Insights into the Recent Progress and Advanced Materials for Photocatalytic Nitrogen Fixation for Ammonia (NH3) Production
Abstract
1. Introduction
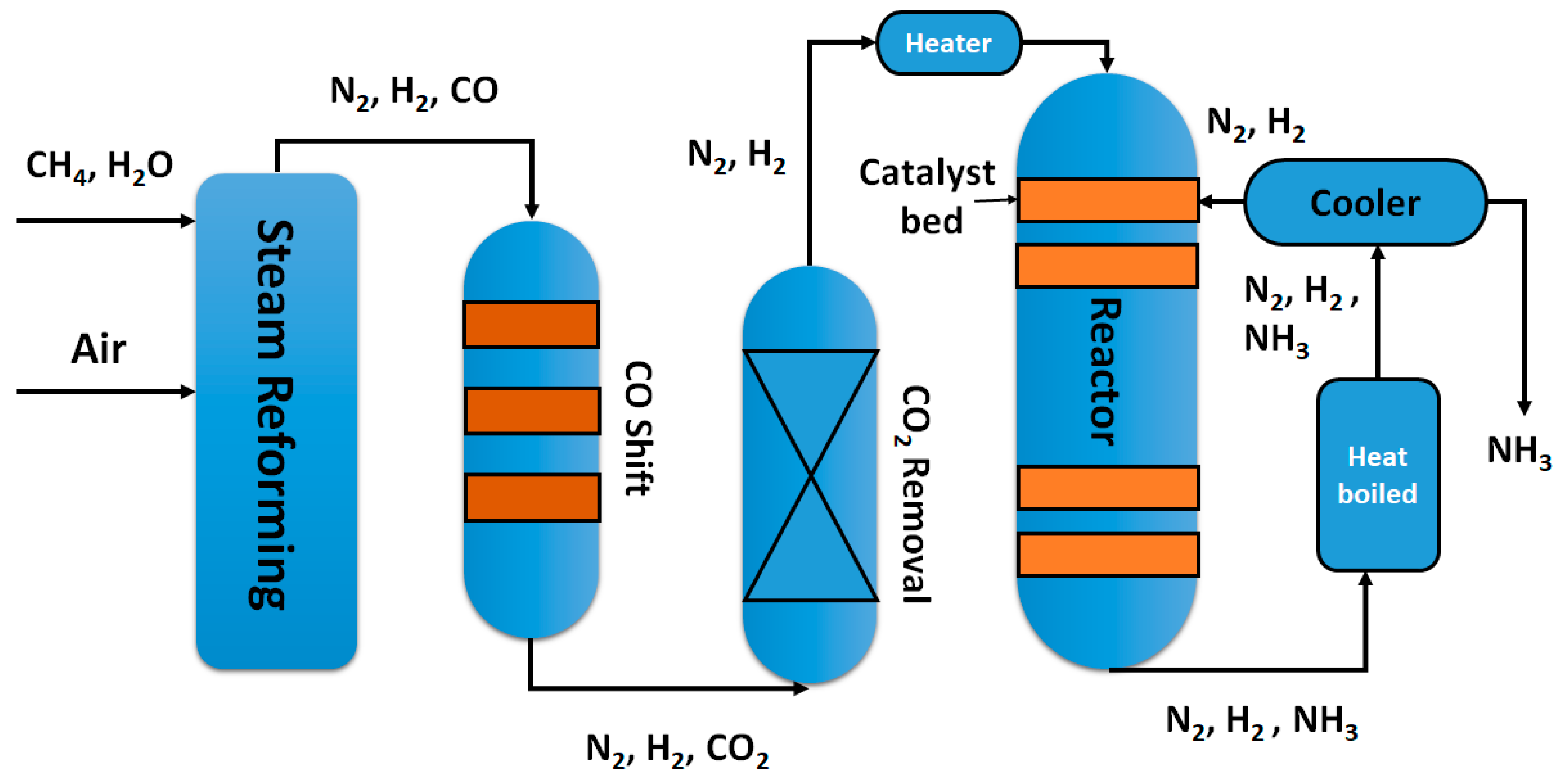
2. A Brief Insight into the Haber-Bosch Process
2.1. Equilibrium Considerations and Reaction Rate
2.2. Catalyst and Mechanism
2.3. Separation of the Ammonia
3. Overview: Fundamental of Photocatalytic Nitrogen Fixation
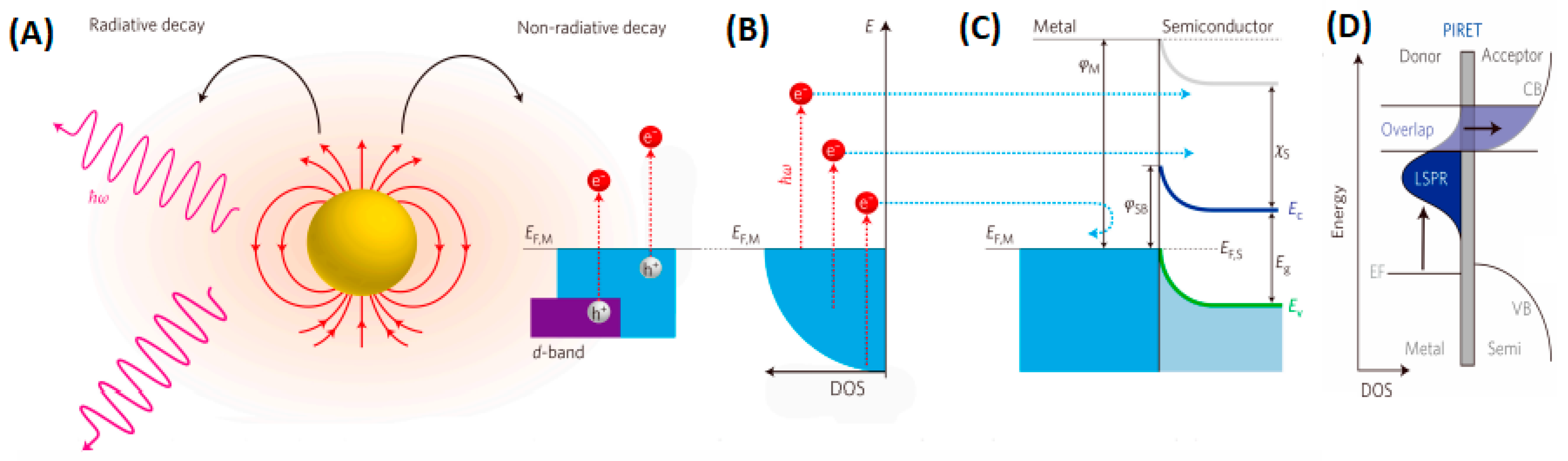
3.1. The Principle of Photocatalysis on Semiconductors
3.2. Quantum Yield (QY)
3.3. Materials for Photocatalysis
3.4. Co-Catalyst Loading
3.5. Localized Surface Plasmon Resonance in Photocatalysis
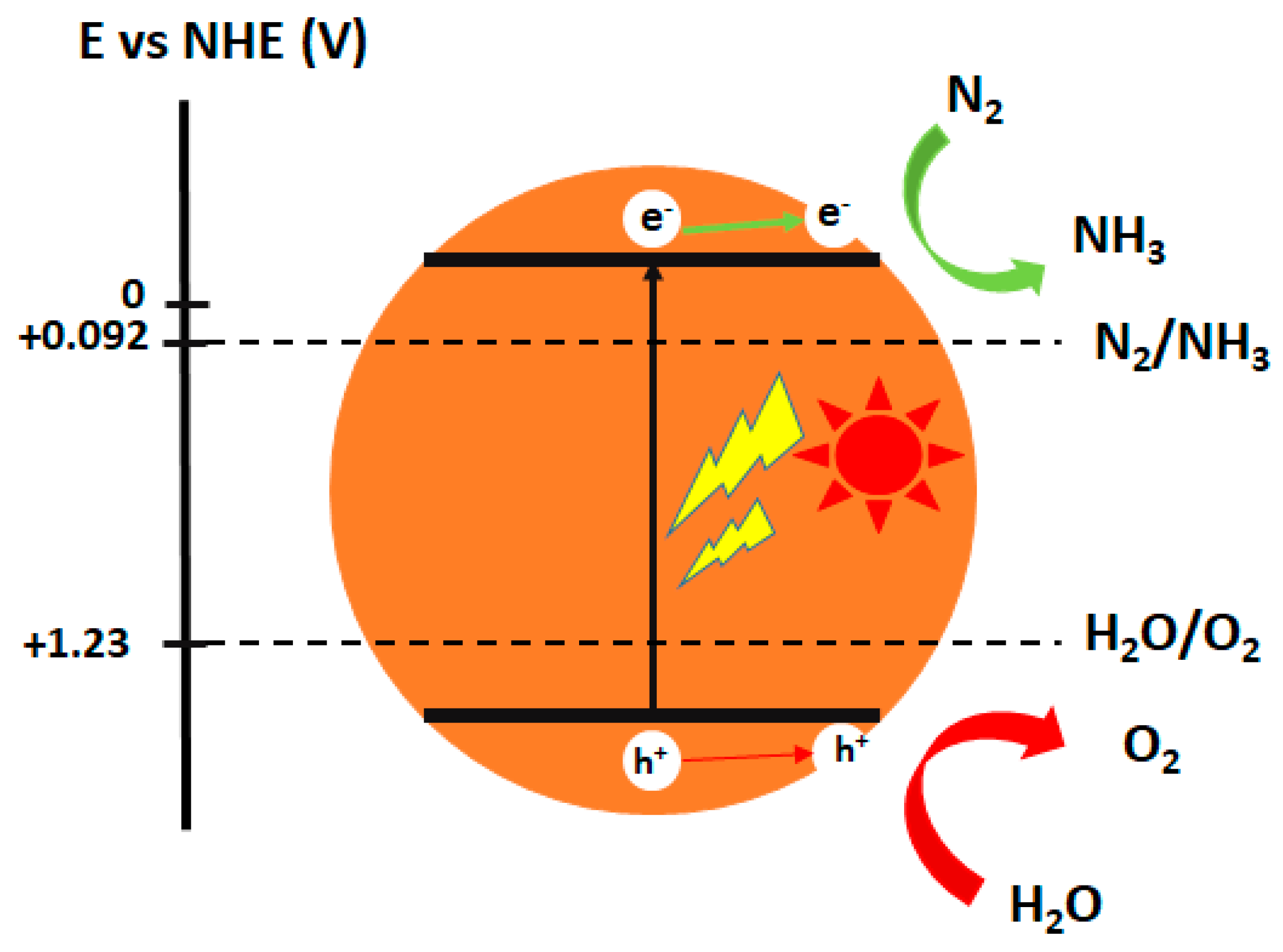
3.6. Fundamentals of Photocatalytic Nitrogen Fixation Principle
4. Classification of Photocatalysts for N2 Fixation Based on Active Sites
4.1. Metal Active Sites
4.1.1. Iron Active Sites
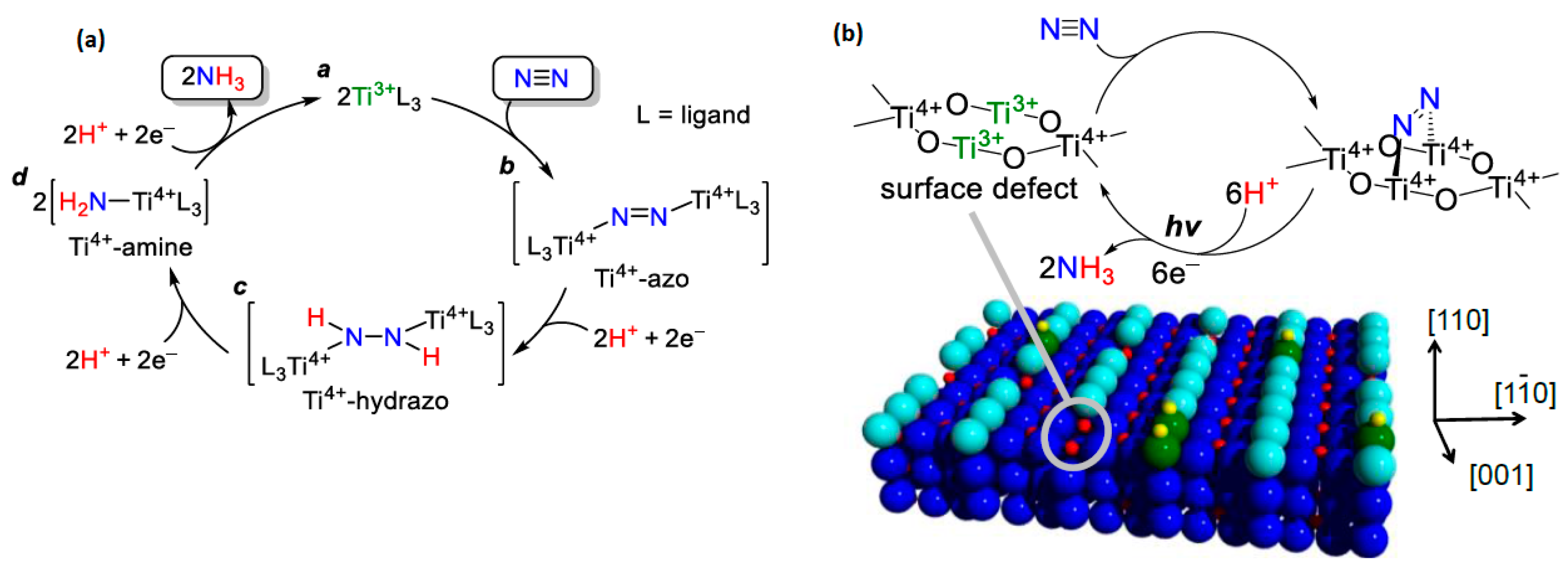
4.1.2. Titanium Active Sites
4.1.3. Molybdenum Active Sites
4.1.4. Nickel Active Sites
4.2. Non-Metal Vacancies
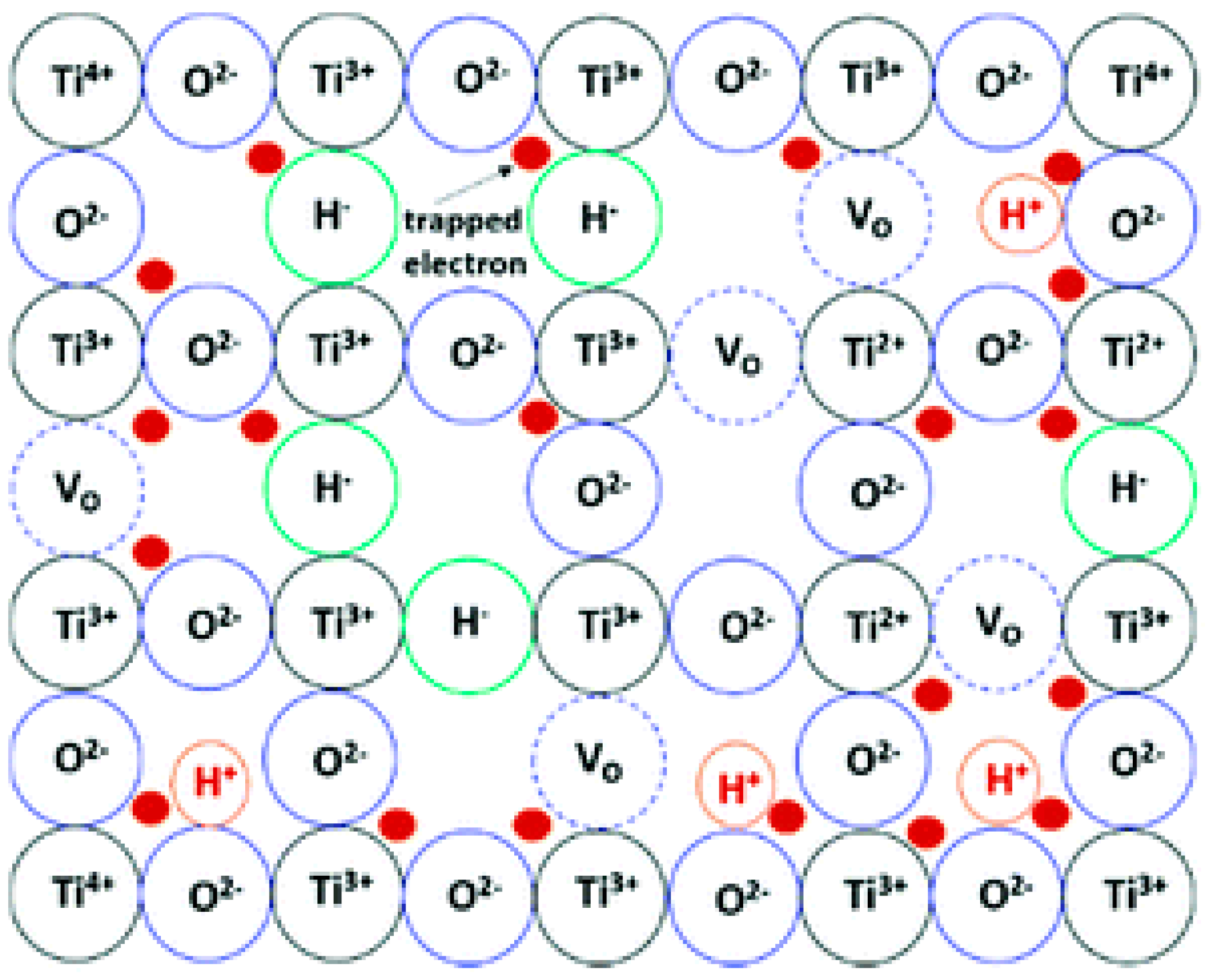
4.2.1. Oxygen Vacancies
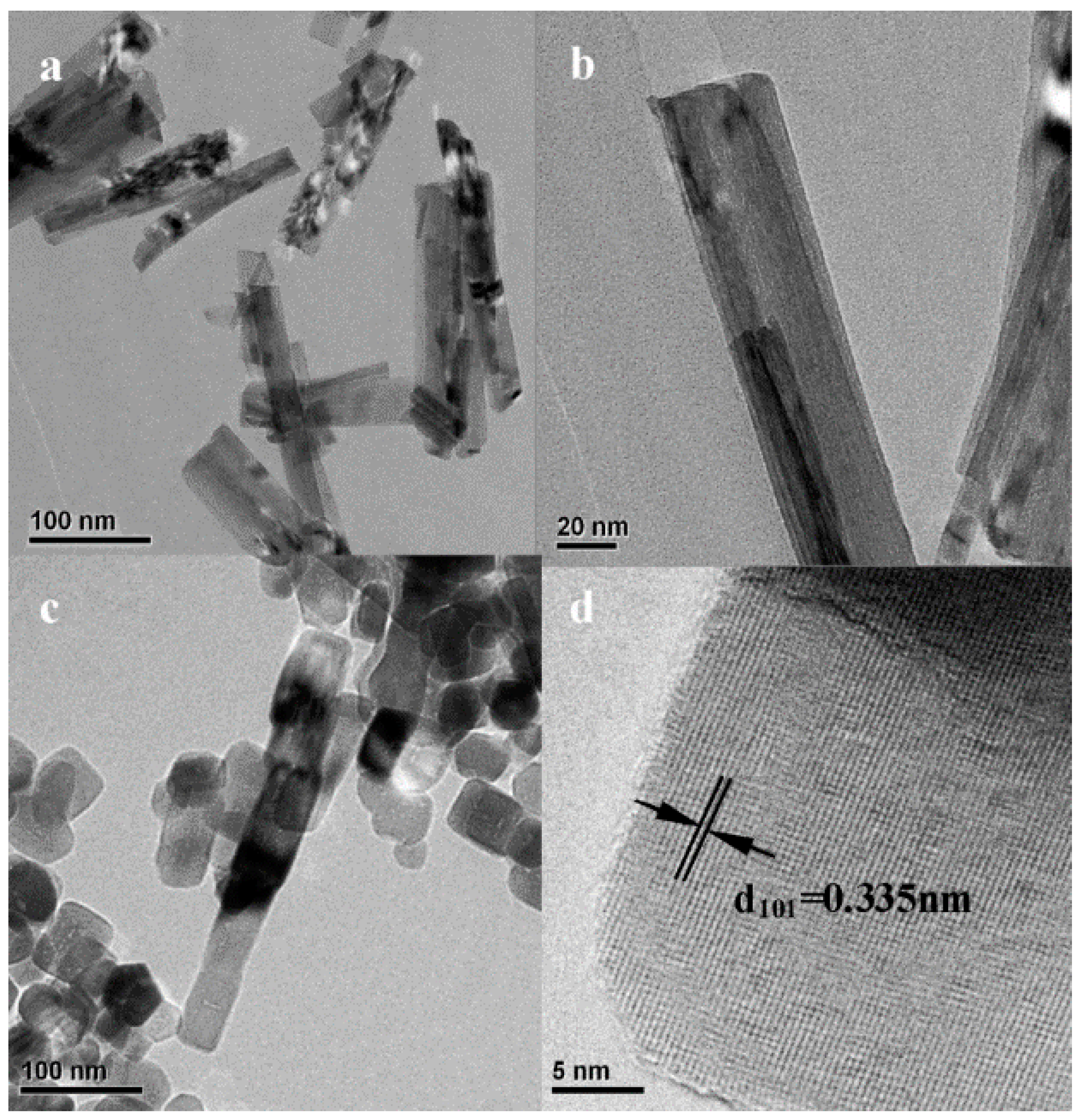
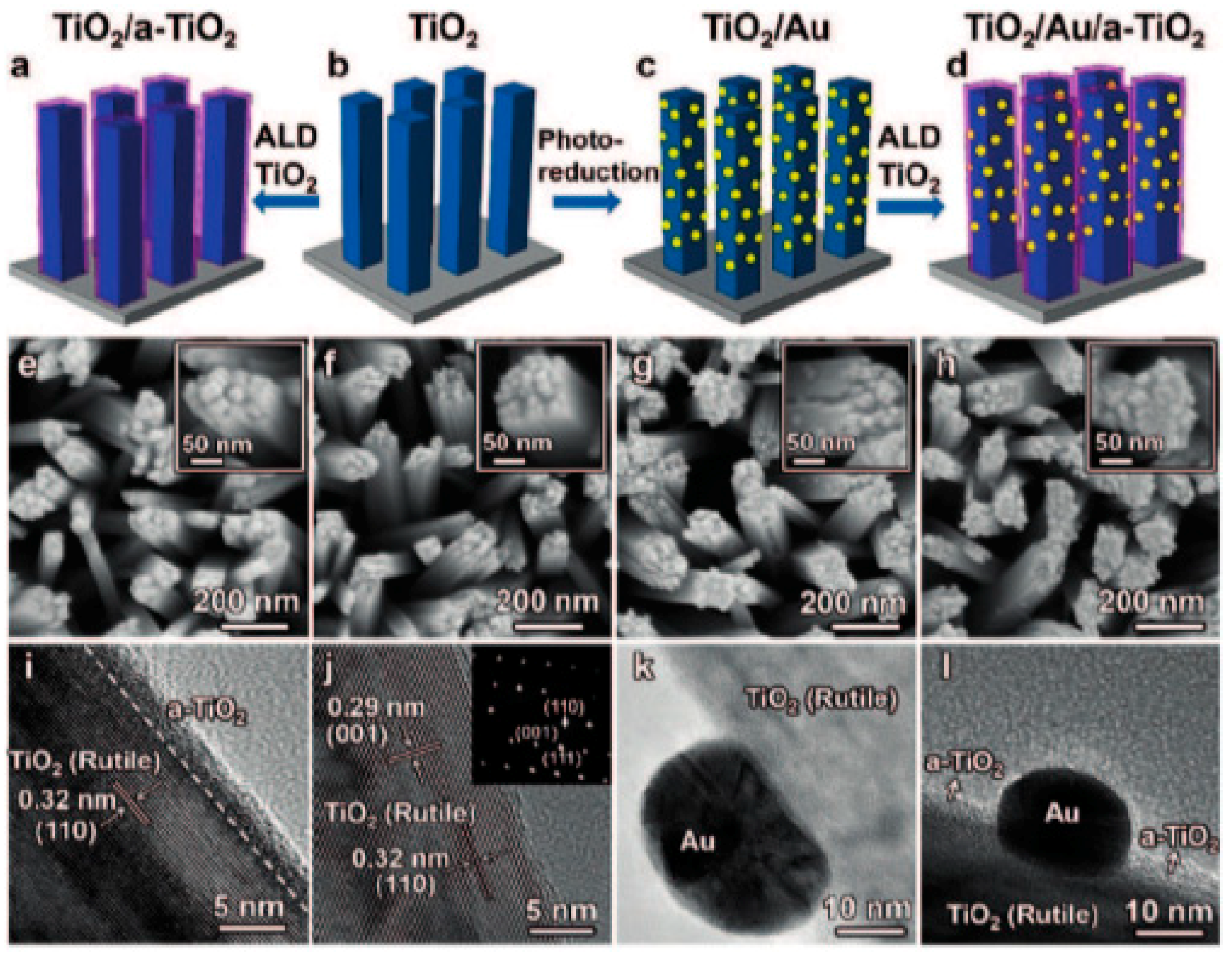
Oxygen Vacancies Based on Titanium Dioxide
Oxygen Vacancies Based on Bismuth Oxyhalide
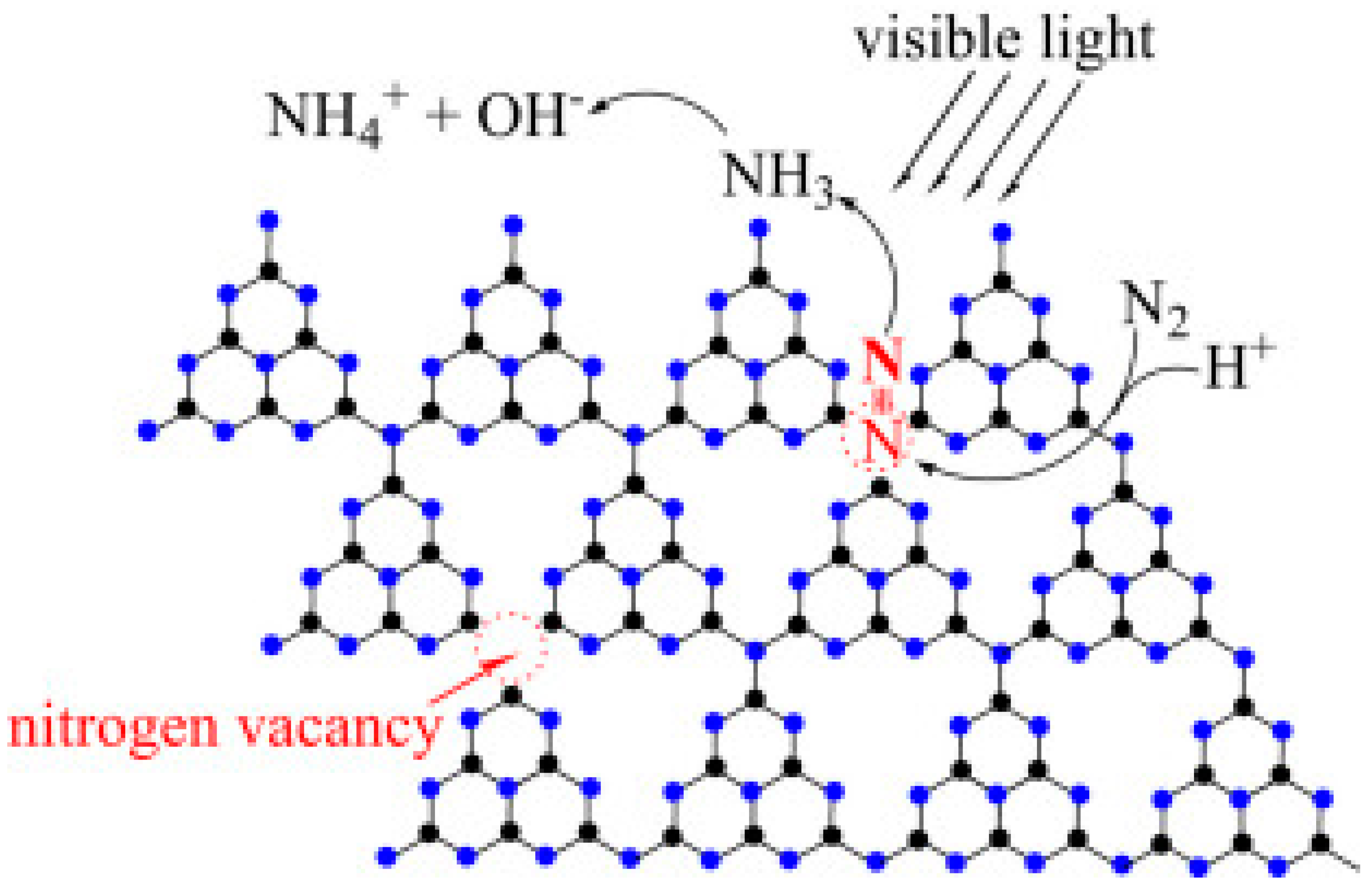
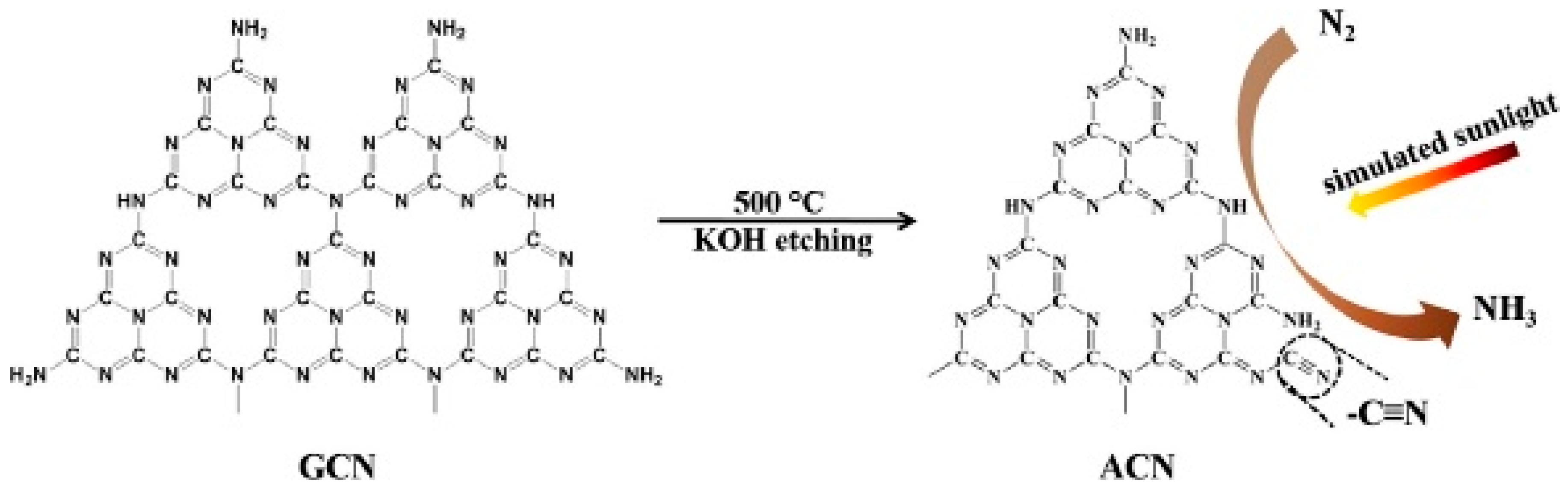
4.2.2. Nitrogen Vacancies
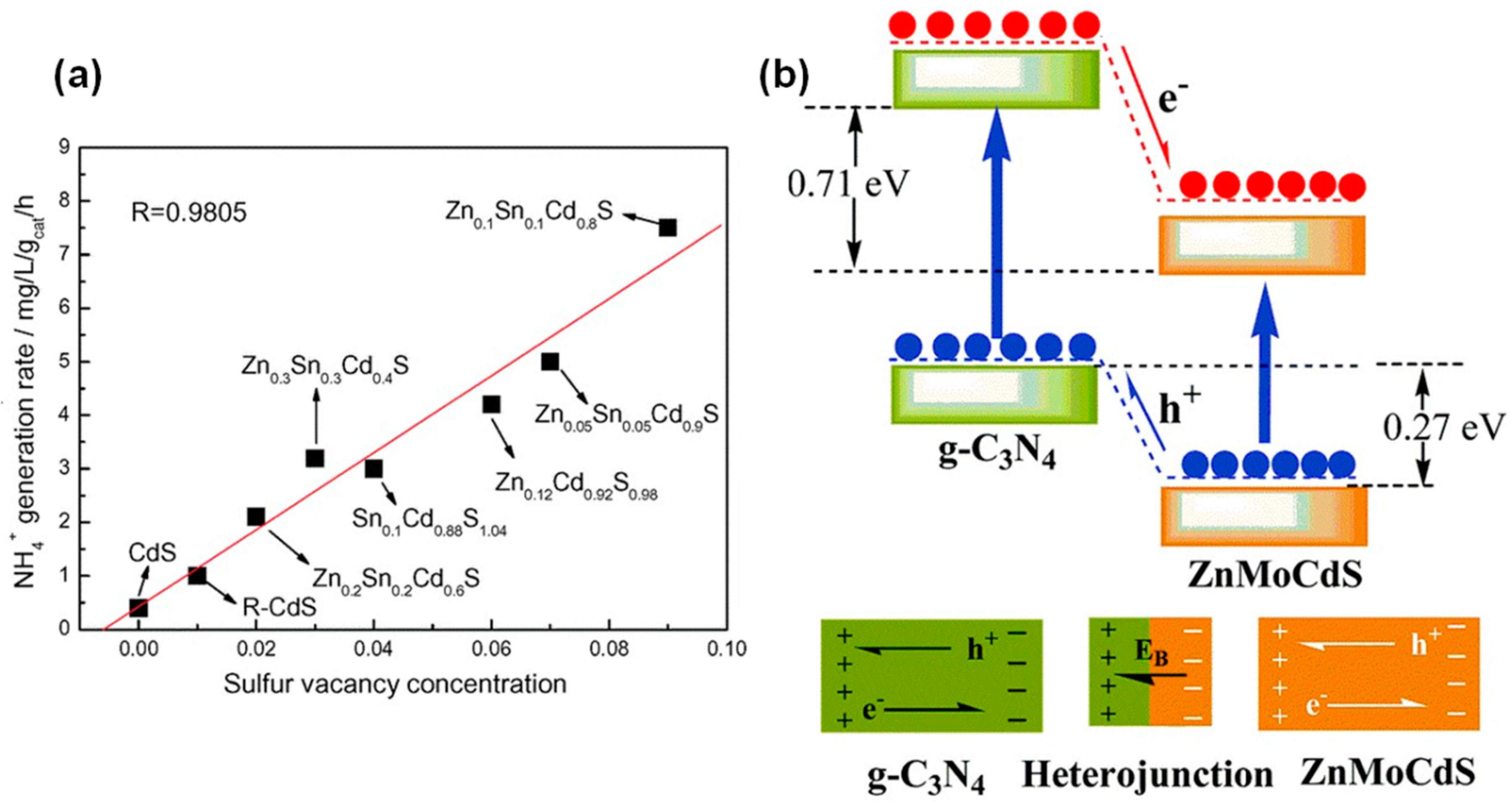
4.2.3. Sulfur Vacancies
4.3. Metal Cocatalyst and Plasmon Enhancement
4.3.1. Metal Cocatalyst
4.3.2. Plasmon Enhancement
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Service, R.F. New recipe produces ammonia from air, water, and sunlight. Science 2014, 345, 610. [Google Scholar] [CrossRef] [PubMed]
- Licht, S.; Cui, B.; Wang, B.; Li, F.-F.; Lau, J.; Liu, S. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 2014, 345, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Datta, R.S.; Ou, J.Z.; Mohiuddin, M.; Carey, B.J.; Zhang, B.Y.; Khan, H.; Syed, N.; Zavabeti, A.; Haque, F.; Daeneke, T.; et al. Two dimensional PbMoO4: A photocatalytic material derived from a naturally non-layered crystal. Nano Energy 2018, 49, 237–246. [Google Scholar]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar]
- Kisch, H. Semiconductor Photocatalysis-Mechanistic and Synthetic Aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Furube, A.; Asahi, T.; Masuhara, H.; Yamashita, H.; Anpo, M. Charge Carrier Dynamics of Standard TiO2 Catalysts Revealed by Femtosecond Diffuse Reflectance Spectroscopy. J. Phys. Chem. B 1999, 103, 3120–3127. [Google Scholar] [CrossRef]
- Habisreutinger Severin, N.; Schmidt-Mende, L.; Stolarczyk Jacek, K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Schiavello, M.; Augugliaro, V.; Palmisano, L. An experimental method for the determination of the photon flow reflected and absorbed by aqueous dispersions containing polycrystalline solids in heterogeneous photocatalysis. J. Catal. 1991, 127, 332–341. [Google Scholar] [CrossRef]
- Kisch, H.; Bahnemann, D. Best Practice in Photocatalysis: Comparing Rates or Apparent Quantum Yields? J. Phys. Chem. Lett. 2015, 6, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Buriak, J.M.; Kamat, P.V.; Schanze, K.S. Best Practices for Reporting on Heterogeneous Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 11815–11816. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L. A Novel Method for the Preparation of a Highly Stable and Active CdS Photocatalyst with a Special Surface Nanostructure. J. Phys. Chem. B 2006, 110, 11139–11145. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, J.Z.; Hong, J.; Li, H.Y.; Chen, H.Z.; Wang, M. Carbon Nanotube/CdS Core–Shell Nanowires Prepared by a Simple Room-Temperature Chemical Reduction Method. Adv. Mater. 2004, 16, 84–87. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Lu, G.Q.; Cheng, H.-M. Stable photocatalytic hydrogen evolution from water over ZnO–CdS core–shell nanorods. Int. J. Hydrog. Energy 2010, 35, 8199–8205. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Chen, Z.-G.; Li, F.; Wang, L.; Lu, G.Q.; Cheng, H.-M. Enhanced photocatalytic hydrogen evolution by prolonging the lifetime of carriers in ZnO/CdS heterostructures. Chem. Commun. 2009, 23, 3452–3454. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Kan, W.; Jiao, S.; Zhu, H.; Kumar, R.V. Efficient visible-light-driven photocatalytic hydrogen production using CdS@TaON core-shell composites coupled with graphene oxide nanosheets. J. Mater. Chem. 2012, 22, 7291–7299. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Majumder, S.A. Optimization of p-silicon surface by etching and electrodeposition of Pt and Ni for photosplitting of water. Int. J. Hydrog. Energy 1989, 14, 653–660. [Google Scholar] [CrossRef]
- Akikusa, J.; Khan, S.U.M. Photoelectrolysis of water to hydrogen in p-SiC/Pt and p-SiC/ n-TiO2 cells. Int. J. Hydrog. Energy 2002, 27, 863–870. [Google Scholar] [CrossRef]
- Gurunathan, K. Photocatalytic hydrogen production using transition metal ions-doped γ-Bi2O3 semiconductor particles. Int. J. Hydrog. Energy 2004, 29, 933–940. [Google Scholar] [CrossRef]
- Jang, J.S.; Yoon, K.Y.; Xiao, X.; Fan, F.-R.F.; Bard, A.J. Development of a Potential Fe2O3-Based Photocatalyst Thin Film for Water Oxidation by Scanning Electrochemical Microscopy: Effects of Ag−Fe2O3 Nanocomposite and Sn Doping. Chem. Mater. 2009, 21, 4803–4810. [Google Scholar] [CrossRef]
- Jang, J.S.; Choi, S.H.; Kim, H.G.; Lee, J.S. Location and State of Pt in Platinized CdS/TiO2 Photocatalysts for Hydrogen Production from Water under Visible Light. J. Phys. Chem. C 2008, 112, 17200–17205. [Google Scholar] [CrossRef]
- Maeda, K.; Xiong, A.; Yoshinaga, T.; Ikeda, T.; Sakamoto, N.; Hisatomi, T.; Takashima, M.; Lu, D.; Kanehara, M.; Setoyama, T.; et al. Photocatalytic Overall Water Splitting Promoted by Two Different Cocatalysts for Hydrogen and Oxygen Evolution under Visible Light. Angew. Chem. Int. Ed. 2010, 49, 4096–4099. [Google Scholar] [CrossRef] [PubMed]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95. [Google Scholar] [CrossRef]
- Ma, X.-C.; Dai, Y.; Yu, L.; Huang, B.-B. Energy transfer in plasmonic photocatalytic composites. Light Sci. Appl. 2016, 5, e16017. [Google Scholar] [CrossRef]
- Mubeen, S.; Hernandez-Sosa, G.; Moses, D.; Lee, J.; Moskovits, M. Plasmonic Photosensitization of a Wide Band Gap Semiconductor: Converting Plasmons to Charge Carriers. Nano Lett. 2011, 11, 5548–5552. [Google Scholar] [CrossRef]
- Fan, W.; Leung, M. Recent Development of Plasmonic Resonance-Based Photocatalysis and Photovoltaics for Solar Utilization. Molecules 2016, 21, 180. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic Activity Enhanced by Plasmonic Resonant Energy Transfer from Metal to Semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient Visible Light Nitrogen Fixation with BiOBr Nanosheets of Oxygen Vacancies on the Exposed {001} Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-J.; Chen, J.-J.; Lin, Z.-Q.; Li, W.-W.; Sheng, G.-P.; Yu, H.-Q. Nitrate formation from atmospheric nitrogen and oxygen photocatalysed by nano-sized titanium dioxide. Nat. Commun. 2013, 4, 2249. [Google Scholar] [CrossRef] [PubMed]
- Bozso, F.; Ertl, G.; Grunze, M.; Weiss, M. Interaction of nitrogen with iron surfaces: I. Fe(100) and Fe(111). J. Catal. 1977, 49, 18–41. [Google Scholar] [CrossRef]
- Tennakone, K.; Wickramanayake, S.; Fernando, C.A.N.; Ileperuma, O.A.; Punchihewa, S. Photocatalytic nitrogen reduction using visible light. J. Chem. Soc. Chem. Commun. 1987, 14, 1078–1080. [Google Scholar] [CrossRef]
- Khader, M.M.; Lichtin, N.N.; Vurens, G.H.; Salmeron, M.; Somorjai, G.A. Photoassisted catalytic dissociation of water and reduction of nitrogen to ammonia on partially reduced ferric oxide. Langmuir 1987, 3, 303–304. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Zhu, X.; Zhang, M.; Tang, J.; Tan, M.; Wang, Y. Enhanced nitrogen photofixation on Fe-doped TiO2 with highly exposed (101) facets in the presence of ethanol as scavenger. Appl. Catal. B Environ. 2014, 144, 468–477. [Google Scholar] [CrossRef]
- Soria, J.; Conesa, J.C.; Augugliaro, V.; Palmisano, L.; Schiavello, M.; Sclafani, A. Dinitrogen photoreduction to ammonia over titanium dioxide powders doped with ferric ions. J. Phys. Chem. 1991, 95, 274–282. [Google Scholar] [CrossRef]
- Rusina, O.; Linnik, O.; Eremenko, A.; Kisch, H. Nitrogen Photofixation on Nanostructured Iron Titanate Films. Chem. A Eur. J. 2003, 9, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Linnik, O.; Kisch, H. On the mechanism of nitrogen photofixation at nanostructured iron titanate films. Photochem. Photobiol. Sci. 2006, 5, 938–942. [Google Scholar] [CrossRef]
- Lashgari, M.; Zeinalkhani, P. Photocatalytic N2 conversion to ammonia using efficient nanostructured solar-energy-materials in aqueous media: A novel hydrogenation strategy and basic understanding of the phenomenon. Appl. Catal. A General 2017, 529, 91–97. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Li, Q.; Li, F.; Fan, Z.; Wang, H.; Wang, Y.; Zheng, B.; Wu, G. Fe3+ doping promoted N2 photofixation ability of honeycombed graphitic carbon nitride: The experimental and density functional theory simulation analysis. Appl. Catal. B Environ. 2017, 201, 58–69. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Photocatalytic reactions. 1. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Tennakone, K.; Bandara, J.M.S.; Thaminimulla, C.T.K.; Jayatilake, W.D.W.; Ketipearachchi, U.S.; Ileperuma, O.A.; Priyadarshana, M.K.A. Photoreduction of dinitrogen to ammonia by ultrafine particles of iron hydroxide oxide (Fe(O)OH) formed by photohydrolysis of iron(II) bicarbonate. Langmuir 1991, 7, 2166–2168. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Zhang, T.; Ge, Z.; Chang, H.; Xiao, P.; Xie, Y.; Hua, L.; Li, Q.; Li, H.; et al. Photoprompted Hot Electrons from Bulk Cross-Linked Graphene Materials and Their Efficient Catalysis for Atmospheric Ammonia Synthesis. ACS Nano 2016, 10, 10507–10515. [Google Scholar] [CrossRef] [PubMed]
- Tennakone, K.; Fernando, C.A.N.; Wickramanayake, S.; Damayanthi, M.W.P.; Silva, L.H.K.; Wijeratne, W.; Illeperuma, O.A.; Punchihewa, S. Photocatalytic reduction of nitrogen to ammonia with coprecipitated Fe(III) and Ti(IV) hydrous oxides. Sol. Energy Mater. 1988, 17, 47–53. [Google Scholar] [CrossRef]
- Ileperuma, O.A.; Kiridena, W.C.B.; Dissanayake, W.D.D. Photoreduction of nitrogen and water on montmorillonite clays loaded with hydrous ferric oxide. J. Photochem. Photobiol. A Chem. 1991, 59, 191–197. [Google Scholar] [CrossRef]
- Baumann, R.; Stumpf, R.; Davis, W.M.; Liang, L.-C.; Schrock, R.R. Titanium and Zirconium Complexes That Contain the Tridentate Diamido Ligands [(i-PrN-o-C6H4)2O]2-([i-PrNON]2-) and [(C6H11N-o-C6H4)2O]2-([CyNON]2-). J. Am. Chem. Soc. 1999, 121, 7822–7836. [Google Scholar] [CrossRef]
- Van Tamelen, E.E.; Fechter, R.B.; Schneller, S.W. Conversion of molecular nitrogen to hydrazine. J. Am. Chem. Soc. 1969, 91, 7196. [Google Scholar] [CrossRef]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic Conversion of Nitrogen to Ammonia with Water on Surface Oxygen Vacancies of Titanium Dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Wang, W.; Zhang, L.; Sun, X. Photocatalytic robust solar energy reduction of dinitrogen to ammonia on ultrathin MoS2. Appl. Catal. B Environ. 2017, 200, 323–329. [Google Scholar] [CrossRef]
- Banerjee, A.; Yuhas, B.D.; Margulies, E.A.; Zhang, Y.; Shim, Y.; Wasielewski, M.R.; Kanatzidis, M.G. Photochemical Nitrogen Conversion to Ammonia in Ambient Conditions with FeMoS-Chalcogels. J. Am. Chem. Soc. 2015, 137, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kelley, M.S.; Wu, W.; Banerjee, A.; Douvalis, A.P.; Wu, J.; Zhang, Y.; Schatz, G.C.; Kanatzidis, M.G. Nitrogenase-mimic iron-containing chalcogels for photochemical reduction of dinitrogen to ammonia. Proc. Natl. Acad. Sci. USA 2016, 113, 5530–5535. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.-Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Han, C.; Ma, Z.; Leng, Y.; Li, J.; Ji, X.; Bi, D.; Xie, H.; Huang, Z. Ni2P loading on Cd0.5Zn0.5S solid solution for exceptional photocatalytic nitrogen fixation under visible light. Chem. Eng. J. 2017, 307, 311–318. [Google Scholar] [CrossRef]
- Wang, S.; Hai, X.; Ding, X.; Chang, K.; Xiang, Y.; Meng, X.; Yang, Z.; Chen, H.; Ye, J. Light-Switchable Oxygen Vacancies in Ultrafine Bi5O7Br Nanotubes for Boosting Solar-Driven Nitrogen Fixation in Pure Water. Adv. Mater. 2017, 29, 1701774. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhao, Z.J.; Yang, W.; Li, J.F.; Li, A.; Yang, Z.; Ozin Geoffrey, A.; Gong, J. Promoted Fixation of Molecular Nitrogen with Surface Oxygen Vacancies on Plasmon-Enhanced TiO2 Photoelectrodes. Angew. Chem. Int. Ed. 2018, 57, 5278–5282. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; An, Q.; Wang, W.; Zhang, L.; Liu, J.; Goddard Iii, W.A. Efficient photocatalytic reduction of dinitrogen to ammonia on bismuth monoxide quantum dots. J. Mater. Chem. A 2017, 5, 201–209. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Zou, J. Nitrogen reduction utilizing solvated electrons produced by thermal excitation of trapped electrons in reduced titanium oxide. New J. Chem. 2018, 42, 6084–6090. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Shi, J.; Zhao, K.; Zhang, L. Facet-dependent solar ammonia synthesis of BiOCl nanosheets via a proton-assisted electron transfer pathway. Nanoscale 2016, 8, 1986–1993. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Chen, T.; Wang, L.; Shi, X.; Zhang, X.; Chen, D. Facet-Dependent Photocatalytic N2 Fixation of Bismuth-Rich Bi5O7I Nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 27661–27668. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Waterhouse, G.I.N.; Zheng, L.; Cao, X.; Teng, F.; Wu, L.-Z.; Tung, C.-H.; O’Hare, D.; Zhang, T. Layered-Double-Hydroxide Nanosheets as Efficient Visible-Light-Driven Photocatalysts for Dinitrogen Fixation. Adv. Mater. 2017, 29, 1703828. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Dong, X.; Zhai, S.; Ma, H.; Wang, X.; Zhang, X. Hydrogenated Bismuth Molybdate Nanoframe for Efficient Sunlight-Driven Nitrogen Fixation from Air. Chem. A Eur. J. 2016, 22, 18722–18728. [Google Scholar] [CrossRef] [PubMed]
- Comer, B.M.; Medford, A.J. Analysis of Photocatalytic Nitrogen Fixation on Rutile TiO2(110). ACS Sustain. Chem. Eng. 2018, 6, 4648–4660. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Zhan, G.; Zhang, L. Solar Water Splitting and Nitrogen Fixation with Layered Bismuth Oxyhalides. Acc. Chem. Res. 2017, 50, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed. 2017, 57, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Ho, W.; Wang, C. Selective photocatalytic N2 fixation dependent on g-C3N4 induced by nitrogen vacancies. J. Mater. Chem. A 2015, 3, 23435–23441. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Hu, S.; Li, Q.; Bai, J.; Wang, F. Infrared ray assisted microwave synthesis: A convenient method for large-scale production of graphitic carbon nitride with outstanding nitrogen photofixation ability. RSC Adv. 2016, 6, 45931–45937. [Google Scholar] [CrossRef]
- Ma, H.; Shi, Z.; Li, Q.; Li, S. Preparation of graphitic carbon nitride with large specific surface area and outstanding N2 photofixation ability via a dissolve-regrowth process. J. Phys. Chem. Solids 2016, 99, 51–58. [Google Scholar] [CrossRef]
- Zhou, N.; Qiu, P.; Chen, H.; Jiang, F. KOH etching graphitic carbon nitride for simulated sunlight photocatalytic nitrogen fixation with cyano groups as defects. J. Taiwan Inst. Chem. Eng. 2018, 83, 99–106. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Li, Q.; Zhao, Y.; Mao, W. Effect of sulfur vacancies on the nitrogen photofixation performance of ternary metal sulfide photocatalysts. Catal. Sci. Technol. 2016, 6, 5884–5890. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, S.; Fan, Z.; Liu, D.; Zhao, Y.; Ma, H.; Li, F. Preparation of g-C3N4/ZnMoCdS hybrid heterojunction catalyst with outstanding nitrogen photofixation performance under visible light via hydrothermal post-treatment. Dalton Trans. 2016, 45, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hu, S.; Li, F.; Fan, Z.; Bai, J.; Lu, G.; Wang, Q. Photofixation of atmospheric nitrogen to ammonia with a novel ternary metal sulfide catalyst under visible light. RSC Adv. 2016, 6, 49862–49867. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Li, F.; Fan, Z.; Ma, H.; Li, W.; Kang, X. Construction of g-C3N4/Zn0.11Sn0.12Cd0.88S1.12 Hybrid Heterojunction Catalyst with Outstanding Nitrogen Photofixation Performance Induced by Sulfur Vacancies. ACS Sustain. Chem. Eng. 2016, 4, 2269–2278. [Google Scholar] [CrossRef]
- Miyama, H.; Fujii, N.; Nagae, Y. Heterogeneous photocatalytic synthesis of ammonia from water and nitrogen. Chem. Phys. Lett. 1980, 74, 523–524. [Google Scholar] [CrossRef]
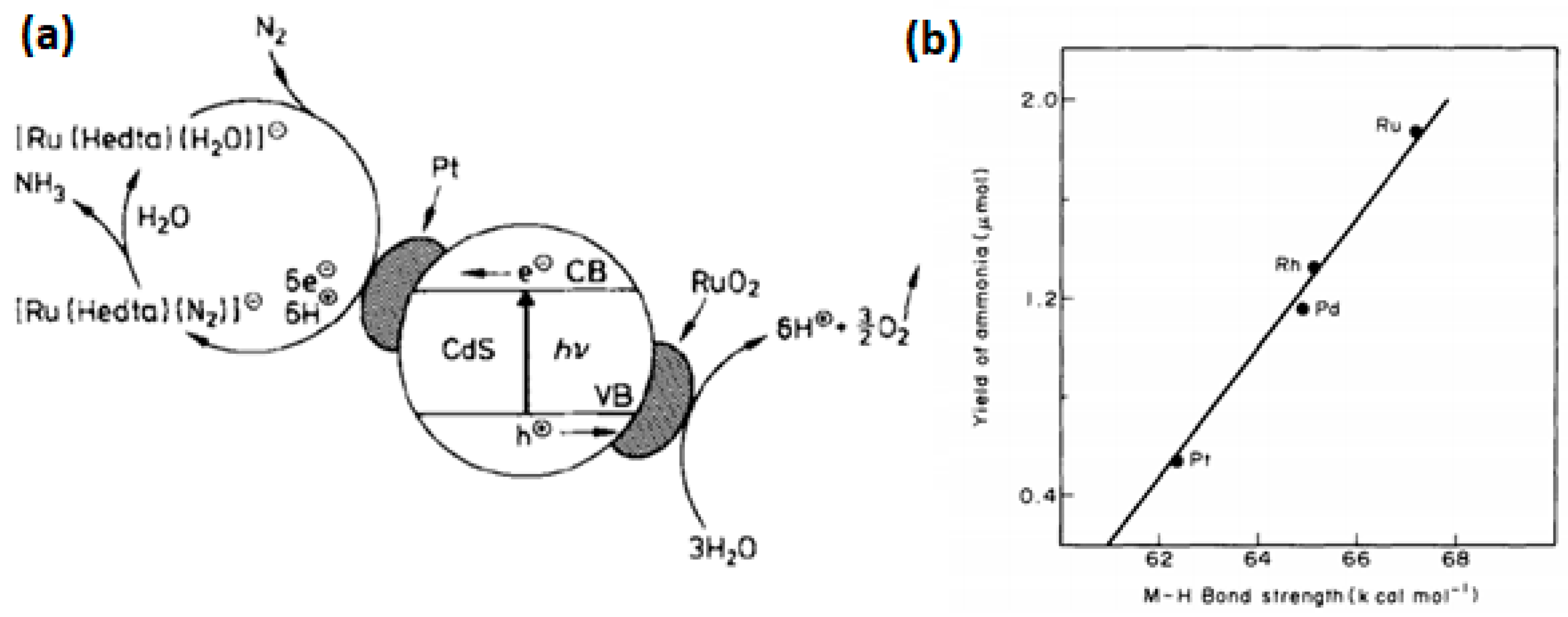
- Khan Mirza, M.T.; Bhardwaj Ramesh, C.; Bhardwaj, C. Catalytic Fixation of Nitrogen by the Photocatalytic CdS/Pt/RuO2 Particulate System in the Presence of Aqueous [Ru(Hedta)N2]⊖ Complex. Angew. Chem. Int. Ed. Engl. 1988, 27, 923–925. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Varadarajan, T.K.; Viswanathan, B. Photocatalytic reduction of dinitrogen to ammonia over noble-metal-loaded TiO2. J. Photochem. Photobiol. A Chem. 1996, 96, 181–185. [Google Scholar] [CrossRef]
- Rao, N.N.; Dube, S.; Manjubala; Natarajan, P. Photocatalytic reduction of nitrogen over (Fe, Ru or Os) /TiO2 catalysts. Appl. Catal. B Environ. 1994, 5, 33–42. [Google Scholar] [CrossRef]
- Medford, A.J.; Hatzell, M.C. Photon-Driven Nitrogen Fixation: Current Progress, Thermodynamic Considerations, and Future Outlook. ACS Catal. 2017, 7, 2624–2643. [Google Scholar] [CrossRef]
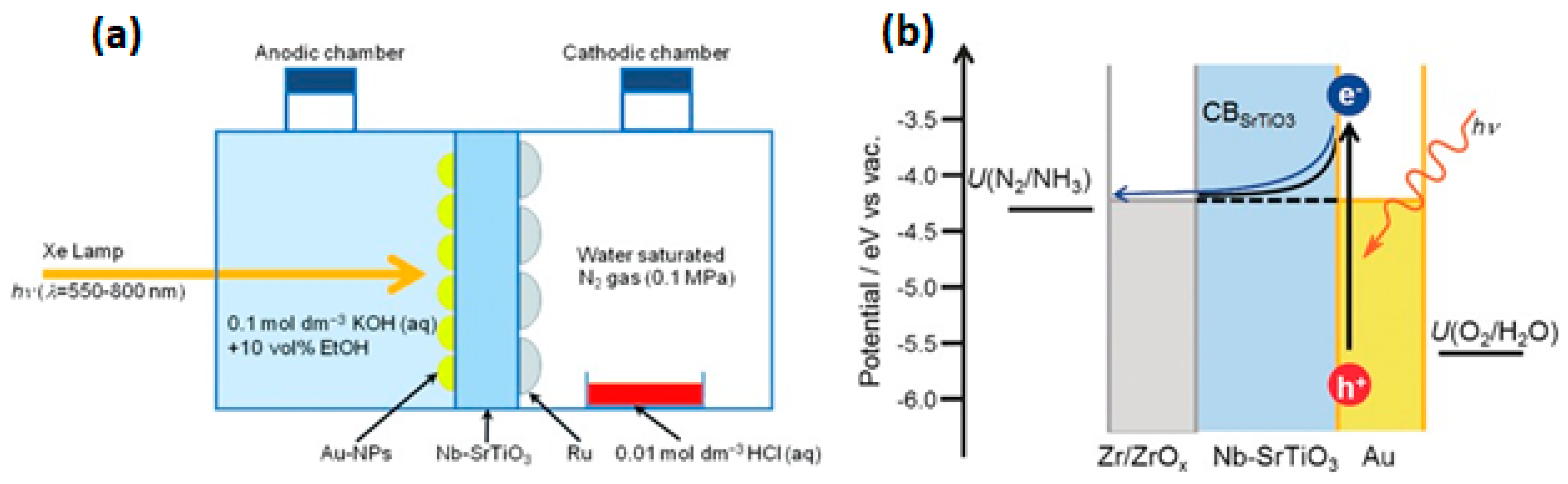
- Zeng, H.; Terazono, S.; Tanuma, T. A novel catalyst for ammonia synthesis at ambient temperature and pressure: Visible light responsive photocatalyst using localized surface plasmon resonance. Catal. Commun. 2015, 59, 40–44. [Google Scholar] [CrossRef]
- Oshikiri, T.; Ueno, K.; Misawa, H. Plasmon-Induced Ammonia Synthesis through Nitrogen Photofixation with Visible Light Irradiation. Angew. Chem. Int. Ed. 2014, 53, 9802–9805. [Google Scholar] [CrossRef]
- Oshikiri, T.; Ueno, K.; Misawa, H. Selective Dinitrogen Conversion to Ammonia Using Water and Visible Light through Plasmon-induced Charge Separation. Angew. Chem. 2016, 128, 4010–4014. [Google Scholar] [CrossRef]
- Ali, M.; Zhou, F.; Chen, K.; Kotzur, C.; Xiao, C.; Bourgeois, L.; Zhang, X.; MacFarlane, D.R. Nanostructured photoelectrochemical solar cell for nitrogen reduction using plasmon-enhanced black silicon. Na. Commun. 2016, 7, 11335. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Oshikiri, T.; Shi, X.; Zhong, Y.; Misawa, H. Plasmon-induced artificial photosynthesis. Interface Focus 2015, 5, 20140082. [Google Scholar] [CrossRef] [PubMed]




| Reaction | E° (V) vs. SHE |
|---|---|
| N2 + 2H+ + 2e− ⇌ N2H2 | +0.035 |
| N2 + 4H+ + 4e− ⇌ N2H4 | −0.4 |
| N2 + 6H+ + 6e− ⇌ 2NH3 | −1.22 |
| Catalyst | Light Source | Sacrificial Reagent | NH3 Rate | Ref. |
|---|---|---|---|---|
| 0.2% Fe-doped TiO2 | 390–420 nm | - | 10 µmolg−1h−1 | [42] |
| 0.5% Fe-doped TiO2 | UV | - | 6 µmolg−1h−1 | [37] |
| Fe-doped TiO2 | 254 nm | Ethanol | 400 µM·h−1 | [36] |
| Partially reduce Fe2O3 | UV-vis | - | 10 µmolg−1h−1 | [34] |
| Fe2O3 | UV-vis | Ethanol | 1362.5 µM·h−1 | [40] |
| Fe2O3·nH2O | Visible | - | 6 µM·h−1 | [34] |
| Fe(O)OH | Vis | - | 9.25 µM·h−1 | [43] |
| Fe doped C3N4 | Vis | Ethanol | 120 µM·h−1 | [41] |
| Fe-load 3D Graphene | UV | - | 24 µmolg−1h−1 | [44] |
| Hydrous oxide of Fe and Ti | Vis | - | 22 µM·h−1 | [45] |
| Iron loaded bentonite | UV | - | 1.33 µM·h−1 | [46] |
| Iron titanate thin film | >320 nm | Ethanol | 0.57 µM·h−1cm−2 | [38] |
| Catalyst | Light Source | Sacrificial Reagent | NH3 Rate | Ref |
|---|---|---|---|---|
| BiOBr nanosheets | UV-Vis/Vis | - | 104.2 µmolg−1h−1 | [31] |
| Bi5O7Br nanotubes | Vis | - | 1.38 mmol·g1h−1 | [55] |
| TiO2/Au/a-TiO2 | Vis | - | 13.4 nmol cm−2h−1 | [56] |
| BiO quantum dots | UV-Vis | - | 1226 µmolg−1h−1 | [57] |
| Reduced TiO2 | Infrared light | - | 3.33 µmolg−1h−1 | [58] |
| Rutile TiO2 | λ > 280 nm | 2-Propanol | 16.67µM·g1h−1 | [49] |
| BiOCl nanosheets | Solar Light | Ethanol | 45 µM·h−1 | [59] |
| Bi5O7I nanosheets | 280–800 nm | Ethanol | 120 µM·h−1 | [60] |
| CuCr–LDH | Vis | - | 57.1 μmolg−1h−1 | [61] |
| Hydrogenated Bi2MoO6 | Solar light | - | 1.3 mmol·g−1h−1 | [62] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, M.-H.; Sakar, M.; Do, T.-O. Insights into the Recent Progress and Advanced Materials for Photocatalytic Nitrogen Fixation for Ammonia (NH3) Production. Catalysts 2018, 8, 621. https://doi.org/10.3390/catal8120621
Vu M-H, Sakar M, Do T-O. Insights into the Recent Progress and Advanced Materials for Photocatalytic Nitrogen Fixation for Ammonia (NH3) Production. Catalysts. 2018; 8(12):621. https://doi.org/10.3390/catal8120621
Chicago/Turabian StyleVu, Manh-Hiep, M. Sakar, and Trong-On Do. 2018. "Insights into the Recent Progress and Advanced Materials for Photocatalytic Nitrogen Fixation for Ammonia (NH3) Production" Catalysts 8, no. 12: 621. https://doi.org/10.3390/catal8120621
APA StyleVu, M.-H., Sakar, M., & Do, T.-O. (2018). Insights into the Recent Progress and Advanced Materials for Photocatalytic Nitrogen Fixation for Ammonia (NH3) Production. Catalysts, 8(12), 621. https://doi.org/10.3390/catal8120621







