Chiral Catalyst Deactivation during the Asymmetric Hydrogenation of Acetophenone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Complexes
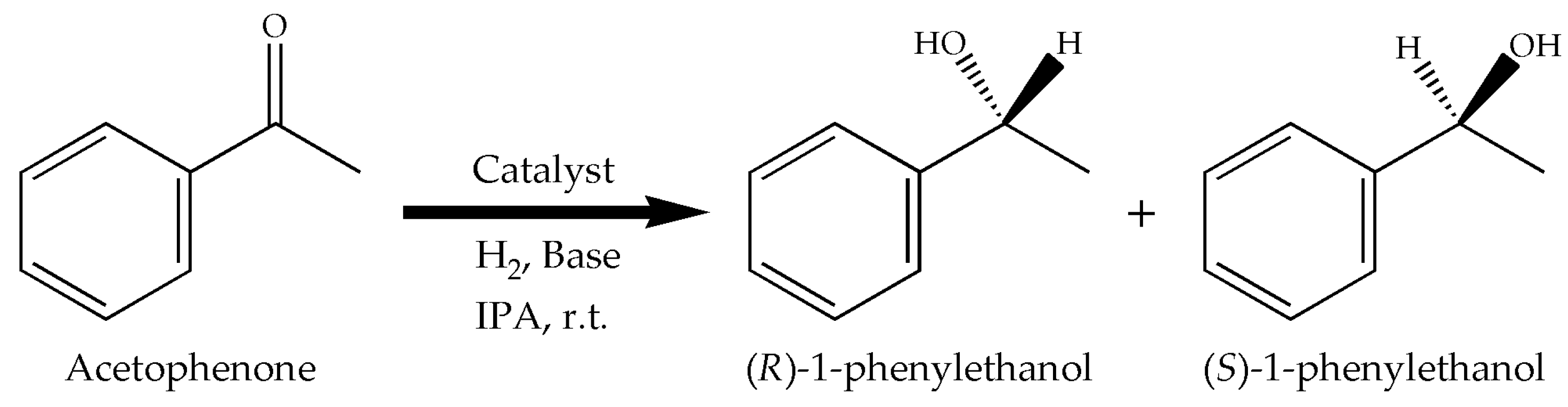
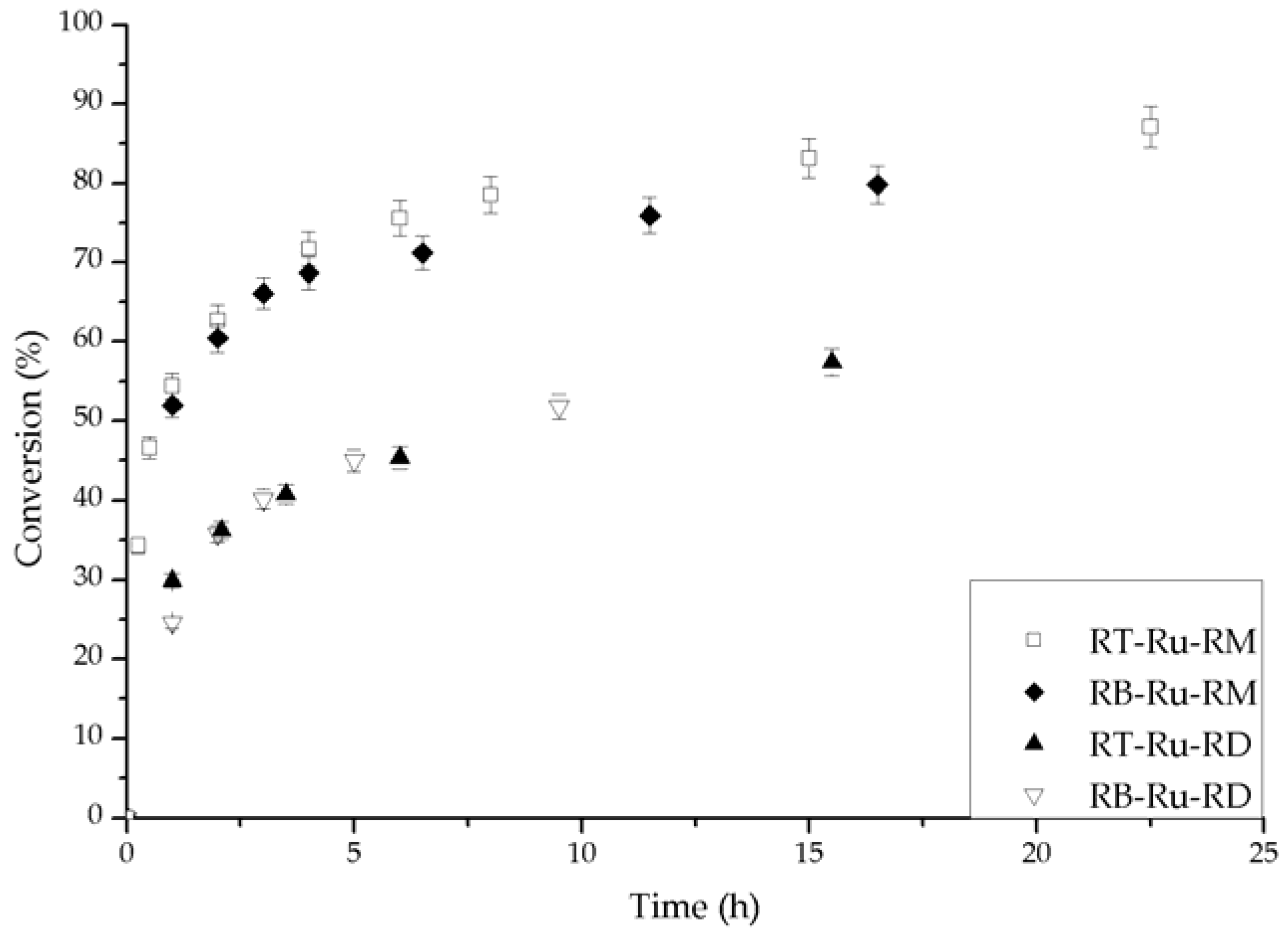
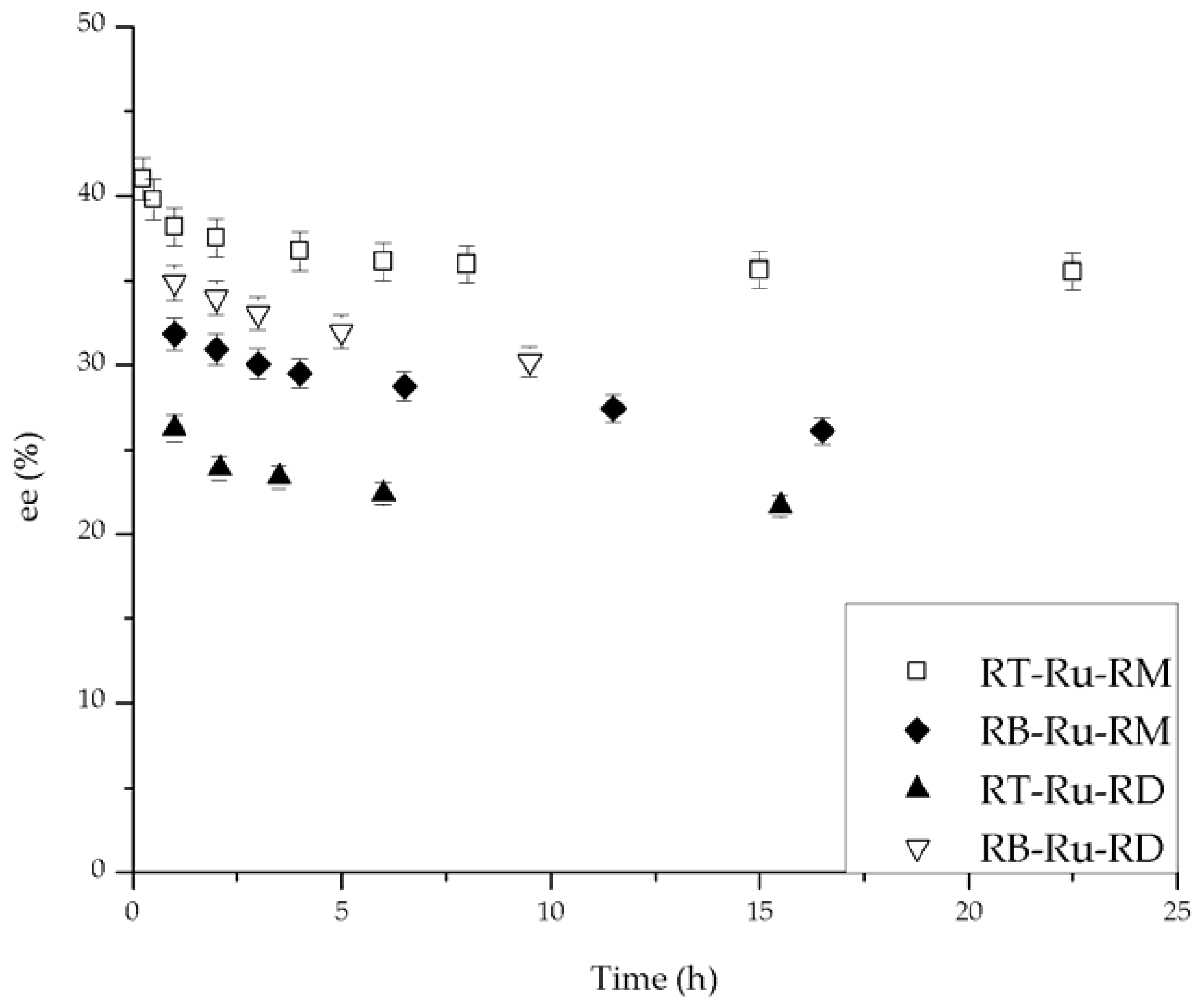
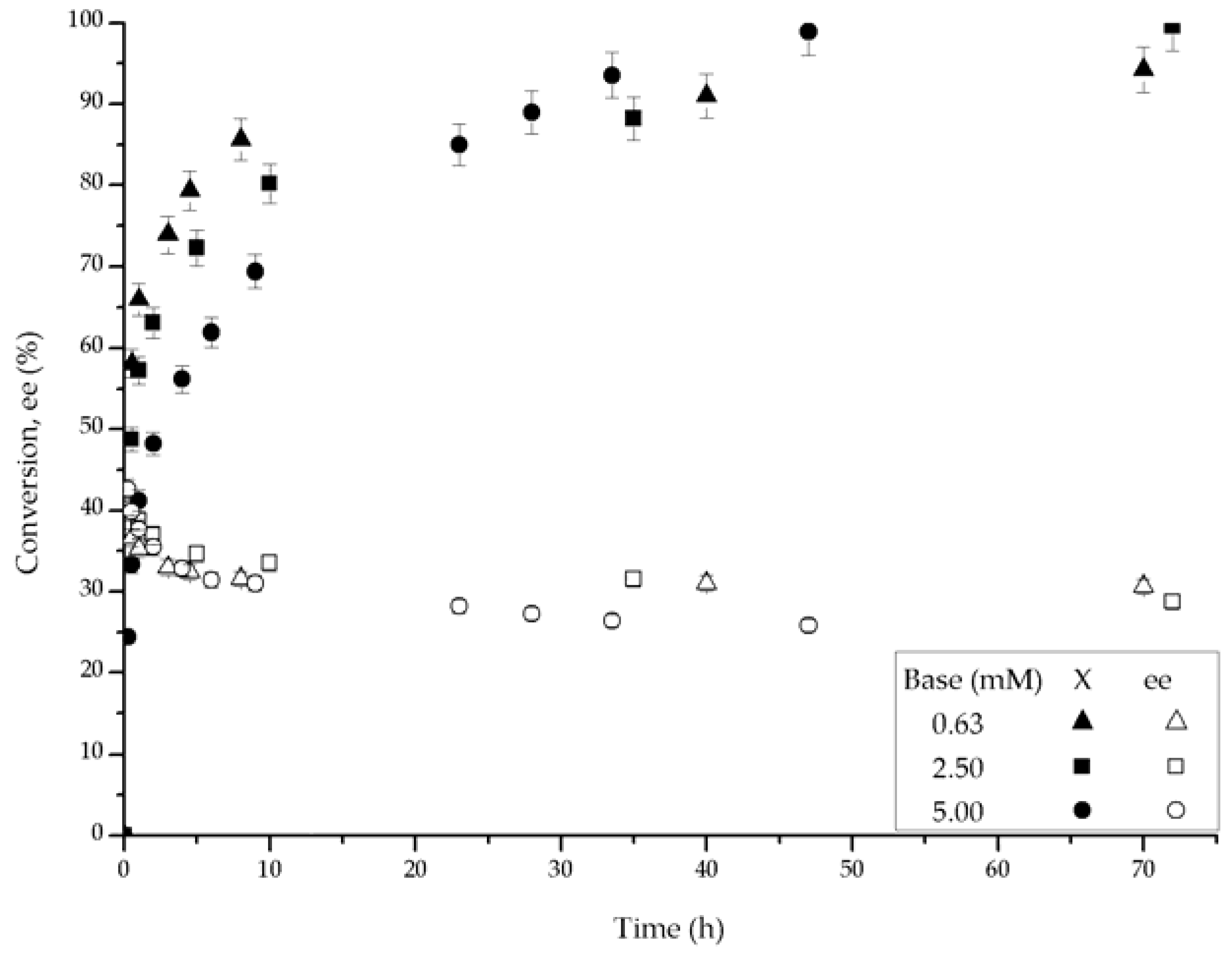
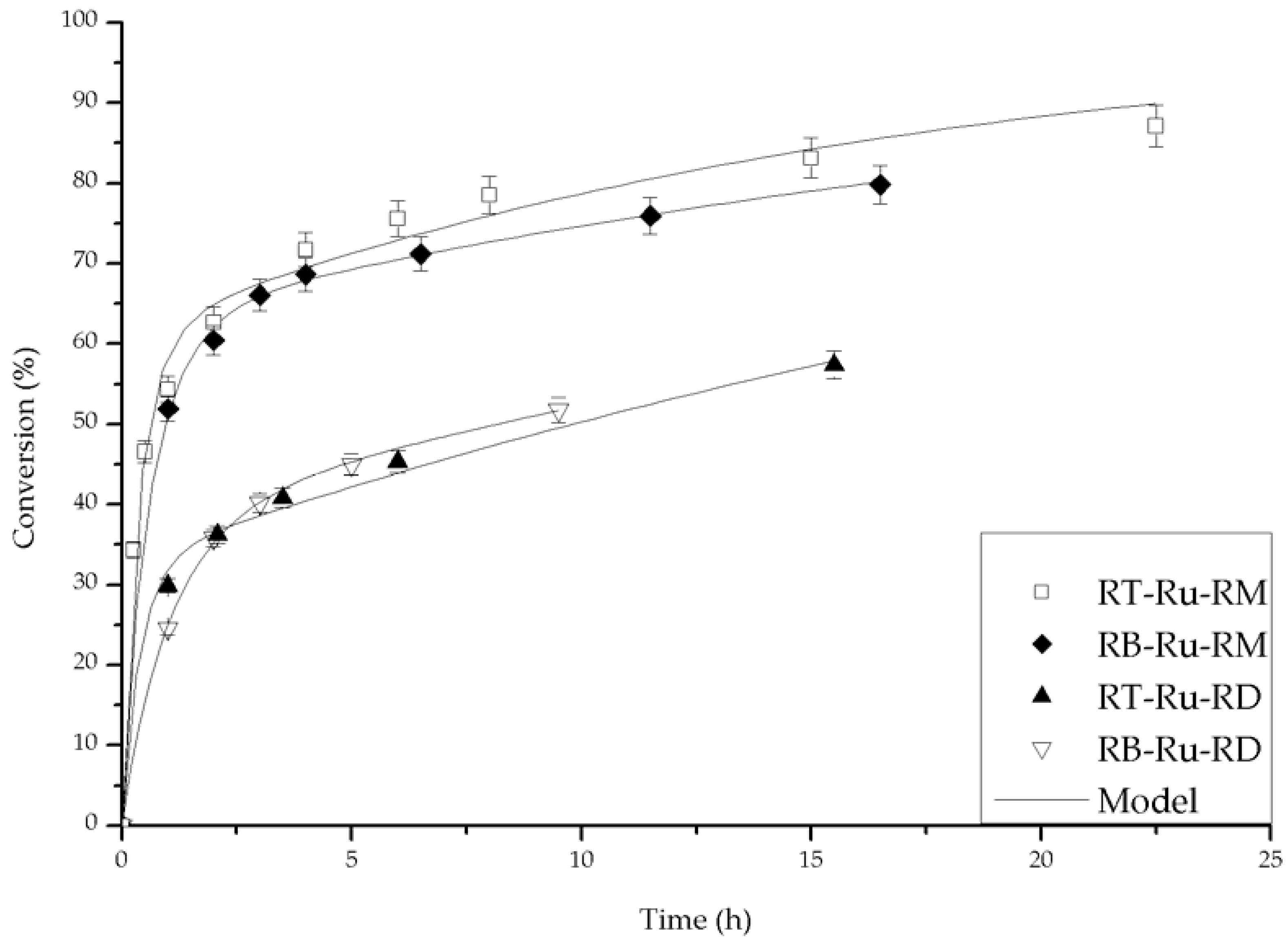
2.2. Catalytic Performance
2.3. Kinetic Model with Deactivation
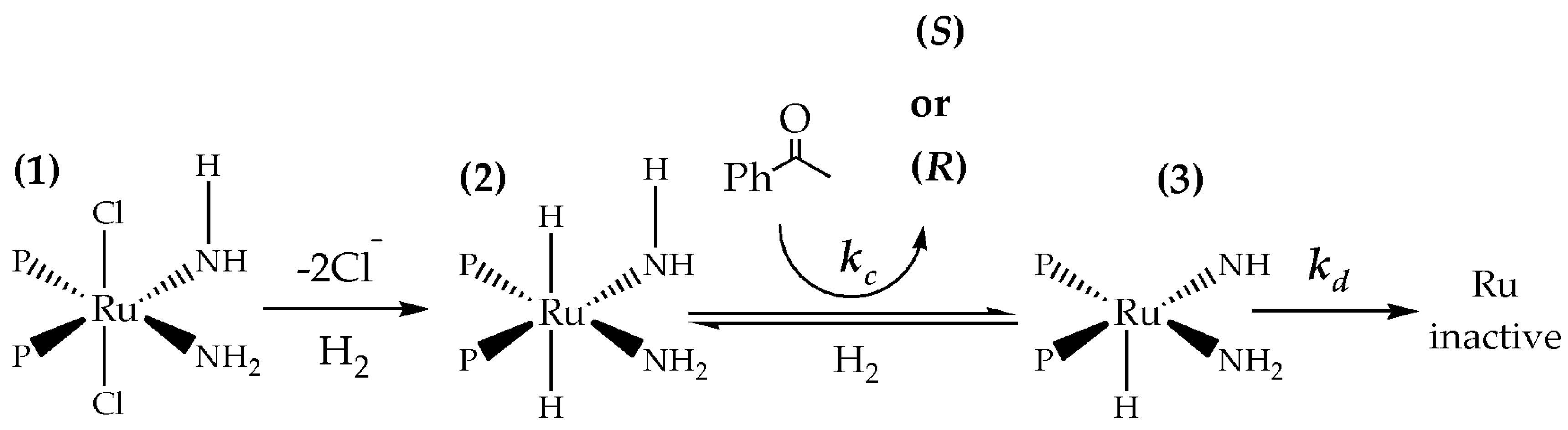
2.4. Mechanistic Considerations
3. Materials and Methods
3.1. Synthesis of the Complexes
3.2. Procedure for the Hydrogenation of Acetophenone
3.3. Parameter Estimation for the Kinetic Model with Catalyst Deactivation
4. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Noyori, R. Facts are the enemy of truth-reflections on serendipitous discovery and unforeseen developments in asymmetric catalysis. Angew. Chem. Int. Ed. 2013, 52, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R.; Ohkuma, T. Asymmetric catalysis by architectural and functional molecular engineering: Pactical chemo- and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 2001, 40, 40–73. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamasaki, K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 2003, 103, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R. Asymmetric catalysis: Science and opportunities (Nobel Lecture). Angew. Chem. Int. Ed. 2002, 41, 2008–2022. [Google Scholar]
- Jones, C.W. On the stability and recyclability of supported metal-ligand complex catalysts: Myths, misconceptions and critical research needs. Top. Catal. 2010, 53, 942–952. [Google Scholar]
- Van Leeuwen, P.W.N.M. Decomposition pathways of homogeneous catalysts. Appl. Catal. A-Gen. 2001, 212, 61–81. [Google Scholar] [CrossRef]
- Grasa, G.A.; Zanotti-Gerosa, A.; Medlock, J.A.; Hems, W.P. Asymmetric hydrogenation of isobutyrophenone using a [(diphosphine) RuCl2 (1,4-diamine)] catalyst. Org. Lett. 2005, 7, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Abdur-Rashid, K.; Clapham, S.E.; Harvey, J.N.; Lough, A.J.; Morris, R.H. Mechanism of the hydrogenation of ketones catalyzed by trans-dihydrido(diamine)ruthenium(II) complexes. J. Am. Chem. Soc. 2002, 124, 15104–15118. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Baumann, W.; Drexler, H.J.; Heller, D. Unusual deactivation in the asymmetric hydrogenation of itaconic acid. J. Organomet. Chem. 2011, 696, 1760–1767. [Google Scholar] [CrossRef]
- Proutiere, F.; Aufiero, M.; Schoenebeck, F. Reactivity and stability of dinuclear Pd(I) complexes: Studies on the active catalytic species, insights into precatalyst activation and deactivation, and application in highly selective cross-coupling reactions. J. Am. Chem. Soc. 2012, 134, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Dub, P.A.; Béthegnies, A.; Poli, R. DFT and experimental studies on the PtX2/X–catalyzed olefin hydroamination: Effect of halogen, amine basicity, and olefin on activity, regioselectivity, and catalyst deactivation. Organometallics 2012, 31, 294–305. [Google Scholar] [CrossRef]
- Matsunami, A.; Kuwata, S.; Kayaki, Y. A bifunctional iridium catalyst modified for persistent hydrogen generation from formic acid: Understanding deactivation via cyclometalation of a 1,2-Diphenylethylenediamine motif. ACS Catal. 2017, 7, 4479–4484. [Google Scholar] [CrossRef]
- Rivera, V.M.; Ruelas-Leyva, J.P.; Fuentes, G.A. Pd and Ru complexes bearing axially chiral ligands for the asymmetric hydrogenation of C=C and C=O double bonds. Catal. Today 2013, 213, 109–114. [Google Scholar] [CrossRef]
- Missaghi, M.N.; Galloway, J.M.; Kung, H.H. Bis(pyridyl)siloxane-Pd(II) complex catalyzed oxidation of alcohol to aldehyde: Effect of ligand tethering on catalytic activity and deactivation behavior. Appl. Catal. A-Gen. 2011, 391, 297–304. [Google Scholar] [CrossRef]
- Sandoval, C.A.; Ohkuma, T.; Muñiz, K.; Noyori, R. Mechanism of asymmetric hydrogenation of ketones catalyzed by BINAP/1,2-diamine-Ruthenium(II) complexes. J. Am. Chem. Soc. 2003, 125, 13490–13503. [Google Scholar] [CrossRef] [PubMed]
- Dub, P.A.; Gordon, J.C. The mechanism of enantioselective ketone reduction with Noyori and Noyori-Ikariya bifunctional catalysts. Dalton Trans. 2016, 45, 6756–6781. [Google Scholar] [CrossRef] [PubMed]
- Abbel, R.; Abdur-Rashid, K.; Faatz, M.; Hadzovic, A.; Lough, A.J.; Morris, R.H. A succession of isomers of Ruthenium dihydride complexes. Which one is the ketone hydrogenation catalyst? J. Am. Chem. Soc. 2005, 127, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Argyle, M.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Argyle, M. Advances in catalyst deactivation and regeneration. Catalysts 2015, 5, 949–954. [Google Scholar] [CrossRef]
- Fuentes, G.A. Catalyst deactivation and steady state activity: A generalized power-law equation. Appl. Catal. 1985, 15, 33–40. [Google Scholar] [CrossRef]
- Li, L.; Pan, Y.; Lei, M. The enantioselectivity in asymmetric ketone hydrogenation catalyzed by RuH2(diphosphine)(diamine) complexes: Insights from a 3D-QSSR and DFT study. Catal. Sci. Technol. 2016, 6, 4450–4457. [Google Scholar] [CrossRef]
- Ugi, I.; Marquarding, D.; Klusacek, H.; Gillespie, P.; Ramirez, F. Berry pseudorotation and turnstile rotation. Acc. Chem. Res. 1971, 4, 288–296. [Google Scholar] [CrossRef]
- Pérez, C.; Pérez, S.; Fuentes, G.A.; Corma, A. Preparation and use of a chiral amine Ruthenium hydrogenation catalyst supported on mesoporous silica. J. Mol. Catal. A-Chem. 2003, 197, 275–281. [Google Scholar] [CrossRef]
- Uehara, A.; Kubota, T.; Tsuchiya, R. New atropisomeric chiral bisphosphine, (S)-6-6′-dimethyl-2-2′-bis(diphenylphosphinamino)biphenyl, and asymmetric hydrogenation using the Rh(I) complex thereof. Chem. Lett. 1983, 13, 441–444. [Google Scholar] [CrossRef]
- Ohkuma, T.; Hattori, T.; Hirohito, O.; Inoue, T.; Noyori, R. BINAP/1,4-diamine-Ruthenium(II) complexes for efficient asymmetric hydrogenation of 1-tetralones and analogues. Org. Lett. 2004, 6, 2681–2683. [Google Scholar] [CrossRef] [PubMed]
- Bertero, N.M.; Apesteguía, C.R.; Marchi, A.J. Catalytic and kinetic study of the liquid-phase hydrogenation of acetophenone over Cu/SiO2 catalysts. Appl. Catal. A-Gen. 2008, 349, 100–109. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mewada, R.K. Selective hydrogenation of acetophenone to 1-phenyl ethanol over nanofibrous Ag-OMS-2 catalysts. Catal. Today 2012, 198, 330–337. [Google Scholar] [CrossRef]
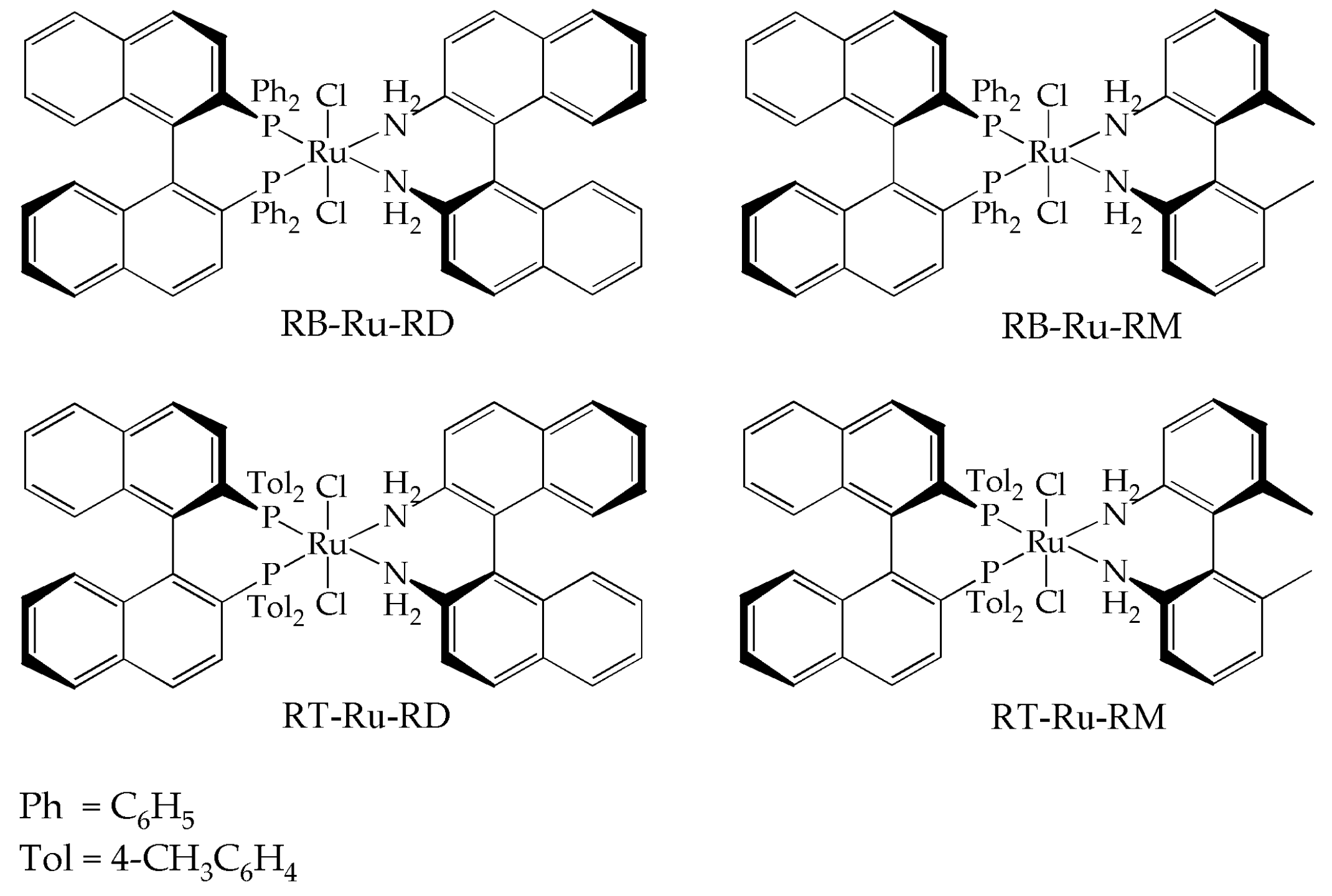

| Entry | Complex | IPA mL | Base (mM) | kc (h−1) | kd (h−1) | ass | TOF (h−1) 1 |
|---|---|---|---|---|---|---|---|
| 1 | RB-Ru-RD | 20 | 1.250 | 0.402 | 0.773 | 0.063 | 40.2 |
| 2 | RT-Ru-RD | 20 | 1.250 | 0.913 | 2.22 | 0.033 | 91.3 |
| 3 | RB-Ru-RM | 20 | 1.250 | 1.183 | 1.151 | 0.032 | 118.3 |
| 4 | RT-Ru-RM | 20 | 1.250 | 1.935 | 1.98 | 0.031 | 193.5 |
| 5 | RB-Ru-RM | 20 | 0.500 | 1.627 | 2.222 | 0.003 | 162.7 |
| 6 | RB-Ru-RM | 20 | 2.500 | 0.383 | 0.644 | 0.019 | 38.3 |
| 7 | RT-Ru-RM | 40 | 0.625 | 2.422 | 1.697 | 0.012 | 242.2 |
| 8 | RT-Ru-RM | 40 | 2.500 | 1.907 | 1.806 | 0.021 | 190.7 |
| 9 | RT-Ru-RM | 40 | 5.000 | 1.213 | 2.047 | 0.052 | 121.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruelas-Leyva, J.P.; Fuentes, G.A. Chiral Catalyst Deactivation during the Asymmetric Hydrogenation of Acetophenone. Catalysts 2017, 7, 193. https://doi.org/10.3390/catal7070193
Ruelas-Leyva JP, Fuentes GA. Chiral Catalyst Deactivation during the Asymmetric Hydrogenation of Acetophenone. Catalysts. 2017; 7(7):193. https://doi.org/10.3390/catal7070193
Chicago/Turabian StyleRuelas-Leyva, Jose P., and Gustavo A. Fuentes. 2017. "Chiral Catalyst Deactivation during the Asymmetric Hydrogenation of Acetophenone" Catalysts 7, no. 7: 193. https://doi.org/10.3390/catal7070193
APA StyleRuelas-Leyva, J. P., & Fuentes, G. A. (2017). Chiral Catalyst Deactivation during the Asymmetric Hydrogenation of Acetophenone. Catalysts, 7(7), 193. https://doi.org/10.3390/catal7070193







