Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio
Abstract
:1. Introduction
2. Results
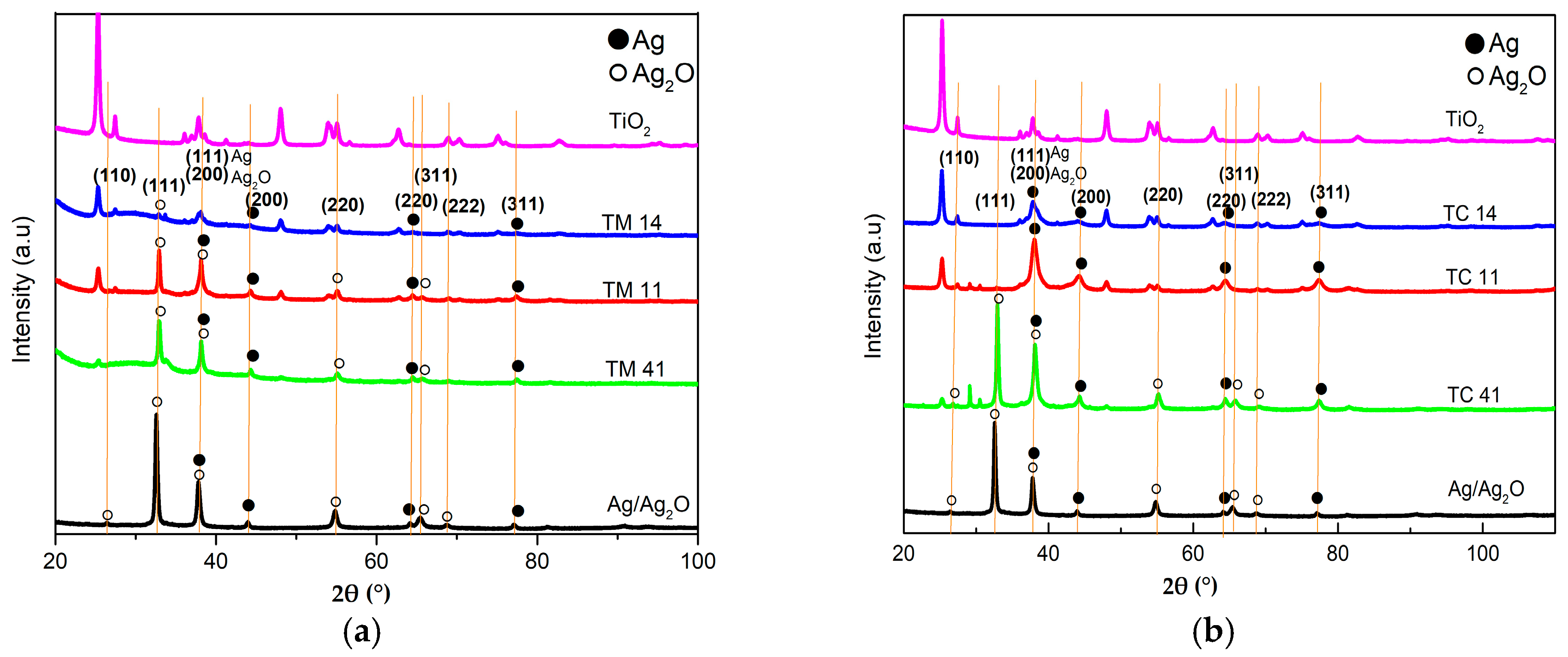
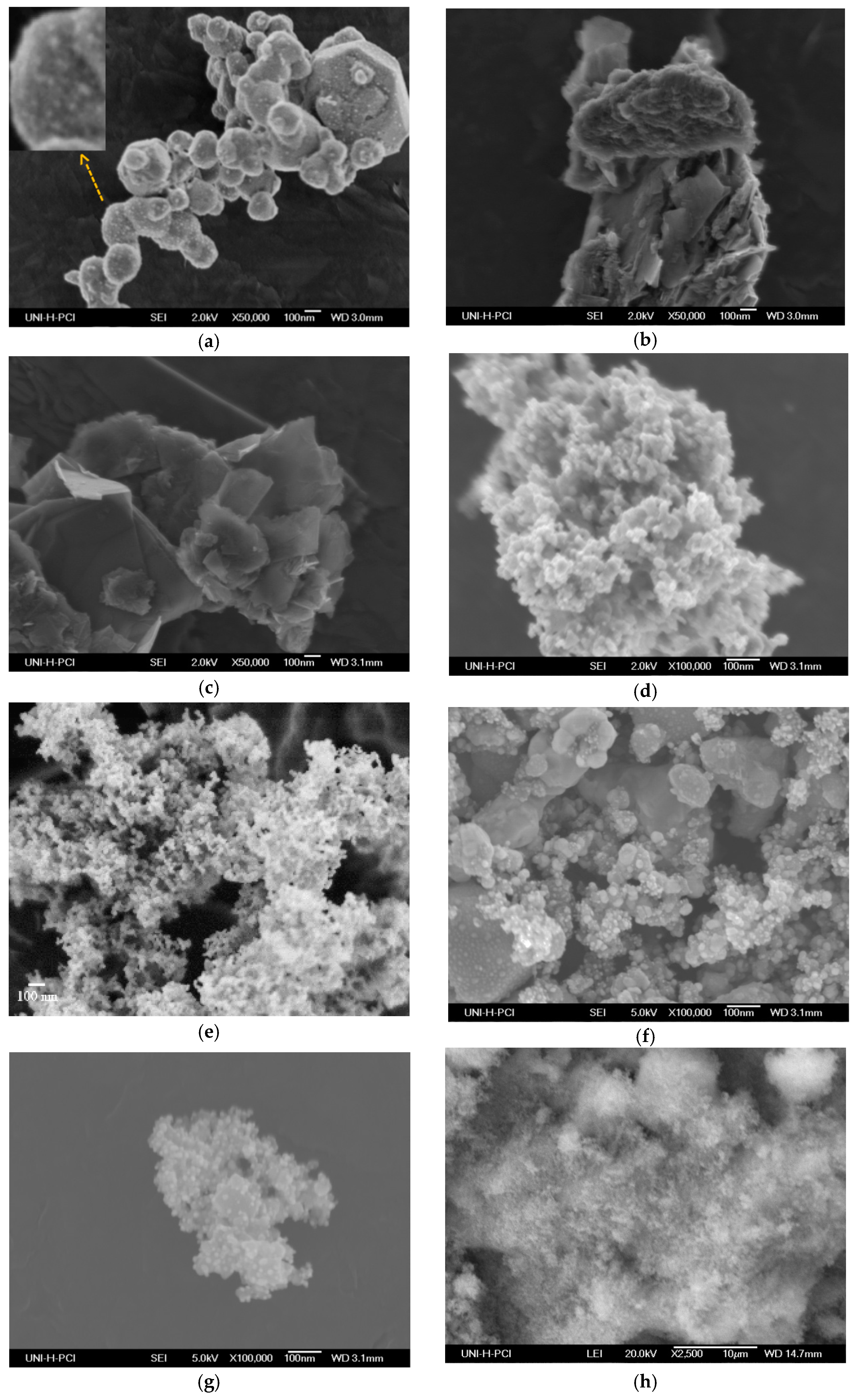
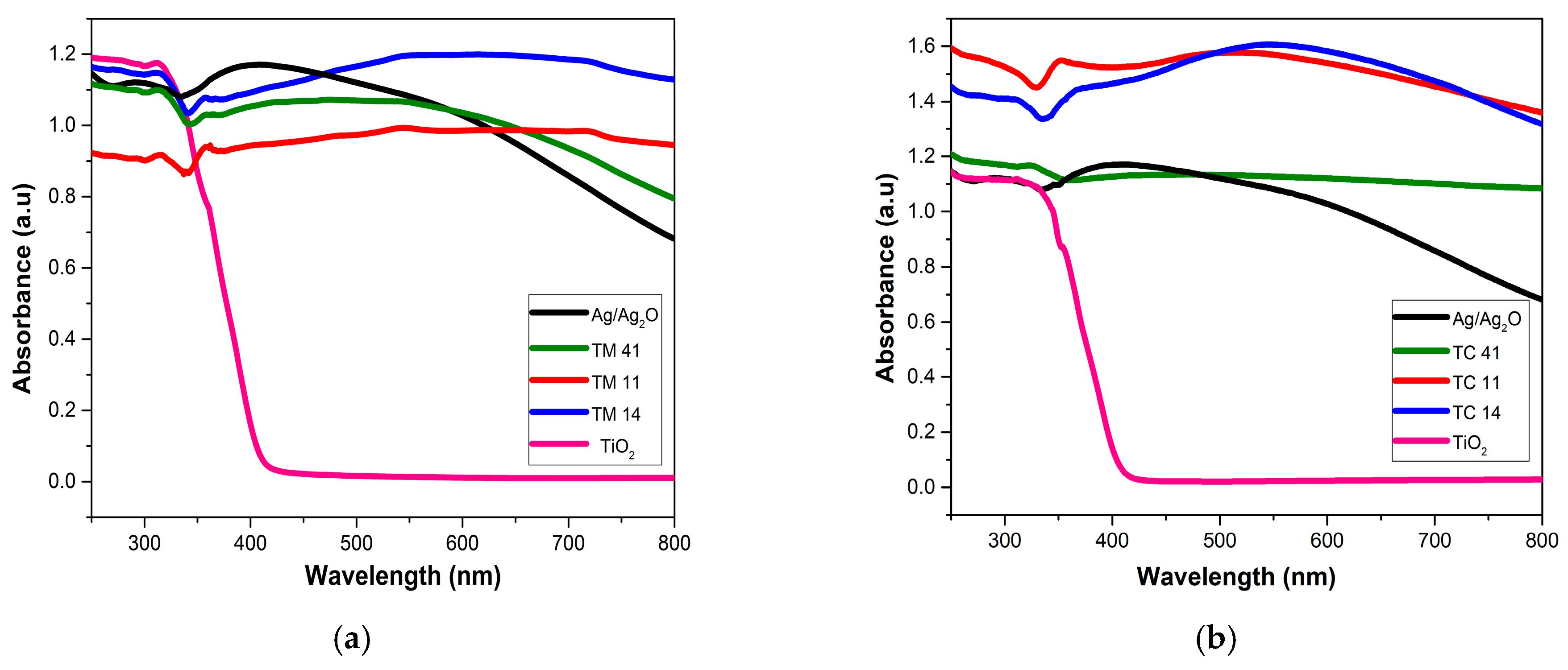
2.1. Characterization of the Prepared Materials
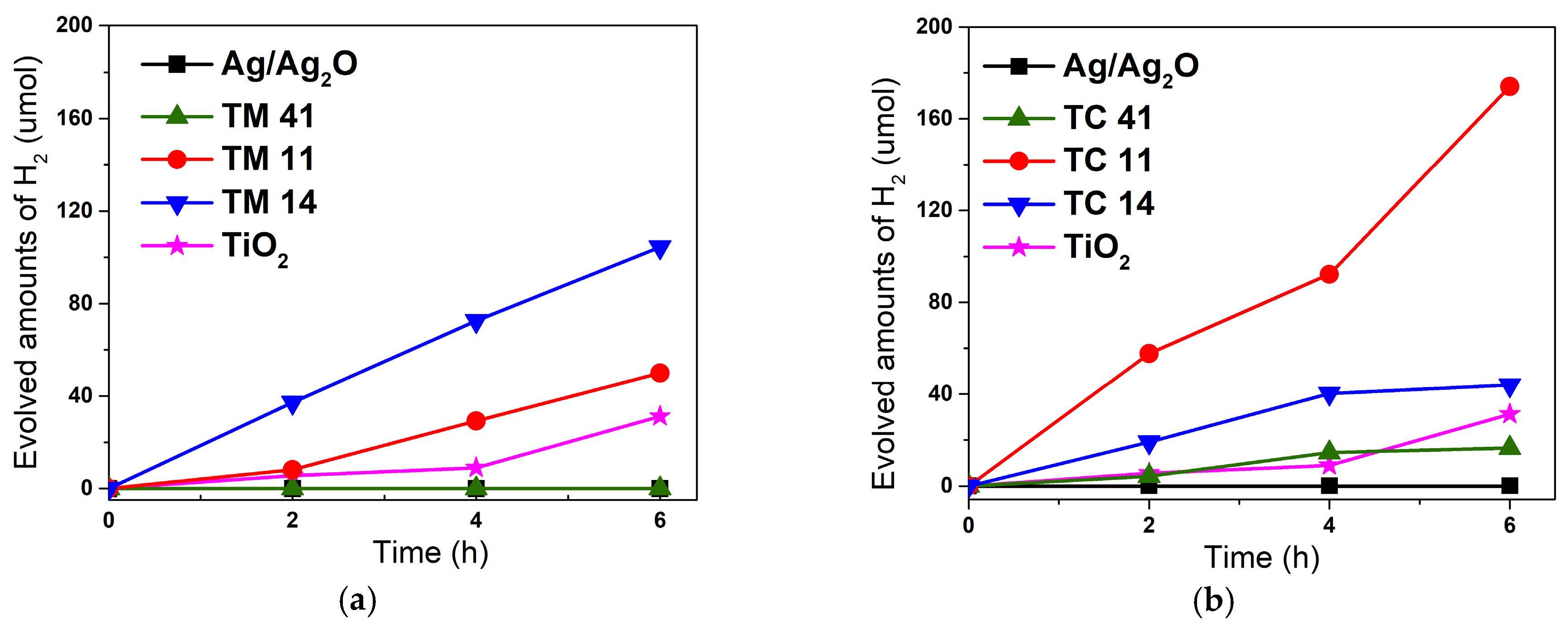
2.2. Photocatalytic Performance of the Materials
3. Discussion
3.1. The Photocatalytic Activity of Ag/Ag2O
3.2. The Photocatalytic Activity of Physical Ag/Ag2O ⁄⁄ TiO2 Mixtures
3.2.1. Bleaching of Methylene Blue
3.2.2. Light-Induced Hydrogen Evolution
3.3. The Photocatalytic Activity of Ag/Ag2O ⁄⁄ TiO2 Composites
3.3.1. Bleaching of Methylene Blue
3.3.2. Light-Induced Hydrogen Evolution
4. Experimental Section
4.1. Materials
4.2. Synthetic Methods
4.2.1. Preparation of Ag/Ag2O
4.2.2. Preparation of TM Mixtures
4.2.3. Preparation of TC Composites
4.3. Characterization of the Materials
4.4. Photocatalytic Measurements
4.4.1. Methylene Blue Degradation
4.4.2. Photocatalytic Hydrogen Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahnemann, D.W. Mechanisms of Organic Transformations on Semiconductor Particles. In Photochemical Conversion and Storage of Solar Energy; Proceedings of the Eighth International Conference on Photochemical Conversion and Storage of Solar Energy, IPS-8, Palermo, Italy, 15–20 July 1990; Pelizzetti, E., Schiavello, M., Eds.; Springer Science/Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 1991; pp. 251–276. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Bahnemann, D.W. Photocatalytic Detoxification of Polluted Waters. In Environmental Photochemistry; Boule, P., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 285–351. [Google Scholar]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Álvarez-Docio, C.M.; Ramírez, V.Z.; Fernández, J.F. Hierarchical Nano ZnO-Micro TiO2 Composites: High UV Protection Yield Lowering Photodegradation in Sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.-F.; Kuang, Q.; Huang, R.-B.; Xie, Z.-X.; Zheng, L.-S. Shape-Dependent Antibacterial Activities of Ag2O Polyhedral Particles. Langmuir 2010, 26, 2774–2778. [Google Scholar] [CrossRef]
- Zou, J.; Xu, Y.; Hou, B.; Wu, D.; Sun, Y. Self-Assembly Ag2O Nanoparticles into Nanowires with the Aid of Amino-Functionalized Silica Nanoparticles. Powder Technol. 2008, 183, 122–126. [Google Scholar] [CrossRef]
- Lalitha, K.; Reddy, J.K.; Phanikrishna Sharma, M.V.; Kumari, V.D.; Subrahmanyam, M. Continuous Hydrogen Production Activity over Finely Dispersed Ag2O/TiO2 Catalysts from Methanol:Water Mixtures under Solar Irradiation: A Structure-Activity Correlation. Int. J. Hydrogen Energy 2010, 35, 3991–4001. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a New Visible-Light Photocatalyst: Self-Stability and High Photocatalytic Activity. Chem. A Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Huang, B.; Cheng, H.; Wang, Z.; Zhan, J.; Qin, X.; Zhang, X.; Dai, Y. Controlled Synthesis of Ag2O Microcrystals with Facet-Dependent Photocatalytic Activities. J. Mater. Chem. 2012, 22, 21189. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Liu, Y.; Fang, P.; Dai, Y. Enhanced Adsorption and Photocatalytic Degradation of High-Concentration Methylene Blue on Ag2O-modified TiO2-based Nanosheet. Chem. Eng. J. 2013, 221, 283–291. [Google Scholar] [CrossRef]
- Hu, X.; Hu, C.; Wang, R. Enhanced Solar Photodegradation of Toxic Pollutants by Long-Lived Electrons in Ag–Ag2O Nanocomposites. Appl. Catal. B Environ. 2015, 176–177, 637–645. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Wu, Z.; Yue, X.; Yuan, S.; Lu, H.; Liang, B. Silver Oxide as Superb and Stable Photocatalyst under Visible and Near-Infrared Light Irradiation and Its Photocatalytic Mechanism. Ind. Eng. Chem. Res. 2015, 54, 832–841. [Google Scholar] [CrossRef]
- Li, J.; Fang, W.; Yu, C.; Zhou, W.; Zhu, L.; Xie, Y. Ag-Based Semiconductor Photocatalysts in Environmental Purification. Appl. Surf. Sci. 2015, 358, 46–56. [Google Scholar] [CrossRef]
- Liu, C.; Cao, C.; Luo, X.; Luo, S. Ag-bridged Ag2O Nanowire Network/TiO2 Nanotube Array p–n Heterojunction as a Highly Efficient and Stable Visible Light Photocatalyst. J. Hazard. Mater. 2015, 285, 319–324. [Google Scholar] [CrossRef]
- Ren, H.-T.; Jia, S.-Y.; Zou, J.-J.; Wu, S.-H.; Han, X. A facile Preparation of Ag2O/P25 Photocatalyst for Selective Reduction of Nitrate. Appl. Catal. B Environ. 2015, 176–177, 53–61. [Google Scholar] [CrossRef]
- Kumar, D.P.; Reddy, N.L.; Karthik, M.; Neppolian, B.; Madhavan, J.; Shankar, M.V. Solar Light Sensitized p-Ag2O/n-TiO2 Nanotubes Heterojunction Photocatalysts for Enhanced Hydrogen Production in Aqueous-Glycerol Solution. Sol. Energy Mater. Sol. Cells 2016, 154, 78–87. [Google Scholar] [CrossRef]
- Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Song, Q.; Zhang, L.; Song, X.; Wang, K.; Zhang, Y.; Li, J.; Wen, J.; Tian, J. Ag2O Nanoparticle/TiO2 Nanobelt Heterostructures with Remarkable Photo-Response and Photocatalytic Properties under UV, Visible and Near-Infrared Irradiation. Appl. Catal. B Environ. 2016, 198, 83–90. [Google Scholar] [CrossRef]
- Yang, H.; Tian, J.; Li, T.; Cui, H. Synthesis of Novel Ag/Ag2O Heterostructures with Solar Full Spectrum (UV, Visible and Near-Infrared) Light-Driven Photocatalytic Activity and Enhanced Photoelectrochemical Performance. Catal. Commun. 2016, 87, 82–85. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Ma, Z. Flower-Like Ag2O/Bi2MoO6 p-n Heterojunction with Enhanced Photocatalytic Activity under Visible Light Irradiation. J. Molec. Catal. A Chem. 2016, 424, 37–44. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, Q.; Deng, X.; Meng, Q.; Cheng, X.; Xie, M.; Li, X.; Cheng, Q.; Liu, H. Fabrication of Ag-Ag2O/reduced TiO2 Nanophotocatalyst and its Enhanced Visible Light Driven Photocatalytic Performance for Degradation of Diclofenac Solution. Appl. Catal. B Environ. 2017, 206, 136–145. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Tian, J.; Li, Y.; Cui, H. Towards Full-Spectrum (UV, Visible, and Near-Infrared) Photocatalysis: Achieving an All-Solid-State Z-Scheme between Ag2O and TiO2 Using Reduced Graphene Oxide as the Electron Mediator. Catal. Sci. Technol. 2017, 7, 4193–4205. [Google Scholar] [CrossRef]
- Kaur, A.; Salunke, D.B.; Umar, A.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Visible Light Driven Photocatalytic Degradation of Fluoroquinolone Levofloxacin Drug Using Ag2O/TiO2 Quantum Dots: A Mechanistic Study and Degradation Pathway. New J. Chem. 2017, 41, 12079–12090. [Google Scholar] [CrossRef]
- Liu, B.; Mu, L.; Han, B.; Zhang, J.; Shi, H. Fabrication of TiO2/Ag2O Heterostructure with Enhanced Photocatalytic and Antibacterial Activities under Visible Light Irradiation. Appl. Surf. Sci. 2017, 396, 1596–1603. [Google Scholar] [CrossRef]
- Mandari, K.K.; Kwak, B.S.; Police, A.K.R.; Kang, M. In-Situ Photo-Reduction of Silver Particles and their SPR Effect in Enhancing the Photocatalytic Water Splitting of Ag2O/TiO2 Photocatalysts under Solar Light Irradiation: A Case Study. Mater. Res. Bull. 2017, 95, 515–524. [Google Scholar] [CrossRef]
- Olya, M.E.; Vafaee, M.; Jahangiri, M. Modeling of Acid Dye Decolorization by TiO2–Ag2O Nano-Photocatalytic Process Using Response Surface Methodology. J. Saudi Chem. Soc. 2017, 21, 633–642. [Google Scholar] [CrossRef]
- Prakoso, S.P.; Taufik, A.; Saleh, R. Ag/Ag2O/TiO2 Nanocomposites: Microwave-Assisted Synthesis, Characterization, and Photosonocatalytic Activities. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012029. [Google Scholar] [CrossRef]
- Ren, H.-T.; Yang, Q. Fabrication of Ag2O/TiO2 with Enhanced Photocatalytic Performances for Dye Pollutants Degradation by a pH-Induced Method. Appl. Surf. Sci. 2017, 396, 530–538. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Chen, Y.; Cheng, Z.; Luo, X.; Mo, S.; Jia, L.; Shu, R.; Lin, P.; Yang, Z.; et al. Efficient Hydrogen Production from Glycerol Photoreforming Over Ag2O–TiO2 Synthesized by a Sol–Gel Method. Int. J. Hydrogen Energy 2017, 42, 17063–17074. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, C.; Xiao, G.; Su, H. Controlled Synthesis and Wastewater Treatment of Ag2O/TiO2 Modified Chitosan-Based Photocatalytic Film. RSC Adv. 2017, 7, 11211–11221. [Google Scholar] [CrossRef]
- Deng, A.; Zhu, Y. Synthesis of TiO2/SiO2/Ag/Ag2O and TiO2/Ag/Ag2O Nanocomposite Spheres with Photocatalytic Performance. Res. Chem. Intermed. 2018, 44, 4227–4243. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Zhang, R.; Zou, B.; Shi, H. Enhanced Photoelectrochemical Water Splitting with TiO2@Ag2O Nanowire Arrays via p-n Heterojunction Formation. Sol. Energy Mater. Sol. Cells 2018, 174, 132–139. [Google Scholar] [CrossRef]
- Liu, R.; Wang, P.; Wang, X.; Yu, H.; Yu, J. UV- and Visible-Light Photocatalytic Activity of Simultaneously Deposited and Doped Ag/Ag(I)-TiO2 Photocatalyst. J. Phys. Chem. C 2012, 116, 17721–17728. [Google Scholar] [CrossRef]
- Kang, J.-G.; Sohn, Y. Interfacial Nature of Ag Nanoparticles Supported on TiO2 Photocatalysts. J. Mater. Sci. 2012, 47, 824–832. [Google Scholar] [CrossRef]
- Jiang, B.; Hou, Z.; Tian, C.; Zhou, W.; Zhang, X.; Wu, A.; Tian, G.; Pan, K.; Ren, Z.; Fu, H. A Facile and Green Synthesis Route Towards Two-Dimensional TiO2@Ag Heterojunction Structure with Enhanced Visible Light Photocatalytic Activity. CrystEngComm 2013, 15, 5821. [Google Scholar] [CrossRef]
- Hammond, J.S.; Gaarenstroom, S.W.; Winograd, N. X-ray Photoelectron Spectroscopic Studies of Cadmium- and Silver-Oxygen Surfaces. Anal. Chem. 1975, 47, 2193–2199. [Google Scholar] [CrossRef]
- Zaccheo, B.A.; Crooks, R.M. Stabilization of Alkaline Phosphatase with Au@Ag2O Nanoparticles. Langmuir 2011, 27, 11591–11596. [Google Scholar] [CrossRef]
- Aeroxide, Aerodisp and Aeroperl. Titanium Dioxide as Photocatalyst. Technical Information. Available online: https://www.aerosil.com/sites/lists/RE/DocumentsSI/TI-1243-Titanium-Dioxide-as-Photocatalyst-EN.pdf (accessed on 16 October 2018).
- Tjeng, L.H.; Meinders, M.B.J.; van Elp, J.; Ghijsen, J.; Sawatzky, G.A.; Johnson, R.L. Electronic Structure of Ag2O. Phys. Rev. B 1990, 41, 3190–3199. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; Del Campo, A.; Fernández, J.F. Enhancement of UV Absorption Behavior in ZnO-TiO2 Composites. Bol. Soc. Esp. Ceram. Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, S.; Quian, B. Semiconductor Properties of Ag2O Film Formed on the Silver Electrode in 1 M NaOH Solution. Electrochim. Acta 1994, 39, 2465–2470. [Google Scholar] [CrossRef]
- Vvedenskii, A.; Grushevskaya, S.; Kudryashov, D.; Ganzha, S. The Influence of the Conditions of the Anodic Formation and the Thickness of Ag(I) Oxide Nanofilm on its Semiconductor Properties. J. Solid State Electrochem. 2010, 14, 1401–1413. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photobleaching of Methylene Blue Sensitised by TiO2: An Ambiguous System? J. Photochem. Photobiol. A Chem. 1999, 127, 123–134. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Fundamentals and Misconceptions in Photocatalysis. J. Photochem. Photobiol. A Chem. 2010, 216, 85–93. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Photocatalysis Fundamentals Revisited to Avoid Several Misconceptions. Appl. Catal. B Environ. 2010, 99, 461–468. [Google Scholar] [CrossRef]
- Bratsch, S.G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Arimi, A.; Megatif, L.; Granone, L.I.; Dillert, R.; Bahnemann, D.W. Visible-Light Photocatalytic Activity of Zinc Ferrites. J. Photochem. Photobiol. A Chem. 2018, 366, 118–126. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The Absolute Energy Positions of Conduction and Valence Bands of Selected Semiconducting Minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M.; Dillert, R.; Bahnemann, D.W. TiO2/Fe3O4/Ag Nanophotocatalysts in Solar Fuel Production: New Approach to Using a Flexible Lightweight Sustainable Textile Fabric. J. Clean. Prod. 2018, 196, 688–697. [Google Scholar] [CrossRef]
- Hamid, S.; Dillert, R.; Bahnemann, D.W. Photocatalytic Reforming of Aqueous Acetic Acid into Molecular Hydrogen and Hydrocarbons over Co-catalyst-Loaded TiO2: Shifting the Product Distribution. J. Phys. Chem. C 2018, 122, 12792–12809. [Google Scholar] [CrossRef]

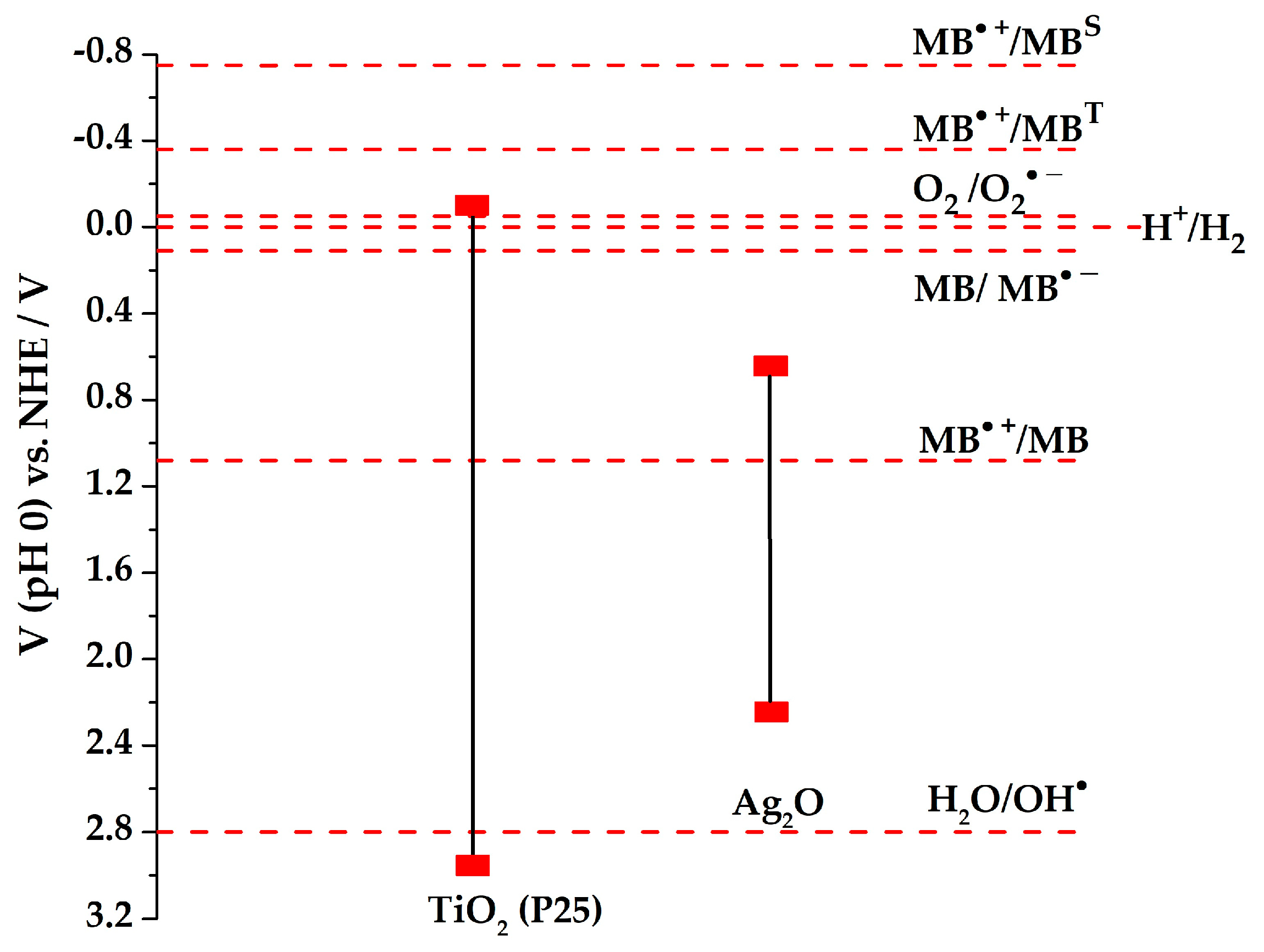


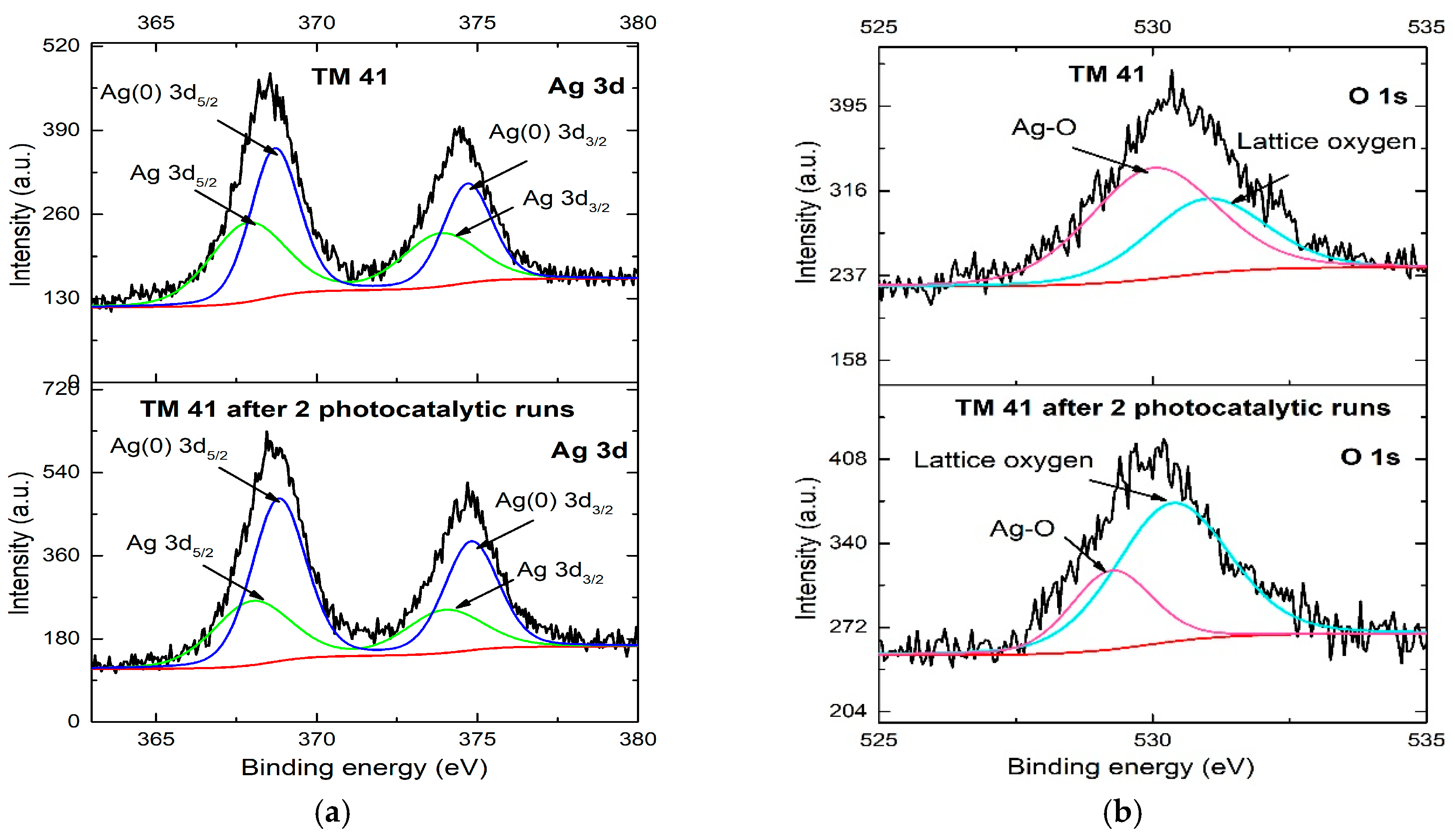


| Sample | Preparation Method | Composition | SSA m2 g−1 | r0 (MB) UV/vis mg L−1 min−1 | r0 (MB) vis mg L−1 min−1 | r (H2) UV/vis μmol h−1 |
|---|---|---|---|---|---|---|
| Photolysis | - | - | - | 0.08 | 0.05 | - |
| Ag/Ag2O | in situ | Ag/Ag2O | 2.7 | 2.64 | 1.17 | - |
| TM 41 | mechanical mixture | Ag/Ag2O ⁄⁄ TiO2 (20% TiO2) | 9.7 | 0.12 | 0.17 | - |
| TM 11 | mechanical mixture | Ag/Ag2O ⁄⁄ TiO2 (50% TiO2) | 22.6 | 0.09 | 0.03 | 9 |
| TM 14 | mechanical mixture | Ag/Ag2O ⁄⁄ TiO2 (80% TiO2) | 38.5 | 0.55 | 0.03 | 17 |
| TiO2 | - | TiO2 | 50 | 3.08 | 0.03 | 5 |
| TC 41 | in situ | Ag/Ag2O ⁄⁄ TiO2 (20% TiO2) | 8.4 | 0.81 | 0.61 | 3 |
| TC 11 | in situ | Ag/Ag2O ⁄⁄ TiO2 (50% TiO2) | 20.1 | 2.03 | 0.27 | 28 |
| TC 14 | in situ | Ag/Ag2O ⁄⁄ TiO2 (80% TiO2) | 22.1 | 1.00 | 0.05 | 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akel, S.; Dillert, R.; Balayeva, N.O.; Boughaled, R.; Koch, J.; El Azzouzi, M.; Bahnemann, D.W. Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts 2018, 8, 647. https://doi.org/10.3390/catal8120647
Akel S, Dillert R, Balayeva NO, Boughaled R, Koch J, El Azzouzi M, Bahnemann DW. Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts. 2018; 8(12):647. https://doi.org/10.3390/catal8120647
Chicago/Turabian StyleAkel, Soukaina, Ralf Dillert, Narmina O. Balayeva, Redouan Boughaled, Julian Koch, Mohammed El Azzouzi, and Detlef W. Bahnemann. 2018. "Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio" Catalysts 8, no. 12: 647. https://doi.org/10.3390/catal8120647
APA StyleAkel, S., Dillert, R., Balayeva, N. O., Boughaled, R., Koch, J., El Azzouzi, M., & Bahnemann, D. W. (2018). Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts, 8(12), 647. https://doi.org/10.3390/catal8120647







