SnSx (x = 1, 2) Nanocrystals as Effective Catalysts for Photoelectrochemical Water Splitting
Abstract
:1. Introduction
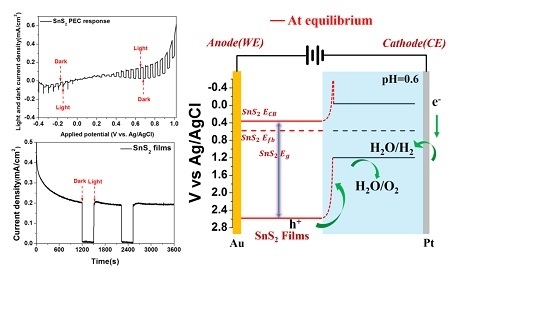
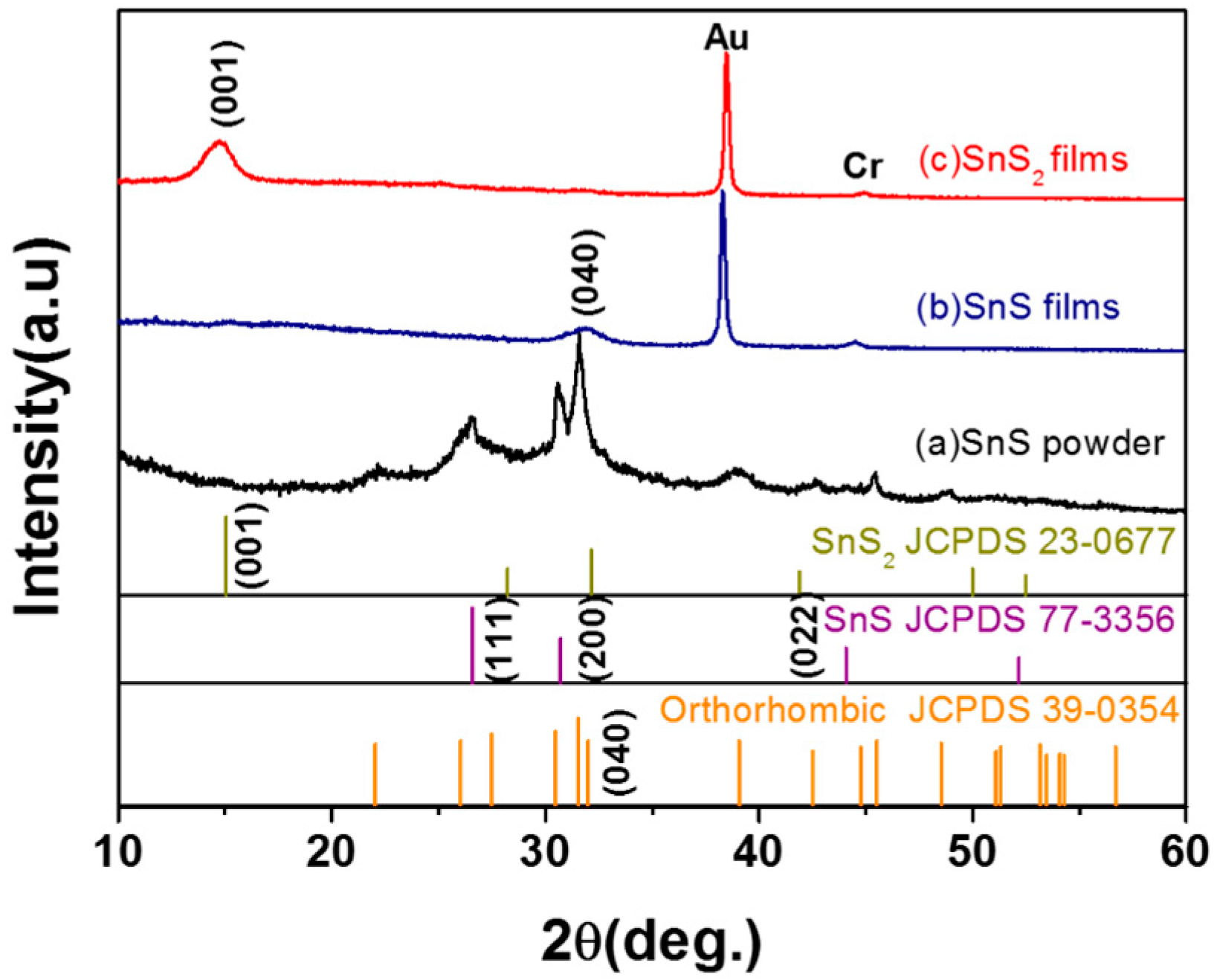
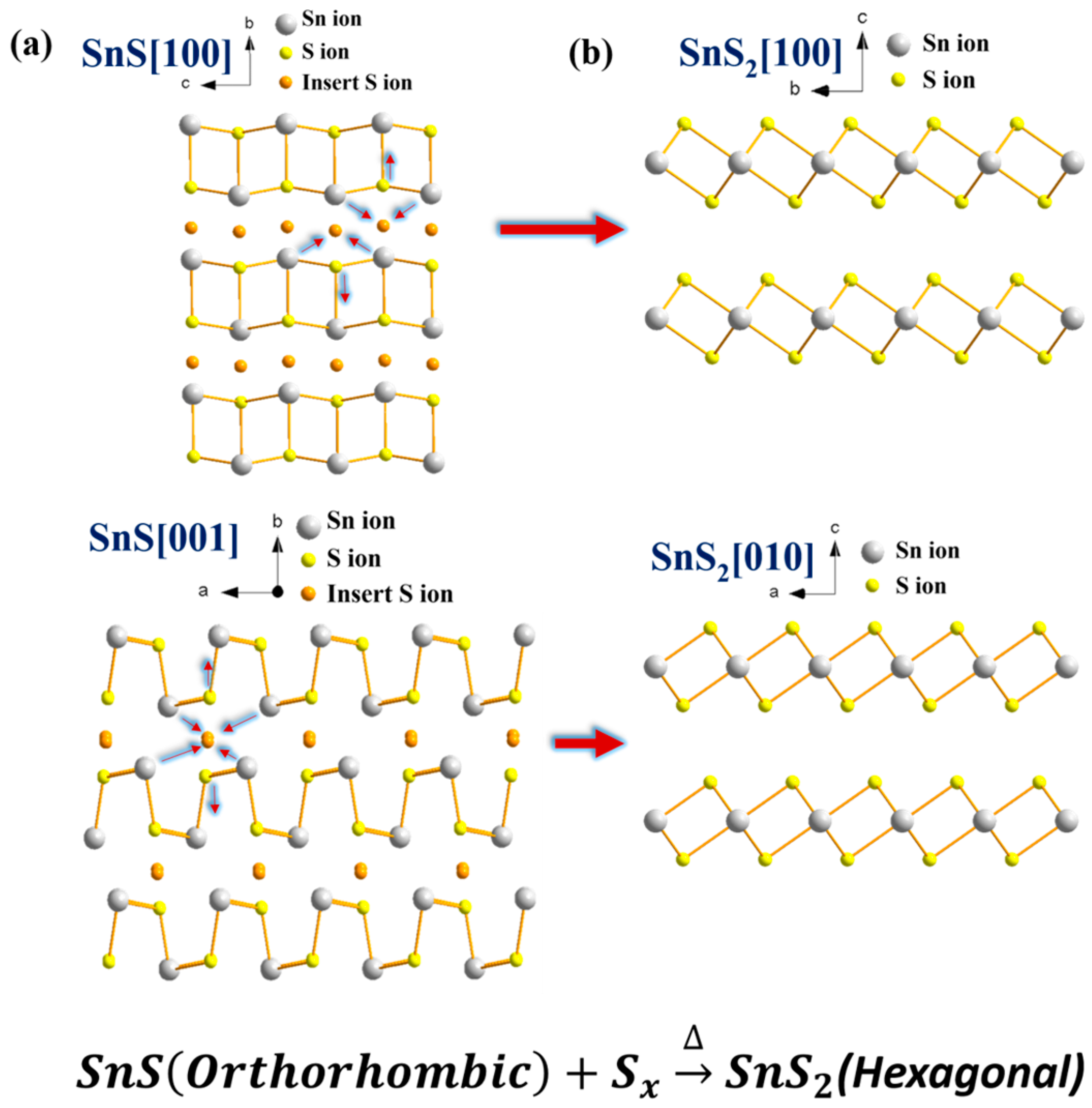
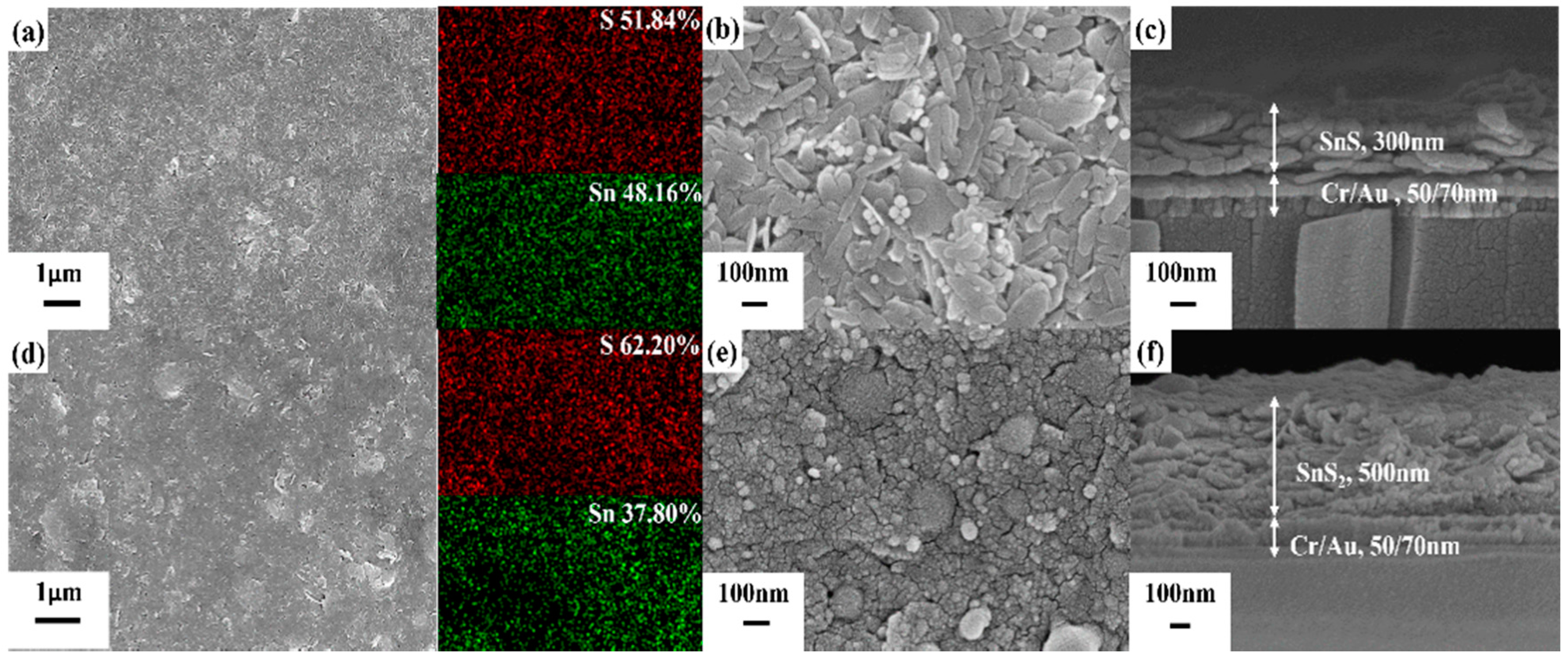
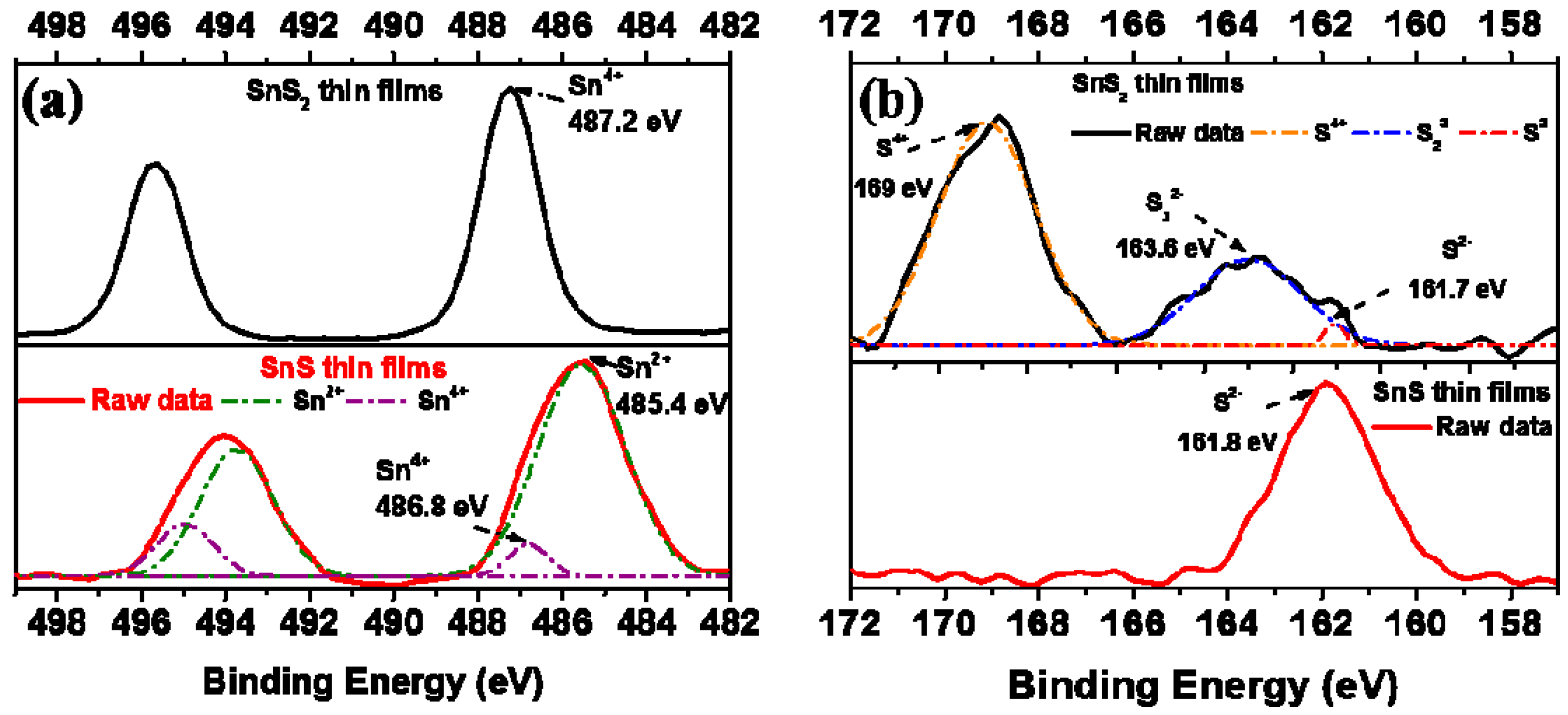
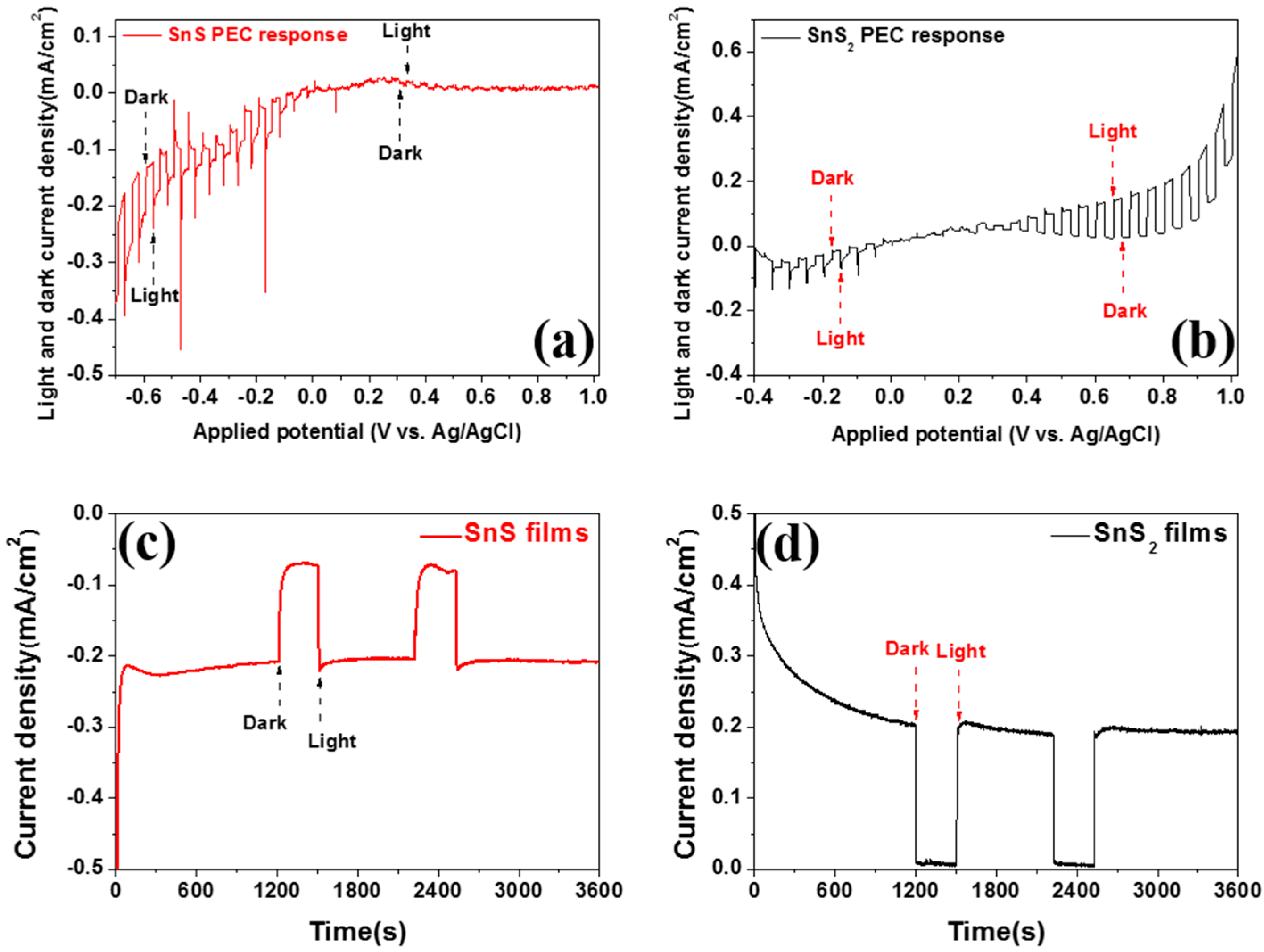
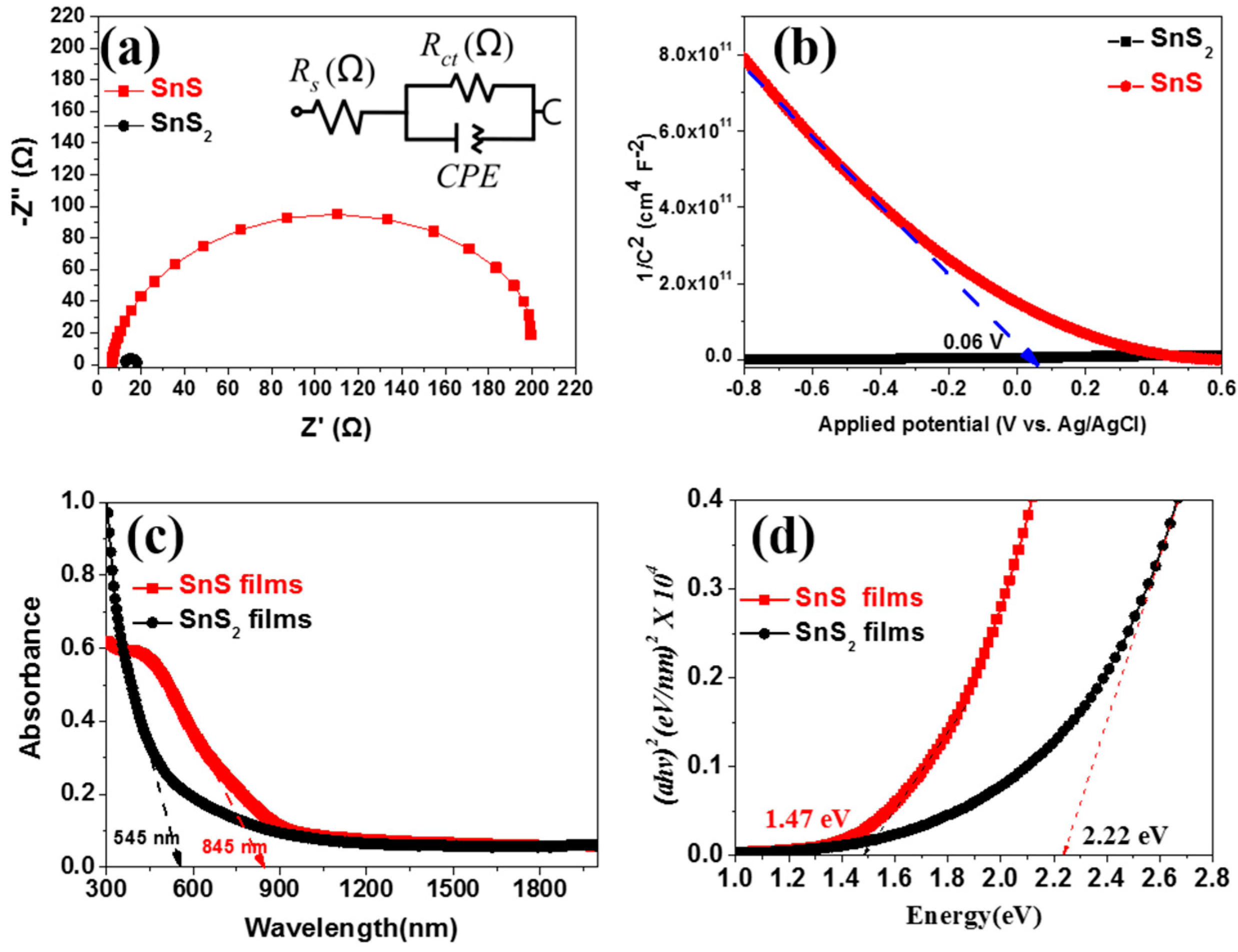
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Mixed-Phase SnS Nanocrystals
3.2. SnS and SnS2 Thin Films Deposition
3.3. SnS and SnS2 Electrochemical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Yu, J.G.; Low, J.X.; Fang, Y.P.; Xiao, J.; Chen, X.B. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Nano Today 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Gao, S.; Lei, F.C.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.Z.; Hollen, S.M.; Cao, L.Y.; Cui, Y.; Gupta, J.A.; Gutierrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.X.; Ismach, A.F.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.Y.; Chen, B.; Bosman, M.; Cao, X.H.; Chen, J.Z.; Zheng, B.; Zhang, H. Au nanoparticle-modified MoS2 nanosheet-based photoelectrochemical cells for water splitting. Small 2014, 10, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.H.; Zhao, Z.H.; Zhao, M.W.; Hao, P.; Leng, Y.H.; Liu, H. From UV to near-infrared, WS2 nanosheet: A novel photocatalyst for full solar light spectrum photodegradation. Adv. Mater. 2015, 27, 363–369. [Google Scholar] [CrossRef]
- Xie, J.F.; Wang, R.X.; Bao, J.; Zhang, X.D.; Zhang, H.; Li, S.; Xie, Y. Zirconium trisulfide ultrathin nanosheets as efficient catalysts for water oxidation in both alkaline and neutral solutions. Inorg. Chem. Front. 2014, 1, 751–756. [Google Scholar] [CrossRef]
- Lei, F.C.; Zhang, L.; Sun, Y.F.; Liang, L.; Liu, K.T.; Xu, J.Q.; Zhang, Q.; Pan, B.C.; Luo, Y.; Xie, Y. Atomic-layer-confined doping for atomic-level insights into visible-light water splitting. Angew. Chem. Int. Ed. 2015, 54, 9266–9270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Sun, Z.H.; Gao, S.; Cheng, H.; Liu, Q.H.; Lei, F.C.; Wei, S.Q.; Xie, Y. All-surface-atomic-metal chalcogenide sheets for high-efficiency visible-light photoelectrochemical water splitting. Adv. Energy Mater. 2014, 4, 1300611. [Google Scholar] [CrossRef]
- Seal, M.; Singh, N.; McFarland, E.W.; Baltrusaitis, J. Electrochemically deposited sb and in doped tin sulfide (SnS) photoelectrodes. J. Phys. Chem. C 2015, 119, 6471–6480. [Google Scholar] [CrossRef]
- Patel, M.; Chavda, A.; Mukhopadhyay, I.; Kim, J.; Ray, A. Nanostructured SnS with inherent anisotropic optical properties for high photoactivity. Nanoscale 2016, 8, 2293–2303. [Google Scholar] [CrossRef]
- Kois, J.; Bereznev, S.; Maricheva, J.; Revathi, N. Electrochemical and photoelectrochemical characterization of SnS photoabsorber films. Mater. Sci. Semicon. Proc. 2017, 58, 76–81. [Google Scholar] [CrossRef]
- Yu, J.; Xu, C.Y.; Ma, F.X.; Hu, S.P.; Zhang, Y.W.; Zhen, L. Monodisperse SnS2 nanosheets for high-performance photocatalytic hydrogen generation. ACS Appl. Mater. Int. 2014, 6, 22370–22377. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Cheng, H.; Gao, S.; Sun, Z.H.; Liu, Q.H.; Liu, Q.; Lei, F.C.; Yao, T.; He, J.F.; Wei, S.Q.; et al. Freestanding tin disulfide single-layers realizing efficient visible-light water splitting. Angew. Chem. Int. Ed. 2012, 51, 8727–8731. [Google Scholar] [CrossRef] [PubMed]
- An, X.Q.; Yu, J.C.; Tang, J.W. Biomolecule-assisted fabrication of copper doped SnS2 nanosheet-reduced graphene oxide junctions with enhanced visible-light photocatalytic activity. J. Mater. Chem. A 2014, 2, 1000–1005. [Google Scholar] [CrossRef]
- Zhou, T.F.; Pang, W.K.; Zhang, C.F.; Yang, J.P.; Chen, Z.X.; Liu, H.K.; Guo, Z.P. Enhanced sodium-ion battery performance by structural phase transition from two-dimensional hexagonal-SnS2 to orthorhombic-SnS. ACS Nano 2014, 8, 8323–8333. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.M.; Mitra, S. Tin sulfide (SnS) nanorods: Structural, optical and lithium storage property study. RSC Adv. 2014, 4, 10358–10366. [Google Scholar] [CrossRef]
- Shinde, S.S.; Sami, A.; Kim, D.H.; Lee, J.H. Nanostructured SnS-n-doped graphene as an advanced electrocatalyst for the hydrogen evolution reaction. Chem. Commun. 2015, 51, 15716–15719. [Google Scholar] [CrossRef] [PubMed]
- Koktysh, D.S.; McBride, J.R.; Geil, R.D.; Schmidt, B.W.; Rogers, B.R.; Rosenthal, S.J. Facile route to SnS nanocrystals and their characterization. Mater. Sci. Eng. B Adv. 2010, 170, 117–122. [Google Scholar] [CrossRef]
- Takeda, N.; Parkinson, B.A. Adsorption morphology, light absorption, and sensitization yields for squaraine dyes on SnS2 surfaces. J. Am. Chem. Soc. 2003, 125, 5559–5571. [Google Scholar] [CrossRef] [PubMed]
- Devika, M.; Reddy, K.T.R.; Reddy, N.K.; Ramesh, K.; Ganesan, R.; Gopal, E.S.R.; Gunasekhar, K.R. Microstructure dependent physical properties of evaporated tin sulfide films. J. Appl. Phys. 2006, 100, 023518. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Kosbar, L.L.; Murray, C.E.; Copel, M.; Afzali, A. High-mobility ultrathin semiconducting films prepared by spin coating. Nature 2004, 428, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.T.R.; Reddy, N.K.; Miles, R.W. Photovoltaic properties of SnS based solar cells. Sol. Energy Mater. Sol. C 2006, 90, 3041–3046. [Google Scholar] [CrossRef]
- Thangaraju, B.; Kaliannan, P. Spray pyrolytic deposition and characterization of SnS and SnS2 thin films. J. Phys. D Appl. Phys. 2000, 33, 1054–1059. [Google Scholar] [CrossRef]
- Avellaneda, D.; Nair, M.T.S.; Nair, P.K. Polymorphic tin sulfide thin films of zinc blende and orthorhombic structures by chemical deposition. J. Electrochem. Soc. 2008, 155, D517–D525. [Google Scholar] [CrossRef]
- Avellaneda, D.; Nair, M.T.S.; Nair, P.K. Photovoltaic structures using chemically deposited tin sulfide thin films. Thin Solid Films 2009, 517, 2500–2502. [Google Scholar] [CrossRef]
- Patel, M.; Ray, A. Evaluation of back contact in spray deposited SnS thin film solar cells by impedance analysis. ACS Appl. Mater. Int. 2014, 6, 10099–10106. [Google Scholar] [CrossRef]
- Kafashan, H.; Azizieh, M.; Vatan, H.N. Ultrasound-assisted electrodeposition of SnS: Effect of ultrasound waves on the physical properties of nanostructured SnS thin films. J. Alloy Compd. 2016, 686, 962–968. [Google Scholar] [CrossRef]
- Chaki, S.H.; Chaudhary, M.D.; Deshpande, M.P. Effect of indium and antimony doping on SnS photoelectrochemical solar cells. Chin. Phys. Lett. 2014, 31, 106102. [Google Scholar] [CrossRef]
- Liang, B.Y.J.; Shen, Y.M.; Wang, S.C.; Huang, J.L. The influence of reaction temperatures and volume of oleic acid to synthesis SnS nanocrystals by using thermal decomposition method. Thin Solid Films 2013, 549, 159–164. [Google Scholar] [CrossRef]
- Huang, P.C.; Huang, J.L.; Wang, S.C.; Shaikh, M.O.; Lin, C.Y. Photoelectrochemical properties of orthorhombic and metastable phase sns nanocrystals synthesized by a facile colloidal method. Thin Solid Films 2015, 596, 135–139. [Google Scholar] [CrossRef]
- Huang, P.C.; Shaikh, M.O.; Wang, S.C. Structural and optoelectronic properties of alloyed snxmn1-xs thin films. Adv. Powder Technol. 2016, 27, 964–970. [Google Scholar] [CrossRef]
- Reddy, N.K. Growth-temperature dependent physical properties of SnS nanocrystalline thin films. ECS J. Solid State Sci. Technol. 2013, 2, P259–P263. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, S.; Cheon, J. Synthesis and structural transformations of colloidal 2d layered metal chalcogenide nanocrystals. Chem. Soc. Rev. 2013, 42, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Herron, S.M.; Tanskanen, J.T.; Roelofs, K.E.; Bent, S.F. Highly textured tin(ii) sulfide thin films formed from sheetlike nanocrystal inks. Chem. Mater. 2014, 26, 7106–7113. [Google Scholar] [CrossRef]
- Freeman, T.L.; Evans, S.D.; Ulman, A. Xps studies of self-assembled multilayer films. Langmuir 1995, 11, 4411–4417. [Google Scholar] [CrossRef]
- Koroteev, V.O.; Bulusheva, L.G.; Asanov, I.P.; Shlyakhova, E.V.; Vyalikh, D.V.; Okotrub, A.V. Charge transfer in the MoS2/carbon nanotube composite. J. Phys. Chem. C 2011, 115, 21199–21204. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wu, F.Y.; Chen, T.Y.; Hsu, C.L.; Chen, C.H.; Wiryo, F.; Wei, K.H.; Chiang, C.Y.; Li, L.J. Three- dimensional molybdenum sulfi de sponges for electrocatalytic water splitting. Small 2014, 10, 895–900. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lee, C.S.; Lee, C.T. Investigation of accumulated carrier mechanism on sulfurated gan layers. J. Appl. Phys. 2003, 93, 5321–5324. [Google Scholar] [CrossRef]
- Wang, G.M.; Ling, Y.C.; Wang, H.Y.; Yang, X.Y.; Wang, C.C.; Zhang, J.Z.; Li, Y. Hydrogen-treated WO3 nanoflakes show enhanced photostability. Energy Environ. Sci. 2012, 5, 6180–6187. [Google Scholar] [CrossRef]
- Gedi, S.; Reddy, V.R.M.; Park, C.; Chan-Wook, J.; Reddy, K.T.R. Comprehensive optical studies on SnS layers synthesized by chemical bath deposition. Opt. Mater. 2015, 42, 468–475. [Google Scholar] [CrossRef]
- Jakhar, A.; Jamdagni, A.; Bakshi, A.; Verma, T.; Shukla, V.; Jain, P.; Sinha, N.; Arun, P. Refractive index of SnS thin nano-crystalline films. Solid State Commun. 2013, 168, 31–35. [Google Scholar] [CrossRef]
- Yang, X.Y.; Wolcott, A.; Wang, G.M.; Sobo, A.; Fitzmorris, R.C.; Qian, F.; Zhang, J.Z.; Li, Y. Nitrogen-doped zno nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2009, 9, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Umezawa, N.; Srinivasan, N.; Koyasu, S.; Sakai, E.; Miyauchi, M. A metal sulfide photocatalyst composed of ubiquitous elements for solar hydrogen production. Chem. Commun. 2016, 52, 7470–7473. [Google Scholar] [CrossRef] [PubMed]
- Shown, I.; Hsu, H.C.; Chang, Y.C.; Lin, C.H.; Roy, P.K.; Ganguly, A.; Wang, C.H.; Chang, J.K.; Wu, C.I.; Chen, L.C.; et al. Highly efficient visible light photocatalytic reduction of CO2 to hydrocarbon fuels by cu-nanoparticle decorated graphene oxide. Nano Lett. 2014, 14, 6097–6103. [Google Scholar] [CrossRef] [PubMed]
- Kibria, M.G.; Zhao, S.; Chowdhury, F.A.; Wang, Q.; Nguyen, H.P.T.; Trudeau, M.L.; Guo, H.; Mi, Z. Tuning the surface fermi level on p-type gallium nitride nanowires for efficient overall water splitting. Nat. Commun. 2014, 5, 3825–3831. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Wang, H.I.; Brahma, S.; Wang, S.C.; Huang, J.L. Synthesis and characteristics of layered SnS2 nanostructures via hot injection method. J. Cryst Growth 2017, 468, 162–168. [Google Scholar] [CrossRef]
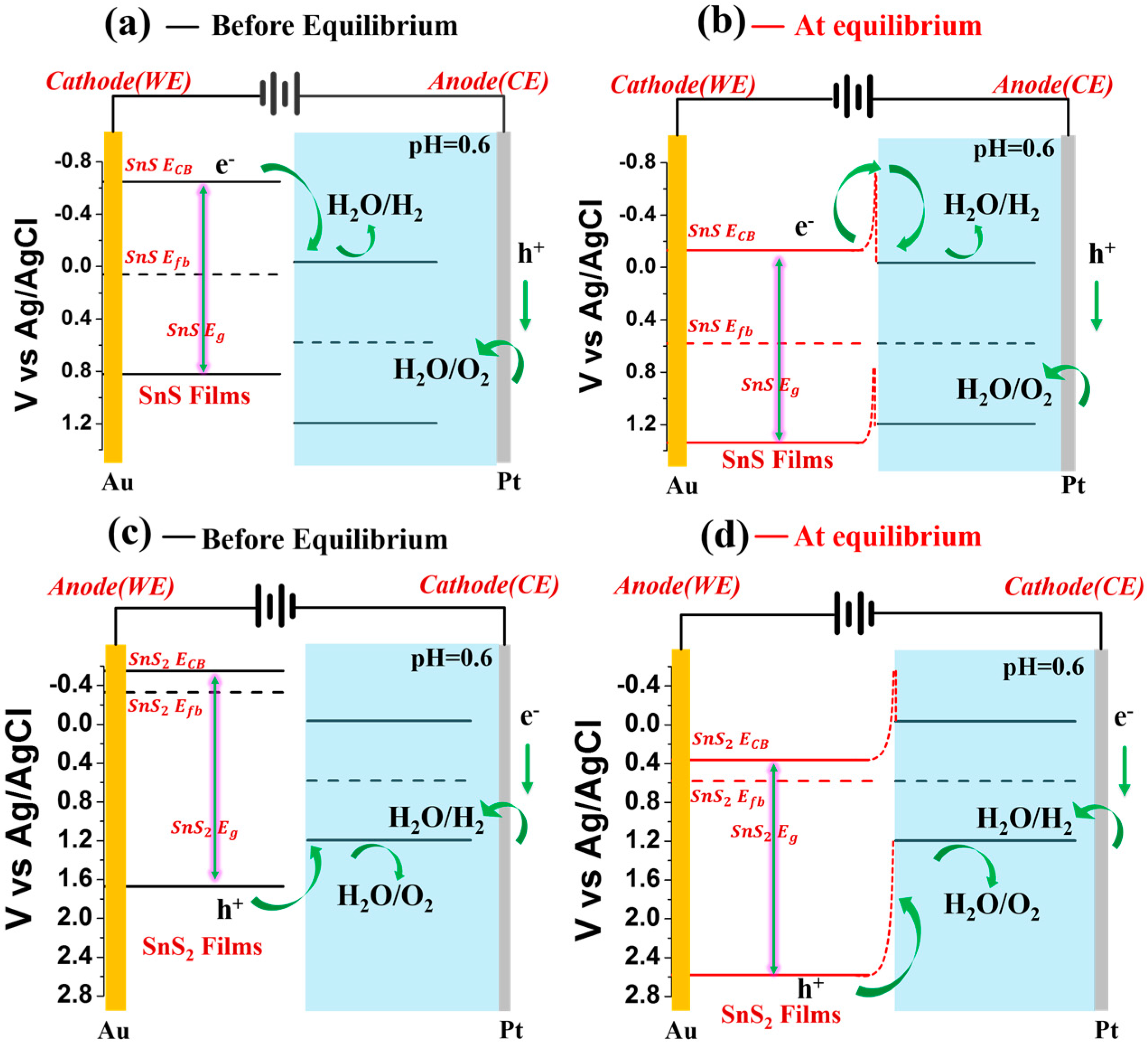
| Before Equilibrium (V vs. Ag/AgCl) | At Equilibrium (V vs. Ag/AgCl) | |||||
|---|---|---|---|---|---|---|
| Conduction Band () | Band Gap () | Flat Band () | Conduction Band () | Band Gap () | Flat Band ( | |
| SnS | −0.65 V | 1.47 eV | 0.06 V | −0.13 | 1.47 eV | 0.58 V |
| SnS2 | −0.55 V | 2.22 eV | −0.33 V | 0.36 V | 2.22 eV | 0.58 V |
| Reduction Potential () | Band Gap () | Fermi Level () | Reduction Potential () | Band Gap () | Fermi Level () | |
| H2SO4 (pH = 0.6) | −0.035 V | 1.23 eV | 0.58 V | −0.035 V | 1.23 eV | 0.58V |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-C.; Shen, Y.-M.; Brahma, S.; Shaikh, M.O.; Huang, J.-L.; Wang, S.-C. SnSx (x = 1, 2) Nanocrystals as Effective Catalysts for Photoelectrochemical Water Splitting. Catalysts 2017, 7, 252. https://doi.org/10.3390/catal7090252
Huang P-C, Shen Y-M, Brahma S, Shaikh MO, Huang J-L, Wang S-C. SnSx (x = 1, 2) Nanocrystals as Effective Catalysts for Photoelectrochemical Water Splitting. Catalysts. 2017; 7(9):252. https://doi.org/10.3390/catal7090252
Chicago/Turabian StyleHuang, Po-Chia, Yu-Min Shen, Sanjaya Brahma, Muhammad Omar Shaikh, Jow-Lay Huang, and Sheng-Chang Wang. 2017. "SnSx (x = 1, 2) Nanocrystals as Effective Catalysts for Photoelectrochemical Water Splitting" Catalysts 7, no. 9: 252. https://doi.org/10.3390/catal7090252
APA StyleHuang, P.-C., Shen, Y.-M., Brahma, S., Shaikh, M. O., Huang, J.-L., & Wang, S.-C. (2017). SnSx (x = 1, 2) Nanocrystals as Effective Catalysts for Photoelectrochemical Water Splitting. Catalysts, 7(9), 252. https://doi.org/10.3390/catal7090252