Abstract
Mesoporous alumina has been successfully prepared using sucrose as templates. Mesoporous alumina-based catalysts, neat and impregnated with metal chlorides, were tested for gas phase methyl chloride synthesis from methanol and HCl. The catalysts were characterized with Transmission electron microscope (TEM), N2-physisorption, X-ray diffraction (XRD), Fourier Transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) to identify the relationship between the catalyst characteristics and their activity and selectivity. Experimental studies revealed that the alumina acidity decreases with increasing calcination temperature, and the catalytic activity is strongly related to the acidity. The catalytic activity of mesoporous alumina (named as Al2O3-500 °C) is higher than that of the commercial alumina under the same experimental conditions. The metal chlorides-modified alumina has more Lewis acid sites than the neat alumina. Impregnation by alcohol is more effective for increasing the amount of Lewis acid sites than impregnation by water. The total Lewis acid concentration of the modified alumina decreases in the following order: ZnCl2/Al2O3-E > ZnCl2/Al2O3-W > FeCl3/Al2O3-E > FeCl3/Al2O3-W, Where E and W respectively indicate that the catalyst impregnation solution is ethanol and water, which agrees well with the catalytic performance order. The effect of ethanol as a solvent in the impregnation could be due to the inhibition of the hydrolysis of metal chloride. The catalyst delivered a stable performance during a 100 h test that was significantly higher than that of commercial alumina.
1. Introduction
Methyl chloride is an important material for the production of higher chlorinated products, silicones, rubber and methyl cellulose and is also applied as a methylating agent. Two processes are used in the commercial production of methyl chloride, i.e., the chlorination of methane and the hydrochlorination of methanol [1,2,3,4]. The hydrochlorination of the methanol pathway has attracted much more attention in industry. This process can be carried out in the liquid phase catalytically in the presence of zinc chloride or in the gas phase over alumina catalysts.
Alumina is an inexpensive porous metal oxide widely used as catalyst or as support materials in chemical industry. The hydrochlorination of methanol over alumina catalysts is believed to be caused by the presence of acid centres on the catalyst surface. However, it is less active and requires higher reaction temperatures. Mesoporous alumina has attracted much more attention due to its moderately high surface area, adequate pore volume for metal loadings, narrow pore size distribution and acid-base properties. Alumina with a mesostructure should show excellent catalytic performance on methyl chloride synthesis, needing further study.
Metal chlorides often act as a Lewis acid catalyst in homogeneous or heterogeneous catalytic reaction. Typical Lewis acid catalysts include AlCl3, FeCl3, FeBr3, and ZnCl2. The catalysts modified with zinc chloride are more active and selective towards methyl chloride than neat alumina [5,6,7]. In addition, neat form and ZnCl2 modified zeolite, including H-Beta and H-ZSM5 catalysts with varying Si/Al ratios, were also studied. ZnCl2 modified zeolite catalysts were found to be highly active, albeit less selective than the ZnCl2 modified alumina [8]. However, the drawback of the zeolite catalysts is their relatively high cost and the susceptibility to deactivation by coking, especially for the low Si/Al ratios [8].
To understand the catalytic properties of the ZnCl2 modified catalysts, it is very critical to know the chemical state of zinc on the catalyst surface. Pillai et al. [9], applying 1H Magic angle spinning-Nuclear magnetic resonance (1H MAS-NMR) and XPS analysis of ZnCl2-modified Al2O3 and unmodified Al2O3, suggested that zinc species replace the terminal basic hydroxide groups on the alumina surface. Meanwhile, the Zn–Cl bond is retained. Other studies [10,11,12] reported that the chlorine-to-zinc ratio was reduced compared to [Cl]/[Zn] stoichiometry, suggesting the formation of an S–O–Zn–Cl species, where S stands for the support. In the case of the ZnCl2-modified alumina or zeolites, the state of the Zn species on the catalyst surface is also under debate. In addition, zinc chloride itself is hygroscopic and even deliquescent in ambient air. It is difficult to fully dehydrate zinc chloride by heating in air because zinc chloride hydrates undergo hydrolytic decomposition with the evolution of hydrogen chloride rather than dehydration. Products such as Zn5(OH)8Cl2·H2O and Zn2OCl2·2H2O were formed during the hydrolysis process [13,14]. Considering the hydrolytic nature of zinc chloride and the interaction between zinc chloride and support, it is difficult to study the chemical state of zinc on the catalyst surface and relevant catalytic performance by water impregnation method. Since certain hydrous metal chloride cannot be simply dehydrated by drying or calcining without the decomposition. Alcohols for the dehydration have been presented [15]. Loading metal chloride with different solvent provide convenience to study the difference of chemical state of zinc on alumina and relevant catalytic performance on methyl chloride synthesis. In this work, mesoporous alumina was synthesized using sucrose as templates and aluminium isopropoxide as aluminium source to understand whether the mesostructure will lead to excellent catalytic performance on methyl chloride synthesis or not. The catalysts were characterized with Fourier Transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and N2-physisorption to identify the relationship between the catalyst characteristics and their activity and selectivity in methyl chloride synthesis. The effect of the calcination temperature on the catalytic activity of mesoporous alumina was also investigated. Mesoporous γ-alumina was modified by FeCl3 and ZnCl2 using water and ethanol as solvents to understand the effect of the solvent on the chemical state of zinc and iron and the relevant catalytic performance.
2. Results and Discussion
2.1. Characterization Results of Mesoporous Alumina
2.1.1. Characterization by Nitrogen Physisorption
Figure 1 shows the isotherms for the Al2O3 samples calcined in the temperature range of 500 to 800 °C. The N2 adsorption-desorption isotherms of Al2O3 are assigned to type IV isotherms with the H2 type of hysteresis according to IUPAC classification, which is characteristic of a mesoporous material. These mesoporous aluminas have relative narrow pore size distributions. It was also found that increasing calcination temperature could widen the pore size distribution of mesoporous alumina and leads to the formation of larger pores. However, the pore size distribution of mesoporous alumina is narrow compared to the commercial alumina.
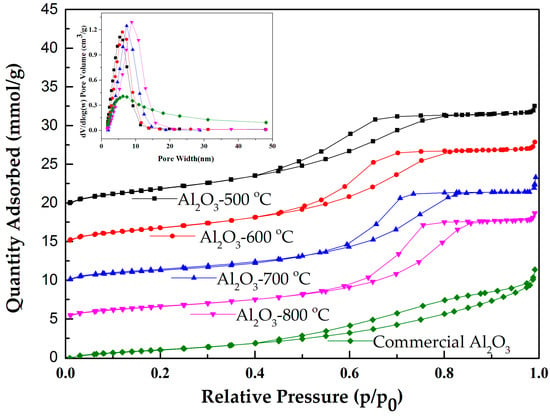
Figure 1.
Nitrogen adsorption-desorption isotherms curves and pore distribution of mesoporous alumina with different calcination temperature. Isotherms curves are on the same scale and offset vertically for clarity.
The specific surface areas and pore volumes of the catalysts were determined. The results are summarized in Table 1. The calcination temperature appears to significantly affect the surface area and pore diameter. A higher calcination temperature leads to the decrease of the surface area of mesoporous alumina. Al2O3-500 °C has a large BET surface area of 343 m2/g. Meanwhile, the specific surface area is reduced to 216 m2/g for Al2O3-800 °C. However, the average pore diameter is increased with increasing calcination temperature. Overall, the specific surface area of mesoporous alumina is larger than that of commercial alumina. Since the mesoporous alumina was modified by zinc chloride and ferric chloride, it is quite clear that the specific surface area and pore volume were reduced because of the blockage in the pores. Pore widths of water impregnated catalyst are increased. It may be due to the dehydration of hydroxyl groups between alumina surface and hydrated metal chloride.

Table 1.
Specific surface area (m2/g) and pore volume (cm3/g) of catalysts
2.1.2. X-ray Powder Diffraction (XRD)
Figure 2 shows the XRD patterns of mesoporous alumina calcined at different temperatures. For comparison, all catalysts exhibited the typical patterns of γ-alumina (JCPDS Card No. 10-0425). With the increment of the calcination temperature, the reflection of γ-alumina became more narrow and intense, as a result of a characteristic change in the average crystallite size. Characteristic reflection of FeCl2·2H2O (JCPDS Card No. 25-1040) were observed in the FeCl3/Al2O3-500 °C (C2H6O) catalyst. This is because ferric chloride can be reduced to ferric(II) chloride by ethanol under light [12]. Ferric(II) chloride can easily absorb the moisture in air and become hydrated. In addition, the reflection of zinc chloride and ferric chloride were not observed in other catalysts.
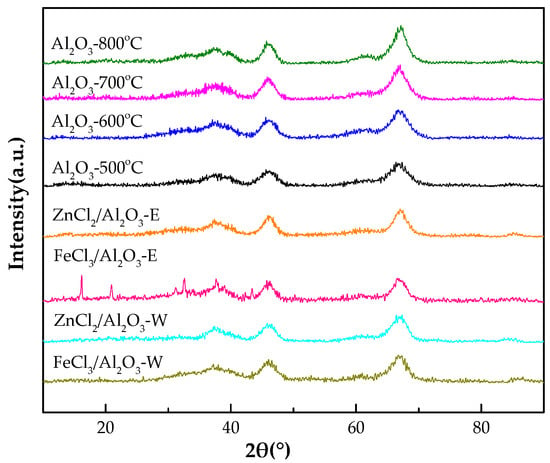
Figure 2.
X-ray diffraction patterns of mesoporous alumina and the metal chlorides-modified mesoporous alumina.
2.1.3. Quantification of Lewis Acid Sites by FTIR
The catalyst acidities were measured by FTIR by using pyridine as the probe molecule. The results are summarized in Figure S1. The Lewis acidity was determined from the adsorption band at 1455 cm−1. The strength of the acid sites is determined from the amount of pyridine desorbed within a defined temperature interval according to the work of Schmidt [7]. Table 2 shows the Lewis acid sites for the alumina samples. The acidity of the investigated alumina decreases with increasing calcination temperature in the temperature range of 500–800 °C. During dehydration of the surface, adjacent hydroxyl ions combine to form water molecules. A13+ Lewis acid sites were created upon the dehydroxylation of A12O3. Thermal dehydroxylation studies of γ-A12O3 were carried out by IR spectroscopy in several studies to understand the coordinatively unsaturated Al3+ sites [16]. However, no correlation was observed between the extent of dehydroxylation and the production of Lewis acid sites as a function of the treatment temperature. The metal chlorides-modified alumina has a higher number of Lewis acid sites than the neat alumina. In addition, impregnation by alcohol is more effective for increasing Lewis acid sites than that by water for both ZnCl2 and FeCl3. The total Lewis acid concentration of modified alumina decreases in the following order: ZnCl2/Al2O3-E > ZnCl2/Al2O3-W > FeCl3/Al2O3-E > FeCl3/Al2O3-W.

Table 2.
The Lewis acidity of the prepared catalysts.
2.2. Catalytic Performance
2.2.1. Effect of Calcination Temperature on Catalytic Performance
The activities of the catalysts treated with different calcination temperatures were tested as shown in Figure 3. The catalytic performance was tested at the temperature of 280 °C and methanol space velocity of 4.75 g MeOH·g−1 (cat.)·h−1. The conversion of methanol is decreased with the increase of the calcination temperature and the selectivity of methyl chloride changed little. The catalytic activity site is often related to the specific surface area of the catalyst. It is clear that the catalytic activity of alumina (shown in Figure 3) is correlated with the specific surface area (data shown in Table 1). This is because the reduction in the specific surface area leads to a decrease in the active sites on the catalyst surface for the increase of the calcination temperature. Moreover, the activity of alumina for dehydration is believed to be caused by the presence of acid centres on the catalyst surface. FTIR investigations using pyridine as the probe molecule showed that the total amount of Lewis acid sites decreased with increasing temperature. A collapse in the alumina structure is observed with the increase of the calcination temperature, leading to a decline in the catalytic activity towards dehydration reaction. The catalytic activity can be related to the number and strength of the Lewis acid sites as shown in Table 2.
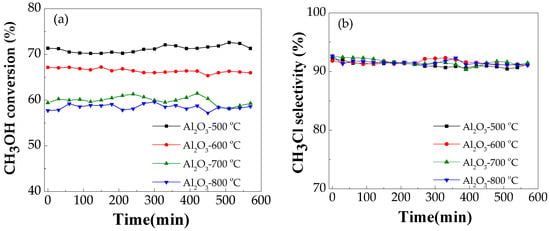
Figure 3.
The methanol conversion (a) and the methyl chloride selectivity (b) over mesoporous alumina with different calcination temperatures (Conditions: 4.75 g MeOH·g−1 (cat.)·h−1, temperature 280 °C).
2.2.2. Effect of Methanol Space Velocity on Catalytic Performance
To compare the catalytic activity of Al2O3-500 with that of commercial alumina, the reaction was performed in the fixed bed reactor at 280 °C with the methanol space velocity of 1.58 g MeOH·g−1 (cat.)·h−1. Figure 4 shows the methanol conversion and selectivity of different catalysts. Compared to commercial alumina, Al2O3-500 exhibits a very high conversion of approximately 92% with nearly the same selectivity. Even though the methanol space velocity increased to 4.75 g MeOH·g−1 (cat.)·h−1, the methanol conversion of Al2O3-500 was 71%, which was significantly higher than that of commercial alumina. The catalytic activity of mesoporous alumina prepared using sucrose as the template is higher than that of the commercial γ-alumina catalyst under the same experimental conditions.
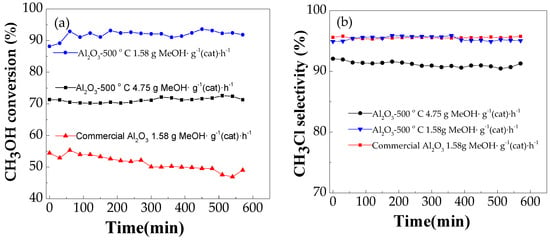
Figure 4.
The methanol conversion (a) and the methyl chloride selectivity (b) over commercial alumina and mesoporous alumina (Conditions: temperature 280 °C).
2.2.3. Effect of Modification on Catalytic Performance
Mesoporous alumina was modified by FeCl3 and ZnCl2 by the impregnation method using water as solvent. To compare the effect of the modification method, another catalyst was also prepared using ethanol as solvent and calcination in HCl atmosphere. All catalytic performances were investigated in the same conditions. Figure 5 shows the catalytic performance of mesoporous alumina, metal chlorides-modified mesoporous alumina using water as solvent and metal chlorides-modified mesoporous alumina using ethanol as solvent and calcination in HCl atmosphere. The modification of alumina with FeCl3 and ZnCl2 significantly changes its catalytic activity (Figure 5). The methanol conversion values of ZnCl2/Al2O3-500 °C (H2O) and FeCl3/Al2O3-500 °C (H2O) are 82% and 73%, respectively, which are obviously higher than that of the unmodified catalyst. In particular, even higher conversions were achieved over alumina modified by metal chlorides, using ethanol as solvent and calcined in HCl atmosphere. The highest conversion of 85% was observed over ZnCl2-modified mesoporous alumina using ethanol as solvent and calcination in HCl atmosphere. The metal chlorides-modified mesoporous alumina enhances not only the conversion of methanol but also the selectivity of methyl chloride formation. The highest selectivity of greater than 95% was achieved by using ZnCl2 as the modifier, ethanol as solvent and calcination in HCl atmosphere.
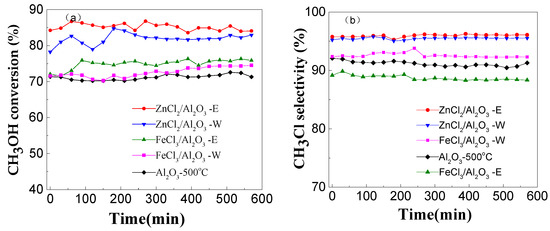
Figure 5.
The methanol conversion (a) and the methyl chloride selectivity (b) over modified mesoporous alumina (Conditions: 4.75 g MeOH·g−1 (cat.)·h−1, temperature 280 °C).
During the dehydration on alumina, the Lewis acid on the surface is the active site. Modification by FeCl3 and ZnCl2 could obviously increase the Lewis acid concentration on the catalyst surface (Table 2). Furthermore, the total Lewis acid concentration on the catalyst surface decreases in the following order: ZnCl2/Al2O3-E > ZnCl2/Al2O3-W > FeCl3/Al2O3-E > FeCl3/Al2O3-W > Al2O3-500, which agrees well with the methanol conversion sequence displayed in Figure 5a. The best results obtained showed that FeCl3- and ZnCl2-modified aluminas are more active than the unmodified alumina and the use of ethanol as the solvent instead of water and calcination in HCl atmosphere is more effective.
Metal chlorides were often used as a Lewis acid for the modification of a solid acid catalyst. However, the hydrolysis of metal chlorides at the temperature rise period may strongly affect the catalytic activity. Thus, the content of metal and chlorine in the modified catalysts was determined by ICP and UV, respectively. Metal and chlorine content in the catalyst surface was also evaluated by XPS for comparison. The calculated atomic ratios of chlorine to metal for each catalyst are shown in Table 3. The Cl/Zn atomic ratio of ZnCl2/Al2O3-E was 1.60, which was lower than the stoichiometric ratio. However, it is higher than the Cl/Zn atomic ratio of ZnCl2/Al2O3-W. The same rule has also been found to be true for the iron(III) chloride modified alumina.

Table 3.
The ratio of Cl/Zn in the prepared catalysts determined by UV, ICP and XPS.
Hydrous metal chloride can be easily hydrolysed at a high temperature. Zinc chloride is mostly found in the hydrated form at room temperature. The dehydration of zinc chloride occurs by the conversion to the Zn5(OH)8Cl2·2H2O over the temperature range of 300–350 °C and Zn2OCl2·2H2O was formed at a higher temperature. Upon the increase of the temperature to above 380 °C, Zn2OCl2·2H2O is further decomposed into ZnO. Hydrogen chloride gas continually escaped in the hydrolysis process [13]. Ethanol can replace water to form coordinates with ZnCl2, making the dehydration of zinc chloride much easier. For iron(III) chloride, the conversion of the hydrate to anhydrous iron(III) chloride is not accomplished by heating, because hydrogen chloride gas and iron oxychlorides are produced. In addition, anhydrous iron(III) chloride can be decomposed to give iron(II) chloride and chlorine gas. When ethanol is used as the solvent, it can also be reduced to ferric(II) chloride by ethanol under light. To summarize, the use of ethanol as the solvent in the impregnation process could inhibit the hydrolysis of metal chloride. This could significantly increase the amount of the Lewis acid active sites of alumina.
The zinc chlorides-modified mesoporous alumina obtained using different solvents were analysed by XPS. The obtained spectra were similar and representative XPS spectra for zinc obtained from modified mesoporous alumina are shown in Figure 6. Table 4 lists the binding energies of the zinc species in the pure zinc compound and in the zinc-supported alumina catalysts. Because of the state of the Zn species on the catalyst surface is still under debate, it is difficult to identify the zinc species and determine their relative amounts in the catalysts. The study by Pillai et al. shows that zinc is likely to interact with the surface functional groups of the catalyst support. In the case of alumina, the most likely modification is Al–O–Zn–Cl [8]. Since water is used as the solvent in the impregnation, the Al–O–Zn–Cl species probably formed by the dehydration of hydroxyl groups on the alumina surfaces and the hydroxyl groups on the Zn species. The binding energies of zinc 2p1/2 and the 2p3/2 in ZnCl2/Al2O3-E are 1022.88 eV and 1046.08 eV, respectively. The ∆Eb of Zn 2p1/2 and Zn 2p3/2 between ZnCl2/Al2O3-E and pure ZnCl2 is −0.12 eV. However, the ∆Eb of Zn 2p1/2 and Zn 2p3/2 between ZnCl2/Al2O3-W and pure ZnCl2 is −0.71 eV. It appears that Zn 2p1/2 and Zn 2p3/2 of ZnCl2/Al2O3-W show significant chemical shifts through the formation of the Zn–O species. However, it is important to note that the measured stoichiometry [Cl]/[Zn] ratio of ZnCl2/Al2O3-W is lower than 1. This shows that the Zn–Cl bond is partly retained in the ZnCl2/Al2O3-W catalyst surface. Which could probably confirm the Al–O–Zn–Cl species on the mesoporous alumina. This is caused by the dehydration of the hydroxyl groups on the alumina surfaces and the hydroxyl groups on the Zn species. As for ZnCl2/Al2O3-E, it seems that ZnCl2 is the predominant zinc species on alumina from the results of XPS and Cl/Zn molar ratio. The different zinc species on alumina is responsible for the activity and selectivity of different catalysts.
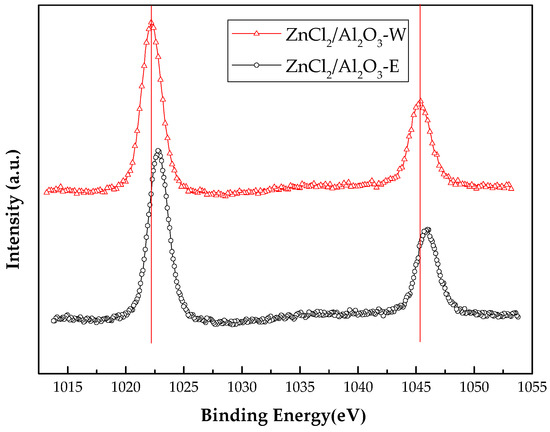
Figure 6.
XPS spectra of Zn 2p in ZnCl2/Al2O3-E and ZnCl2/Al2O3-W.

Table 4.
Binding energies of Zn 2p1/2 and Zn 2p3/2 of pure zinc species and zinc chloride modified alumina.
2.2.4. Catalyst Stability
To study the long-term catalytic stability of alumina and metal chlorides-modified alumina, a long-term experiment of 100 h was performed at 280 °C with the methanol space velocity of 4.75 g MeOH·g−1 (cat.)·h−1. The results are displayed in Figure 7. All catalysts are stable and show no decline of conversion or selectivity. The ZnCl2/Al2O3-E catalyst shows the highest conversion and selectivity.
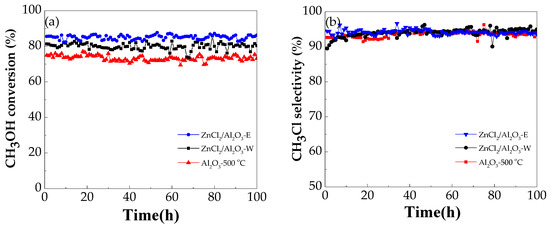
Figure 7.
Lifetime test of the methanol conversion (a) and the methyl chloride selectivity (b) over neat and modified alumina (Conditions: 4.75 g MeOH·g−1 (cat.)·h−1, temperature 280 °C).
3. Experimental
3.1. Catalyst Preparation
Sucrose, commercial γ-alumina, anhydrous zinc chloride and anhydrous iron(III) chloride were purchased from Beijing Chemical Reagent (Beijing, China). Aluminium iso-propoxide was purchased from Sigma-Aldrich (99.99%, Shanghai, China). All other chemicals were used as received without purification. Deionized water was used in all syntheses.
In a typical procedure, 17.1 g of sucrose was dissolved in 90 mL of deionized water under vigorous stirring at room temperature until all sucrose dissolved; 10.2 g of aluminium iso-propoxide was added to the solution and stirred magnetically. Then, nitric acid was added to adjust the pH to 5.5. The mixture was covered with a polyethylene film, stirred at room temperature for approximately 48 h and then placed into a drying oven at 80 °C to undergo the solvent evaporation process [17]. At different temperatures, calcination was carried out using the temperature ramp of 3 °C/min in air for 4 h. The alumina was named as Al2O3-500 °C, Al2O3-600 °C, Al2O3-700 °C and Al2O3-800 °C, indicating the alumina catalysts that were calcined in air at 500, 600, 700 and 800 °C, respectively.
The modified alumina catalysts were prepared via the incipient wetness impregnation method. An aqueous ZnCl2 (or FeCl3) solution with pH adjusted to 3.0 was added into the alumina (calcined in air at 500 °C) at room temperature under stirring, followed by incubation at 65 °C for 10 h and was then evaporated at 80 °C under vacuum. The molar ratio of Zn/Al(Fe/Al) was 1/20. The catalyst was calcined for 2 h at 400 °C in air, and designated ZnCl2/Al2O3-W and FeCl3/Al2O3-W, respectively. For comparison, another modified mesoporous alumina catalyst was also prepared using ethanol as a solution instead of water and calcined in the HCl atmosphere, which were designated ZnCl2/Al2O3-E and FeCl3/Al2O3-E.
3.2. Catalyst Characterization
3.2.1. Nitrogen-Physisorption
The specific surface areas and pore size distributions were obtained from nitrogen adsorption-desorption isotherms measured using a Micromeritics ASAP 2020C adsorption analyser (Micromeritics Instrument Co., Norcross, GA, USA) at −196 °C. All samples were degassed at 200 °C in vacuum for 6 h prior to adsorption measurements. The surface areas were calculated by the Brunauer–Emmett–Teller (BET) method, and the pore size distributions and total pore volume were determined by the Barrett-Joyner-Halenda (BJH) method from the desorption branch of the isotherms.
3.2.2. X-ray Diffraction
The X-ray diffraction (XRD) patterns of the samples were recorded with a Bruker D8 Advance diffractometer (Bruker, Billerica, MA, USA) using Cu Kα radiation (λ = 1.5418 Å) with the step size of 0.019605 in the 2θ range from 10° to 90°. The accelerating voltage and applied current were 40 kV and 40 mA, respectively.
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
The amount of Lewis and Brønsted acid sites on the catalysts was quantified by Fourier transform infrared spectroscopy (Thermo Nicolet Co., Madison, WI, USA) using pyridine as the probe molecule. The Lewis acidity was determined from the adsorption band at 1455 cm−1 and the Brønsted acidity from the adsorption band at 1545 cm−1 according to Emeis [18].
3.2.4. X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) analysis was also conducted by using a Kratos AXIS Ultra DLD spectrometer (Shimadzu-kratos Co., Kyoto, Japan) with a monochromatised Al-Ka X-ray source (225 W). The binding energy was calibrated with respect to the C 1s level of contaminated carbon at 284.80 eV.
3.2.5. UV
The chlorine content in the catalyst was determined using a UV spectrophotometer. Absorbance was obtained using a UV spectrophotometer (Shanghai Jingke 752 N, Shanghai Lengguang Technology Co., Ltd., Shanghai, China). The sample was treated by the standard method, and the sample was measured with the blank reagent as the reference. The absorbance of the sample was measured by a beam with the wavelength of 460 nm.
3.2.6. ICP
The content of iron and zinc in the catalyst was measured by ICP (Agilent Technologies Inc., Santa Clara, CA, USA). ZnCl2 was diluted with different quantity of deionized water as water samples of known Zn2+ concentration. The consistency of the water samples was measured. Then based on the result, a standard curve was plotted. After testing the absorbance of the sample, the concentration of metal ions in the sample was obtained from the standard curve.
3.3. Catalytic Performance Evaluation
The reaction was carried out in a stainless steel tubular reactor with the length of 400 mm and the inner diameter of 10 mm. Experiments were performed for at least 600 min under the following conditions: 280 °C, 20 mL/min CH4/N2 mixture gas, 6 mL/min HCl and 0.01 mL/min MeOH, corresponding to the stoichiometric HCl:MeOH ratio of 1:1. The catalyst mass used in the experiments was 0.1 g for mesoporous γ-Al2O3. The amount of the commercial alumina catalyst was increased to 0.3 g owing to its lower activity. Methanol was fed from a tank containing liquid methanol using a LabAlliance HPLC pump (series II, Scientific Systems, Inc., Philadelphia, PA, USA). HCl was fed from gas cylinders. A mixture of 1.003% methane in nitrogen was fed from gas cylinders. The methane of the mixture gas was used as the internal standard substance. The mixture gas was added as an inert gas to suppress problematic corrosion. After the reactor, a neutralization column filled with calcium oxide was installed to remove HCl and water at 120 °C. Thus, the corrosion problem was minimized and HCl and water injections into GC were prevented. The gas samples were analysed by a gas chromatographic analysis using a Fuli 9790 II instrument (Zhejiang Fuli Analytical Instrument Co., Ltd., Taizhou, China) equipped with an FID and a GDX-302 Column (2 m, I.D. 3 mm, T = 50 °C). The retention times of the components were: CH4: 0.819 min; MeCl: 11.255 min; DME: 17.812 min; MeOH: 23.985 min. The system was calibrated for methyl chloride, dimethyl ether and methanol using mixtures of known concentrations.
The methanol conversion and the methyl chloride selectivity were calculated as follows (no products were present in the feed [7]).
4. Conclusions
The alumina synthesized with sucrose as the template and aluminium iso-propoxide as the aluminium source have high surface areas, uniform pore structures and narrow pore-size distributions. The acidity of the mesoporous alumina decreases with increasing calcination temperature. The catalytic activity of the mesoporous alumina is higher than that of the commercial alumina catalyst under the same conditions. The metal chlorides-modified alumina has a higher amount of Lewis acid sites than the neat alumina. In addition, impregnation by alcohol is more effective for increasing the amount of Lewis acid sites than impregnation by water for both ZnCl2 and FeCl3. The total Lewis acid concentration of the modified alumina decreases in the following order: ZnCl2/Al2O3-E > ZnCl2/Al2O3-W > FeCl3/Al2O3-E > FeCl3/Al2O3-W, which is in good agreement with the order of the observed catalytic performance. Ethanol used as the solvent in impregnation inhibits the hydrolysis of metal chloride. ZnCl2 is the predominant zinc species in ZnCl2/Al2O3-E which is responsible for the higher catalytic performance. A long-term stability test indicates that the catalysts are stable.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/8/3/99/s1, Figure S1: FTIR spectra of pyridine adsorbed on mesoporous aluminas and metal chlorides modified aluminas. Spectra are on the same scale and offset vertically for clarity.
Acknowledgments
This research was performed with the support of the Doctor Foundation of Xinjiang Bingtuan (No. 2014BB001), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT_15R46), and Yangtze River scholar research project of Shihezi University (No. CJXZ201601).
Author Contributions
Jianshu Zhang conceived and designed the experiments; Yuwen Ji and Feilong Zhang performed the experiments; Jinli Zhang analyzed the data; Feng Yu contributed reagents/materials/analysis tools; Jianshu Zhang and Yuwen Ji wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thyagarajan, M.S.; Kumar, R.; Kuloor, N.R. Hydrochlorination of methanol to methyl chloride in fixed catalyst beds. Ind. Eng. Chem. Proc. Des. Dev. 1966, 5, 209–213. [Google Scholar] [CrossRef]
- Podkolzin, S.G.; Stangland, E.E.; Jones, M.E.; Peringer, E.; Lercher, J.A. Methyl chloride production from methane over lanthanum-based catalysts. J. Am. Chem. Soc. 2007, 129, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Grenouillet, P.; Bornette, F.; Bellefon, C. Switching from water to ionic liquids for the production of methylchloride: Catalysis and reactor issues. Chem. Eng. J. 2009, 145, 441–445. [Google Scholar] [CrossRef]
- Becerra, A.M.; Castro Luna, A.E.; Ardissone, D.E.; Ponzi, M.I. Kinetics of the catalytic hydrochlorination of methanol to methyl chloride. Ind. Eng. Chem. Res. 1992, 31, 1045–1050. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Kumar, N.; Reinsdorf, A.; Eränen, K.; Wärnå, J.; Murzin, D.Y.; Salmi, T. Methyl chloride synthesis over Al2O3 catalyst coated microstructured reactor-Thermodynamics, kinetics and mass transfer. Chem. Eng. Sci. 2013, 95, 232–245. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Peurla, M.; Narendra Kumar, N.; Eränen, K.; Murzin, D.Y.; Salmi, T. Preparation of selective ZnCl2/alumina catalysts for methyl chloride synthesis: Influence of pH, precursor and zinc loading. Appl. Catal. A Gen. 2015, 490, 117–127. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Kumar, N.; Zhang, B.; Eranen, K.; Murzin, D.Y.; Salmi, T. Preparation and characterization of alumina-based microreactors for application in methyl chloride synthesis. Ind. Eng. Chem. Res. 2012, 51, 4545–4555. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Kumar, N.; Shchukarev, A.; Eränen, K.; Mikkola, J.-P.; Murzin, D.Y.; Salmi, T. Preparation and characterization of neat and ZnCl2 modified zeolites and alumina for methyl chloride synthesis. Appl. Catal. A Gen. 2013, 468, 120–134. [Google Scholar] [CrossRef]
- Pillai, S.K.; Hamoudi, S.; Belkacemi, K. Functionalized value-added products via metathesis of methyloleate over methyltrioxorhenium supported on ZnCl2-promoted mesoporous alumina. Fuel 2013, 110, 32–39. [Google Scholar] [CrossRef]
- Aoun, M.; Chater, M. Préparation et caractérisation des catalyseurs Rh/Al2O3 et Rh/ZnO-Al2O3. C. R. Chim. 2007, 10, 644–651. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mantri, K. Thermal activation of a clayzic catalyst useful for Friedel-Crafts reactions: HCl evolved with creation of active sites in different thermal treatments to ZnCl2/Mont-K10. Catal. Lett. 2002, 81, 163–168. [Google Scholar] [CrossRef]
- Conte, M.; Davies, T.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Selective formation of chloroethane by the hydrochlorination of ethane using zinc catalysts. J. Catal. 2007, 252, 23–29. [Google Scholar] [CrossRef]
- Ma, J.H.; Yang, H.Q.; Li, L.; Xie, X.L.; Liu, B.; Zhang, L.H.; Zhang, L.N. Synthesis of aligned ZnO submicron rod arrays by heating zinc foil covered with ZnCl2 solution. Acta Chim. Sin. 2009, 67, 1515–1522. [Google Scholar]
- Ma, Y.H.; Wang, Q.H.; Wang, X.N.; Sun, X.H.; Wang, X.Q. A comprehensive study on activated carbon prepared from spent shiitake substrate via pyrolysis with ZnCl2. J. Porous Mater. 2015, 22, 157–169. [Google Scholar] [CrossRef]
- Zhang, D.; Azakami, T.; Yazawa, A. Utilization of alcohols for the dehydration of magnesium chloride. Can. Metall. Q. 2013, 31, 189–194. [Google Scholar] [CrossRef]
- Ballinger, T.H.; Yates, J.T. IR spectroscopic detection of Lewis acid sites on Al2O3 using adsorbed CO Correlation with Al-OH group removal. Langmuir 1991, 7, 3041–3045. [Google Scholar] [CrossRef]
- Xu, B.J.; Long, J.; Yan, Z.F.; Zhu, Y.X.; Tian, H.P. Synthesis of mesoporous alumina using sucrose as the template. Ind. Catal. 2010, 18, 31–35. [Google Scholar]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).