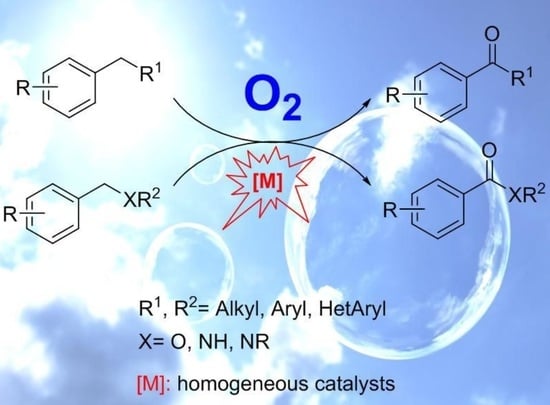
Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds
Abstract
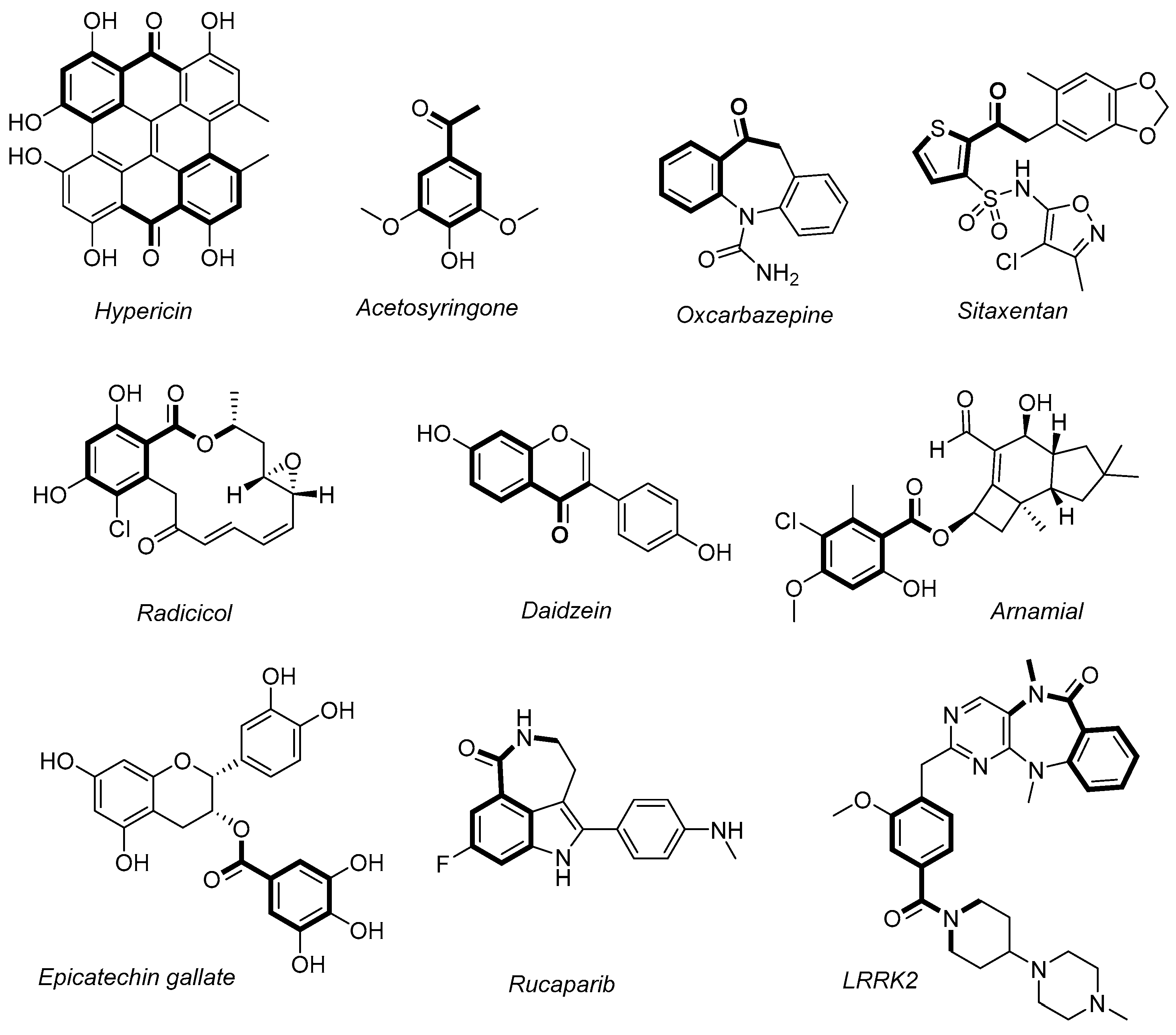
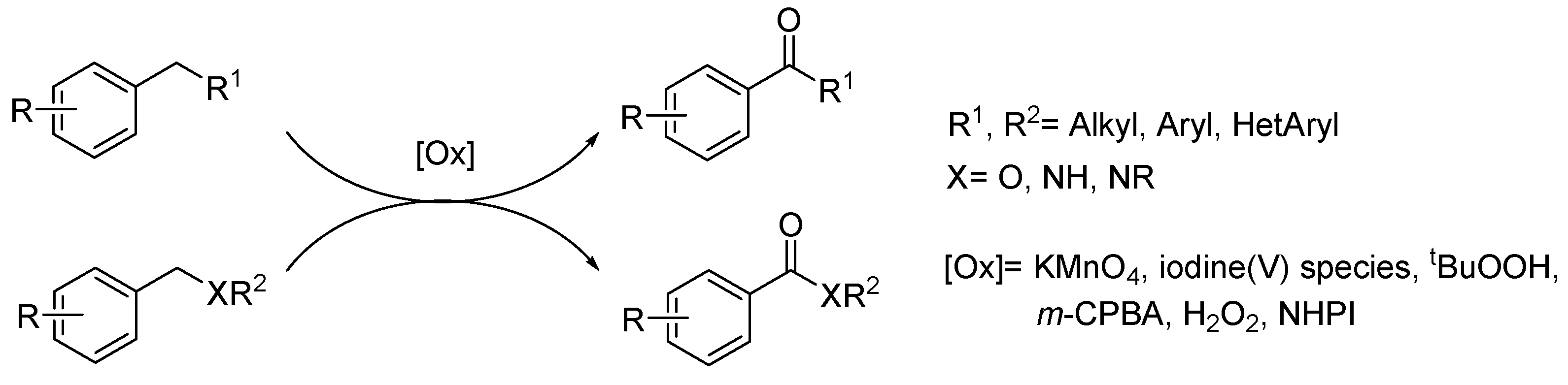
1. Introduction
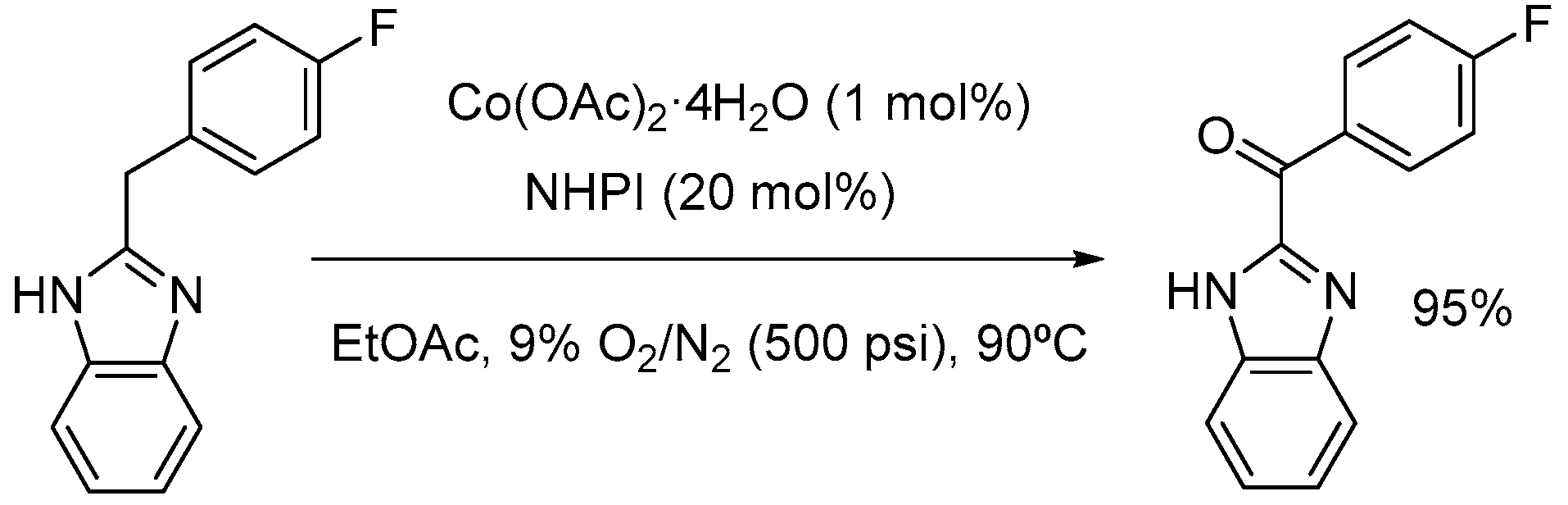
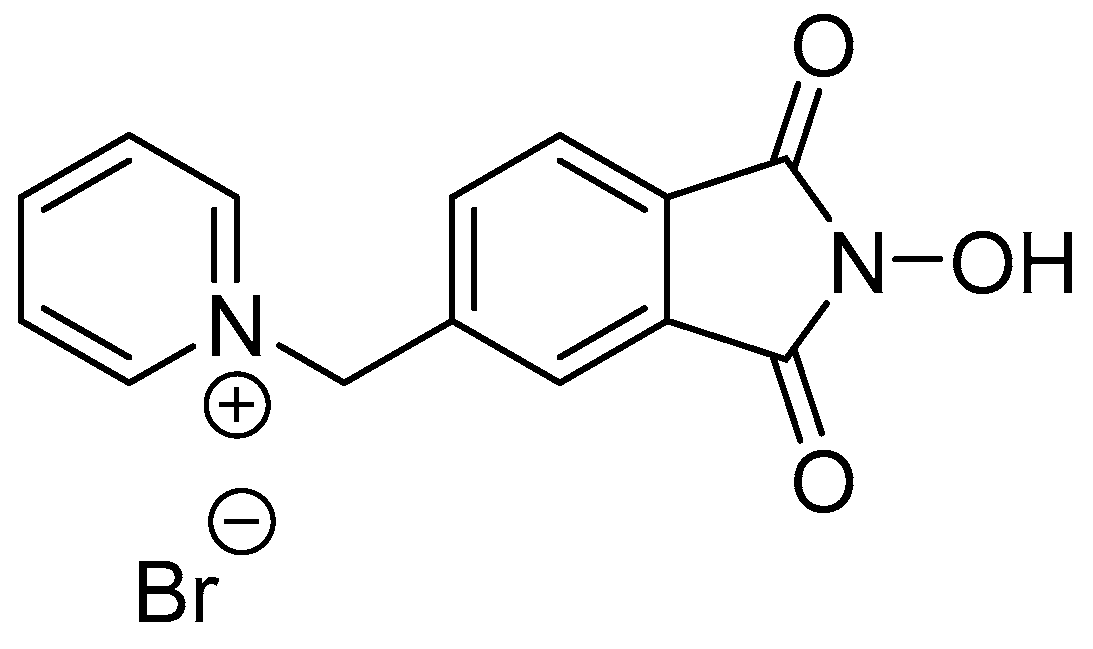
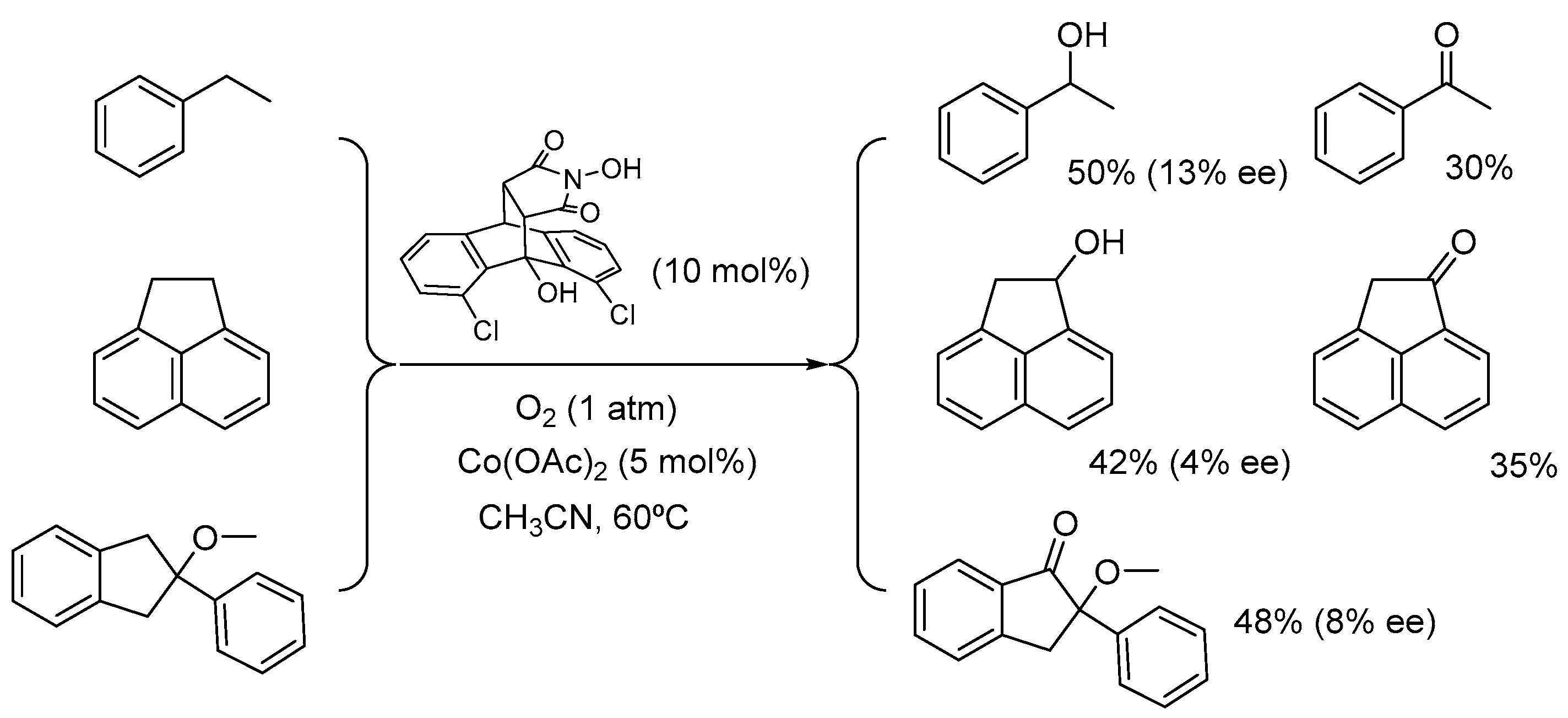
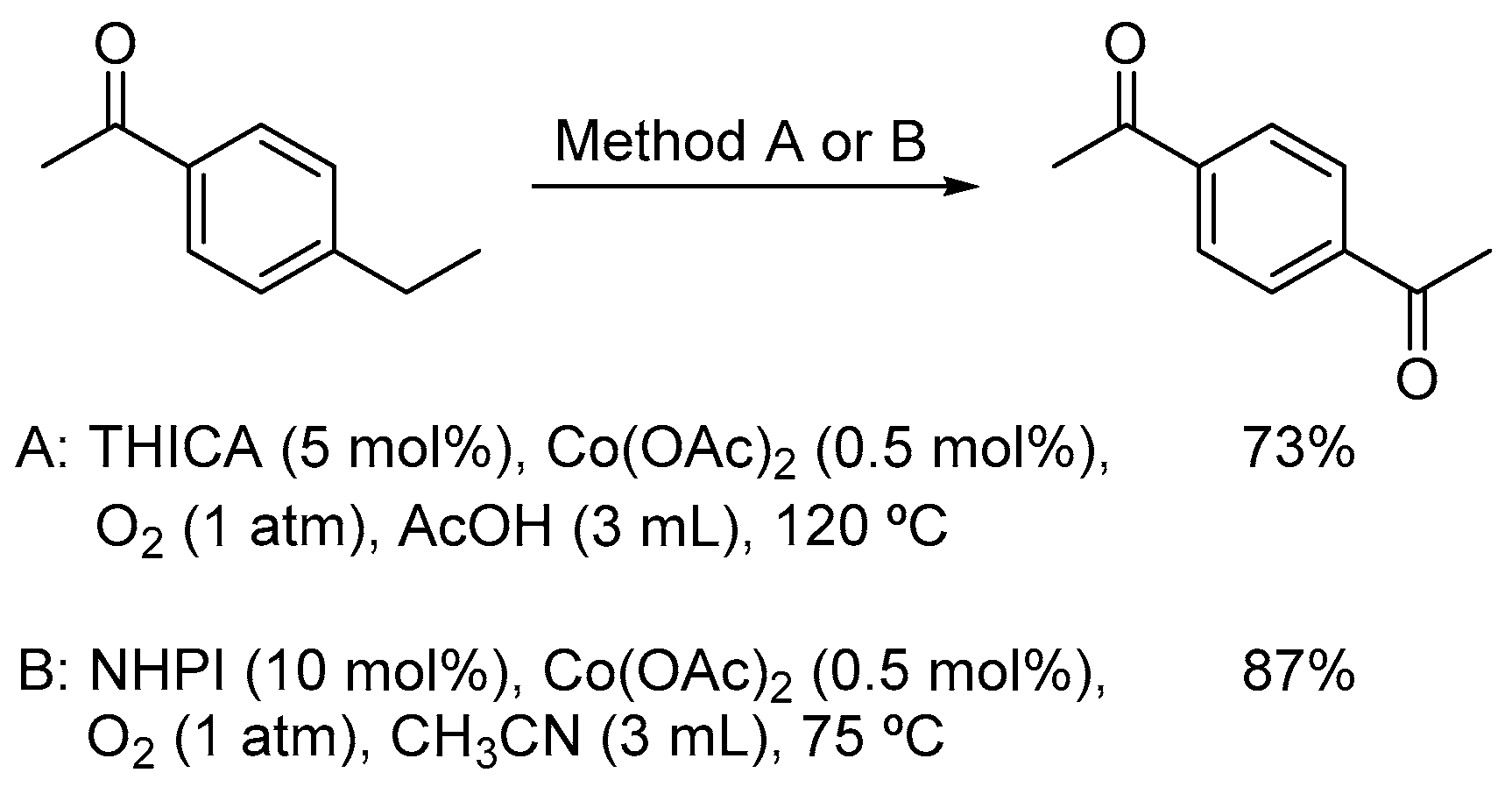
2. Cobalt Catalysts
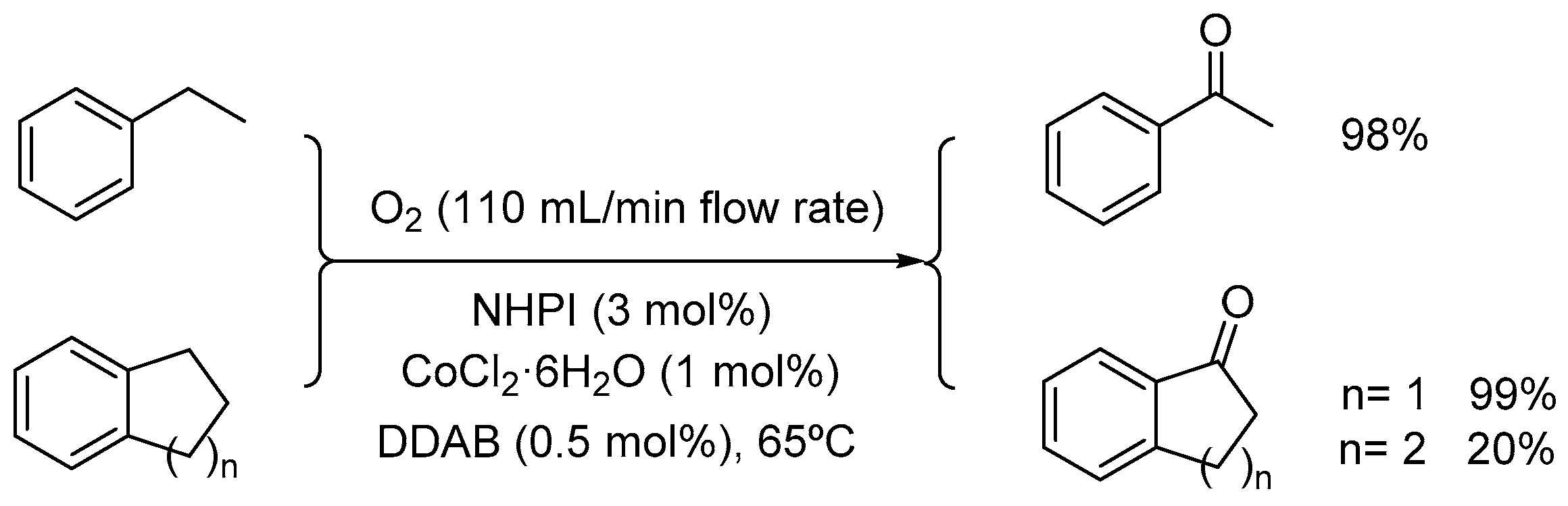
2.1. Co(II)-NHPI
2.2. Co(II) without Hydroxyimides
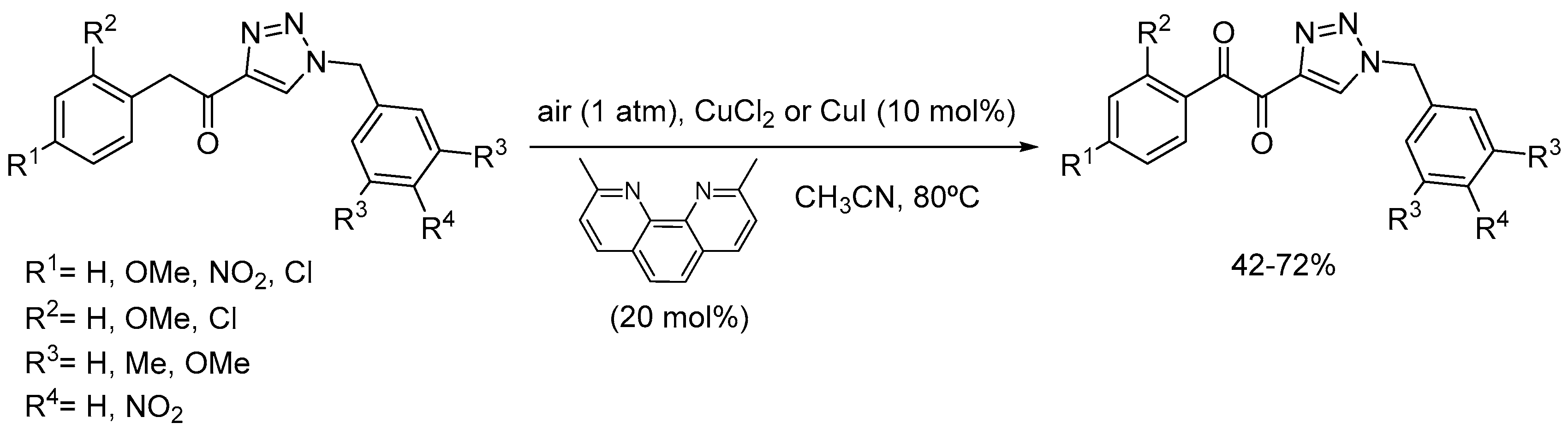
3. Copper Catalysts
3.1. Cu-NHPI
3.2. Additive-Free Procedures
3.3. With Ligand and/or Additives
4. Iron Catalysts
4.1. Fe-NHPI
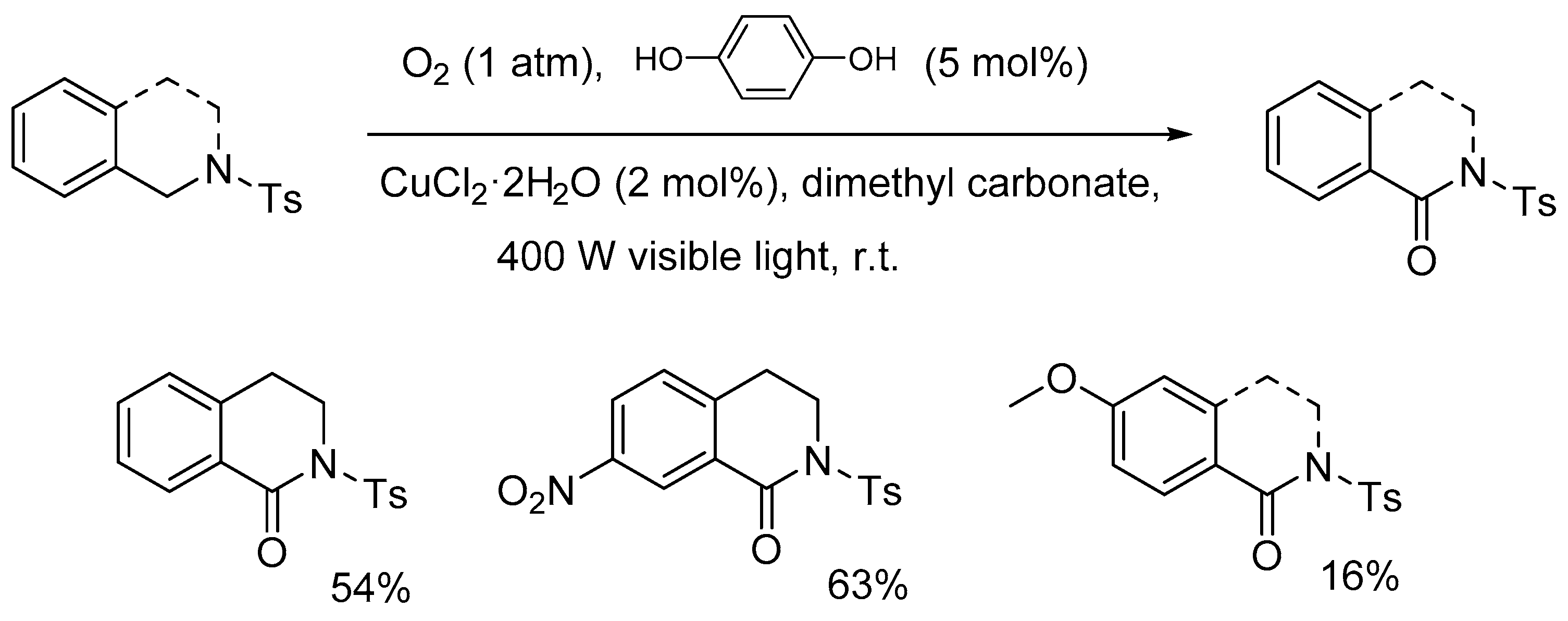
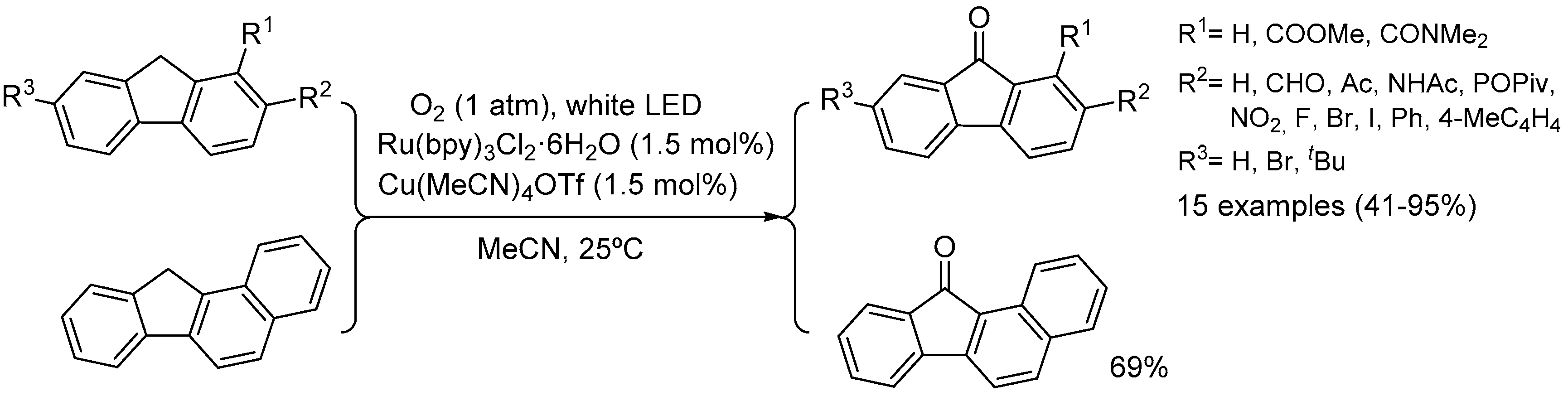
4.2. Light-Induced Oxidations
4.3. Other Approaches
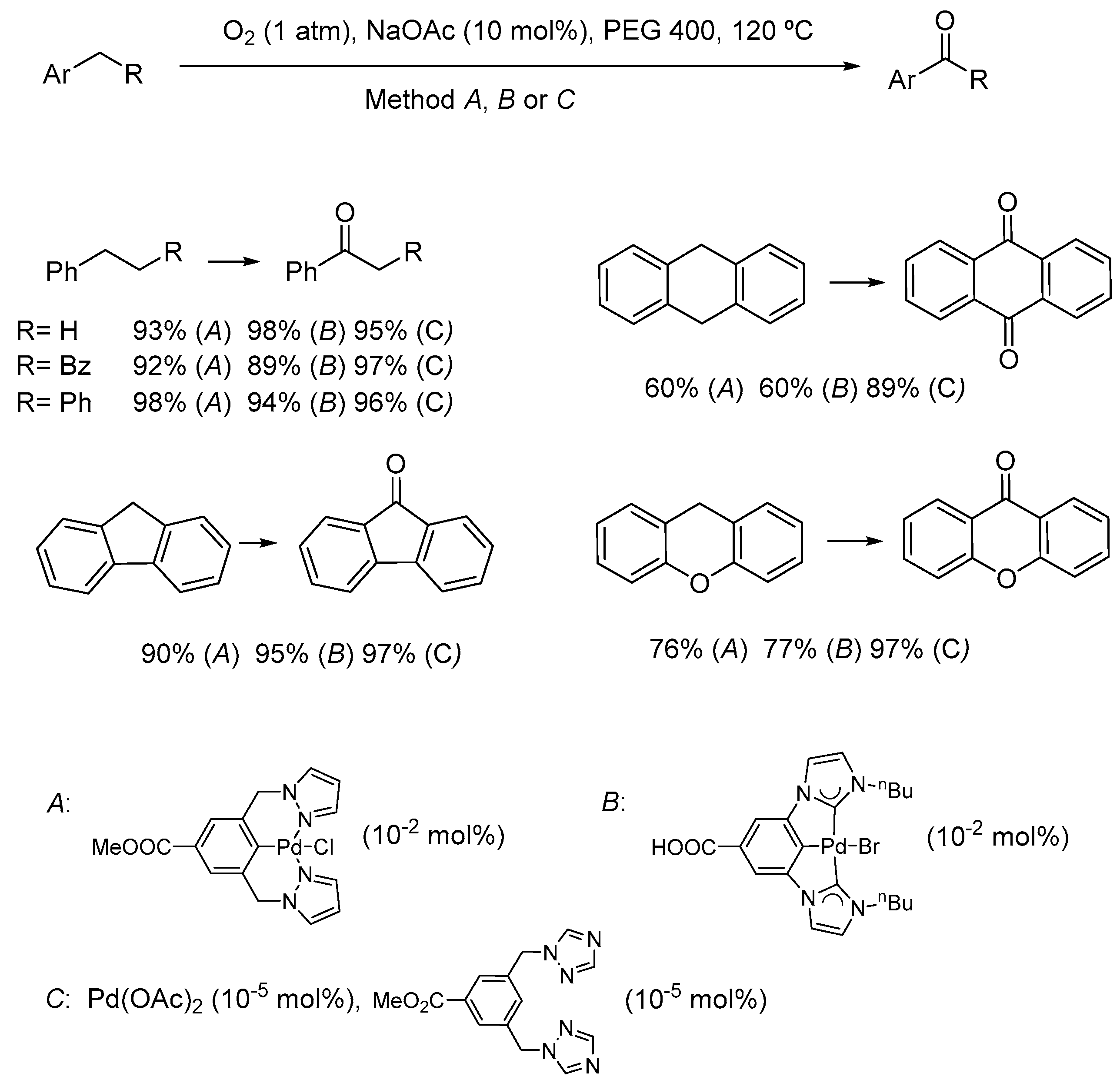
5. Palladium Catalysts
6. Other Metal Catalysts
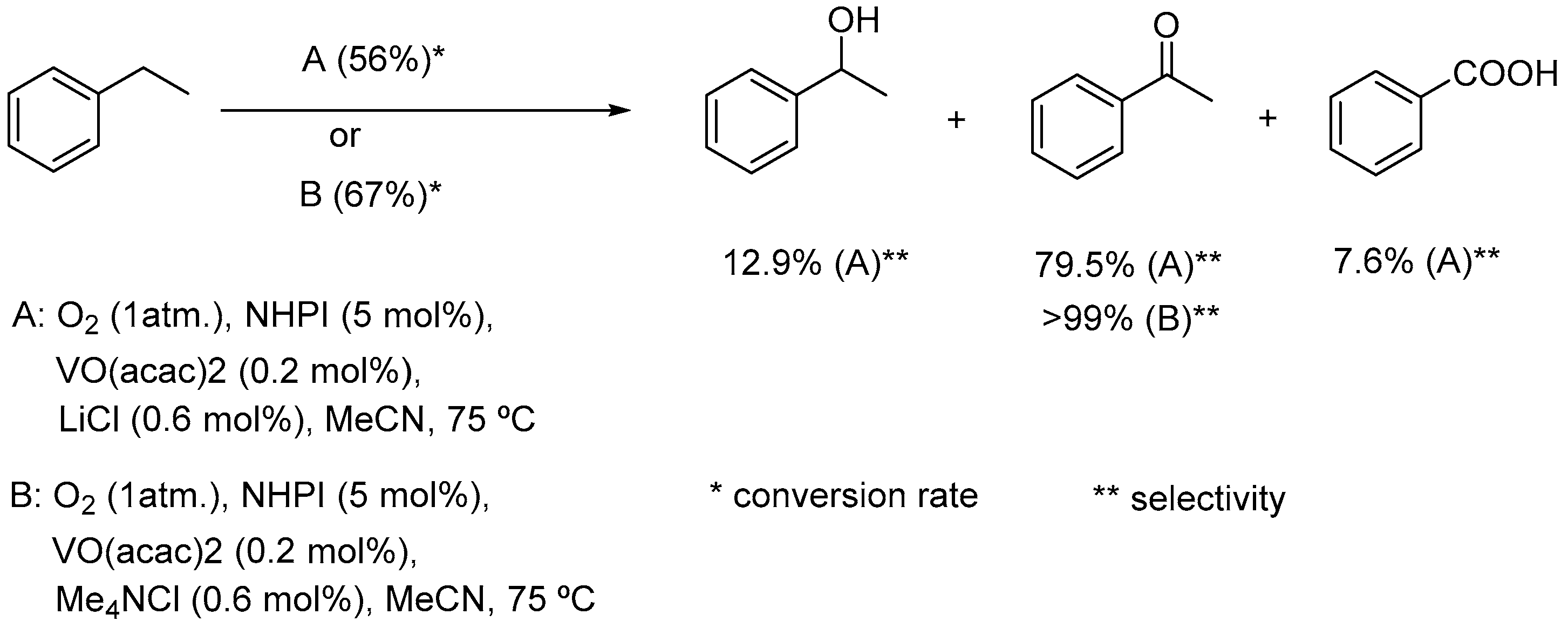
6.1. Mg/V-NHPI
6.2. Other Approaches
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- van de Putte, M.; Roskams, T.; Vandenheede, J.R.; Agostinis, P.; de Witte, P.A.M. Elucidation of the tumoritropic principle of hypericin. Br. J. Cancer 2005, 92, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.M.; Pascual, D.; Meruelo, D.; Liebes, L.; Mazur, Y.; Dubovi, E.; Mandel, M.; Lavie, G. Strategies for Evaluation of Enveloped Virus Inactivation in Red Cell Concentrates Using Hypericin. Photochem. Photobiol. 2000, 71, 188–195. [Google Scholar] [CrossRef]
- Rahimipour, S.; Palivan, C.; Barbosa, F.; Bilkis, I.; Koch, Y.; Weiner, L.; Fridkin, M.; Mazur, Y.; Gescheidt, G. Chemical and Photochemical Electron Transfer of New Helianthrone Derivatives: Aspects of Their Photodynamic Activity. J. Am. Chem. Soc. 2003, 125, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem. 1983, 26, 1158–1163. [Google Scholar] [CrossRef]
- Morton, J.G.M.; Draghici, C.; Kwon, L.D.; Njadarson, J.T. Rapid Assembly of Vinigrol’s Unique Carbocyclic Skeleton. Org. Lett. 2009, 11, 4492–4495. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Mishra, B.R.; Jowhar, J.; Mohapatra, D.; Parida, S.; Bisoi, D. Effect of Oxcarbazepine on Serum Brain Derived Neurotrophic Factor in Bipolar Mania: An Exploratory Study. Clin. Psychopharmacol. Neurosci. 2017, 15, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Bang, L.M.; Goa, K.L. Spotlight on oxcarbazepine in epilepsy. CNS Drugs 2004, 18, 57–61. [Google Scholar] [CrossRef]
- Liu, M.; Yanagihara, N.; Toyohira, Y.; Tsutsui, M.; Ueno, S.; Shinohara, Y. Dual Effects of Daidzein, a Soy Isoflavone, on Catecholamine Synthesis and Secretion in Cultured Bovine Adrenal Medullary Cells. Endocrinology 2007, 148, 5348–5354. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.-H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2018, 18, 4163–4174. [Google Scholar] [CrossRef]
- Scott, L.J. Sitaxentan: In Pulmonary Arterial Hypertension. Drugs 2007, 67, 761–770. [Google Scholar] [CrossRef]
- Erve, J.C.L.; Gauby, S.; Maynard, J.W., Jr.; Svensson, M.A.; Tonn†, G.; Quinn, K.P. Bioactivation of Sitaxentan in Liver Microsomes, Hepatocytes, and Expressed Human P450s with Characterization of the Glutathione Conjugate by Liquid Chromatography Tandem Mass Spectrometry. Chem. Res. Toxicol. 2013, 26, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.T.; Joshi, B.K.; Gamble, W.R.; Gloer, J.B.; Dowd, P.F. Antifungal Metabolites (Monorden, Monocillin IV, and Cerebrosides) from Humicola fuscoatra Traaen NRRL 22980, a Mycoparasite of Aspergillus flavus Sclerotia. Appl. Environ. Microbiol. 1998, 64, 4482–4484. [Google Scholar] [PubMed]
- Moulin, E.; Zoete, V.; Barluenga, S.; Karplus, M.; Winssinger, N. Design, Synthesis, and Biological Evaluation of HSP90 Inhibitors Based on Conformational Analysis of Radicicol and Its Analogues. J. Am. Chem. Soc. 2005, 127, 6999–7004. [Google Scholar] [CrossRef] [PubMed]
- Misiek, M.; Williams, J.; Schmich, K.; Hüttel, W.; Merfort, I.; Salomon, C.E.; Aldrich, C.C.; Hoffmeister, D. Structure and Cytotoxicity of Arnamial and Related Fungal Sesquiterpene Aryl Esters. J. Nat. Prod. 2009, 72, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Misiek, M.; Hoffmeister, D. Sesquiterpene aryl ester natural products in North American Armillaria species. Mycol. Prog. 2012, 11, 7–15. [Google Scholar] [CrossRef]
- Hajime Kobori, H.; Sekiya, A.; Suzuki, T.; Choi, J.-H.; Hirai, H.; Kawagishi, H. Bioactive Sesquiterpene Aryl Esters from the Culture Broth of Armillaria sp. J. Nat. Prod. 2015, 78, 163–167. [Google Scholar] [CrossRef]
- Shiota, S.; Shimizu, M.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis). Biol. Pharm. Bull. 1999, 22, 1388–1390. [Google Scholar] [CrossRef]
- Hughes, D.L. Patent Review of Manufacturing Routes to Recently Approved PARP Inhibitors: Olaparib, Rucaparib, and Niraparib. Org. Process Res. Dev. 2017, 21, 1227–1244. [Google Scholar] [CrossRef]
- Merin, B.; Josini, C.J. Rucaparib: A PARP inhibitor for the treatment of advanced ovarian cancer. Int. J. Basic Clin. Pharmacol. 2017, 6, 1018–1019. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- White, A.W.; Almassy, R.; Calvert, A.H.; Curtin, N.J.; Griffin, R.J.; Hostomsky, Z.; Maegley, K.; Newell, D.R.; Srinivasan, S.; Golding, B.T. Resistance-Modifying Agents. 9.1 Synthesis and Biological Properties of Benzimidazole Inhibitors of the DNA Repair Enzyme Poly(ADP-ribose) Polymerase. J. Med. Chem. 2000, 43, 4084–4097. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Dzamko, N.; Prescott, A.; Davies, P.; Liu, Q.; Yang, Q.; Lee, J.-D.; Patricelli, M.P.; Nomanbhoy, T.K.; Alessi, D.R.; et al. Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nat. Chem. Biol. 2011, 7, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Howden, A.J.M.; Sarhan, A.R.; Lis, P.; Ito, G.; Martinez, T.N.; Brockmann, K.; Gasser, T.; Alessi, D.R.; Sammler, E.M. Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem. J. 2018, 475, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, U.; Mir, A.A.; Emrick, T. Soluble, Allyl-Functionalized Deoxybenzoin Polymers. Macromolecules 2017, 50, 3772–3778. [Google Scholar] [CrossRef]
- Chena, C.-H.; Lina, C.-H.; Hona, J.-M.; Wanga, M.-W.; Juang, T.-Y. First halogen and phosphorus-free, flame-retardant benzoxazine thermosets derived from main-chain type bishydroxydeoxybenzoin-based benzoxazine polymers. Polymer 2018, 154, 35–41. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Nonhalogen flame retarded poly(butylene terephthalate) composite using aluminum phosphinate and phosphorus-containing deoxybenzoin polymer. J. Appl. Polym. Sci. 2017, 134, 45537–45546. [Google Scholar] [CrossRef]
- Moon, S.; Ku, B.-C.; Emrick, T.; Coughlin, B.E.; Farris, R.J. Flame Resistant Electrospun Polymer Nanofibers from Deoxybenzoin-based Polymers. J. Appl. Polym. Sci. 2009, 111, 301–307. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Zhong, Y.; Zhu, S.; Wang, Z.; Zou, Z. Synergistic Effects of BHDB-IPC with AlPi/MCA on Flame Retarding TPEE. RSC Adv. 2015, 5, 87609–87615. [Google Scholar] [CrossRef]
- Reis, D.C.; Pinto, M.C.X.; Souza-Fagundes, E.M.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. Antimony(III) complexes with 2-benzoylpyridine-derived thiosemicarbazones: Cytotoxicity against human leukemia cell lines. Eur. J. Med. Chem. 2010, 45, 3904–3910. [Google Scholar] [CrossRef]
- Hochgürtel, M.; Biesinger, R.; Kroth, H.; Piecha, D.; Hofmann, M.W.; Krause, S.; Schaaf, O.; Nicolau, C.; Eliseev, A.V. Ketones as Building Blocks for Dynamic Combinatorial Libraries: Highly Active Neuraminidase Inhibitors Generated via Selection Pressure of the Biological Target. J. Med. Chem. 2003, 46, 356–358. [Google Scholar] [CrossRef]
- Rong, F.; Chow, S.; Yan, S.; Larson, G.; Hong, Z.; Wu, J. Structure–activity relationship (SAR) studies of quinoxalines as novel HCV NS5B RNA-dependent RNA polymerase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Zhang, L.; Sun, X.; Xu, Y.; Krishnamurthy, D.; Senanayake, C.H. Addition of Grignard Reagents to Aryl Acid Chlorides: An Efficient Synthesis of Aryl Ketones. Org. Lett. 2005, 7, 5593–5595. [Google Scholar] [CrossRef] [PubMed]
- Urgoitia, G.; SanMartin, R.; Herrero, M.T.; Domínguez, E. An Aerobic Alternative to Oxidative Ozonolysis of Styrenes. Adv. Synth. Catal. 2016, 358, 1150–1156. [Google Scholar] [CrossRef]
- Cheng, L.-J.; Mankad, N.P. Cu-Catalyzed Hydrocarbonylative C−C Coupling of Terminal Alkynes with Alkyl Iodides. J. Am. Chem. Soc. 2017, 139, 10200–10203. [Google Scholar] [CrossRef] [PubMed]
- Iranpoor, N.; Firouzabadi, H.; Khalili, D.; Motevalli, S. Easily Prepared Azopyridines As Potent and Recyclable Reagents for Facile Esterification Reactions. An Efficient Modified Mitsunobu Reaction. J. Org. Chem. 2008, 73, 4882–4887. [Google Scholar] [CrossRef] [PubMed]
- But, T.Y.S.; Toy, P.H. Organocatalytic Mitsunobu Reactions. J. Am. Chem. Soc. 2006, 128, 9636–9637. [Google Scholar] [CrossRef] [PubMed]
- Ratchadapiban, K.; Praserthdam, P.; Tungasmita, D.N.; Tangku, C.; Anutrasakda, W. Effect of Surface Modifications of SBA-15 with Aminosilanes and 12-Tungstophosphoric Acid on Catalytic Properties in Environmentally Friendly Esterification of Glycerol with Oleic Acid to Produce Monoolein. Catalysts 2018, 8, 360. [Google Scholar] [CrossRef]
- Makihara, M.; Aoki, H.; Komura, K. Reaction Profiles of High Silica MOR Zeolite Catalyzed Friedel–Crafts Acylation of Anisole Using Acetic Anhydride in Acetic Acid. Catal. Lett. 2018, 148, 2974–2979. [Google Scholar] [CrossRef]
- Li, L.-H.; Niua, Z.-J.; Liang, Y.-M. New Friedel–Crafts strategy for preparing 3-acylindoles. Org. Biomol. Chem. 2018, 16, 7792–7796. [Google Scholar] [CrossRef]
- Suchand, B.; Satyanarayana, G. Palladium-Catalyzed Direct Acylation: One-Pot Relay Synthesis of Anthraquinones. Synthesis 2018, 50. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Singh, B.; Chankeshwara, S.V.; Patel, A.R. Protic Acid Immobilized on Solid Support as an Extremely Efficient Recyclable Catalyst System for a Direct and Atom Economical Esterification of Carboxylic Acids with Alcohols. J. Org. Chem. 2009, 74, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Moumne, R.; Lavielle, S.; Karoyan, P. Efficient Synthesis of β2-Amino Acid by Homologation of α-Amino Acids Involving the Reformatsky Reaction and Mannich-Type Imminium Electrophile. J. Org. Chem. 2006, 71, 3332–3334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wen, Y.; Fu, Y.; Chen, H.; Ye, M.; Luo, G. Graphene Oxide: An Efficient Acid Catalyst for the Construction of Esters from Acids and Alcohols. Synlett 2017, 28, 981–985. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rokhum, L. A microwave-assisted highly practical chemoselective esterification and amidation of carboxylic acids. RSC Adv. 2016, 6, 93729–93740. [Google Scholar] [CrossRef]
- Jiang, L.; Yu, J.; Niu, F.; Zhang, D.; Sun, X. A high-efficient method for the amidation of carboxylic acids promoted by triphenylphosphine oxide and oxalyl chloride. Heteroat. Chem. 2017, 28, e21364. [Google Scholar] [CrossRef]
- Moon, H.K.; Sung, G.S.; Kim, B.R.; Park, J.K.; Yoon, Y.-J.; Yoon, H.J. One for Many: A Universal Reagent for Acylation Processes. Adv. Synth. Catal. 2016, 358, 1725–1730. [Google Scholar] [CrossRef]
- Partenheimer, W. Methodology and scope of metal/bromide autoxidation of hydrocarbons. Catal. Today 1995, 23, 69–158. [Google Scholar] [CrossRef]
- Bäckvall, J.-E. Modern Oxidation Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004; ISBN 978-3-527-63203-9. [Google Scholar]
- Bahador Karami, B.; Montazerozohori, M. Tungstate Sulfuric Acid (TSA)/KMnO4 as a Novel Heterogeneous System for Rapid Deoximation. Molecules 2006, 11, 720–725. [Google Scholar] [CrossRef]
- Lounisa, Z.; Riahi, A.; Djafri, F.; Muzart, J. Chromium-exchanged zeolite (CrE-ZSM-5) as catalyst for alcohol oxidation and benzylic oxidation with t-BuOOH. Appl. Catal. A Gen. 2006, 309, 270–272. [Google Scholar] [CrossRef]
- Moghadam, M.; Mirkhani, V.; Tangestaninejad, S.; Mohammdpoor-Baltorka, I.; Kargar, H. Silica supported Mn(Br8TPP)Cl and Mn(TPP)Cl as efficient and reusable catalysts for selective hydrocarbon oxidation under various reaction conditions: The effect of substituted bromines on the catalytic activity and reusability. J. Mol. Catal. A Chem. 2008, 288, 116–124. [Google Scholar] [CrossRef]
- Singh, S.J.; Jayaram, R.V. Oxidation of alkylaromatics to benzylic ketones using TBHP as an oxidant over LaMO3 (M = Cr, Co, Fe, Mn, Ni) perovskites. Catal. Commun. 2009, 10, 2004–2007. [Google Scholar] [CrossRef]
- Mayilmurugan, R.; Stoeckli-Evans, H.; Suresh, E.; Palaniandavar, M. Chemoselective and biomimetic hydroxylation of hydrocarbons by non-heme μ-oxo-bridged diiron(III) catalysts using m-CPBA as oxidant. Dalton Trans. 2009, 26, 5101–5114. [Google Scholar] [CrossRef] [PubMed]
- Yusubov, M.S.; Nemykin, V.N.; Zhdankin, V.Z. Transition metal-mediated oxidations utilizing monomeric iodosyl- and iodylarene species. Tetrahedron 2010, 66, 5745–5752. [Google Scholar] [CrossRef]
- Zhou, M.; Schley, N.D.; Crabtree, R.H. Cp* Iridium Complexes Give Catalytic Alkane Hydroxylation with Retention of Stereochemistry. J. Am. Chem. Soc. 2010, 132, 12550–12551. [Google Scholar] [CrossRef] [PubMed]
- Srour, H.; Maux, P.L.; Simonneaux, G. Enantioselective Manganese-Porphyrin-Catalyzed Epoxidation and C–H Hydroxylation with Hydrogen Peroxide in Water/Methanol Solutions. Inorg. Chem. 2012, 51, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.-H.; Yang Li, Y.; Jiangwei Li, J.; Gu, Y.-J.; Wang, B.; Liu, Z.-T.; Liu, Z.-W.; Lu, J. Selective oxidation of arylalkanes with N-Graphitic-Modified cobalt nanoparticles in water. Catal. Commun. 2017, 97, 130–133. [Google Scholar] [CrossRef]
- Zhang, Q.; Gorden, J.D.; Goldsmith, C.R. C–H Oxidation by H2O2 and O2 Catalyzed by a Non-Heme Iron Complex with a Sterically Encumbered Tetradentate N-Donor Ligand. Inorg. Chem. 2013, 52, 13546–13554. [Google Scholar] [CrossRef]
- Stepovik, L.P.; Potkina, A.Y. Oxidation of Alkylarene C–H Bonds by tert-Butyl Hydroperoxide in the Presence of Cobalt, Chromium, and Vanadium Acetylacetonates. Russ. J. Gen. Chem. 2013, 83, 1047–1059. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Zhang, H.; Yu, B.; Zhao, Y.; Wang, H.; Ji, G.; Chen, Y.; Liu, X.; Liu, Z. N-Doped porous carbon nanotubes: synthesis and application in catalysis. Chem. Commun. 2017, 53, 929–932. [Google Scholar] [CrossRef]
- Ottenbacher, R.V.; Talsi, E.P.; Bryliakov, K.P. Mechanism of Selective C–H Hydroxylation Mediated by Manganese Aminopyridine Enzyme Models. ACS Catal. 2015, 5, 39–44. [Google Scholar] [CrossRef]
- Miyamoto, M.; Minami, Y.; Ukaji, Y.; Kinoshita, H.; Inomata, K. A New Aerobic Oxidation System Using Pd-Cu Catalysts in the Presence of CO. Chem. Lett. 1994, 1149–1152. [Google Scholar] [CrossRef]
- Ishii, Y.; Nakayama, K.; Takeno, M.; Sakaguchi, S.; Iwahama, T.; Nishiyama, Y. Yasutaka Ishii, Kouichi Nakayama, Mitsuhiro Takeno, Satoshi Sakaguchi, Takahiro Iwahama, and Yutaka Nishiyama. J. Org. Chem. 1995, 60, 3934–3935. [Google Scholar] [CrossRef]
- Ishii, Y.; Iwahama, T.; Sakaguchi, S.; Nakayama, K.; Nishiyama, Y. Alkane Oxidation with Molecular Oxygen Using a New Efficient Catalytic System: N-Hydroxyphthalimide (NHPI) Combined with Co(acac)n (n = 2 or 3). J. Org. Chem. 1996, 61, 4520–4526. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Hayashi, Y.; Iwahama, T.; Sakaguchi, S.; Ishii, Y. Catalytic Oxidation of Alkylbenzenes with Molecular Oxygen under Normal Pressure and Temperature by N-Hydroxyphthalimide Combined with Co(OAc)2. J. Org. Chem. 1997, 62, 6810–6813. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakaguchi, S. A new strategy for alkane oxidation with O2 Using N-hydroxyphthalimide (NHPI) as a radical catalyst. Catal. Surv. Jpn. 1999, 3, 27–35. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakaguchi, S.; Iwahama, T. Innovation of Hydrocarbon Oxidation with Molecular Oxygen and Related Reactions. Adv. Synth. Catal. 2001, 343, 393–427. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E. Organocatalytic Oxidations Mediated by Nitroxyl Radicals. Adv. Synth. Catal. 2004, 346, 1051–1071. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Miao, H.; Tong, X.; Xu, J. Selective Organocatalytic Oxygenation of Hydrocarbons by Dioxygen Using Anthraquinones and N-Hydroxyphthalimide. Org. Lett. 2005, 7, 263–266. [Google Scholar] [CrossRef]
- Matsushita, Y.; Sugamoto, K.; Iwakiri, Y.; Yoshida, S.; Chaen, T.; Matsui, T. Aerobic oxidation of 8,11,13-abietatrienes catalyzed by N-hydroxyphthalimide combined with 2,20-azobis(4-methoxy-2,4-dimethylvaleronitrile) and its application to synthesis of naturally occurring diterpenes. Tetrahedron Lett. 2010, 51, 3931–3934. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I.W.C.E.; Buehler, K.; Schallmey, A.; Bühler, B. Enzyme-mediated oxidations for the chemist. Green Chem. 2011, 13, 226–265. [Google Scholar] [CrossRef]
- Rickert, A.; Krombach, V.; Hamers, O.; Zorn, H.; Maison, W. Enzymatic allylic oxidations with a lyophilisate of the edible fungus Pleurotus sapidus. Green Chem. 2012, 14, 639–644. [Google Scholar] [CrossRef]
- Weidmann, V.; Schaffrath, M.; Zorn, H.; Rehbein, J.; Maison, W. Elucidation of the regio- and chemoselectivity of enzymatic allylic oxidations with Pleurotus sapidus–conversion of selected spirocyclic terpenoids and computational analysis. Beilstein J. Org. Chem. 2013, 9, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Kexian Chen, K.; Zhang, P.; Wang, Y.; Li, H. Metal-free allylic/benzylic oxidation strategies with molecular oxygen: Recent advances and future prospects. Green Chem. 2014, 16, 2344–2374. [Google Scholar] [CrossRef]
- Ren, L.; Wang, L.; Lv, Y.; Li, G.; Gao, S. Synergistic H4NI−AcOH Catalyzed Oxidation of the Csp3−H Bonds of Benzylpyridines with Molecular Oxygen. Org. Lett. 2015, 17, 2078–2081. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Huang, H.; Tan, J.; Xu, K. KOtBu-Promoted Oxidation of (Hetero)benzylic Csp3−H to Ketones with Molecular Oxygen. Org. Lett. 2016, 18, 5680–5683. [Google Scholar] [CrossRef]
- Jin, W.; Zheng, P.; Wing-Tak Wong, W.-T.; Law, G.-L. Efficient Selenium-Catalyzed Selective C(sp3)-H Oxidation of Benzylpyridines with Molecular Oxygen. Adv. Synth. Catal. 2017, 359, 1588–1593. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Wang, Y.-Q.; Huang, L.-T.; Zhu, L.-Q.; Feng, Y.-Y.; Lu, Y.-M.; Zhao, Q.; Wang, X.-Q.; Wang, Z. NaI-mediated divergent synthesis of isatins and isoindigoes: A new protocol enabled by an oxidation relay strategy. Chem. Commun. 2018, 54, 8265–8268. [Google Scholar] [CrossRef]
- Gao, B.; Kong, D.; Zhang, Y. Preparation and catalytic activity of P4VP–Cu(II)complex supported on silica gel. J. Mol. Catal. A Chem. 2008, 286, 143–148. [Google Scholar] [CrossRef]
- Shaabani, A.; Rahmati, A. Aerobic oxidation of alkyl arenes using a combination of N-hydroxy phthalimide and recyclable cobalt(II) tetrasulfophthalocyanine supported on silica. Catal. Commun. 2008, 9, 1692–1697. [Google Scholar] [CrossRef]
- Murwani, I.K.; Scheurell, K.; Kemnitz, E. Liquid phase oxidation of ethylbenzene on pure and metal doped HS-AlF3. Catal. Commun. 2008, 10, 227–231. [Google Scholar] [CrossRef]
- Shaabani, A.; Hezarkhani, Z.; Badali, E. Wool supported manganese dioxide nano-scale dispersion: A biopolymer based catalyst for the aerobic oxidation of organic compounds. RSC Adv. 2015, 5, 61759–61767. [Google Scholar] [CrossRef]
- Chauhan, P.; Yan, N. Nanocrystalline cellulose grafted phthalocyanine: A heterogeneous catalyst for selective aerobic oxidation of alcohols and alkyl arenes at room temperature in a green solvent. RSC Adv. 2015, 5, 37517–37520. [Google Scholar] [CrossRef]
- Rezaeifard, A.; Khoshyan, A.; Jafarpour, M.; Pourtahmasb, M. Selective aerobic benzylic C–H oxidation co-catalyzed by N-hydroxyphthalimide and Keplerate {Mo72V30} nanocluster. RSC Adv. 2017, 7, 15754–15761. [Google Scholar] [CrossRef]
- Lin, X.; Nie, Z.; Zhang, L.; Mei, S.; Chen, Y.; Zhang, B.; Zhu, R.; Liu, Z. Nitrogen-doped carbon nanotubes encapsulate cobalt nanoparticles as efficient catalysts for aerobic and solvent-free selective oxidation of hydrocarbons. Green Chem. 2017, 19, 2164–2173. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yoshimura, Y.; Yamashita, H. Liquid-phase oxidation of alkylaromatics to aromatic ketones with molecular oxygen over a Mn-based metal–organic framework. Dalton Trans. 2017, 46, 8415–8421. [Google Scholar] [CrossRef] [PubMed]
- Mouchaham, G.; Abeykoon, B.; Giménez-Marqués, M.; Navalon, S.; Santiago-Portillo, A.; Affram, M.; Guillou, N.; Martineau, C.; Garcia, H.; Fateeva, A.; et al. Adaptability of the metal(III, IV) 1,2,3-trioxobenzene rod secondary building unit for the production of chemically stable and catalytically active MOFs. Chem. Commun. 2017, 53, 7661–7664. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, Q.; Li, H.; Guo, H.; He, W. Ag/C nanoparticles catalysed aerobic oxidation of diaryl and aryl(hetero) methylenes into ketones. Nano Res. 2017, 10, 3261–3267. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Wang, Y.; Fan, H.; Wu, J.; Huang, C.; Hou, H. Cu(I) Coordination Polymers as the Green Heterogeneous Catalysts for Direct C–H Bonds Activation of Arylalkanes to Ketones in Water with Spatial Confinement Effect. Inorg. Chem. 2017, 56, 13329–13336. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, D.; Feng, P.; Shi, S.; Wang, C.; Zheng, A.; Lüa, G.; Tiana, Z. Synthesis of zeolite Beta containing ultra-small CoO particles for ethylbenzene oxidation. Chin. J. Catal. 2017, 38, 1207–1215. [Google Scholar] [CrossRef]
- Sharma, A.S.; Kaur, H. Sustainable Protocol for Benzylic -CH2 Oxidation with Dioxygen to Phenones Using AuNPs@ Resin Beads. Chem. Sel. 2017, 31, 10112–10117. [Google Scholar] [CrossRef]
- Dai, X.; Chen, D.; Zhou, W.; Yang, S.; Sun, F.; Qian, J.; He, M.; Chen, Q. Aromatization of hydrocarbons by oxidative dehydrogenation catalyzed by nickel porphyrin with molecular oxygen. Catal. Commun. 2018, 117, 85–89. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, J.-X.; Zhou, X.-T.; Chen, H.-Y.; Ji, H.-B. Mechanistic Understanding towards the Role of Cyclohexene in Enhancing the Efficiency of Manganese Porphyrin-Catalyzed Aerobic Oxidation of Diphenylmethane. Eur. J. Inorg. Chem. 2018, 2018, 2666–2674. [Google Scholar] [CrossRef]
- Wentzel, B.B.; Donners, M.P.J.; Alsters, P.L.; Feiters, M.C.; Nolte, R.J.M. N-Hydroxyphthalimide/Cobalt(II) Catalyzed Low Temperature Benzylic Oxidation Using Molecular Oxygen. Tetrahedron 2000, 56, 7797–7803. [Google Scholar] [CrossRef]
- Patil, R.D.; Fuchs, B.; Taha, N.; Sasson, Y. Solvent–free and Selective Autooxidation of Alkylbenzenes Catalyzed by Co/NHPI under Phase Transfer Conditions. Chem. Sel. 2016, 1, 3791–3796. [Google Scholar] [CrossRef]
- Hruszkewycz, D.P.; Miles, K.S.; Thiel, O.R.; Stahl, S.S. Co/NHPI-mediated aerobic oxygenation of benzylic C–H bonds in pharmaceutically relevant molecules. Chem. Sci. 2017, 8, 1282–1287. [Google Scholar] [CrossRef]
- Wang, J.-R.; Liu, L.; Wang, Y.-F.; Zhang, Y.; Deng, W.; Guo, Q.-X. Aerobic oxidation with N-hydroxyphthalimide catalysts in ionic liquid. Tetrahedron Lett. 2005, 46, 4647–4651. [Google Scholar] [CrossRef]
- Shen, J.; Tan, C.-H. Anthrone-derived NHPI analogues as catalysts in reactions using oxygen as an oxidant. Org. Biomol. Chem. 2008, 6, 4096–4098. [Google Scholar] [CrossRef]
- Baucherel, X.; Gonsalvi, L.; Arends, I.W.C.E.; Ellwood, S.; Sheldon, R.A. Aerobic Oxidation of Cycloalkanes, Alcohols and Ethylbenzene Catalyzed by the Novel Carbon Radical Chain Promoter NHS (N-Hydroxysaccharin). Adv. Synth. Catal. 2004, 346, 286–296. [Google Scholar] [CrossRef]
- Nakamura, R.; Obora, Y.; Ishii, Y. Selective Oxidation of Acetophenones Bearing Various Functional Groups to Benzoic Acid Derivatives with Molecular Oxygen. Adv. Synth. Catal. 2009, 351, 1677–1684. [Google Scholar] [CrossRef]
- Shaabani, A.; Farhangi, E.; Rahmati, A. Aerobic oxidation of alkyl arenes and alcohols using cobalt(II) phthalocyanine as a catalyst in 1-butyl-3-methyl-imidazolium bromide. Appl. Catal. A Gen. 2008, 338, 14–19. [Google Scholar] [CrossRef]
- Wang, P.; She, Y.; Fu, H.; Zhao, W.; Wang, M. Oxidation of alkylaromatics to aromatic ketones catalyzed by metalloporphyrins under the special temperature control method. Can. J. Chem. 2014, 92, 1059–1065. [Google Scholar] [CrossRef]
- Gutmann, B.; Elsner, P.; Roberge, D.; Kappe, C.O. Homogeneous Liquid-Phase Oxidation of Ethylbenzene to Acetophenone in Continuous Flow Mode. ACS Catal. 2013, 3, 2669–2676. [Google Scholar] [CrossRef]
- Wang, J.-Q.; He, L.-N. Polyethylene glycol radical-initiated benzylic C–H bond oxygenation in compressed carbon dioxide. New J. Chem. 2009, 33, 1637–1640. [Google Scholar] [CrossRef]
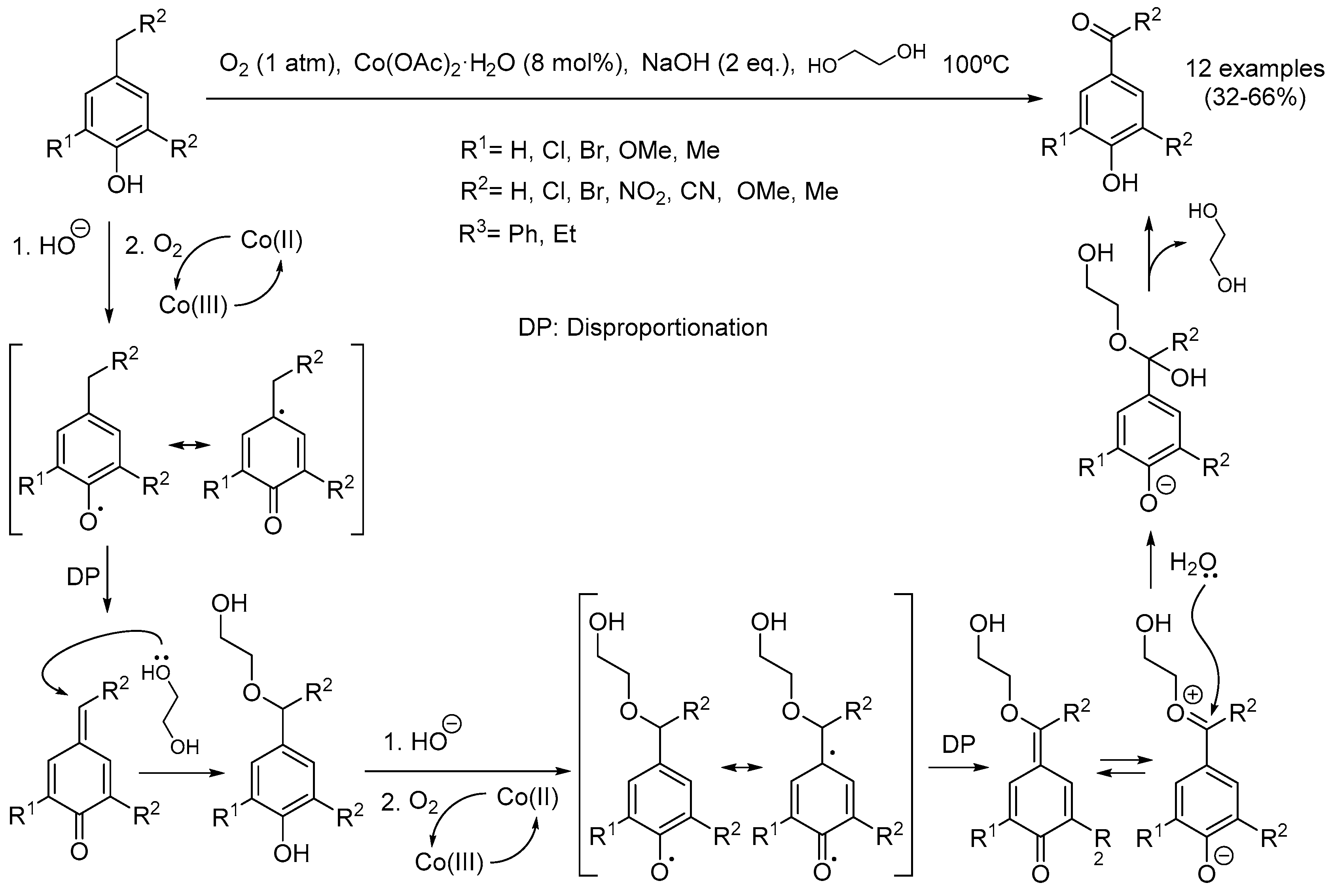
- Huang, J.-G.; Guo, Y.; Jiang, J.-A.; Liu, H.-W.; Ji, Y.-F. An eco-friendly Co(OAc)2-catalyzed aerobic oxidation of 4-benzylphenols into 4-hydroxybenzophenones. Res. Chem. Intermed. 2015, 41, 7115–7124. [Google Scholar] [CrossRef]
- Orlińska, B.; Romanowska, I. N-Hydroxyphthalimide and transition metal salts as catalysts of the liquid-phase oxidation of 1-methoxy-4-(1-methylethyl)benzene with oxygen. Cent. Eur. J. Chem. 2011, 9, 670–676. [Google Scholar] [CrossRef]
- Chen, L.; Tang, R.; Li, Z.; Liang, S. An Efficient and Mild Oxidation of α-Isophorone to Ketoisophorone Catalyzed by N-Hydroxyphthalimide and Copper Chloride. Bull. Korean Chem. Soc. 2012, 33, 459–463. [Google Scholar] [CrossRef]
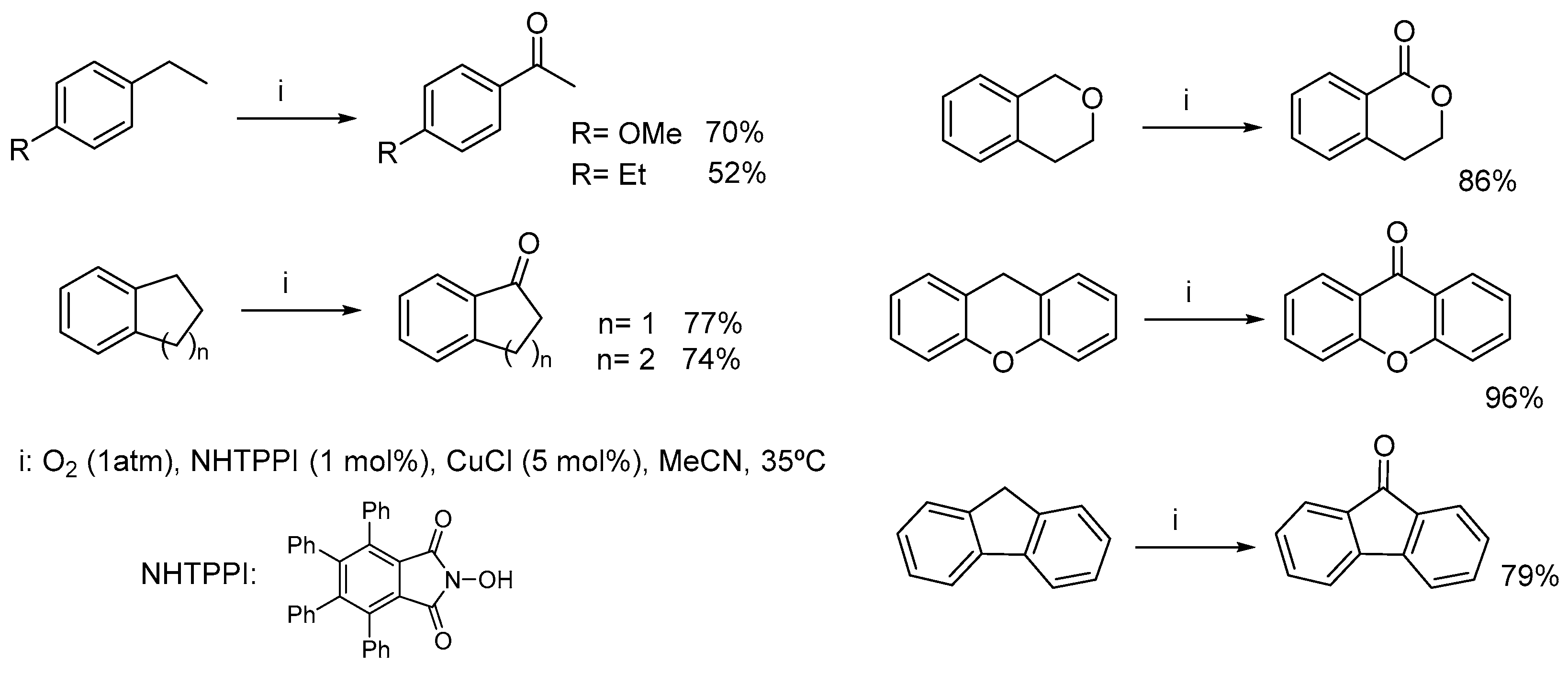
- Nechab, M.; Einhorn, C.; Einhorn, J. New aerobic oxidation of benzylic compounds: Efficient catalysis by N-hydroxy-3,4,5,6-tetraphenylphthalimide (NHTPPI)/CuCl under mild conditions and low catalyst loading. Chem. Commun. 2004, 1500–1501. [Google Scholar] [CrossRef]
- Abe, T.; Tanaka, S.; Ogawa, A.; Tamura, M.; Sato, K.; Itoh, S. Copper-Catalyzed Selective Oxygenation of Methyl and Benzyl Substituents in Pyridine with O2. Chem. Lett. 2017, 46, 348–350. [Google Scholar] [CrossRef]
- Jiang, J.-A.; Chen, C.; Huang, J.-G.; Liu, H.-W.; Cao, S.; Ji, Y.-F. Cu(OAc)2-catalyzed remote benzylic C(sp3)–H oxyfunctionalization for C=O formation directed by the hindered para-hydroxyl group with ambient air as the terminal oxidant under ligand- and additive-free conditions. Green Chem. 2014, 16, 1248–1254. [Google Scholar] [CrossRef]
- Zhang, L.; Ang, G.Y.; Chiba, S. Copper-Catalyzed Benzylic C–H Oxygenation under an Oxygen Atmosphere via N-H Imines as an Intramolecular Directing Group. Org. Lett. 2011, 13, 1622–1625. [Google Scholar] [CrossRef]
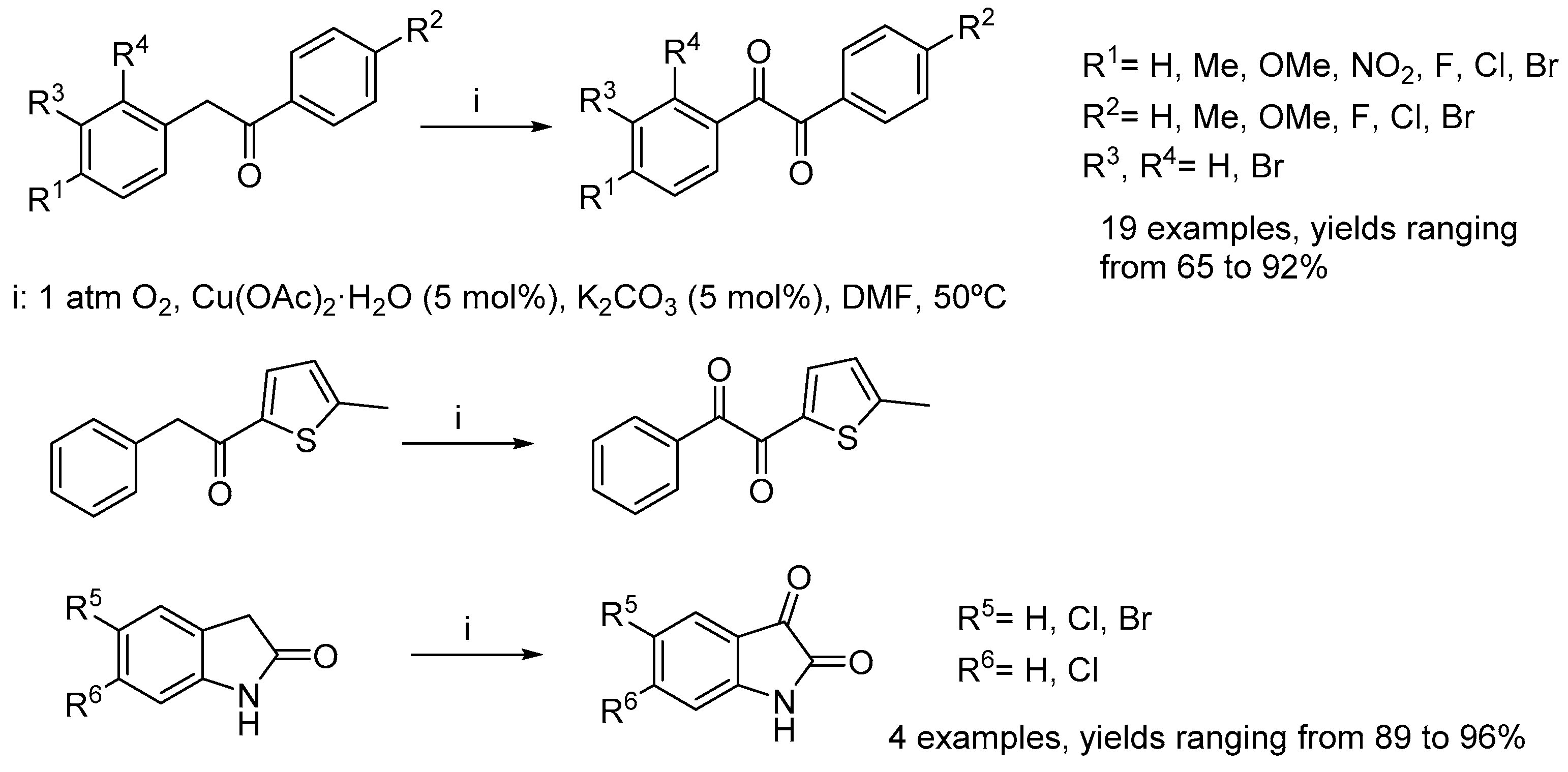
- Yu, J.-W.; Mao, S.; Wang, Y.-Q. Copper-catalyzed base-accelerated direct oxidation of C–H bond to synthesize benzils, isatins, and quinoxalines with molecular oxygen as terminal oxidant. Tetrahedron Lett. 2015, 56, 1575–1580. [Google Scholar] [CrossRef]
- De Houwer, J.; Tehrani, K.A.; Maes, B.U.W. Synthesis of Aryl(di)azinyl Ketones through Copper- and Ironcatalyzed Oxidation of the Methylene Group of Aryl(di)azinylmethanes. Angew. Int. Chem. Ed. 2012, 51, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
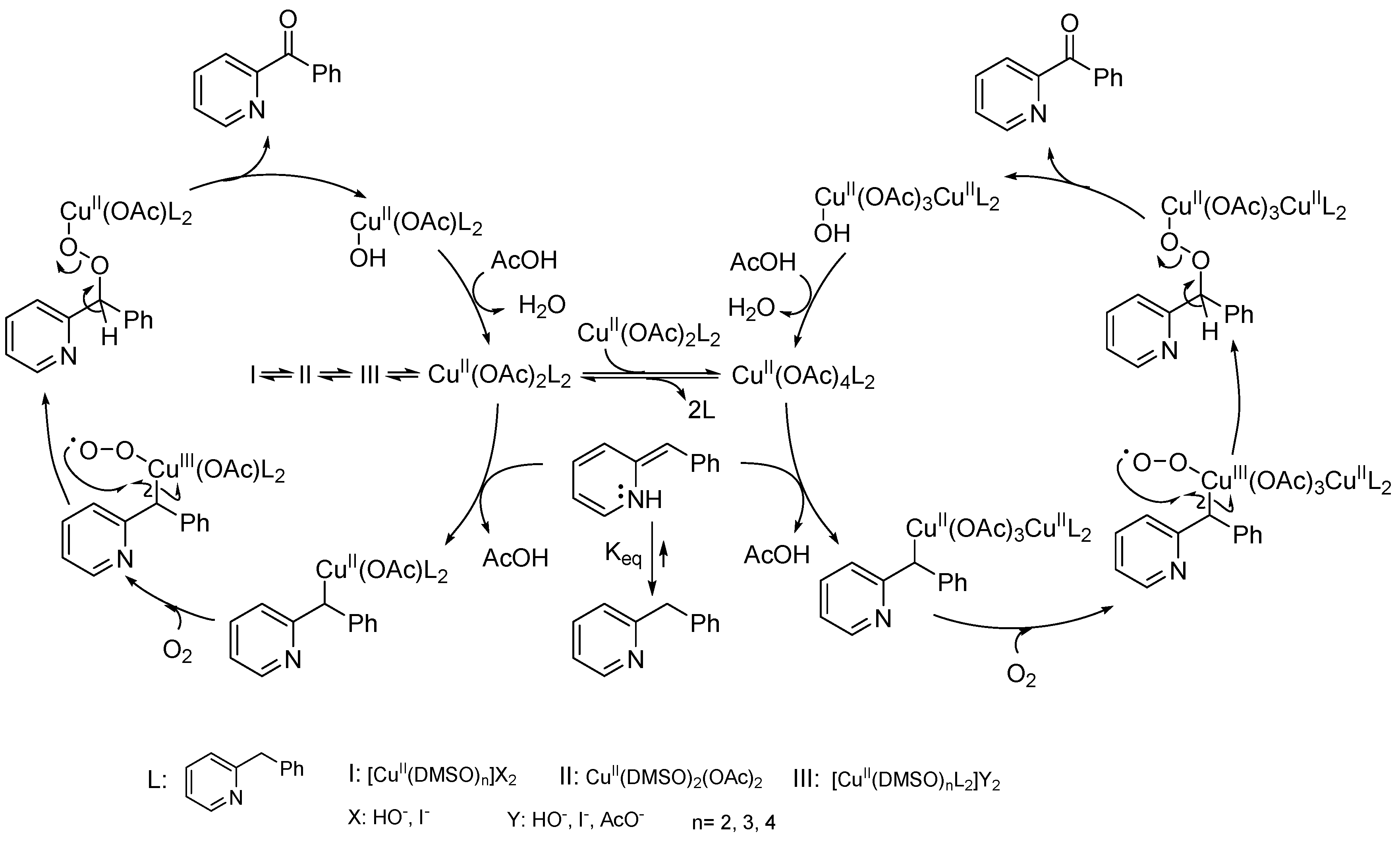
- Sterckx, H.; De Houwer, J.; Mensch, C.; Caretti, I.; Tehrani, K.A.; Herrebout, W.A.; Van Doorslaerb, S.; Maes, B.U.W. Mechanism of the CuII-catalyzed benzylic oxygenation of (aryl)(heteroaryl)methanes with oxygen. Chem. Sci. 2016, 7, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Yi, H.; Liu, C.; Liu, R.; Zhang, H.; Zhuo, K.; Lei, A. Chloroacetate-Promoted Selective Oxidation of Heterobenzylic Methylenes under Copper Catalysis. Angew. Chem. Int. Ed. 2015, 54, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Komiya, N.; Suzuki, K.; Murahashi, S.-I. Copper-catalyzed aerobic oxidative functionalization of C–Hbonds of alkanes in the presence of acetaldehyde under mild conditions. Tetrahedron Lett. 2013, 54, 2706–2709. [Google Scholar] [CrossRef]
- Finney, L.C.; Mitchell, L.J.; Moody, C.J. Visible light mediated oxidation of benzylic sp3 C–H bonds using catalytic 1,4-hydroquinone, or its biorenewable glucoside, arbutin, as a pre-oxidant. Green Chem. 2018, 20, 2242–2249. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A.; Verdiglione, R. Copper-Catalyzed Oxidation of Deoxybenzoins to Benzils under Aerobic Conditions. Synthesis 2013, 45, 1701–1707. [Google Scholar]
- Menendez, C.; Gau, S.; Ladeira, S.; Lherbet, C.; Baltas, M. Synthesis of α,β-Diketotriazoles by Aerobic Copper-Catalyzed Oxygenation with Triazole as an Intramolecular Assisting Group. Eur. J. Org. Chem. 2012, 2012, 409–416. [Google Scholar] [CrossRef]
- Navarro, M.; Escobar, A.; Landaeta, V.R.; Visbal, G.; Lopez-Linares, F.; Luis, M.L.; Fuentes, A. Catalytic oxidation of tetralin by biologically active copper and palladium complexes. Appl. Catal. A Gen. 2009, 363, 27–31. [Google Scholar] [CrossRef]
- Miao, C.; Zhao, H.; Zhao, Q.; Xiaa, C.; Sun, W. NHPI and ferric nitrate: A mild and selective system for aerobic oxidation of benzylic methylenes. Catal. Sci. Technol. 2016, 6, 1378–1383. [Google Scholar] [CrossRef]
- Lesieur, M.; Genicot, C.; Pasau, P. Development of a Flow Photochemical Aerobic Oxidation of Benzylic C−H Bonds. Org. Lett. 2018, 20, 1987–1990. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Gong, Z.; Han, W.; Sun, X. Visible light mediated aerobic photocatalytic activation of CAH bond by riboflavin tetraacetate and N-hydroxysuccinimide. Tetrahedron Lett. 2018, 59, 658–662. [Google Scholar] [CrossRef]
- Li, S.; Zhu, B.; Lee, R.; Qiao, B.; Jiang, Z. Visible light-induced selective aerobic oxidative transposition of vinyl halides using a tetrahalogenoferrate(III) complex catalyst. Org. Chem. Front. 2018, 5, 380–385. [Google Scholar] [CrossRef]
- Sterckx, H.; De Houwer, J.; Mensch, C.; Herrebout, W.; Tehrani, K.A.; Maes, B.U.W. Base metal catalyzed benzylic oxidation of (aryl)(heteroaryl)methanes with molecular oxygen. Beilstein J. Org. Chem. 2016, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Pieber, B.; Kappe, C.O. Direct aerobic oxidation of 2-benzylpyridines in a gas–liquid continuous-flow regime using propylene carbonate as a solvent. Green Chem. 2013, 15, 320–324. [Google Scholar] [CrossRef]
- Li, S.-J.; Wang, Y.-G. A novel and selective catalytic oxidation of hydrocarbons to ketones using chloramine-T/O2/Fe(TPP)Cl system. Tetrahedron Lett. 2005, 46, 8013–8015. [Google Scholar] [CrossRef]
- Klopstra, M.; Hage, R.; Kelloggc, R.M.; Feringa, B.L. Non-heme iron catalysts for the benzylic oxidation: A parallel ligand screening approach. Tetrahedron Lett. 2003, 44, 4581–4584. [Google Scholar] [CrossRef]
- Urgoitia, G.; SanMartin, R.; Herrero, M.T.; Domínguez, E. Palladium NCN and CNC pincer complexes as exceptionally active catalysts for aerobic oxidation in sustainable media. Green Chem. 2011, 13, 2161–2166. [Google Scholar] [CrossRef]
- Urgoitia, G.; Maiztegi, A.; SanMartin, R.; Herrero, M.T.; Domízguez, E. Aerobic oxidation at benzylic positions catalyzed by a simple Pd(OAc)2/bis-triazole system. RSC Adv. 2015, 5, 103210–103217. [Google Scholar] [CrossRef]
- Dos Santos, A.; Kaïm, L.E.; Grimaud, L. Metal-free aerobic oxidation of benzazole derivatives. Org. Biomol. Chem. 2013, 11, 3282–3287. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, L.; Chen, Y.; Chen, C.; Su, Y.; Miao, H.; Xu, J. A promotion effect of alkaline-earth chloride on N-hydroxyphthalimide-catalyzed aerobic oxidation of hydrocarbons. Catal. Commun. 2009, 11, 171–174. [Google Scholar] [CrossRef]
- Figiel, P.J.; Sobczak, J.M. Aerobic oxidation of alcohols and alkylaromatics with dioxygen catalysed by N-hydroxyphthalimide with vanadium co-catalysts. New J. Chem. 2007, 31, 1668–1673. [Google Scholar] [CrossRef]
- Gao, J.; Tong, X.; Li, X.; Miao, H.; Xu, J. The efficient liquid-phase oxidation of aromatic hydrocarbons by molecular oxygen in the presence of MnCO3. J. Chem. Technol. Biotechnol. 2007, 82, 620–625. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Yan, M.; Li, X.; Zeng, W. Iridium-catalyzed aerobic oxidation of alkylarenes with excellentturnover numbers. Catal. Commun. 2013, 35, 64–67. [Google Scholar] [CrossRef]
- Urgoitia, G.; SanMartin, R.; Herrero, M.T.; Domínguez, E. An outstanding catalyst for the oxygen-mediated oxidation of arylcarbinols, arylmethylene and arylacetylene compounds. Chem. Commun. 2015, 51, 4799–4802. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Oisaki, K.; Kanai, M. Chemoselective aerobic photo-oxidation of 9H-fluorenes for the synthesis of 9-fluorenones. Tetrahedron Lett. 2014, 55, 4736–4738. [Google Scholar] [CrossRef]

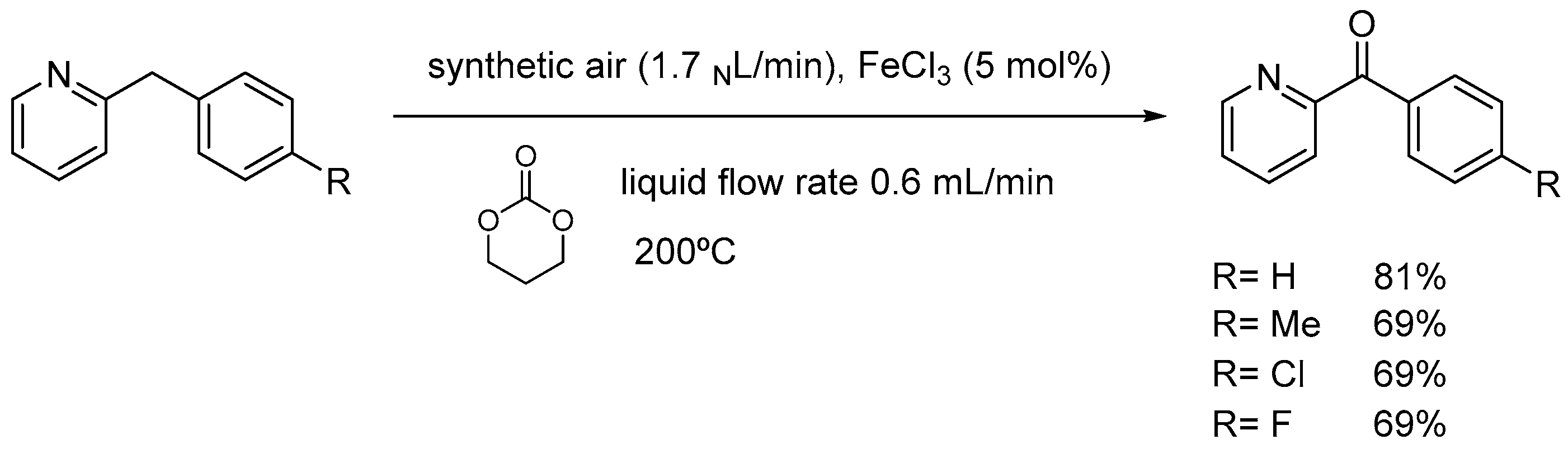
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urgoitia, G.; SanMartin, R.; Herrero, M.T.; Domínguez, E. Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds. Catalysts 2018, 8, 640. https://doi.org/10.3390/catal8120640
Urgoitia G, SanMartin R, Herrero MT, Domínguez E. Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds. Catalysts. 2018; 8(12):640. https://doi.org/10.3390/catal8120640
Chicago/Turabian StyleUrgoitia, Garazi, Raul SanMartin, María Teresa Herrero, and Esther Domínguez. 2018. "Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds" Catalysts 8, no. 12: 640. https://doi.org/10.3390/catal8120640
APA StyleUrgoitia, G., SanMartin, R., Herrero, M. T., & Domínguez, E. (2018). Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds. Catalysts, 8(12), 640. https://doi.org/10.3390/catal8120640