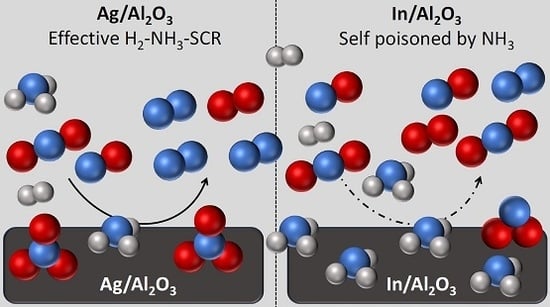
Surface Species and Metal Oxidation State during H2-Assisted NH3-SCR of NOx over Alumina-Supported Silver and Indium
Abstract
1. Introduction
2. Results
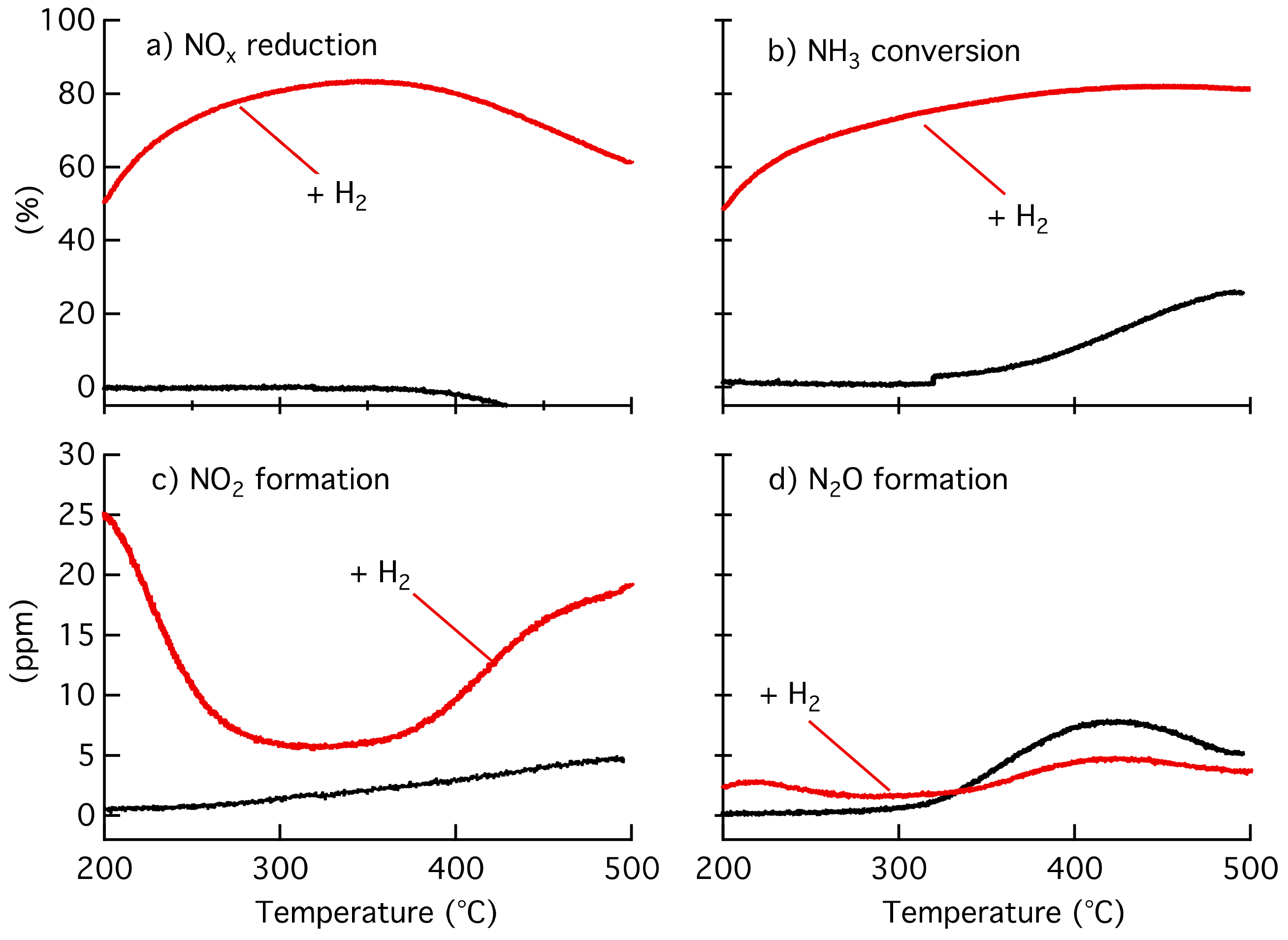
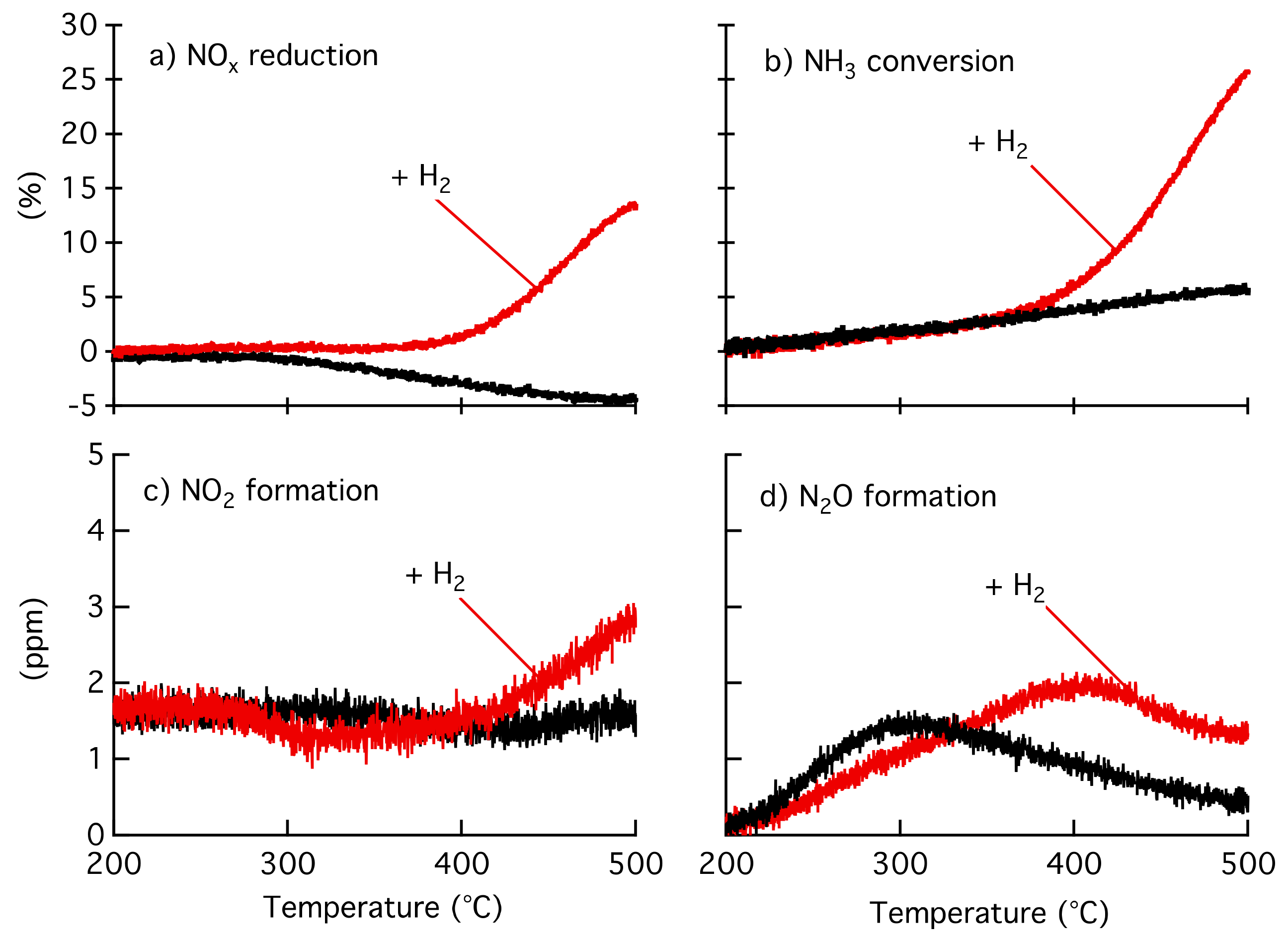
2.1. Catalytic Activity
2.2. Surface Acidity
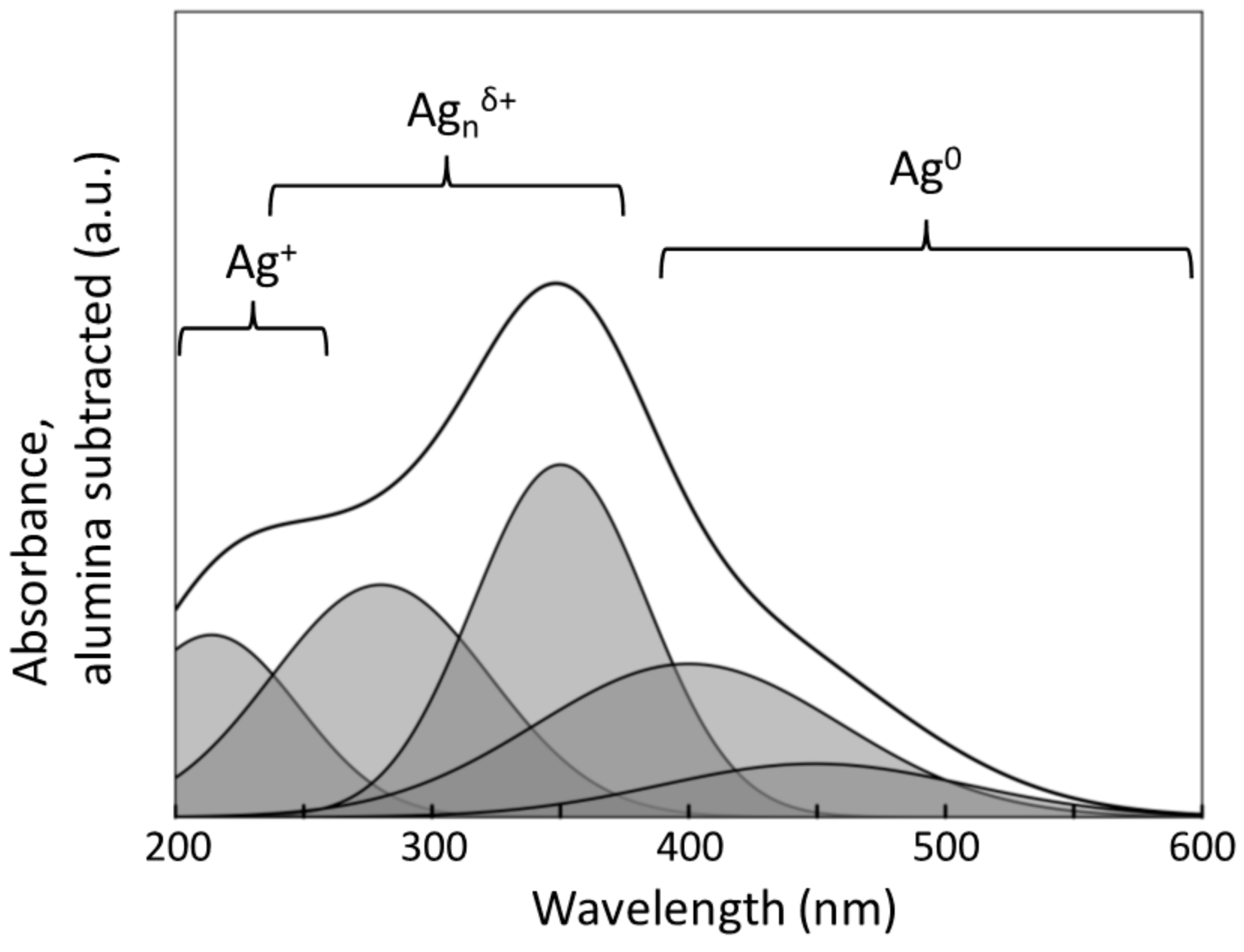
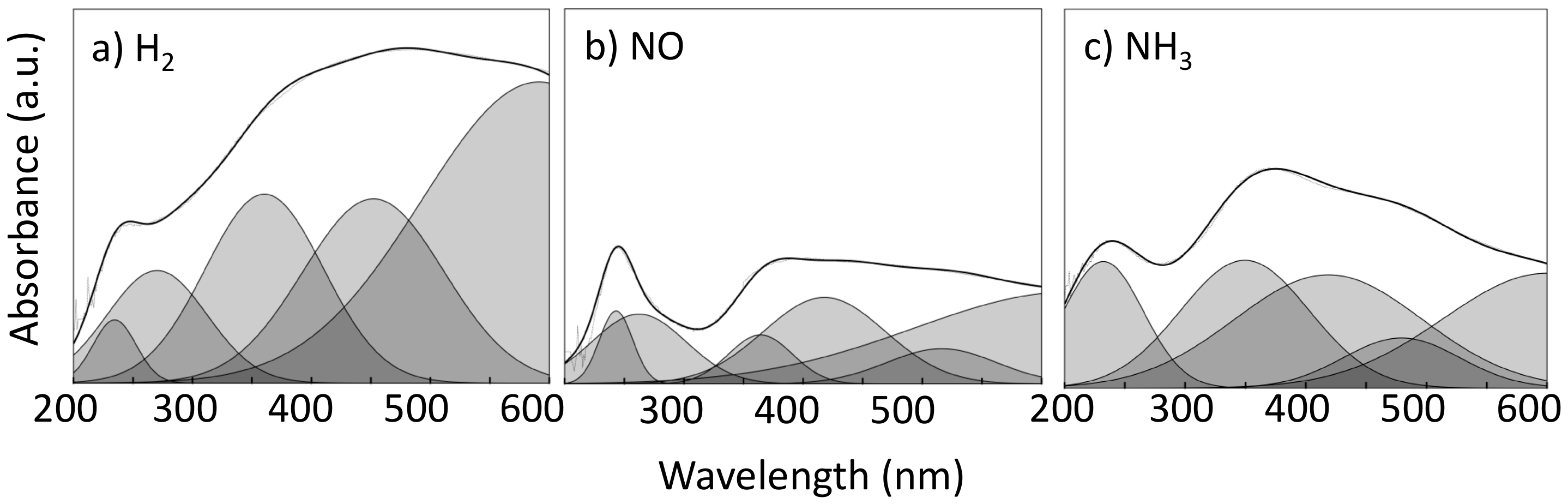
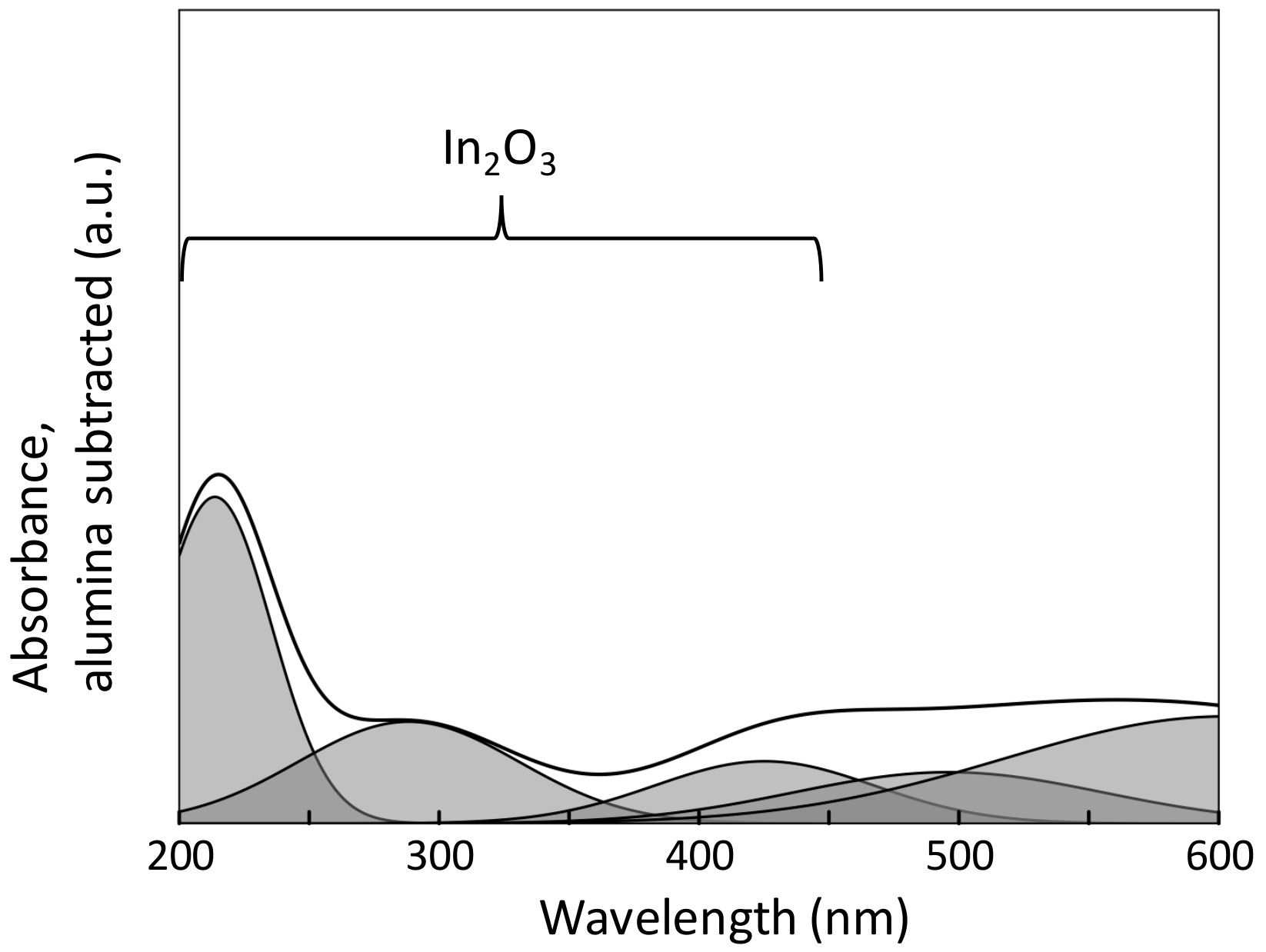
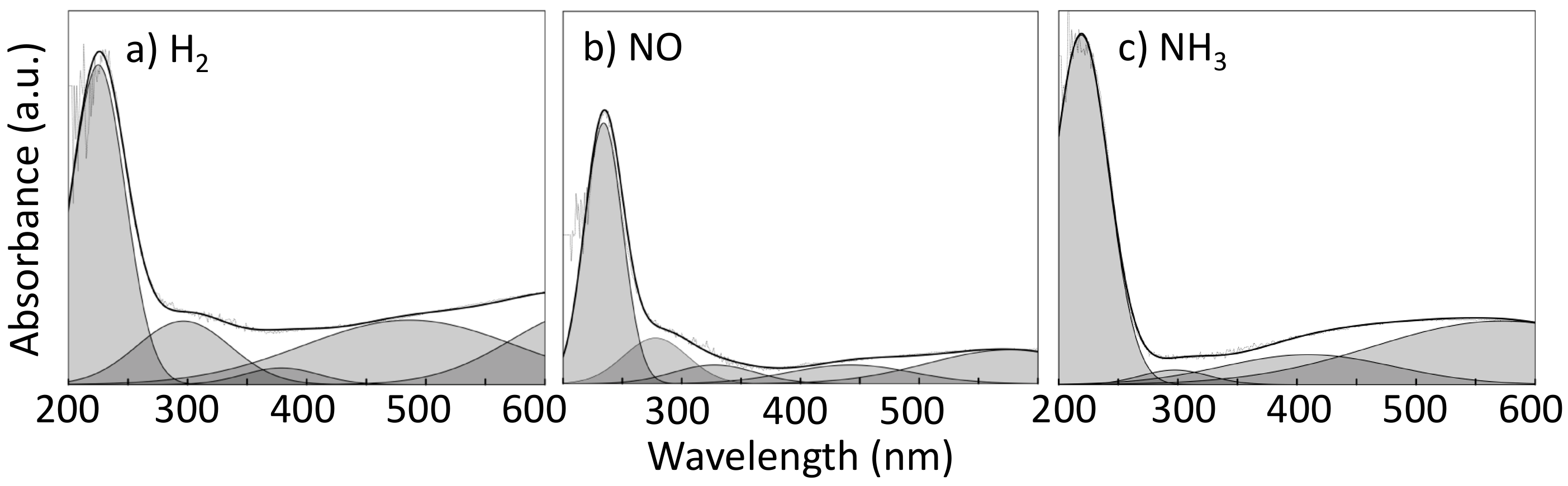
2.3. Characterization of the Oxidation State of the Active Phase
2.4. Evaluation of Surface Species
2.4.1. Assignment of NO Absorption Bands
2.4.2. Assignment of NH3 Absorption Bands
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation and Basic Characterization
4.2. Lean NOx Reduction Experiments
4.3. UV-Vis Spectroscopy
4.4. In Situ DRIFTS
4.5. NH3-TPD
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.H.; Zhao, H.W.; Haller, G.; Li, Y.D. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Shishkin, A.; Shwan, S.; Pingel, T.N.; Olsson, E.; Clemens, A.; Carlsson, P.A.; Härelind, H.; Skoglundh, M. Functionalization of SSZ-13 and Fe-Beta with copper by NH3 and NO facilitated solid-state ion-exchange. Catalysts 2017, 7, 232. [Google Scholar] [CrossRef]
- Clemens, A.K.S.; Shishkin, A.; Carlsson, P.A.; Skoglundh, M.; Martinez-Casado, F.J.; Matej, Z.; Balmes, O.; Härelind, H. Reaction-driven ion exchange of copper into zeolite SSZ-13. ACS Catal. 2015, 5, 6209–6218. [Google Scholar] [CrossRef]
- Gunnarsson, F.; Pihl, J.A.; Toops, T.J.; Skoglundh, M.; Härelind, H. Lean NOx reduction over Ag/alumina catalysts via ethanol-SCR using ethanol/gasoline blends. Appl. Catal. B Environ. 2017, 202, 42–50. [Google Scholar] [CrossRef]
- Matarrese, R.; Ingelsten, H.H.; Skoglundh, M. Aspects of reducing agent and role of amine species in the reduction of NO over H-ZSM-5 in oxygen excess. J. Catal. 2008, 258, 386–392. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Satokawa, S. Enhancing the NO/C3H8/O2 reaction by using H2 over Ag/Al2O3 catalysts under lean-exhaust conditions. Chem. Lett. 2000, 29, 294–295. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Hardacre, C.; Hill, C.J. Structural investigation of the promotional effect of hydrogen during the selective catalytic reduction of NOx with hydrocarbons over Ag/Al2O3 catalysts. J. Phys. Chem. B 2005, 109, 4805–4807. [Google Scholar] [CrossRef] [PubMed]
- Satokawa, S.; Shibata, J.; Shimizu, K.; Atsushi, S.; Hattori, T. Promotion effect of H2 on the low temperature activity of the selective reduction of NO by light hydrocarbons over Ag/Al2O3. Appl. Catal. B Environ. 2003, 42, 179–186. [Google Scholar] [CrossRef]
- Kannisto, H.; Ingelsten, H.H.; Skoglundh, M. Aspects of the role of hydrogen in H2-assisted HC-SCR over Ag-Al2O3. Top. Catal. 2009, 52, 1817. [Google Scholar] [CrossRef]
- Shibata, J.; Takada, Y.; Shichi, A.; Satokawa, S.; Satsuma, A.; Hattori, T. Ag cluster as active species for SCR of NO by propane in the presence of hydrogen over Ag-MFI. J. Catal. 2004, 222, 368–376. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R. A review of the effect of the addition of hydrogen in the selective catalytic reduction of NOx with hydrocarbons on silver catalysts. Top. Catal. 2006, 39, 53–58. [Google Scholar] [CrossRef]
- Boutros, M.; Starck, J.; de Tymowski, B.; Trichard, J.-M.; Da Costa, P. On the effect of poor metals (Al, Ga, In) on the NOx conversion in ethanol selective catalytic reduction. Top. Catal. 2009, 52, 1786. [Google Scholar] [CrossRef]
- Li, J.H.; Hao, J.M.; Cui, X.Y.; Fu, L.X. Influence of preparation methods of In2O3/Al2O3 catalyst on selective catalytic reduction of NO by propene in the presence of oxygen. Catal. Lett. 2005, 103, 75–82. [Google Scholar] [CrossRef]
- Maunula, T.; Kintaichi, Y.; Inaba, M.; Haneda, M.; Sato, K.; Hamada, H. Enhanced activity of in and Ga-supported sol-gel alumina catalysts for NO reduction by hydrocarbons in lean conditions. Appl. Catal. B Environ. 1998, 15, 291–304. [Google Scholar] [CrossRef]
- Maunula, T.; Kintaichi, Y.; Haneda, M.; Hamada, H. Preparation and reaction mechanistic characterization of sol-gel indium/alumina catalysts developed for NOx reduction by propene in lean conditions. Catal. Lett. 1999, 61, 121–130. [Google Scholar] [CrossRef]
- Park, P.W.; Ragle, C.S.; Boyer, C.L.; Balmer, M.L.; Engelhard, M.; McCready, D. In2O3/Al2O3 catalysts for NOx reduction in lean condition. J. Catal. 2002, 210, 97–105. [Google Scholar] [CrossRef]
- Erkfeldt, S.; Petersson, M.; Palmqvist, A. Alumina-supported In2O3, Ga2O3 and B2O3 catalysts for lean NOx reduction with dimethyl ether. Appl. Catal. B Environ. 2012, 117, 369–383. [Google Scholar] [CrossRef]
- Doronkin, D.E.; Fogel, S.; Tamm, S.; Olsson, L.; Khan, T.S.; Bligaard, T.; Gabrielsson, P.; Dahl, S. Study of the “Fast SCR”-like mechanism of H2-assisted SCR of NOx with ammonia over Ag/Al2O3. Appl. Catal. B Environ. 2012, 113, 228–236. [Google Scholar] [CrossRef]
- Ström, L.; Carlsson, P.-A.; Skoglundh, M.; Härelind, H. Hydrogen-assisted SCR of NOx over alumina-supported silver and indium catalysts using C2-hydrocarbons and oxygenates. Appl. Catal. B Environ. 2016, 181, 403–412. [Google Scholar] [CrossRef]
- Tamm, S.; Vallim, N.; Skoglundh, M.; Olsson, L. The influence of hydrogen on the stability of nitrates during H2-assisted SCR over Ag/Al2O3 catalysts—A drift study. J. Catal. 2013, 307, 153–161. [Google Scholar] [CrossRef]
- Sadokhina, N.A.; Doronkin, D.E.; Baeva, G.N.; Dahl, S.; Stakheev, A.Y. Reactivity of surface nitrates in H2-assisted SCR of NOx over Ag/Al2O3 catalyst. Top. Catal. 2013, 56, 737–744. [Google Scholar] [CrossRef]
- Kim, P.S.; Kim, M.K.; Cho, B.K.; Nam, I.-S.; Oh, S.H. Effect of H2 on denox performance of HC-SCR over Ag/Al2O3: Morphological, chemical and kinetic changes. J. Catal. 2013, 301, 65–76. [Google Scholar] [CrossRef]
- Thomas, C. On an additional promoting role of hydrogen in the H2-assisted C3H6-SCR of NOx on Ag/Al2O3: A lowering of the temperature of formation-decomposition of the organo-NOx intermediates? Appl. Catal. B Environ. 2015, 162, 454–462. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Kondratenko, V.A.; Richter, M.; Fricke, R. Influence of O2 and H2 on NO reduction by NH3 over Ag/Al2O3: A transient isotopic approach. J. Catal. 2006, 239, 23–33. [Google Scholar] [CrossRef]
- Burch, R.; Breen, J.P.; Meunier, F.C. A review of the selective reduction of NOx, with hydrocarbons under lean-burn conditions with non-zeolitic oxide and platinum group metal catalysts. Appl. Catal. B Environ. 2002, 39, 283–303. [Google Scholar] [CrossRef]
- Gunnarsson, F.; Granlund, M.Z.; Englund, M.; Dawody, J.; Pettersson, L.J.; Härelind, H. Combining HC-SCR over Ag/Al2O3 and hydrogen generation over Rh/CeO2-ZrO2 using biofuels: An integrated system approach for real applications. Appl. Catal. B Environ. 2015, 162, 583–592. [Google Scholar] [CrossRef]
- Shimizu, K.; Shibata, J.; Yoshida, H.; Satsuma, A.; Hattori, T. Silver-alumina catalysts for selective reduction of NO by higher hydrocarbons: Structure of active sites and reaction mechanism. Appl. Catal. B Environ. 2001, 30, 151–162. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Meunier, F.C.; Avalos-Borja, M.; Breen, J.P.; Pestryakov, A. On the nature of the silver phases of Ag/Al2O3 catalysts for reactions involving nitric oxide. Appl. Catal. B Environ. 2002, 36, 287–297. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Hungria, A.B.; Martinez-Arias, A.; Fuerte, A.; Fernandez-Garcia, M.; Anderson, J.A.; Conesa, J.C.; Soria, J. Nature and catalytic role of active silver species in the lean NOx reduction with C3H6 in the presence of water. J. Catal. 2003, 217, 310–323. [Google Scholar] [CrossRef]
- Kannisto, H.; Ingelsten, H.H.; Skoglundh, M. Ag-Al2O3 catalysts for lean NOx reduction-influence of preparation method and reductant. J. Mol. Catal. A Chem. 2009, 302, 86–96. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Satsuma, A. Reaction mechanism of H2-promoted selective catalytic reduction of NO with NH3 over Ag/Al2O3. J. Phys. Chem. C 2007, 111, 2259–2264. [Google Scholar] [CrossRef]
- Shibata, J.; Shimizu, K.; Takada, Y.; Shichia, A.; Yoshida, H.; Satokawa, S.; Satsuma, A.; Hattori, T. Structure of active Ag clusters in Ag zeolites for SCR of NO by propane in the presence of hydrogen. J. Catal. 2004, 227, 367–374. [Google Scholar] [CrossRef]
- Meunier, F.C.; Breen, J.P.; Zuzaniuk, V.; Olsson, M.; Ross, J.R.H. Mechanistic aspects of the selective reduction of NO by propene over alumina and silver-alumina catalysts. J. Catal. 1999, 187, 493–505. [Google Scholar] [CrossRef]
- Tamm, S.; Fogel, S.; Gabrielsson, P.; Skoglundh, M.; Olsson, L. The effect of the gas composition on hydrogen-assisted NH3-SCR over Ag/Al2O3. Appl. Catal. B Environ. 2013, 136, 168–176. [Google Scholar] [CrossRef]
- Richter, M.; Fricke, R.; Eckelt, R. Unusual activity enhancement of NO conversion over Ag/Al2O3 by using a mixed NH3/H2 reductant under lean conditions. Catal. Lett. 2004, 94, 115–118. [Google Scholar] [CrossRef]
- Kondratenko, V.A.; Bentrup, U.; Richter, M.; Hansen, T.W.; Kondratenko, E.V. Mechanistic aspects of N2O and N2 formation in no reduction by NH3 over Ag/Al2O3: The effect of O2 and H2. Appl. Catal. B Environ. 2008, 84, 497–504. [Google Scholar] [CrossRef]
- Poisson, R.; Brunelle, J.-P.; Nortier, P. Catalyst Supports and Supported Catalysts. Theoretical and Applied Concepts; Stiles, A.B., Ed.; Stoneham: Butterworth, Malasia, 1987; pp. 44–47. [Google Scholar]
- Knozinger, H.; Ratnasamy, P. Catalytic aluminas—Surface models and characterization of surface sites. Catal. Rev. Sci. Eng. 1978, 17, 31–70. [Google Scholar] [CrossRef]
- Digne, M.; Sautet, P.; Raybaud, P.; Euzen, P.; Toulhoat, H. Use of DFT to achieve a rational understanding of acid-basic properties of gamma-alumina surfaces. J. Catal. 2004, 226, 54–68. [Google Scholar] [CrossRef]
- Sazama, P.; Capek, L.; Drobna, H.; Sobalik, Z.; Dedecek, J.; Arve, K.; Wichterlova, B. Enhancement of decane-SCR-NOx over Ag/alumina by hydrogen. Reaction kinetics and in situ FTIR and UV-vis study. J. Catal. 2005, 232, 302–317. [Google Scholar] [CrossRef]
- Shi, C.; Cheng, M.J.; Qu, Z.P.; Bao, X.H. Investigation on the catalytic roles of silver species in the selective catalytic reduction of NOx with methane. Appl. Catal. B Environ. 2004, 51, 171–181. [Google Scholar] [CrossRef]
- Miao, S.J.; Wang, Y.; Ma, D.; Zhu, Q.J.; Zhou, S.T.; Su, L.L.; Tan, D.L.; Bao, X.H. Effect of Ag+ cations on nonoxidative activation of methane to C2-hydrocarbons. J. Phys. Chem. B 2004, 108, 17866–17871. [Google Scholar] [CrossRef]
- Musi, A.; Massiani, P.; Brouri, D.; Trichard, J.-M.; Da Costa, P. On the characterisation of silver species for SCR of NOx with ethanol. Catal. Lett. 2009, 128, 25–30. [Google Scholar] [CrossRef]
- Sato, K.; Yoshinari, T.; Kintaichi, Y.; Haneda, M.; Hamada, H. Remarkable promoting effect of rhodium on the catalytic performance of Ag/Al2O3 for the selective reduction of NO with decane. Appl. Catal. B Environ. 2003, 44, 67–78. [Google Scholar] [CrossRef]
- Männikkö, M.; Skoglundh, M.; Ingelsten, H.H. Selective catalytic reduction of NOx with methanol over supported silver catalysts. Appl. Catal. B Environ. 2012, 119, 256–266. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Davydov, A.A. Study of supported silver states by the method of electron spectroscopy of diffuse reflectance. J. Electron Spectrosc. Relat. Phenom. 1995, 74, 195–199. [Google Scholar] [CrossRef]
- She, X.; Flytzani-Stephanopoulos, M. The role of AgOAl species in silver-alumina catalysts for the selective catalytic reduction of NOx with methane. J. Catal. 2006, 237, 79–93. [Google Scholar] [CrossRef]
- Lv, J.; Kako, T.; Li, Z.; Zou, Z.; Ye, J. Synthesis and photocatalytic activities of NaNbO3 rods modified by In2O3 nanoparticles. J. Phys. Chem. C 2010, 114, 6157–6162. [Google Scholar] [CrossRef]
- Yang, X.; Xu, J.; Wong, T.; Yang, Q.; Lee, C.-S. Synthesis of In2O3-In2S3 core-shell nanorods with inverted type-I structure for photocatalytic H2 generation. Phys. Chem. Chem. Phys. 2013, 15, 12688–12693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Guo, L.; Shen, X.; Ji, Z.; Chen, K.; Zhou, H. Monodispersed In2O3 mesoporous nanospheres: One-step facile synthesis and the improved gas-sensing performance. Sens. Actuators B Chem. 2015, 220, 977–985. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Zhao, Q.; Zhang, Q.; Tade, M.; Liu, S. Fabrication of α-Fe2O3/In2O3 composite hollow microspheres: A novel hybrid photocatalyst for toluene degradation under visible light. J. Colloid Interface Sci. 2015, 457, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Z.; Huang, S.B.; Jian, Z.C.; Pan, M.L.; Zhang, Y.Q.; Fei, Z.B.; Xu, X.R. Enhancement of the visible light photocatalytic activity of heterojunction In2O3/BiVO4 composites. Appl. Phys. A Mater. Sci. Process. 2015, 120, 1529–1535. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Liu, R.; Mao, G. Controlled synthesis of monodispersed sub-50 nm nanoporous In2O3 spheres and their photoelectrochemical performance. Eur. J. Inorg. Chem. 2015, 2015, 845–851. [Google Scholar] [CrossRef]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Underwood, G.M.; Miller, T.M.; Grassian, V.H. Transmission FT-IR and knudsen cell study of the heterogeneous reactivity of gaseous nitrogen dioxide on mineral oxide particles. J. Phys. Chem. A 1999, 103, 6184–6190. [Google Scholar] [CrossRef]
- Satsuma, A.; Shimizu, K. In situ FT/IR study of selective catalytic reduction of NO over alumina-based catalysts. Prog. Energy Combust. Sci. 2003, 29, 71–84. [Google Scholar] [CrossRef]
- Adams, E.C.; Skoglundh, M.; Gabrielsson, P.; Carlsson, P.A. Passive SCR: The effect of H2 to NO ratio on the formation of NH3 over alumina supported platinum and palladium catalysts. Top. Catal. 2016, 59, 970–975. [Google Scholar] [CrossRef]
- Adams, E.C.; Skoglundh, M.; Folic, M.; Bendixen, E.C.; Gabrielsson, P.; Carlsson, P.-A. Ammonia formation over supported platinum and palladium catalysts. Appl. Catal. B Environ. 2015, 165, 10–19. [Google Scholar] [CrossRef]
- Acke, F.; Westerberg, B.; Skoglundh, M. Selective reduction of no by HNCO over PT promoted Al2O3. J. Catal. 1998, 179, 528–536. [Google Scholar] [CrossRef]
- Centeno, M.A.; Carrizosa, I.; Odriozola, J.A. In situ drifts study of the SCR reaction of NO with NH3 in the presence of O2 over lanthanide doped V2O5/Al2O3 catalysts. Appl. Catal. B Environ. 1998, 19, 67–73. [Google Scholar] [CrossRef]
- Wallin, M.; Grönbeck, H.; Spetz, A.L.; Skoglundh, M. Vibrational study of ammonia adsorption on Pt/SiO2. Appl. Surf. Sci. 2004, 235, 487–500. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, Q.; Zhang, S. The enhancement for SCR of NO by NH3 over the H2 or CO pretreated Ag/gamma-Al2O3 catalyst. Phys. Chem. Chem. Phys. 2014, 16, 12560–12566. [Google Scholar] [CrossRef] [PubMed]
- Tamm, S. The role of silver for the H2-effect in H2-assisted selective catalytic reduction of NOx with NH3 over Ag/Al2O3. Catal. Lett. 2013, 143, 957–965. [Google Scholar] [CrossRef]
- Anderson, J.R.; Pratt, K.C. Introduction to Characterization and Testing of Catalysts; Academic Press Inc.: Melbourne, Australia, 1985. [Google Scholar]
- Kannisto, H.; Karatzas, X.; Edvardsson, J.; Pettersson, L.J.; Ingelsten, H.H. Efficient low temperature lean NOx reduction over Ag/Al2O3—A system approach. Appl. Catal. B Environ. 2011, 104, 74–83. [Google Scholar] [CrossRef]
- Wang-Hansen, C.; Kamp, C.J.; Skoglundh, M.; Andersson, B.; Carlsson, P.-A. Experimental method for kinetic studies of gas-solid reactions: Oxidation of carbonaceous matter. J. Phys. Chem. C 2011, 115, 16098–16108. [Google Scholar] [CrossRef]
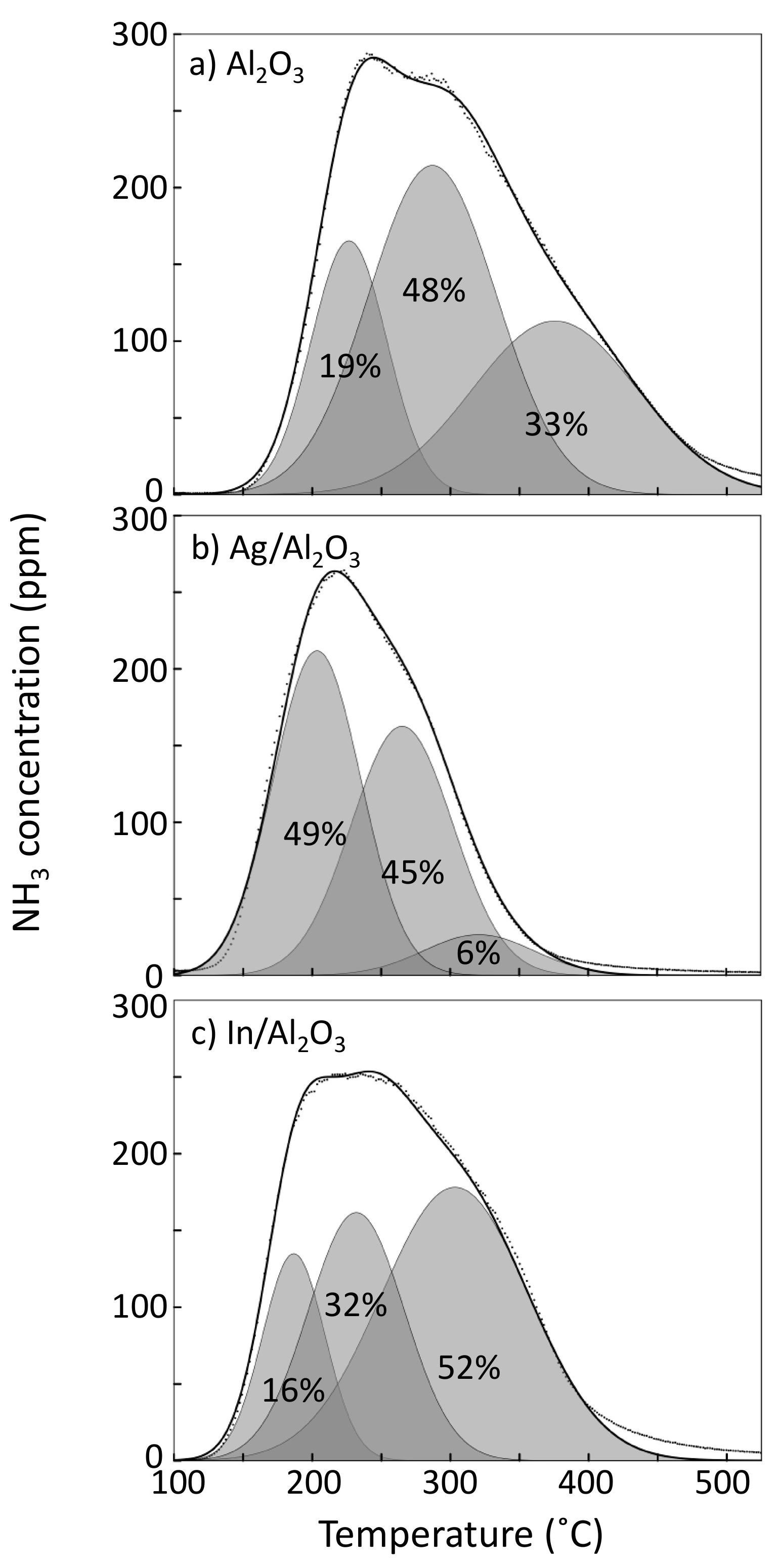
| Sample | Desorbed NH3 (mmol/cm2) |
|---|---|
| γ-Al2O3 | 11.2 |
| Ag/Al2O3 | 7.4 |
| In/Al2O3 | 9.5 |
| Species | Wavelength [nm] | Reference |
|---|---|---|
| Isolated Ag+-ions | 192–250 | [32] |
| 196, 212, 224 | [42] | |
| 220 | [43] | |
| 215–240 | [44] | |
| 212, 260 | [44] | |
| Agnδ+-clusters | 260–370 | [37] |
| 238–272 | [45,46] | |
| 322 | [42] | |
| 350, 285 | [32] | |
| Ag0 | >390 | [28,29,32,42,46,47,48] |
| Sequence | NO | H2 | NH3 |
|---|---|---|---|
| 1 | NO | ||
| 2 | NO | H2 | |
| 3 | NO | H2 | NH3 |
| 4 | H2 | NH3 | |
| 5 | NH3 | ||
| 6 | NO | NH3 | |
| 7 | NO | H2 | NH3 |
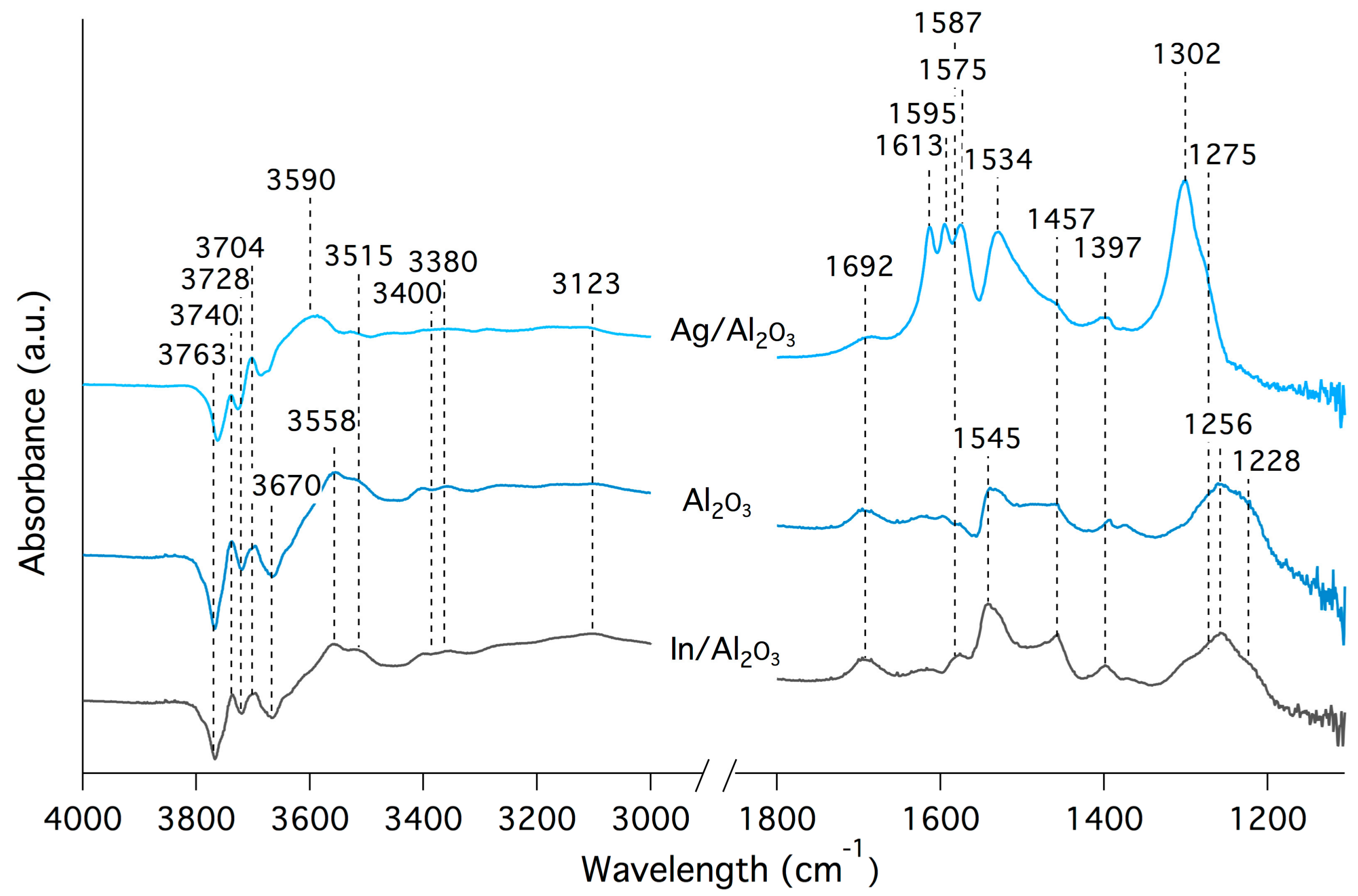
| Wavenumber (cm−1) | Appears in Sample | Surface Species | References |
|---|---|---|---|
| 1228 | Al2O3, In/Al2O3 | Nitrite | [21] |
| 1256 | Al2O3, In/Al2O3 | Bidentate nitrate | [21,58] |
| 1302 | Ag/Al2O3 | Monodentate nitrate | [21] |
| 1534 | Ag/Al2O3 | Monodentate nitrate | [21] |
| 1545 | Al2O3, In/Al2O3 | Monodentate nitrate | [21] |
| 1575 | Ag/Al2O3 | Monodentate nitrate | [21] |
| 1595 | Ag/Al2O3 | Bidentate nitrate | [21] |
| 1613 | Ag/Al2O3 | Bridged nitrate | [21,59,60] |
| Wavenumber (cm−1) | Vibration | Reference |
|---|---|---|
| 1275 | Symmetric bending of surface coordinated NH3 | [61] |
| 1397 | NH4+ ions at Brønstedt acidic site | [62,63] |
| 1587 | Asymmetric bending of surface coordinated NH3 | [61] |
| 1692 | NH4+ ions at Brønstedt acidic site | [62,63] |
| 3380 | Symmetric NH stretching vibrations of NH3 hydrogen bonded to surface OH | [62,63] |
| 3400 | Asymmetric NH stretching vibrations of NH3 hydrogen bonded to surface OH | [63] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ström, L.; Carlsson, P.-A.; Skoglundh, M.; Härelind, H. Surface Species and Metal Oxidation State during H2-Assisted NH3-SCR of NOx over Alumina-Supported Silver and Indium. Catalysts 2018, 8, 38. https://doi.org/10.3390/catal8010038
Ström L, Carlsson P-A, Skoglundh M, Härelind H. Surface Species and Metal Oxidation State during H2-Assisted NH3-SCR of NOx over Alumina-Supported Silver and Indium. Catalysts. 2018; 8(1):38. https://doi.org/10.3390/catal8010038
Chicago/Turabian StyleStröm, Linda, Per-Anders Carlsson, Magnus Skoglundh, and Hanna Härelind. 2018. "Surface Species and Metal Oxidation State during H2-Assisted NH3-SCR of NOx over Alumina-Supported Silver and Indium" Catalysts 8, no. 1: 38. https://doi.org/10.3390/catal8010038
APA StyleStröm, L., Carlsson, P.-A., Skoglundh, M., & Härelind, H. (2018). Surface Species and Metal Oxidation State during H2-Assisted NH3-SCR of NOx over Alumina-Supported Silver and Indium. Catalysts, 8(1), 38. https://doi.org/10.3390/catal8010038