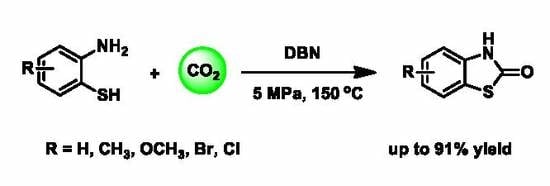
Organic Base-Catalyzed C–S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones
Abstract
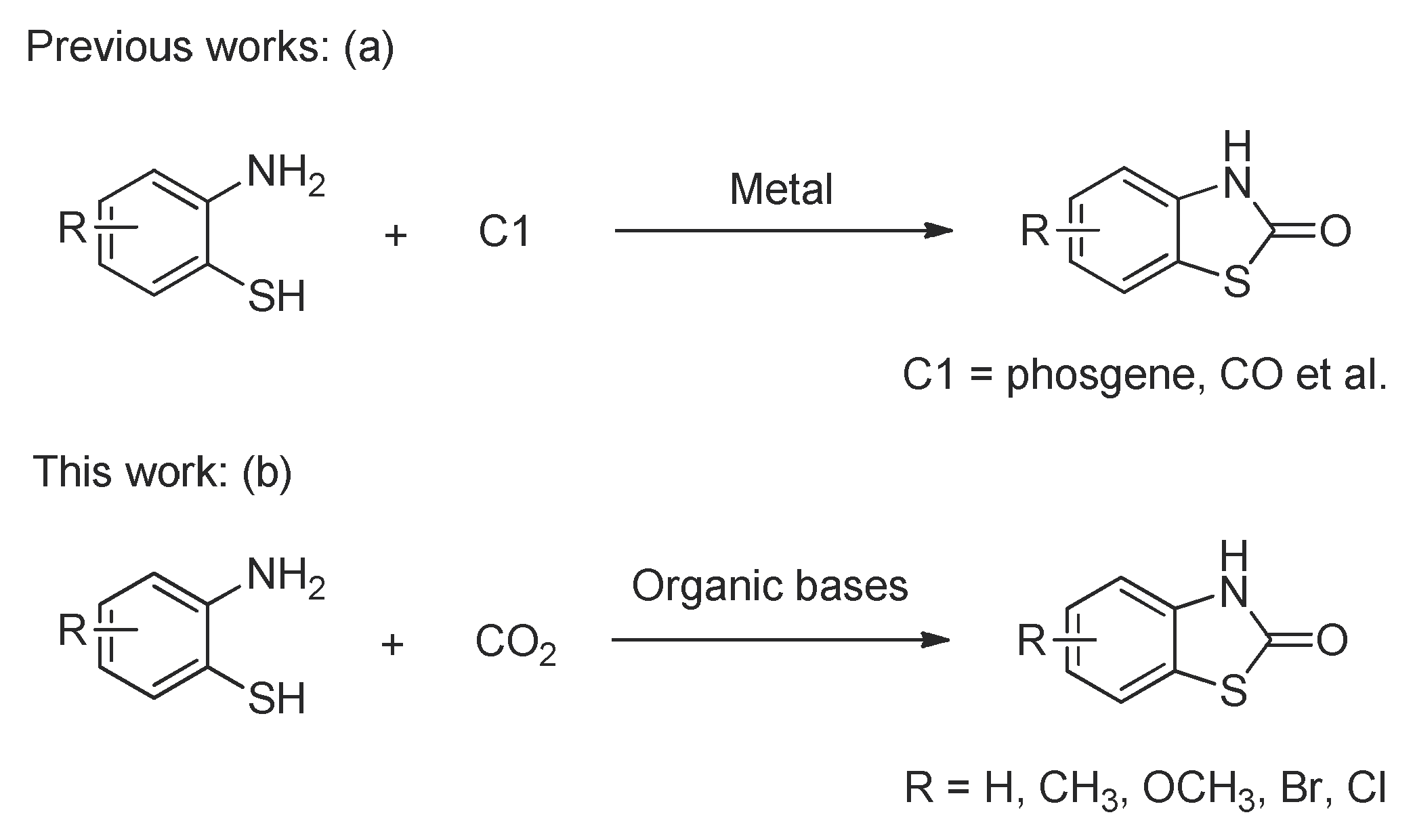
1. Introduction
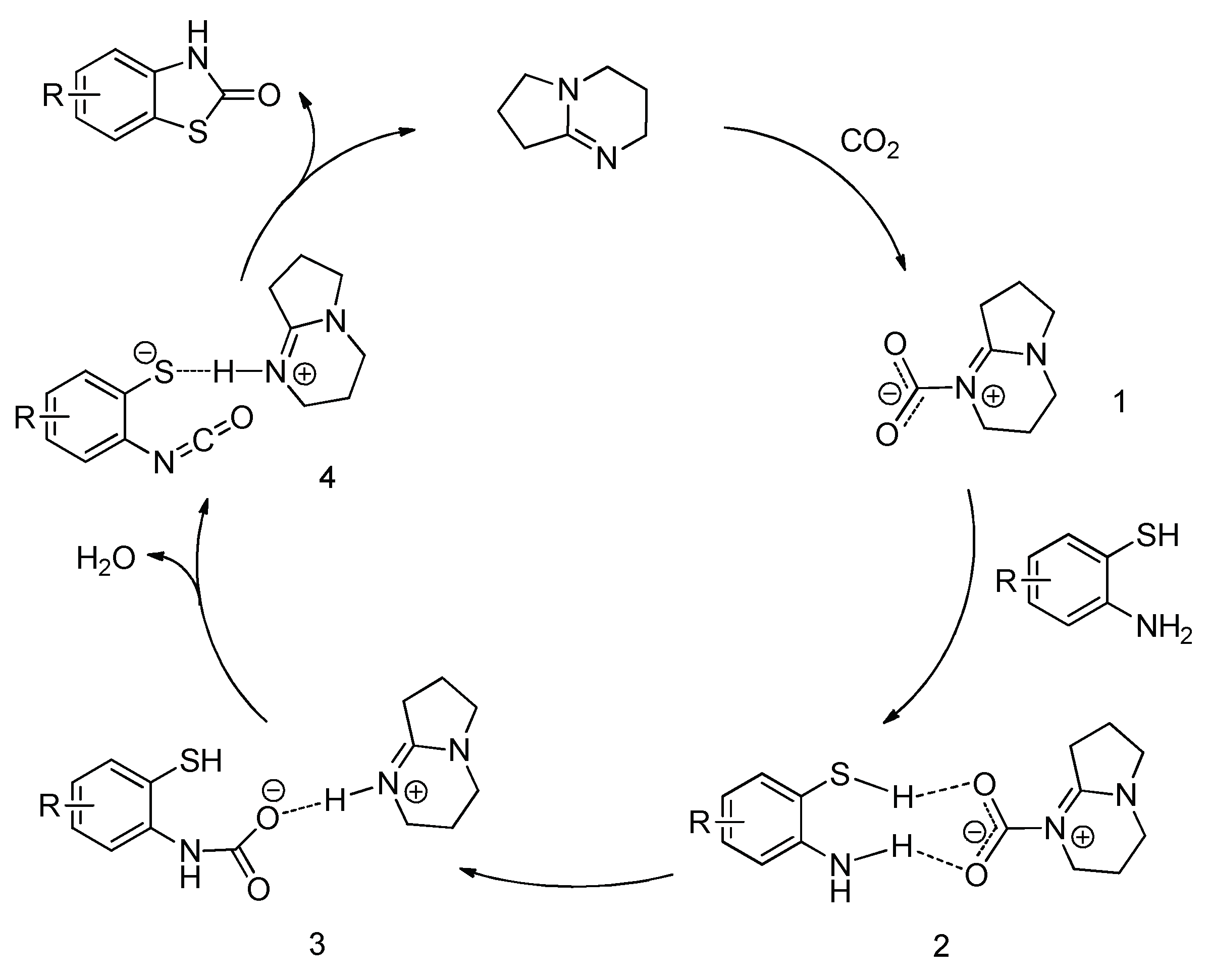
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. General Procedure for the Synthesis of 2-Aminothiophenol Substrates
3.3. Typical Procedure for the Synthesis of Benzothiazolones
3.4. NMR Spectral Data of the Substrates and Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Wang, X.; Ling, G.; Xue, Y.; Lu, S. Selenium-Catalyzed Reductive Carbonylation of 2-Nitrophenols to2-Benzoxazolones. Eur. J. Org. Chem. 2005, 1675–1679. [Google Scholar] [CrossRef]
- Murru, S.; Mondal, P.; Yella, R.; Patel, B.K. Copper(I)-Catalyzed Cascade Synthesis of 2-Substituted 1,3-Benzothiazoles: Direct Access to Benzothiazolones. Eur. J. Org. Chem. 2009, 2009, 5406–5413. [Google Scholar] [CrossRef]
- Ciba, M.; Kaynak, F.; Ozgen, S.; Sahin, M.; Onkol, T. Microwave Synthesis and Antimicrobial Evaluation of Mannich Bases of 6-Benzoyl-2(3H)-benzothiazolone. Asian J. Chem. 2010, 22, 5685–5693. [Google Scholar]
- Aydın, A.; Akkurt, M.; Uzun, L.; Yıldırım, L.; Önkol, T. Synthesis, Crystal Structure and Spectroscopic Characterization of {6-[2-(2-chlorophenyl)-1,3-thiazol-4-yl]-2-oxo-1,3-benzothiazol-3(2H)-yl}acetic acid. J. Chem. Crystallogr. 2010, 40, 816–820. [Google Scholar] [CrossRef]
- Weng, J.; Huang, H.; Yu, Z.; Zhang, H.; Tan, C.; Liu, X. Synthesis and Fungicidal Activity of Hydrazone Derivatives Containing 2(3H)-Benzothiazolone Moiety. Asian J. Chem. 2012, 24, 561–564. [Google Scholar]
- Sridhar, R.; Perumal, P.T. A Novel Method for the Synthesis of 2(3H)-Benzimidazolones, 2(3H)-Benzoxazolone, and 2(3H)-Benzothiazolone via In Situ Generated Ortho Substituted Benzoic Acid Azides: Application of Ammonium Azide and Vilsmeier Complex for Acid Azide Generation. Synth. Commun. 2004, 34, 735–742. [Google Scholar] [CrossRef]
- Velikorodov, A.V.; Kuanchalieva, A.K.; Melent’eva, E.A.; Titova, O.L. Synthesis of 1,3-benzothiazol-2(3H)-one and some its derivatives. Russ. J. Org. Chem. 2011, 47, 1375–1379. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, P.; Yang, Y.; Zeng, Z.; Liu, J.; Yi, H.; Lei, A. Room-Temperature Copper-Catalyzed Oxidation of Electron-Deficient Arenes and Heteroarenes Using Air. Angew. Chem. Int. Ed. 2012, 51, 4666–4670. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Jiang, Y.; Ma, D. CuI-catalyzed one-pot synthesis of benzothiazolones from 2-iodoanilines-derived carbamates and sodium sulfide. Tetrahedron Lett. 2012, 53, 2511–2513. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zeng, W.; Huang, H.; Liang, Y. Copper catalyzed three-component synthesis of benzothiazolones from o-iodoanilines, DMF, and potassium sulfide. RSC Adv. 2014, 4, 6090–6093. [Google Scholar] [CrossRef]
- Patonay, T.; Hegedüs, L.; Mogyoródi, F.; Zolnai, L. α-Haloalkyl Haloformates and Related Compounds. II. Synthesis of Dichloromethyl Carbonates and Their Transformation to Carbamates. Synth. Commun. 1994, 24, 2507–2513. [Google Scholar] [CrossRef]
- Troisi, L.; Granito, C.; Perrone, S.; Rosato, F. Synthesis of benzo-fused five- and six-membered heterocycles by palladium-catalyzed cyclocarbonylation. Tetrahedron Lett. 2011, 52, 4330–4332. [Google Scholar] [CrossRef]
- Singh, M.; Singh, P.; Singh, S. Synthesis of benzoxazole-2-ones, benzothiazole-2-ones and their 2-thione derivatives: Efficient conversion of 2-thione to 2-oxo derivatives. Indian J. Chem. 2007, 46, 1666–1671. [Google Scholar] [CrossRef]
- Fu, Y.; Baba, T.; Ono, Y. Carbonylation of o-Phenylenediamine and o-Aminophenol with Dimethyl Carbonate Using Lead Compounds as Catalysts. J. Catal. 2001, 197, 91–97. [Google Scholar] [CrossRef]
- Schwiebert, K.E.; Chin, D.N.; MacDonald, J.C.; Whitesides, G.M. Engineering the Solid State with 2-Benzimidazolones. J. Am. Chem. Soc. 1996, 118, 4018–4029. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, R.; Lin, Y.; Zhou, X. Lanthanide-catalyzed cyclocarbonylation and cyclothiocarbonylation: A facile synthesis of benzannulated 1,3-diheteroatom five- and six-membered heterocycles. Sci. China Chem. 2014, 57, 1117–1125. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kuhn, F.E. Transformation of Carbon Dioxide with Homogeneous Transition-Metal Catalysts: A Molecular Solution to a Global Challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Sun, Y.; Han, B. Green carbon science: scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: from CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [PubMed]
- Yu, B.; He, L.-N. Upgrading carbon dioxide by incorporation into heterocycles. Using carbon dioxide as a building block in organic synthesis. ChemSusChem 2015, 8, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933–5947. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Carbon–heteroatom bond formation catalysed by organometallic complexes. Nature 2008, 455, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, P.F.; Sun, Q.; Bai, S.Q.; Hor, T.S.A.; Liu, X.G. Recent advances in C–S bond formation via C–H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, B.; Zhao, Y.F.; Hao, L.D.; Liu, Z.M. Hydrosilane-promoted cyclization of 2-aminothiophenols by CO2 to benzothiazoles. RSC Adv. 2014, 4, 56957–56960. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Yang, Z.Z.; Zhao, Y.F.; Zhong, H.Y.; Hao, L.D.; Han, B.X.; Liu, Z.M. Ionic Liquid-Catalyzed C–S Bond Construction using CO2 as a C1 Building Block under Mild Conditions: A Metal-Free Route to Synthesis of Benzothiazoles. ACS Catal. 2015, 5, 6648–6652. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Yuan, G.; Hao, L.; Gao, X.; Yang, Z.; Yu, B.; Zhang, H.; Liu, Z. Azole-Anion-Based Aprotic Ionic Liquids: Functional Solvents for Atmospheric CO2 Transformation into Various Heterocyclic Compounds. Chem. Asian J. 2016, 11, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, H.; Zhao, Y.; Chen, S.; Xu, J.; Hao, L.; Liu, Z. DBU-Based Ionic-Liquid-Catalyzed Carbonylation of o-Phenylenediamines with CO2 to 2-Benzimidazolones under Solvent-Free Conditions. ACS Catal. 2013, 3, 2076–2082. [Google Scholar] [CrossRef]
- Endo, T.; Nagai, D.; Monma, T.; Yamaguchi, H.; Ochiai, B. A novel construction of a reversible fixation− release system of carbon dioxide by amidines and their polymers. Macromolecules 2004, 37, 2007–2009. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Jessop, P.G.; Thomas, C.A.; Eckert, C.A.; Liotta, C.L. Liotta, The Reaction of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) with Carbon Dioxide. J. Org. Chem. 2005, 70, 5335–5338. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.S.; deAzevedo, E.R.; da Silva, E.F.; Bonagamba, T.J.; Agostíni, D.L.d.; Magalhães, A.; Job, A.E.; González, E.R.P. Study of the carbon dioxide chemical fixation-activation by guanidines. Tetrahedron 2008, 64, 10097–10106. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Zhao, Y.-N.; Li, B. CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion. Energy Environ. Sci. 2011, 4, 3971–3975. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Yang, Z.-Z.; Luo, S.-H.; He, L.-N. Design of task-specific ionic liquids for catalytic conversion of CO2 with aziridines under mild conditions. Catal. Today 2013, 200, 2–8. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, W.; Yang, Z.; Wang, J.; Xu, T.; Xin, J.; Zhang, S. Superbase/cellulose: An environmentally benign catalyst for chemical fixation of carbon dioxide into cyclic carbonates. Green Chem. 2014, 16, 3071–3078. [Google Scholar] [CrossRef]
- Gregory, G.L.; Ulmann, M.; Buchard, A. Synthesis of 6-membered cyclic carbonates from 1, 3-diols and low CO2 pressure: a novel mild strategy to replace phosgene reagents. RSC Adv. 2015, 5, 39404–39408. [Google Scholar] [CrossRef]
- Wang, L.; Kodama, K.; Hirose, T. DBU/benzyl bromide: An efficient catalytic system for the chemical fixation of CO2 into cyclic carbonates under metal-and solvent-free conditions. Catal. Sci. Technol. 2016, 6, 3872–3877. [Google Scholar] [CrossRef]
- Cecchi, L.; de Sarlo, F.; Machetti, F. 1,4-Diazabicyclo[2.2.2]octane (DABCO) as an Efficient Reagent for the Synthesis of Isoxazole Derivatives from Primary Nitro Compounds and Dipolarophiles: The Role of the Base. Eur. J. Org. Chem. 2006, 2006, 4852–4860. [Google Scholar] [CrossRef]
- Heller, S.T.; Schultz, E.E.; Sarpong, R. Chemoselective N-Acylation of Indoles and Oxazolidinones with Carbonylazoles. Angew. Chem. Int. Ed. 2012, 51, 8304–8308. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, E.; Angelini, A.; Carafa, M.; Dibenedetto, A.; Mele, V. Carbonic Acid Diester Activation by Polymer-Bound DBU and Its Relevance to Catalytic N-Carbonylation of N-Heteroaromatics: Direct Evidence for an Elusive N-Carboxy-Substituted Amidinium Cation Intermediate. ACS Catal. 2014, 4, 195–202. [Google Scholar] [CrossRef]
- Arico, F.; Evaristo, S.; Tundo, P. Synthesis of five-and six-membered heterocycles by dimethyl carbonate with catalytic amounts of nitrogen bicyclic bases. Green Chem. 2015, 17, 1176–1185. [Google Scholar] [CrossRef]
- Shim, Y.N.; Lee, J.K.; Im, J.K.; Mukherjee, D.K.; Nguyen, D.Q.; Cheong, M.; Kim, H.S. Ionic liquid-assisted carboxylation of amines by CO2: A mechanistic consideration. Phys. Chem. Chem. Phys. 2011, 13, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ma, X.; Zhou, Y.; Liang, S.; Zhang, J.; Han, B. Solvent-free synthesis of substituted ureas from CO2 and amines with a functional ionic liquid as the catalyst. Green Chem. 2008, 10, 465–469. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Miao, C.-X.; Chanfreau, S. Lewis Basic Ionic Liquids-Catalyzed Conversion of Carbon Dioxide to Cyclic Carbonates. Adv. Synth. Catal. 2010, 352, 2233–2240. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Peng, S.-Y.; Liu, A.-H. Lewis basic ionic liquids-catalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 under solvent-free conditions. Green Chem. 2010, 12, 1850–1854. [Google Scholar] [CrossRef]
- Kee, C.W.; Peh, K.Q.E.; Wong, M.W. Coupling Reactions of Alkynyl Indoles and CO2 by Bicyclic Guanidine: Origin of Catalytic Activity? Chem. Asian J. 2017, 12, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
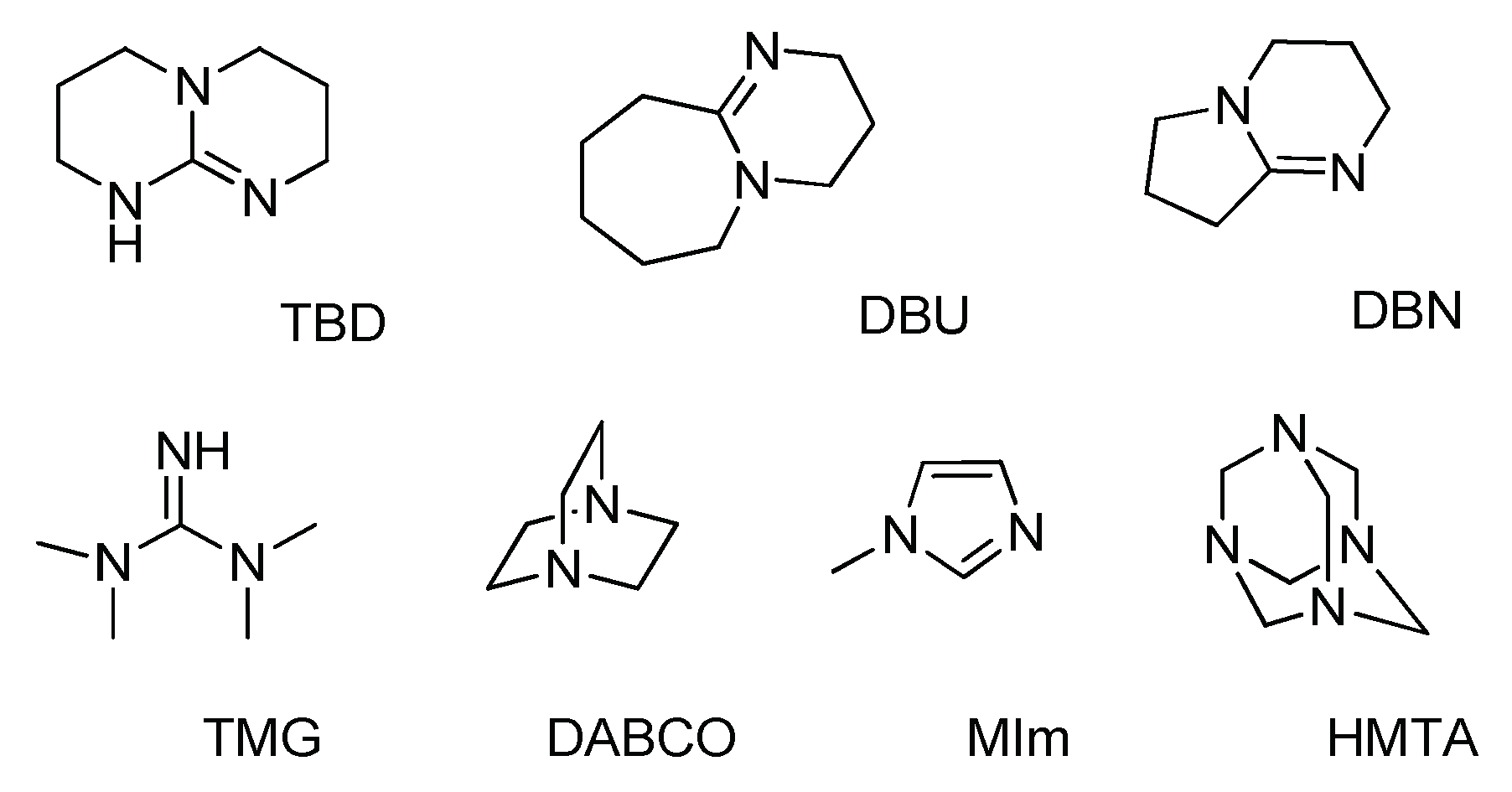
| Entry | Catalyst | pKa of the Conjugated Acid b | Solvent | Yield d (%) |
|---|---|---|---|---|
| 1 | None | --- | NMP | 0 |
| 2 | NaOH | 15.7 c | NMP | 0 |
| 3 | TBD | 26 | NMP | 87 |
| 4 | DBU | 24.3 | NMP | 84 |
| 5 | DBN | 23.8 | NMP | 91 |
| 6 | TMG | 23.3 | NMP | 30 |
| 7 | DABCO | (8.7) | NMP | 7 |
| 8 | MIm | (7.1) | NMP | 4 |
| 9 | HMTA | 6.2 | NMP | 0 |
| 10 | DBN | 23.8 | DMF | 57 |
| 11 | DBN | 23.8 | CH3CN | 32 |
| 12 | DBN | 23.8 | DMSO | 16 |
| Entry | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| CO2 pressure (MPa) | 1 | 3 | 5 | 7 | 9 |
| Yield b (%) | 28 | 77 | 91 | 83 | 79 |
| Entry | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Temperature (oC) | 110 | 120 | 130 | 140 | 150 | 160 |
| Yield b (%) | 37 | 42 | 54 | 78 | 91 | 91 |
| Entry | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Amount of catalyst (mmol) | 0.5 | 1 | 1.5 | 2 | 2.5 |
| Yield b (%) | 41 | 71 | 78 | 91 | 92 |
| Entry | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Time (h) | 12 | 18 | 24 | 30 | 36 |
| Yield b (%) | 66 | 80 | 91 | 96 | 99 |

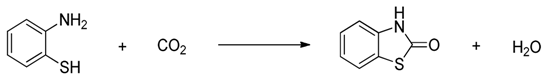
| Entry | Substrate | Product | Yield b(%) |
|---|---|---|---|
| 1 | 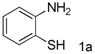 |  | 91(88) |
| 2 | 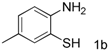 | 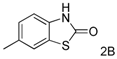 | 70(61) |
| 3 | 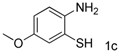 |  | 45(36) |
| 4 | 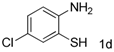 | 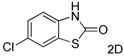 | 36 c |
| 5 | 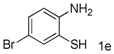 | 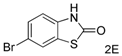 | 23 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Deng, Y.; Lu, C.; Zhang, L.; Wang, X.; Yu, B. Organic Base-Catalyzed C–S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones. Catalysts 2018, 8, 271. https://doi.org/10.3390/catal8070271
Gao X, Deng Y, Lu C, Zhang L, Wang X, Yu B. Organic Base-Catalyzed C–S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones. Catalysts. 2018; 8(7):271. https://doi.org/10.3390/catal8070271
Chicago/Turabian StyleGao, Xiang, Yuehua Deng, Changyu Lu, Lei Zhang, Xintao Wang, and Bo Yu. 2018. "Organic Base-Catalyzed C–S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones" Catalysts 8, no. 7: 271. https://doi.org/10.3390/catal8070271
APA StyleGao, X., Deng, Y., Lu, C., Zhang, L., Wang, X., & Yu, B. (2018). Organic Base-Catalyzed C–S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones. Catalysts, 8(7), 271. https://doi.org/10.3390/catal8070271