Methanation of CO2 on Ni/Al2O3 in a Structured Fixed-Bed Reactor—A Scale-Up Study
Abstract
:1. Introduction
2. Results and Discussion
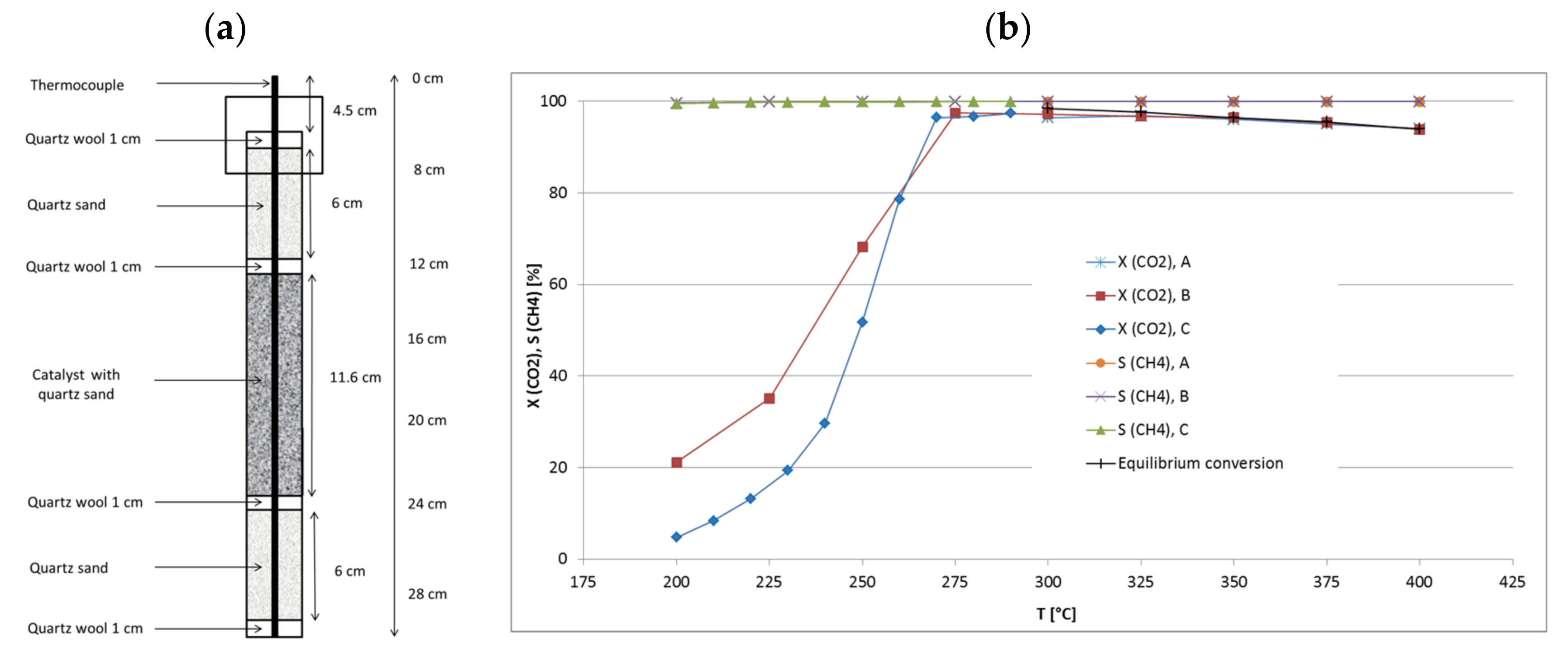
2.1. Catalytic Test Runs
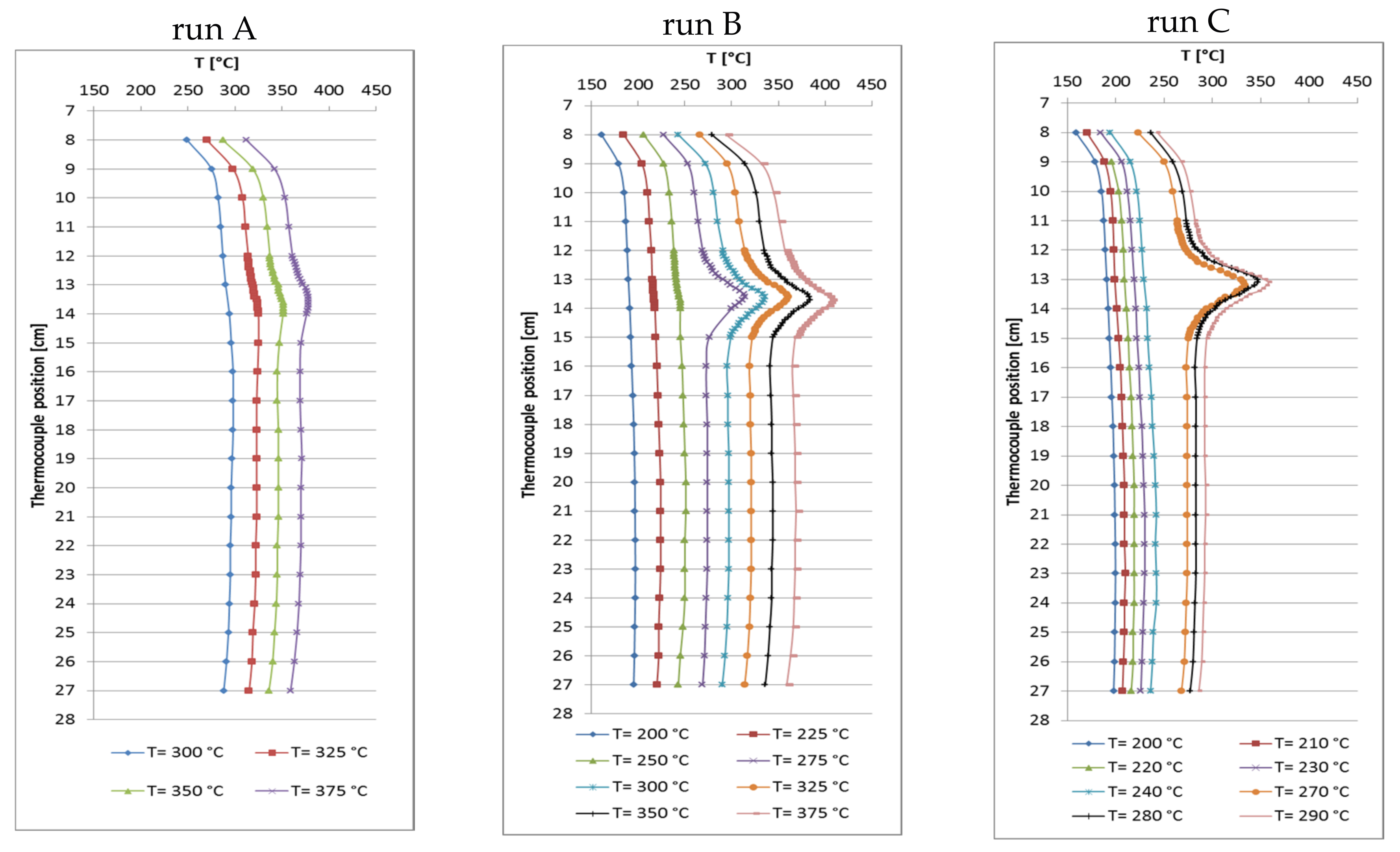
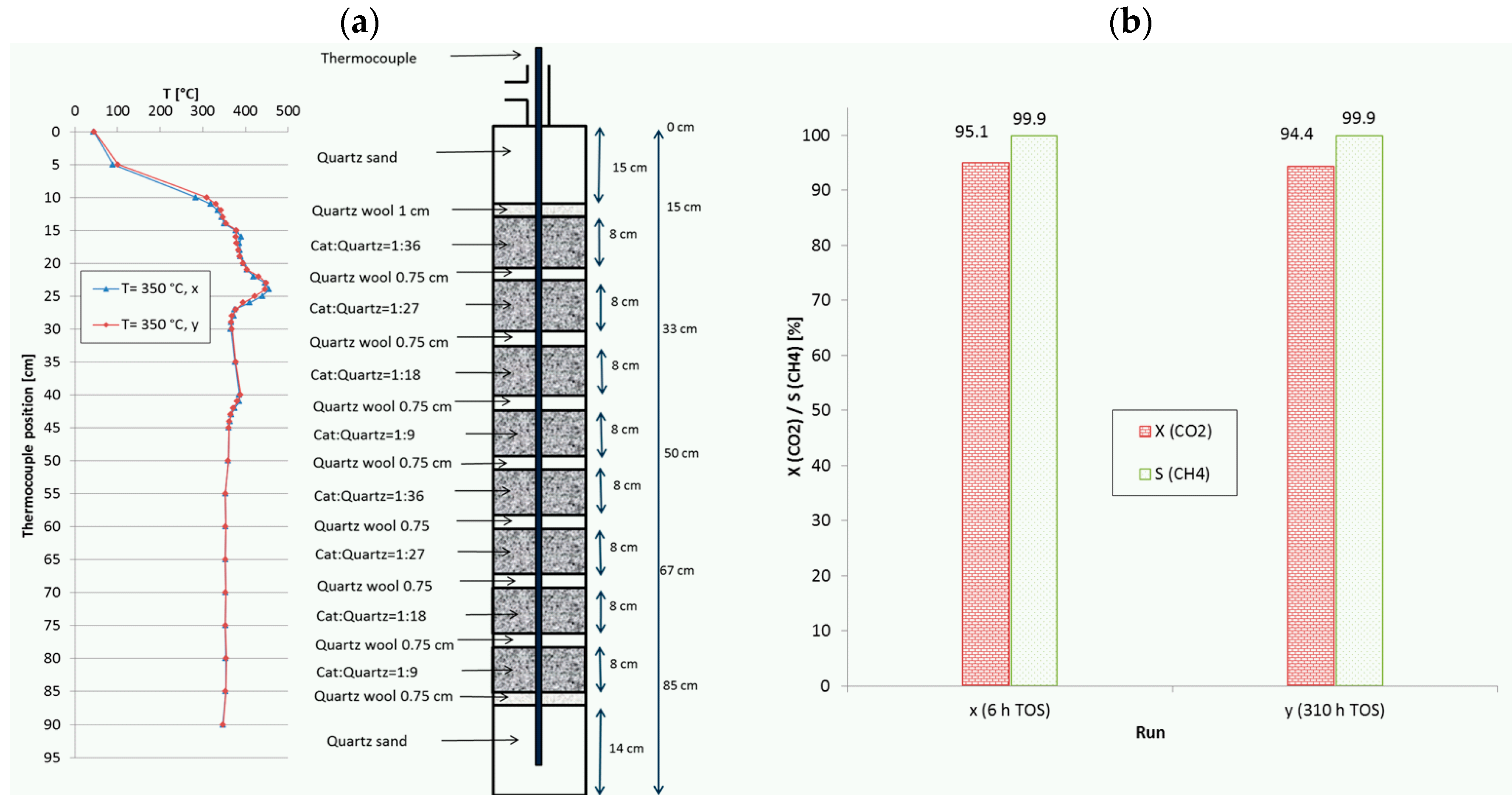
2.2. Temperature Profiles in the Small Tube Reactor
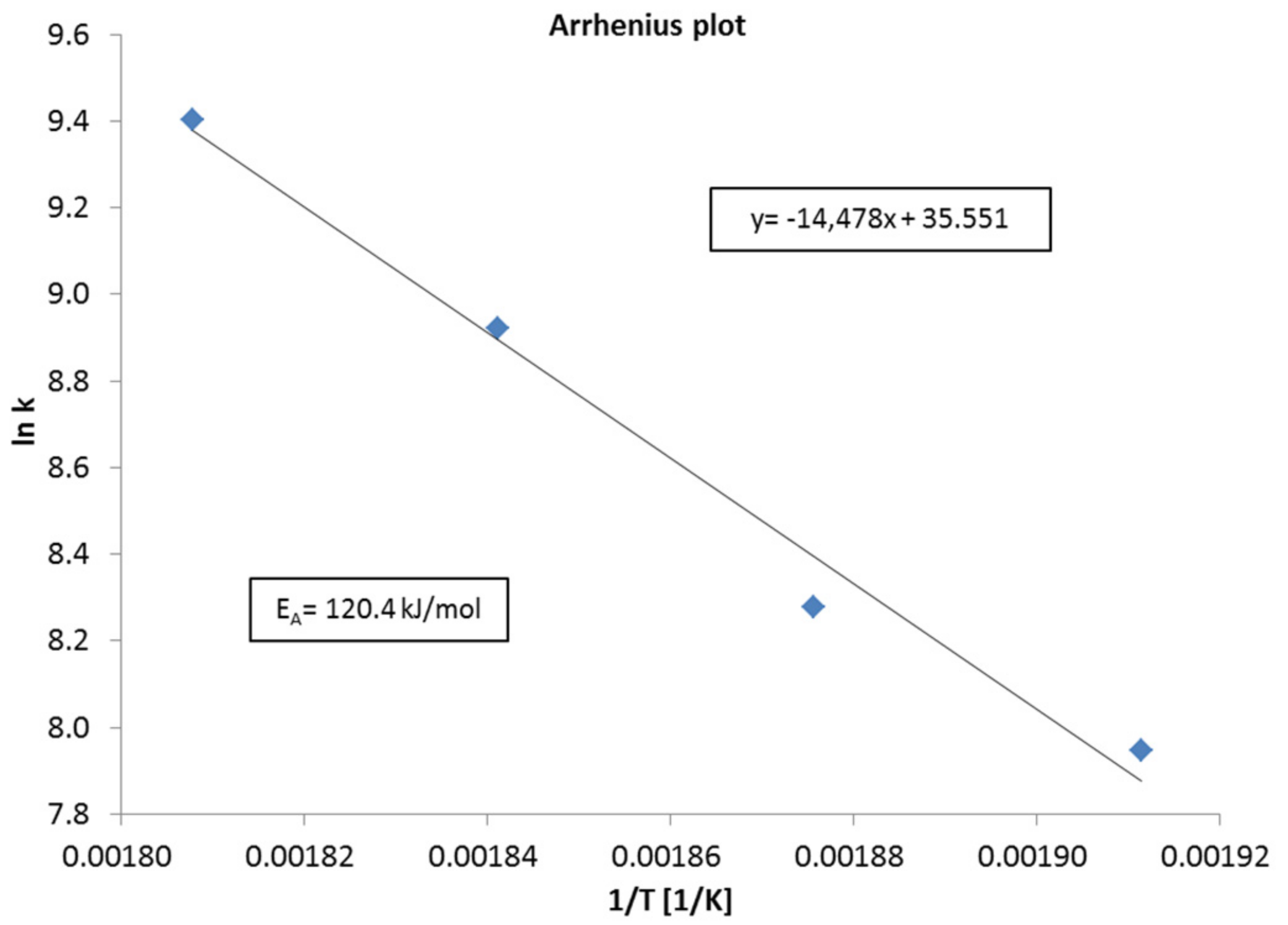
2.3. Activation Energy
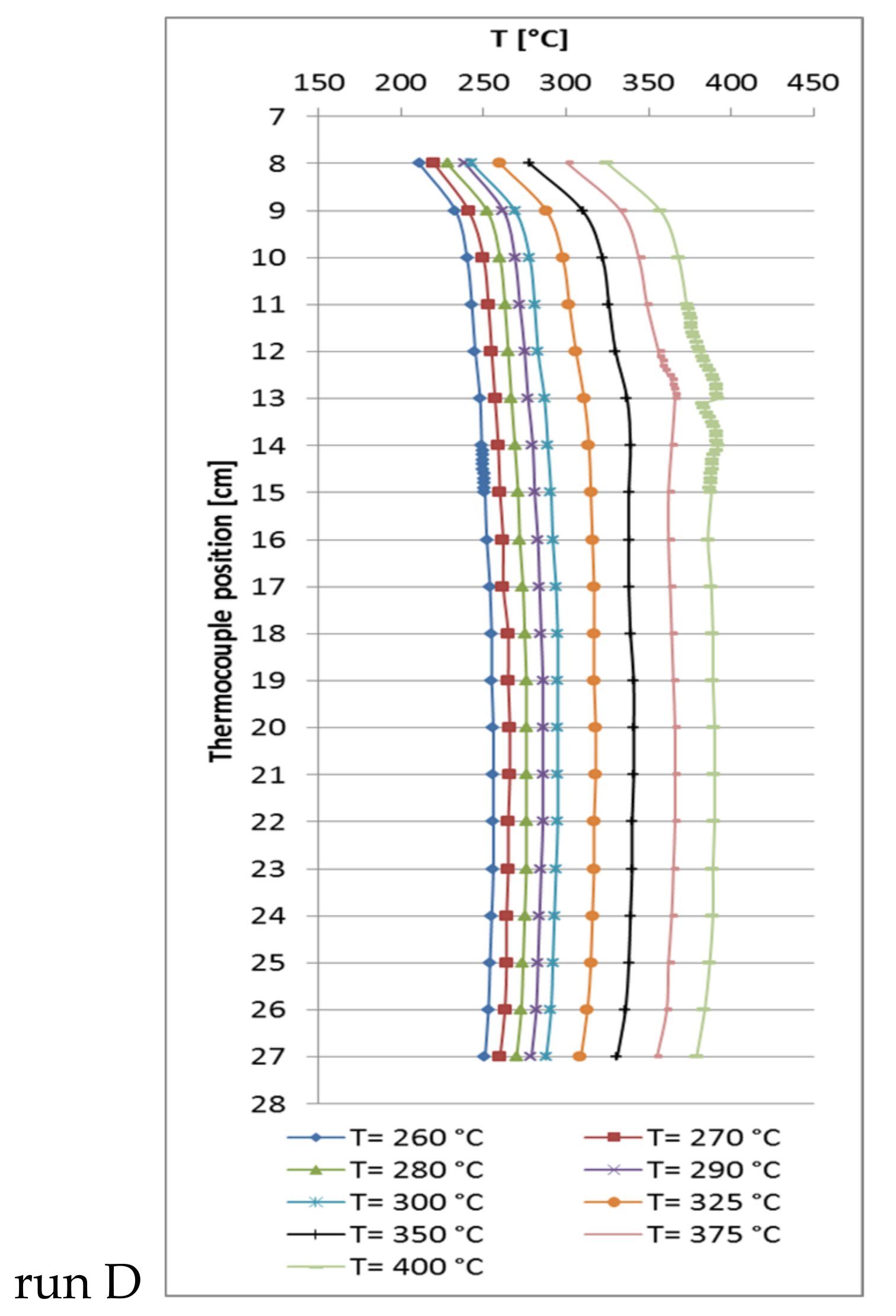
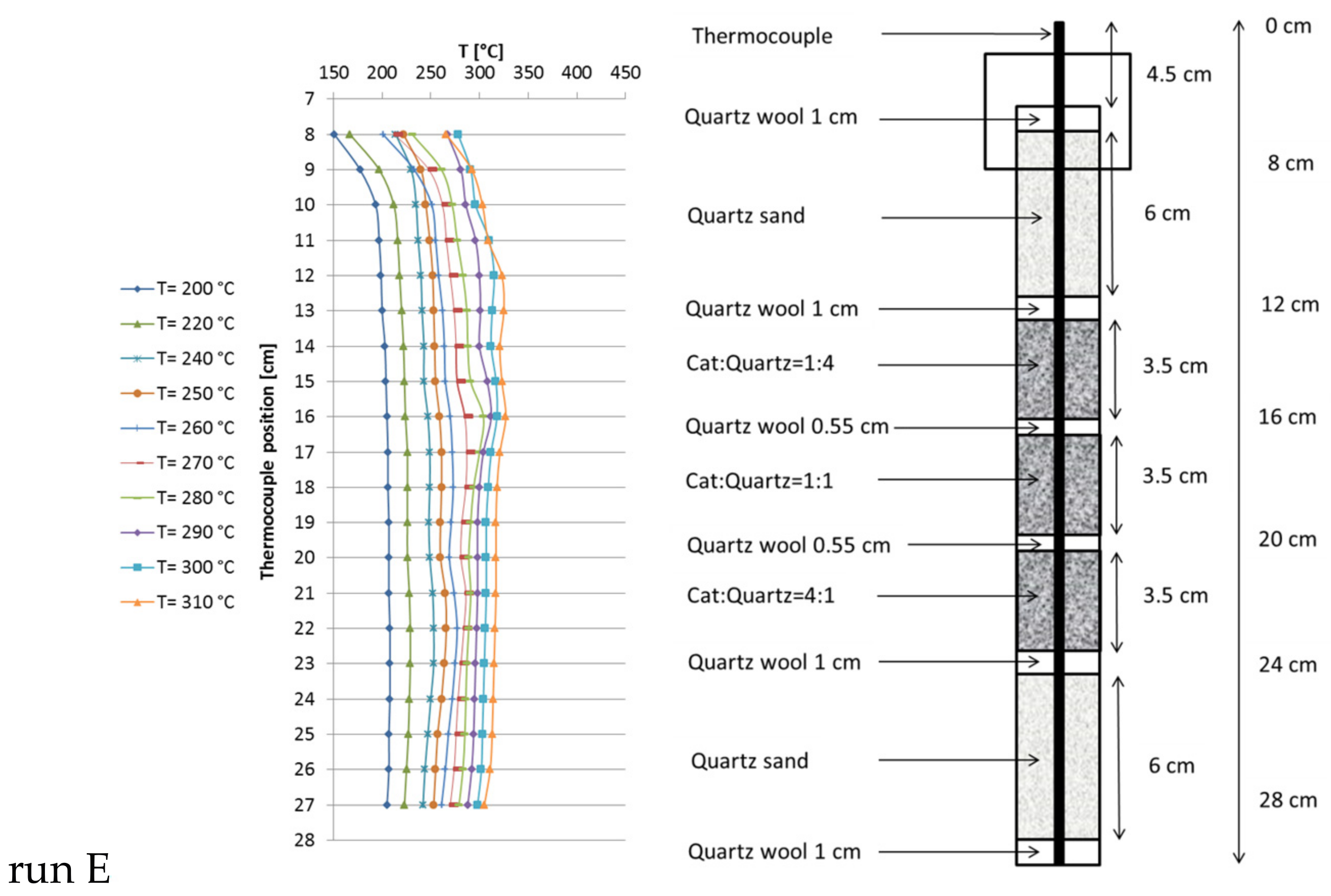
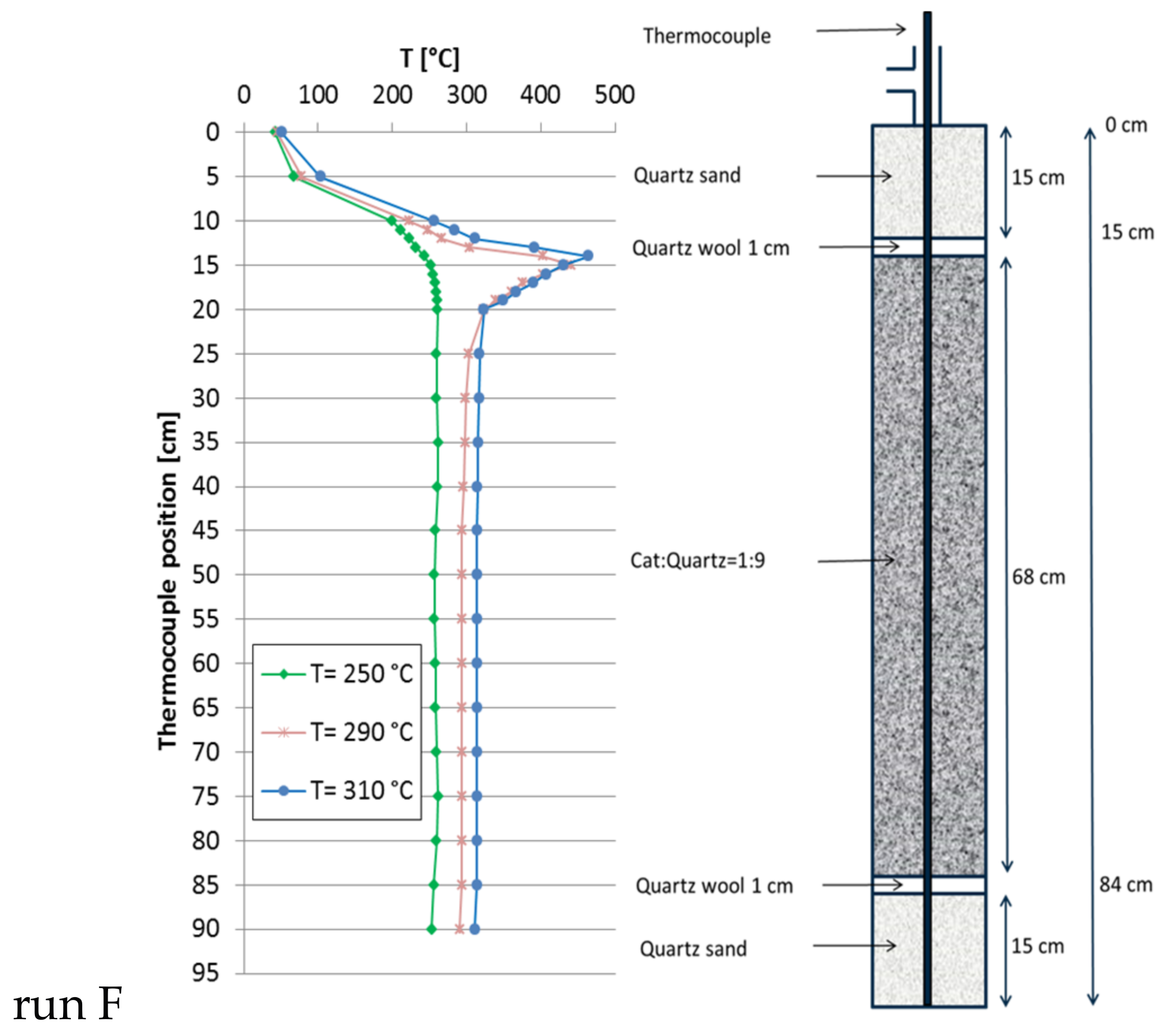
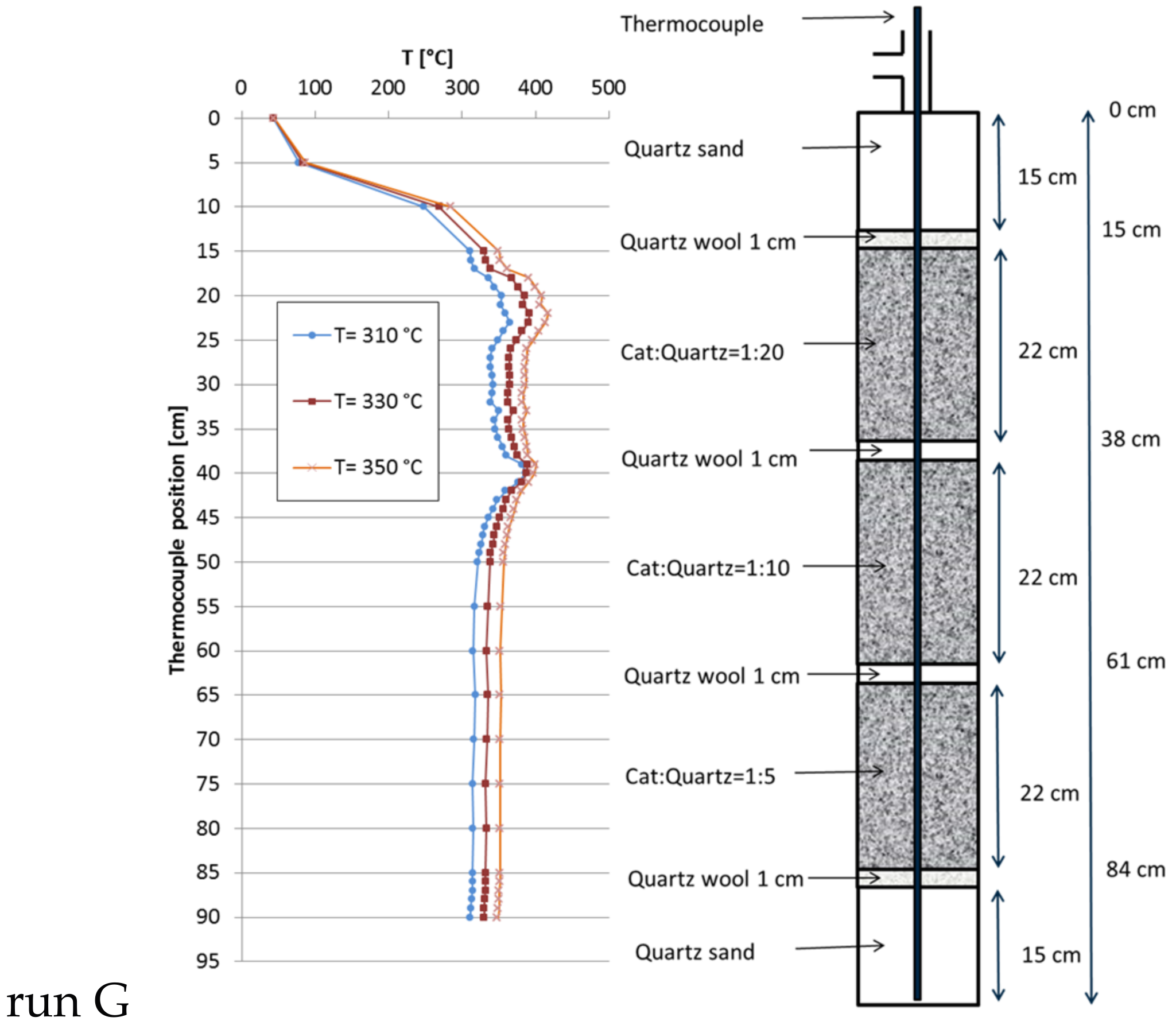
2.4. Temperature Profiles in the Large Tube Reactor
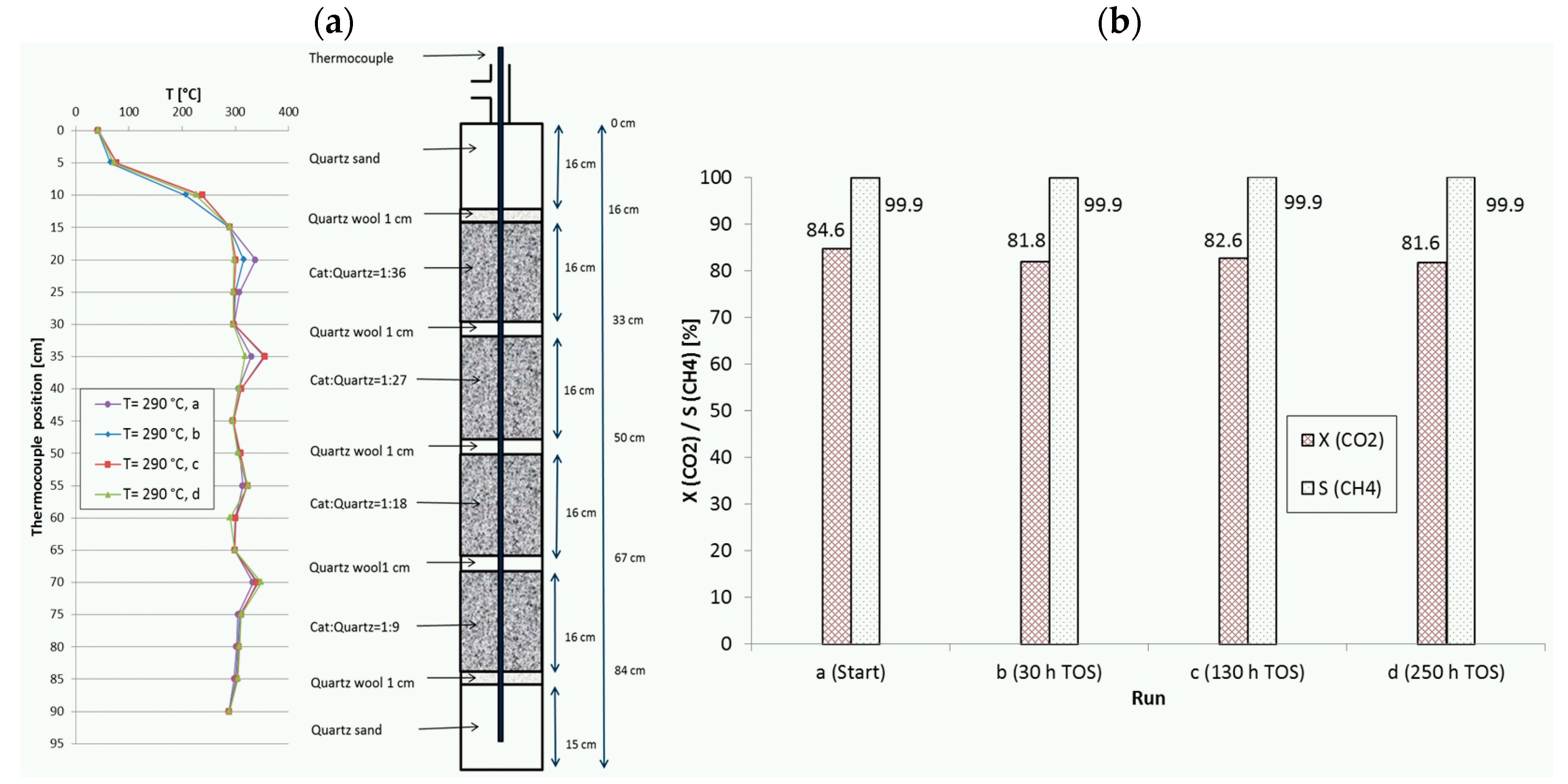
2.5. Catalyst Stability
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- German Government on the Change of Energy Supply. Available online: https://www.bundesregierung.de/Content/DE/tatischeSeiten/Breg/Energiekonzept/0-Buehne/ma%C3%9Fnahmen-im-ueberblick.html (accessed on 1 March 2017).
- BP Statistical Review of World Energy, June 2016. Available online: http://www.bp.com/content/dam/bp/pdf/energy-economics/statistical-review-2016/bp-statistical-review-of-world-energy-2016-full-report.pdf (accessed on 1 March 2017).
- Bundesministerium für Wirtschaft und Energie (BMWI), Federal Ministry of Economics and Energy, Germany. Dossier Erneuerbare Energien. Available online: https://www.bmwi.de/Redaktion/DE/Dossier/erneuerbare-energien.html (accessed on 1 March 2017).
- Eurostat Statistics Explained. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Energy_production_and_imports/de (accessed on 1 March 2017).
- Bajohr, S.; Götz, M.; Graf, F.; Ortloff, F. Speicherung von regenerativ erzeugter elektrischer Energie in der Erdgasinfrastruktur. Gwf-Gas Erdgas 2011, 152, 200–210. [Google Scholar]
- Valentin, F.; von Bredow, H. Power-to-Gas: Rechtlicher Rahmen für Wasserstoff und synthetisches Gas aus erneuerbaren Energien. Energiewirtschaftliche Tagesfragen 2011, 61, 99–105. [Google Scholar]
- Sabatier, P.; Senderens, J.B. Nouvelles syntheses du methane. C. R. Acad. Sci. 1902, 134, 514–516. [Google Scholar]
- Sabatier, P.; Senderens, J.B. Hydrogénation directe des oxydes du carbone en présence de divers métaux divisés. C. R. Acad. Sci. 1902, 134, 689–691. [Google Scholar]
- Nørskov, J.K.; Bligaard, T.; Hvolbæk, B.; Abild-Pedersen, F.; Chorkendorff, I.; Christensen, C.H. The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 2008, 37, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/Al2O3 catalysts at atmospheric pressure: Catalysts activation, behavior and stability. Int. J. Hydrog. Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Peebles, D.E.; Goodman, D.W.; White, J.M. Methanation of carbon dioxide on nickel(100) and the effects of surface modifiers. J. Phys. Chem. 1983, 27, 4378–4387. [Google Scholar] [CrossRef]
- Wang, W.; Gong, J. Methanation of carbon dioxide: an overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar]
- Van der Laan, G.P.; Beenackers, A.A.C.M. Kinetics and Selectivity of the Fischer-Tropsch Synthesis: A Literature Review. Catal. Rev. Sci. Eng. 1999, 41, 255–318. [Google Scholar] [CrossRef]
- Yang, J.; Ma, W.; Chen, D.; Holmen, A.; Davis, B.H. Fischer-Tropsch synthesis: A review of the effect of CO conversion on methane selectivity. Appl. Catal. A Gen. 2014, 470, 250–260. [Google Scholar] [CrossRef]
- Renewable Energy Concepts. Available online: http://www.renewable-energy-concepts.com/german/bioenergie/biogas-basiswissen/biogaszusammensetzung.html (accessed on 1 March 2017).
- European Biogas Association. EBA Biomethane & Biogas Report 2015. Available online: http://european-biogas.eu/2015/12/16/biogasreport2015/ (accessed on 2 March 2017).
- Fachverband Biogas e.V. Available online: http://www.biogas.org/edcom/webfvb.nsf/id/DE-Zahlen-und-Fakten (accessed on 2 March 2016).
- Mills, G.A.; Steffgen, F.W. Catalytic methanation. Catal. Rev. Sci. Eng. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Garbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrog. Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Car Manufacturer Audi: Audi E-Gas Project. Available online: http://www.audi-cr2014.de/uploads/files/901411328702714676-umweltbilanz-e-gas-project.pdf (accessed on 2 March 2017).
- Clariant Supplies Catalyst for Audi’s German Methanation Plant. Available online: http://www.chemicals-technology.com/news/news-clariant-catalyst-audi-german-methanation-plant (accessed on 2 March 2017).
- World’s Largest SNG Plant Goes On-Stream in China with Catalysts and Process Technology from Haldor Topsoe A/S. Available online: http://www.topsoe.com/content/world%E2%80%99s-largest-sng-plant-goes-stream-china-catalysts-and-process-technology-haldor-tops%C3%B8e (accessed on 2 March 2017).
- Younas, M.; Kong, L.L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Kienberger, T.; Karl, J. Substitute natural gas (SNG)—Stand der Technik, theoretische Grundlagen, Forschung am Institut für Wärmetechnik. In Proceedings of the 11th Symposium Energieinnovation, Graz, Austria, 10–12 February 2010. [Google Scholar]
- Sudiro, M.; Bertucco, A. Natural Gas; Sciyo: Rijeka, Croatia, 2010; pp. 105–126. [Google Scholar]
- Hydrogen Energy. Available online: http://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/coal-to-sng (accessed on 2 March 2017).
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 2007, 62, 1161–1170. [Google Scholar] [CrossRef]
- Schoder, M.; Armbruster, U.; Martin, A. Heterogen-katalysierte Hydrierung von CO2 zu Methan unter erhöhten Drücken. Chem. Ing. Tech. 2013, 82, 344–352. [Google Scholar] [CrossRef]
- Lange, F.; Armbruster, U.; Martin, A. Heterogeneously-catalyzed hydrogenation of carbon dioxide to methane over RuNi-bimetallic catalysts. Energy Technol. 2015, 3, 55–62. [Google Scholar] [CrossRef]
- Martin, A.; Türks, D.; Mena, H.; Armbruster, U. Hydrogenation of Carbon Dioxide to Synthetic Natural Gas: Impact of Catalyst Bed Arrangement. JECM 2016, 3, 25–30. [Google Scholar]
- Reschetilowski, W. Einführung in Die Heterogene Katalyse; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Weatherbee, G.D.; Bartholomew, C.D. Hydrogenation of CO2 on group VIII metals. J. Catal. 1981, 68, 67–76. [Google Scholar] [CrossRef]
- Dautzenberg, F.M. Ten Guidelines for Catalyst Testing. In Characterization and Catalyst Development; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1989; Volume 411, pp. 99–119. [Google Scholar]
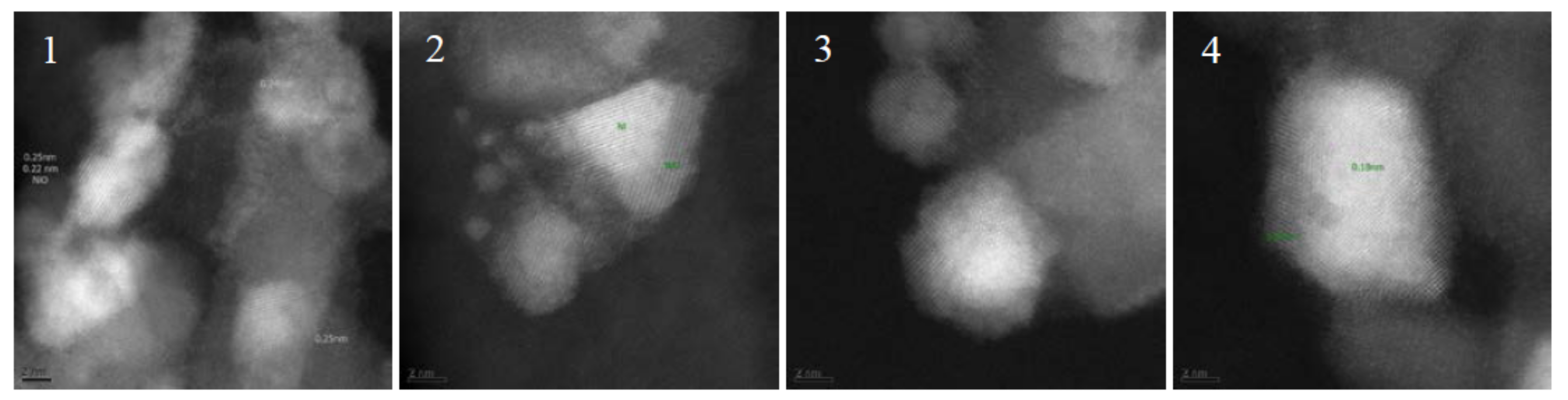

| Temperature (°C) | Xeq (CO2) (%) | X1 bar (CO2) (%) | X10 bar (CO2) (%) | S1 bar (CH4) (%) | S10 bar (CH4) (%) |
|---|---|---|---|---|---|
| 300 | 98.5 | 19.5 | 96.7 | 99.0 | 99.8 |
| 325 | 97.7 | 38.5 | 96.8 | 99.5 | 99.9 |
| 350 | 96.5 | 54.3 | 95.9 | 99.6 | 100 |
| 375 | 95.5 | 66.0 | 94.6 | 99.2 | 99.9 |
| 400 | 94.0 | 71.1 | 93.2 | 98.9 | 99.9 |
| Set Point Temperature | XCO2 (%) | SCH4 (%) | Hot Spot (K) 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (°C) | A | B | C | A | B | C | A | B | C |
| 270 | - | - | 96.5 | - | - | 99.9 | - | - | 64 |
| 280 | - | - | 96.7 | - | - | 99.9 | - | - | 67 |
| 290 | - | - | 97.4 | - | - | 99.9 | - | - | 68 |
| 300 | 96.5 | 97.3 | - | 99.9 | 99.9 | - | - | 36 | - |
| 325 | 96.9 | 96.8 | - | 99.9 | 99.9 | - | - | 36 | - |
| 350 | 96.1 | 96.5 | - | 99.9 | 99.9 | - | 1 | 34 | - |
| 375 | 95.1 | 95.4 | - | 99.9 | 99.9 | - | 3 | 31 | - |
| Temperature | XCO2 (%) | SCH4 (%) | Hot Spot (K) 1 | |||
|---|---|---|---|---|---|---|
| (°C) | D | E | D | E | D | E |
| 250 | - | 72.6 | - | 99.9 | - | 16 |
| 260 | 43.6 | 90.9 | 99.9 | 99.9 | - | 17 |
| 270 | 55.3 | 93.6 | 99.9 | 99.9 | - | 16 |
| 280 | 68.6 | 97.2 | 99.9 | 99.9 | - | 20 |
| 290 | 81.9 | 97.5 | 99.9 | 99.9 | - | 23 |
| 300 | 85.5 | 97.5 | 99.9 | 99.9 | - | 18 |
| 310 | - | 97.5 | - | 99.9 | - | 17 |
| 325 | 96.3 | - | 99.9 | - | - | - |
| 350 | 96.6 | - | 99.9 | - | - | - |
| 375 | 95.8 | - | 99.9 | - | - | - |
| 400 | 94.7 | - | 99.9 | - | - | - |
| Temperature | XCO2 (%) | SCH4 (%) | Hot Spot (K) 1 | |||
|---|---|---|---|---|---|---|
| (°C) | F | G | F | G | F | G |
| 250 | 43.0 | - | 99.9 | - | - | - |
| 290 | 96.1 | - | 99.9 | - | 160 | - |
| 310 | 96.2 | 93.1 | 99.9 | 99.9 | 160 | 78 |
| 325 | - | 92.7 | - | 99.9 | - | 62 |
| 330 | - | 91.1 | - | 99.9 | - | 68 |
| Run | Vcat | Vdil | GHSV | T | XCO2,max | ∆Tmax | STY | Productivity |
|---|---|---|---|---|---|---|---|---|
| (mL) | (mL) | (h−1) | (°C) | (%) | (K) | (L/(mLcat × h)) | (L/h) | |
| A | 1 | 4 | 6000 | 300 | 96.5 | 5 | 1.1 | 1.1 |
| B | 2.5 | 2.5 | 6000 | 275 | 97.4 | 40 | 1.1 | 2.6 |
| C | 4 | 1 | 6588 | 270 | 96.5 | 65 | 1.2 | 4.6 |
| D | 0.5 | 4.5 | 12,000 | 350 | 96.6 | - | 2.2 | 1.1 |
| E | 2.3 | 2.3 | 6000 | 280 | 97.2 | 20 | 1.1 | 2.4 |
| F | 30 | 270 | 8000 | 310 | 96.2 | 160 | 1.4 | 42.5 |
| G | 30 | 271 | 10,000 | 350 | 92.5 | 62 | 1.7 | 50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türks, D.; Mena, H.; Armbruster, U.; Martin, A. Methanation of CO2 on Ni/Al2O3 in a Structured Fixed-Bed Reactor—A Scale-Up Study. Catalysts 2017, 7, 152. https://doi.org/10.3390/catal7050152
Türks D, Mena H, Armbruster U, Martin A. Methanation of CO2 on Ni/Al2O3 in a Structured Fixed-Bed Reactor—A Scale-Up Study. Catalysts. 2017; 7(5):152. https://doi.org/10.3390/catal7050152
Chicago/Turabian StyleTürks, Daniel, Hesham Mena, Udo Armbruster, and Andreas Martin. 2017. "Methanation of CO2 on Ni/Al2O3 in a Structured Fixed-Bed Reactor—A Scale-Up Study" Catalysts 7, no. 5: 152. https://doi.org/10.3390/catal7050152
APA StyleTürks, D., Mena, H., Armbruster, U., & Martin, A. (2017). Methanation of CO2 on Ni/Al2O3 in a Structured Fixed-Bed Reactor—A Scale-Up Study. Catalysts, 7(5), 152. https://doi.org/10.3390/catal7050152






