Electrochemical Oxidation of Amines Using a Nitroxyl Radical Catalyst and the Electroanalysis of Lidocaine
Abstract
1. Introduction
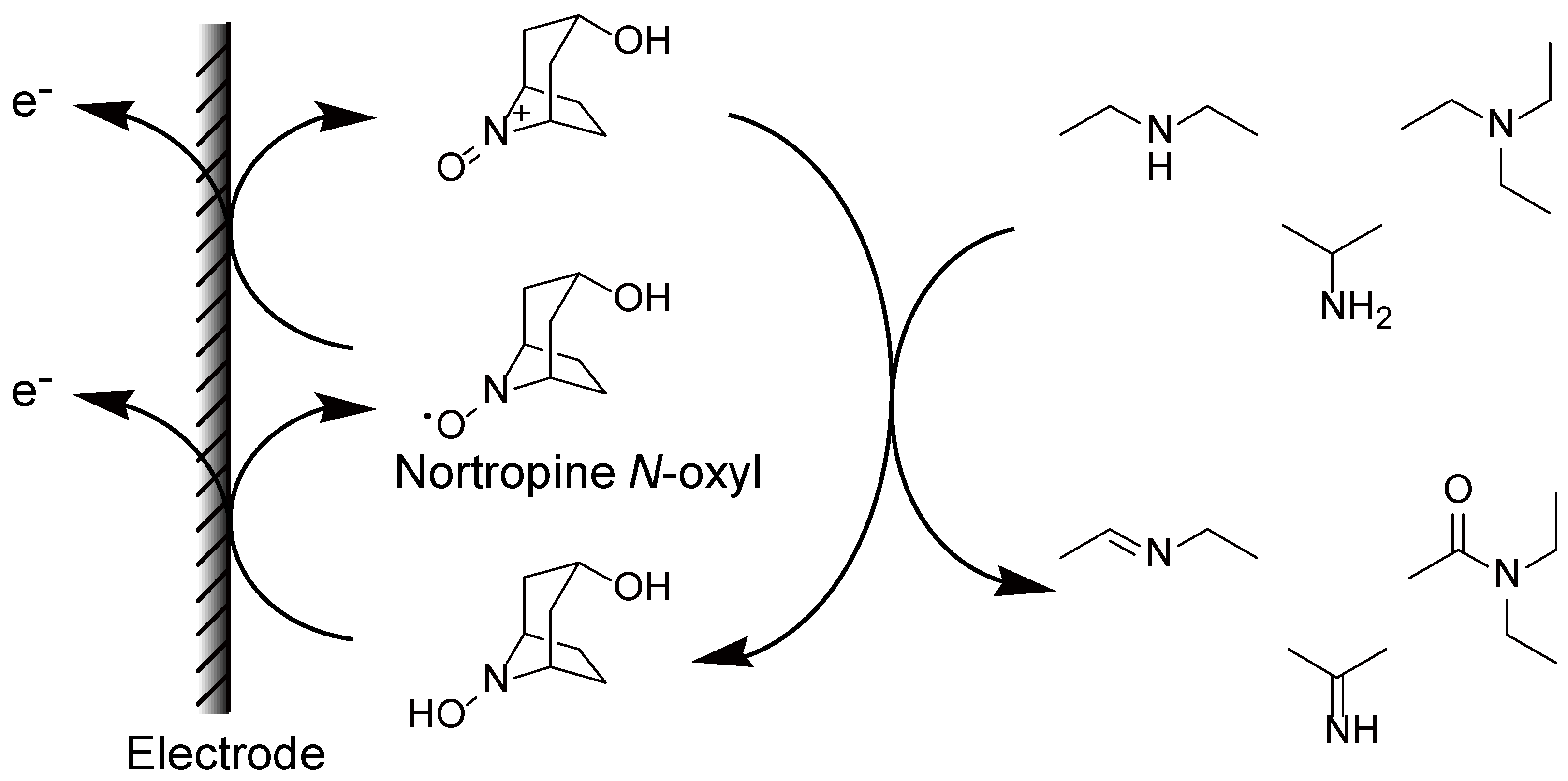
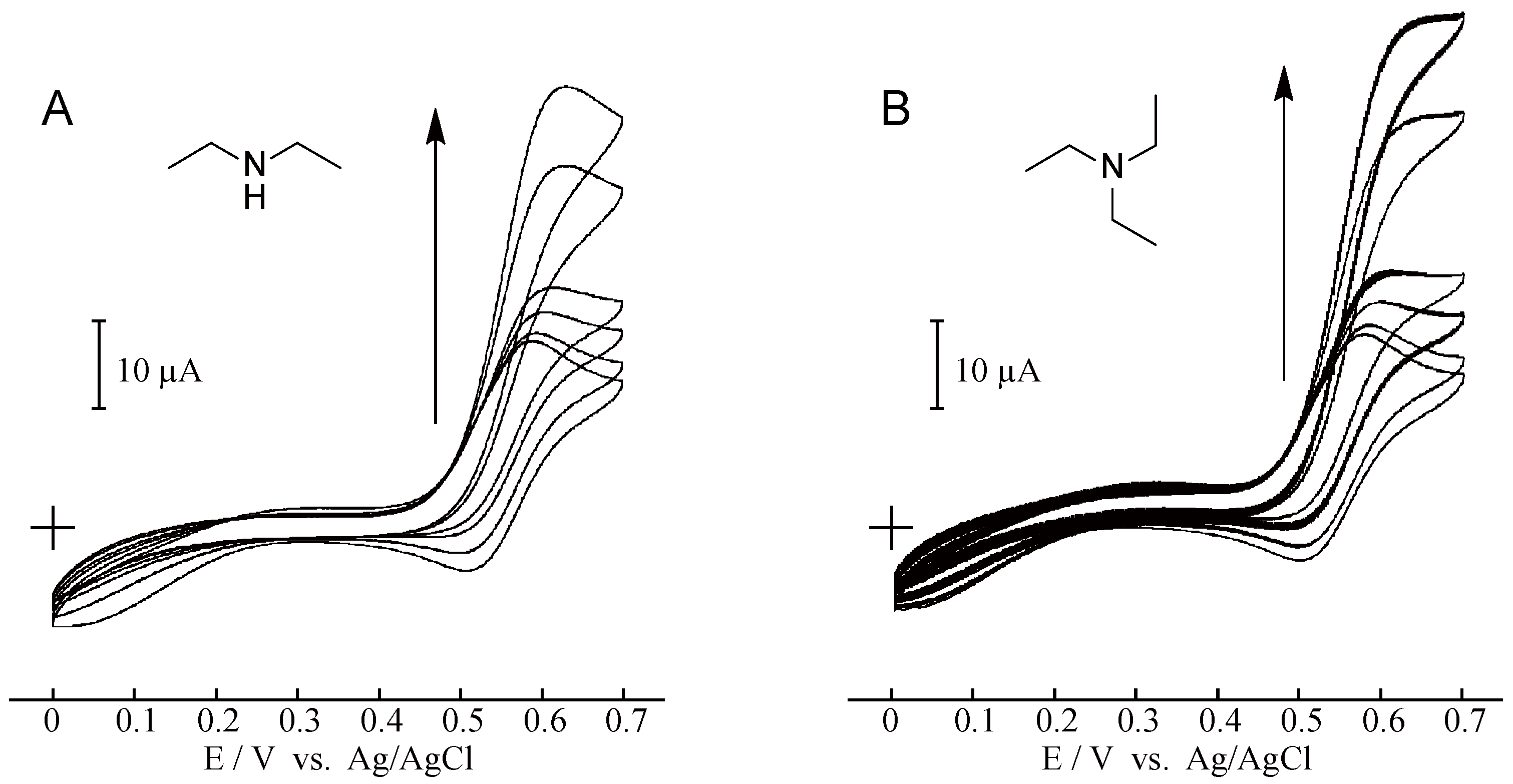
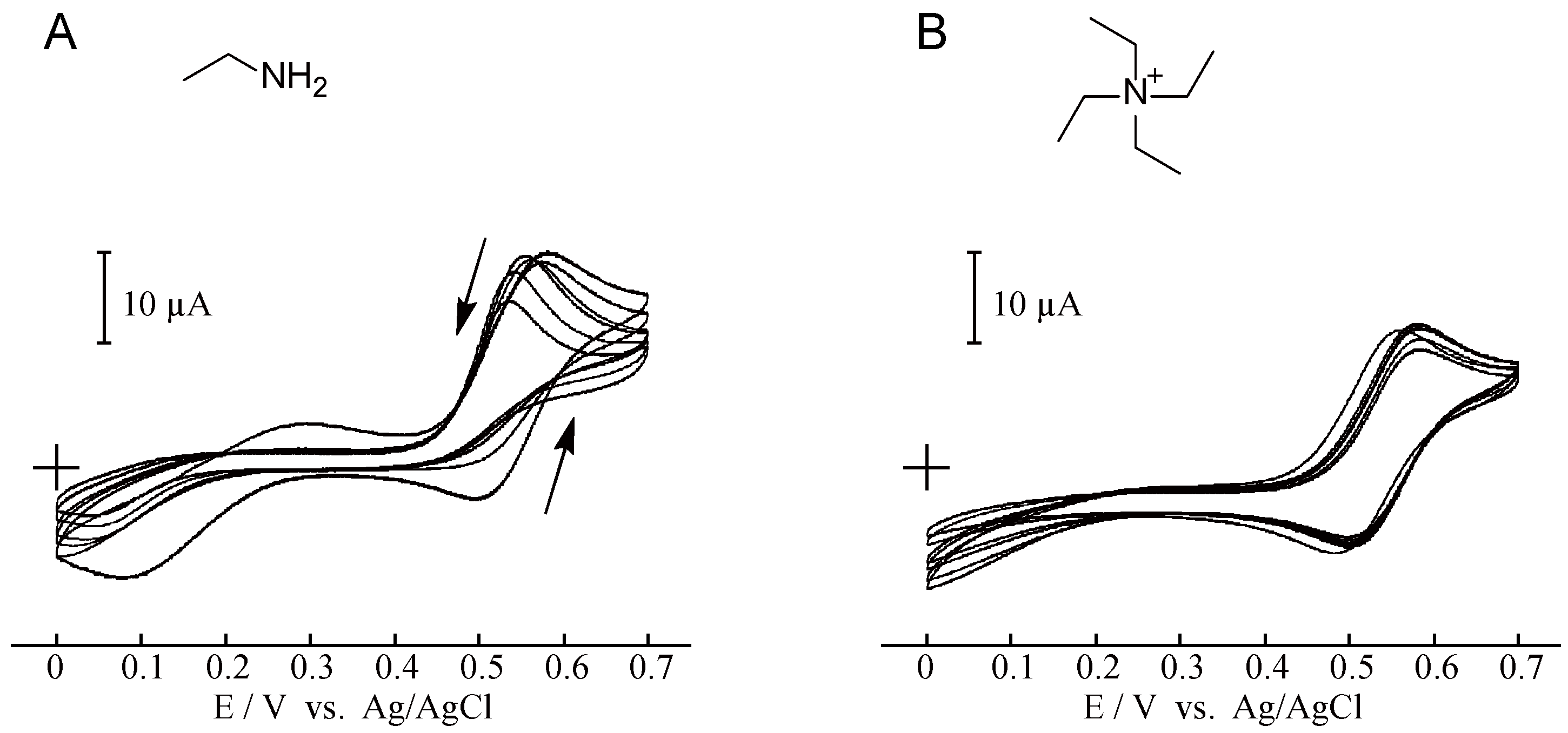
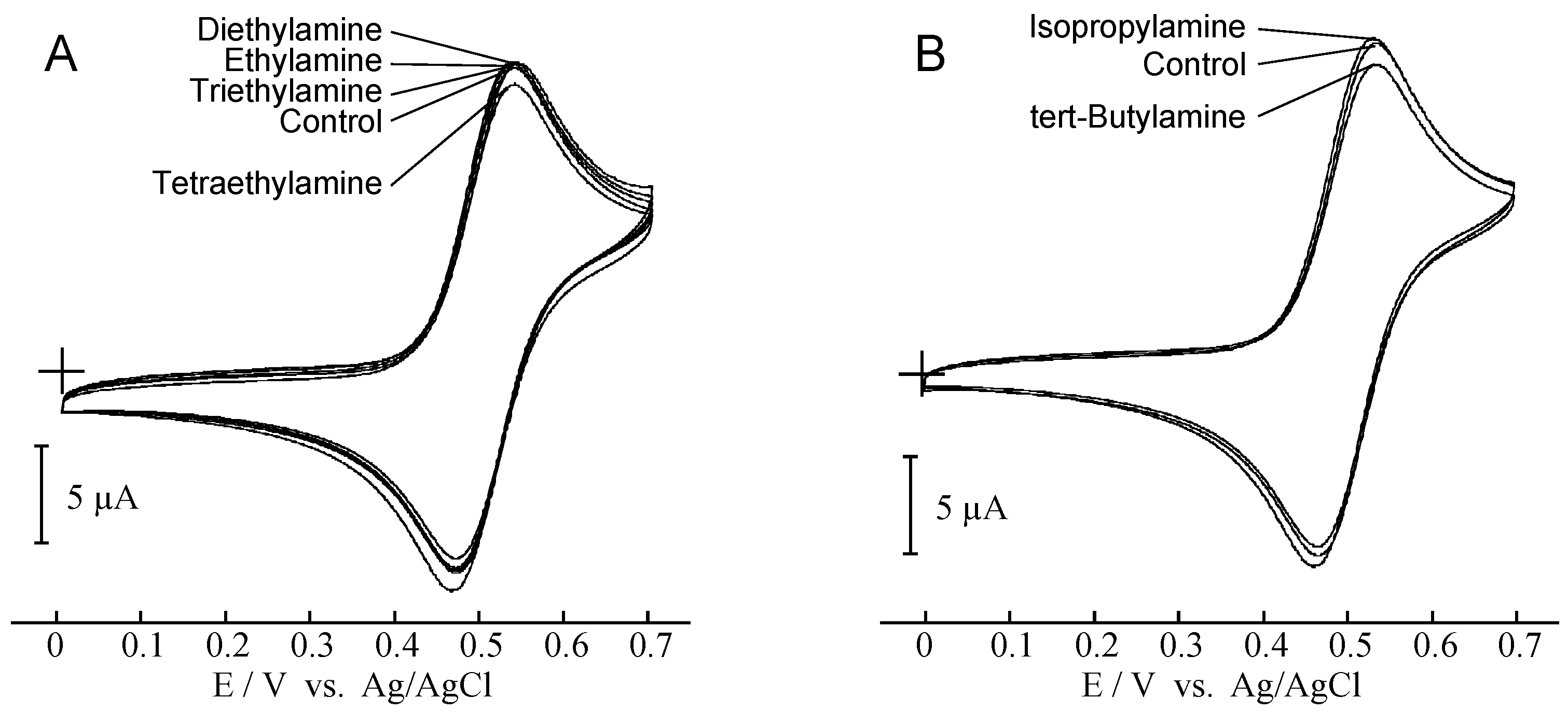
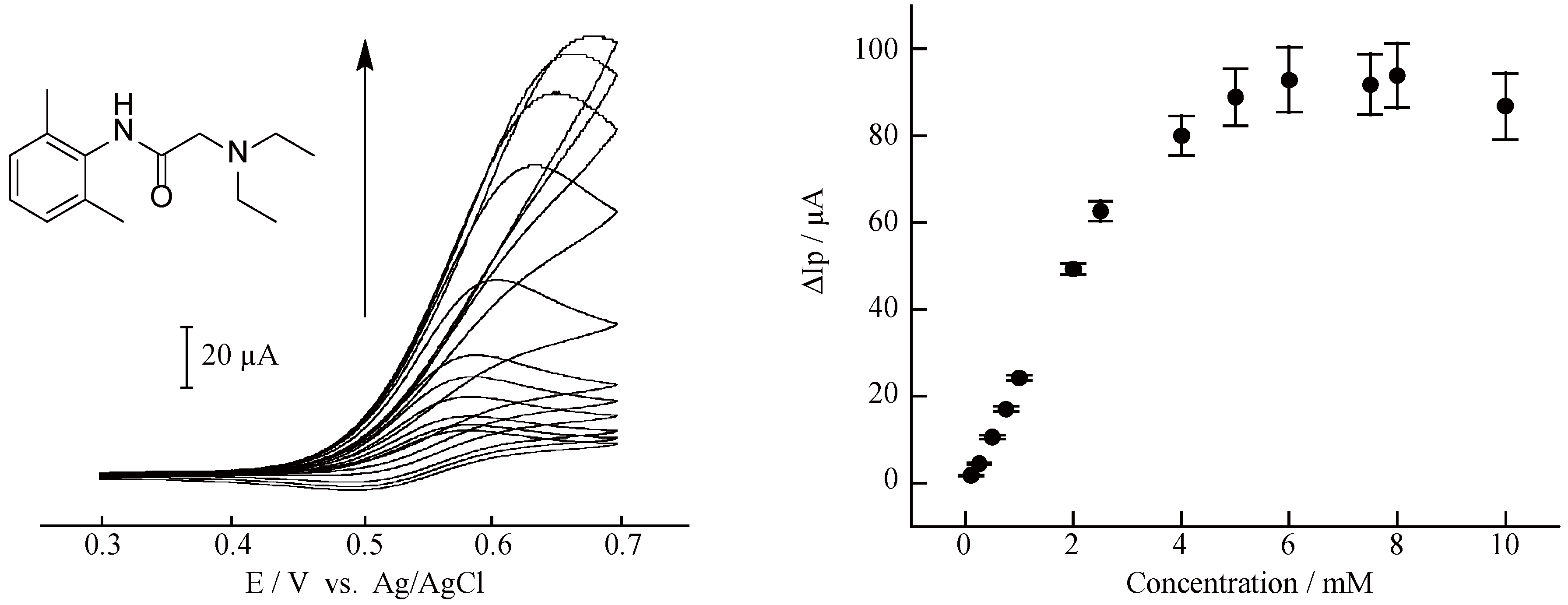
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakker, E.; Qin, Y. Electrochemical sensors. Anal. Chem. 2006, 78, 3965–3983. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Chatard, C.; Meiller, A.; Marinesco, S. Microelectrode biosensors for in vivo analysis of brain interstitial fluid. Electroanalysis 2018, 30, 977–998. [Google Scholar] [CrossRef]
- Asal, M.; Özen, Ö.; Şahinler, M.; Polatoğlu, İ. Recent developments in enzyme, DNA and immuno-based biosensors. Sensors 2018, 18, 1924. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kamijo, T.; Takahashi, T.; Sato, T. Comparison of NAD with NADP-dependent Glutamate Dehydrogenase, and CNT with rGO-modified Electrodes, for the Construction of Glutamate Sensors. Electroanalysis 2018, 30, 2237–2240. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Maeda, H.; Omori, H. Amperometric detection of alcohols and carbohydrates coupled with their electrocatalytic oxidation by 2, 2, 6, 6-Tetramethylpiperidinyl-1-oxy (TEMPO). Chem. Pharm. Bull. 1996, 44, 1021–1025. [Google Scholar] [CrossRef]
- Koga, T.; Taniguchi, I. Electrochemical oxidation of glucose to glucarate using TEMPO as a mediator in an alkaline solution. Electrochemistry 2004, 72, 858–860. [Google Scholar]
- Ono, T.; Sato, K.; Shimizu, S.; Yoshida, K.; Dairaku, T.; Suzuki, Y.; Kashiwagi, Y. Electrocatalytic Oxidation of Carbohydrates Mediated by Nitroxyl Radical-Modified Electrodes in Aqueous Solution. Electroanalysis 2018, 30, 24–26. [Google Scholar] [CrossRef]
- Tebben, L.; Studer, A. Nitroxides: Applications in Synthesis and in Polymer Chemistry. Angew. Chem. Int. Ed. 2011, 50, 5034–5068. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Org. Process. Res. Dev. 2010, 14, 245–251. [Google Scholar] [CrossRef]
- Vogler, T.; Studer, A. Applications of TEMPO in Synthesis. Synthesis 2008, 1979–1993. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E. Catalytic oxidations mediated by metal ions and nitroxyl radicals. J. Mol. Catal. A Chem. 2006, 251, 200–214. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E. Organocatalytic Oxidations Mediated by Nitroxyl Radicals. Adv. Synth. Catal. 2004, 346, 1051–1071. [Google Scholar] [CrossRef]
- Adam, W.; Saha-Moller, C.R.; Ganeshpure, P.A. Synthetic Applications of Nonmetal Catalysts for Homogeneous Oxidations. Chem. Rev. 2001, 101, 3499–3548. [Google Scholar] [CrossRef] [PubMed]
- De Nooy, A.E.J.; Besemer, A.C.; van Bekkum, H. On the Use of Stable Organic Nitroxyl Radicals for the Oxidation of Primary and Secondary Alcohols. Synthesis 1996, 1153–1176. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Schmid, C.R.; Cortes, D.A.; Chou, C.S. Nitroxyl-mediated electrooxidation of alcohols to aldehydes and ketones. J. Am. Chem. Soc. 1983, 105, 4492–4494. [Google Scholar] [CrossRef]
- Deronzier, A.; Limosin, D.; Moutet, J.-C. Electrochemical oxidation of carbinols mediated by nitroxyl radicals in solution or bonded to polypyrrolic coatings on platinum and carbon electrodes. Electrochim. Acta 1987, 32, 1643–1647. [Google Scholar] [CrossRef]
- Bobbitt, J.M.; Flores, M.C.L. Organic Nitrosonium Salts as Oxidants in Organic Chemistry. Heterocycles 1988, 27, 509–533. [Google Scholar] [CrossRef]
- Inokuchi, T.; Matsumoto, S.; Nishiyama, T.; Torii, S. A selective and efficient method for alcohol oxidations mediated by N-oxoammonium salts in combination with sodium bromite. J. Org. Chem. 1990, 55, 462–466. [Google Scholar] [CrossRef]
- Ma, Z.; Bobbitt, J.M. Organic oxoammonium salts. 3. A new convenient method for the oxidation of alcohols to aldehydes and ketones. J. Org. Chem. 1991, 56, 6110–6114. [Google Scholar] [CrossRef]
- Davis, N.J.; Flitsch, S.L. Selective oxidation of monosaccharide derivatives to uronic acids. Tetrahedron Lett. 1993, 34, 1181–1184. [Google Scholar] [CrossRef]
- Iwabuchi, Y. Discovery and Exploitation of AZADO: The Highly Active Catalyst for Alcohol Oxidation. Chem. Pharm. Bull. 2013, 61, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Sasano, Y.; Nagasawa, S.; Shibuya, M.; Iwabuchi, Y. 9-Azanoradamantane N-Oxyl (NorAZADO): A Highly Active Organocatalyst for Alcohol Oxidation. Chem. Pharm. Bull. 2011, 59, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Sasano, Y.; Tomizawa, M.; Hamada, T.; Kozawa, M.; Nagahama, N.; Iwabuchi, Y. Practical Preparation Methods for Highly Active Azaadamantane-Nitroxyl-Radical-Type Oxidation Catalysts. Synthesis 2011, 3418–3425. [Google Scholar] [CrossRef]
- Shibuya, M.; Tomizawa, M.; Sasano, Y.; Iwabuchi, Y. An Expeditious Entry to 9-Azabicyclo[3.3.1]nonane N-Oxyl (ABNO): Another Highly Active Organocatalyst for Oxidation of Alcohols. J. Org. Chem. 2009, 74, 4619–4622. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Tomizawa, M.; Suzuki, I.; Iwabuchi, Y. 2-Azaadamantane N-Oxyl (AZADO) and 1-Me-AZADO: Highly Efficient Organocatalysts for Oxidation of Alcohols. J. Am. Chem. Soc. 2006, 128, 8412–8413. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ono, T.; Yoshida, K.; Ito, T.; Kashiwagi, Y. Electrochemical Determination of D-Glucose Using Nortropine-N-oxyl under Physiological Conditions. Electroanalysis 2015, 27, 2272–2274. [Google Scholar] [CrossRef]
- Granot, E.; Tel-Vered, R.; Lioubashevski, O.; Willner, I. Stereoselective and Enantioselective Electrochemical Sensing of Monosaccharides Using Imprinted Boronic Acid-Functionalized Polyphenol Films. Adv. Funct. Mater. 2008, 18, 478–484. [Google Scholar] [CrossRef]
- Arimori, S.; Ushiroda, S.; Peter, L.M.; Jenkins, A.T.A.; James, T.D. A modular electrochemical sensor for saccharides. Chem. Commun. 2002, 2368–2369. [Google Scholar] [CrossRef]
- Shoji, E.; Freund, M.S. Potentiometric Saccharide Detection Based on the pKa Changes of Poly(aniline boronic acid). J. Am. Chem. Soc. 2002, 124, 12486–12493. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Shibata, H.; Heo, Y.J.; Okitsu, T.; Matsunaga, Y.; Kawanishia, T.; Takeuchi, S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, T.; Romey, M.A.; Zhu, P.C.; Holody, M.Z.; Shinkai, S. A study of boronic acid based fluorescent glucose sensors. J. Fluoresc. 2004, 14, 499–512. [Google Scholar] [CrossRef]
- Sato, K.; Nakajima, T.; Yasukawa, Y.; Anzai, J. Sugar-dependent solubility and fluorescence property of copolymers consisting of phenylboronic acid and 2-hydroxyethyl methacrylate moieties. Polym. Bull. 2010, 65, 807–814. [Google Scholar] [CrossRef]
- Bartelson, A.L.; Lambert, K.M.; Bobbitt, J.M.; Bailey, W.F. Recent Developments in the Nitroxide-Catalyzed Oxidation of Amines: Preparation of Imines and Nitriles. Chem. Cat. Chem. 2016, 8, 3421–3430. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Schmid, C.R. Nitroxyl-mediated electro-oxidation of amines to nitriles and carbonyl compounds. J. Am. Chem. Soc. 1983, 105, 6732–6734. [Google Scholar] [CrossRef]
- MacCorquodale, F.; Crayston, J.A.; Walton, J.C.; Worsfold, D.J. Synthesis and electrochemical characterisation of poly(tempoacrylate). Tetrahedron Lett. 1990, 31, 771–774. [Google Scholar] [CrossRef]
- Wu, X.-F.; Bheeter, C.B.; Neumann, H.; Dixneuf, P.H.; Beller, M. Lewis acid-catalyzed oxidation of benzylamines to benzamides. Chem. Commun. 2012, 48, 12237–12239. [Google Scholar] [CrossRef]
- Majji, G.; Rajamanickam, S.; Khatun, N.; Santra, S.K.; Patel, B.K. Generation of bis-Acyl Ketals from Esters and Benzyl Amines Under Oxidative Conditions. J. Org. Chem. 2015, 80, 3440–3446. [Google Scholar] [CrossRef]
- Rao, S.N.; Reddy, N.N.K.; Samanta, S.; Adimurthy, J.S. I2-Catalyzed Oxidative Amidation of Benzylamines and Benzyl Cyanides under Mild Conditions. Org. Chem. 2017, 82, 13632–13642. [Google Scholar] [CrossRef]
- Zhanga, Y.; Gao, Z.; Zhang, W.; Wang, W.; Chang, J.; Kaia, J. Fluorescent carbon dots as nanoprobe for determination of lidocaine hydrochloride. Sens. Actuators B 2018, 262, 928–937. [Google Scholar] [CrossRef]
- Doran, G.S.; Smith, A.K.; Rothwell, J.T.; Edwards, S.H. Direct detection of glucuronide metabolites of lidocaine in sheep urine. J. Chromatogr. B 2018, 1076, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zargar, B.; Hatamie, A. Hollow fiber liquid based microextraction combined with high-performance liquid-chromatography for the analysis of lidocaine in biological and pharmaceutical samples. Anal. Methods 2014, 6, 2506–2511. [Google Scholar] [CrossRef]
- Chu, B.; Lou, D.; Yu, P.; Hu, S.; Shen, A. Development of an on-column enrichment technique based on C18-functionalized magnetic silica nanoparticles for the determination of lidocaine in rat plasma by high performance liquid chromatography. J. Chromatogr. A 2011, 1218, 7248–7253. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Jiao, Z.; Zhong, M.; Shi, X.; Zhang, J.; Li, Z.; Cui, X. Simultaneous determination of procaine, lidocaine, ropivacaine, tetracaine and bupivacaine in human plasma by high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Plossl, F.; Giera, M.; Bracher, F. Multiresidue analytical method using dispersive solid-phase extraction and gas chromatography/ion trap mass spectrometry to determine pharmaceuticals in whole blood. J. Chromatogr. A 2006, 1135, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Namera, A.; Yashiki, M.; Iwasaki, Y.; Kojima, T. Simple analysis of local anaesthetics in human blood using headspace solid-phase microextraction and gas chromatography-mass spectrometry-electron impact ionization selected ion monitoring. J. Chromatogr. B Biomed. Sci. Appl. 1998, 709, 225–232. [Google Scholar] [CrossRef]
- Hamdah, M.A.N.; Matthew, P.; James, S.G.; Dion, R.B. A High-Performance Liquid Chromatography Assay Method for the Determination of Lidocaine in Human Serum. Pharmaceutics 2017, 9, 52. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhang, Y.; Yu, F.; Wang, F.; Peng, Z.; Li, Y. Voltammetric lidocaine sensor by using a glassy carbon electrode modified with porous carbon prepared from a MOF, and with a molecularly imprinted polymer. Microchim. Acta 2018, 185, 78–83. [Google Scholar] [CrossRef]
- Yanga, G.; Zhao, F. A novel electrochemical sensor for the determination of lidocaine based on surface-imprinting on porous three-dimensional film. J. Mater. Chem. C 2014, 2, 10201–10208. [Google Scholar] [CrossRef]
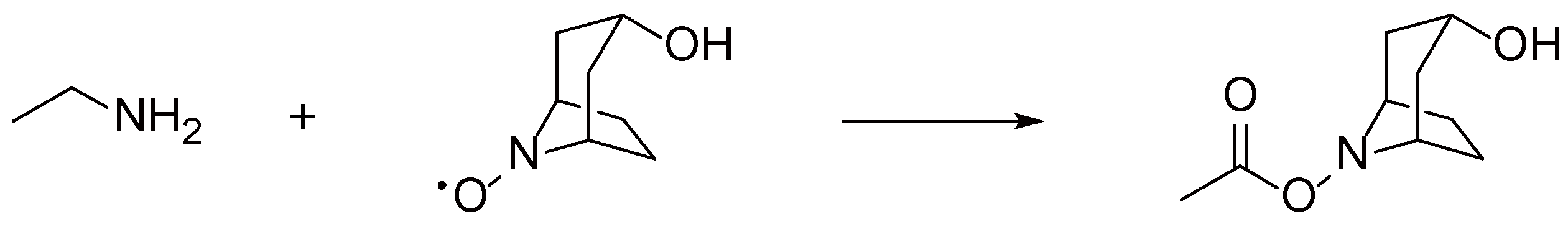

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Ono, T.; Sasano, Y.; Sato, F.; Kumano, M.; Yoshida, K.; Dairaku, T.; Iwabuchi, Y.; Kashiwagi, Y. Electrochemical Oxidation of Amines Using a Nitroxyl Radical Catalyst and the Electroanalysis of Lidocaine. Catalysts 2018, 8, 649. https://doi.org/10.3390/catal8120649
Sato K, Ono T, Sasano Y, Sato F, Kumano M, Yoshida K, Dairaku T, Iwabuchi Y, Kashiwagi Y. Electrochemical Oxidation of Amines Using a Nitroxyl Radical Catalyst and the Electroanalysis of Lidocaine. Catalysts. 2018; 8(12):649. https://doi.org/10.3390/catal8120649
Chicago/Turabian StyleSato, Katsuhiko, Tetsuya Ono, Yusuke Sasano, Fumiya Sato, Masayuki Kumano, Kentaro Yoshida, Takenori Dairaku, Yoshiharu Iwabuchi, and Yoshitomo Kashiwagi. 2018. "Electrochemical Oxidation of Amines Using a Nitroxyl Radical Catalyst and the Electroanalysis of Lidocaine" Catalysts 8, no. 12: 649. https://doi.org/10.3390/catal8120649
APA StyleSato, K., Ono, T., Sasano, Y., Sato, F., Kumano, M., Yoshida, K., Dairaku, T., Iwabuchi, Y., & Kashiwagi, Y. (2018). Electrochemical Oxidation of Amines Using a Nitroxyl Radical Catalyst and the Electroanalysis of Lidocaine. Catalysts, 8(12), 649. https://doi.org/10.3390/catal8120649




