Asphalts as inhibitors of radical polymerization. J. Appl. Polym. Sci. 1983, 28, 1001–1009. [Google Scholar] [CrossRef]
- Herrington, P.R. Effect of Concentration on the Rate of Reaction of Asphaltenes with Oxygen. Energy Fuels 2004, 18, 1573–1577. [Google Scholar] [CrossRef]
- Guth, E.; Diaz, A. Method for Removing Sulfur and Nitrogen in Petroleum Oils. US3847800A, 12 November 1974. [Google Scholar]
- Gürü, M. Oxidative Desulfurization of Aşkale Coal by Nitric Acid Solution. Energy Source Part A Recover. Util. Environ. Eff. 2007, 29, 463–469. [Google Scholar] [CrossRef]
- Irum, S.; Akhtar, J.; Sheikh, N.; Munir, S. Oxidative desulfurization of Chakwal coal using potassium permanganate, ferric sulfate, and sodium hypochlorite. Energy Source Part A Recover. Util. Environ. Eff. 2017, 39, 426–432. [Google Scholar] [CrossRef]
- Wei, S.; He, H.; Cheng, Y.; Yang, C.; Zeng, G.; Qiu, L. Performances, kinetics and mechanisms of catalytic oxidative desulfurization from oils. RSC Adv. 2016, 6, 103253–103269. [Google Scholar] [CrossRef]
- Aida, T.; Funakoshi, I. Method of recovering organic sulfur compound from liquid oil. EP 1993, 565324, 10–13. [Google Scholar]
- Fedorova, E.V.; Zhirkov, N.; Tarakanova, A.V.; Ivanov, A.A.; Senyavin, V.; Anisimov, A.V.; Tulyakova, E.V.; Surin, S.A. Oxidative desulfurization of diesel fuel with hydrogen peroxide in the presence of vanadium peroxo complexes. Pet. Chem. 2002, 42, 253–256. [Google Scholar]
- Koseoglu, O.R.; Bourane, A. System and Process for Integrated Oxidative Desulfurization, Desalting and Deasphalting of Hydrocarbon Feedstocks. US8980080B2, 17 March 2015. [Google Scholar]
- Tam, P.S.; Kittrell, J.R.; Eldridge, J.W. Desulfurization of fuel oil by oxidation and extraction. 1. Enhancement of extraction oil yield. Ind. Eng. Chem. Res. 1990, 29, 321–324. [Google Scholar] [CrossRef]
- Yazu, K.; Yamamoto, Y.; Furuya, T.; Miki, K.; Ukegawa, K. Oxidation of Dibenzothiophenes in an Organic Biphasic System and Its Application to Oxidative Desulfurization of Light Oil. Energy Fuels 2001, 15, 1535–1536. [Google Scholar] [CrossRef]
- Sikarwar, P.; Kumar, U.K.A.; Gosu, V.; Subbaramaiah, V. Catalytic Oxidative Desulfurization of DBT Using Green Catalyst (Mo/MCM-41) Derived from Coal Fly Ash. JECE 2018. [Google Scholar] [CrossRef]
- Bakar, W.A.W.A.; Ali, R.; Kadir, A.A.A.; Mokhtar, W.N.A.W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process. Technol. 2012, 101, 78–84. [Google Scholar] [CrossRef]
- Akbari, A.; Omidkhah, M.; Towfighi Darian, J. Facilitated and selective oxidation of thiophenic sulfur compounds using MoOx/Al2O3–H2O2 system under ultrasonic irradiation. Ultrason. Sonochem. 2015, 23, 231–237. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; García, P.; Murrieta-Guevara, F.; Jiménez-Cruz, F. Ultra-deep oxidative desulfurization of diesel fuel by the Mo/Al2O3-H2O2 system: The effect of system parameters on catalytic activity. Appl. Catal. A Gen. 2008, 334, 366–373. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, K.; Cheng, Y.; Zeng, G.; Zhang, M.; Shao, J.; Lu, L. Catalytic oxidative desulfurization of BT and DBT from n-octane using cyclohexanone peroxide and catalyst of molybdenum supported on 4A molecular sieve. Sep. Purif. Technol. 2016, 163, 153–161. [Google Scholar] [CrossRef]
- Tomskii, I.S.; Vishnetskaya, M.V.; Vakhrushin, P.A.; Tomskaya, L.A. Oxidative desulfurization of straight-run diesel fraction on vanadium–molybdenum catalysts. Pet. Chem. 2017, 57, 908–913. [Google Scholar] [CrossRef]
- Cedeño, L.; Gomez-Bernal, H.; Fraustro-Cuevas, A.; Guerra-Gomez, H.D.; Cuevas-García, R. Oxidative desulfurization of synthetic diesel using supported catalysts: Part III. Support effect on vanadium-based catalysts. Catal. Today 2008, 133, 244–254. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.C.; Perez-Presas, P.; Campos-Martin, J.M.; Fierro, J.L.G. Highly efficient deep desulfurization of fuels by chemical oxidation. Catal. Today 2010, 157, 390–396. [Google Scholar] [CrossRef]
- Abdalla, Z.E.A.; Li, B.; Tufail, A. Preparation of phosphate promoted Na2WO4/Al2O3 catalyst and its application for oxidative desulfurization. J. Ind. Eng. Chem. 2009, 15, 780–783. [Google Scholar] [CrossRef]
- Long, Z.; Yang, C.; Zeng, G.; Peng, L.; Dai, C.; He, H. Catalytic oxidative desulfurization of dibenzothiophene using catalyst of tungsten supported on resin D152. Fuel 2014, 130, 19–24. [Google Scholar] [CrossRef]
- Dumont, V.; Oliviero, L.; Maugé, F.; Houalla, M. Oxidation of dibenzothiophene by a metal-oxygen-aldehyde system. Catal. Today 2008, 130, 195–198. [Google Scholar] [CrossRef]
- Chang, J.; Wang, A.; Liu, J.; Li, X.; Hu, Y. Oxidation of dibenzothiophene with cumene hydroperoxide on MoO3/SiO2 modified with alkaline earth metals. Catal. Today 2010, 149, 122–126. [Google Scholar] [CrossRef]
- Jiang, Z.; Lü, H.; Zhang, Y.; Li, C. Oxidative Desulfurization of Fuel Oils. Chin. J. Catal. 2011, 32, 707–715. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Srinivas, D.; Knözinger, H. Active Sites and Reactive Intermediates in Titanium Silicate Molecular Sieves. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2004; pp. 1–169. [Google Scholar]
- Yan, X.-M.; Mei, P.; Lei, J.; Mi, Y.; Xiong, L.; Guo, L. Synthesis and characterization of mesoporous phosphotungstic acid/TiO2 nanocomposite as a novel oxidative desulfurization catalyst. J. Mol. Catal. A Chem. 2009, 304, 52–57. [Google Scholar] [CrossRef]
- Xiao, J.; Wu, L.; Wu, Y.; Liu, B.; Dai, L.; Li, Z.; Xia, Q.; Xi, H. Effect of gasoline composition on oxidative desulfurization using a phosphotungstic acid/activated carbon catalyst with hydrogen peroxide. Appl. Energy 2014, 113, 78–85. [Google Scholar] [CrossRef]
- Kocal, J.A. Process for the Desulfurization of a Hydrocarbonaceous Oil. US6277271B1, 21 August 2001. [Google Scholar]
- Karas, L.J.; Han, Y.-Z.; Leyshon, D.W. Organosulfur Oxidation Process. US7270742B2, 18 September 2007. [Google Scholar]
- Palomeque-Santiago, J.F.; López-Medina, R.; Oviedo-Roa, R.; Navarrete-Bolaños, J.; Mora-Vallejo, R.; Montoya-de la Fuente, J.A.; Martínez-Magadán, J.M. Deep oxidative desulfurization with simultaneous oxidative denitrogenation of diesel fuel and straight run gas oil. Appl. Catal. B:Environ. 2018. [Google Scholar] [CrossRef]
- Kocal, J.A.; Brandvold, T.A. Removal of Sulfur-Containing Compounds from Liquid Hydrocarbon Streams. US6368495B1, 9 April 2002. [Google Scholar]
- Corma Canós, A.; Domine, M.E.; Martinez, C. Method and Catalysts for the Elimination of Sulphur Compounds from the Diesel Fraction. US7371318B2, 13 May 2008. [Google Scholar]
- Corma Canós, A.; Domine, M.E.; Martinez Sánchez, C. Process and Catalysts for Eliminating Sulphur Compounds from the Gasoline Fraction. US6846406B2, 25 January 2005. [Google Scholar]
- Corma Canós, A.; Domine, M.E.; Martinez, C. Method of Oxidising Sulphur Compounds Present in Gasoline, Kerosene and Diesel Fractions. WO/2003/044129, 31 May 2003. [Google Scholar]
- Ja’fari, M.; Ebrahimi, S.L.; Khosravi-Nikou, M.R. Ultrasound-assisted oxidative desulfurization and denitrogenation of liquid hydrocarbon fuels: A critical review. Ultrason. Sonochem. 2018, 40, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.; Venkateswaran, N.; Uppara, P.; Iyengar, S.B.; Katti, S.S. Current knowledge and potential applications of cavitation technologies for the petroleum industry. Ultrason. Sonochem. 2018, 42, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.R.; Sobati, M.A. Intensification of oxidative desulfurization of gas oil by ultrasound irradiation: Optimization using Box–Behnken design (BBD). Appl. Therm. Eng. 2017, 111, 1158–1170. [Google Scholar] [CrossRef]
- Gonzalez, L.A.; Kracke, P.; Green, W.H.; Tester, J.W.; Shafer, L.M.; Timko, M.T. Oxidative Desulfurization of Middle-Distillate Fuels Using Activated Carbon and Power Ultrasound. Energy Fuels 2012, 26, 5164–5176. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, D.; Qi, Y. Sono-desulfurization oxidation reactivities of FCC diesel fuel in metal ion/H2O2 systems. Ultrason. Sonochem. 2011, 18, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.A.; de Mello, P.A.; Bizzi, C.A.; Nunes, M.A.G.; Moreira, E.M.; Alencar, M.S.; Motta, H.N.; Dressler, V.L.; Flores, É.M.M. Sulfur removal from hydrotreated petroleum fractions using ultrasound-assisted oxidative desulfurization process. Fuel 2011, 90, 2158–2164. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Carnaroglio, D.; Boffa, L.; Cravotto, G.; Moreira, E.M.; Nunes, M.A.G.; Dressler, V.L.; Flores, E.M.M. Efficient H2O2/CH3COOH oxidative desulfurization/denitrification of liquid fuels in sonochemical flow-reactors. Ultrason. Sonochem. 2014, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.E.S.; Roces, S.; Dugos, N.; Wan, M.-W. Mixing-assisted oxidative desulfurization of model sulfur compounds using polyoxometalate/H2O2 catalytic system. Sustain. Environ. Res. 2016, 26, 184–190. [Google Scholar] [CrossRef]
- Gogate, P.R. Intensification of chemical processing applications using ultrasonic and microwave irradiations. Curr. Opin. Chem. Eng. 2017, 17, 9–14. [Google Scholar] [CrossRef]
- Wan, M.-W.; Yen, T.-F. Portable Continuous Ultrasound-Assisted Oxidative Desulfurization Unit for Marine Gas Oil. Energy Fuels 2008, 22, 1130–1135. [Google Scholar] [CrossRef]
- Bin Wan Kamal, W.M.I.; Okawa, H.; Kato, T.; Sugawara, K. Ultrasound-assisted oxidative desulfurization of bitumen. Jpn. J. Appl. Phys. 2017, 56, 07JE03. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Van Rantwijk, F.; Sheldon, R.A. Biocatalysis in ionic liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Abro, R.; Gao, S.; Abbas, T.; Chen, X.; Yu, G. Oxidative desulfurization of fuel oils using ionic liquids: A review. J. Taiwan Inst. Chem. Eng. 2016, 62, 84–97. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic Liquids in Selective Oxidation: Catalysts and Solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Baker, G.A. Oxidative desulfurization of fuels using ionic liquids: A review. Front. Chem. Sci. Eng. 2015, 9, 262–279. [Google Scholar] [CrossRef]
- Muzart, J. Ionic Liquids as Solvents for Catalyzed Oxidations of Organic Compounds. J. Adv. Synth. Catal. 2006, 348, 275–295. [Google Scholar] [CrossRef]
- Gharnati, L.; Doring, M.; Arnold, U. Catalytic Oxidation with Hydrogen Peroxide in Ionic Liquids. Curr. Org. Synth. 2009, 6, 342–361. [Google Scholar] [CrossRef]
- Jian-long, W.; Zhao, D.; Zhou, E.; Dong, Z. Desulfurization of gasoline by extraction with N-alkyl-pyridinium-based ionic liquids. J. Fuel Chem. Techno. 2007, 35, 293–296. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, S.; Liu, X.; Nie, Y.; Zhang, X.; Zhang, S. Extractive desulfurization of fuel using N-butylpyridinium-based ionic liquids. RSC Adv. 2015, 5, 30234–30238. [Google Scholar] [CrossRef]
- Holbrey, J.D.; López-Martin, I.; Rothenberg, G.; Seddon, K.R.; Silvero, G.; Zheng, X. Desulfurisation of oils using ionic liquids: Selection of cationic and anionic components to enhance extraction efficiency. Green Chem. 2008, 10, 87–92. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Sugawara, K. Removal of Organic Sulfur from Hydrocarbon Resources Using Ionic Liquids. Energy Fuels 2008, 22, 3303–3307. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.-Z.; Jiang, S.-G.; Wu, K.-C.; Liu, Y.-J.; Tian, L.-X.; Zhang, Y.-Q.; Niu, J. Dietary supplementation of honeysuckle improves the growth, survival and immunity of Penaeus monodon. Fish Shellfish Immunol. 2013, 35, 161–169. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, H.; Zhu, W.; Guo, J.; Jiang, X.; Lu, J.; Yan, Y. Deep Oxidative Desulfurization of Fuels Using Peroxophosphomolybdate Catalysts in Ionic Liquids. Ind. Eng. Chem. Res. 2008, 47, 6890–6895. [Google Scholar] [CrossRef]
- Zhu, W.; Li, H.; Jiang, X.; Yan, Y.; Lu, J.; Xia, J. Oxidative Desulfurization of Fuels Catalyzed by Peroxotungsten and Peroxomolybdenum Complexes in Ionic Liquids. Energy Fuels 2007, 21, 2514–2516. [Google Scholar] [CrossRef]
- Leng, Y.; Ge, H.; Zhou, C.; Wang, J. Direct hydroxylation of benzene with hydrogen peroxide over pyridine–heteropoly compounds. Chem. Eng. J. 2008, 145, 335–339. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Li, X.; Ma, X. Oxidative desulfurization of dibenzothiophene and diesel over [Bmim]3PMo12O40. J. Catal. 2011, 279, 269–275. [Google Scholar] [CrossRef]
- Riisager, A.; Eriksen, K.M.; Wasserscheid, P.; Fehrmann, R. Propene and 1-Octene Hydroformylation with Silica-Supported, Ionic Liquid-Phase (SILP) Rh-Phosphine Catalysts in Continuous Fixed-Bed Mode. Catal. Lett. 2003, 90, 149–153. [Google Scholar] [CrossRef]
- Xun, S.; Zhu, W.; Zheng, D.; Li, H.; Jiang, W.; Zhang, M.; Qin, Y.; Zhao, Z.; Li, H. Supported ionic liquid [Bmim]FeCl4/Am TiO2 as an efficient catalyst for the catalytic oxidative desulfurization of fuels. RSC Adv. 2015, 5, 43528–43536. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.Y.; Lin, T.-Y.; Yen, T.F. Superoxides: Alternative Oxidants for the Oxidative Desulfurization Process. Energy Fuels 2008, 22, 3326–3328. [Google Scholar] [CrossRef]
- Foote, C.S.; Valentine, J.S.; Greenberg, A.; Liebman, J.F. Active Oxygen in Chemistry; Springer Science & Business Media: Berlin, Germany, 2012; Volume 2. [Google Scholar]
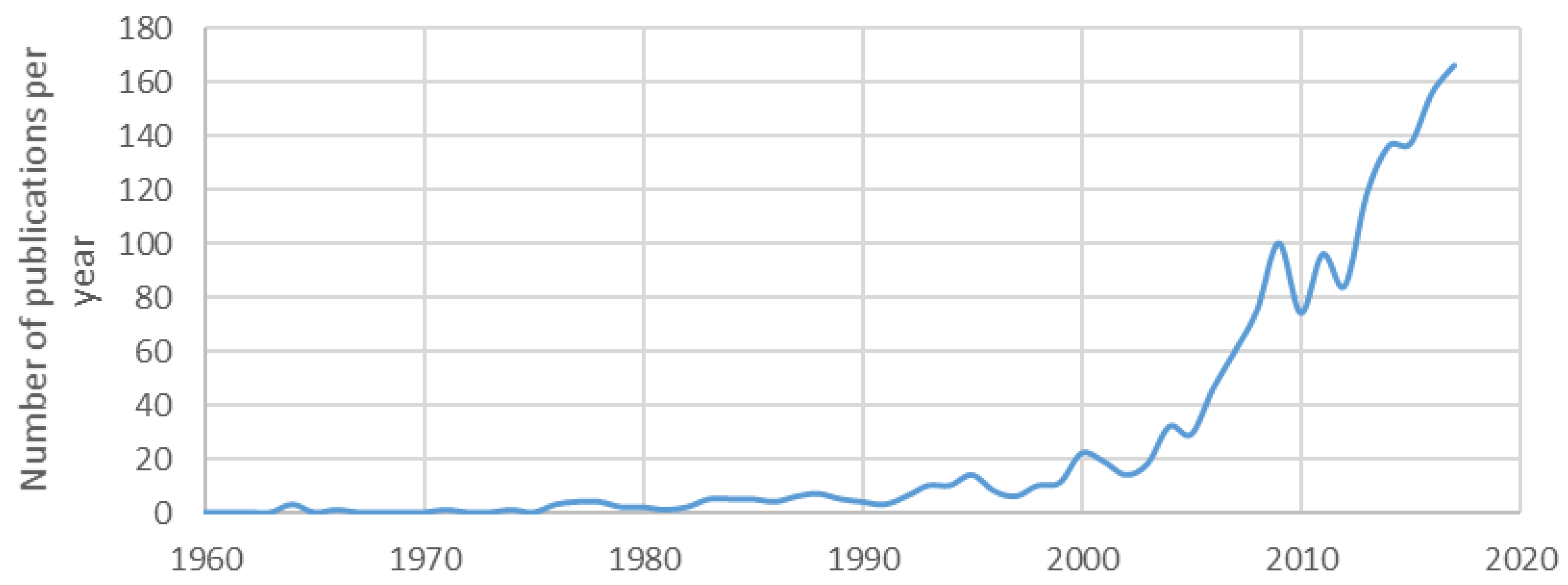
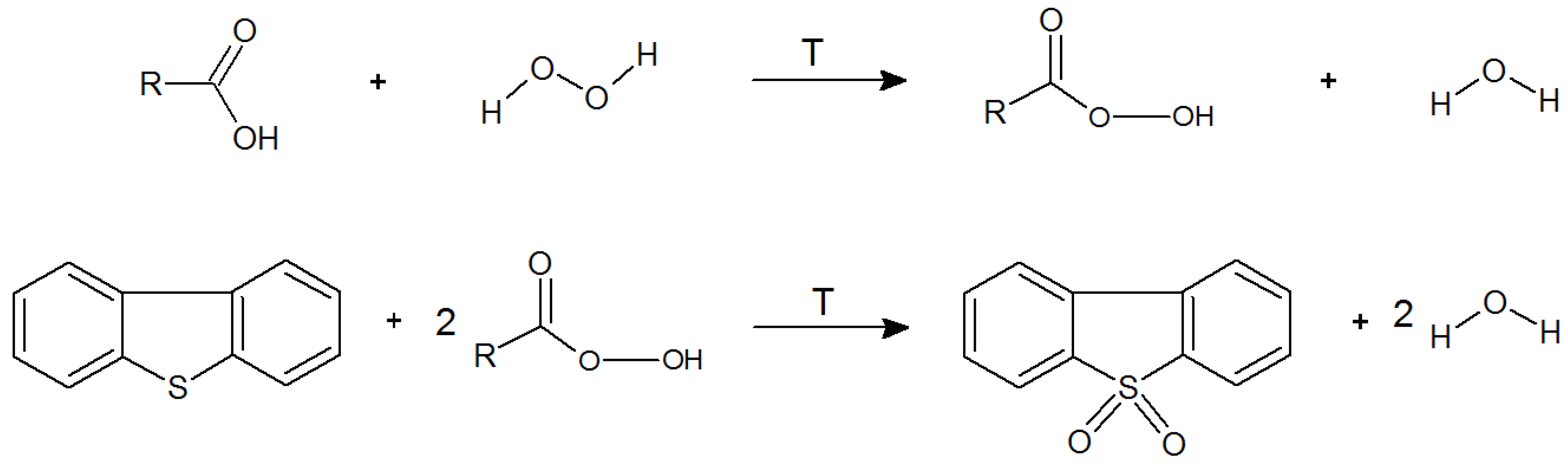
| Fraction | Designation Used in Article | Sulfur Content (%) | References |
|---|---|---|---|
| Gasoline | Naphtha | 2.00 | Imtiaz et al. 2013 [8] |
| Gasoline | 1.30 | Kulkarni et Afonso. 2010 [9] | |
| Diesel | Light Gas Oil (LGO) SR-LGO | 0.005 1.33 | Estephane et al. 2018 [3] Otsuki et al. 2000 [10] |
| Light Cycle Oil (LCO) | 0.80 | Prasad et al. 2008 [11] | |
| Diesel fuel | 0.86 | Anisimov et al. 2003 [12] | |
| LCO | 0.63 | Toteva et al. 2009 [13] | |
| LGO | 1.20 | Imtiaz et al. 2013 [8] | |
| Heavy Gas Oil | 4.00 | ||
| Straight Run Diesel Fraction | 1.19 | Krivtsov et al. 2013 [14] | |
| Gas Oil | 0.57 | Trakarnpruk et al. 2009 [15] | |
| Gas Oil | 2.87 | Zannikos et al. 1995 [16] | |
| Straight Run Gas Oil (SRGO) | 1.56 | Hassan et al. 2013 [17] | |
| Marine Diesel | 0.02 | Oh et al. 2017 [4] | |
| Crude Diesel | 0.83 | Mokhtar et al. 2016 [18] | |
| Diesel Fuel | 0.72 | Najafi et al. 2011 [19] | |
| Marine Diesel Oil | 0.17 | Wan et Yen. 2007 [20] | |
| Diesel Oil | 0.77 | Mei et al. 2003 [21] | |
| Hydrotreated Petroleum Product | 3.60 | Mello et al. 2009 [22] | |
| Vaccum gas oil | Vaccum Gas Oil (VGO) | 2.17 | Otsuki et al. 2000 [10] |
| Diesel Mixture (VGO+diesel) | 0.63 | Rakhmanov et al. 2015 [23] | |
| Vaccum Gas Oil | 1.98 | Shiraishi et Hirai. 2004 [24] | |
| Heavy fuel oil | Heavy Fuel Oil (HFO) | 1.80 | Ogunlaja et al. 2017 [25] |
| HFO | 2.75 | Farshi et Shiralizadeh. 2015 [26] | |
| HFO | 3.85 | Flores et al. 2004 [27] | |
| Bunker C Oil | 3.17 | Tang et al. 2013 [28] | |
| Residual Oil | Residual Fuel | 2.86 | Kazakov et al. 2016 [29] |
| Residual Oil | 0.04 | Tetrisyanda et al. 2017 [30] | |
| Crude Oil | Crude Oil | 1.60 | Mohammed et al. 2012 [31] |
| Crude Oil | 1.12 | Abu Bakar et Ahmed. 2016 [32] | |
| Crude Oil | 0.10 | Rafiee et al. 2016 [33] | |
| Crude Oil | 2.50 | Otaibi et al. 2015 [34] | |
| Crude Oil | 5.20 | Hosseini et Hamidi. 2014 [35] | |
| Crude Oil | 1.19 | Haruna et al. 2018 [36] |
| Extraction Solvent. | Dipole Moment (Debye) | Dielectric Constant | Solvent to Oil Ratio S/O | Petroleum Fraction | Sulfur Content (ppm) | D.R. (%) | Extraction Steps | Reference |
|---|---|---|---|---|---|---|---|---|
| N, N-dimethylformamide (DMF) | 3.24 | 36.71 | 1 | VGO + Diesel Fraction | 6300 | 90% | 3 | Rakhmanov et al. 2015 [23] |
| Acetonitrile | 3.53 | 35.94 | 1 | HFO | 27,500 | 59% | 1 | Farshi et Shiralizadeh. 2015 [26] |
| Acetonitrile + water (80:20) followed by methanol-water (80:20) | - | - | 1 | Naphtha | 20,000 | 83% | 1 | Imtiaz et al. 2013 [8] |
| - | - | - | LGO | 12,000 | 85% | 1 | - | |
| - | - | - | HGO | 40,000 | 69% | 1 | - | |
| - | - | - | Bitumen | 50,000 | 64% | 1 | - | |
| n-Heptane | - | - | 2 | Crude oil | 14,940 | 85% | 1 | Mohammed et al. 2012 [31] |
| Methanol | 2.87 | 32.66 | 1 | Gas oil | 24,000 | 90% | 1 | Zannikos et al. 1995 [16] |
| Acetonitrile | 3.53 | 35.94 | 1 | VGO | 19,800 | 89% | 1 | Shiraishi et Hirai.2004 [24] |
| DMF | 3.24 | 36.71 | 1 | SR-LGO | 13,500 | 92% | - | Otsuki et al. 2000 [10] |
| DMF | 3.24 | 36.71 | 1 | VGO | 21,700 | 100% | 10 | - |
| Acetonitrile | 3.53 | 35.94 | 0.5 | Diesel | 7,744 | 99% | 1 | Mei et al. 2003 [21] |
| Acetonitrile | 3.53 | 35.94 | 1 | HFO | 38,500 | 35% | 1 | Flores et al. 2004 [27] |
| DMF | 3.24 | 36.71 | 1 | Diesel | 8,286 | 90% | 4 | Mokhtar et al. 2016 [18] |
| Sulfur Compound | Structure | Electron Density |
|---|---|---|
| Thiophene | 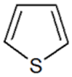 | 5.696 |
| Benzothiophene | 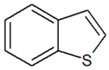 | 5.739 |
| Dibenzothiophene | 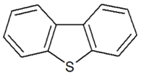 | 5.758 |
| 4,6-dimethyldibenzothiophene | 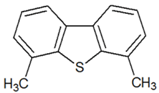 | 5.76 |
| Tetrahydrodibenzothiophene | 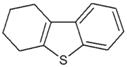 | 5.923 |
| Tetrahydrobenzonaphthothiophene | 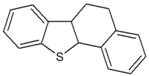 | 5.921 |
| Octahydrodinaphthothiophene | 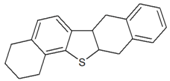 | 5.926 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts 2018, 8, 344. https://doi.org/10.3390/catal8090344
Houda S, Lancelot C, Blanchard P, Poinel L, Lamonier C. Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts. 2018; 8(9):344. https://doi.org/10.3390/catal8090344
Chicago/Turabian StyleHouda, Sara, Christine Lancelot, Pascal Blanchard, Line Poinel, and Carole Lamonier. 2018. "Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review" Catalysts 8, no. 9: 344. https://doi.org/10.3390/catal8090344
APA StyleHouda, S., Lancelot, C., Blanchard, P., Poinel, L., & Lamonier, C. (2018). Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts, 8(9), 344. https://doi.org/10.3390/catal8090344





