Enzymatic Synthesis of Novel Glycyrrhizic Acid Glucosides Using a Promiscuous Bacillus Glycosyltransferase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Glycosylation of GA with Bs-YjiC
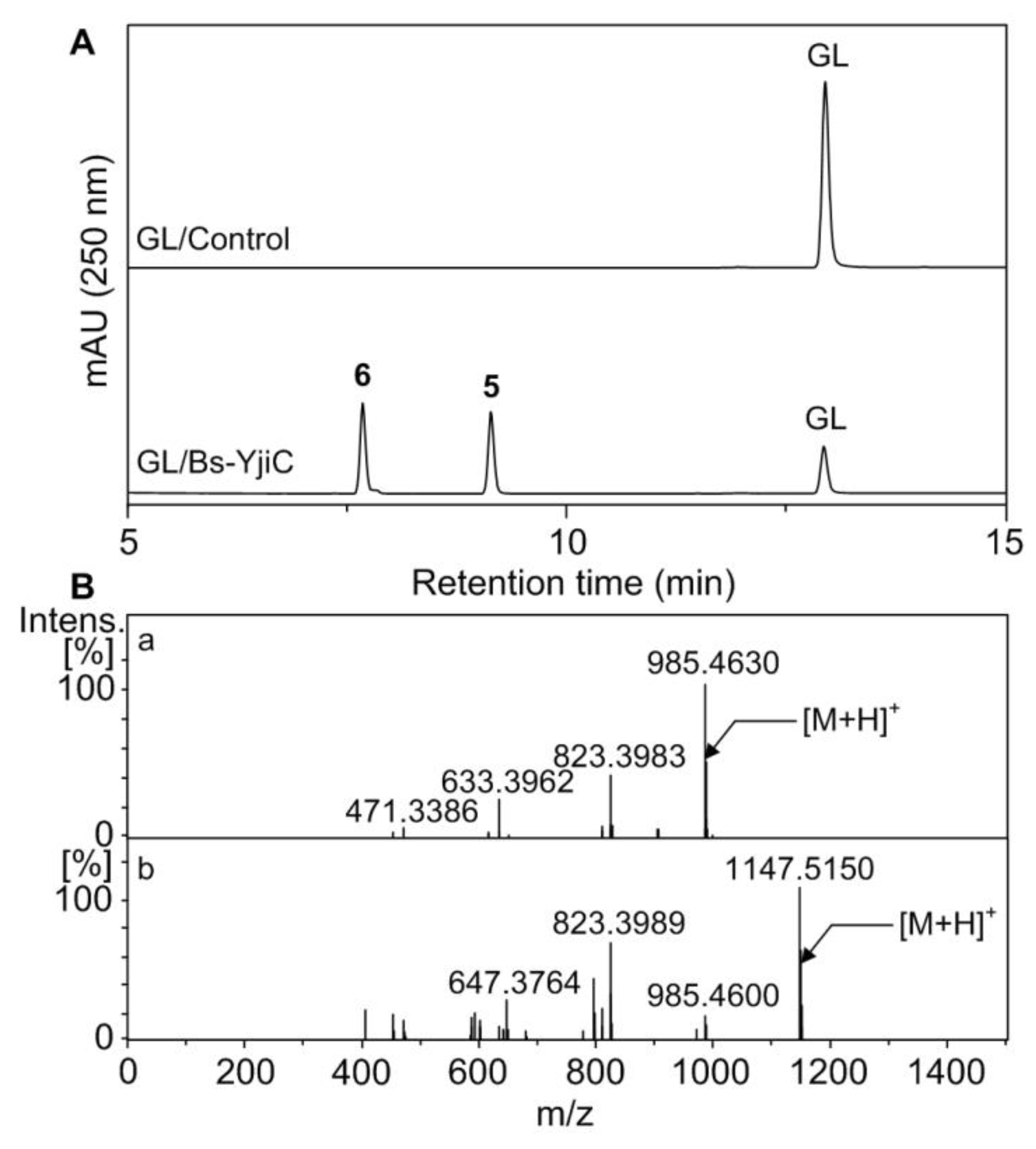
2.2. Glycosylation of GL with Bs-YjiC
2.3. Kinetic Parameters of Bs-YjiC toward GA and GL
2.4. Solubility of the Glycosylated Products
2.5. In Vitro Cytotoxicity of the Glycosylated Products
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. In Vitro Enzymatic Glycosylation
3.3. Kinetic Parameters of Bs-YjiC
3.4. Structural Analysis of GA and GL Glucosides
3.5. Water Solubility Tests
3.6. In Vitro Cytotoxic Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seki, H.H.; Tamura, K.; Muranaka, T. P450s and UGTs: Key players in the structural diversity of triterpenoid saponins. Plant Cell Physiol. 2015, 56, 1463. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Nishimura, K.; Yasuyama, N.; Ebizuka, Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine Max. FEBS Lett. 2010, 584, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Dixon, R.A. Genomic and coexpression analyses predict multiple genes involved in triterpene saponin biosynthesis in Medicago Truncatula. Plant Cell 2010, 22, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.Q.; Qu, H.H.; Kong, H.; Zhang, Y.; Liu, S.C.; Cheng, J.J.; Yan, X.; Zhao, Y. The Effects of sweet foods on the pharmacokinetics of glycyrrhizic acid by iceELISA. Molecules 2017, 22, 498. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Pollier, J.; Almagro, L.; Buyst, D.; Van, M.M.; Pedreño, M.A.; Martins, J.C.; Thevelein, J.M.; Goossens, A. Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiae using a C-16α hydroxylase from Bupleurum Falcatum. Proc. Natl. Acad. Sci. USA 2014, 111, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, P.P.; Wei, Y.J.; Liu, Q.F.; Yang, C.S.; Zhao, G.P.; Yue, J.M.; Yan, X.; Zhou, Z.Z. Characterization of Panax ginseng UDP-Glycosyltransferases catalyzing protopanaxatriol and biosyntheses of bioactive ginsenosides F1 and Rh1 in metabolically engineered yeasts. Mol. Plant 2015, 8, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Dai, L.H.; Yang, J.G.; Liu, C.; Men, Y.; Zeng, Y.; Cai, Y.; Zhu, Y.M.; Sun, Y.X. Oxidation of cucurbitadienol catalyzed by CYP87D18 in the biosynthesis of mogrosides from Siraitia Grosvenorii. Plant Cell Physiol. 2016, 57, 1000. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, C.X.; Sun, W.T.; Zhou, A.Q.; Wang, Y.; Zhang, G.L.; Zhou, X.H.; Huo, Y.X.; Li, C. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants. Metab. Eng. 2017, 45, 43. [Google Scholar] [CrossRef]
- Li, L.; Shin, S.Y.; Lee, S.J.; Moon, J.S.; Im, W.T.; Han, N.S. Production of ginsenoside F2 by using Lactococcus lactis with enhanced expression of β-glucosidase gene from Paenibacillus Mucilaginosus. J. Agric. Food Chem. 2016, 64, 2506. [Google Scholar] [CrossRef]
- Mochida, K.; Sakurai, T.; Seki, H.; Yoshida, T.; Takahagi, K.; Sawai, S.; Uchiyama, H.; Muranaka, T.; Saito, K. Draft genome assembly and annotation of Glycyrrhiza uralensis, a medicinal legume. Plant J. 2017, 89, 181–194. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, A.Z.; Yen, G.C. Protective effects of glycyrrhizic acid and 18β-glycyrrhetinic acid against cisplatin-induced nephrotoxicity in BALB/c mice. J. Agric. Food Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Lv, B.; Feng, X.D.; Li, C. A Perspective on biotransformation and de novo biosynthesis of licorice constituents. J. Agric. Food Chem. 2017, 65, 11147–11156. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Nikolic, D.; van Breemen, R.B. Identification and chemical standardization of licorice raw materials and dietary supplements using UHPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, N.; Karimi, M.H.; Amirghofran, Z. The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cell. Immunol. 2012, 280, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Li, C. Biosynthesis of plant triterpenoid saponins in microbial cell factories. J. Agric. Food Chem. 2018, 66, 12155–12165. [Google Scholar] [CrossRef]
- Kim, J.S.; Cheon, S.; Kim, S.W.; Kim, B.; Kim, H.; Park, K.D.; Kim, S.M. Glycyrrhizic acid prevents astrocyte death by neuromyelitis optica-specific IgG via inhibition of C1q binding. Biochem. Biophys. Res. Commun. 2016, 478, 553–558. [Google Scholar] [CrossRef]
- Zhang, G.L.; Cao, Q.; Liu, J.Z.; Liu, B.Y.; Li, J.; Li, C. Refactoring β-amyrin synthesis in Saccharomyces Cerevisiae. AIChE J. 2015, 61, 3172–3179. [Google Scholar] [CrossRef]
- Chen, K.; He, J.; Hu, Z.; Song, W.; Yu, L.; Li, K.; Qiao, X.; Ye, M. Enzymatic glycosylation of oleanane-type triterpenoids. J. Asian Nat. Prod. Res. 2018, 1–9. [Google Scholar] [CrossRef]
- He, J.B.; Chen, K.; Hu, Z.M.; Li, K.; Song, W.; Yu, L.Y.; Leung, C.H.; Ma, D.L.; Qiao, X.; Ye, M. UGT73F17, a new glycosyltransferase from Glycyrrhiza uralensis, catalyzes the regiospecific glycosylation of pentacyclic triterpenoids. Chem. Commun. 2018. [Google Scholar] [CrossRef]
- Xu, G.J.; Cai, W.; Gao, W.; Liu, C.S. A novel glucuronosyltransferase has an unprecedented ability to catalyse continuous two-step glucuronosylation of glycyrrhetinic acid to yield glycyrrhizin. New Phytol. 2016, 212, 123. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Zhang, L.; Feng, X.D.; Lv, B.; Li, C. Biosynthesis of glycyrrhetinic acid-3-O-monoglucose using glycosyltransferase UGT73C11 from Barbar. Ind. Eng. Chem. Res. 2017, 56, 14949–14958. [Google Scholar] [CrossRef]
- Chang, T.S.; Chiang, C.M.; Wang, T.Y.; Lee, C.H.; Lee, Y.W.; Wu, J.Y. New triterpenoid from novel triterpenoid 15-O-glycosylation on ganoderic acid A by intestinal bacteria of Zebrafish. Molecules 2018, 23, 2345. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.H.; Liu, C.; Li, J.; Dong, C.X.; Yang, J.G.; Dai, Z.B.; Zhang, X.L.; Sun, Y.X. One-pot synthesis of ginsenoside Rh2 and bioactive unnatural ginsenoside by coupling promiscuous glycosyltransferase from Bacillus subtilis 168 to sucrose Synthase. J. Agric. Food Chem. 2018, 66. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.H.; Li, J.; Yang, J.G.; Zhu, Y.M.; Men, Y.; Zeng, Y.; Cai, Y.; Dong, C.X.; Dai, Z.B.; Zhang, X.L.; et al. Use of a promiscuous glycosyltransferase from Bacillus subtilis 168 for the enzymatic synthesis of novel protopanaxatriol-type ginsenosides. J. Agric. Food Chem. 2018, 66, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.H.; Li, J.; Yao, P.Y.; Zhu, Y.M.; Men, Y.; Zeng, Y.; Yang, J.G.; Sun, Y.X. Exploiting the aglycon promiscuity of glycosyltransferase Bs-YjiC from Bacillus subtilis and its application in synthesis of glycosides. J. Biotechnol. 2017, 248, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Song, M.C.; Kim, E.; Ban, Y.H.; Yoo, Y.J.; Kim, E.J.; Park, S.R.; Pandey, R.P.; Sohng, J.K.; Yoon, Y.J. Achievements and impacts of glycosylation reactions involved in natural product biosynthesis in prokaryotes. Appl. Microbiol. Biotechnol. 2013, 97, 5691–5704. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Fukui, H.; Tabata, M. Biotransformation of 18 β-glycyrrhetinic acid by cell suspension cultures of Glycyrrhiza Glabra. Phytochemistry 1990, 29, 2149–2152. [Google Scholar] [CrossRef]
- Dai, L.H.; Liu, C.; Zhu, Y.M.; Zhang, J.S.; Men, Y.; Zeng, Y.; Sun, Y.X. Functional characterization of cucurbitadienol synthase and triterpene glycosyltransferase involved in biosynthesis of mogrosides from Siraitia Grosvenorii. Plant Cell Physiol. 2015, 56, 1172. [Google Scholar] [CrossRef]
- Schmölzer, K.; Gutmann, A.; Diricks, M.; Desmet, T.; Nidetzky, B. Sucrose synthase: A unique glycosyltransferase for biocatalytic glycosylation process development. Biotechnol. Adv. 2015, 34, 88–111. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045. [Google Scholar] [CrossRef]
- Thibodeaux, C.J.; Melan, C.E.; Liu, H.W. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew. Chem. Int. Ed. 2009, 47, 9814–9859. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Ohyama, K.; Sawai, S.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Akashi, T.; Aoki, T.; Saito, K.; Muranaka, T. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc. Natl. Acad. Sci. USA 2008, 105, 14204. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Sawai, S.; Ohyama, K.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Fukushima, E.O.; Akashi, T.; Aoki, T.; Saito, K. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 2011, 23, 4112–4123. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, G.; Walmagh, M.; Diricks, M.; Lepak, A.; Gutmann, A.; Nidetzky, B.; Desmet, T. Screening of recombinant glycosyltransferases reveals the broad acceptor specificity of stevia UGT-76G1. J. Biotechnol. 2016, 233, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, D.; Janeczko, T.; Suslow, E.K. Glycosylation of 3-hydroxyflavone, 3-methoxyflavone, quercetin and baicalein in fungal cultures of the Genus Isaria. Molecules 2018, 23, 2477. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Kim, Y.M.; Walsh, M.K.; Wee, Y.J.; Yang, K.Y.; Ko, J.; Han, S.; Nguyen, T.T.H.; Ji, Y.K.; Kim, D. Synthesis and functional characterization of caffeic acid glucoside using Leuconostoc mesenteroides dextransucrase. J. Agric. Food Chem. 2017, 65. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.D.; Dewitte, G.; Dirkshofmeister, M.E.; Laet, S.D.; Pelantová, H.; Kren, V.; Desmet, T. Enzymatic glycosylation of phenolic antioxidants: Phosphorylase mediated synthesis and characterization. J. Agric. Food Chem. 2015, 63, 10131–10139. [Google Scholar] [CrossRef] [PubMed]
- Bruyn, F.D.; Maertens, J.; Beauprez, J.; Soetaert, W.; Mey, M.D. Biotechnological advances in UDP-sugar based glycosylation of small molecules. Biotechnol. Adv. 2015, 33, 288–302. [Google Scholar] [CrossRef]
- Guo, L.C.; Katiyo, W.; Lu, L.S.; Zhang, X.; Wang, M.M.; Yan, J.A.; Mao, X.Y.; Yang, R.J.; Zou, L.; Zhao, W. Glycyrrhetic acid 3-O-mono-β-d-glucuronide (GAMG): An innovative high-potency sweetener with improved biological activities. Compr. Rev. Food Sci. Food Saf. 2018. [Google Scholar] [CrossRef]
- Chen, J.Y.; Kaleem, I.; He, D.M.; Liu, G.Y.; Li, C. Efficient production of glycyrrhetic acid 3-O-mono-β-d-glucuronide by whole-cell biocatalysis in an ionic liquid/buffer biphasic system. Process. Biochem. 2012, 47, 908–913. [Google Scholar] [CrossRef]
- Lepak, A.; Gutmann, A.; Kulmer, S.T.; Nidetzky, B. Creating a water-soluble resveratrol-based antioxidant by site-selective enzymatic glucosylation. ChemBioChem 2015, 16, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, Y.; Dai, L.H.; Cai, T.Y.; Zhu, Y.M.; Dou, D.Q.; Ma, L.Q.; Sun, Y.X. Mogrol represents a novel leukemia therapeutic, via ERK and STAT3 inhibition. Am. J. Cancer Res. 2015, 5, 1308. [Google Scholar] [PubMed]


| Substrate | Km (μM) | kcat (s−1) | kcat/Km (s−1 M−1) |
|---|---|---|---|
| GA | 245.00 ± 23.03 | 3.36 ± 0.13 | 1.37 × 104 |
| GL | 58.73 ± 4.07 | 0.31 ± 0.01 | 0.53 × 104 |
| Compound | Solubility | Relative Solubility |
|---|---|---|
| GA | 29 ± 2 | 1 |
| GL | 1526 ± 322 | 53 |
| 1 | 58 ± 2 | 2 |
| 2 | 242 ± 20 | 8 |
| 3 | 2022 ± 192 | 70 |
| 4 | 98,326 ± 5022 | 3390 |
| 5 | 136,698 ± 8425 | 4714 |
| 6 | ND | ND |
| Compound | HepG2 | MCF-7 |
|---|---|---|
| GL b | 37.3 ± 2.5 | 113.7 ± 10.4 |
| 1 | 7.2 ± 0.6 | 7.7 ± 0.6 |
| 2 | 25.4 ± 1.6 | 13.0 ± 1.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Li, J.; Yang, J.; Men, Y.; Zeng, Y.; Cai, Y.; Sun, Y. Enzymatic Synthesis of Novel Glycyrrhizic Acid Glucosides Using a Promiscuous Bacillus Glycosyltransferase. Catalysts 2018, 8, 615. https://doi.org/10.3390/catal8120615
Dai L, Li J, Yang J, Men Y, Zeng Y, Cai Y, Sun Y. Enzymatic Synthesis of Novel Glycyrrhizic Acid Glucosides Using a Promiscuous Bacillus Glycosyltransferase. Catalysts. 2018; 8(12):615. https://doi.org/10.3390/catal8120615
Chicago/Turabian StyleDai, Longhai, Jiao Li, Jiangang Yang, Yan Men, Yan Zeng, Yi Cai, and Yuanxia Sun. 2018. "Enzymatic Synthesis of Novel Glycyrrhizic Acid Glucosides Using a Promiscuous Bacillus Glycosyltransferase" Catalysts 8, no. 12: 615. https://doi.org/10.3390/catal8120615
APA StyleDai, L., Li, J., Yang, J., Men, Y., Zeng, Y., Cai, Y., & Sun, Y. (2018). Enzymatic Synthesis of Novel Glycyrrhizic Acid Glucosides Using a Promiscuous Bacillus Glycosyltransferase. Catalysts, 8(12), 615. https://doi.org/10.3390/catal8120615





