Abstract
Ru-Zn catalysts were synthesized via a precipitation method, and the mechanism of NaOH modifying Ru-Zn catalysts on the selective hydrogenation of benzene to cyclohexene was thoroughly investigated. Fresh as well as used catalysts were characterized via X-ray diffraction (XRD), X-ray Fluorescence (XRF), transmission electron microscope (TEM), scanning electron microscope (SEM), Auger electron spectroscopy (AES), X-ray photoelectron spectroscopy (XPS), and density functional theory (DFT), respectively. Before catalytic experiments, metallic Ru and rodlike ZnO were detected from fresh Ru-Zn catalysts. Notably, with the increasing concentration of NaOH added into the reaction medium (e.g., from 0 to 0.6 mol·L−1), the dispersion of ZnO on the Ru surface significantly improved, resulting in the enhancement Ruδ+ species of electron deficiency. The catalytic activity towards benzene conversion was therefore retarded and the selectivity towards cyclohexene was improved. When the added NaOH concentration reached 0.6 mol·L−1, the atomic ratio of Zn/Ru decreased from 0.27 (when no NaOH was added) to 0.16, benzene conversion of 45.3%, and cyclohexene selectivity of 89.3% was achieved using a batch reactor after 25 min of reaction time. However, with continually increasing the NaOH concentration, i.e., to 1.2 mol·L−1, parts of ZnO could react with the over-added NaOH, leading to the unfavorable consumption of uniformly dispersed ZnO. This causes the increasing of catalytic activity towards benzene conversion, as well as the decreasing of the selectivity towards cyclohexene. Moreover, no loss of catalytic activity and selectivity towards cyclohexene formation from selective hydrogenation of benzene was observed after 10 times of catalytic experiments without any regeneration.
1. Introduction
Caprolactam and adipic acid are the most common monomers for the production of nylon 6, nylon 66, polyamide, and polyester, which are considered as the raw material of modern chemical industry [1,2,3,4]. It has attracted great attention for the production of caprolactam and adipic acid via selective hydrogenation of benzene. This is mainly attributed to the fact that it is safety, energy conservation, environmental friendly, and high carbon atom economy [5,6,7,8]. However, the complete hydrogenation of benzene to cyclohexane, the only by-product, is more thermodynamically favorable (∆G = −98 kJ mol−1, 25 °C) than that needed for selective hydrogenation to cyclohexene (∆G = −23 kJ mol−1, 25 °C) [9,10,11,12]. Therefore, the development of the catalysts with high selectivity to cyclohexene is still great urgent at this stage.
Utilizing additives is usually selected as one of the most promised way to enhance catalytic selectivity towards cyclohexene over the heterogeneous catalytic system. For instance, the cyclohexene selectivity could be significantly improved over Ru catalysts by adding ZnSO4. 40% of benzene conversion, as well as 80% of cyclohexene selectivity, was achieved over a Ru-Zn catalyst with ZnSO4 as a reaction additive by Asahi in 1989 [13,14]. Moreover, Liu et al. reported that 53% of cyclohexene yield were obtained over Ru-Ce/SBA-15 catalyst using ZnSO4 as a reaction additive [15]. It was also demonstrated by Zhou et al. that 48% of cyclohexene yield was observed over a Ru/B-ZrO2 catalyst with addition of ZnSO4 [16]. In addition, 46% of cyclohexene yield was achieved by Liao et al. over a Ru-La/MCM-41 catalyst by adding ZnSO4 [17]. Ning et al. utilized ZnSO4 as a reaction additive for the Ru-SiO2 catalyst, over which, 42% of cyclohexene yield was shown [18]. ZnSO4 was also selected by Yan et al. over the Ru-Zn/ZrO2 catalyst, which gave 49% of cyclohexene yield [19]. However, drawbacks of employing ZnSO4 as a reaction additive are unavoidable in terms of industrial operability. The main disadvantage is that the acidic condition that was generated by the hydrolysis of ZnSO4 caused the corrosion of reactor [6]. Moreover, salts loading are typically high (~25 times higher than the amount of catalysts), reducing the economic viability of the process [14].
Currently, development of less-corrosive additives for selective hydrogenation of benzene is in urgent need for scientific research, from which, NaOH draws great attentions. For instance, it was exhibited by Liu et al. that 49.4% of cyclohexene yield and 68.3% of cyclohexene selectivity could be obtained over Ru-Cu/ZnO by applying NaOH as a reaction additive [20]. Furthermore, Zhang et al. reported that 47.3% selectivity and 33% of yield towards cyclohexene could be achieved over a Ru-Zn catalyst supported on hydroxyapatite [21]. They suggested that the effect of using NaOH as an additive mainly includes: (i) OH− of NaOH is chemisorbed on the Ru surface, leading to the improvement of hydrophicility of the Ru catalysts. Since benzene is more soluble than cyclohexene in water, the generated stagnant water layer could slow the hydrogenation of cyclohexene to cyclohexane. In addition, water might have competition with cyclohexene to adsorb on the Ru surface, thus disadvantage its hydrogenation to cyclohexane. (ii) OH− of NaOH could cover parts of the Ru active sites that benefits the hydrogenation of cyclohexene to retard its further hydrogenation, and thus increase the selectivity to cyclohexene. In general, for hydrogenation reactions, the role of Ru active sites is addressed to dissociate molecular hydrogen and activate the hydrogenation process. On the other hand, 41.5% of cyclohexene yield and 57.7% of selectivity towards cyclohexene were reported by Wu et al. [22]. They suggested that Na2Zn(OH)4 was generated via the reaction of ZnO and NaOH, which was chemisorbed on the Ru surface and inhibit the further hydrogenation of cyclohexene to cyclohexane. Therefore, it is clear that the mechanism of NaOH affecting selective hydrogenation of benzene for cyclohexene synthesis is still controversial.
ZnSO4, in our previous work, as the reaction additive for selective hydrogenation of benzene to cyclohexene over Ru-Zn catalysts [23,24], Ru-Mn catalysts [25], Ru-La catalysts [26], as well as Re-Ce catalysts [27] have been systematically investigated. Besides, how PEG-10000 [28] and amines [29] improving the catalytic selectivity towards cyclohexene generation were also studied. Based on the understanding of aforementioned research, the mechanism of NaOH as a reaction additive affecting cyclohexene formation from the selective hydrogenation of benzene is investigated in this work.
2. Results
2.1. Catalyst Characterization
XRD patterns for Ru-Zn (0.27) and Ru catalysts before and after catalytic experiments were illustrated in Figure 1. Figure 1a indicates that Ru and Zn exist as metallic Ru and ZnO, respectively. It was suggested by Zhou et al. that Zn exists as ZnO for Ru-Zn/ZrO2 [30]. Yan et al. also reported that Zn exists as Zn(OH)2 for Ru-Zn/ZrO2 [19]. Furthermore, the particle size of fresh Ru-Zn (0.27) catalyst was calculated to be 4.2 nm (Table 1) from the half peak width of diffraction peaks at 43.7° for Ru (101). XRD profiles for Ru-Zn (0.27) catalyst after catalytic experiments with addition of different concentration of NaOH (e.g., 0.000, 0.075, 0.150, 0.300, 0.600, and 1.200 mol L−1) were demonstrated in Figure 1b. Characteristic diffractions of Ru could be detected over all the tested samples, suggesting that Ru maintains metallic state through the whole reaction. On the other hand, it is noticed that ZnO peaks could be observed when the added NaOH concentration is lower than 0.075 mol L−1. However, with increasing the concentration of NaOH, ZnO peaks disappeared. This is attributed to that soluble Na2Zn(OH)4 was generated during the reaction, according to Equation 1 [22]. Furthermore, the particle size of Ru-Zn (0.27) catalyst after hydrogenation reaction remains close to 4.2 nm without obvious change by adding different amount of NaOH in the reaction medium. Correspondingly, Figure 1c shows that only metallic Ru exists in monometallic Ru. Figure 1d indicates that metallic Ru did not change through the whole catalytic experiments with addition of different concentration of NaOH (e.g., 0.000, 0.075, 0.150, 0.300, 0.600, and 1.200 mol L−1).
ZnO + 2NaOH + H2O ⇌ Na2Zn(OH)4
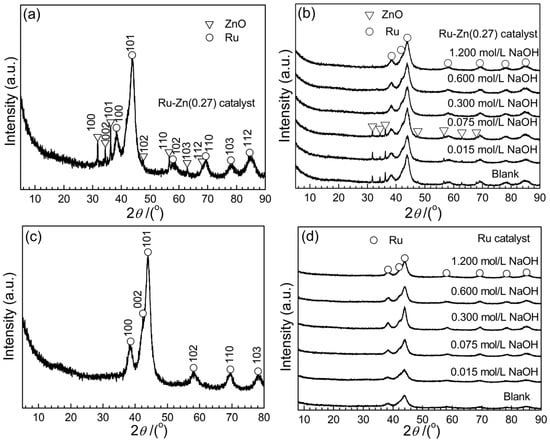
Figure 1.
X-ray diffraction (XRD) profiles for Ru-Zn (0.27) catalysts and monometallic Ru catalysts before and after catalytic reactions. (a) Fresh Ru-Zn (0.27) catalyst; (b) used Ru-Zn (0.27) catalysts with different concentration of NaOH as an additive; (c) fresh monometallic Ru catalyst; (d) used monometallic Ru catalyst with different concentration of NaOH as an additive.

Table 1.
Molar ratio of Zn to Ru as well as Na to Ru particle size of the Ru-Zn (0.27) catalysts and monometallic Ru catalysts with the addition of different concentration of NaOH.
Table 1 shows the molar ratio of Zn to Ru, as well as Na to Ru of the Ru-Zn (0.27) catalysts and monometallic Ru catalysts with the addition of different concentration of NaOH. Without adding NaOH, n(Zn)/n(Ru) molar ratio of Ru-Zn (0.27) has no change. However, the n(Zn)/n(Ru) molar ratio decreases along with increasing the concentration of added NaOH (e.g., 0.26 of n(Zn)/n(Ru) with 0.075 mol L−1 NaOH vs. 0.14 of n(Zn)/n(Ru) with 1.200 mol L−1 NaOH), suggesting the decline of ZnO content in Ru-Zn (0.27). This is rationalized in terms that with increasing the NaOH concentration, more ZnO will be consumed by forming the soluble Na2Zn(OH)4 via Equation (1). In addition, no Na+ was observed over both Ru-Zn (0.27) and monometallic Ru catalysts after catalytic experiments, demonstrating that NaOH and Na2Zn(OH)4 can hardly be chemisorbed on the Ru surface.
TEM images for Ru-Zn (0.27) after catalytic experiments with adding different concentrations of NaOH were shown in Figure 2. When NaOH concentration was lower than 0.300 mol L−1, Ru particles, as well as the rodlike ZnO could be clearly observed (Figure 2a–c). This indicates that most parts of ZnO are difficult to disperse on the Ru surface with applying a low concentration of NaOH. However, no rodlike ZnO was demonstrated when 0.600 mol L−1 (Figure 2d) and 1.200 mol L−1 (Figure 2f) of NaOH was utilized, indicating that ZnO was highly dispersed on the Ru surface. In addition, via energy dispersive spectrometer (EDS), the n(Zn)/n(Ru) molar ratio was determined to be 0.16 and 0.14 by adding 0.600 and 1.200 mol L−1 of NaOH as additive, respectively. This is consistent to that observed from Table 1.
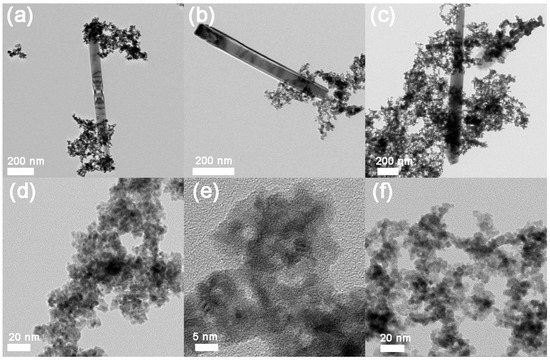
Figure 2.
Transmission electron microscope (TEM) images for Ru-Zn (0.27) catalysts after catalytic experiments with adding different concentration of NaOH. (a) Blank; (b) 0.150 mol L−1 NaOH; (c) 0.300 mol L−1 NaOH; (d,e) 0.600 mol L−1 NaOH; (f) 1.200 mol L−1 NaOH.
SEM, as well as element mapping images of Ru-Zn (0.27) catalysts after catalytic experiments with adding different concentration of NaOH, was illustrated in Figure 3. Rodlike ZnO can be observed (Figure 3a,b) by adding 0.150 mol L−1 and 0.300 mol L−1 of NaOH, respectively. In addition, as shown in Figure 3c,d, when the added NaOH concentration is no less than 0.600 mol L−1, the rodlike ZnO is no longer observed, indicating that ZnO is uniformly dispersed on the Ru surface. These results imply that the dispersion of ZnO on the Ru surface could be improved by utilizing NaOH (higher concentration) as the reaction additive.
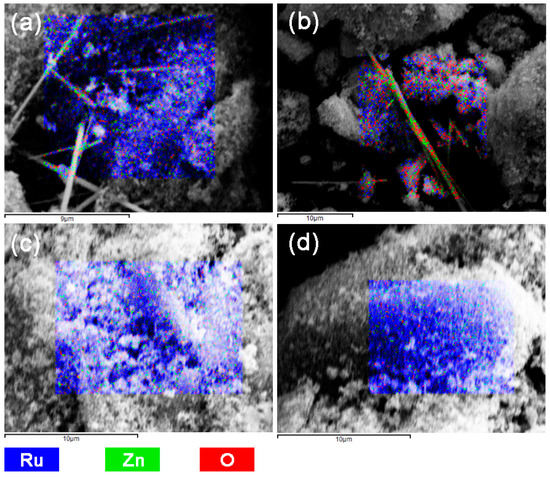
Figure 3.
Scanning electron microscope (SEM) images as well as element mapping images of Ru-Zn (0.27) catalysts after catalytic experiments with adding different concentration of NaOH. (a) 0.150 mol L−1; (b) 0.300 mol L−1; (c) 0.600 mol L−1; (d) 1.200 mol L−1.
Notably, since the melting point of Ru (2606 K) is higher than that of ZnO (2247 K), Ru with higher surface bond enthalpy is tended to be replaced by ZnO. This helps to reduce the Ru surface enthalpy (ΔH < 0). Meanwhile, with increasing the concentration of NaOH, the highly ordered ZnO with rodlike shape becomes more dispersed and less ordered, resulting in the enhancement of entropy (ΔS > 0). According to the formula of Gibbs free energy (ΔG = ΔH − TΔS), the free enthalpy change is negative. Therefore, ZnO could be dispersed on the Ru surface spontaneously. However, it still needs activation energy for ZnO to be diffused on the Ru surface, for which a relative high temperature is of great necessity. This is the key reason why the non-dispersed rodlike ZnO could be observed (e.g., Figure 2a–c as well as Figure 3a,b).
Based on aforementioned discussion, a plausible mechanism of how NaOH promotes the dispersion of ZnO on the Ru surface was proposed in Figure 4. Firstly, ZnO on the Ru surface could react with NaOH in the aqueous phase to form Na2Zn(OH)4, which generates an area with a relative high concentration of Na2Zn(OH)4 (area A). Subsequently, the concentration gradient forces the diffusion of Na2Zn(OH)4 to that with the lower concentration (area B), where ZnO was generated again from the decomposition of Na2Zn(OH)4. The generated ZnO was then chemisorbed on the Ru surface. When NaOH is sufficient, this procedure could continuously take place until ZnO was uniformly dispersed on the Ru surface (e.g., Figure 2d, Figure 3c,d). It is worth mentioning that, because ZnO was already uniformly dispersed on the Ru surface when adding 0.600 mol L−1 NaOH, thus, with increasing the NaOH concentration to 1.200 mol L−1, parts of the highly dispersed ZnO was consumed unfavorably.
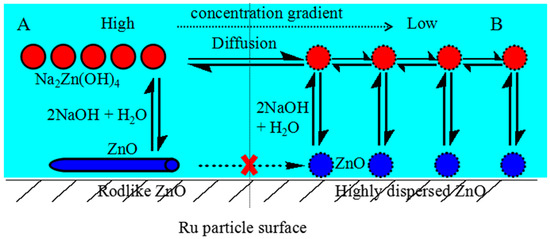
Figure 4.
Schematic diagram of the NaOH effect for the dispersion of ZnO on the Ru surface.
Table 2 gives the texture properties of Ru-Zn (0.27) catalysts by using NaOH with different concentration as a reaction additive. It is found that, with increasing the added NaOH concentration, the specific surface area and the pore volume declined, but the average pore diameter increased. This can be rationalized in terms that ZnO becomes more dispersed on the Ru surface with enhancing the amount of applied NaOH, and the highly dispersed ZnO might cover some micro pores of Ru particles. This is in a good agreement with the discussion before. Additionally, it was also reported by Zhou et al. that the increased pore diameter might benefit the escape of cyclohexene from the Ru pores, thus improving its selectivity [30].

Table 2.
Specific surface area (SBET), pore volume (Vp), and average pore diameter (Dp) of the Ru-Zn (0.27) catalysts with the addition of different concentration of NaOH.
Figure 5 shows XPS and AES spectra of Ru-Zn (0.27) catalyst before and after hydrogenation with the addition of 0.600 mol L−1 NaOH as a reaction additive. Figure 5a gives the electronic binding energy (BE) of Ru 3p3/2 (461.0 eV), as well as Ru 3p1/2 (483.0 eV) for Ru-Zn (0.27) catalyst before reaction. This reveals the existence of metallic Ru state [31]. Moreover, the BE of 1022.0 eV for Zn 2p3/2 and the kinetic energy of 987.7 eV for Zn LMM were presented in Figure 5c,e, which is consistent with the reported ZnO [32,33]. On the other hand, the electronic BE of 462.4 eV for Ru 3p3/2 was observed in Figure 5b, indicating that the metallic Ru still existed after the reaction [34]. Besides, due to the BE for Ru 3p3/2 after reaction is 1.4 eV higher than that of the fresh sample, it is deemed that Ruδ+ species is generated. The BE of 1021.5 eV for Zn 2p3/2 and the kinetic energy of 988.4 eV for Zn LMM were illustrated in Figure 5d,f, suggesting that Zn of Ru-Zn (0.27) still exists as ZnO after the reaction [35,36]. Furthermore, the BE of Zn 2p3/2 after the catalytic experiment is 0.5 eV lower than that of the fresh sample, implying that the electrons were transferred from Ru to ZnO. These results indicate that there is a critical electronic effect between the Ru and ZnO during the catalytic hydrogenation of benzene to cyclohexene.
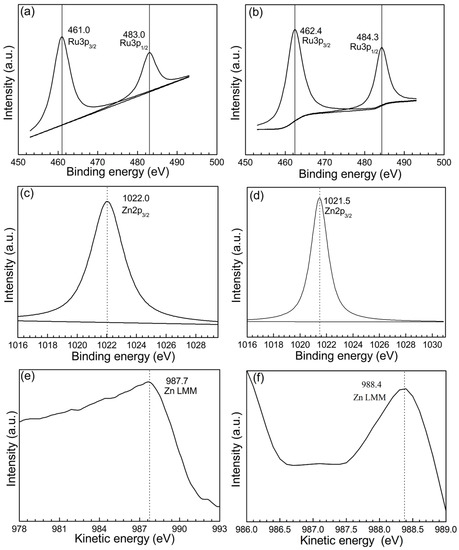
Figure 5.
X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) spectra of Ru-Zn (0.27) catalyst before and after catalytic experiments with addition of 0.600 mol L−1 of NaOH as additive (Before catalytic experiments: (a) Ru 3p3/2 and Ru 3p1/2, (c) Zn 2p3/2, and (e) Zn LMM. After catalytic experiments: (b) Ru 3p3/2 and Ru 3p1/2, (d) Zn 2p3/2, and (f) Zn LMM).
Optimized structure of the Ru-Zn cluster that is formed by one Zn2+ and three Ru atoms, as well as their positive electrical charge numbers were demonstrated in Figure 6a. Theoretically, the positive charge of Zn2+ is supposed to be 2. However, the vitreous electricity of Zn2+ was 0.716, and the three Ru atoms were calculated to be 0.470, 0.470, and 0.344, respectively. It is obvious that the positive electrical charge of Zn2+ was partly transferred to Ru. In addition, as can be seen from Figure 6b, electrons from two closest Ru atoms were transferring to the nucleus of Zn2+. Therefore, it is concluded that part of electrons from Ru were transferred to Zn2+, resulting in forming the Ruδ+ species of electron deficiency. This is consistent with the XPS results.
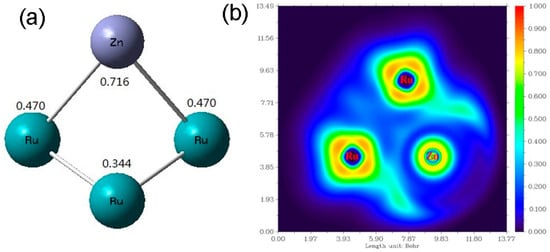
Figure 6.
(a) Optimized structure of the Ru-Zn cluster formed by one Zn2+ and three Ru atoms as well as their positive electrical charges; (b) electron density map of one Zn2+ and two closest Ru atoms.
2.2. Catalytic Performance
Catalytic activity towards benzene conversion and cyclohexene selectivity over Ru-Zn (0.27) catalyst utilizing different concentration of NaOH as a reaction additive were illustrated in Figure 7a,b, respectively. It is found that NaOH as a reaction additive could retard the catalytic activity and significantly improve the cyclohexene selectivity over Ru-Zn (0.27) catalyst. Without adding NaOH, 75.4% of benzene conversion and 12.4% of cyclohexene selectivity were obtained at 5 min. Furthermore, the great enhancement of selectivity towards cyclohexene, as well as a decline of the catalytic activity with increasing the NaOH concentration to 0.600 mol L−1, and 45.3% of benzene conversion and 89.3% of cyclohexene selectivity was achieved at 25 min (0.600 mol L−1 of NaOH applied). This is greatly advantageous in comparison to the current level of industrialization of cyclohexene production (40% of benzene conversion and 80% of selectivity to cyclohexene) [14]. It is worth mentioning that a 10% increment of selectivity towards cyclohexene would drastically reduce the obstacle of isolating cyclohexene from the mixture of products. This is because the boiling point of benzene, cyclohexene, and cyclohexane is extremely comparable (e.g., 353, 356, and 353.7 K, respectively), while the extraction is mostly applied for separating cyclohexene from the mixture of products. Therefore, it is much more convenient to obtain the target product with higher selectivity towards cyclohexene [37]. Notably, in comparison to ZnSO4, NaOH as a reaction additive causes less corrosive to the reactor [21]. Further, the reaction was conducted under the optimized conditions (i.e., 0.600 mol L−1 of NaOH) until benzene was completely conversed (Figure 7c). 66.2% of cyclohexene yield was obtained after 55 min of catalytic experiment, which is one of the highest yield of cyclohexene reported by far [15,28]. However, it was noticed that with increasing concentration of NaOH from 0.600 to 1.200 mol L−1, the catalytic activity increased and the selectivity to cyclohexene decreased. On the contrary, it was observed that the catalytic activity showed slight discrepancy over pure Ru catalyst by raising NaOH concentration from 0.000 to 1.200 mol L−1. Besides, no cyclohexene was detected throughout the reaction process over Ru samples. This suggests that NaOH has no effect on the monometallic Ru catalyst.
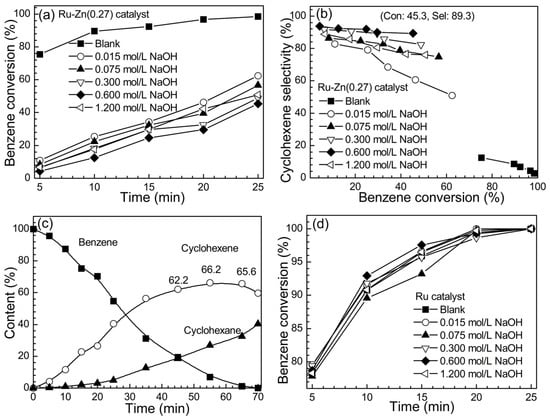
Figure 7.
Catalytic activity towards selective hydrogenation of benzene over Ru-Zn (0.27) and monometallic Ru catalysts with different concentration of NaOH as a reaction additive (mcat = 2.0 g, vH2O = 280 mL, vbenzene = 140 mL, T = 423 K, pH2 = 5.0 MPa). (a) Benzene conversion and (b) cyclohexene selectivity over Ru-Zn (0.27) catalyst; (c) reaction course under optimized conditions (0.600 mol L−1 of NaOH); (d) benzene conversion over Ru catalyst.
When combined with characterization as well as the hydrogenation results, it is obvious that NaOH as a reaction additive has no effect on improving the cyclohexene selectivity over monometallic Ru catalyst, and promoter ZnO with a specific amount can only feebly increase the catalytic selectivity. Besides, as proven by XRF and XRD results, Na2Zn(OH)4 cannot be adsorbed on the Ru surface, and no change of the particle size of the catalysts was observed after catalytic experiments. However, NaOH as a reaction additive effectively improve the catalytic selectivity towards cyclohexene over Ru-Zn (0.27) by improving the dispersion of ZnO on the Ru surface, with forming Na2Zn(OH)4 during the catalytic experiments. Three main roles of highly dispersed ZnO are addressed, as follows: (1) parts of electrons of Ru could be transferred to ZnO, causing the generation of Ruδ+ species of electron deficiency. It was suggested by Mazzieri et al. [38] and Zhou et al. [30] that Ruδ+ has weaker capability of cyclohexene adsorption in comparison to Ru, which accelerates the desorption of cyclohexene; (2) the atomic radius of Zn2+ from highly dispersed ZnO is similar to that of Ru, which could firstly adsorbed on Ru surface and cover part of the Ru active sites that unsuitable for cyclohexene formation [39]; and, (3) a stable complex could be formed between the Zn2+ of highly dispersed ZnO and cyclohexene, which could stabilize the synthesized cyclohexene and prohibit its further hydrogenation to cyclohexane, and thus improve the cyclohexene selectivity [40,41].
Therefore, when no NaOH was employed, rodlike ZnO can hardly disperse on the Ru surface, thus no aforementioned effect could be actually realized. Hence, relative high benzene conversion and low cyclohexene selectivity were expected over Ru-Zn (0.27) catalyst (e.g., 75.4% of benzene conversion and 12.4% of cyclohexene selectivity were obtained in 5 min). In comparison, with enhancing the utilized NaOH concentration, ZnO became uniformly dispersed on the Ru surface, which leads to the improvement selectivity towards cyclohexene (Figure 7). When the NaOH concentration reached 0.600 mol L−1, the lower conversion of benzene (45.3%) and the highest selectivity to cyclohexene (89.3%) were obtained over Ru-Zn (0.27) catalyst within 25 min.
The reusability of Ru-Zn (0.27) catalyst with the addition of 0.600 mol L−1 of NaOH was tested under the same reaction conditions without further regeneration (Figure 8). It can be observed that benzene conversion stays approximately 45% and cyclohexene selectivity maintains around 88% after 10 times of catalytic experiments. Thus, the catalytic system over Ru-Zn (0.27) and NaOH as a reaction additive shows a good reusability for selective hydrogenation of benzene towards cyclohexene. Since NaOH causes much less corrosion of reactor, this catalytic system possesses a great potential for industrial application on selective hydrogenation of benzene towards cyclohexene production.
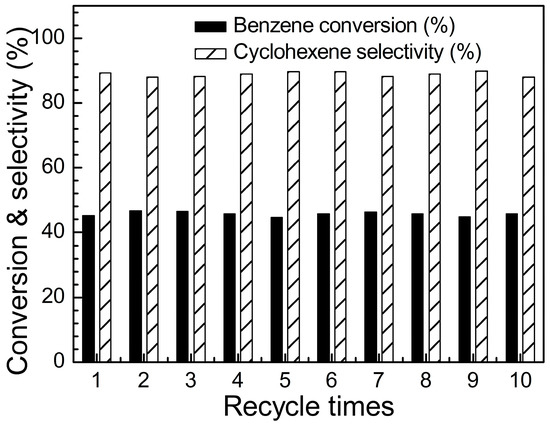
Figure 8.
Reusability of Ru-Zn (0.27) for selective hydrogenation of benzene to cyclohexene by using 0.600 mol L−1 of NaOH as a reaction additive, including benzene conversion as well as cyclohexene selectivity (mcat = 2.0 g, vH2O = 280 mL, vbenzene = 140 mL, T = 423 K, pH2 = 5.0 MPa, treaction = 25 min).
3. Materials and Methods
3.1. Chemicals
RuCl3·3H2O was commercially obtained from Sino-Platinum Co. Ltd. (Kunming, China). ZnSO4·7H2O was purchased from Fuchen Chemical Reagent Factory (Tianjin, China). NaOH and benzene were delivered from Kemiou Chemical Reagent Co. Ltd. (Tianjin, China). All of the chemicals were of analytical grade without further purification and distilled water was applied in all of the experiments.
3.2. Preparation of Catalysts
Ru-Zn catalysts were prepared, as following: 20.0 g RuCl3·3H2O precursor and 5.9 g of ZnSO4·7H2O were dissolved in 200 mL of distilled water. Then, 200 mL of NaOH (5 wt.%) aqueous solution was added with continuous stirring at 353 K for 30 min, followed by transferring all of the solids and solutions in a 1000 mL Hastelloy autoclave to conduct a reduction procedure under 5.0 MPa of H2 and a stirring speed of 800 rpm at 423 K for 3 h. After the reduction procedure, the sample was then cooled down to the room temperature, and the fresh catalysts were obtained by washing with distilled water to neutral and vacuum-dried. The prepared Ru-Zn catalysts were denoted as Ru-Zn(x), where x stands for the molar ratio of Zn to Ru. Similarly, monometallic Ru catalysts were synthesized through the aforementioned procedure, just without the addition of ZnSO4·7H2O.
3.3. Catalytic Experimental Procedure
All of the catalytic experiments were carried out in a 1000 mL GS-1 type Hastelloy autoclave. In a typical reaction, 2.0 g Ru-Zn catalyst and 280 mL of NaOH solution with different concentration (e.g., 0.000, 0.075, 0.150, 0.300, 0.600, and 1.200 mol L−1) were added to the autoclave. Then, the reactor was purified by N2 for 3 times and followed by purification with H2 for another four times. Then, the reactor was heated under 5.0 MPa of H2 with a stirring speed of 800 rpm. When the temperature reached to 423 K, the autoclave was charged with 140 mL of benzene and adjusting the stirring speed to 1400 rpm to eliminate the mass transfer limitation. Subsequently, the liquid samples were taken periodically from the reactor every 5 min. All of the withdrawn samples were analyzed by gas chromatography equipped with a flame ionization detector (FID). The benzene conversion and cyclohexene yield were obtained with calibration area normalization method. Specifically, cyclohexene selectivity was calculated, according to the following Equation (2). After each reaction, the organic phase was removed via a separating funnel and the solid Ru-Zn catalyst together with the aqueous solution were recharged into the autoclave to investigate the reusability of the catalysts through aforementioned experimental procedures.
3.4. Catalysts Characterization
X-ray diffraction (XRD) patterns were recorded on an X’Pert Pro instrument from Philips (Amsterdam, The Netherlands) using diffracted intensity of Cu-Kα radiation (λ = 0.154 nm) was measured at the range of 2θ between 5° and 90°, with a step size of 0.03°. The particle size of the catalysts was calculated via Scherrer’s equation. In addition, elemental analysis was obtained via X-ray Fluorescence (XRF) using a S4 Pioneer instrument from Bruker AXS (Karlsruhe, Germany). Furthermore, JEOL JEM 2100 transmission electron microscope (TEM) combined with energy dispersive spectrometer (EDS) (Akishima, Tokyo, Japan) was utilized to identify the surface composition of the selected samples. The catalyst surface element scanning was tested by a ∑IGMA scanning electron microscope (SEM) from Carl Zeiss AG (Oberkochen, Germany). Textural properties were analyzed by the Nova 1000e-Physisorption Analyzer. All of the samples were evacuated at 523 K under the vacuum pressure for 2 h prior to the measurements and the isotherms were taken at 77 K. The specific surface area (SBET) was determined by the Brunauer–Emmett–Teller (BET) model. Moreover, the valence state of Ru and Zn on the catalyst surface was analyzed by X-ray photoelectron spectroscopy (XPS) using a PHI Quantera SXM Instrument from Ulvac-Phi (Kanagawa, Japan). The kinetic energy of Zn LMM electrons was determined via Auger electron spectroscopy (AES) from ULVAC-PHI (PHI-700 type, Chigasaki, Japan). Additionally, a density functional theory (DFT) study was conducted in order to obtain the optimized structure of the Ru-Zn cluster formed by one Zn2+ and three Ru atoms, from which LANL2DZ and 6-311+G(d) was applied for Ru and Zn, respectively. The electron density map was obtained via Multiwfn.
4. Conclusions
Ru and Zn exist as metallic Ru and rodlike ZnO for Ru-Zn (0.27) catalyst, respectively. Rodlike ZnO can hardly disperse on Ru surface. When NaOH was utilized as a reaction additive, dispersion of ZnO on the Ru surface could be significantly improved via the formation of Na2Zn(OH)4. Furthermore, electrons from Ru were transferred to the nucleus of Zn2+, which leads to the generation of Ruδ+ species of electron deficiency. This causes the enhancement of catalytic selectivity to cyclohexene and decline of activity towards benzene conversion. When the added NaOH concentration increased from 0.000 to 0.600 mol L−1, the selectivity to cyclohexene improved from 2.7% to 89.3%, and the benzene conversion decreased from 98.5% to 45.3% over the Ru-Zn (0.27) catalyst after 25 min of catalytic experiments, from which the highest yield of cyclohexene (40.5%) was achieved. Noteworthy, when the NaOH concentration continuously increased to 1.200 mol L−1, the catalytic activity towards benzene conversion over Ru-Zn (0.27) catalyst stopped to increase and selectivity to cyclohexene declined. This is due to the further unfavorable consumption of the uniformly dispersed ZnO. In addition, no obvious loss of catalytic activity and selectivity towards cyclohexene was observed after 10 times of catalytic experiments.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (21273205), the Key Scientific Research Project of Henan Province (18A180018), and the Environmental Catalysis Innovative Research Team of Zhengzhou Normal University.
Author Contributions
Haijie Sun, Zhihao Chen and Zhikun Peng conceived and designed the experiments; Chenggang Li performed the experiments; Zhongyi Liu and Shouchang Liu analyzed the data; Lingxia Chen contributed reagents/materials/analysis tools; Haijie Sun wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Z.B.; Zhang, Q.; Lu, X.F.; Chen, S.J.; Liu, C.J. Ru-Zn catalysts for selective hydrogenation of benzene using coprecipitation in low alkalinity. Chin. J. Catal. 2015, 36, 400–407. [Google Scholar] [CrossRef]
- Fan, G.F.; Li, R.X.; Li, X.J.; Chen, H. Effect of organic on partial hydrogenation of benzene. Catal. Commun. 2008, 9, 1394–1397. [Google Scholar] [CrossRef]
- Yuan, P.Q.; Wang, B.Q.; Ma, Y.M.; He, H.M.; Cheng, Z.M.; Yuan, W.K. Hydrogenation of cyclohexene over Ru-Zn/Ru(0001) surface alloy: A first principles density functional study. J. Mol. Catal. A Chem. 2009, 301, 140–145. [Google Scholar] [CrossRef]
- He, H.M.; Yuan, P.Q.; Ma, Y.M.; Cheng, Z.M.; Yuan, W.K. Theoretical and experimental study on the partial hydrogenation of benzene over Ru-Zn/ZrO2 catalyst. Chin. J. Catal. 2009, 30, 312–318. [Google Scholar]
- Peng, Z.K.; Liu, X.; Lin, H.N.; Wang, Z.; Li, Z.J.; Li, B.J.; Liu, Z.Y.; Liu, S.C. Surface engineering on a nanocatalyst: Basic zinc salt nanoclusters improve catalytic performances of Ru nanoparticles. J. Mater. Chem. A 2016, 4, 17694–17703. [Google Scholar] [CrossRef]
- Fan, G.Y.; Jiang, W.D.; Wang, J.B.; Li, R.X.; Chen, H.; Li, X.J. Selective hydrogenation of benzene to cyclohexene over RuCoB/γ-Al2O3 without additive. Catal. Commun. 2008, 10, 98–102. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, L.X.; Huang, Z.X.; Sun, L.L.; Li, Y.Y.; Liu, S.C.; Liu, Z.Y. Effect of reduction medium and reduction temperature on the performance of Ru-Zn catalysts for selective hydrogenation of benzene to cyclohexene. Chem. Ind. Eng. Prog. 2017, 36, 2962–2970. [Google Scholar]
- Sun, H.J.; Li, Y.Y.; Li, S.H.; Zhang, Y.X.; Liu, S.C.; Liu, Z.Y.; Ren, B.Z. ZnSO4 and La2O3 as Co-Modifier of the Monoclinic Ru Catalyst for Selective Hydrogenation of Benzene to Cyclohexene. Acta Phys. Chim. Sin. 2014, 30, 1332–1340. [Google Scholar]
- Foppa, L.; Dupont, J. Benzene partial hydrogenation: Advances and perspectives. Chem. Soc. Rev. 2015, 44, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Chen, L.X.; Huang, Z.X.; Liu, S.C.; Liu, Z.Y. Particle Size Effect of Ru-Zn Catalysts on Selective Hydrogenation of Benzene to Cyclohexene. Chem. J. Chin. Univ. 2015, 36, 1969–1976. [Google Scholar]
- Sun, H.J.; Chen, X.L.; Huang, Z.X.; Liu, Z.Y.; Liu, S.C. Effect of NaOH concentration on performance of Ru-Zn catalyst for selective hydrogenation of benzene to cyclohexene. CIESC J. 2016, 67, 1324–1332. [Google Scholar]
- Sun, H.J.; Chen, J.J.; Huang, Z.X.; Liu, Z.Y.; Liu, S.C. Selective hydrogenation of benzene to cyclohexene over the nano-sized Ru-Zn catalyst modified by Rrabic gum. Chin. J. Inorg. Chem. 2016, 32, 202–210. [Google Scholar]
- Nagahara, H.; Ono, M.; Konishi, M.; Fukuoka, Y. Partial hydrogenation of benzene to cyclohexene. Appl. Surf. Sci. 1997, 121–122, 448–451. [Google Scholar] [CrossRef]
- Wu, J.M.; Yang, Y.F.; Chen, J.L. Study on the causes of catalyst inactivation of benzene semi-hydrogenation. Chem. Ind. Eng. Prog. 2003, 22, 295–297. [Google Scholar]
- Liu, J.L.; Zhu, L.J.; Pei, Y.; Zhuang, J.H.; Li, H.; Li, H.X.; Qiao, M.H.; Fan, K.N. Ce-promoted Ru/SBA-15 catalysts prepared by a “two solvent” impregnation method for selective hydrogenation of benzene to cyclohexene. Appl. Catal. A Gen. 2009, 353, 282–287. [Google Scholar] [CrossRef]
- Zhou, G.B.; Pei, Y.; Jiang, Z.; Fan, K.N.; Qiao, M.H.; Sun, B.; Zong, B.N. Doping effects of B in ZrO2 on structural and catalytic properties of Ru/B-ZrO2 catalysts for benzene partial hydrogenation. J. Catal. 2014, 311, 393–403. [Google Scholar] [CrossRef]
- Liao, H.G.; Ouyang, D.H.; Zhang, J.; Xiao, Y.J.; Liu, P.L.; Hao, F.; You, K.Y.; Luo, H.A. Benzene hydrogenation over oxide-modified MCM-41 supported ruthenium-lanthanum catalyst: The influence of zirconia crystal form and surface hydrophilicity. Chem. Eng. J. 2014, 243, 207–216. [Google Scholar] [CrossRef]
- Ning, J.B.; Xu, J.; Liu, J.; Lu, F. Selective hydrogenation of benzene to cyclohexene over colloidal ruthenium catalyst stabilized by silica. Catal. Lett. 2006, 109, 175–180. [Google Scholar] [CrossRef]
- Yan, X.H.; Zhang, Q.; Zhu, M.Q.; Wang, Z.B. Selective hydrogenation of benzene to cyclohexene over Ru-Zn/ZrO2 catalysts prepared by a two step impreganation method. J. Mol. Catal. A Chem. 2016, 413, 85–93. [Google Scholar] [CrossRef]
- Liu, H.Z.; Liang, S.G.; Wang, W.T.; Jiang, T.; Han, B.X. The partial hydrogenation of benzene to cyclohexene over Ru-Cu catalyst supported on ZnO. J. Mol. Catal. A Chem. 2011, 341, 35–41. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, T.B.; Jiang, T.; Wang, W.T.; Liu, H.Z.; Fan, H.L.; Zhang, Z.F.; Han, B.X. Ru-Zn supported on hydroxyapatite as an effective catalyst for partial hydrogenation of benzene. Green Chem. 2013, 15, 152–159. [Google Scholar] [CrossRef]
- Wu, T.B.; Zhang, P.; Jiang, T.; Yang, D.X.; Han, B.X. Enhancing the selective hydrogenation of benzene to cyclohexene over Ru/TiO2 catalyst in the presence of a very small amount of ZnO. Sci. China Chem. 2015, 58, 93–100. [Google Scholar] [CrossRef]
- Sun, H.J.; Wang, H.X.; Jiang, H.B.; Li, S.H.; Liu, S.C.; Liu, Z.Y.; Yuan, X.M.; Yang, K.J. Eeffect of (Zn(OH)2)3(ZnSO4)(H2O)5 on the performance of Ru-Zn catalyst for benzene selective hydrogenation to cyclohexene. Appl. Catal. A 2013, 450, 160–168. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhang, X.D.; Chen, Z.H.; Zhou, X.L.; Guo, W.; Liu, Z.Y.; Liu, S.C. Monolayer dispersed Ru-Zn Catalyst and its performance in the Selective Hydrogenation of Benzene to Cyclohexene. Chin. J. Catal. 2011, 32, 224–230. [Google Scholar] [CrossRef]
- Sun, H.J.; Jiang, H.B.; Li, S.H.; Wang, H.X.; Pan, Y.J.; Dong, Y.Y.; Liu, S.C.; Liu, Z.Y. Selective hydrogenation of benzene to cyclohexene over nanocomposite Ru-Mn/ZrO2 catalyst. Chin. J. Catal. 2013, 34, 684–694. [Google Scholar] [CrossRef]
- Sun, H.J.; Dong, Y.Y.; Li, S.H.; Jiang, H.B.; Zhang, Y.X.; Liu, Z.Y.; Liu, S.C. The role of La in improving the selectivity to cyclohexene of Ru catalyst for hydrogenation of benzene. J. Mol. Catal. A Chem. 2013, 368–369, 119–124. [Google Scholar] [CrossRef]
- Sun, H.J.; Pan, Y.J.; Li, S.H.; Zhang, Y.X.; Dong, Y.Y.; Liu, S.C.; Liu, Z.Y. Selective hydrogenation of benzene to cyclohexene over Ce-promoted Ru catalysts. J. Energy Chem. 2013, 22, 710–716. [Google Scholar] [CrossRef]
- Sun, H.J.; Jiang, H.B.; Dong, Y.Y.; Wang, H.X.; Pan, Y.J.; Liu, S.C.; Tang, M.S.; Liu, Z.Y. Effect of alcohols as additives on the performance of a nano-sized Ru-Zn(2.8%) catalyst for selective hydrogenation of benzene to cyclohexene. Chem. Eng. J. 2013, 218, 415–424. [Google Scholar] [CrossRef]
- Sun, H.J.; Pan, Y.J.; Wang, H.X.; Dong, Y.Y.; Liu, Z.Y.; Liu, S.C. Selective hydrogenation of benzene to cyclohexene over a Ru-Zn Catalyst with diethanolamine as an additive. Chin. J. Catal. 2012, 33, 610–620. [Google Scholar] [CrossRef]
- Zhou, G.B.; Wang, H.; Pei, Y.; Qiao, M.H.; Sun, B.; Zong, B.N. Pore size effect of Ru-Zn/ZrO2 catalyst on partial hydrogenation of benzene to cyclohexene. Acta Chim. Sin. 2017, 75, 321–328. [Google Scholar] [CrossRef]
- Kötz, R.; Lewerenz, H.J.; Stucki, S. XPS studies of oxygen evolution on Ru and RuO2 anodes. J. Electrochem. Soc. 1983, 130, 825–829. [Google Scholar] [CrossRef]
- Wehner, P.S.; Mercer, P.N.; Apai, G. Interaction of H2 and CO with Rh4(CO)12 supported on ZnO. J. Catal. 1983, 84, 244–247. [Google Scholar] [CrossRef]
- Gaarenstroom, S.W.; Winograd, N. Initial and final state effects in the ESCA spectra of cadmium and silver oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Folkesson, B. ESCA Studies on the Charge Distribution in Some Dinitrogen Complexes of Rhenium, Iridium, Ruthenium, and Osmium. Acta Chem. Scand. 1973, 27, 287–302. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Firsov, M.N.; Shaplygin, I.S. Electronic structures of MRhO2, MRh2O4, RhMO4 and Rh2MO6 on the basis of X-ray spectroscopy and ESCA data. J. Electron Spectrosc. Relat. Phenom. 1982, 26, 65–78. [Google Scholar] [CrossRef]
- Schӧn, G. Auger and direct electron spectra in X-ray photoelectron studies of zinc, zinc oxide, gallium and gallium oxide. J. Electron Spectrosc. Relat. Phenom. 1973, 2, 75–86. [Google Scholar] [CrossRef]
- Sun, H.J.; Guo, W.; Zhou, X.L.; Chen, Z.H.; Liu, Z.Y.; Liu, S.C. Process in Ru-based Amorphous Alloy Catalysts for Benzene Selective Hydrogenation to Cyclohexene. Chin. J. Catal. 2011, 32, 1–16. [Google Scholar] [CrossRef]
- Mazzieri, V.A.; L’Argentire, P.C.; Coloma-Pascual, F.; Fgoli, N.S. Effect of Chlorine on the Properties of Ru/Al2O3. Ind. Eng. Chem. Res. 2003, 42, 2269–2272. [Google Scholar] [CrossRef]
- Struijk, J.; Moene, R.; Kamp, T.V.D.; Scholten, J.J.F. Partial liquid phase hydrogenation of benzene to cyclohexene over ruthenium catalysts in the presence of an aqueous salt solution II. Influence of various salts on the performance of the catalyst. Appl. Catal. A Gen. 1992, 89, 77–102. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhu, Y.; Liu, J.; Pei, Y.; Li, Z.H.; Li, H.; Li, H.X.; Qiao, M.H.; Fan, K.N. Discrimination of the roles of CdSO4 and ZnSO4 in liquid phase hydrogenation of benzene to cyclohexene. J. Catal. 2009, 268, 100–105. [Google Scholar] [CrossRef]
- Sun, H.J.; Qin, H.A.; Huang, Z.X.; Su, M.F.; Li, Y.Y.; Liu, S.C.; Liu, Z.Y. Effect of reaction modifier ZnSO4 and pretreatment on performance of Ru-Zn catalyst for selective hydrogenation of benzene to cyclohexene. Chin. J. Inorg. Chem. 2017, 33, 73–80. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).