Flower-Like Au–CuO/Bi2WO6 Microsphere Catalysts: Synthesis, Characterization, and Their Catalytic Performances for CO Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD
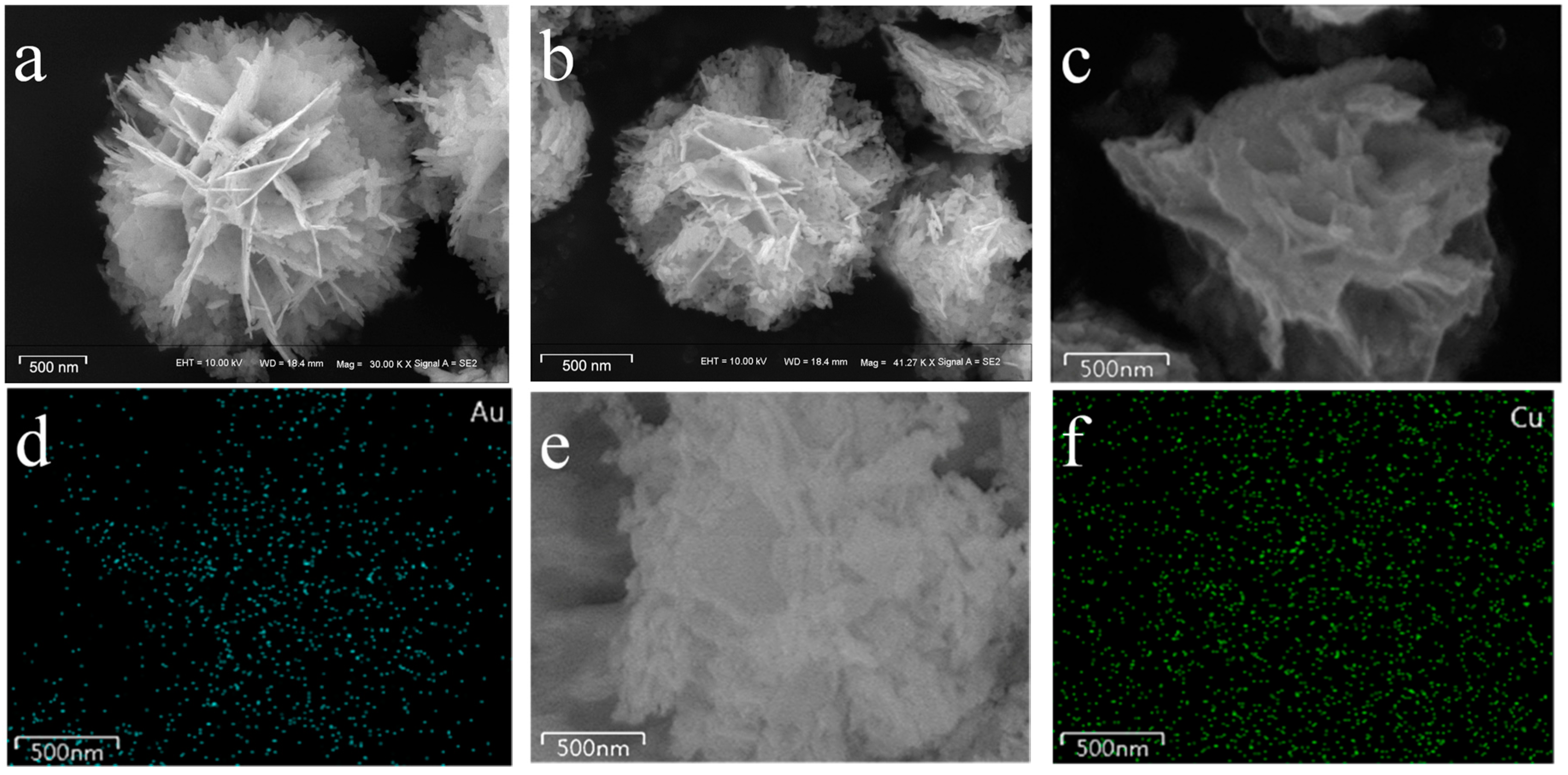
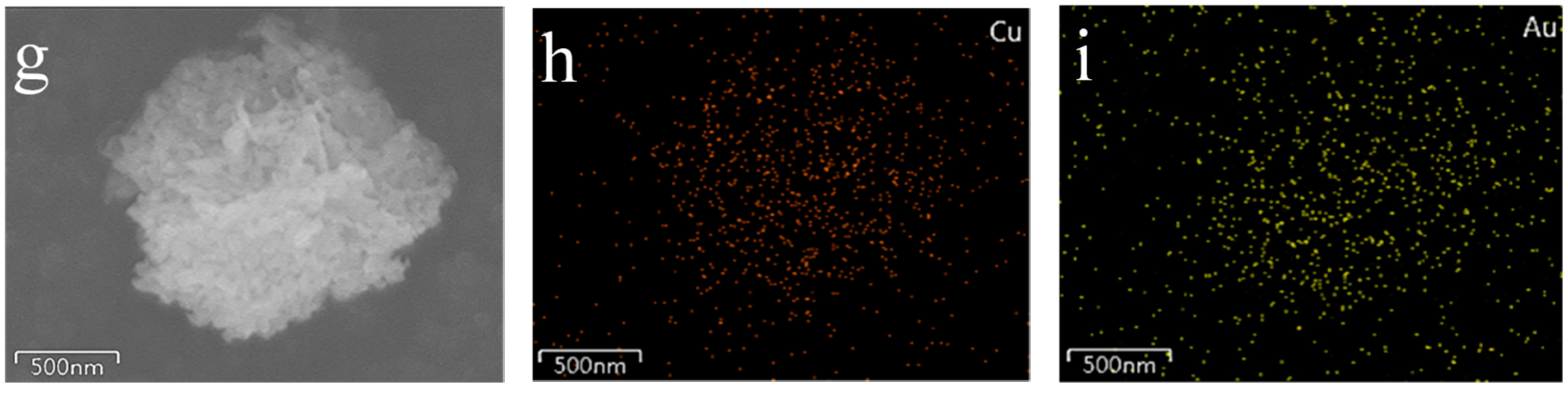
2.2. SEM
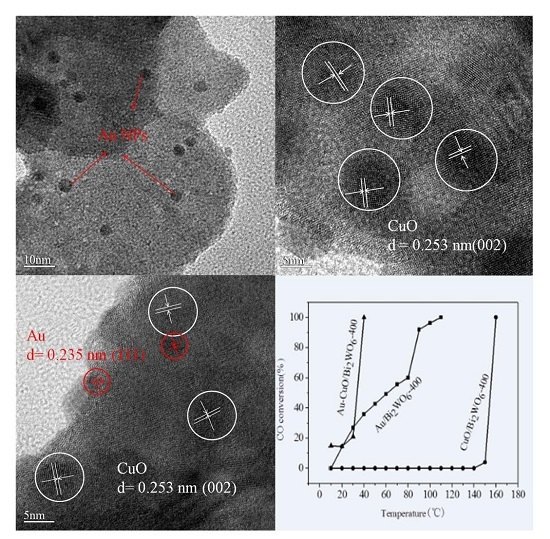
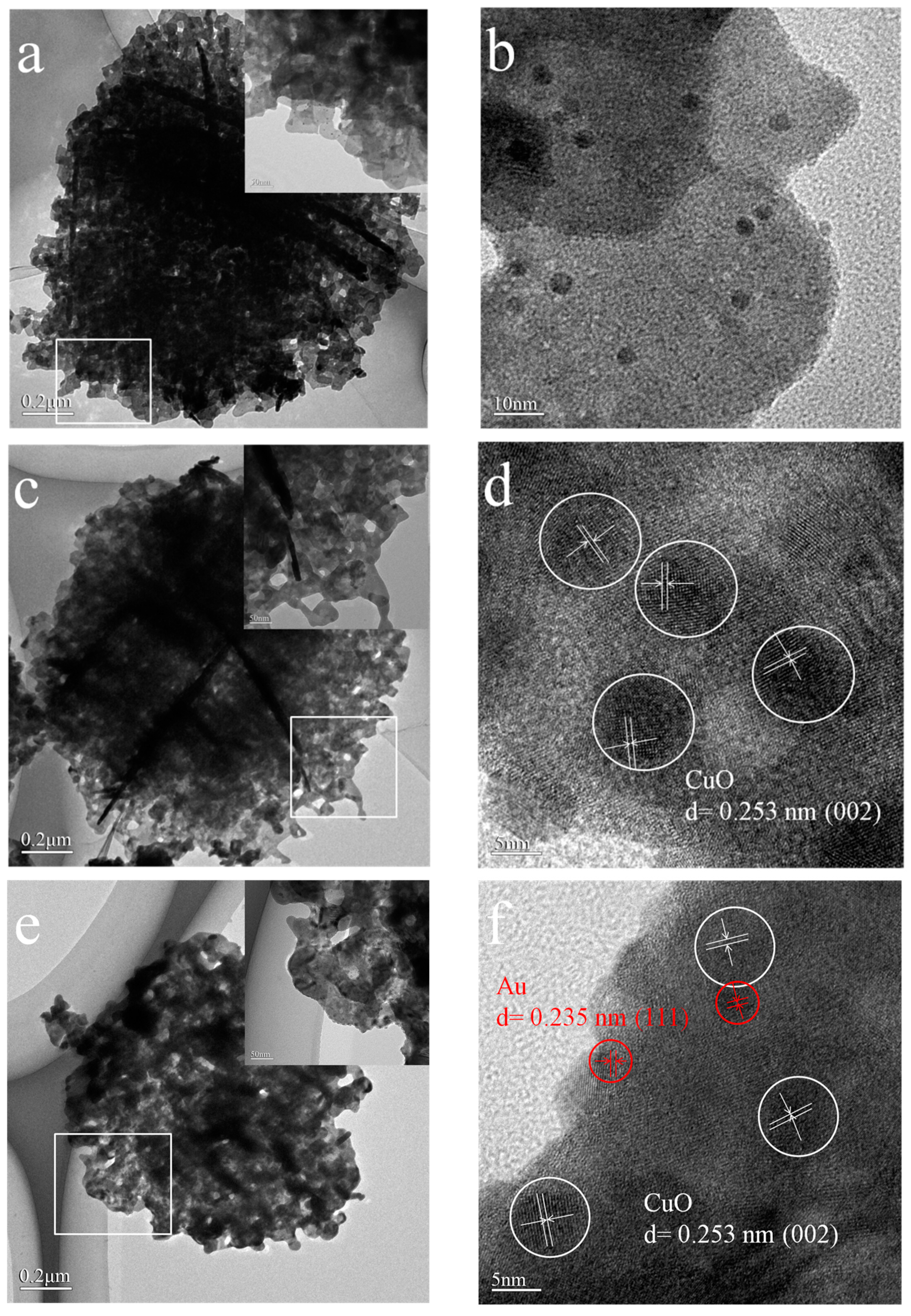
2.3. TEM
2.4. UV–Vis Spectra
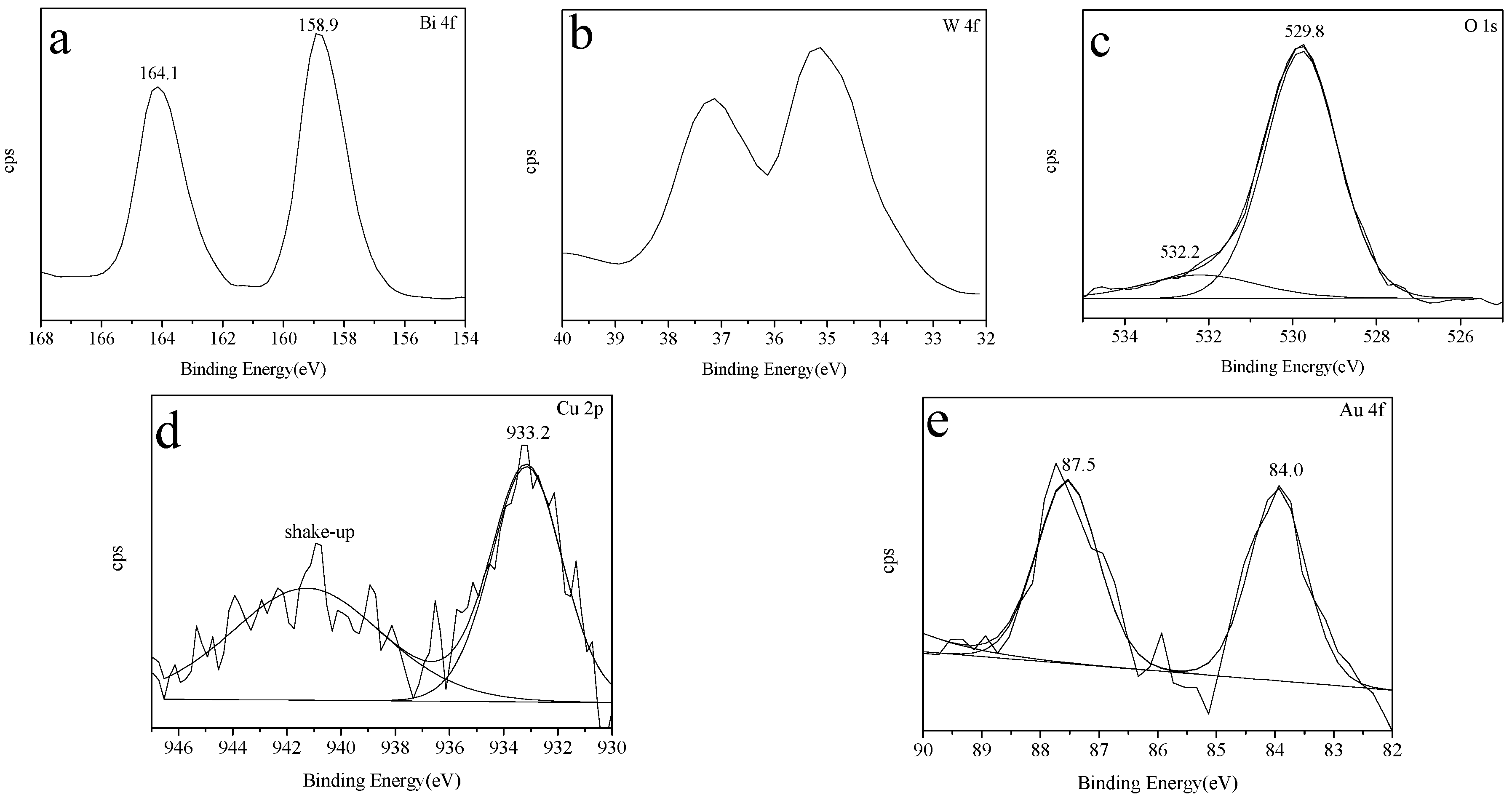
2.5. XPS Spectra
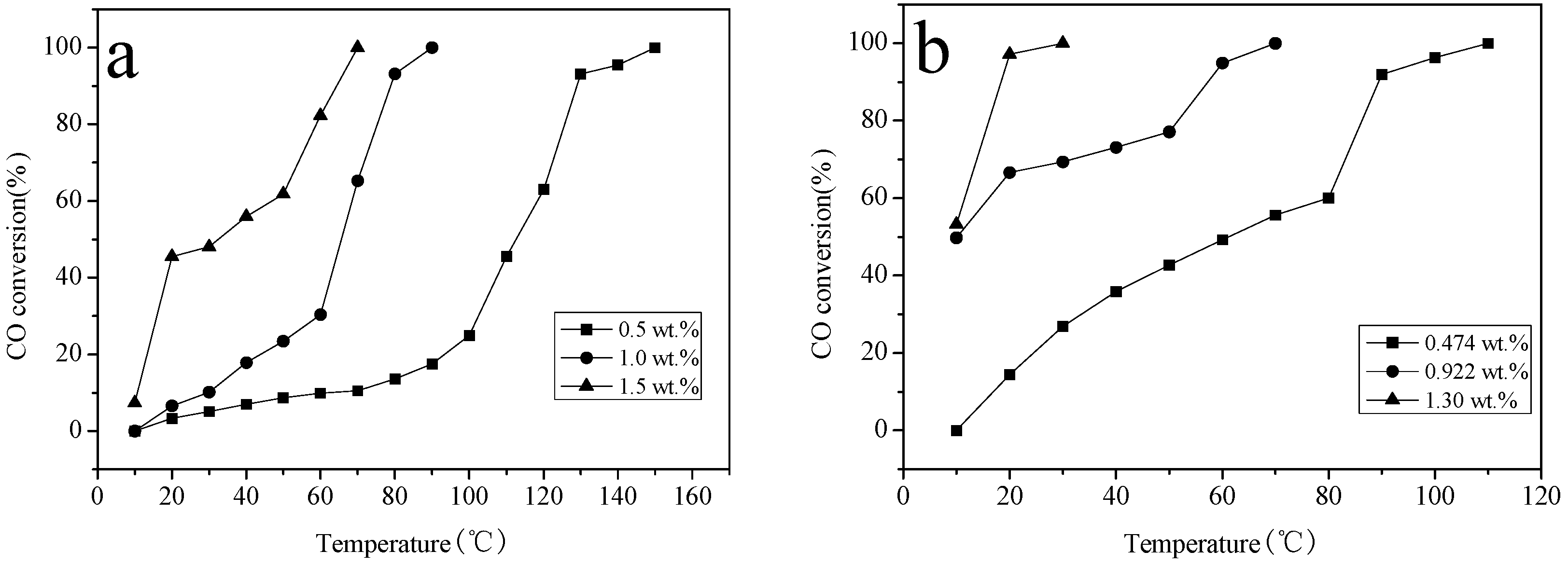
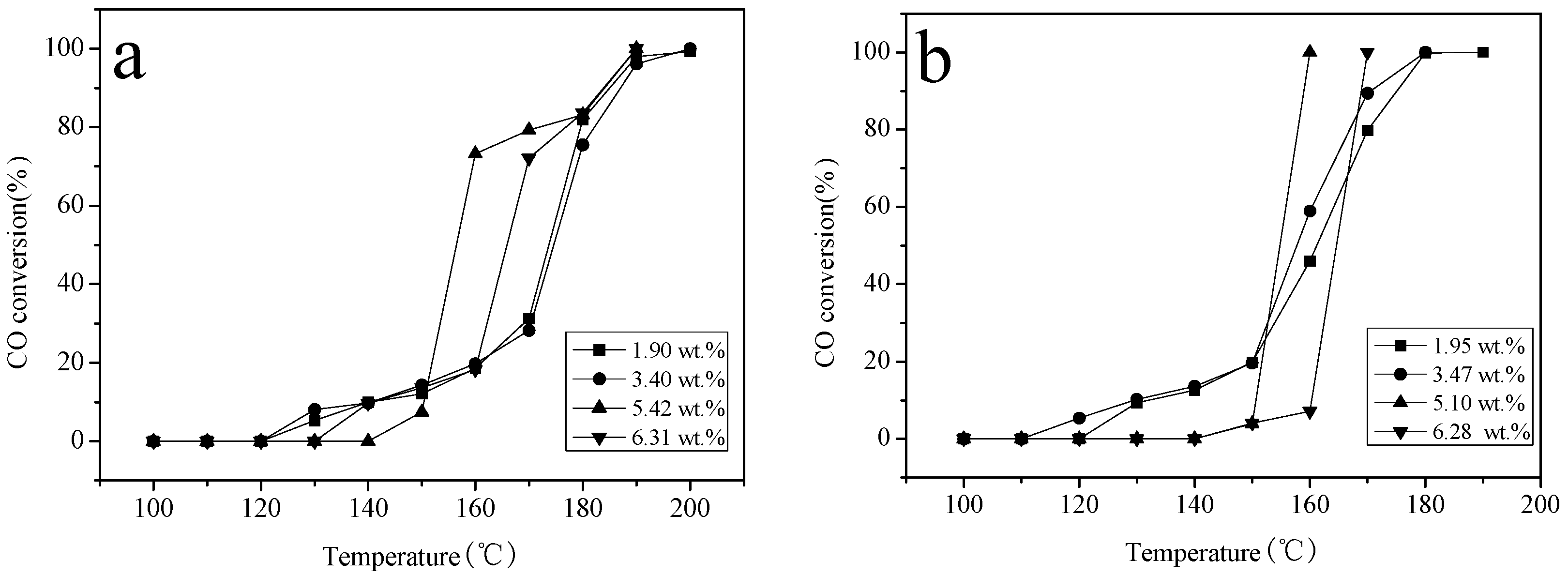
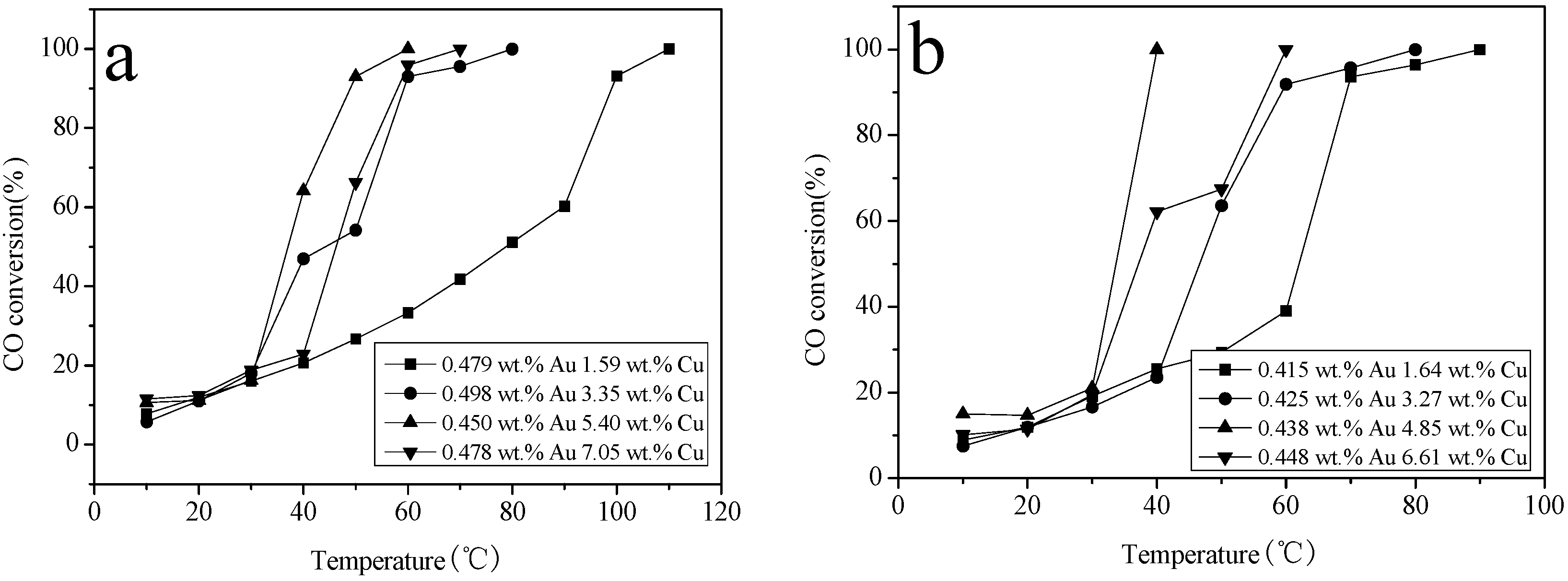
2.6. Catalytic Activity Studies
3. Experimental
3.1. Synthesis of Flower-Like Bi2WO6 Microspheres
3.2. Synthesis of Au/Bi2WO6
3.3. Synthesis of CuO/Bi2WO6
3.4. Synthesis of Au–CuO/Bi2WO6
3.5. Characterization
3.6. Evaluation of Catalytic Performance of Catalysts for Catalytic CO Oxidation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, J.Q.; Sun, C.X.; Li, N.; Jia, A.P.; Luo, M.F. Kinetic study of CO oxidation over CuO/MO2 (M = Si, Ti and Ce) catalysts. Appl. Surf. Sci. 2013, 287, 124–134. [Google Scholar] [CrossRef]
- Qian, K.; Qian, Z.X.; Hua, Q.; Jiang, Z.Q.; Huang, W.X. Structure-activity relationship of CuO/MnO2 catalysts in CO oxidation. Appl. Surf. Sci. 2013, 273, 357–363. [Google Scholar] [CrossRef]
- Deng, X.Q.; Zhu, B.; Li, X.S.; Liu, J.L.; Zhu, X.B.; Zhu, A.M. Visible-light photocatalytic oxidation of CO over plasmonic Au/TiO2: Unusual features of oxygen plasma activation. Appl. Catal. B Environ. 2016, 188, 48–55. [Google Scholar] [CrossRef]
- Tomita, A.; Shimizu, K.; Tai, Y. Effect of metal oxide promoters on low temperature CO oxidation over water-pretreated Pt/alumina catalysts. Catal. Lett. 2014, 144, 1689–1695. [Google Scholar] [CrossRef]
- Wang, C.; Wen, C.; Lauterbach, J.; Sasmaz, E. Superior oxygen transfer ability of Pd/MnOx–CeO2 for enhanced low temperature CO oxidation activity. Appl. Catal. B Environ. 2017, 206, 1–8. [Google Scholar] [CrossRef]
- Inui, T.; Ono, Y.; Takagi, Y.; Kim, J.B. Oxygen spillover effects induced by Rh-modification on the low-temperature oxidation of CO over Cu-incorporated zeolite A studied by the forced oscillating reaction method. Appl. Catal. A Gen. 2000, 202, 215–222. [Google Scholar] [CrossRef]
- Margitfalvi, J.L.; Hegedúś, M.; Szegedi, Á.; Sajó, I. Modification of Au/MgO catalysts used in low temperature CO oxidation with Mn and Fe. Appl. Catal. A Gen. 2004, 272, 87–97. [Google Scholar] [CrossRef]
- Li, S.N.; Zhu, H.Q.; Qin, Z.F.; Wang, G.F.; Zhang, Y.G.; Wu, Z.W.; Li, Z.K.; Chen, G.; Dong, W.W.; Wu, Z.H.; et al. Morphologic effects of nano CeO2-TiO2 on the performance of Au/CeO2-TiO2 catalysts in low-temperature CO oxidation. Appl. Catal. B Environ. 2014, 144, 498–506. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 2, 405–408. [Google Scholar] [CrossRef]
- Albadi, J.; Alihoseinzadeh, A.; Razeghi, A. Novel metal oxide nanocomposite of Au/CuO–ZnO for recyclable catalytic aerobic oxidation of alcohols in water. Catal. Commun. 2014, 49, 1–5. [Google Scholar] [CrossRef]
- Harzandi, A.M.; Tiwari, J.N.; Lee, H.S.; Jeon, H.; Cho, W.J.; Lee, G.; Baik, J.; Kwak, J.H.; Kim, K.S. Efficient CO oxidation by 50-facet Cu2O nanocrystals coated with CuO nanoparticles. ACS Appl. Mater. Interface 2017, 9, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.L.; Wang, Y.; Yu, X.L.; Wang, S.R.; Wu, S.H.; Yuan, Z.Y. Mesoporous CuO–Fe2O3 composite catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. B Environ. 2008, 79, 26–34. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; El-Wahab, M.M.M.A.; Goda, M.N. Synthesis and characterization of pure and (Ce, Zr, Ag) doped mesoporous CuO-Fe2O3 as highly efficient and stable nanocatalysts for CO oxidation at low temperature. Appl. Surf. Sci. 2016, 390, 649–665. [Google Scholar] [CrossRef]
- Zheng, X.C.; Zhang, X.L.; Wang, X.Y.; Wang, S.R.; Wu, S.H. Preparation and characterization of CuO/CeO2 catalysts and their applications in low-temperature CO oxidation. Appl. Catal. A Gen. 2005, 295, 142–149. [Google Scholar] [CrossRef]
- Zhu, P.F.; Li, J.; Zuo, S.F.; Zhou, R.X. Preferential oxidation properties of CO in excess hydrogen over CuO–CeO2 catalyst prepared by hydrothermal method. Appl. Surf. Sci. 2008, 255, 2903–2909. [Google Scholar] [CrossRef]
- Zeng, S.H.; Chen, T.J.; Liu, K.W.; Su, H.Q. Promotion effect of metal oxides on inverse CeO2/CuO catalysts for preferential oxidation of CO. Catal. Commun. 2014, 45, 16–20. [Google Scholar] [CrossRef]
- Zeng, S.H.; Zhang, W.L.; Śliwa, M.; Su, H.Q. Comparative study of CeO2/CuO and CuO/CeO2 catalysts on catalytic performance for preferential CO oxidation. Int. J. Hydrog. Energy 2013, 38, 3597–3605. [Google Scholar] [CrossRef]
- Huang, J.; Kang, Y.F.; Wang, L.W.; Yang, T.L.; Wang, Y.; Wang, S.R. Mesoporous CuO/TixZr1−xO2 catalysts for low-temperature CO oxidation. Catal. Commun. 2011, 15, 41–45. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Gurbani, A.; Gonźalez-Marcos, M.P.; Gutiérrez-Ortiz, M.A. CO oxidation on CexZr1−xO2-supported CuO catalysts: Correlation between activity and support composition. Appl. Catal. A Gen. 2010, 387, 119–128. [Google Scholar] [CrossRef]
- Gamboa-Rosales, N.K.; Ayastuy, J.L.; Boukha, Z.; Bion, N.; Duprez, D.; Pérez-Omil, J.A.; del Río, E.; Gutiérrez-Ortiz, M.A. Ceria-supported Au-CuO and Au–Co3O4 catalysts for CO oxidation: An 18O/16O isotopic exchange study. Appl. Catal. B Environ. 2015, 168, 87–97. [Google Scholar] [CrossRef]
- Pongstabodee, S.; Monyanon, S.; Luengnaruemitchai, A. Applying a face-centered central composite design to optimize the preferential CO oxidation over a PtAu/CeO2–ZnO catalyst. Int. J. Hydrog. Energy 2012, 37, 4749–4761. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.Q.; Yang, X.F.; Zhang, T.; Mou, C.Y.; Su, D.S.; Li, J. Synthesis of thermally stable and highly active bimetallic Au–Ag nanoparticles on inert supports. Chem. Mater. 2009, 21, 410–418. [Google Scholar] [CrossRef]
- Sandova, A.; Louis, C.; Zanella, R. Improved activity and stability in CO oxidation of bimetallic Au–Cu/TiO2 catalysts prepared by deposition-precipitation with urea. Appl. Catal. B Environ. 2013, 140, 363–377. [Google Scholar] [CrossRef]
- Xu, Z.C.; Lai, E.C.; Shao-Horn, Y.; Hamad-Schifferli, K. Compositional dependence of the stability of AuCu alloy nanoparticles. Chem. Commun. 2012, 48, 5626–5628. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, A.Q.; Wang, X.D.; Mou, C.Y.; Zhang, T. Au–Cu Alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem. Commun. 2008, 3187–3189. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.W.; Lee, D.G.; Kim, Y.K.; Baek, K.K.; Kim, K.; Jin, T.W.; Shim, J.H.; Park, J.Y.; Lee, I.S. Mechanistic insight into the conversion chemistry between Au–CuO heterostructured nanocrystals confined inside SiO2 nanospheres. Chem. Mater. 2017, 29, 1788–1795. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.B.; Figueiredo, J.L. Gold supported on metal oxides for carbon monoxide oxidation. Nano Res. 2011, 4, 180–193. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, W.Z.; Shang, M.; Yin, W.Z. Low-temperature combustion synthesis of Bi2WO6 nanoparticles as a visible-light-driven photocatalyst. J. Hazard. Mater. 2010, 177, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Bai, Y.; Chen, J.C.; Zhou, W.Q.; He, H.B.; Yu, J.C.; Zhu, L.H.; Xue, S.S. Pt/Bi2WO6 composite microflowers: High visible light photocatalytic performance and easy recycle. Sep. Purif. Technol. 2015, 154, 115–122. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Feng, Y.; Wu, Q.S.; Xu, Y.Y.; Gao, D.Z. Facile fabrication of flower-shaped Bi2WO6 superstructures and visible-light-driven photocatalytic performance. Mater. Res. Bull. 2012, 47, 1919–1924. [Google Scholar] [CrossRef]
- Dittmer, A.; Menze, J.; Mei, B.; Strunk, J.; Luftman, H.S.; Gutkowski, R.; Wachs, I.E.; Schuhmann, W.; Muhler, M. Surface structure and photocatalytic properties of Bi2WO6 nanoplatelets modified by molybdena islands from chemical vapor deposition. J. Phys. Chem. C 2016, 120, 18191–18200. [Google Scholar] [CrossRef]
- Cui, Y.M.; Li, H.Q.; Hong, W.S.; Fan, S.H.; Zhu, L.J. The effect of carbon content on the structure and photocatalytic activity of nano-Bi2WO6 powder. Powder Technol. 2013, 247, 151–160. [Google Scholar] [CrossRef]
- Xie, H.D.; Shen, D.Z.; Wang, X.Q.; Shen, G.Q. Microwave hydrothermal synthesis and visible-light photocatalytic activity of Bi2WO6 nanoplates. Mater. Chem. Phys. 2007, 103, 334–339. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.Z.; Ren, J.; Sun, S.M.; Wang, L.; Zhang, L. A practical visible-light-driven Bi2WO6 nanofibrous mat prepared by electrospinning. J. Mater. Chem. 2009, 19, 6213–6218. [Google Scholar] [CrossRef]
- Wu, D.X.; Zhu, H.T.; Zhang, C.Y.; Chen, L. Novel synthesis of bismuth tungstate hollow nanospheres in water-ethanol mixed solvent. Chem. Commun. 2010, 46, 7250–7252. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Huang, S.M.; Chen, W.X.; Hu, S.W.; Shi, F.F.; Fan, K.L. Self-assembled three-dimensional hierarchical umbilicate Bi2WO6 microspheres from nanoplates: Controlled synthesis, photocatalytic activities, an wettability. J. Phys. Chem. C 2009, 113, 4369–4374. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhan, H.; Li, F.; Zhu, L.Y. Degradation and mineralization of bisphenol a by mesoporous Bi2WO6 under simulated solar light irradiation. Environ. Sci. Technol. 2010, 44, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Chen, Y.W. Au/CuO–CeO2 catalyst for preferential oxidation of CO in hydrogen-rich stream: Effect of CuO content. Int. J. Hydrog. Energy 2016, 41, 3605–3612. [Google Scholar] [CrossRef]
- Lei, J.H.; Liu, Y.; Wang, X.Y.; Hu, P.; Peng, X.S. Au/CuO nanosheets composite for glucose sensor and CO oxidation. RSC Adv. 2015, 5, 9130–9137. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E.; Tavares, P.B.; Moreira, J.A.; Romaguera-Barcelay, Y.; Figueiredo, J.L. Effect of preparation method on the solid state properties and the deN2O performance of CuO-CeO2 oxides. Catal. Sci. Technol. 2015, 5, 3714–3727. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Bogdanchikova, N.; Tavares, P.B.; Figueiredo, J.L. Nanostructured iron oxide catalysts with gold for the oxidation of carbon monoxide. RSC Adv. 2012, 2, 2957–2965. [Google Scholar] [CrossRef]
- Hu, Z.P.; Zhu, Y.P.; Gao, Z.M.; Wang, G.X.; Liu, Y.P.; Liu, X.Y.; Yuan, Z.Y. CuO catalysts supported on activated red mud for efficient catalytic carbon monoxide oxidation. Chem. Eng. J. 2016, 302, 23–32. [Google Scholar] [CrossRef]
- Zhong, K.; Xue, J.J.; Mao, Y.C.; Wang, C.S.; Zhai, T.; Liu, P.; Xia, X.D.; Li, H.H.; Tong, Y.X. Facile synthesis of CuO nanorods with abundant adsorbed oxygen concomitant with high surface oxidation states for CO oxidation. RSC Adv. 2012, 2, 11520–11528. [Google Scholar] [CrossRef]
- Liu, H.; Guo, K.; Duan, C.Y.; Chen, X.J.; Zhu, Z.F. A novel biosensor based on the direct electrochemistry of horseradish peroxidase immobilized in the three-dimensional flower-like Bi2WO6 microspheres. Mater. Sci. Eng. C 2016, 64, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Huang, S.Y.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Preparation and characterization of mesoporous TiO2-sphere-supported Au-nanoparticle catalysts with high activity for CO oxidation at ambient temperature. J. Nanopart. Res. 2016, 18, 323. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhu, B.; Zhang, S.; Huang, W. Flower-Like Au–CuO/Bi2WO6 Microsphere Catalysts: Synthesis, Characterization, and Their Catalytic Performances for CO Oxidation. Catalysts 2017, 7, 266. https://doi.org/10.3390/catal7090266
Wang L, Zhu B, Zhang S, Huang W. Flower-Like Au–CuO/Bi2WO6 Microsphere Catalysts: Synthesis, Characterization, and Their Catalytic Performances for CO Oxidation. Catalysts. 2017; 7(9):266. https://doi.org/10.3390/catal7090266
Chicago/Turabian StyleWang, Lili, Baolin Zhu, Shoumin Zhang, and Weiping Huang. 2017. "Flower-Like Au–CuO/Bi2WO6 Microsphere Catalysts: Synthesis, Characterization, and Their Catalytic Performances for CO Oxidation" Catalysts 7, no. 9: 266. https://doi.org/10.3390/catal7090266
APA StyleWang, L., Zhu, B., Zhang, S., & Huang, W. (2017). Flower-Like Au–CuO/Bi2WO6 Microsphere Catalysts: Synthesis, Characterization, and Their Catalytic Performances for CO Oxidation. Catalysts, 7(9), 266. https://doi.org/10.3390/catal7090266