Abstract
The metathesis of 2-butene (Trans and Cis) to propene was investigated over W-based catalysts. Thermodynamic calculations for metathesis and isomerization were carried out at various temperatures to test the reactions. The results showed that the WO3/MCM-48 catalyst had good catalytic activity. The metathesis activity depended on the acidity of the catalyst and the dispersity of the WO3 on the supports. High temperatures promoted the isomerization of 2-butene to 1-butene. According to thermodynamic analysis, however, this is adverse to the metathesis reaction, making it important to determine an appropriate reaction temperature.
1. Introduction
Olefin metathesis, which is the reaction of olefin carbon–carbon double bond cleavage and intermolecular rearrangement to form new olefins, can convert low-value olefins to high-value olefins; for example, the by-product of 2-butene into propene of market demand [1] has great prospects in the petrochemical industry and in organic synthesis. Generally, transition metal compounds are used as metathesis catalysts, which are classified into two main groups: (a) heterogeneous catalysts—transition metal oxides or carbonyls deposited on high-surface-area supports, (b) homogeneous catalysts—transition metal salts or coordination compounds in combination with selected organometallic derivatives, or Lewis acids [2]. The most successful heterogeneous catalysts for olefin metathesis are rhenium-based [3], molybdenum-based [4], and tungsten-based [5] catalysts.
Now, many olefin metathesis routes [6,7,8] have been reported to produce propene. Inclusive, we have reported a new propene synthesis route with 2-butene [9].
Most metathesis catalysts are sensitive to oxygen and/or moisture, particularly Re-based and Mo-based catalysts, so metathesis reactions have to be performed in an inert atmosphere. Tungsten-based catalysts have been established to be low cost, poison resistant [10], and regenerative [11]. Accordingly, tungsten-based catalysts have been investigated for decades [12,13]. The support material is one of the key factors influencing the performance of catalysts. WO3-SiO2-Al2O3 metathesis catalysts were reported to be yielded by one-step preparation methods [14]. Chaemchuen et al. [15] reported that the relationship between the reactivity of catalysts and temperature calcined. Porous materials offer special possibilities, such as large surface areas, large void volume, and so on. Wu et al. [8] reported that tungsten incorporated ordered, mesoporous silicates as a metathesis catalyst. Super-microporous WO3/SiO2 olefin metathesis catalysts were prepared by a one-pot aerosol-assisted Sol-gel method [16]. Isomerization and metathesis of 2-butene, however, was less reported.
In the contribution, 2-butene was used as feed stock to study isomerization on MgO. In order to know the effects of supports on the metathesis performance of catalysts, MCM-48, MCM-41 and SiO2 were used as supports. Four main independent reactions were selected to calculate their thermodynamic properties. The experiments were carried out under different conditions and thermodynamic analyses.
2. Results and Discussion
Figure 1 demonstrates the effects of loading on the structural characteristics of the catalysts. Figure 1a shows the XRD (X-ray diffraction) patterns of the catalysts. The characteristic peak of SiO2 at 10–80° (2-Theta), was only observed for MCM-48. The weak peaks at 23–26° (2-Theta), corresponded to the crystalline phase of WO3 and were observed for 8WO3/MCM-48. When loading was more than 8 wt.%, strong peaks at 23–26° and 30–25° (2-Theta) were found; this indicated that the dispersity of WO3 had worsened. Figure 1b shows the UV-vis DRS of catalysts. There were two absorption bands at ca. 225 and 270 nm for all W-based catalysts. There was an additional absorption band at ca. 400 nm for W-based catalysts with W-loading beyond 4 wt.%. As shown in Figure 1b, MCM-48 does not possess the band. This indicates that the loading of tungsten on supports results in an absorption band at 400 nm. The band at 225 nm is attributed to the charge transfer of the ligand-to-metal which stems from the isolated tetrahedral species [8,9,17]. The band at ca. 270 nm is due to the charge transfer (O2− → W6+) which is attributed to the octahedral polytungstate WO3 species [8,9,17]. The absorption band at ca. 400 nm is attributed to the bulk WO3 species [8]. Uniquely, the band intensities at ca. 225 and 270 nm changed with WO3 loading which indicated that the populations of active W species were different. Both the intensity of the absorption band at ca. 400 nm and the populations of inactive bulk WO3 species were higher. Figure 1c shows the isotherms of nitrogen adsorption and pore size distribution for W-based catalysts. The textural properties of catalysts and supports were listed in Table 1. The nitrogen adsorption–desorption isotherms of reversible type IV can also be found in Figure 1c. Pore size distribution was ca. 2.5 nm. The specific surface areas of MCM-48 and catalysts were 1302.1 and ca. 880 m2/g, respectively. The textural properties of supports and catalysts are different for the squash technique. Table 1 shows the acidity of WO3-based catalysts with different loadings. The acidity of MCM-48 was low; however, after the WO3 was loaded on MCM-48, the acidity of the catalysts became strong enough for interaction between supports and WO3 to form acidic sites. The peak at 453–473 K was attributed to a weak acid peak and the peak shifted to slightly higher temperatures with loading. It was interesting to note the increased acidity of the catalysts with tungsten loading.
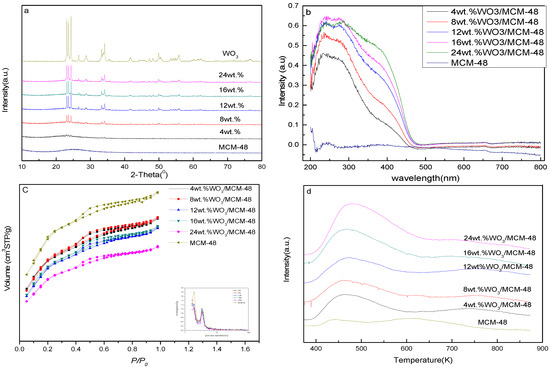
Figure 1.
Structural characteristics of catalysts with different loading. (a) XRD patterns of catalysts, (b) UV-vis DRS of catalysts, (c) N2 adsorption-desorption isotherms of catalysts, (d) NH3-TPD of catalysts.

Table 1.
Characteristics of WO3/MCM-48.
Figure 2a shows the XRD spectrum of catalysts with various supports. The intensity of peaks at 22–25° increased as follows: 16 wt.%WO3/MCM-48 < 16 wt.%WO3/MCM-41 < 16 wt.%WO3/SiO2, indicating that dispersion of WO3 on supports worsened. Figure 2b shows the UV-vis DRS of all catalysts. The population of tetrahedral tungsten oxide species was more than that of octahedral W species in the cases of both WO3/MCM-48 and WO3/MCM-41. These cases are opposite to WO3/SiO2. The intensity of peaks at ca. 400 nm increased as follows: 16 wt.%WO3/MCM-48 < 16 wt.%WO3/MCM-41 < 16 wt.%WO3/SiO2, showing that the dispersity of WO3 is different on supports, and the result is in accordance with that of XRD. It was found that peak shifts occurred in the case of 16 wt.%WO3/SiO2 for a band at 400 nm. Figure 2c shows the reduction profiles of all catalysts. The position and area of peaks for each catalyst were different. There were two discriminated peaks, one at ca. 853 and the other at 1223 K. The peak at ca. 853 K was attributed to surface WO3 species (tetrahedral tungsten oxide species) [15], and this peak area qualitatively expressed the population of relevant species. The acid characteristics of catalysts are shown in Figure 2d. Peaks at 473 and 823 K correspond to weak and strong acidic sites respectively. The data are detailed in Table 2.
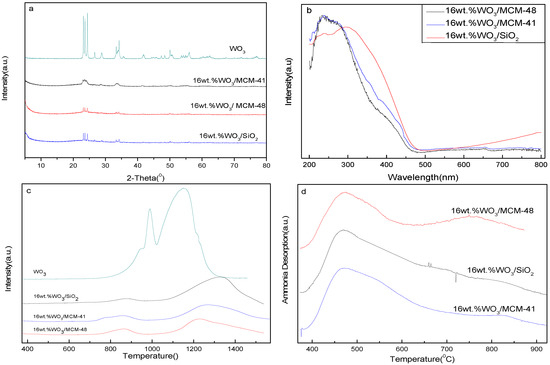
Figure 2.
Effect of supports on structural characteristics of catalysts. (a) XRD patterns of catalysts, (b) UV-vis DRS of catalysts, (c) TPR of catalysts, (d) NH3-TPD of catalysts.

Table 2.
Characteristics of W-based catalysts.
Figure 3 shows the effect of loadings on the performance of catalysts. Butene conversion increased with loadings of WO3 and catalysts with loadings of 16 wt.% held the highest activity. The conversion of butene decreased with further increase of loadings. Catalytic activity of metathesis catalysts is related to surface tungsten oxide species (tetrahedral) [12,18], which depend on the loading of WO3. Here, UV-DRS results showed that catalysts with loadings of 16 wt.% held a relatively high proportion of tetrahedral tungsten oxide species at ca. 225 nm. The population of octahedral tungsten oxide species and bulk WO3 increased exponentially with loading above 16 wt.%, even tetrahedral tungsten oxide species converted into tungsten oxide species without metathesis activity. Catalytic performance also depended on the acidity of the catalyst. Catalysts with more acidic sites showed higher performance. Loading had little effect on propene selectivity.
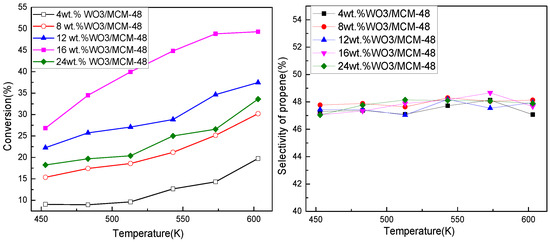
Figure 3.
Propene selectivity and butene conversion over catalysts with different loadings. Reaction conditions: P = 0.8 MPa; WHSV = 3.2 h−1.
Figure 4 shows the effect of reaction conditions on the performance of a 16 wt.%WO3/MCM-48 catalyst. As shown in Figure 4a, butene conversion and propene selectivity change slightly with pressure in an equimolar reaction. In Figure 4b, the performance of the catalyst decreases with WHSV, which is attributed to reduced residence time on the catalyst at a temperature. Butene conversion and propene selectivity increase with temperature and are highest at ca. 693 K.
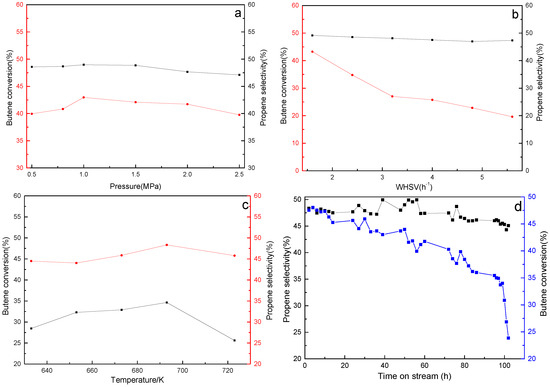
Figure 4.
Propene selectivity and butene conversion under different conditions. (a) T = 573 K, WHSV = 3.2 h−1, (b) T = 573 K, P = 0.8 MPa, (c) WHSV = 3.2 h−1, P = 0.8 MPa, (d) T = 573 K, WHSV = 3.2 h−1, P = 0.8 MPa.
It is found that supports significantly affect the performance of catalyst. In Figure 5, propene selectivity is considerable on 16 wt.%WO3/MCM-48 and 16 wt.%WO3/MCM-41 catalysts but is lower on 16 wt.%WO3/SiO2. Butene conversion has the same trend as propene selectivity. Bronsted acidity and surface tetrahedral tungsten species play an important role in the metathesis reaction. There is a direct relation between Bronsted acidity and surface tetrahedral tungsten species in line with the above results characteristic of catalysts.
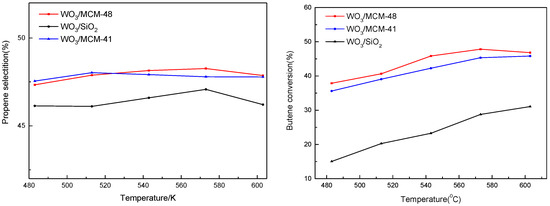
Figure 5.
Propene selectivity and butene conversion over catalysts. Reaction conditions: WHSV = 3.2 h−1, P = 0.8 Mpa.
Analysis of Thermodynamics of n-Butene
Isomerization of 2-butene to 1-butene is a key reaction. The 2-butene isomerization mainly consists of the double-bond positional and trans/cis isomerization (Scheme 1). The isomerization is endothermic (Table 3), therefore, high temperatures elicit favorable reactions, and ΔG of the reaction positively indicates that the reactions are not spontaneous.
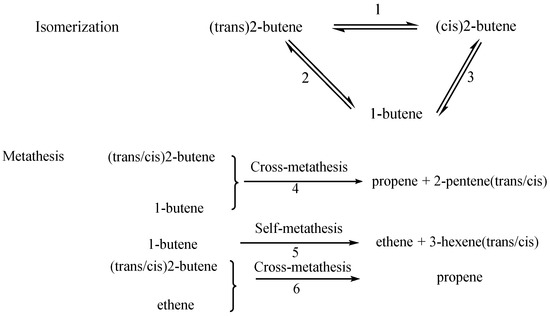
Scheme 1.
Routes of n-butene.

Table 3.
Thermodynamic parameters of various temperatures.
Isomerization of butene was carried out over 2 g MgO at 473 K in a stainless reactor. The results are shown in Figure 6. Other products were not found, indicating that only isomerization occurred. As can be seen in Figure 6a–c, the rate of cis-2-butene as a reactant was higher than that of tras-2-butene as a reactant; the rate of isomerization of 1-butene was the highest while the reverse reaction was the slowest. At equilibrium, ratios of trans-2-butene to 1-butene and cis-2-butene to 1-butene were ca. 0.38 and 0.61, respectively. The ratios were near equilibrium at 473 K (Figure 6d, data from HSC 6.0). The above results show that high temperatures are favorable for the formation of 1-butene.
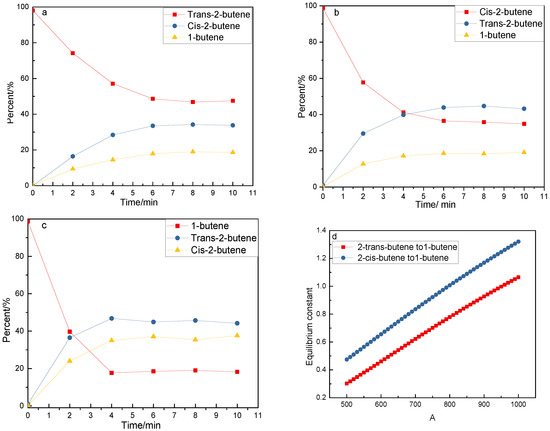
Figure 6.
Isomerization on time stream and equilibrium constant on temperature. (a)Isomerization of Trans-butene (b) Isomerization of Cis-butene (c) Isomerization of 1-butene (d) Equilibrium constants of Trans-2-butene to 1-butene and Cis-2-butene to 1-butene.
Metathesis of butene is complex; the detailed reactions are shown in Scheme 1. Thermodynamic parameters of all metathesis reactions and their temperatures are listed in Table 3. For metathesis of 2-butene and 1-butene (route 4), all reactions are exothermal except the reaction of T to C (trans-2-butene to cis-2-pentene), high temperatures are not favorable to them; only the ΔG of C to C is positive, showing that it is not spontaneous. Self-metathesis of 1-butene is exothermal, and high temperatures are favorable. For second metathesis of ethene and n-butene, it is exothermic and ΔG is negative, indicating the reactions are spontaneous. For route 5, whatever product is trans-2-butene or cis-2-butene, ΔH and ΔG of the processes are positive, indicating that the reaction is exothermic and spontaneous and that high temperature is not favorable. However, the case is contrary for route 6. Using only thermodynamic analysis, the main and limited reactions are not definite during metathesis.
3. Experimental Section
3.1. Preparation of Catalyst
All the materials are from Shanghai Civi Chemical Technology Co. (Shanghai, China). Tetraethylorthosilicate was mixed with sodium hydroxide solution at 308 K, and then CTAB and NaF was added sequentially into the mixture. The molar composition of the final mixture was SiO2:0.70CTAB:60H2O:0.5NaOH:0.1NaF. The as-synthesized mixture was kept for 24 h at 393 K in an autoclave. The product was filtrated, washed with distilled water, and dried at 373 K. Finally, the synthesized samples were calcined in air at 823 K for 5 h.
Catalysts with different tungsten loadings were prepared using a wet impregnation method. The ratio (weight) of WO3 to support was 4.0, 8.0, 12.0, 16.0, and 24 wt.%, respectively. Composition of catalysts were listed in Table 4. The impregnated products were kept for 12 h at 353 K; then, catalysts were treated at 823 K for 4 h in a furnace (1 K/min).

Table 4.
Composition of catalysts.
3.2. Characterization of Catalysts
Specific surface area, pore volume, and pore size of catalysts were obtained with a Micromeritics ASAP 2010 automatic N2 adsorption analyzer (Micromeritics, Norcross, GA, USA). The acidic number of samples was determined by the temperature-programmed desorption of ammonia (NH3-TPD, chemical adsorption-desorption device 6080A, Xianquan company, Tianjin, China). Temperature-programmed reduction of H2(TPR-H2) was recorded on chemical adsorption-desorption device 6080A (Xianquan conpany, Tianjin, China). X-ray powder diffraction patterns of W-based catalysts were recorded on a Bruker D8 ADVANCE diffractometer (Bruker, Billerica, MA, USA), by CuKα (1.5406 Å) radiation in the 2-Theta range of 10–80° with a scanning rate of 1°/min. The UV–vis DRS (diffuse reflectance spectroscopy) of catalyst was recorded in the range of 200–800 nm against the support as a reference, on a Hitachi U-4100 spectrophotometer (Hitachi, Tokyo, Japan) equipped with an integration sphere diffuse reflectance attachment. The 244.0 nm line from a He–Cd laser was used as the excitation source. The spectra resolution is estimated to be 4.0 cm−1. The tungsten and silicon contents of the catalysts were determined by inductively coupled plasma optical emission spectrometry (ICP-OES) using a JY 2000 instrument (Jobin Yvon, Horiba, France).
3.3. Test of Catalyst
The isomerization of 2-butene was carried out on MgO (2 g) in a fixed-bed stainless-steel micro reactor (10 mm i.d.). Butenes (1-butene, Trans-2-butene and Cis-2-butene) were from Huter gas in Nanchang, China.
MgO (2 g) and W-based catalysts (0.375 g) were blended mechanically and loaded into the fixed-bed stainless-steel micro reactor. 2-butene (ca. 81 wt.%, Table 5) from Shanghai Gaoqiao Petrochemical co. LTD in China was a by-product of the addition of isobutene and methanol into MTBE (methyl tert-butyl ether). Organic oxides and other trace contaminants, which can cause catalyst deactivation, were removed by BASF adsorbents (Selexsorb CD and COS). After the catalyst was treated in situ with a mixture of N2 and H2 for 30 min at 693K, C4 stream was fed into the reactor. Main reactions of n-butene over W-based catalysts are shown in Scheme 1. All products were analyzed using a gas chromatograph SP3420 equipped with a flame ionization detector (FID) and a PONA capillary column (50 m × 0.20 mm).

Table 5.
Composition of feed stock.
3.4. Thermodynamic Analysis
For the isomerization and metathesis of 2-butene, isomerization and metathesis reactions will occur sequentially, and metathesis reactions are described in Scheme 1.
1-butene are mainly from Equations (2) and (3). Isomerization reactions consist of Equations (1)–(3), two of them are independent. The equilibrium constants are marked as K1, K2, K3, K4, K5, and K6, respectively. The equilibrium constant of Equation (1) can be described by K1 = K2/K3. Each of Equations (4)–(6) contains four reactions, their equilibrium constants are described by Ki,CT (Cis-reactants to Trans-products).
Equilibrium constants are calculated using the following formulas [19]:
4. Conclusions
Isomerization and metathesis of n-butene were studied over MgO and WO3/MCM-48 catalysts. The results show that pressure had little effect on butene conversion; 16 wt.% WO3/MCM-48 catalyst held the highest activity under the same conditions. With the conditions, the catalyst showed outstanding stability on stream with a 2-butene conversion of 47% for 95 h; and then the performance of the catalyst decreased notably. In all factors, temperature and loading played key roles. Except for the above-mentioned factors, the support was also an important factor. Catalytic activity increased as follows: WO3/SiO2 < WO3/MCM-41 < WO3/MCM-48, which was attributed to the dispersity of WO3 on the support and acidic sites of the catalysts. The dispersity of WO3 on MCM-48 was better than the others, and acidic sites were greatest on MCM-48, as evidenced from TPD-NH3. It was found, using thermodynamic analyses, that high-temperatures benefit in forming 1-butene, which promotes the formation of propene from its dynamics; however, metathesis is complex with 2-butene as a stock feed, and the feasible temperature is 693 K, where all reactions reach optimum. Next, we will investigate an effect of geometric isomerism on products.
Author Contributions
Conceptualization, D.H., Z.Z. and Q.H.; methodology, J.L.; software, X.L.; validation, D.H., Z.Z. and J.L.; formal analysis, D.H., Z.Z. and Q.H.; investigation, D.H. and Z.Z.; resources, Y.X.; data curation, H.X. and Q.H.; writing—original draft preparation, M.L.; writing—review and editing, D.H.; visualization, J.Y.; supervision, D.H.; project administration, D.H.; funding acquisition, Y.Y.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21466001, 21461002, 21566001, and Open Foundation of Key Laboratory of Jiangxi University for Functional Materials Chemistry (FMC17301).
Conflicts of Interest
All authors declare no conflict of interest.
References
- Zhang, D.Z.; Li, X.J.; Liu, S.L.; Zhu, X.X.; Chen, F.C.; Xu, L.Y. Investigations into the C4 olefin metathesis over Mo/Al2O3: Effects of support nature and pretreatment conditions on the product distribution. Appl. Catal. A Gen. 2014, 472, 92–100. [Google Scholar] [CrossRef]
- Calderon, N. Olefin metathesis reaction. Acc. Chem. Res. 1972, 5, 127–132. [Google Scholar] [CrossRef]
- Mahmood, C.S.; Yarmo, M.A.; Hamid, S.B.D.-A. Determination of active centres in Re2O7–Al2O3 metathesis catalysts by titration method. J. Mol. Catal. A Chem. 2000, 161, 11–16. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Liu, S.; Xie, S.; Zhu, X.; Bao, X.; Xu, L. Promoting effect of Mg in supported Mo/HBeta–Al2O3 catalyst for cross-metathesis of ethene and butene-2 to propene. J. Mol. Catal. A Chem. 2009, 313, 38–43. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Praserthdam, P.; Suriye, K.; Devillers, M.; Debecker, D.P. WO3-based catalysts prepared by non-hydrolytic sol-gel for the production of propene by cross-metathesis of ethene and 2-butene. Appl. Catal. A Gen. 2014, 488, 200–207. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.-L.; Gao, J.; Xu, C. Effect of tungsten oxide loading on metathesis activity of ethene and 2-butene over WO3/SiO2 catalysts. Trans. Met. Chem. 2009, 34, 621–627. [Google Scholar] [CrossRef]
- Limsangkass, W.; Praserthdam, P.; Phatanasri, S.; Panpranot, J.; Chaemchuen, S.; Jareewatchara, W.; Ayudhya, S.K.N.; Suriye, K. Influence of micro- and nano-sized SiO2 excess support on the metathesis of ethylene and trans-2-butene to propylene over silica-supported tungsten catalysts. Reac. Kinet. Mech. Cat. 2014, 113, 225–240. [Google Scholar]
- Wu, J.-F.; Ramanathan, A.; Snavely, W.K.; Zhu, H.; Rokicki, A.; Subramaniam, B. Enhanced metathesis of ethylene and 2-butene on tungsten incorporated ordered mesoporous silicates. Appl. Catal. A Gen. 2016, 528, 142–149. [Google Scholar] [CrossRef]
- Hua, D.; Chen, S.-L.; Yuan, G.; Wang, Y.; Zhao, Q.; Wang, X.; Fu, B. Metathesis of butene to propene and pentene over WO3/MTS-9. Micropor. Mesopor. Mat. 2011, 143, 320–325. [Google Scholar] [CrossRef]
- Hua, D.; Chen, S.; Zhou, Z.; Lu, X.; Li, J. Poisoning Deactivation of Mesoporous Titanosilicate-Supported WO3 During Metathesis of Butene to Propene. Catal. Surv. Asia 2016, 20, 53–58. [Google Scholar] [CrossRef]
- Schalkwyk, C.V.; Spamer, A.; Moodley, D.J.; Dube, T.; Reynhardt, J.; Botha, J.M. Application of a WO3/SiO2 catalyst in an industrial environment: part I. Appl. Catal. A Gen. 2003, 255, 121–131. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Debecker, D.P.; Praserthdam, P.; Panpranot, J.; Suriye, K.; Ayudhya, S.K.N. NaOH modified WO3/SiO2 catalysts for propylene production from 2-butene and ethylene metathesis. Chin. J. Catal. 2014, 35, 232–241. [Google Scholar] [CrossRef]
- Lwin, S.; Li, Y.; Frenkel, A.I.; Wachs, I.E. Nature of WOx Sites on SiO2 and Their Molecular Structure–Reactivity/Selectivity Relationships for Propylene Metathesis. ACS Catal. 2016, 6, 3061–3071. [Google Scholar] [CrossRef]
- Debecker, D.P.; Stoyanova, M.; Rodemerck, U.; Colbeau-Justin, F.; Boissère, C.; Chaumonnot, A.; Bonduelle, A.; Sanchez, C. Aerosol route to nanostructured WO3-SiO2-Al2O3 metathesis catalysts: Toward higher propene yield. Appl. Catal. A Gen. 2014, 470, 458–466. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Phatanasri, S.; Verpoort, F.; Sae-ma, N.; Suriye, K. The structure-reactivity relationship for metathesis reaction between ethylene and 2-butene on WO3/SiO2 catalysts calcinated at different temperatures. Kinet. Catal. 2012, 53, 247–252. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Praserthdam, P.; Suriye, K.; Debecker, D.P. Preparation of super-microporous WO3–SiO2 olefin metathesis catalysts by the aerosol-assisted sol–gel process. Micropor. Mesopor. Mat. 2015, 213, 125–133. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, R.; Li, P.; Feng, S.; Zhou, G.; Yu, C.; Zhou, H.; Xu, C.; Xu, Q. Metathesis and isomerization of n-butene and ethylene over WO3/SiO2 and MgO catalysts: Thermodynamic and experimental analysis. Appl. Catal. A Gen. 2016, 517, 227–235. [Google Scholar] [CrossRef]
- Bhuiyan, T.I.; Arudra, P.; Akhtar, M.N.; Aitani, A.M.; Abudawoud, R.H.; Al-Yami, M.A.; Al-Khattaf, S.S. Metathesis of 2-butene to propylene over W-mesoporous molecular sieves: A comparative study between tungsten containing MCM-41 and SBA-15. Appl. Catal. A Gen. 2013, 467, 224–234. [Google Scholar] [CrossRef]
- Huang, S.; Chen, F.; Liu, S.; Zhu, Q.; Zhu, X.; Xin, W.; Feng, Z.; Li, C.; Wang, Q.; Xu, L. The influence of preparation procedures and tungsten loading on the metathesis activity of ethene and 2-butene over supported WO3 catalysts. J. Mol. Catal. A Chem. 2007, 267, 224–233. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).