Expanding the Scope of Cu(I) Catalyzed “Click Chemistry” with Abnormal NHCs: Three-Fold Click to Tris-Triazoles
Abstract
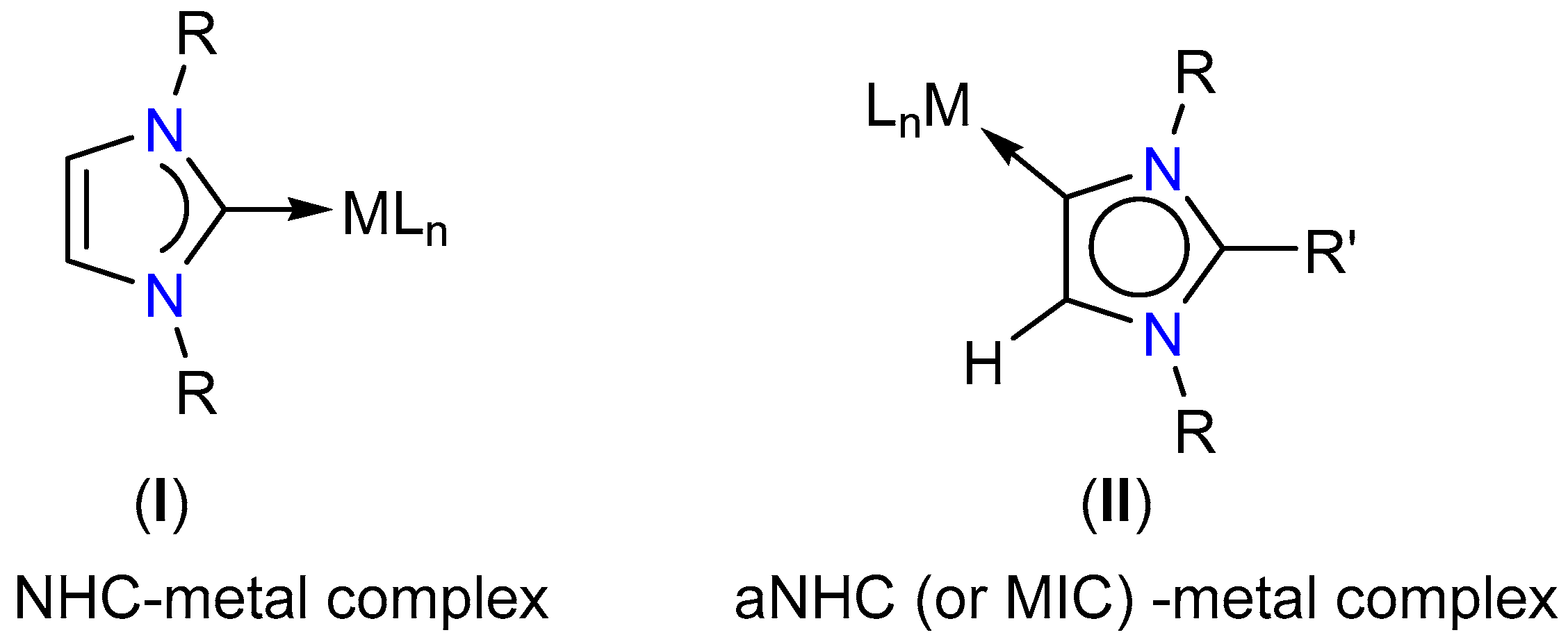
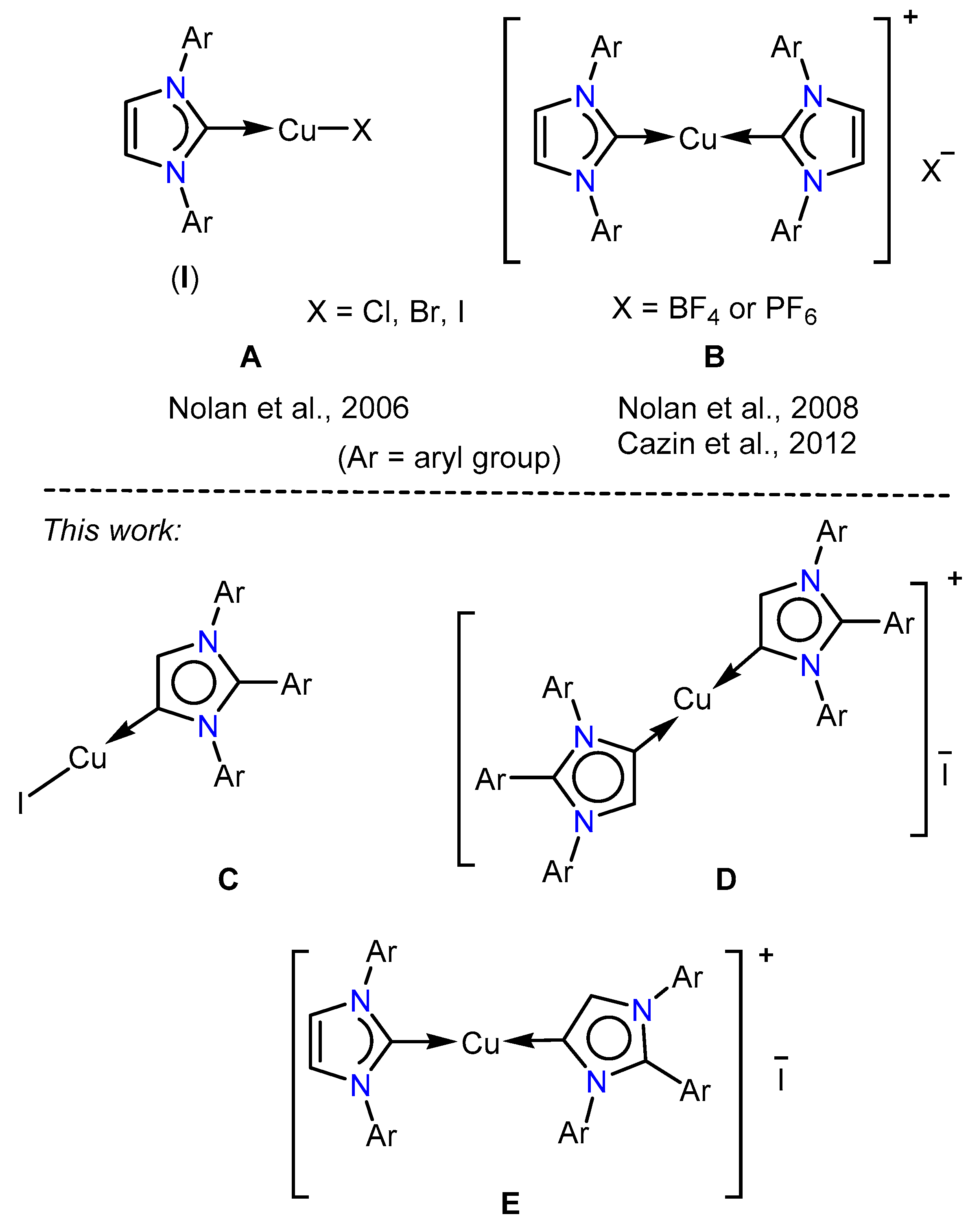
1. Introduction
2. Results and Discussion
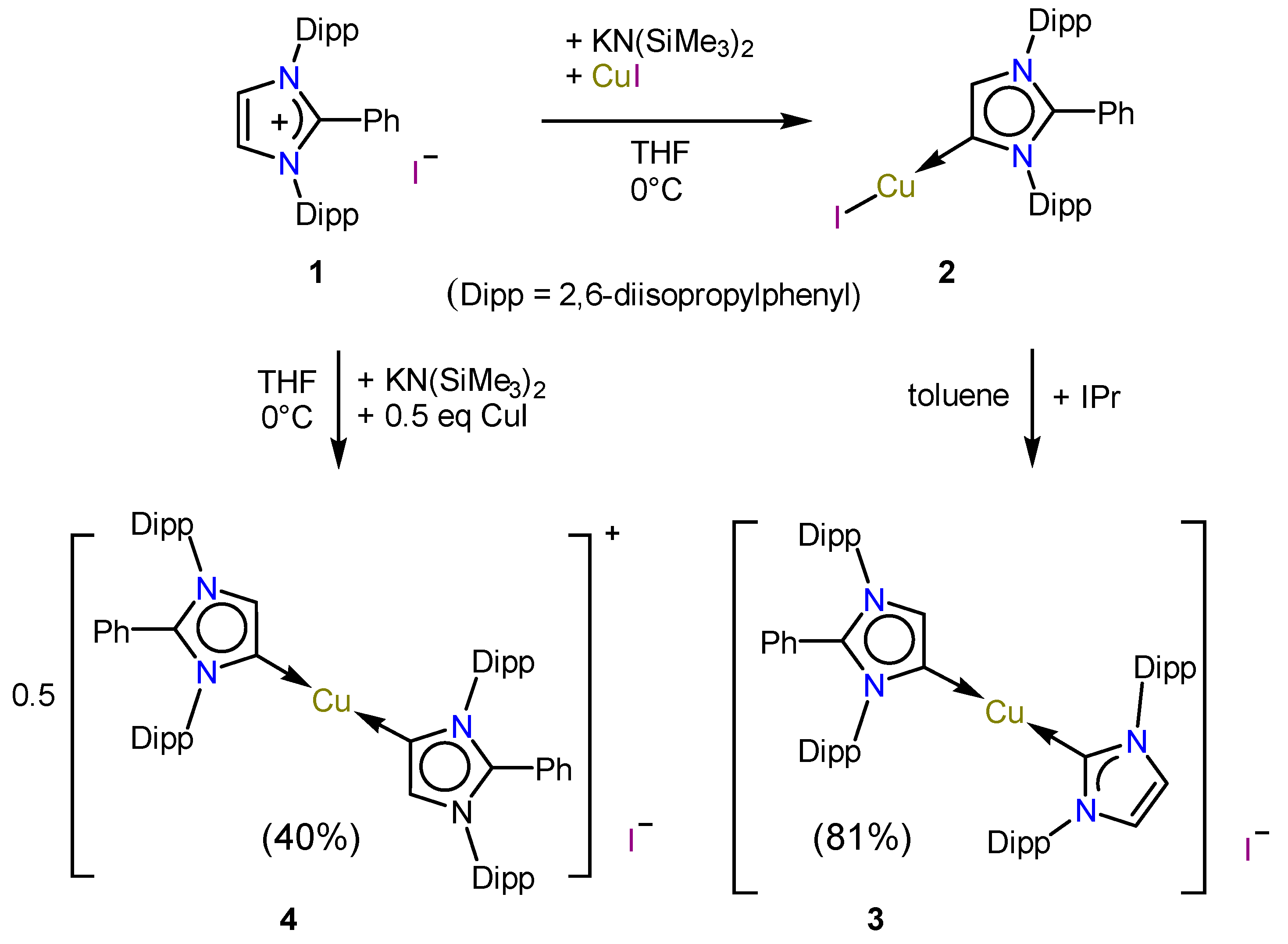
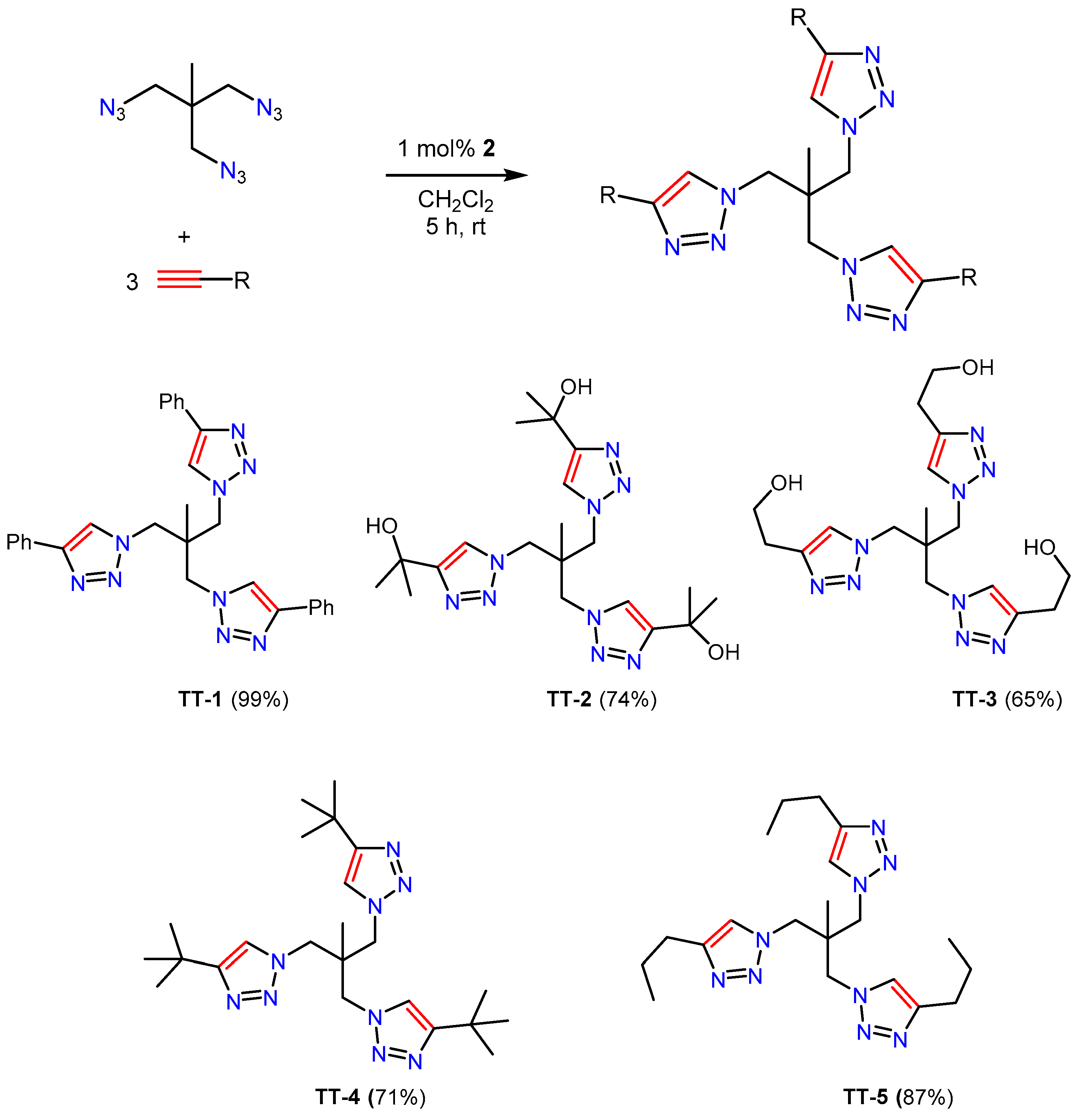
2.1. Synthesis
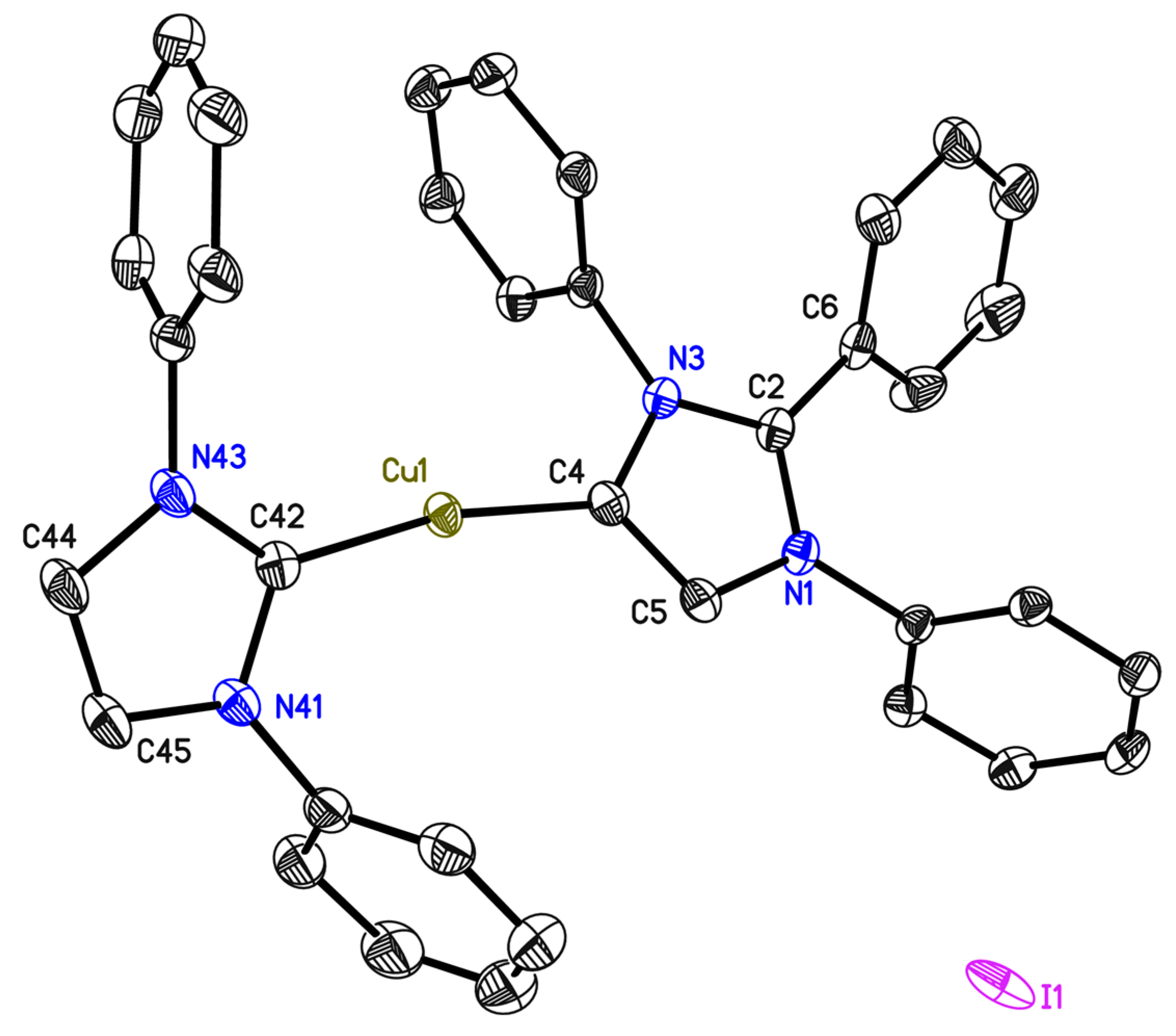
2.2. Characterization
2.3. Catalysis
3. Materials and Methods
3.1. Synthesis of Complexes 3 and 4
3.2. Crystal Structure Determinations
3.3. General Procedure for Catalysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, S.; Marion, N.; Nolan, S.P. N-Heterocyclic carbenes in late transition metal catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef] [PubMed]
- Glorius, F. N-Heterocyclic Carbenes in Transition Metal Catalysis; Springer: Berlin/Heidelberg, Germany, 2007; Volume 21. [Google Scholar]
- Herrmann, W.A. N-Heterocyclic Carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes in Synthesis; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Nolan, S.P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Lazreg, F.; Nahra, F.; Cazin, C.S.J. Copper–NHC complexes in catalysis. Coord. Chem. Rev. 2015, 293, 48–79. [Google Scholar] [CrossRef]
- Enders, D.; Balensiefer, T. Nucleophilic Carbenes in Asymmetric Organocatalysis. Acc. Chem. Res. 2004, 37, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Cesar, V.; Bellemin-Laponnaz, S.; Gade, L.H. Chiral N-heterocyclic carbenes as stereodirecting ligands in asymmetric catalysis. Chem. Soc. Rev. 2004, 33, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Boeda, F.; Nolan, S.P. N-Heterocyclic carbene-containing complexes in catalysis. Annu. Rep. Prog. Chem. Sect. B Org. Chem. 2008, 104, 184–210. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Bantreil, X.; Nolan, S.P. Synthesis of N-heterocyclic carbene ligands and derived ruthenium olefin metathesis catalysts. Nat. Protoc. 2011, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, A.; Enders, D. N-Heterocyclic carbene catalyzed domino reactions. Angew. Chem. Int. Ed. 2012, 51, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Soleilhavoup, M.; Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): Stable carbenes on the rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Robinson, G.H. N-heterocyclic carbene-main-group chemistry: A rapidly evolving field. Inorg. Chem. 2014, 53, 11815–11832. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.D.; Soleilhavoup, M.; Bertrand, G. Carbene-stabilized main group radicals and radical ions. Chem. Sci. 2013, 4, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Ghadwal, R.S.; Azhakar, R.; Roesky, H.W. Dichlorosilylene: A high temperature transient species to an indispensable building block. Acc. Chem. Res. 2013, 46, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Bidal, Y.D.; Santoro, O.; Melaimi, M.; Cordes, D.B.; Slawin, A.M.Z.; Bertrand, G.; Cazin, C.S.J. Generalization of the copper to late-transition-metal transmetallation to carbenes beyond N-heterocyclic carbenes. Chem. Eur. J. 2016, 22, 9404–9409. [Google Scholar] [CrossRef] [PubMed]
- Ghadwal, R.S. Carbon-based two electron sigma-donor ligands beyond classical N-heterocyclic carbenes. Dalton Trans. 2016, 45, 16081–16095. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, C.; Maraval, V.; Canac, Y.; Chauvin, R. On the nature of the dative bond: Coordination to metals and beyond. The carbon case. Coord. Chem. Rev. 2016, 308, 59–75. [Google Scholar] [CrossRef]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable cyclic carbenes and related species beyond diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [Google Scholar] [CrossRef] [PubMed]
- Schuster, O.; Yang, L.; Raubenheimer, H.G.; Albrecht, M. Beyond conventional N-heterocyclic carbenes: Abnormal, remote and other classes of NHC ligands with reduced heteroatom stabilization. Chem. Rev. 2009, 109, 3445–3478. [Google Scholar] [CrossRef] [PubMed]
- Ghadwal, R.S.; Rottschäfer, D.; Blomeyer, S.; Neumann, B.; Stammler, H.-G. Silylene-functionalized N-heterocyclic carbene (Si-NHC). Chem. Eur. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schnee, G.; Nieto Faza, O.; Specklin, D.; Jacques, B.; Karmazin, L.; Welter, R.; Silva López, C.; Dagorne, S. Normal-to-abnormal NHC rearrangement of AlIII, GaIII, and InIII trialkyl complexes: Scope, mechanism, reactivity studies, and H2 activation. Chem. Eur. J. 2015, 21, 17959–17972. [Google Scholar] [CrossRef] [PubMed]
- Poulain, A.; Iglesias, M.; Albrecht, M. Abnormal NHC palladium complexes: Synthesis, structure, and reactivity. Curr. Org. Chem. 2011, 15, 3325–3336. [Google Scholar] [CrossRef]
- Xu, X.; Xu, B.; Li, Y.; Hong, S.H. Abnormal N-heterocyclic carbene promoted Suzuki−Miyaura coupling reaction: A comparative study. Organometallics 2010, 29, 6343–6349. [Google Scholar] [CrossRef]
- Aldeco-Perez, E.; Rosenthal, A.J.; Donnadieu, B.; Parameswaran, P.; Frenking, G.; Bertrand, G. Isolation of a C5-deprotonated imidazolium, a crystalline “Abnormal” N-heterocyclic carbene. Science 2009, 326, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Crittall, M.R.; Ellul, C.E.; Mahon, M.F.; Saker, O.; Whittlesey, M.K. Abnormal coordination of Arduengo’s carbene upon reaction with M3 (CO) 12 (M = Ru, Os). Dalton Trans. 2008, 4209–4211. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.E.; Jennings, M.C.; Pomeroy, R.K.; Clyburne, J.A.C. Normal and abnormal NHC coordination in [Os4(μ-H)4(CO)11(IMes)] and exhaustive dehydrogenation of an IMes methyl group. Organometallics 2007, 26, 6059–6062. [Google Scholar] [CrossRef]
- Arnold, P.L.; Pearson, S. Abnormal N-heterocyclic carbenes. Coord. Chem. Rev. 2007, 251, 596–609. [Google Scholar] [CrossRef]
- Chianese, A.R.; Kovacevic, A.; Zeglis, B.M.; Faller, J.W.; Crabtree, R.H. Abnormal C5-bound N-heterocyclic carbenes: Extremely strong electron donor ligands and their iridium(I) and iridium(III) complexes. Organometallics 2004, 23, 2461–2468. [Google Scholar] [CrossRef]
- Grundemann, S.; Kovacevic, A.; Albrecht, M.; Faller, J.W.; Crabtree, R.H. Abnormal binding in a carbene complex formed from an imidazolium salt and a metal hydride complex. Chem. Commun. 2001, 2274–2275. [Google Scholar] [CrossRef]
- Droge, T.; Glorius, F. The measure of all rings-N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940–6952. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Guisado-Barrios, G.; Bouffard, J.; Donnadieu, B.; Bertrand, G. Crystalline 1H-1,2,3-triazol-5-ylidenes: New stable mesoionic carbenes (MICs). Angew. Chem. Int. Ed. 2010, 49, 4759–4762. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. Abnormal, mesoionic and remote N-heterocyclic carbene complexes. Coord. Chem. Rev. 2013, 257, 755–766. [Google Scholar] [CrossRef]
- Heckenroth, M.; Kluser, E.; Neels, A.; Albrecht, M. Neutral ligands with exceptional donor ability for palladium-catalyzed alkene hydrogenation. Angew. Chem. Int. Ed. 2007, 46, 6293–6296. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. C4-bound imidazolylidenes: From curiosities to high-impact carbene ligands. Chem. Commun. 2008, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Melaimi, M.; Soleilhavoup, M.; Bertrand, G. A brief survey of our contribution to stable carbene chemistry. Organometallics 2011, 30, 5304–5313. [Google Scholar] [CrossRef] [PubMed]
- Prades, A.; Viciano, M.; Sanau, M.; Peris, E. Preparation of a series of “Ru(p-cymene)” complexes with different N-heterocyclic carbene ligands for the catalytic β-alkylation of secondary alcohols and dimerization of phenylacetylene. Organometallics 2008, 27, 4254–4259. [Google Scholar] [CrossRef]
- Saha, S.; Ghatak, T.; Saha, B.; Doucet, H.; Bera, J.K. Steric control at the wingtip of a bis-N-heterocyclic carbene ligand: Coordination behavior and catalytic responses of its ruthenium compounds. Organometallics 2012, 31, 5500–5505. [Google Scholar] [CrossRef]
- Keitz, B.K.; Bouffard, J.; Bertrand, G.; Grubbs, R.H. Protonolysis of a ruthenium-carbene bond and applications in olefin metathesis. J. Am. Chem. Soc. 2011, 133, 8498–8501. [Google Scholar] [CrossRef] [PubMed]
- Bidal, Y.D.; Lesieur, M.; Melaimi, M.; Nahra, F.; Cordes, D.B.; Athukorala Arachchige, K.S.; Slawin, A.M.Z.; Bertrand, G.; Cazin, C.S.J. Copper(I) complexes bearing carbenes beyond classical N-heterocyclic carbenes: Synthesis and catalytic activity in “Click chemistry”. Adv. Synth. Catal. 2015, 357, 3155–3161. [Google Scholar] [CrossRef]
- Sau, S.C.; Roy, S.R.; Sen, T.K.; Mullangi, D.; Mandal, S.K. An abnormal N-heterocyclic carbene-copper(I) complex in click chemistry. Adv. Synth. Catal. 2013, 355, 2982–2991. [Google Scholar] [CrossRef]
- Ghadwal, R.S.; Reichmann, S.O.; Herbst-Irmer, R. Palladium-catalyzed direct C2-arylation of an N-heterocyclic carbene: An atom-economic route to mesoionic carbene ligands. Chem. Eur. J. 2015, 21, 4247–4251. [Google Scholar] [CrossRef] [PubMed]
- Ghadwal, R.S.; Ho, N.K.; Neumann, B.; Stammler, G.; Menezes da Silva, V.H.; Watanabe, D.; Braga, A.A.C. Nickel-catalysed direct C2-arylation of N-heterocyclic carbene. Dalton Trans. 2017. [Google Scholar] [CrossRef]
- Rottschäfer, D.; Schürmann, C.J.; Lamm, J.-H.; Paesch, A.N.; Neumann, B.; Ghadwal, R.S. Abnormal-NHC palladium(II) complexes: Rational synthesis, structural elucidation, and catalytic activity. Organometallics 2016, 35, 3421–3429. [Google Scholar] [CrossRef]
- Ghadwal, R.S.; Rottschäfer, D.; Schürmann, C.J. Expedient access to normal- and abnormal- N-heterocyclic carbene (NHC) magnesium compounds from imidazolium salts. Z. Anorg. Allg. Chem. 2016, 642, 1236–1240. [Google Scholar] [CrossRef]
- Ghadwal, R.S.; Lamm, J.-H.; Rottschafer, D.; Schurmann, C.J.; Demeshko, S. Facile routes to abnormal-NHC-cobalt(II) complexes. Dalton Trans. 2017, 46, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide−alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.G.; Fokin, V.V. Click chemistry: Function follows form. Chem. Soc. Rev. 2010, 39, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide–alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef] [PubMed]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Schulze, B.; Schubert, U.S. Beyond click chemistry—Supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Straub, B.; Holm, S.; Siegle, A.; Loos, C.; Rominger, F. Preparation and N-alkylation of 4-aryl-1,2,4-triazoles. Synthesis 2010, 2010, 2278–2286. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Yu, S.; Cornilleau, T.; Ruiz, J.; Salmon, L.; Astruc, D. Design and applications of an efficient amphiphilic “Click” CuI catalyst in water. ACS Catal. 2016, 6, 5424–5431. [Google Scholar] [CrossRef]
- Wang, D.; Deraedt, C.; Salmon, L.; Labrugère, C.; Etienne, L.; Ruiz, J.; Astruc, D. A tris(triazolate) ligand for a highly active and magneticall recoverable palladium catalyst of selective alcohol oxidation using air at atmospheric pressure. Chem. Eur. J. 2015, 21, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Shreeve, J.N.M. Energetic materials with promising properties: Synthesis and characterization of 4,4′-bis(5-nitro-1,2,3-2H-triazole) derivatives. Angew. Chem. Int. Ed. 2015, 54, 6260–6264. [Google Scholar] [CrossRef] [PubMed]
- Dippold, A.A.; Klapötke, T.M. A study of dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: Design of high-performance insensitive energetic materials by the introduction of N-oxides. J. Am. Chem. Soc. 2013, 135, 9931–9938. [Google Scholar] [CrossRef] [PubMed]
- Brantley, J.N.; Wiggins, K.M.; Bielawski, C.W. Unclicking the click: Mechanically facilitated 1,3-dipolar cycloreversions. Science 2011, 333, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Juricek, M.; Kouwer, P.H.J.; Rowan, A.E. Triazole: A unique building block for the construction of functional materials. Chem. Commun. 2011, 47, 8740–8749. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, S.; Escudero-Adan, E.C.; Benet-Buchholz, J.; Stevens, E.D.; Slawin, A.M.Z.; Nolan, S.P. [(NHC)CuX] complexes: Synthesis, characterization and catalytic activities in reduction reactions and click chemistry. On the advantage of using well-defined catalytic systems. Dalton Trans. 2010, 39, 7595–7606. [Google Scholar] [CrossRef] [PubMed]
- Makarem, A.; Berg, R.; Rominger, F.; Straub, B.F. A fluxional copper acetylide cluster in CuAAC catalysis. Angew. Chem. Int. Ed. 2015, 54, 7431–7435. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, S.; Nolan, S.P. [(NHC)2Cu]X Complexes as efficient catalysts for azide-alkyne click chemistry at low catalyst loadings. Angew. Chem. Int. Ed. 2008, 47, 8881–8884. [Google Scholar] [CrossRef] [PubMed]
- Diaz Velazquez, H.; Ruiz Garcia, Y.; Vandichel, M.; Madder, A.; Verpoort, F. Water-soluble NHC-Cu catalysts: Applications in click chemistry, bioconjugation and mechanistic analysis. Org. Biomol. Chem. 2014, 12, 9350–9356. [Google Scholar] [CrossRef] [PubMed]
- Hohloch, S.; Sarkar, B.; Nauton, L.; Cisnetti, F.; Gautier, A. Are Cu(I)-mesoionic NHC carbenes associated with nitrogen additives the best Cu-carbene catalysts for the azide-alkyne click reaction in solution? A case study. Tetrahedron Lett. 2013, 54, 1808–1812. [Google Scholar] [CrossRef]
- Nakamura, T.; Terashima, T.; Ogata, K.; Fukuzawa, S. Copper(I) 1,2,3-triazol-5-ylidene complexes as efficient catalysts for click reactions of azides with alkynes. Org. Lett. 2011, 13, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, S.; Stevens, E.D.; Nolan, S.P. A [(NHC)CuCl] complex as a latent click catalyst. Chem. Commun. 2008, 4747–4749. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, S.; Nolan, S.P. N-heterocyclic carbene-copper(I) complexes in homogeneous catalysis. Synlett 2007, 2007, 2158–2167. [Google Scholar] [CrossRef]
- Diez-Gonzalez, S.; Correa, A.; Cavallo, L.; Nolan, S.P. (NHC)copper(I)-catalyzed [3+2] cycloaddition of azides and mono- or disubstituted alkynes. Chem. Eur. J. 2006, 12, 7558–7564. [Google Scholar] [CrossRef] [PubMed]
- Bidal, Y.D.; Lesieur, M.; Melaimi, M.; Cordes, D.B.; Slawin, A.M.; Bertrand, G.; Cazin, C.S. A simple access to transition metal cyclopropenylidene complexes. Chem. Commun. 2015, 51, 4778–4781. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.C.; Roy, S.R.; Mandal, S.K. One-pot consecutive catalysis by integrating organometallic catalysis with organocatalysis. Chem. Asian. J. 2014, 9, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.R.; Sau, S.C.; Mandal, S.K. Chemoselective reduction of the carbonyl functionality through hydrosilylation: Integrating click catalysis with hydrosilylation in one pot. J. Org. Chem. 2014, 79, 9150–9160. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Castro-Rodriguez, I.; Meyer, K. A bis-carbene alkenyl copper(I) complex from a tripodal tris-carbene ligand. Organometallics 2003, 22, 3016–3018. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Cosimi, E.; Lefort, L.; Conley, M.P.; Coperet, C.; Lutz, M.; Hensen, E.J.M.; Pidko, E.A. Lutidine-derived Ru-CNC hydrogenation pincer catalysts with versatile coordination properties. ACS Catal. 2014, 4, 2667–2671. [Google Scholar] [CrossRef]
- Bitzer, M.J.; Pothig, A.; Jandl, C.; Kühn, F.E.; Baratta, W. Ru-Ag and Ru-Au dicarbene complexes from an abnormal carbene ruthenium system. Dalton Trans. 2015, 44, 11686–11689. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A.; Albrecht, M. Rhodium carbene complexes as versatile catalyst precursors for Si-H bond activation. Chem. Eur. J. 2012, 18, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.; Haller, L.J.L.; Muller-Bunz, H.; Serada, O.; Neels, A.; Macgregor, S.A.; Albrecht, M. Smooth C(alkyl)-H bond activation in rhodium complexes comprising abnormal carbene ligands. Dalton Trans. 2011, 40, 9911–9920. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Braunstein, P. N-Heterocyclic dicarbene iridium(III) pincer complexes featuring mixed NHC/abnormal NHC ligands and their applications in the transfer dehydrogenation of cyclooctane. Organometallics 2012, 31, 2606–2615. [Google Scholar] [CrossRef]
- Tang, C.Y.; Smith, W.; Vidovic, D.; Thompson, A.L.; Chaplin, A.B.; Aldridge, S. Sterically encumbered Iridium bis(N-heterocyclic carbene) systems: Multiple C-H activation processes and isomeric normal/abnormal carbene complexes. Organometallics 2009, 28, 3059–3066. [Google Scholar] [CrossRef]
- Tan, K.V.; Dutton, J.L.; Skelton, B.W.; Wilson, D.J.D.; Barnard, P.J. Nickel(II) and palladium(II) complexes with chelating N-heterocyclic carbene amidate ligands: Interplay between normal and abnormal coordination modes. Organometallics 2013, 32, 1913–1923. [Google Scholar] [CrossRef]
- Lebel, H.; Janes, M.K.; Charette, A.B.; Nolan, S.P. Structure and reactivity of “unusual” N-heterocyclic carbene (NHC) palladium complexes synthesized from imidazolium salts. J. Am. Chem. Soc. 2004, 126, 5046–5047. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Tsoureas, N.; Wright, J.A.; Light, M.E. N-Heterocyclic pincer dicarbene complexes of iron(II): C-2 and C-5 Metalated carbenes on the same metal center. Organometallics 2004, 23, 166–168. [Google Scholar] [CrossRef]
- Varonka, M.S.; Warren, T.H. Three-coordinate N-heterocyclic carbene nickel nitrosyl complexes. Organometallics 2010, 29, 717–720. [Google Scholar] [CrossRef]
- Santoro, O.; Lazreg, F.; Cordes, D.B.; Slawin, A.M.Z.; Cazin, C.S.J. Homoleptic and heteroleptic bis-NHC Cu(I) complexes as carbene transfer reagents. Dalton Trans. 2016, 45, 4970–4973. [Google Scholar] [CrossRef] [PubMed]
- Lazreg, F.; Slawin, A.M.Z.; Cazin, C.S.J. Heteroleptic bis(N-heterocyclic carbene) Copper(I) complexes: Highly efficient systems for the [3+2] cycloaddition of azides and alkynes. Organometallics 2012, 31, 7969–7975. [Google Scholar] [CrossRef]
- Weisser, F.; Stevens, H.; Klein, J.; van der Meer, M.; Hohloch, S.; Sarkar, B. Tailoring Ru(II) pyridine/triazole oxygenation catalysts and using photoreactivity to probe their electronic properties. Chem. Eur. J. 2015, 21, 8926–8938. [Google Scholar] [CrossRef] [PubMed]
- Schweinfurth, D.; Sommer, M.G.; Atanasov, M.; Demeshko, S.; Hohloch, S.; Meyer, F.; Neese, F.; Sarkar, B. The ligand field of the azido ligand: Insights into bonding parameters and magnetic anisotropy in a Co(II)-azido complex. J. Am. Chem. Soc. 2015, 137, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Maity, R.; van der Meer, M.; Hohloch, S.; Sarkar, B. Di- and trinuclear iridium(III) complexes with poly-mesoionic carbenes synthesized through selective base-dependent metalation. Organometallics 2015, 34, 3090–3096. [Google Scholar] [CrossRef]
- Maity, R.; van der Meer, M.; Sarkar, B. Redox-active multinuclear Pd(II) complexes with bis- and tris-mesoionic carbenes. Dalton Trans. 2015, 44, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Etayo, P.; Ayats, C.; Pericas, M.A. Synthesis and catalytic applications of C3-symmetric tris(triazolyl)methanol ligands and derivatives. Chem. Commun. 2016, 52, 1997–2010. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Schulz, T.; Meindl, K.; Leusser, D.; Stern, D.; Graf, J.; Michaelsen, C.; Ruf, M.; Sheldrick, G.M.; Stalke, D. A comparison of a microfocus X-ray source and a conventional sealed tube for crystal structure determination. J. Appl. Cryst. 2009, 42, 885–891. [Google Scholar] [CrossRef]
- Bruker AXS Inc. In Bruker Apex CCD, SAINT v8.30C; Bruker AXS Inst. Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Stalke, D. An empirical correction for the influence of low-energy contamination. J. Appl. Cryst. 2015, 48, 1907–1913. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hubschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, N.K.T.; Reichmann, S.O.; Rottschäfer, D.; Herbst-Irmer, R.; Ghadwal, R.S. Expanding the Scope of Cu(I) Catalyzed “Click Chemistry” with Abnormal NHCs: Three-Fold Click to Tris-Triazoles. Catalysts 2017, 7, 262. https://doi.org/10.3390/catal7090262
Ho NKT, Reichmann SO, Rottschäfer D, Herbst-Irmer R, Ghadwal RS. Expanding the Scope of Cu(I) Catalyzed “Click Chemistry” with Abnormal NHCs: Three-Fold Click to Tris-Triazoles. Catalysts. 2017; 7(9):262. https://doi.org/10.3390/catal7090262
Chicago/Turabian StyleHo, Nga Kim T., Sven O. Reichmann, Dennis Rottschäfer, Regine Herbst-Irmer, and Rajendra S. Ghadwal. 2017. "Expanding the Scope of Cu(I) Catalyzed “Click Chemistry” with Abnormal NHCs: Three-Fold Click to Tris-Triazoles" Catalysts 7, no. 9: 262. https://doi.org/10.3390/catal7090262
APA StyleHo, N. K. T., Reichmann, S. O., Rottschäfer, D., Herbst-Irmer, R., & Ghadwal, R. S. (2017). Expanding the Scope of Cu(I) Catalyzed “Click Chemistry” with Abnormal NHCs: Three-Fold Click to Tris-Triazoles. Catalysts, 7(9), 262. https://doi.org/10.3390/catal7090262



