Influence of Dissolved Ions on the Water Purification Performance of TiO2-Impregnated Porous Silica Tubes
Abstract
:1. Introduction
2. Results and Discussion
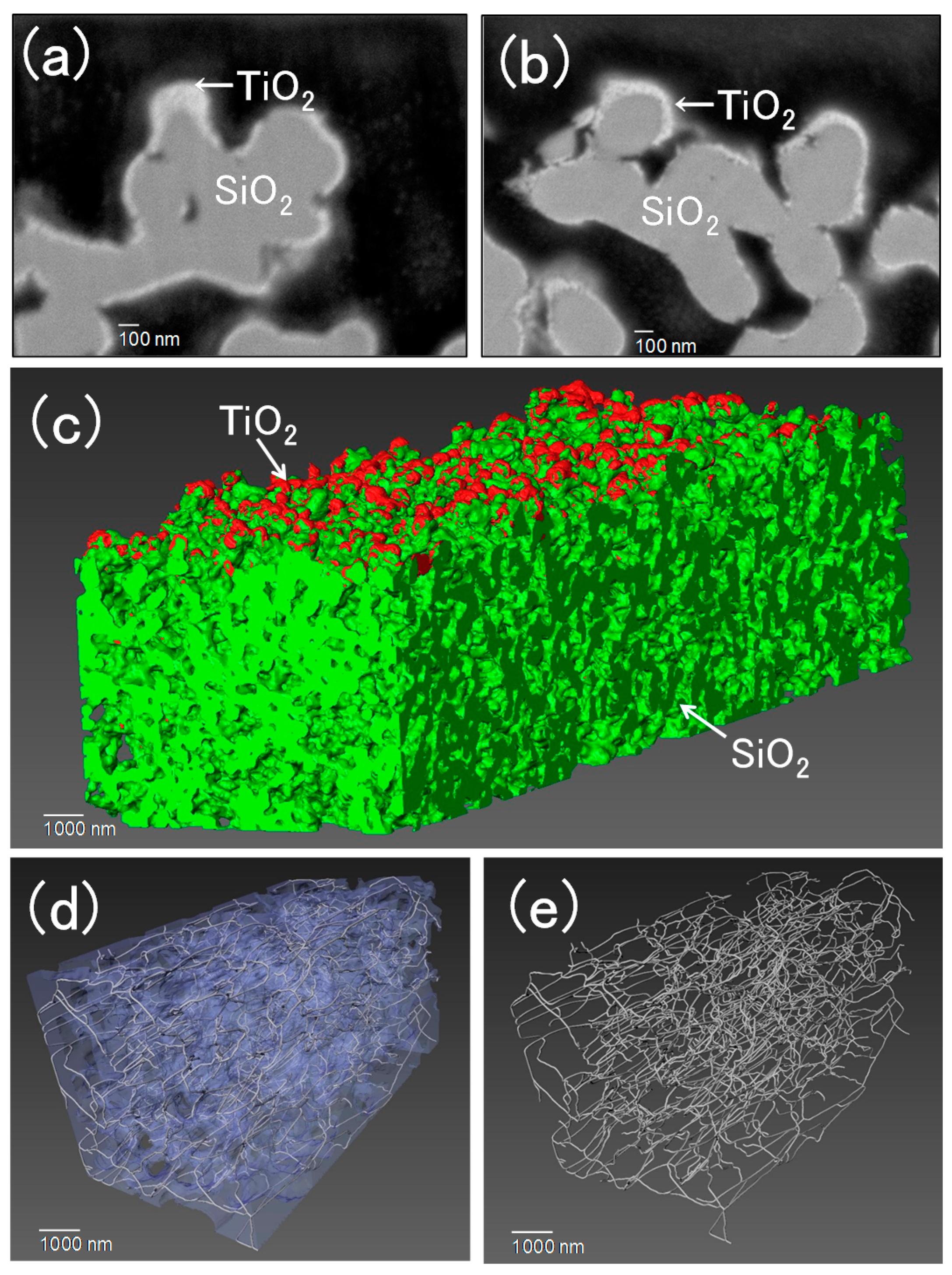
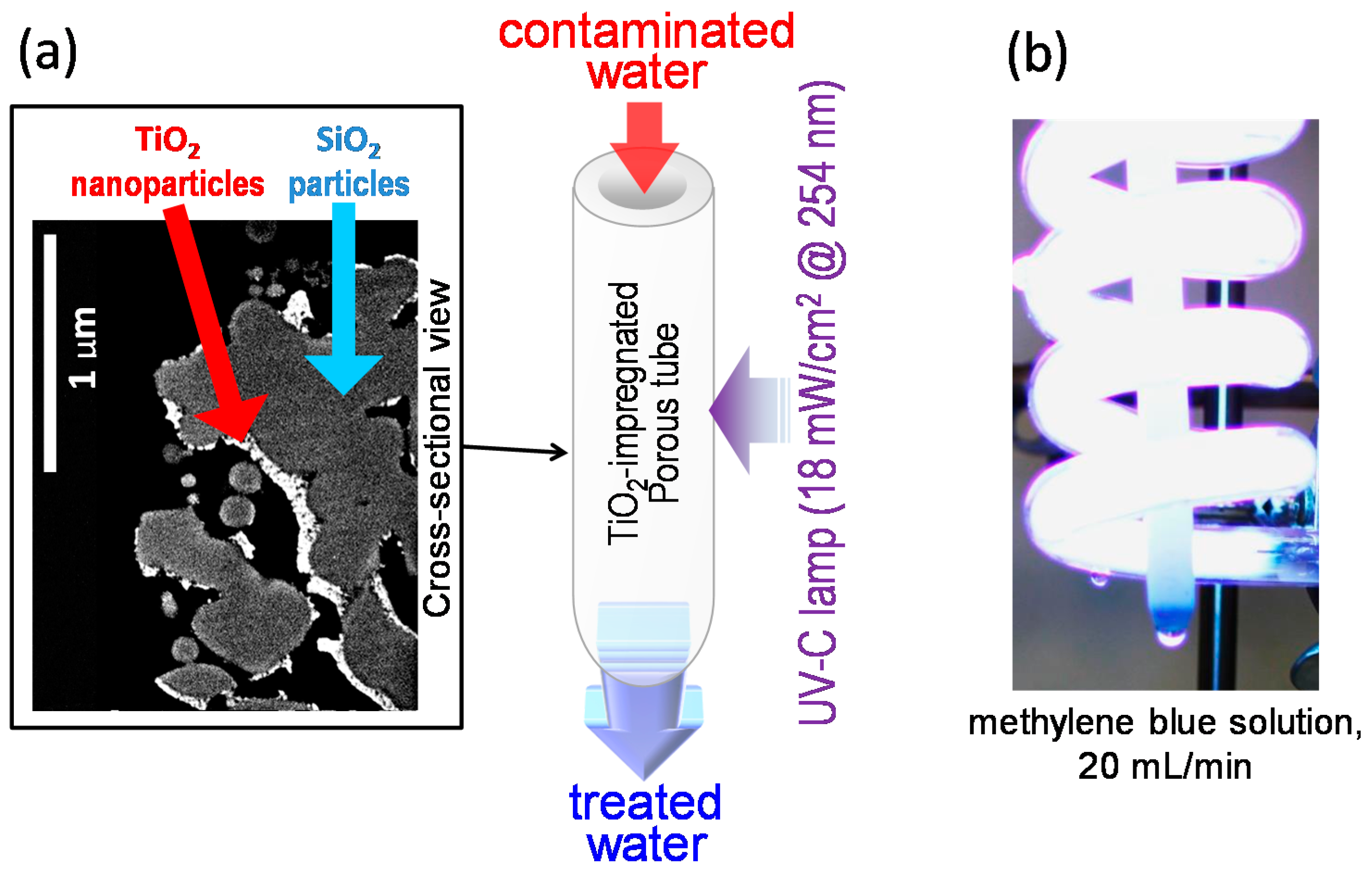
2.1. Characterization of Porous a-Silica Tubes
2.2. Properties of the Methylene Blue Solutions
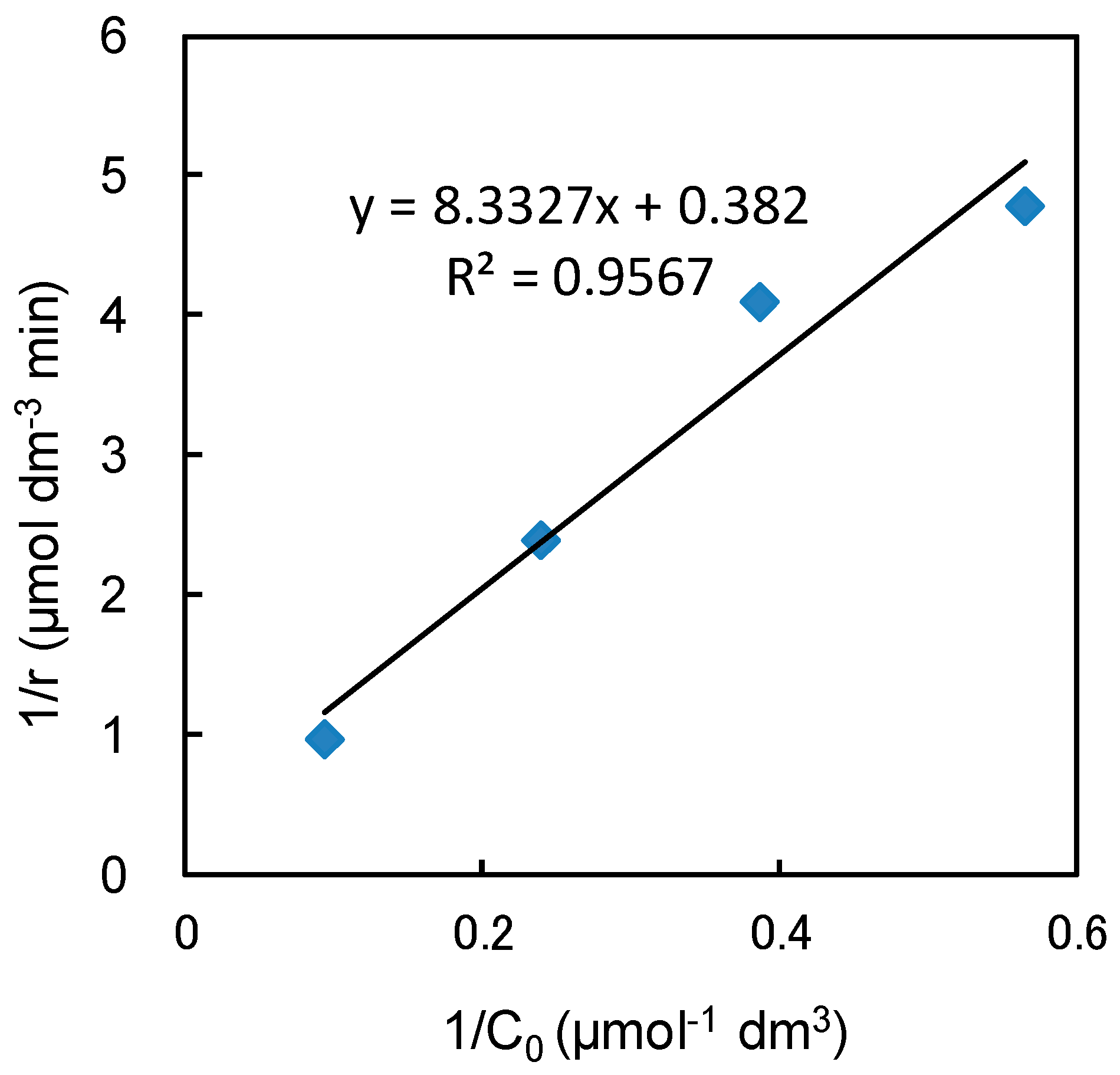
2.3. Effect of Initial Methylene Blue Concentration
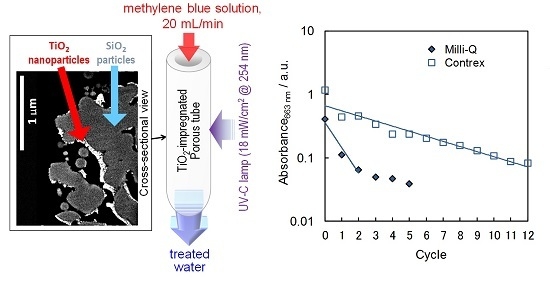
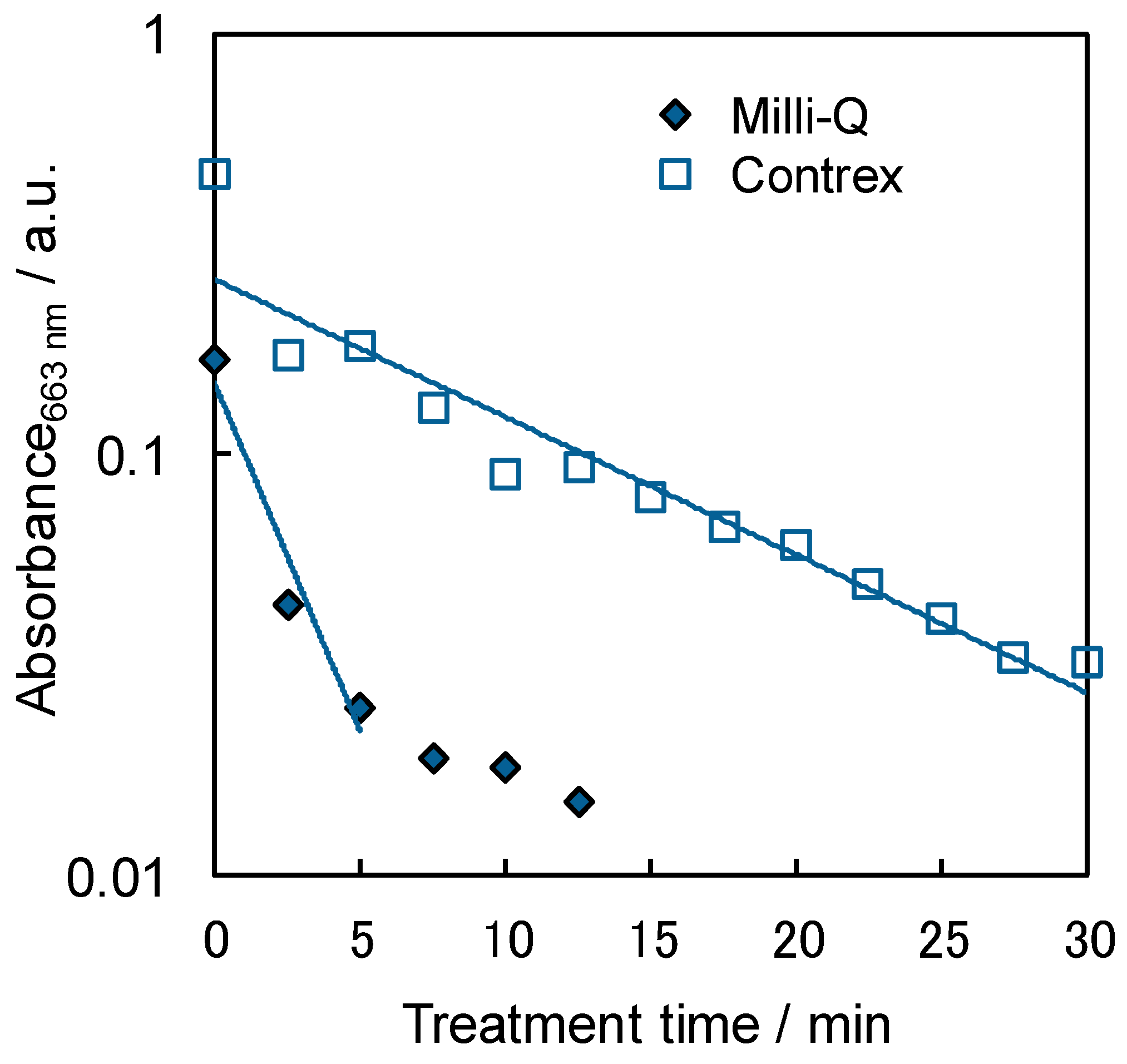
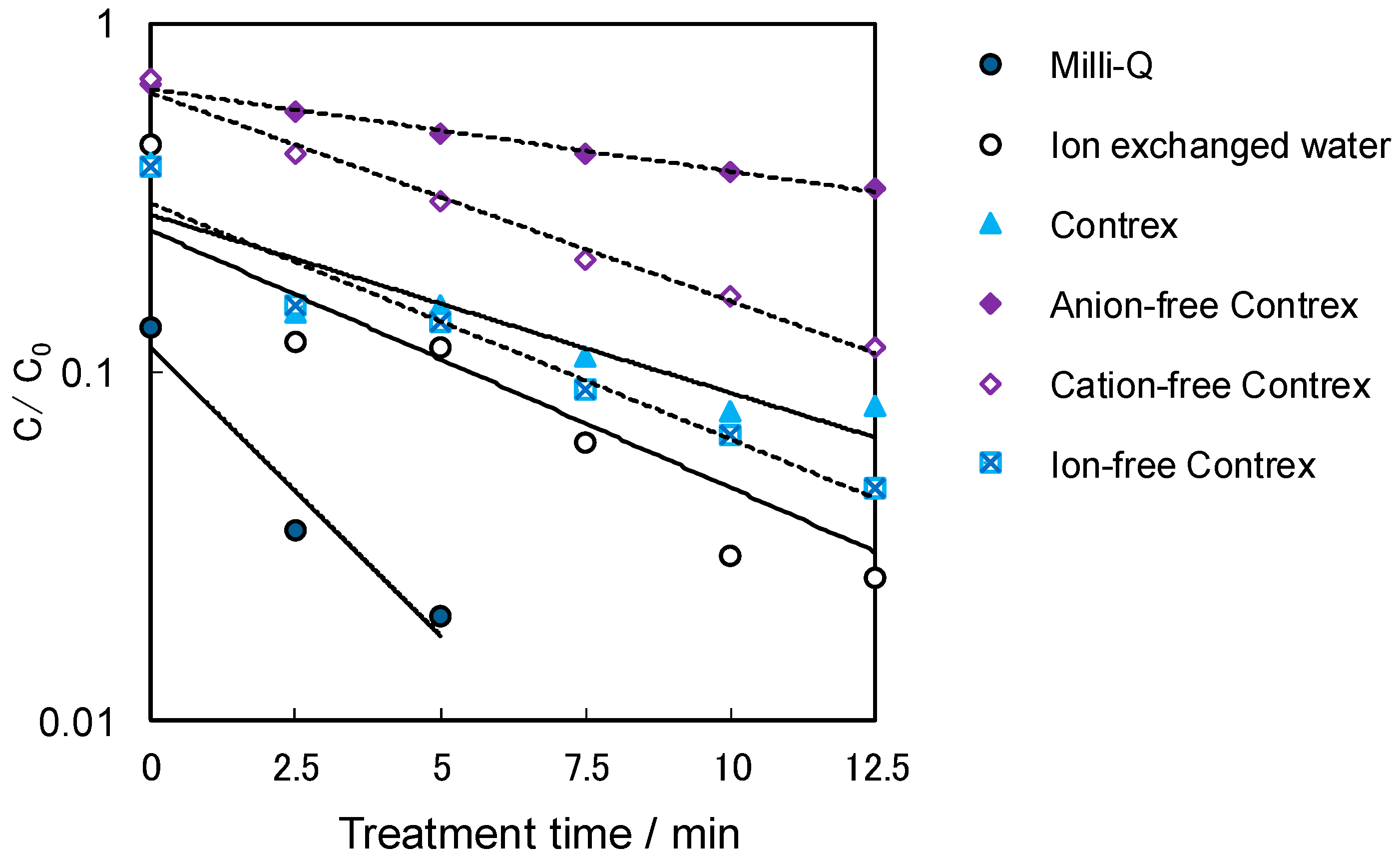
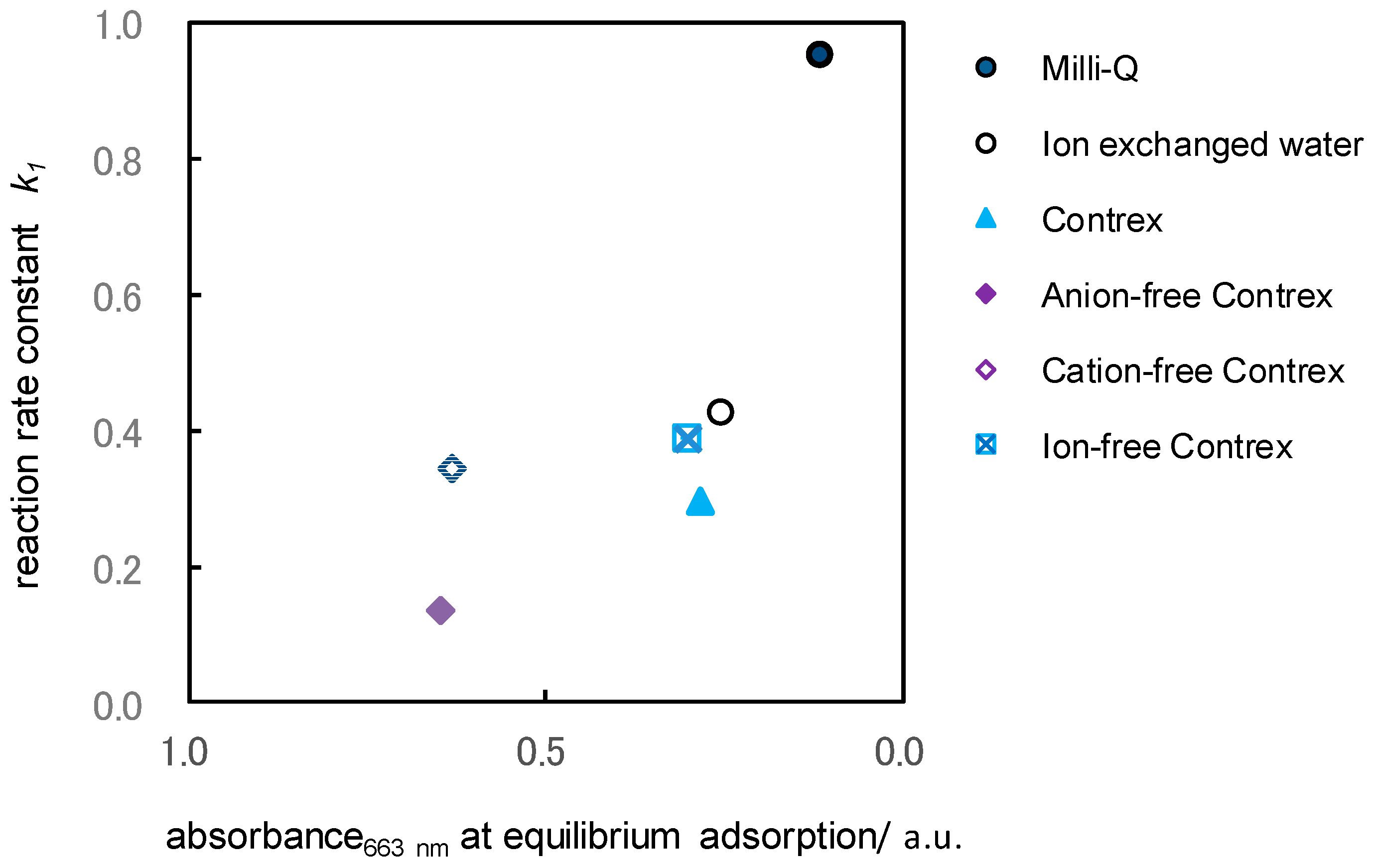
2.4. Influence of the Dissolved Ions
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Liu, B.; Nakata, K.; Sakai, M.; Saito, H.; Ochiai, T.; Murakami, T.; Takagi, K.; Fujishima, A. Hierarchical TiO2 spherical nanostructures with tunable pore size, pore volume, and specific surface area: Facile preparation and high-photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1933–1939. [Google Scholar] [CrossRef]
- Reddy, K.R.; Nakata, K.; Ochiai, T.; Murakami, T.; Tryk, D.A.; Fujishima, A. Facile fabrication and photocatalytic application of Ag nanoparticles-TiO2 nanofiber composites. J. Nanosci. Nanotechnol. 2011, 11, 3692–3695. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Tago, S.; Tawarayama, H.; Hosoya, T.; Ishiguro, H.; Fujishima, A. Fabrication of a porous TiO2-coated silica glass tube and its application for a handy water purification unit. Int. J. Photoenergy 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Guillard, C.; Puzenat, E.; Lachheb, H.; Houas, A.; Herrmann, J.-M. Why inorganic salts decrease the TiO2 photocatalytic efficiency. Int. J. Photoenergy 2005, 7, 1–9. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zahraa, O.; Bouchy, M. Inhibition of the adsorption and photocatalytic degradation of an organic contaminant in an aqueous suspension of TiO2 by inorganic ions. J. Photochem. Photobiol. A 1997, 108, 37–44. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Y.; Lu, Z.; Huo, P.; Xing, W.; He, M.; Li, J.; Yan, Y. Influence of inorganic ions and pH on the photodegradation of 1-methylimidazole-2-thiol with TiO2 photocatalyst based on magnetic multi-walled carbon nanotubes. Bull. Korean Chem. Soc. 2014, 35, 76–82. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Weber, J.-V. Photocatalytic mineralization of humic acids with TiO2: Effect of pH, sulfate and chloride anions. Int. J. Photoenergy 2003, 5, 69–74. [Google Scholar] [CrossRef]
- Gjipalaj, J.; Alessandri, I. Easy recovery, mechanical stability, enhanced adsorption capacity and recyclability of alginate-based TiO2 macrobead photocatalysts for water treatment. J. Environ. Chem. Eng. 2017, 5, 1763–1770. [Google Scholar] [CrossRef]
- Ochiai, T.; Tago, S.; Hayashi, M.; Tawarayama, H.; Hosoya, T.; Fujishima, A. TiO2-impregnated porous silica tube and its application for compact air- and water-purification units. Catalysts 2015, 5, 1498–1506. [Google Scholar] [CrossRef]
- Salehi, M.; Hashemipour, H.; Mirzaee, M. Experimental study of influencing factors and kinetics in catalytic removal of methylene blue with TiO2 nanopowder. AJEE 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Peiró, A.M.; Ayllon, J.A.; Peral, J.; Domenech, X. TiO2-photocatalyzed degradation of phenol and ortho-substituted phenolic compounds. Appl. Catal. B 2001, 30, 359–373. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of liquid phase photocatalyzed reactions: An illuminating approach. J. Phys. Chem. B 2005, 109, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B. Chapter 10-Photocatalysis by inorganic solid materials: Revisiting its definition, concepts, and experimental procedures. In Advanced Inorganic Chemistry: A Comprehensive Text; van Eldik, R., Stochel, G., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 63, pp. 395–430. ISBN 0898-8838. [Google Scholar]
- Sardar, S.; Sarkar, S.; Myint, M.T.; Al-Harthi, S.; Dutta, J.; Pal, S.K. Role of central metal ions in hematoporphyrin-functionalized titania in solar energy conversion dynamics. Phys. Chem. Phys. 2013, 15, 18562–18570. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Nakata, K.; Murakami, T.; Fujishima, A.; Yao, Y.Y.; Tryk, D.A.; Kubota, Y. Development of solar-driven electrochemical and photocatalytic water treatment system using a boron-doped diamond electrode and TiO2 photocatalyst. Water Res. 2010, 44, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, D.; Zhang, G.; Cai, C.; Zhang, C.; Qiu, G.; Zheng, K.; Wu, Z. Adsorption of methylene blue from aqueous solution onto multiporous palygorskite modified by ion beam bombardment: Effect of contact time, temperature, pH and ionic strength. Appl. Clay Sci. 2013, 83–84, 137–143. [Google Scholar] [CrossRef]
- Shaban, Y.A. Enhanced photocatalytic removal of methylene blue from seawater under natural sunlight using carbon-modified n-TiO2 nanoparticles. Environ. Pollut. 2013, 3, 41–50. [Google Scholar] [CrossRef]
- Tolosa, N.C.; Lu, M.-C.; Mendoza, H.D.; Rollon, A.P. Factors affecting the photocatalytic oxidation of 2,4-dichlorophenol using modified titanium dioxide TiO2/KAl(SO4)2 catalyst under visible light. Sustain. Environ. Res. 2011, 21, 381–387. [Google Scholar]

| Milli-Q | Contrex | Ion Exchanged Water | Anion-Free Contrex | Cation-Free Contrex | Ion-Free Contrex | |
|---|---|---|---|---|---|---|
| Dissolved ion [mg/L] | ||||||
| Ca2+ | 0.0960 | 486 (468) | 0.232 | 95.2 | 0.262 | 0.0143 |
| Mg2+ | n.d. | 82.2 (74.5) | 0.005 | n.d. | 0.040 | 0.0255 |
| Na+ | 0.0328 | 8.0 (9.4) | 0.037 | 3.00 | 0.774 | 0.021 |
| K+ | n.d. | 2.95 (2.8) | 0.021 | 1.08 | n.d. | 0.010 |
| SO42− | n.d. | 1356 (1121) | n.d. | 1.30 | 1406 | 0.115 |
| NO42− | n.d. | 2.76 (2.9) | n.d. | n.d. | 2.51 | n.d. |
| HCO3− | Unmeasured | Unmeasured (327) | Unmeasured | Unmeasured | Unmeasured | Unmeasured |
| Cl− | n.d. | 9.17 (7.6) | n.d. | 3.29 | 11.0 | n.d. |
| Electrical conductivity [µS/cm] | 0.91 | 1987 | 3.54 | 934 | >1999 | 3.41 |
| pH | 7 | 7 | 7 | 11 | 2 | 7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M.; Ochiai, T.; Tago, S.; Tawarayama, H.; Hosoya, T.; Yahagi, T.; Fujishima, A. Influence of Dissolved Ions on the Water Purification Performance of TiO2-Impregnated Porous Silica Tubes. Catalysts 2017, 7, 158. https://doi.org/10.3390/catal7050158
Hayashi M, Ochiai T, Tago S, Tawarayama H, Hosoya T, Yahagi T, Fujishima A. Influence of Dissolved Ions on the Water Purification Performance of TiO2-Impregnated Porous Silica Tubes. Catalysts. 2017; 7(5):158. https://doi.org/10.3390/catal7050158
Chicago/Turabian StyleHayashi, Mio, Tsuyoshi Ochiai, Shoko Tago, Hiromasa Tawarayama, Toshifumi Hosoya, Tsukaho Yahagi, and Akira Fujishima. 2017. "Influence of Dissolved Ions on the Water Purification Performance of TiO2-Impregnated Porous Silica Tubes" Catalysts 7, no. 5: 158. https://doi.org/10.3390/catal7050158
APA StyleHayashi, M., Ochiai, T., Tago, S., Tawarayama, H., Hosoya, T., Yahagi, T., & Fujishima, A. (2017). Influence of Dissolved Ions on the Water Purification Performance of TiO2-Impregnated Porous Silica Tubes. Catalysts, 7(5), 158. https://doi.org/10.3390/catal7050158