Catalytic Copyrolysis of Cork Oak and Waste Plastic Films over HBeta
Abstract
:1. Introduction
2. Results and Discussion
2.1. TG Analysis
2.2. TMR-GC/MS/FID
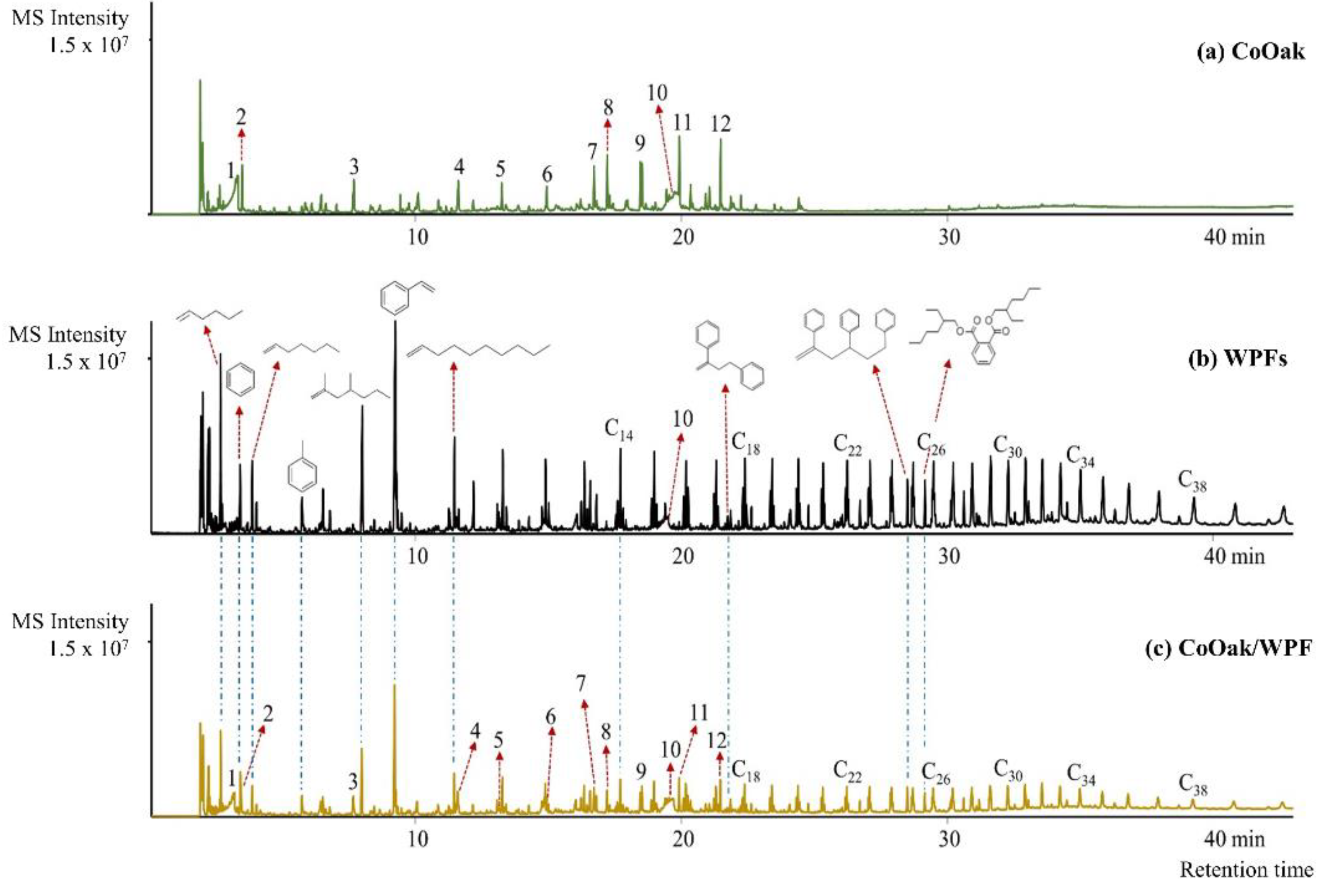
2.2.1. Non-Catalytic Pyrolysis of CoOak, WPFs, and CoOak/WPFs
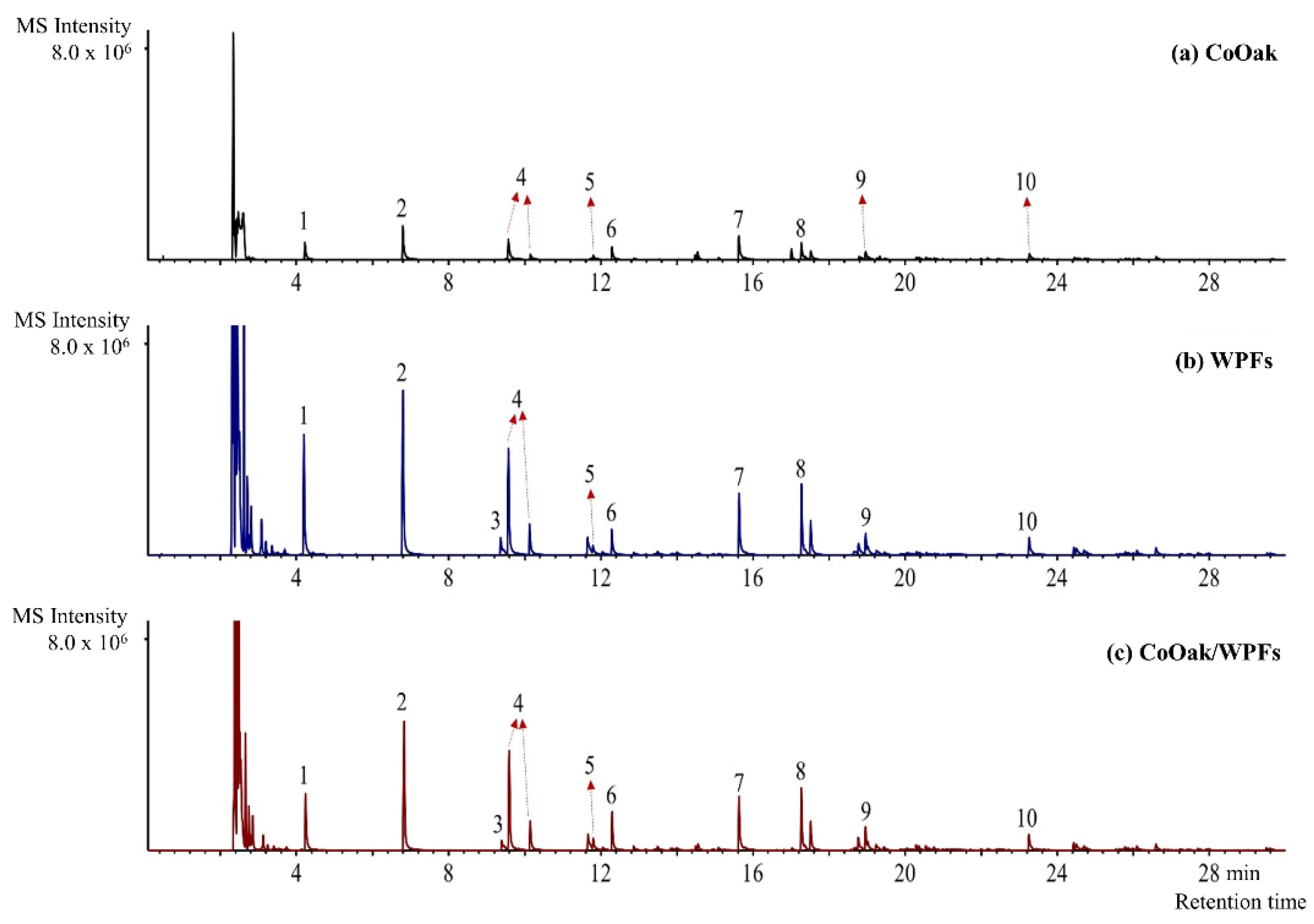
2.2.2. Catalytic Pyrolysis of CoOak and WPFs
3. Materials and Methods
3.1. CoOak and WPFs
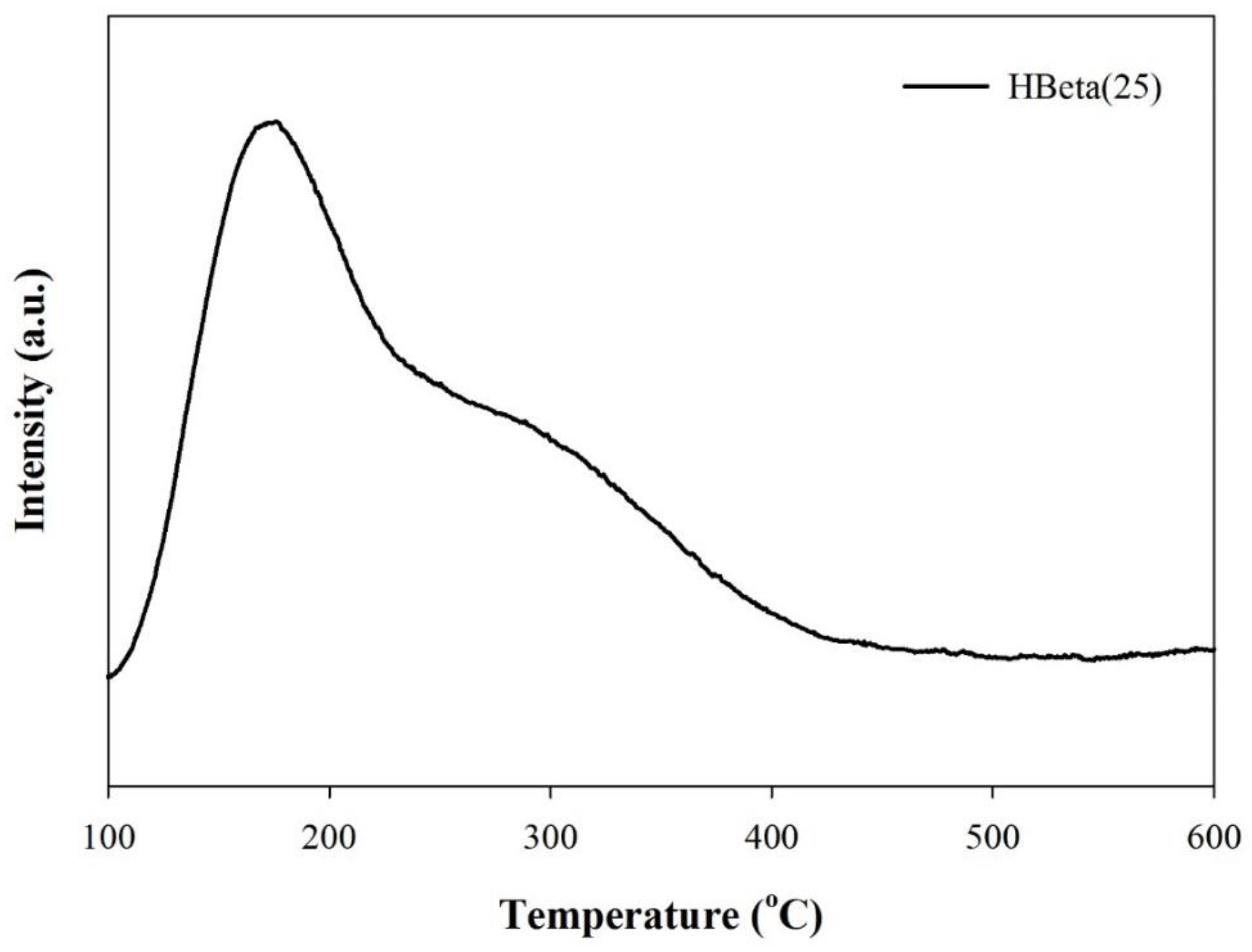
3.2. Catalyst
3.3. TG Analysis
3.4. TMR-GC/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.; Park, R.S.; Lee, H.W.; Hong, Y.; Lee, Y.; Park, S.H.; Jung, S.-C.; Yoo, K.-S.; Jeon, J.-K.; Park, Y.-K. Adsorptive removal of atmospheric pollutants over Pyropia tenera chars. Carbon Lett. 2016, 19, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Han, T.U.; Hwang, B.; Lee, B.; Lee, H.W.; Park, Y.-K.; Kim, S. Pyrolysis kinetics and product properties of softwoods, hardwoods, and the nut shell of softwood. Korean J. Chem. Eng. 2016, 33, 2350–2358. [Google Scholar] [CrossRef]
- Lee, Y.; Shafaghat, H.; Kim, J.; Jeon, J.-K.; Jung, S.-C.; Lee, I.-G.; Park, Y.-K. Upgrading of pyrolysis bio-oil using WO3/ZrO2 and Amberlyst catalysts: Evaluation of acid number and viscosity. Korean J. Chem. Eng. 2017, 34, 2180–2187. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.-M.; Lee, I.-G.; Jeon, J.-K.; Jung, S.-C.; Chung, J.D.; Choi, W.G.; Park, Y.-K. Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J. Chem. Eng. 2016, 33, 3299–3315. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.; Kang, K. Performance and emission characteristics of a diesel engine operated with wood pyrolysis oil. Proc. Inst. Mech. Eng. D J. Automob. Eng. 2014, 228, 180–189. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim, B.-S.; Chea, K.-S.; Jo, T.S.; Kim, S.; Park, Y.K. Ex-Situ catalytic pyrolysis of Korean native oak tree over microporous zeolites. Appl. Chem. Eng. 2016, 27, 407–414. [Google Scholar] [CrossRef]
- Mochizuki, T.; Chen, S.-Y.; Toba, M.; Yoshimura, Y. Pyrolyzer-GC/MS system-based analysis of the effects of zeolite catalysts on the fast pyrolysis of Jatropha husk. Appl. Catal. A 2013, 10, 174–181. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud, W.M.A.W. Aromatic hydrocarbon production by catalytic pyrolysis of palm kernel shell waste using a bifunctional Fe/HBeta catalyst: Effect of lignin-derived phenolics on zeolite deactivation. Green Chem. 2016, 18, 1684–1693. [Google Scholar] [CrossRef]
- Antonakou, E.; Lappas, A.; Nilsen, M.H.; Bouzga, A.; Stöcker, M. Evaluation of various types of Al-MCM-41 materials as catalysts in biomass pyrolysis for the production of bio-fuels and chemicals. Fuel 2006, 85, 2202–2212. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.K.; Hameed, B.H. Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour. Technol. 2016, 221, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Dorado, C.; Mullen, C.A.; Boateng, A.A. H-ZSM5 catalyzed co-pyrolysis of biomass and plastics. ACS Sustain. Chem. Eng. 2014, 2, 301–311. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouadi, M.; Jaeger, N.; Greenhalf, C.; Santos, J.; Conti, R.; Hornung, A. Thermo-catalytic reforming of municipal solid waste. Waste Manag. 2017, 68, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Marcilla, M.; Gómez-Siurana, A.; Odjo, A.O.; Navarro, R.; Berenger, N.D. Characterization of vacuum gas oil-low density polyethylene blends by thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 723–730. [Google Scholar] [CrossRef]
- Sirous-Rezaei, P.; Jae, J.; Ha, J.-M.; Ko, C.H.; Kim, J.M.; Jeon, J.K.; Park, Y.-K. Mild hydrodeoxygenation of phenolic lignin model compounds over a FeReOx/ZrO2 catalyst: Zirconia and rhenium oxide as efficient dehydration promoters. Green Chem. 2018, 20, 1472–1483. [Google Scholar] [CrossRef]
- Han, T.U.; Kim, Y.-M.; Watanabe, C.; Teramae, N.; Park, Y.-K.; Kim, S.; Lee, Y. Analytical pyrolysis properties of waste medium-density fiberboard and particle board. J. Ind. Eng. Chem. 2015, 32, 245–352. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kim, Y.-M.; Jae, J.; Watanabe, C.; Kim, S.; Jung, S.-C.; Kim, S.C.; Park, Y.-K. Pyrolysis and catalytic upgrading of Citrus unshiu peel. Bioresour. Technol. 2015, 194, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Kim, S.; Han, T.U.; Park, Y.-K.; Watanabe, C. Pyrolysis reaction characteristics of Korean pine (Pinus Koraiensis) nut shell. J. Anal. Appl. Pyrolysis 2014, 110, 435–441. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jae, J.; Kim, B.-S.; Hong, Y.; Jung, S.-C.; Park, Y.-K. Catalytic co-pyrolysis of torrefied yellow poplar and high-density polyethylene using microporous HZSM-5 and mesoporous Al-MCM-41 catalysts. Energy Convers. Manag. 2017, 149, 966–973. [Google Scholar] [CrossRef]
- Vega, D.; Villar, M.A.; Failla, M.D.; Vallés, E.M. Thermogravimetric analysis of starch-based biodegradable blends. Polym. Bull. 1996, 37, 229–235. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, B.; Han, T.U.; Kim, S.; Yu, T.-U.; Bang, B.Y.; Kim, J.-S.; Park, Y.-K. Research on pyrolysis properties of waste plastic films. Appl. Chem. Eng. 2017, 28, 23–28. [Google Scholar]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Oromiehie, A.R.; Taherzadeh lari, T.; Rabiee, A. Physical and thermal mechanical properties of corn starch/LDPE composites. Appl. Polym. 2013, 127, 1128–1134. [Google Scholar] [CrossRef]
- El-Sabbagh, A. Effect of coupling agent on natural fibre in natural fibre/polypropylene composites on mechanical and thermal behaviour. Compos. B Eng. 2014, 57, 126–135. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Jordá-Vilaplana, A.; Balart, R.; Garcia-Sanoguera, D. Development and characterization of green composites from bio-based polyethylene and peanut shell. Appl. Polym. 2016, 133, 43940. [Google Scholar] [CrossRef]
- Marcilla, A.; Go’mez-Siurana, A.; Valde’s, F. Catalytic pyrolysis of LDPE over H-beta and HZSM-5 zeolites in dynamic conditions Study of the evolution of the process. J. Anal. Appl. Pyrolysis 2007, 79, 433–442. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Product distribution from fast pyrolysis of glucose-based carbohydrates. J. Anal. Appl. Pyrolysis 2009, 86, 323–330. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kim, Y.-M.; Lee, H.W.; Jae, J.; Kim, D.H.; Jung, S.-C.; Watanabe, C.; Park, Y.-K. Catalytic copyrolysis of cellulose and thermoplastics over HZSM-5 and HY. ACS Sustain. Chem. Eng. 2016, 4, 1354–1363. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Li, J.; Su, L.; Zuo, J.; Komarneni, S.; Wang, Y. Improving the aromatic production in catalytic fast pyrolysis of cellulose by co-feeding low-density polyethylene. Appl. Catal. A Gen. 2013, 455, 114–121. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, Y.; Rezaei, P.S.; Kim, B.-S.; Jeon, J.-K.; Jae, J.; Jung, S.-C.; Kim, S.C.; Park, Y.-K. In-Situ catalytic copyrolysis of cellulose and polypropylene over desilicated ZSM-5. Catal. Today 2017, 293–294, 151–158. [Google Scholar] [CrossRef]
- To, A.T.; Resasco, D.E. Role of a phenolic pool in the conversion of m-cresol to aromatics over HY and HZSM-5 zeolites. Appl. Catal. A Gen. 2014, 487, 62–71. [Google Scholar] [CrossRef]
- Xue, Y.; Kelkar, A.; Bai, X. Catalytic co-pyrolysis of biomass and polyethylene in a tandem micropyrolyzer. Fuel 2016, 166, 227–236. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eränen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Widayatno, W.B.; Guan, G.; Rizkiana, J.; Du, X.; Hao, X.; Zhang, Z.; Abudula, A. Selective catalytic conversion of bio-oil over high-silica zeolites. Bioresour. Technol. 2015, 179, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.-T.; Huber, G.W. Production of targeted aromatics by using Diels–Alder classes of reactions with furans and olefins over ZSM-5. Green Chem. 2012, 14, 3114–3125. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, D.; Kim, Y.-M.; Jae, J.; Jung, S.-C.; Jeon, J.-K.; Kim, S.C.; Park, Y.-K. Catalytic copyrolysis of cork oak and bio-oil distillation residue. Appl. Surf. Sci. 2018, 429, 95–101. [Google Scholar] [CrossRef]


| Cork Oak | WPFs | CoOak/WPFs | |
|---|---|---|---|
| Theoretical Value | Experimental Value | ||
| 2.90 | 11.75 | 7.31 | 7.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-K.; Lee, B.; Watanabe, A.; Lee, H.W.; Lee, J.Y.; Kim, S.; Han, T.U.; Kim, Y.-M. Catalytic Copyrolysis of Cork Oak and Waste Plastic Films over HBeta. Catalysts 2018, 8, 318. https://doi.org/10.3390/catal8080318
Park Y-K, Lee B, Watanabe A, Lee HW, Lee JY, Kim S, Han TU, Kim Y-M. Catalytic Copyrolysis of Cork Oak and Waste Plastic Films over HBeta. Catalysts. 2018; 8(8):318. https://doi.org/10.3390/catal8080318
Chicago/Turabian StylePark, Young-Kwon, Boram Lee, Atsushi Watanabe, Hyung Won Lee, Ji Young Lee, Seungdo Kim, Tae Uk Han, and Young-Min Kim. 2018. "Catalytic Copyrolysis of Cork Oak and Waste Plastic Films over HBeta" Catalysts 8, no. 8: 318. https://doi.org/10.3390/catal8080318
APA StylePark, Y.-K., Lee, B., Watanabe, A., Lee, H. W., Lee, J. Y., Kim, S., Han, T. U., & Kim, Y.-M. (2018). Catalytic Copyrolysis of Cork Oak and Waste Plastic Films over HBeta. Catalysts, 8(8), 318. https://doi.org/10.3390/catal8080318