Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview
Abstract
1. Introduction
2. Catalytic Studies for the Transformation of Glucose
- (a)
- Routes through 5-HMF as a building block for further transformations to furan-based products. Such pathways require prior isomerization of glucose to fructose, from which 5-HMF can be produced. Figure 2 presents a scheme of a number of products and intermediates in some cases that can be synthesized via pathways from 5-HMF starting from glucose through isomer fructose. Additionally, this figure also shows the derivation of glucose from lignocellulosic material.
- (b)
- Reactions to products obtained by non-5-HMF related routes, thus avoiding isomerization and dehydration as the first steps of the corresponding conversion. These are schematized in Figure 3.
2.1. Isomerization to Fructose and Reactions to Products through 5-HMF Related Routes
2.2. Other Routes from Glucose to Value-Added Products
3. Catalytic Studies for the Transformation of Fructose
3.1. Transformations of Fructose through 5-HMF Related Routes
3.2. Additional Routes from Fructose to Further Products
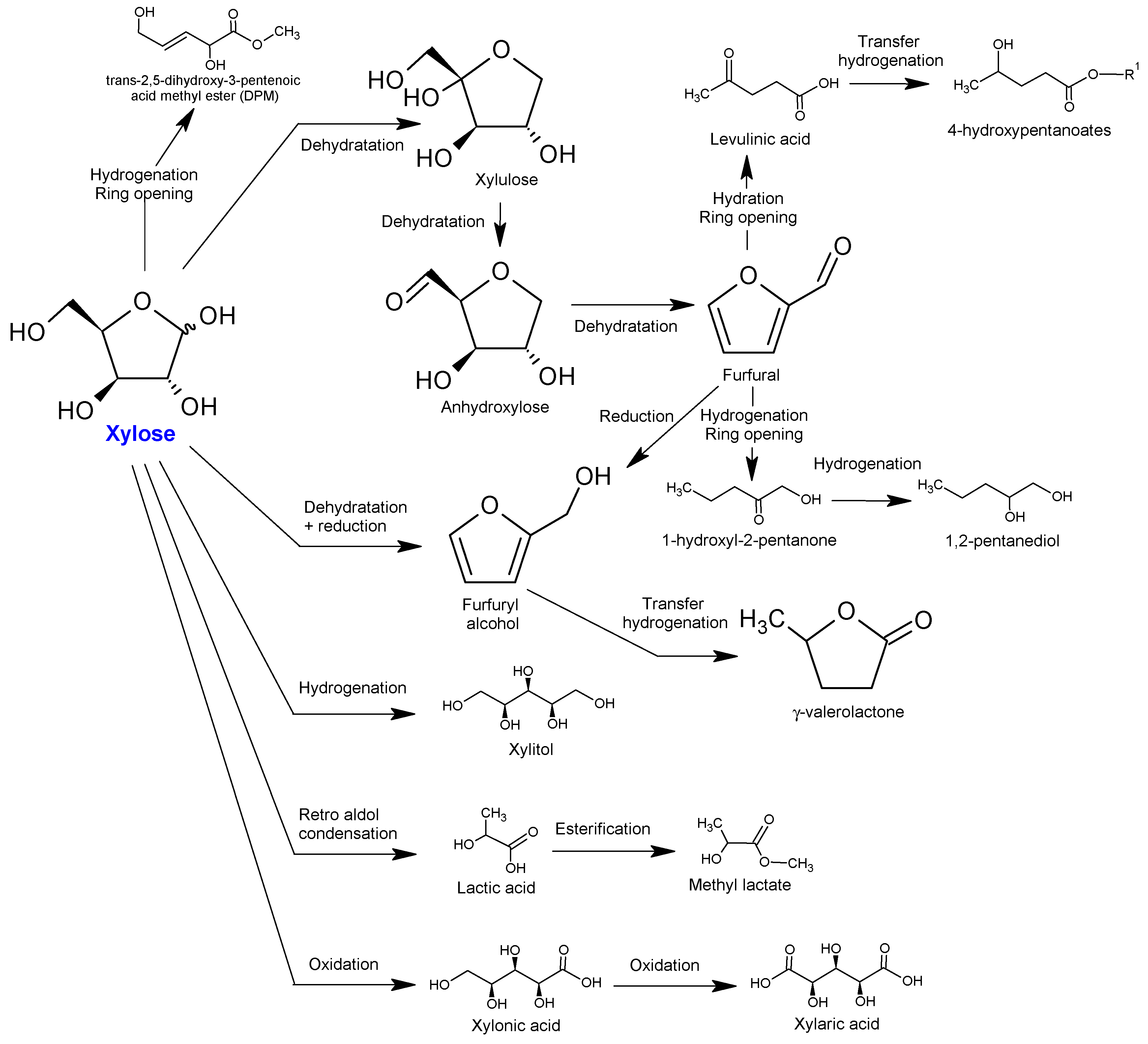
4. Catalytic Routes from Xylose
4.1. Dehydration of Xylose to Furfural
4.2. Direct Reactions of Xylose to Alcohols, Acids and Polymers
5. Catalytic Transformation of Mannose
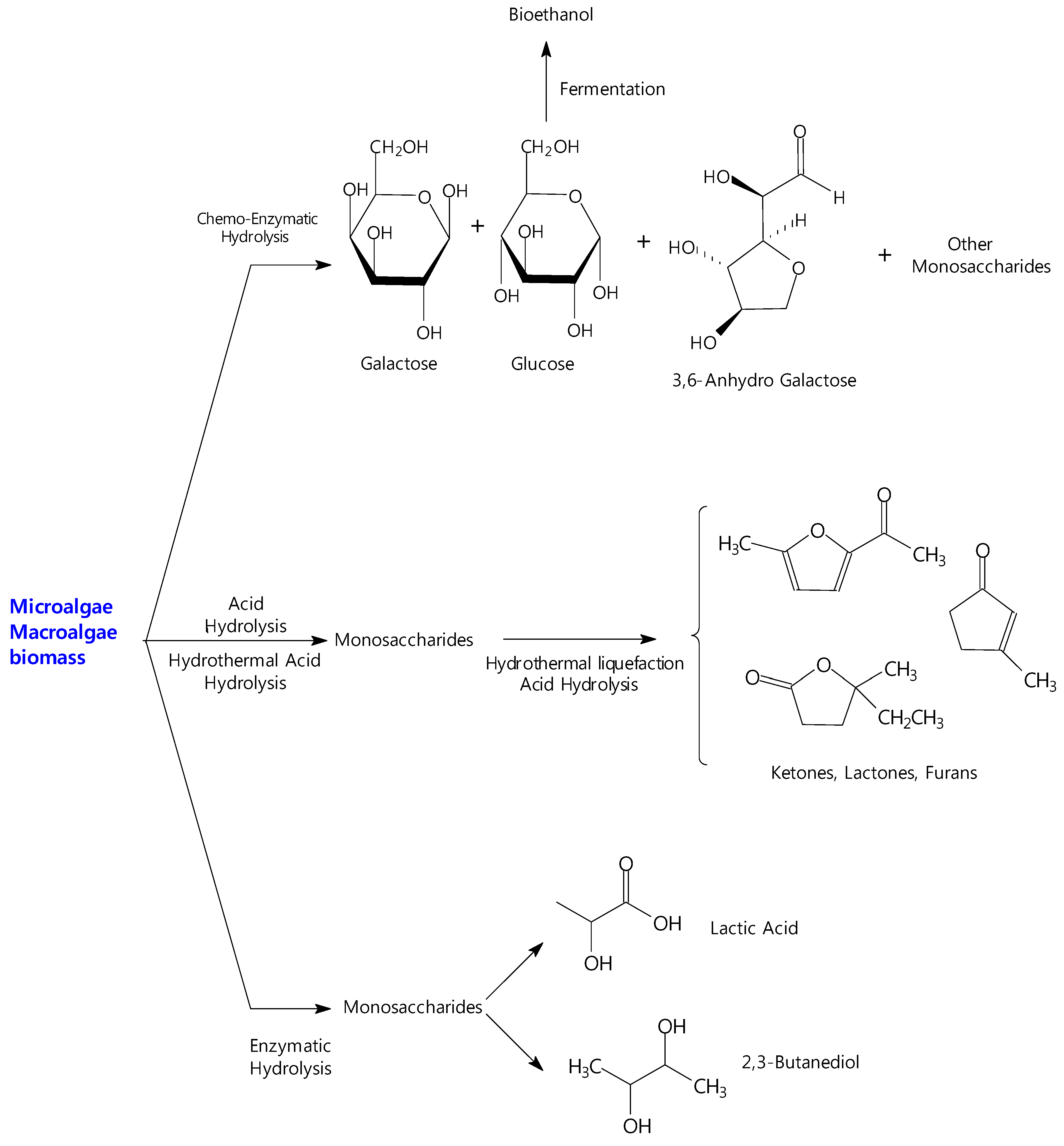
6. Catalytic Studies for the Transformation of Biomass from Micro and Macro-Algae
- (a)
- Processes based on mineral catalysis, mainly acid catalysis, to obtain hydrolysis products.
- (b)
- Processes based on the combination of mineral catalysis and enzymatic catalysis.
- (c)
- Hydrothermal processes combined with acid catalysis.
6.1. Production of Biosugars and Value-Added Products by Acid Hydrolysis Processes
6.2. Production of Biosugars and Value-Added Products by Chemo-Enzymatic Processes
6.3. Production of Biosugars and Value-Added Products by Catalytic Hydrothermal Processes
7. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Jayamuthunagai, J.; Kumar, R.P. Integrated Biorefinery for Bioenergy and Platform Chemicals. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 417–435. [Google Scholar]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- US Energy Information Administration. Annual Energy Outlook 2018: With Projections to 2050. 2018. Available online: https://www.eia.gov/outlooks/aeo/pdf/AEO2018.pdf (accessed on 28 October 2018).
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Snehesh, A.S.; Mukunda, H.S.; Mahapatra, S.; Dasappa, S. Fischer-Tropsch route for the conversion of biomass to liquid fuels—Technical and economic analysis. Energy 2017, 130, 182–191. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic Transfer Hydrogenolysis as an Effective Tool for the Reductive Upgrading of Cellulose, Hemicellulose, Lignin, and Their Derived Molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.M.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Horvath, I.S.; Taherzadeh, M.J. Biochemicals from food waste and recalcitrant biomass via syngas fermentation: A review. Bioresour. Technol. 2018, 248, 113–121. [Google Scholar] [CrossRef]
- Karinen, R.; Vilonen, K.; Niemela, M. Biorefining: Heterogeneously Catalyzed Reactions of Carbohydrates for the Production of Furfural and Hydroxymethylfurfural. Chemsuschem 2011, 4, 1002–1016. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef]
- Shen, F.; Smith, R.L.; Li, L.Y.; Yan, L.L.; Qi, X.H. Eco-friendly Method for Efficient Conversion of Cellulose into Levulinic Acid in Pure Water with Cellulase-Mimetic Solid Acid Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2421–2427. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Ahamd, P.; Muhammad, N. Dicationic ionic liquids as sustainable approach for direct conversion of cellulose to levulinic acid. J. Clean. Prod. 2018, 170, 591–600. [Google Scholar] [CrossRef]
- Luo, Y.P.; Li, Z.; Li, X.L.; Liu, X.F.; Fan, J.J.; Clark, J.H.; Hu, C.W. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Dhepe, P.L. Effects of cations, anions and H+ concentration of acidic ionic liquids on the valorization of polysaccharides into furfural. New J. Chem. 2017, 41, 6137–6144. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, J.P.; Liu, H.F.; Luo, N.C.; Zhao, Z.T.; Wang, F. Sustainable Productions of Organic Acids and Their Derivatives from Biomass via Selective Oxidative Cleavage of C-C Bond. Acs Catal. 2018, 8, 2129–2165. [Google Scholar] [CrossRef]
- Agarwal, B.; Kailasam, K.; Sangwan, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv Fractionation of Softwood Biomass for Biofuel and Biorefinery Applications. Energies 2018, 11, 50. [Google Scholar] [CrossRef]
- Song, J.L.; Fan, H.L.; Ma, J.; Han, B.X. Conversion of glucose and cellulose into value-added products in water and ionic liquids. Green Chem. 2013, 15, 2619–2635. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Huang, Y.B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Yoo, C.G.; Li, N.; Swannell, M.; Pan, X.J. Isomerization of glucose to fructose catalyzed by lithium bromide in water. Green Chem. 2017, 19, 4402–4411. [Google Scholar] [CrossRef]
- Mensah, J.B.; Delidovich, I.; Hausoul, P.J.C.; Weisgerber, L.; Schrader, W.; Palkovits, R. Mechanistic Studies of the Cu(OH)(+)-Catalyzed Isomerization of Glucose into Fructose in Water. Chemsuschem 2018, 11, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Saravanamurugan, S.; Riisager, A.; Taarning, E.; Meier, S. Combined Function of Bronsted and Lewis Acidity in the Zeolite-Catalyzed Isomerization of Glucose to Fructose in Alcohols. Chemcatchem 2016, 8, 3107–3111. [Google Scholar] [CrossRef]
- Pienkoss, F.; Ochoa-Hernandez, C.; Theyssen, N.; Leitner, W. Kaolin: A Natural Low-Cost Material as Catalyst for Isomerization of Glucose to Fructose. ACS Sustain. Chem. Eng. 2018, 6, 8782–8789. [Google Scholar] [CrossRef]
- Parveen, F.; Upadhyayula, S. Efficient conversion of glucose to HMF using organocatalysts with dual acidic and basic functionalities—A mechanistic and experimental study. Fuel Process. Technol. 2017, 162, 30–36. [Google Scholar] [CrossRef]
- Hou, Q.D.; Zhen, M.N.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.Q.; Li, W.Z.; Ju, M.T. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B-Environ. 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Cui, M.; Wu, Z.J.; Huang, R.L.; Qi, W.; Su, R.X.; He, Z.M. Integrating chromium-based ceramic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Renew. Energy 2018, 125, 327–333. [Google Scholar] [CrossRef]
- Insyani, R.; Verma, D.; Kim, S.M.; Kim, J. Direct one-pot conversion of monosaccharides into high-yield 2,5-dimethylfuran over a multifunctional Pd/Zr-based metal-organic framework@sulfonated graphene oxide catalyst. Green Chem. 2017, 19, 2482–2490. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Qi, W.; Su, R.X.; He, Z.M. 3D Flower-like Micro/Nano Ce-Mo Composite Oxides as Effective Bifunctional Catalysts for One-Pot Conversion of Fructose to 2,5-Diformylfuran. ACS Sustain. Chem. Eng. 2017, 5, 4179–4187. [Google Scholar] [CrossRef]
- Zhao, J.; Anjali, J.; Yan, Y.B.; Lee, J.M. Cr-MIL-101-Encapsulated Keggin Phosphomolybdic Acid as a Catalyst for the One-Pot Synthesis of 2,5-Diformylfuran from Fructose. Chemcatchem 2017, 9, 1187–1191. [Google Scholar] [CrossRef]
- Wang, J.; Yao, G.D.; Jin, F.M. One-pot catalytic conversion of carbohydrates into alkyl lactates with Lewis acids in alcohols. Mol. Catal. 2017, 435, 82–90. [Google Scholar] [CrossRef]
- Tosi, I.; Riisager, A.; Taarning, E.; Jensen, P.R.; Meier, S. Kinetic analysis of hexose conversion to methyl lactate by Sn-Beta: Effects of substrate masking and of water. Catal. Sci. Technol. 2018, 8, 2137–2145. [Google Scholar] [CrossRef]
- Wei, W.Q.; Wu, S.B. Experimental and kinetic study of glucose conversion to levulinic acid in aqueous medium over Cr/HZSM-5 catalyst. Fuel 2018, 225, 311–321. [Google Scholar] [CrossRef]
- Cui, J.L.; Tan, J.J.; Deng, T.S.; Cui, X.J.; Zhu, Y.L.; Li, Y.W. Conversion of carbohydrates to furfural via selective cleavage of the carbon-carbon bond: The cooperative effects of zeolite and solvent. Green Chem. 2016, 18, 1619–1624. [Google Scholar] [CrossRef]
- Zhang, L.X.; Xi, G.Y.; Chen, Z.; Jiang, D.; Yu, H.B.; Wang, X.C. Highly selective conversion of glucose into furfural over modified zeolites. Chem. Eng. J. 2017, 307, 868–876. [Google Scholar] [CrossRef]
- Li, K.; Du, M.M.; Ji, P.J. Multifunctional Tin-Based Heterogeneous Catalyst for Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2018, 6, 5636–5644. [Google Scholar] [CrossRef]
- Lari, G.M.; Groninger, O.G.; Li, Q.; Mondelli, C.; Lopez, N.; Perez-Ramirez, J. Catalyst and Process Design for the Continuous Manufacture of Rare Sugar Alcohols by Epimerization-Hydrogenation of Aldoses. Chemsuschem 2016, 9, 3407–3418. [Google Scholar] [CrossRef]
- Deng, W.P.; Wang, P.; Wang, B.J.; Wang, Y.L.; Yan, L.F.; Li, Y.Y.; Zhang, Q.H.; Cao, Z.X.; Wang, Y. Transformation of cellulose and related carbohydrates into lactic acid with bifunctional Al(III)-Sn(II) catalysts. Green Chem. 2018, 20, 735–744. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F.; Moreno-Marrodan, C. Selective direct conversion of C-5 and C-6 sugars to high added-value chemicals by a bifunctional, single catalytic body. Green Chem. 2016, 18, 2935–2940. [Google Scholar] [CrossRef]
- Lin, S.Y.; Guo, X.; Qin, K.; Feng, L.; Zhang, Y.H.; Tang, Y. Efficient Production of Biomass-Derived C-4 Chiral Synthons in Aqueous Solution. Chemcatchem 2017, 9, 4179–4184. [Google Scholar] [CrossRef]
- Zhou, B.W.; Song, J.L.; Zhang, Z.R.; Jiang, Z.W.; Zhang, P.; Han, B.X. Highly selective photocatalytic oxidation of biomass-derived chemicals to carboxyl compounds over Au/TiO2. Green Chem. 2017, 19, 1075–1081. [Google Scholar] [CrossRef]
- Ding, G.D.; Su, J.; Zhang, C.; Tang, K.; Yang, L.S.; Lin, H.F. Coupling Glucose Dehydrogenation with CO2 Hydrogenation by Hydrogen Transfer in Aqueous Media at Room Temperature. Chemsuschem 2018, 11, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Payormhorm, J.; Chuangchote, S.; Kiatkittipong, K.; Chiarakorn, S.; Laosiripojana, N. Xylitol and gluconic acid productions via photocatalytic-glucose conversion using TiO2 fabricated by surfactant-assisted techniques: Effects of structural and textural properties. Mater. Chem. Phys. 2017, 196, 29–36. [Google Scholar] [CrossRef]
- Rizescu, C.; Podolean, I.; Albero, J.; Parvulescu, V.I.; Coman, S.M.; Bucur, C.; Puche, M.; Garcia, H. N-Doped graphene as a metal-free catalyst for glucose oxidation to succinic acid. Green Chem. 2017, 19, 1999–2005. [Google Scholar] [CrossRef]
- Ventura, M.; Williamson, D.; Lobefaro, F.; Jones, M.D.; Mattia, D.; Nocito, F.; Aresta, M.; Dibenedetto, A. Sustainable Synthesis of Oxalic and Succinic Acid through Aerobic Oxidation of C6 Polyols Under Mild Conditions. Chemsuschem 2018, 11, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Rodygin, K.S.; Werner, I.; Ananikov, V.P. A Green and Sustainable Route to Carbohydrate Vinyl Ethers for Accessing Bioinspired Materials with a Unique Microspherical Morphology. Chemsuschem 2018, 11, 292–298. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Qi, W.; Huang, R.L.; Fang, J.; Su, R.X.; He, Z.M. Functionalized silica nanoparticles for conversion of fructose to 5-hydroxymethylfurfural. Chem. Eng. J. 2016, 296, 209–216. [Google Scholar] [CrossRef]
- Fan, C.; Huang, B.H.; Pan, C.; Zhang, J.S.; Wen, H.N.; Yang, J.Y.; Sun, Y. Synthesis of flake-like mesoporous silicate having multiple metal centers and catalytic application for conversion of D-(-)-fructose into fine chemicals. Mater. Chem. Phys. 2017, 200, 295–307. [Google Scholar] [CrossRef]
- Guo, X.W.; Tang, J.Q.; Xiang, B.; Zhu, L.F.; Yang, H.Q.; Hu, C.W. Catalytic Dehydration of Fructose into 5-Hydroxymethylfurfural by a DMSO-like Polymeric Solid Organocatalyst. Chemcatchem 2017, 9, 3218–3225. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Len, C.; Varma, R.S. Sustainable pathway to furanics from biomass via heterogeneous organo-catalysis. Green Chem. 2017, 19, 164–168. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ding, G.Q.; Yang, X.H.; Zheng, H.Y.; Zhu, Y.L.; Li, Y.W. Selectively convert fructose to furfural or hydroxymethylfurfural on Beta zeolite: The manipulation of solvent effects. Appl. Catal. B-Environ. 2018, 235, 150–157. [Google Scholar] [CrossRef]
- Xiang, X.M.; Cui, J.L.; Ding, G.Q.; Zheng, H.Y.; Zhu, Y.L.; Li, Y.W. One-Step Continuous Conversion of Fructose to 2,5-Dihydroxymethylfuran and 2,5-Dimethylfuran. ACS Sustain. Chem. Eng. 2016, 4, 4506–4510. [Google Scholar] [CrossRef]
- Wu, W.P.; Xu, Y.J.; Zhu, R.; Cui, M.S.; Li, X.L.; Deng, J.; Fu, Y. Selective Conversion of 5-Hydroxymethylfuraldehyde Using Cp*Ir Catalysts in Aqueous Formate Buffer Solution. Chemsuschem 2016, 9, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Rathod, P.V.; Jadhav, V.H. Efficient Method for Synthesis of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural and Fructose Using Pd/CC Catalyst under Aqueous Conditions. ACS Sustain. Chem. Eng. 2018, 6, 5766–5771. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Wang, G.X.; Chen, F.; Zhu, J.; Wang, C.T.; Bian, C.Q.; Pan, S.X.; Xiao, F.S. Hierarchical Sn-Beta Zeolite Catalyst for the Conversion of Sugars to Alkyl Lactates. ACS Sustain. Chem. Eng. 2017, 5, 3123–3131. [Google Scholar] [CrossRef]
- Nemoto, K.; Hirano, Y.; Hirata, K.; Takahashi, T.; Tsuneki, H.; Tominaga, K.; Sato, K. Cooperative In-Sn catalyst system for efficient methyl lactate synthesis from biomass-derived sugars. Appl. Catal. B-Environ. 2016, 183, 8–17. [Google Scholar] [CrossRef]
- Yang, X.M.; Liu, Y.; Li, X.X.; Ren, J.X.; Zhou, L.P.; Lu, T.L.; Su, Y.L. Synthesis of Sn-Containing Nanosized Beta Zeolite As Efficient Catalyst for Transformation of Glucose to Methyl Lactate. ACS Sustain. Chem. Eng. 2018, 6, 8256–8265. [Google Scholar] [CrossRef]
- Fu, J.; Xu, X.X.; Lu, X.L.; Lu, X.Y. Hydrothermal Decomposition of Carbohydrates to Levulinic Acid with Catalysis by Ionic Liquids. Ind. Eng. Chem. Res. 2016, 55, 11044–11051. [Google Scholar] [CrossRef]
- Rao, B.S.; Kumari, P.K.; Lakshmi, D.D.; Lingaiah, N. One pot selective transformation of biomass derived chemicals towards alkyl levulinates over titanium exchanged heteropoly tungstate catalysts. Catal. Today 2018, 309, 269–275. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Nguyen, L.H.; Okorie, N.C.; Jamal, S.M. A two-step efficient preparation of a renewable dicarboxylic acid monomer 5,5′-oxybis(methylene) bis 2-furancarboxylic acid from D-fructose and its application in polyester synthesis. Green Chem. 2017, 19, 1570–1575. [Google Scholar] [CrossRef]
- Morales, G.; Paniagua, M.; Melero, J.A.; Iglesias, J. Efficient production of 5-ethoxymethylfurfural from fructose by sulfonic mesostructured silica using DMSO as co-solvent. Catal. Today 2017, 279, 305–316. [Google Scholar] [CrossRef]
- Antonyraj, C.A.; Haridas, A. A lignin-derived sulphated carbon for acid catalyzed transformations of bio-derived sugars. Catal. Commun. 2018, 104, 101–105. [Google Scholar] [CrossRef]
- Wrigstedt, P.; Keskivali, J.; Perea-Buceta, J.E.; Repo, T. One-Pot Transformation of Carbohydrates into Valuable Furan Derivatives via 5-Hydroxymethylfurfural. Chemcatchem 2017, 9, 4244–4255. [Google Scholar] [CrossRef]
- Yan, D.X.; Wang, G.Y.; Gao, K.; Lu, X.M.; Xin, J.Y.; Zhang, S.J. One-Pot Synthesis of 2,5-Furandicarboxylic Acid from Fructose in Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 1851–1858. [Google Scholar] [CrossRef]
- Zelin, J.; Meyer, C.I.; Regenhardt, S.A.; Sebastian, V.; Garetto, T.F.; Marchi, A.J. Selective liquid-phase hydrogenation of fructose to D-mannitol over copper-supported metallic nanoparticles. Chem. Eng. J. 2017, 319, 48–56. [Google Scholar] [CrossRef]
- Lu, T.N.; Chang, C.C. Synthesis of 3-Deoxy-l-ketohexoses through Group Transfer. J. Org. Chem. 2016, 81, 469–475. [Google Scholar] [CrossRef]
- Zhou, X.W.; Li, W.J.; Mabon, R.; Broadbelt, L.J. A Critical Review on Hemicellulose Pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K. Terephthalic acid from waste PET: An efficient and reusable catalyst for xylose conversion into furfural. Catal. Today 2018. [Google Scholar] [CrossRef]
- Hui, W.; Zhou, Y.; Dong, Y.; Cao, Z.-J.; He, F.-Q.; Cai, M.-Z.; Tao, D.-J. Efficient hydrolysis of hemicellulose to furfural by novel superacid SO4H-functionalized ionic liquids. Green Energy Environ. 2018. [Google Scholar] [CrossRef]
- Xu, S.Q.; Pan, D.H.; Wu, Y.F.; Song, X.H.; Gao, L.J.; Li, W.Q.; Das, L.; Xiao, G.M. Efficient production of furfural from xylose and wheat straw by bifunctional chromium phosphate catalyst in biphasic systems. Fuel Process. Technol. 2018, 175, 90–96. [Google Scholar] [CrossRef]
- Sener, C.; Motagamwala, A.H.; Alonso, D.M.; Dumesic, J.A. Enhanced Furfural Yields from Xylose Dehydration in the gamma-Valerolactone/Water Solvent System at Elevated Temperatures. Chemsuschem 2018, 11, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Hu, X.J.; Lam, F.L.Y. A dual acidic hydrothermally stable MOF-composite for upgrading xylose to furfural. Appl. Catal. A-Gen. 2018, 566, 130–139. [Google Scholar] [CrossRef]
- Sato, O.; Mimura, N.; Masuda, Y.; Shirai, M.; Yamaguchi, A. Effect of extraction on furfural production by solid acid-catalyzed xylose dehydration in water. J. Supercrit. Fluids 2019, 144, 14–18. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Kongparakul, S.; Karnjanakom, S.; Reubroycharoen, P.; Guan, G.Q.; Chanlek, N.; Samart, C. Highly productive xylose dehydration using a sulfonic acid functionalized KIT-6 catalyst. Fuel 2019, 236, 1156–1163. [Google Scholar] [CrossRef]
- Moreno-Marrodan, C.; Barbaro, P.; Caporali, S.; Bossola, F. Low-Temperature Continuous-Flow Dehydration of Xylose over Water-Tolerant Niobia-Titania Heterogeneous Catalysts. Chemsuschem 2018, 11, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Zhu, J.H.; Wu, Y.L.; Xie, R.R.; Wu, K.J.; Yang, M.D. Preparation of packing type catalysts AAO@Al/Meso-SiO2-SO3H for the dehydration of xylose into furfural. Microporous Mesoporous Mater. 2018, 262, 112–121. [Google Scholar] [CrossRef]
- Lin, Q.X.; Zhang, C.H.; Wang, X.H.; Cheng, B.G.; Mai, N.; Ren, J.L. Impact of activation on properties of carbon-based solid acid catalysts for the hydrothermal conversion of xylose and hemicelluloses. Catal. Today 2019, 319, 31–40. [Google Scholar] [CrossRef]
- Lopez-Aguado, C.; Paniagua, M.; Iglesias, J.; Morales, G.; Garcia-Fierro, J.L.; Melero, J.A. Zr-USY zeolite: Efficient catalyst for the transformation of xylose into bio-products. Catal. Today 2018, 304, 80–88. [Google Scholar] [CrossRef]
- Canhaci, S.J.; Perez, R.F.; Borges, L.E.P.; Fraga, M.A. Direct conversion of xylose to furfuryl alcohol on single organic-inorganic hybrid mesoporous silica-supported catalysts. Appl. Catal. B-Environ. 2017, 207, 279–285. [Google Scholar] [CrossRef]
- Wang, N.L.; Chen, Z.P.; Liu, L.C. Acid catalysis dominated suppression of xylose hydrogenation with increasing yield of 1,2-pentanediol in the acid-metal dual catalyst system. Appl. Catal. A-Gen. 2018, 561, 41–48. [Google Scholar] [CrossRef]
- Morales, R.; Campos, C.H.; Fierro, J.L.G.; Fraga, M.A.; Pecchi, G. Stable reduced Ni catalysts for xylose hydrogenation in aqueous medium. Catal. Today 2018, 310, 59–67. [Google Scholar] [CrossRef]
- Delgado Arcaño, Y.; Valmaña García, O.D.; Mandelli, D.; Carvalho, W.A.; Magalhães Pontes, L.A. Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today 2018. [Google Scholar] [CrossRef]
- Liu, D.J.; Kim, K.H.; Sun, J.; Simmons, B.A.; Singh, S. Cascade Production of Lactic Acid from Universal Types of Sugars Catalyzed by Lanthanum Triflate. Chemsuschem 2018, 11, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Fu, J.; Langrish, T.; Lu, X.Y. Simultaneous Catalytic Conversion of C6 and C5 Sugars to Methyl Lactate in Near-critical Methanol with Metal Chlorides. Bioresources 2018, 13, 3627–3641. [Google Scholar] [CrossRef]
- Sadula, S.; Saha, B. Aerobic Oxidation of Xylose to Xylaric Acid in Water over Pt Catalysts. Chemsuschem 2018, 11, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.G.; Andersen, C.; Tolborg, S.; Meier, S.; Sadaba, I.; Daugaard, A.E.; Taarning, E. Synthesis of a novel polyester building block from pentoses by tin-containing silicates. Rsc Adv. 2017, 7, 985–996. [Google Scholar] [CrossRef]
- Lopez-Vidal, E.M.; Gregory, G.L.; Kociok-Kohn, G.; Buchard, A. Polymers from sugars and CS2: Synthesis and ring-opening polymerisation of sulfur-containing monomers derived from 2-deoxy-d-ribose and D-xylose. Polym. Chem. 2018, 9, 1577–1582. [Google Scholar] [CrossRef]
- Jia, S.Y.; He, X.J.; Xu, Z.W. Valorization of an underused sugar derived from hemicellulose: Efficient synthesis of 5-hydroxymethylfurfural from mannose with aluminum salt catalyst in dimethyl sulfoxide/water mixed solvent. Rsc Adv. 2017, 7, 39221–39227. [Google Scholar] [CrossRef]
- Tamura, M.; Yuasa, N.; Cao, J.; Nakagawa, Y.; Tomishige, K. Transformation of Sugars into Chiral Polyols over a Heterogeneous Catalyst. Angew. Chem.-Int. Ed. 2018, 57, 8058–8062. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.M.; Ling, T.C.; Nagarajan, D.; Lee, D.J.; Chang, J.S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Shukla, M.; Kumar, S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew. Sustain. Energy Rev. 2018, 82, 402–414. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Lam, M.K.; Tan, I.S.; Lee, K.T. Utilizing lipid-extracted microalgae biomass residues for maltodextrin production. Chem. Eng. J. 2014, 235, 224–230. [Google Scholar] [CrossRef]
- Mirsiaghi, M.; Reardon, K.F. Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res.-Biomass Biofuels Bioprod. 2015, 8, 145–152. [Google Scholar] [CrossRef]
- Kim, T.H.; Oh, Y.K.; Lee, J.W.; Chang, Y.K. Levulinate production from algal cell hydrolysis using in situ transesterification. Algal Res.-Biomass Biofuels Bioprod. 2017, 26, 431–435. [Google Scholar] [CrossRef]
- Goo, B.G.; Baek, G.; Choi, D.J.; Park, Y.I.; Synytsya, A.; Bleha, R.; Seong, D.H.; Lee, C.G.; Park, J.K. Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Bioresour. Technol. 2013, 129, 343–350. [Google Scholar] [CrossRef]
- Lee, O.K.; Kim, A.L.; Seong, D.H.; Lee, C.G.; Jung, Y.T.; Lee, J.W.; Lee, E.Y. Chemo-enzymatic saccharification and bioethanol fermentation of lipid-extracted residual biomass of the microalga, Dunaliella tertiolecta. Bioresour. Technol. 2013, 132, 197–201. [Google Scholar] [CrossRef]
- Kim, S.W.; Hong, C.H.; Jeon, S.W.; Shin, H.J. High-yield production of biosugars from Gracilaria verrucosa by acid and enzymatic hydrolysis processes. Bioresour. Technol. 2015, 196, 634–641. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Maurya, R.; Bhattacharya, S.; Bachani, P.; Mishra, S. Comparative evaluation of chemical and enzymatic saccharification of mixotrophically grown de-oiled microalgal biomass for reducing sugar production. Bioresour. Technol. 2016, 204, 9–16. [Google Scholar] [CrossRef]
- Overbeck, T.; Steele, J.L.; Broadbent, J.R. Fermentation of de-oiled algal biomass by Lactobacillus casei for production of lactic acid. Bioprocess Biosyst. Eng. 2016, 39, 1817–1823. [Google Scholar] [CrossRef]
- Kim, Y.J.; Joo, H.W.; Park, J.; Kim, D.K.; Jeong, K.J.; Chang, Y.K. Production of 2,3-Butanediol by Klebsiella Oxytoca From Various Sugars in Microalgal Hydrolysate. Biotechnol. Prog. 2015, 31, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Juarez, J.M.; Hernando, A.L.; Torre, R.M.; Lanza, S.B.; Rodriguez, S.B. Saccharification of microalgae biomass obtained from wastewater treatment by enzymatic hydrolysis. Effect of alkaline-peroxide pretreatment. Bioresour. Technol. 2016, 218, 265–271. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, S.K.; Hong, Y.K.; Jeong, G.T. Optimization of the production of platform chemicals and sugars from the red macroalga, Kappaphycus alvarezii. Algal Res.-Biomass Biofuels Bioprod. 2016, 13, 303–310. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.B.; Kim, S.K.; Park, D.H.; Jeong, G.T. Optimization and Evaluation of Sugars and Chemicals Production from Green Macro-algae Enteromorpha intestinalis. BioEnergy Res. 2016, 9, 1155–1166. [Google Scholar] [CrossRef]
- Jeon, W.; Ban, C.; Park, G.; Yu, T.K.; Suh, J.Y.; Woo, H.C.; Kim, D.H. Catalytic hydrothermal conversion of macroalgae-derived alginate: Effect of pH on production of furfural and valuable organic acids under subcritical water conditions. J. Mol. Catal. A-Chem. 2015, 399, 106–113. [Google Scholar] [CrossRef]
- Yang, W.C.; Li, X.G.; Zhang, D.H.; Feng, L.J. Catalytic upgrading of bio-oil in hydrothermal liquefaction of algae major model components over liquid acids. Energy Convers. Manag. 2017, 154, 336–343. [Google Scholar] [CrossRef]
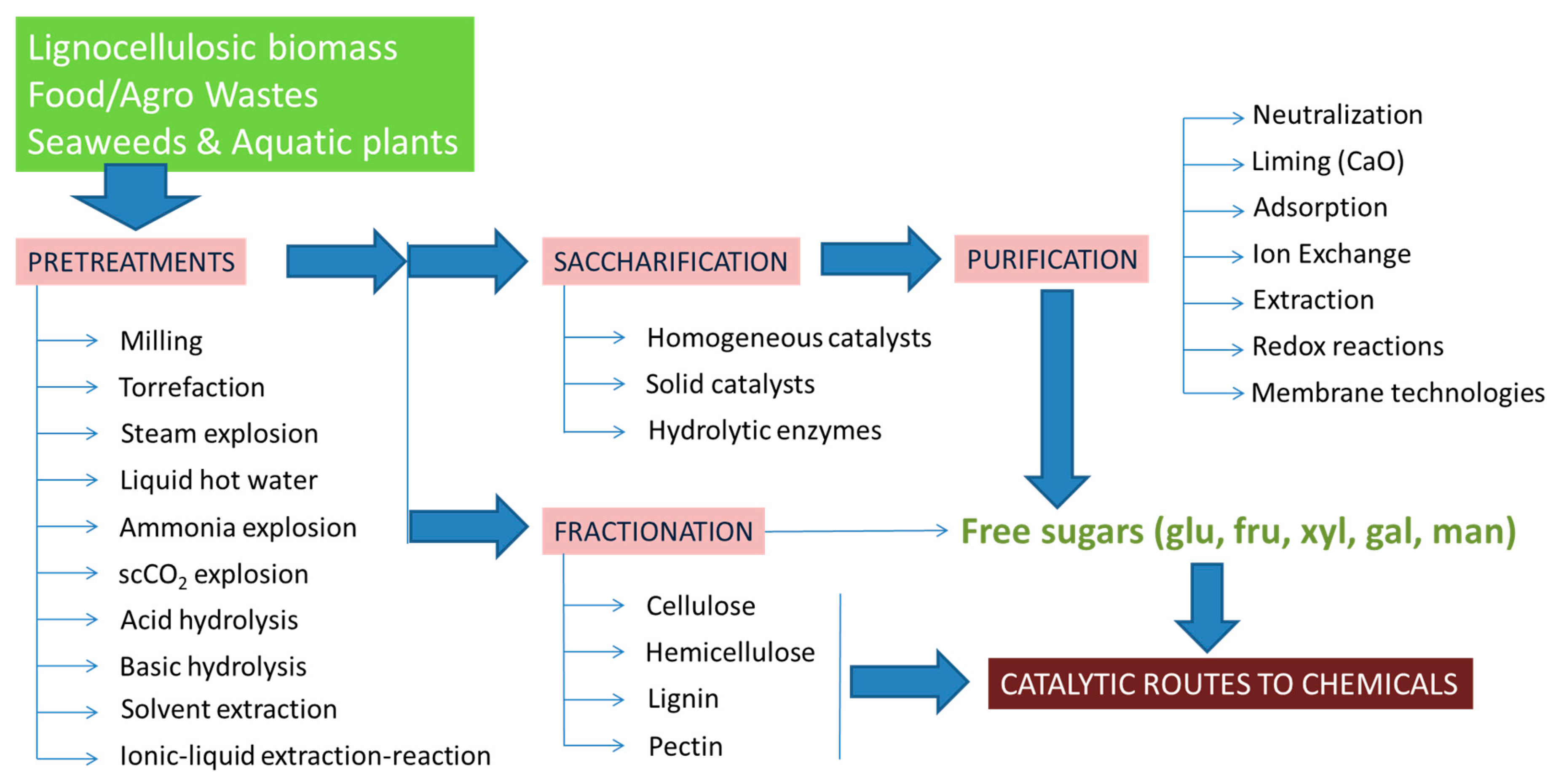
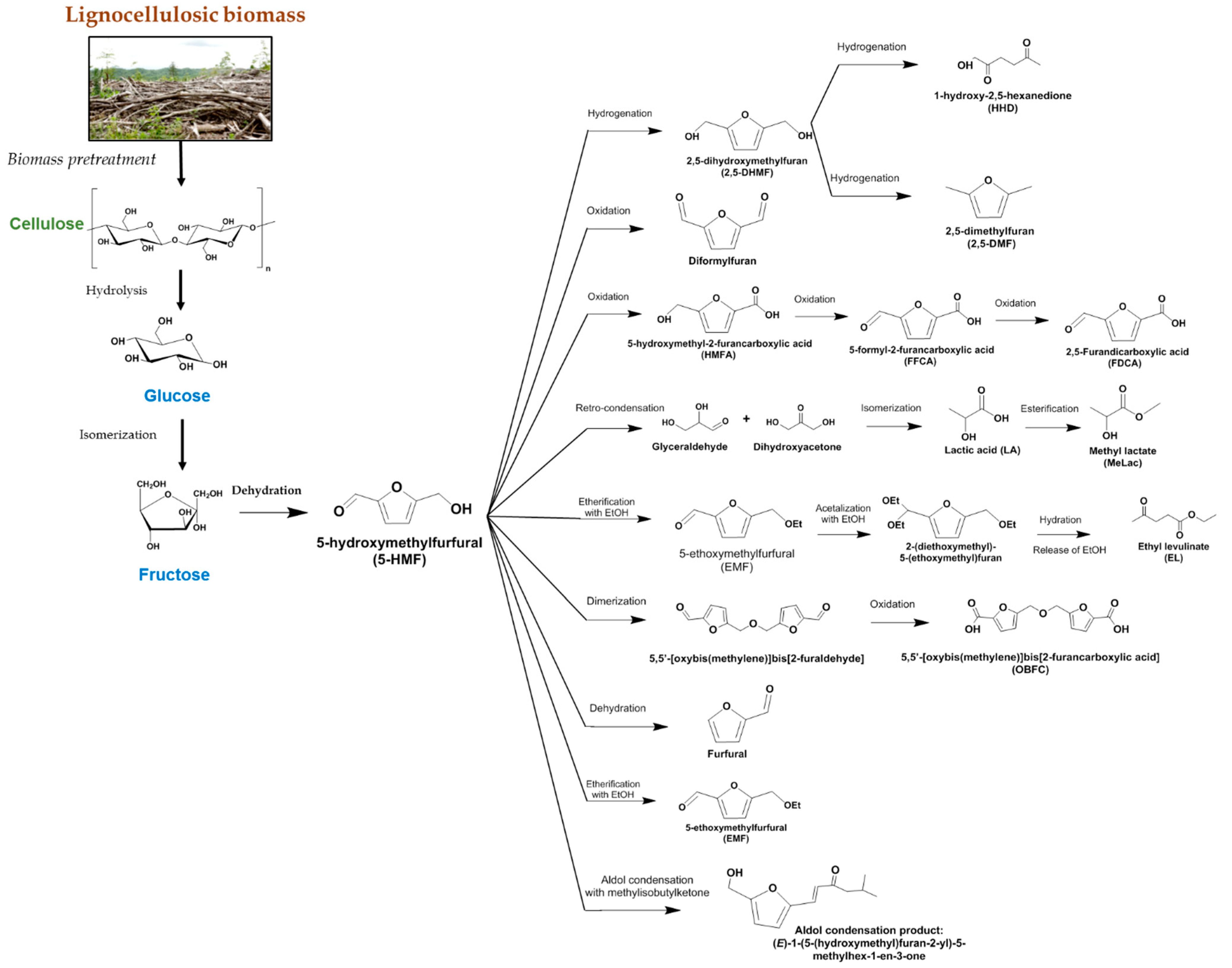
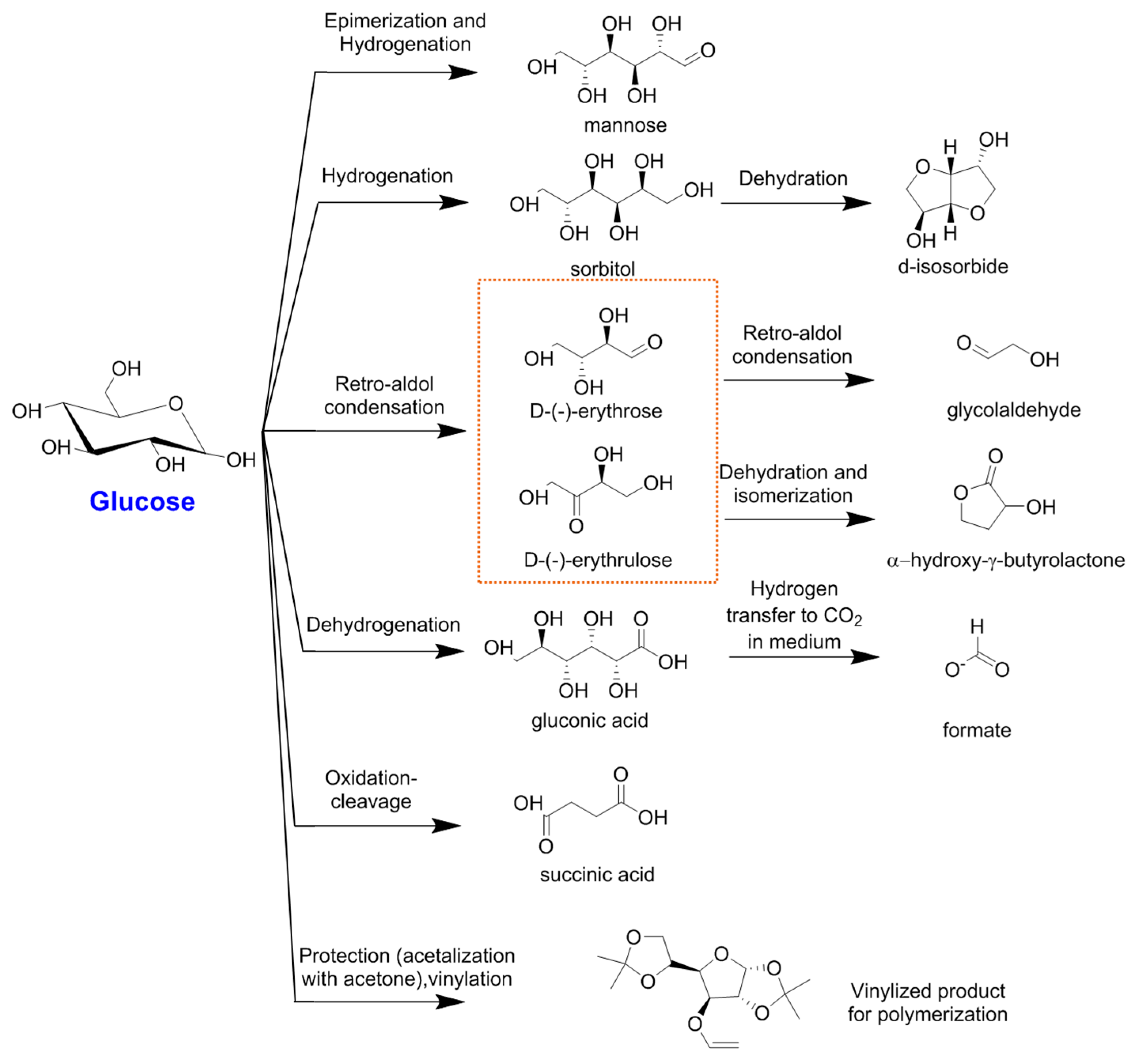
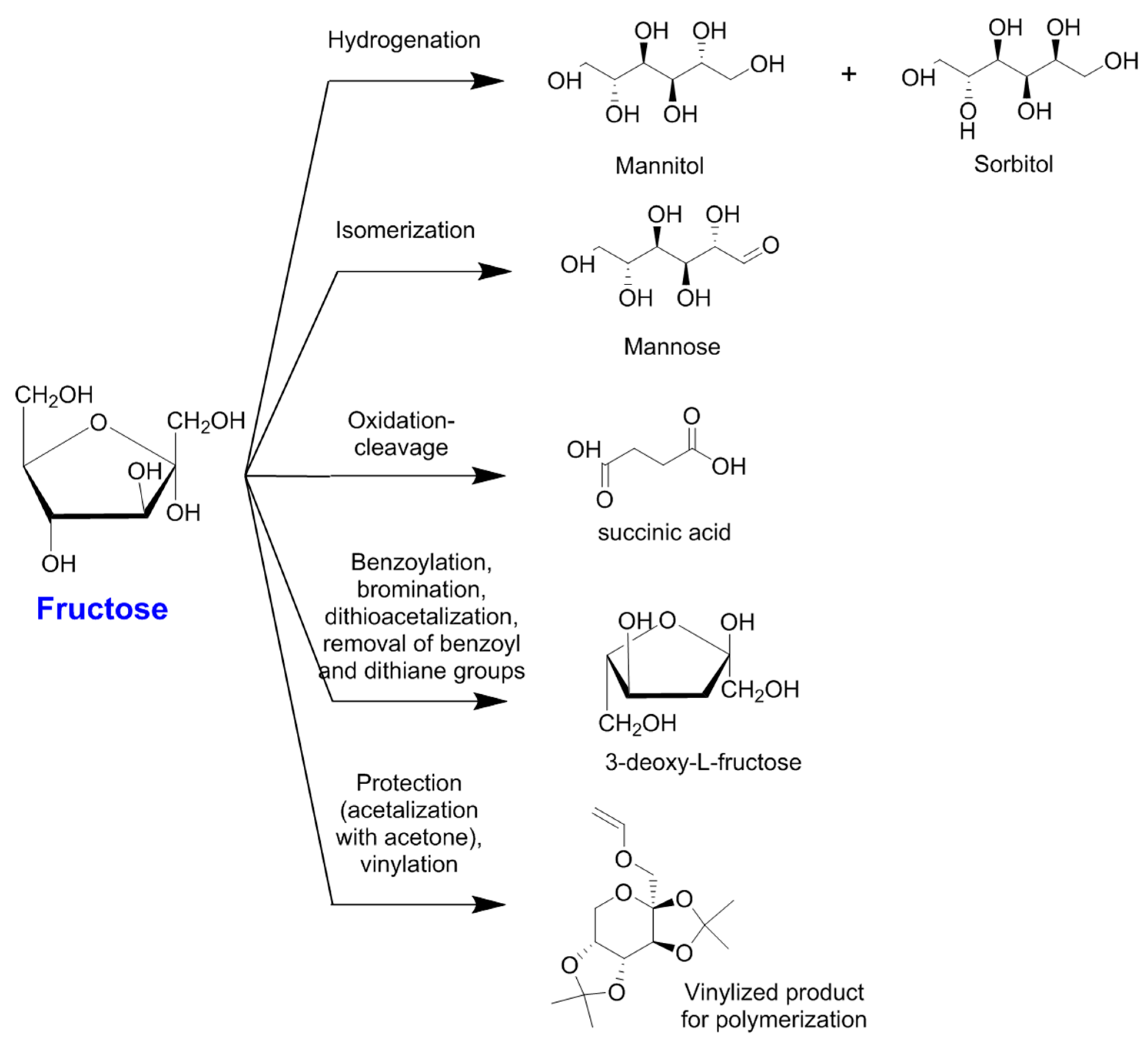
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Isomerization | Fructose (Fru) | Mannose Decomposition products | LiBr | T = 120 °C Cglu = 10 g L−1 in water Ccat = 60% w/w | t = 15 min Xglu = 51.8% Yfru = 30.3% TOF = 0.01 molfru molcat−1 h−1 | [23] |
| Isomerization | Fructose | Mannose | Cu(NO3)2 and other Cu-containing catalysts. | T = 110 °C Cglu = 1% w/w in water Ccat = 60% w/w pH = 5.3 | t = 90 min Xglu = 18% Yfru = 16% TOF = 0.45 molfru molcat−1 h−1 | [24] |
| Isomerization -etherification | Methyl fructoside (MF) | Fructose | Zeolites H-USY, H-Y, H-β (Lewis acidity) Dowex 50WX8-100 (Brønsted acidity) Si/Al ratio = 30 | T = 120 °C Cglu = 3.13% w/w in MeOH Ccat = 60% | t = 60 min Xglu = 83% YMF = 72% TOF = 84.24 molMF gcat−1 h−1 | [25] |
| Isomerization-etherification | Methyl fructoside | Fructose | Kaolin: Strem-2008 and other kaolin samples SBET = 16 m2 g−1 | T = 120 °C Cglu = 3% w/w in MeOH Ccat = 60% w/w | t = 900 min Xglu = 93% YMF = 52% TOF = 1.15 molMF gcat−1 h−1 | [26] |
| Isomerization Dehydration | 5-HMF | Fructose | Sulfanilinic acid, aniline, PTSA, sulphamic acid | T = 160 °C Cglu = 5% mol in H2O/DMSO/MIBK Ccat = 0.01 M | t = 30 min Xglu = 90% YHMF = 44% TOF = 4.4 molHMF molcat−1 h−1 | [27] |
| Isomerization Dehydration | 5-HMF | Fructose | SnPO (from Sn3(PO4)4) SBET = 120.8 m2 g−1 | T = 120 °C Cglu = 20% w/w % in [Emim][Br] Ccat = 10% w/w | t = 180 min Xglu = 94.1% YHMF = 58.3% TOF = 2.16 molHMF gcat−1 h−1 | [28] |
| Isomerization Dehydration | 5-HMF | Fructose | Chitosan nanoparticles doped with Cr(III) and Cr(VI) ions aided by H2SO4 SBET = 30.4 m2 g−1 CCr(III) = 3% | T = 180 °C Cglu = 3.13% w/w in water/DMSO Ccat = 5% w/w | t = 180 min Xglu = 92.3% YHMF = 64.7% TOF = 0.75 molHMF gcat−1 h−1 | [29] |
| Isomerization Dehydration | 5-HMF | Fructose | SnPCP@MnO2 –PDA SBET = 240.6 m2 g−1 | T = 150 °C Cglu = 4% w/w in DMSO Ccat = 1% w/w | t = 300 min Xglu = 92.2% YHMF = 55.8% TOF = 2.47 molHMF gcat−1 h−1 | [38] |
| Isomerization Dehydrations Hydrogenolysis | 2,5-DMF | Fructose, 5-HMF, 5-methylfurfural, 2,5-bis(hydroxymethyl)-furan; 5-methyl-furanmethanol | 4.8Pd/UiO-66@SGO (Pd on a Zr-based MOF deposited on sulfonated graphene oxide) SBET = 715 m2 g−1 CPd = 4.8% | T = 160 °C PH2 = 1 MPa Cglu = 0.025 M in THF Ccat = 0.5% w/w | t = 180 min Xglu = 87.3% YDMF = 45.3% TOF = 0.76 molDMF gcat−1 h−1 | [30] |
| Isomerization Retro-aldol fragmentation Dehydration Acetalization Isomerization | Methyl lactate (MeLac) | Fructose, glyceraldehyde, dihydroxyacetone, pyruvaldehyde, among many others reported | ZnCl2 and other Zn(II) salts | T = 200 °C Cglu = 0.4% w/w in EtOH and water Ccat = 0.004 M | t = 180 min YMeLac = 47.7% TOF = 0.88 molMeLac molcat−1 h−1 | [33] |
| Isomerization Retro-aldol fragmentation Dehydration Acetalization Isomerization | Methyl lactate (MeLac) | Fructose, fructofuranosides, fructopyranosides | Sn-Beta zeolite SBET = 722 m2 g−1 CSn = 0.977% | T = 160 °C Cglu = 0.132 M in EtOH Ccat = 1% w/w | t = 720 min YMeLac = 43% TOF = 0.47 molMeLac gcat−1 h−1 | [34] |
| Dehydration Rehydration | Levulinic acid (LevAc) | 5-HMF, formic acid, humins | Cr-HZSM-5 SBET = 308.9 m2 g−1 CCr = 7.25% | T = 180 °C Cglu = 2% w/w in water Ccat = 0.75% w/w | t = 180 min Xglu = 100% YLevAc = 64.4% TOF = 3.18 molLevAc gcat−1 h−1 | [35] |
| Isomerization, C-C bond cleavage Dehydration | Furfural | Fructose, 2,5-HMF, lactic acid, arabinose | H-β zeolite | T = 150 °C Cglu = 5% w/w in γ-valerolactone and water Ccat = 1% w/w PN2 = 2 MPa | t = 60 min Xglu = 99.9% YFur = 56.5% TOF = 15.68 molFur gcat−1 h−1 | [36] |
| Isomerization Dehydration | Furfural | 5-HMF | Sn, Fe and Zr-β zeolite SBET = 539.9 m2 g−1 CSn = 7.1 µmol g−1 | T = 180 °C Cglu = 0.6% w/w in γ-valerolactone and water Ccat = 2.4% w/w | t = 33 min Xglu = 100% YFur = 69.2% TOF = 1.75 molFur gcat−1 h−1 | [37] |
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Epimerization Hydrogenation | Mannose | Fructose | Epimerization: Cs-HPA/C Hydrogenation: Cs-HPA+Ru/C SBET = 654 m2 g−1 CMo = 16.1% | Continuous flow Cglu = 0.28 M Wcat = 0.2 gcat Epimerization T = 190 °C; Hydrogenation T = 160 °C QH2 = 50 mL min−1 | WHSV = 7.56 gglu gcat−1 h−1 Xglu = 53.9% YFru = 37.7% | [39] |
| Isomerization Retro-aldol fragmentation Dehydration Isomerization | Lactic acid | Glyceraldehyde, dihydroxyacetone and pyruvaldehyde | Bifunctional Al(III)-Sn(II) catalysts Al(III)/Sn(II) ratio = 1/1 | T = 180 °C Cglu = 0.5% w/w in H2O pH = 2.8 Ccat = 0.005 M | t = 120 min Xglu ≈ 100% YLA = 81% TOF = 2.25 molLacAc molcat−1 h−1 | [40] |
| Hydrogenation -Dehydratation | D-isosorbide | Sorbitol, sorbitan | Ru@Dowex-H | T = 190 °C PH2 = 30 bar Cglu = 1 M (H2O) CRu = 0.2% w/w | t = 2880 min Xglu = 100% YISOSORB = 81% TOF = 8.44 molISOSORB gcat−1 h−1 | [41] |
| Retroaldol fragmentation-Dehydration Isomerization | Glycaldehyde and α-hydroxy-γ-valerolactone | d-(-)-erythrose, d-(+)-erythrulose, vinylglycolate | Ammonium tungstate | T = 190 °C Cglu = 1% w/w in water Ccat = 0.2% w/w | t = 1 min Xglu = 94.4% Yerythrose = 11.5% Yerythrulose = 6.3% Yglycaldehde = 52.5% YHBL = 6.3% TOF = 87.42 molGlyAld molcat−1 h−1 | [42] |
| Oxidation | Gluconic acid | - | AuNPs/TiO2 | T = 30 °C Cglu = 0.1 M (H2O) Ccat = 2.5% w/w CAu = 0.075% w/w λ = 420–780 nm 0.3 W cm−2 | t = 240 min Xglu > 99% YGA = 98% TOF = 0.98 molGluAC gcat−1 h−1 | [43] |
| Coupling dehydrogenation and NH4(CO3)2 hydrogenation | Gluconic acid and formate | Sorbitol | Pd/AC and Pt/AC jointly (on activated carbon) | T = 20 °C Cglucose = 6 M in EtOH/H2O CNH4(CO3)2 = 3.3 M CKOH = 6.6 M Ccat = 1.7% w/w CPd/AC = 5% w/w CPt/AC = 5% w/w | t = 1440 min Xglu = 72.6% YGlucAc = 59.5% Yformate = 32.3% TOF = 8.75 molGluAC gcat−1 h−1 | [44] |
| Oxidation to gluconic acid -Decomposition to xylitol | Gluconic acid and xylitol | Arabinose (by decarboxylation of gluconic acid) and formic acid | (a) SG/PEG-TiO2 (b) US/CTAB-TiO2 and other synthesized TiO2 SBET = 5.93 m2 g−1 | T = 20 °C Cglu = 1 g L−1 in ACN/H2O Ccat = 1 g L−1 λmax = 365 nm | t = 120 min Xglu = 26% (a) YGlucAc = 7.6% (a) TOF = 0.02 molGluAc gcat−1 h−1 | [45] |
| Oxidation and cleavage | Succinic acid | Lactic acid, glyceric acid, glycolic acid | N-doped graphene NH2-rGO (3.8) CN = 3.8% | T = 160 °C Cglu = 0.05 M in water Ccat = 2.5 g L−1 PO2 = 18 atm | t = 1200 min Xglu = 100% YSucAc = 68% TOF = 0.006 molSucAc gcat−1 h−1 | [46] |
| Oxidation and cleavage | Oxalic and Succinic acid | Fructose, 2-formyl-5-furancarboxylic acid, formic acid | V-Fe@CNT SBET = 81 m2 g−1 CV = 0.5% | T = 150 °C Cglu = 0.2 M in water Ccat = 4 g L−1 PO2 = 20 bar | t = 12 h Xglu = 96.6% YOxAc = 46.3% YSucAc = 7.8% TOF = 0.002 molOxAc gcat−1 h−1 | [47] |
| Acetalization (for protection) and vinylation | Vinylized monomer | Acetalized intermediate | Acetalization H2SO4 Vinylation CaC2 (KF, KOH) | T = 130 °C Cglu = 0.2 M in DMSO/water CCaC2 = 1.2 M CKOH/KF = 0.22 M | t = 180 min Ymonomer = 86% TOF = 0.74 molmonomer molcat−1 h−1 | [48] |
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Dehydration | 5-HMF | Levulinic acid | SiNP-SO3H-C16 and other functionalized SiO2 nanoparticles | T = 120 °C Cfru = 5% w/w in DMSO/water Ccat = 2.8% w/w | t = 180 min Xfru = 100% YLevA = 87% TOF = 2.87 molHMF gcat−1 h−1 | [49] |
| Dehydration | 5-HMF | Sucrose Methyl 2-furoate 2,3-dihydro-3,5-dihydroxy-6-methylpyran-4-one | Mesoporous silicates + Pluronic 123 + polyvinyl pyrrolidone + cholesterol + -TPA SBET = 372 m2 g−1 | T = 80 °C Cfru = 1.2% w/w in water CZr = 2% w/w | t = 360 min Xfru = 100% YLA = 81% TOF = 14.6 molHMF gcat−1 h−1 | [50] |
| Dehydration | 5-HMF | Glucose, furfural, levulinic acid | Au@(polythio phenepoly thiophene oxides) | T = 140 °C Cfru = 0.2 M in dioxane CS = 0.1 M | t = 240 min Xfru = 100% YLA = 72.6% TOF = 7.88 molHMF gcat−1 h−1 | [51] |
| Dehydration | 5-HMF | 2,5-Diformylfuran | Sulfonated graphitic carbon nitride (Sg-CN) SBET = 10.1 m2 g−1 | T = 100 °C Cfru = 2 M in water Ccat = 1.25% w/w | t = 30 min Xfru = 100% YHMF = 96% TOF = 307.2 molHMF gcat−1 h−1 | [52] |
| Dehydration | 5-HMF or furfural | Glucose, arabinose, formic acid, levulinic acid | H-β zeolite SBET = 525.6 m2 g−1 | T = 150 °C Cfru = 5% w/w Ccat = 1% w/w Solvents: NMP for 5-HMF GBL for furfural | t = 60 min Xfru = 97.4% (NMP) Xfru = 99.9% (GBL) YHMF = 83.3% YFur = 0.2% TOF = 23.12 molHMF gcat−1 h−1 | [53] |
| Dehydration Hydrogenation | 2,5-DHMF and 2,5-DMF | 5-HMF, furfural, levulinic acid | HY zeolite and (HT)-Cu/ZnO/Al2O3 SBET = 73.6 m2 g−1 CCu = 38.37% | Continuous flow: Cfru = 3% w/w in GBL Wcat = 4 gcat QH2 = 15 mL min−1 Dehydration: T = 140 °C Hydrogenation: T = 240 °C | WHSV = 0.02 gglu gcat−1 h−1 Xfru = 100% YDHMF = 48.2% YDMF = 40.6% | [54] |
| Dehydration Oxidation | Diformylfuran | 5-HMF, lactic acid | f-Ce9Mo1Oδ Ce/Mo ratio = 9/0.93 SBET = 66.25 m2 g−1 CMo = 6.36% | T = 120 C Cfru = 45 g L−1 in DMSO Ccat = 6.36% w/w QO2 = 10 mL min−1 | t = 720 min Xfru = 100% YDFF = 74% TOF = 0.26 molDFF gcat−1 h−1 | [31] |
| Dehydration Oxidation | Diformylfuran | 5-HMF | Phosphomolybdic acid encapsulated in MIL-101 | T = 150 °C Cfru = 40 g L−1 in DMSO Ccat = 0.8% w/w QO2 = 20 mL min−1 | t = 420 min Xfru = 100% YDFF = 75.1% TOF = 0.003 molDFF gcat−1 h−1 | [32] |
| Dehydration Oxidation | 2,5-furandicarboxylic acid | 5-HMF 5-hydroxy-methyl-2-furancarboxylic acid, Diformylfuran 5-formyl-2-furancarboxylic acid | Pd/CC derived from glucose SBET = 68.3 m2 g−1 CPd = 5.84% | T = 140 °C Cfructose = 2% w/w in water Ccat = 20% w/w QO2 = 20 mL min−1 | t = 1800 min YFDCA = 64% TOF = 0.011 molFDCA gcat−1 h−1 | [56] |
| Dehydration Oxidation | 2,5-furandicarboxylic acid | 5-HMF 5-hydroxy methyl-2-furancarboxylic acid 5-formyl-2-furancarboxylic acid | Dehydration: Amberlyst-15. Oxidation: Fe0.6Zr0.4O2 SBET = 96 m2 g−1 | T = 160 °C; Cfru = 0.1 M in [Bmim][Cl]; CAmberlyst-15 = 1% w/w CFe0.6Zr0.4O2 = 1% w/w PO2 = 2 MPa | t = 1440 min Xfru = 100% YFDCA = 46.4% TOF = 0.19 molFDCA gcat−1 h−1 | [66] |
| Dehydration Hydrolysis Hydrogenation | 1-hydroxy-2,5-hexanedione | 5-HMF 2,5-bis-(hydroxymethyl) furan | 1st step to 5-HMF: HCl 2nd step to 1-hydroxy-2,5-hexanedione: Cp*IrIII half-sandwich complexes with bipyridine ligands CIr = 3.64 mgL−1 | 1st step to 5-HMF: T = 130 °C; Cfru = 0.5 M in IPA/water CHCl = 0.05 M 2nd step to 1-hydroxy-2,5-hexanedione T = 130 °C C5-HMF = 0.517 M in aq. formate buffer solution (pH = 2.5) Ccat = 0.517 M | t = 180 + 120 min Xfru = 71.9% YHDone = 99% TOF = 0.19 molHDone molcat−1 h−1 | [55] |
| Dehydration, retro-condensation Isomerization Esterification | Methyl lactate | 5-HMF glyceraldehyde DHA fructosides | Hierarchical Sn-β Zeolite SBET = 719 m2 g−1 Si/Al ratio = 12.5 CSn = 3.7% | T = 160 °C; Cfructose = 0.15 M in methanol Ccat = 0.5% w/w PN2 = 1 MPa | t = 1200 min Xfru = 100% YML = 86% TOF = 1.29 molMeLac gcat−1 h−1 | [57] |
| Retro-aldol fragmentation Dehydration Acetalization isomerization | Methyl lactate | Glyceraldehyde, dihydroxyacetone, pyruvaldehyde, among many others reported | ZnCl2 | T = 200 °C; Cfru = 0.4% w/w in EtOH and water Ccat = 0.004 M | t = 180 min YML = 52% TOF = 0.96 molMeLac molcat−1 h−1 | [33] |
| Retro-aldol fragmentation Isomerization Esterification | Methyl lactate | Methyl levulinate and fructosides | InCl3.4H2O/Bu2SnCl2 and other In–Sn catalytic systems In/Sn ratio = 5 | T = 160 °C Cfru = 0.125 M in methanol Ccat = 0.5% w/w PN2 = 0.5 MPa | t = 600 min Xfru = 98% YMeLac = 72% TOF = 1.22 molMeLac molcat−1 h−1 | [58] |
| Mechanism is not discussed | Methyl lactate | Not reported | Sn-β zeolites SBET = 422 m2 g−1 | T = 160 °C Cfructose = 2.5% w/w in methanol; Ccat = 1.6% w/w PN2 = 0.5 MPa | t = 600 min YMeLac = 47% TOF = 0.39 molMeLac gcat−1 h−1 | [59] |
| Hydrothermal Decomposition | Levulinic acid | 5-HMF | [PrSO3HMIm] [Cl] and other ionic liquids | T = 180 °C; Cfru = 2% w/w in water Ccat = 40% w/w | t = 180 min Xfru = 100% YLevAc = 79% TOF = 0.31 molLevAc molcat−1 h−1 | [60] |
| Dehydration Etherification Acetalization Hydration | Ethyl levulinate | 5-HMF, furfural, ethoxyfurfural | Ti0.75TPA and other titanium exchanged heteropoly TPA | T = 120 °C Cfru = 0.25 M in EtOH; Ccat = 2.25% w/w | t = 360 min Xfru = 100% YEtLev = 63% TOF = 1.17 molEtLev gcat−1 h−1 | [61] |
| Dimerization Oxidation | Dicarboxylic acid monomer: 5,5’-[oxybis (methylene)]bis [2-furancarboxylic acid] | 5,5’-[oxybis (methylene)]bis [2-furaldehyde] (OBFA) | Dimerization: Dowex 50 W X8 Oxidation: 5% Pt/C | Dimerization: T = 110 °C Cfru = 45% w/w in DMSO Ccat = 10% w/w. Oxidation: T = 23 °C OBFA = 0.2 M in aq. NaOH (1.5 M) Ccat = 2% w/w; PO2 = 1 atm | t = 1440 + 2880 min Xfru = 100% Ymonomer = 75% | [62] |
| C-C bond cleavage and dehydration | Furfural | Glucose 2,5-HMF Lactic acid Arabinose | H-β zeolite Si/Al ratio = 25 Cacid = 0.366 mmol gcat−1 | T = 150 °C Cfru = 5% w/w in GBL/water Ccat = 1% w/w PN2 = 20 bar | t = 60 min Xfru = 100% YFur = 64% TOF = 1.78 molFur gcat−1 h−1 | [36] |
| Dehydration | Furfural | 5-HMF | Sn, Fe and Zr-Beta zeolite SBET = 539.9 m2 g−1 CSn = 7.1 µmol g−1 | T = 170 °C Cfru = 0.6% w/w in GBL/water Ccat = 2.4% w/w | t = 30 min Xfru = 100% YFur = 69% TOF = 1.92 molFur gcat−1 h−1 | [37] |
| Dehydration Etherification | Ethoxymethyl furfural | 5-HMF Ethyl levulinate | Ar-SO3 H-SBA-15 and other mesoporous silica SBET = 712 m2 g−1 | T = 116 °C, Cfru = 0.2 M in ethanol/DMSO (91.7:8.3 v/v) Ccat = 0.027 M | t = 240 min Xfru = 100% YEMFur = 64% TOF = 1.19 molEMFur gcat−1 h−1 | [63] |
| Dehydration Etherification | Ethoxymethyl furfural | HMF, lactic acid | lignin-derived sulphated carbon SBET = 26 m2 g−1 CS = 36 M | T = 150 °C Cfru = 2% w/w in ethanol Ccat = 0.5% w/w PN2 = 20 bar | t = 180 min Xfru = 100% YEMFur = 64% TOF = 3.03 molEMFur gcat−1 h−1 | [64] |
| Dehydrations Hydrogenations Hydrogenolysis | 2,5-DMF | Fructose, 5-HMF, 5-MFA (5-methylfurfural), 2,5-BHMF (2,5-bis(hydroxymethyl)-furan; 5-MFM (5-methyl-furanmethanol) | 4.8Pd/UiO-66@SGO (Pd on a Zr-based metalorganic framework deposited on sulfonated graphene oxide) SBET = 715 m2 g−1 CPd = 4.8% | T = 160 °C PH2 = 1 MPa Cfru = 0.5% mol in THF Ccat = 0.5% w/w | t = 180 min Xfru = 92% YDMF = 71% TOF = 1.18 molDMF gcat−1 h−1 | [30] |
| Dehydration Aldol condensation with methylisobutyl ketone | (E)-1-(5-(hydroxymethyl) furan-2-yl)-5-methylhex-1-en-3-one | 5-HMF | Dehydration: KBr, H2SO4 Aldol condensation: NaOH | Dehydration: T = 150 °C Cfru = 0.055 M in dioxane CKBr = 0.0375 M CH2SO4 = 0.125 M Aldol condensation: T = 55 °C CMIBK = 1 M in dioxane | t = 1 + 180 min Xfru = 100% Yproduct = 73% TOF = 0.36 molproductr molcat−1 h−1 | [65] |
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Hydrogenation | Mannitol | Sorbitol Glucose | Cu/SiO2-PD and other copper-supported metallic nanoparticles SBET = 225 m2 g−1 CCu = 11.3% | T = 200 °C PH2 = 40 bar Cfru = 0.055 M in EtOH/water Ccat = 0.5% w/w | t = 360 min Xfru = 100% YMan = 78% TOF = 1.43 molmannitol gcat−1 h−1 | [67] |
| Oxidation and cleavage | Oxalic and Succinic acid | Fructose, 2-formyl-5-furancarboxylic acid, formic acid | Fe@CNT SBET = 78 m2 g−1 | T = 140 °C Cglu = 0.05 M in water Ccat = 2.5 g L−1 PO2 = 20 bar | t = 12 h Xglu = 99% YOxAc = 46.8% YSucAc = 21% TOF = 0.003 molOxAc gcat−1 h−1 | [47] |
| Acetalization (for protection) Vinylation | Vinylized monomer | Acetalized intermediate | Acetalization: H2SO4 Vinylation: CaC2 (KF, KOH) | T = 130 °C Cfru = 0.33 M in DMSO/water CCaC2 = 1.2 M CKOH/KF = 0.22 M | t = 180 min Ymonomer = 92% TOF = 1.29 molmonomer molcat−1 h−1 | [48] |
| Retro-aldol fragmentation Dehydration Isomerization | Lactic acid | Glyceraldehyde Dihydroxyacetone Pyruvaldehyde | Bifunctional Al(III)-Sn(II) catalysts | T = 180 °C Cfru = 0.5% w/w in water pH = 2.8 Ccat = 0.005 M Al(III)/Sn(II) ratio = 1/1 | t = 120 min Xfru = 100% YLA = 90% TOF = 2.50 molLacAc molcat−1 h−1 | [40] |
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Dehydratation Resinification or self-polymerization | Furfural | polyfurfural | Terephthalic acid (TPA) | T = 190 °C Cxyl = 8.9% w/w in H2O Vtoluene/VH2O = 2 Ccat = 0.5% w/v | t = 180 min Xxyl = 92% YFur = 72% 100% stable for 5 cycles TOF = 0.05 molFur gcat−1 h−1 | [70] |
| Dehydration Polymerization | Furfural | From xylan: Humins Monosaccharides From xylose: Not indicated (Low conc.) | Ionic liquid [Choline-SO4H][CF3SO3] | T = 120 °C Cxyl = 40% in 1,4-dioxane with 2% H2O (Cxylan identical) Ccat = 2% w/v | From xylan t = 360 min Xxyl = 64% YFur = 62.4% TOF = 2.3⋅10−3 molFur gcat−1 h−1 From xylose t = 600 min Xxyl = 99.5% YFur = 91.5% TOF = 3.36⋅10−3 molFur gcat−1 h−1 | [71] |
| Dehydration | Furfural | From xylose: xylulose | CrPO4 | T = 160 °C Cxyl = 10% w/v in H2O Vtoluene/VH2O = 3 CNaCl = 35% w/w H2O Ccat = 1.5% w/w H2O | t = 60 min Xxyl = 98% YFur = 88% TOF = 0.094 molFur gcat−1 h−1 | [72] |
| Dehydration Polymerization Resinification | Furfural | Humins Furfural polymers | HCl | T = 222 °C Cxyl = 10% w/v in H2O VGVL/VH2O = 4 Ccat = 5 mM Tubular reactor Liquid system | t = 96 s Xxyl = 93% YFur = 82% TOF = 8.57 molFur mmolcat−1 h−1 Maple: t = 30 min Xxyl = 93% YFur = 82% TOF = 0.084 molFur mmolcat−1 h−1 | [73] |
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Dehydratation Isomerization | Furfural | xylulose | Cr-MOF with Sn phosphate nanoparticles SBET = 1000–2820 m2 g−1 Cacid = 0.41–0.62 mmol gcat−1 | T = 150 °C Cxyl = 10% w/w in H2O Vtoluene/VH2O = 7/3 Ccat = 3% w/v H2O CNaCl = 70 ppt | t = 180 min Xxyl = 97% YFur = 95% 100% stable up to 10 cycles TOF = 0.022 molFur gcat−1 h−1 | [74] |
| Dehydratation Polymerization | Furfural | humins | Amberlyts 70 M-20 ZSM-5-30 Cacid = 0.42–4.15 mmol gcat−1 | T = 150 °C Cxyl = 10% w/w in H2O PCO2 = 20 MPa QCO2 = 0.94 g min−1 Ccat = 10% w/v H2O Amberlyst 70 | t = 16 h Xxyl = 91.4% YFur = 50.5% TOF = 1.24 × 10−3 molFur gcat−1 h−1 | [75] |
| Dehydration Polymerization | Furfural | Humins | SO3H-KIT-6 SBET = 265 m2 g−1 Cacid = 0.69–1.53 mmol gcat−1 | T = 170 °C Cxyl = 4% w/v in H2O Ccat = 25% w/w H2O | t = 120 min Xxyl = 97.5% YFur = 94.7% TOF = 1.99 × 10−4 molFur gcat−1 h−1 | [76] |
| Dehydration Polymerization Resinification | Furfural | Humins Anhydroxylose | NbTiO-MNL SBET = 145 m2 g−1 Cacid = 0.69–1.53 mmol gcat−1 | T = 130 °C Cxyl = 20 mM VGVL/VH2O = 9 Wcat = 280 mg | tresidence = 106 s Xxyl = 98% YFur = 29% TOF = 7.26 molFur gcat−1 h−1 | [77] |
| Dehydration Polymerization | Furfural | Humins | FDU and SBA mesoporous catalysts SBET = 500–900 m2 g−1 Cacid = 0.07–0.53 mmol gcat−1 | T = 160 °C PN2 = 2 MPa Cxyl = 5% w/v in H2O Vtoluene/VH2O = 2/1 Ccat = 2.5% w/v H2O FDU-5-7.5E-SO3H | t = 240 min Xxyl = 96.81% YFur = 78.55% TOF = 5.13 × 10−3 molFur gcat−1 h−1 | [78] |
| Dehydration | Furfural | Not indicated | Sulfonated graphitic carbon nitrides SBET = 10–35 m2 g−1 Cacid = 5.47 mmol gcat−1 | T = 100 °C Wxyl = 30 mg in H2O Wcat = 25 mg | t = 30 min Xxyl = 100% YFur = 96% TOF = 5.36 × 10−3 gFur gcat−1 h−1 | [52] |
| Dehydration | Furfural | Not indicated | Sulfonated active carbons (CA) SBET = 620–750 m2 g−1 Cacid = 0.43–0.90 mmol gcat−1 | T = 180 °C Cxyl = 1.4% w/v in H2O Ccat = 0.14% w/w H2O KOH-activated CA | t = 180 min Xxyl = 95% YFur = 60% TOF = 0.0152 molFur gcat−1 h−1 | [79] |
| Dehydration Esterification Reduction Ring opening Translocation Hydrogenation | Furfural | Xylose ethers Furfuryl alcohol Lactones GVL Levulinic acid Hydroxy-pentanoates | Zr-USY zeolites with several Al/Zr ratios SBET = 308–418 m2 g−1 Cacid = 0.137–0.650 mmol gcat−1 | T = 170 °C Cxyl = 1 mol to 50 mol 2-propanol Ccat = 1% w/v Parent H-USY | t = 180 min Xxyl = 100% YFur = 40% TOF = 3.48 × 10−3 molFur gcat−1 h−1 t = 60 min Xxyl = 80% YXylethers = 70% TOF = 8.21 × 10−3 molXylethers gcat−1 h−1 | [80] |
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Dehydratation Reduction | Furfuryl alcohol | Xylulose Xylitol | SBA-15 with sulfonic groups and Pt crystals SBET = 340–614 m2 g−1 Cacid = 0.7–1.3 mmol gcat−1 H+/Pt = 0–28 mmol gcat−1 | T = 130 °C Cxyl = 1.3% w/v Visopropanol/VH2O = 1/1 Ccat = 3% w/v H2O PH2 = 3 MPa | t = 180 min Xxyl = 20% SFur-OH = 83% TOF = 1.68 × 10−4 molFur-OH gcat−1 h−1 | [81] |
| Hydrogenation Dehydration Ring opening | 1,2-Pentanediol | Xylitol 1-hydroxyl-2-pentanone 5-HMF | Ru/C and Niobium phosphate dual catalyst SBET = 226–427 m2 g−1 | T = 150 °C Cxyl = 0.6% w/w Wcyclohexane/WH2O-GVL = 1/1 Ccat-acid = 0.6% w/w Ccat-hydrog = 0.15% w/w PH2 = 3 MPa | t = 240 min Xxyl = 75% S1,2PD = 18% Sxylitol = 23% TOF = 2.41 × 10−4 mol1,2PD gcat−1 h−1 | [82] |
| Hydrogenation Dehydration Ring opening | Xylitol | Xylulose Glycerol Ethyleneglycol | Ni-metal catalyst from mixed oxide precursors with Ce and Ni SBET = 2 m2 g−1 | T = 100 °C Cxyl = 6% w/w Ccat = 0.12% w/w PH2 = 2.5 MPa | t = 240 min Xxyl = 60% Yxylitol = 25% Ybyproducts = 15% TOF = 1.35 × 10−3 molxylitolgcat−1 h−1 | [83] |
| Retro-aldol condensation Dehydration | Lactic acid | Furfural | Lanthanum triflate | T = 250 °C Cxyl = 1% w/w Ccat = 14% molar | t = 60 min Xxyl = 60% Ylactic acid = 61% | [85] |
| Retro-aldol condensation Esterification Isomerization | Methyl lactate | Methyl-xylopyranoside Xylulose Glyceraldehyde 1,3-dihydroxy acetone | LaCl3 Several metal chlorides tested | T = 170 °C Cxyl = 0.6 M Ccat = 30 mM metal ion | t = 360 min Xxyl = 92% YMLA = 33% TOF = 1.84 molMLA molcat−1 h−1 | [86] |
| Oxidation Decarboxylation | Xylaric acid | Xylonic acid Tartaric acid Oxalic acid | Pt/C Precious metals on C tested | T = 60 °C Cxyl = 5% w/w in H2O Ccat = 0.15% w/w | t = 600 min Xxyl = 99% YXylAcid = 43% TOF = 0.0123 molXylAc gcat−1 h−1 | [87] |
| Esterification Dehydration | Trans-2,5-dihydroxy-3-pentenoic acid methyl ester (DPME) | Furfural Other methyl esters | Sn-beta zeolite CSn = 1.25–1.5% w/w | T = 160 °C Cxyl = 8.3% w/w in H2O Ccat = 4.1% w/w | t = 120 min Xxyl = 68% YDPM = 33% TOF = 6.78 × 10−5 molDPME gcat−1 h−1 | [88] |
| Cyclocarbona-tation Addition | Thionocarbo-nate xanthate monomers | Not reported | DBU Et3N | T = 0–25 °C | t = 1 + 12 h Xxyl = 10% Yxanthate = 10% Ythionocarbonate = 15–48% | [89] |
| Reaction | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Epimerization Hydrogenation | Mannitol | Sorbitol | Cs-HPA (Ep) Ru/C (Hyd) SBET = 581–629 m2 g−1 Ru = 5% w/w Metal = 1% w/w | T = 90 °C Cxyl = 1.3% w/v Qliquid = 0.5 mL/min QH2 = 50 mL/min Ccat = 20% v/v FBR PH2 = 1 MPa | Time on stream = 24 h Residence time = 50–60 s Xxyl = 66% SFur-OH = 83% TOF = 0.0118 molDPM gcat−1 h−1 | [39] |
| Dehydratation Polymerization | 5-HMF | Humins | AlCl3⋅6H2O | T = 140 °C Cman = 6% w/v Wcyclohexane/WH2O-GVL = 1/1 Ccat = 10% mol/mol mannose | t = 30 min Xman = 95% YHMF = 60% TOF = 7.33 molHMF molcat−1 h−1 | [90] |
| Epimerization Isomerization Retro aldol condensation | Tetroses and C4-synthons | Xylulose Glycerol Ethyleneglycol | Ammonium tungstate (AT) | T = 190 °C Cman = 1% w/w H2O WAT = 4 g PO2 = 1.7 MPa pH = 10 | t = 10 s MW Xxyl = 46% Yerythrulose = 8% Yerythrose = 38% TOF = 0.013 molerythrose gcat−1 h−1 | [42] |
| Hydrogenation | Dideoxy glycoside | Non-totally reduced species | RuOx-Pd/CeO2 Pd/Re: ¼ Re = 2% w/w | T = 150 °C Cman = 2.5% w/v PH2 = 8 Mpa Ccat = 1–1.5% w/v | t = 51 h Xman = 99% Ydideoxyglycoside = 96% TOF = 5.84 × 10−4 moldideoxyglycoside gcat−1 h−1 | [90,91] |
| Reaction and Biomass Source | Product | Other Products | Catalyst | Reaction Conditions | Main Results | Reference |
|---|---|---|---|---|---|---|
| Acid hydrolysis Nanochloropsis salina | Monosaccharides | Formic acid Levulinic acid 5-HMF Furfural | HCl H2SO4 | T = 90 °C Cbiomass = 10% w/v in 10 mL H2O Ccat = 10% w/w | t = 60 min Ymal = 90% TOF = 0.6 gmalt molcat−1 h−1 | [95] |
| Acid hydrolysis Chlorella vulgaris | Malodextrin | H2SO4 | T = 90 °C Ccarboh = 37.3% Ccat = 0.56 M | t = 5 h Ymonosac = 243 mg/g TOF = 0.052 molsugar gcat−1 h−1 | [96] | |
| Acid hydrolysis Chlorella sp. and Nanochloropsis gaditana | Levulinic ester | H2SO4 | T = 130 °C Cbiomass = 38 g/L alcohol Cglucose = 6.8–28.1% w/w Ccat = 15% w/v | t = 2 h YLevulinate = 40% TOF = 0.0252 mollev molcat−1 h−1 | [97] | |
| Chemo-enzymatic hydrolysis Dunaliella tertiolecta | Glucose | H2SO4 α-amylase and α-glucosidase | T = 50–90 °C Cpolyssac = 0.1–1 g/20–100 mL Ccat = 0.5–1.5% Acid catalyst T = 37 °C | t = 24 h YMF = 90% TOF = 0.136 molgluc molcat−1 h−1 | [98] | |
| Chemo-enzymatic hydrolysis Dunaliella tertiolecta | Glucose | Ethanol | HCl and H2SO4 Saccharomyces cerevisiae | T = 121 °C Cbiomass = 5% w/v Ccat = 0.05–1M Acid catalyst Cenzyme = 0.1–1.0 mL/g T = 35–55 °C pH = 3.5–6.5 | t = 15 min Ysugar = 42.0% Csugar = 21 mg/mL Cethanol = 0.44 g/g glucose TOF = 0.467 molglu molcat−1 h−1 | [99] |
| Chemo-enzymatic hydrolysis Gracilaria verrucosa | Glucose Galactose 3,6-anhydro Galactose | Levilinic acid 5-HMF Ethanol | HCl H2SO4 Cellic Ctec2 | T = 125 °C Cbiomass = 2% w/w Ccat = 0.01–1.5 N Acid catalyst Cenzyme = 150 FPU/mL T = 50 °C pH = 5 | t = 60 min Xcarbohyd = 57.2% Ysugar = 21.3–37.4% TOF = 0.039 molmonosac molcat−1 h−1 | [100] |
| Chemo-enzymatic hydrolysis Scenedesmus sp. | Monosaccharides | Ethanol | HCl Viscozyme L | T = 121 °C Cbiomass = 2% w/v Ccat = 0.5 M Acid catalyst Cenzyme = 20 FBGU/g biomass T = 45 °C pH = 5.5 | t = 45 min Ysugar = 37.9% (HCl) TOF = 0.098 molmonosac molcat−1 h−1 t = 72 h Ysugar = 43.4% (Enz.) | [101] |
| Enzymatic hydrolysis Great Salt lake USU080 | Lactic acid | Lactobacillus casei 12A | T = 37 °C Cbiomass = 15% w/v Cenzyme = 1% v/v 200–250 rpm | t = 3–24 h Ylactic = 11.7g/L | [102] | |
| Enzymatic hydrolysis Nanochloropsis oceanic | 2,3-Butanediol | Klebsiella oxytoca | T = 37 °C Cbiomass = 15% w/v Csugar = 5 g/L 150 rpm | t = 6–7 h Y2,3-BDO = 0.31 g/g sugars 0.0031 mol2,3-BDO L−1 h−1 | [103] | |
| Enzymatic hydrolysis Scenedesmus obliquus | Monosaccharides (Glucose and xylose) | Organic acids | Celluclast1.5L Novozyme 188 Alkaline-peroxide pretreatment | T = 50 °C Cbiomass = 6% w/w Cenzymes = 10FPU/g and 20CBU/g pH = 4.9 300 rpm | t = 6–7 h Ysugrs = 0.098 g/g biomass | [104] |
| Catalytic-hydrothermal process Kappaphycus alvarezzi | Glucose Galactose | Levulinic acid 5-HMF Furfural | H2SO4 | T = 160–175 °C Cbiomass = 2 g/30 mL Ccat = 1% w/w | t = 20 min Ymonosac = 14.5 g/L TOF = 0.364 molmonosac gcat−1 h−1 | [105] |
| Catalytic-hydrothermal process Enteromorpha intestinalis | Glucose Galactose Xylose Mannose | Levulinic acid 5-HMF Furfural | H2SO4 | T = 156 °C Ccat = 1.3% w/w | t = 11 min Ymonosac = 28.6% | [106] |
| Catalytic-hydrothermal process Alginate from macroalgae and cellulose | H+ medium: Furfural, Mannuronic, Guluronic acids -OH medium: Lactic, Fumaric, Malic acids | HCl NaOH | T = 150 °C Calginate = 20 g/L pH = 1–13 | t = 30 min Ymonomers = 43% in acid medium TOF = 0.955 molmonom molcat−1 h−1 | [107] | |
| Catalytic-hydrothermal liquefaction process Chlorella vulgaris | Bi-oil Cyclic ketones Lactones Furans Phenols | H2SO4 CH3CO2H | T = 220–330 °C Cbiomass = 100 g/L in H2O Ccat = 0.01–0.1 mol/L | t = 30 min Yproducts = 16% | [108] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban, J.; Yustos, P.; Ladero, M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts 2018, 8, 637. https://doi.org/10.3390/catal8120637
Esteban J, Yustos P, Ladero M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts. 2018; 8(12):637. https://doi.org/10.3390/catal8120637
Chicago/Turabian StyleEsteban, Jesús, Pedro Yustos, and Miguel Ladero. 2018. "Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview" Catalysts 8, no. 12: 637. https://doi.org/10.3390/catal8120637
APA StyleEsteban, J., Yustos, P., & Ladero, M. (2018). Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts, 8(12), 637. https://doi.org/10.3390/catal8120637







