Abstract
While the catalytic activity of some Ru-based polypyridine complexes in water oxidation is well established, the relationship between their chemical structure and activity is less known. In this work, the single site Ru complex [Ru(bpy)2(H2O)2]2+ (bpy = 2,2′-bipyridine)—which can exist as either a cis isomer or a trans isomer—is investigated. While a difference in the catalytic activity of these two isomers is well established, with cis-[Ru(bpy)2(H2O)2]2+ being much more active, no mechanistic explanation of this fact has been presented. The oxygen evolving capability of both isomers at multiple concentrations has been investigated, with cis-[Ru(bpy)2(H2O)2]2+ showing a second-order dependence of O2 evolution activity with increased catalyst concentration. Measurement of the electron paramagnetic resonance (EPR) spectrum of cis-[Ru(bpy)2(H2O)2]2+, shortly after oxidation with CeIV, showed the presence of a signal matching that of cis,cis-[RuIII(bpy)2(H2O)ORuIV(bpy)2(OH)]4+, also known as “blue dimer”. The formation of dimers is a concentration-dependent process, which could serve to explain the greater than first order increase in catalytic activity. The trans isomer showed a first-order dependence of O2 evolution on catalyst concentration. Behavior of [Ru(bpy)2(H2O)2]2+ isomers is compared with other Ru-based catalysts, in particular [Ru(tpy)(bpy)(H2O)]2+ (tpy = 2,2′;6,2′′-terpyridine).
1. Introduction
The oxidation of water is a vital reaction in nature, occurring during photosynthesis and as a key step in solar energy conversion schemes centered around artificial photosynthesis. There are numerous water oxidation catalysts (WOC’s) that have been reported, many of which are based on transition metals, such as Ir [1,2] and Ru. In attempts to create more economically-viable catalysts, complexes containing more abundant elements such as Fe, Co, and Ni have been discovered [3]. Ru-based WOCs, in particular, have been investigated for decades, and today they are still the most extensively studied group of WOCs. Currently there are many mono-Ru [4,5,6,7] and di-Ru [8,9,10,11] complexes capable of oxidizing water; however, the stability of molecular catalysts in highly oxidizing conditions is still a major issue, with most known catalysts deactivating after some time [12]. Under strongly oxidizing conditions one of the reaction pathways for single-site complexes involves the formation of dinuclear complexes [13,14,15]. In some cases, the dinuclear complexes are more stable than their mononuclear counterparts, with the mononuclear catalyst being converted to a binuclear or multinuclear catalyst [13,15].
This work is focused on the study of water oxidation by the single-site complex cis-[Ru(bpy)2(H2O)2]2+ and electron paramagnetic resonance (EPR) characterization of the products. Both cis- and trans-[Ru(bpy)2(H2O)2]2+ were reported and characterized by Meyer et al. [16,17]. It was found that the cis configuration is more stable in the absence of light, but under illumination the complex undergoes cis-trans isomerization. Later, it was shown that upon addition of CeIV, cis-[Ru(bpy)2(H2O)2]2+ acts as a water oxidation catalyst, yet its performance is limited by having few turnovers [18]. Interestingly, in the same work, trans-[Ru(bpy)2(H2O)2]2+ was shown to be a much less active catalyst than cis-[Ru(bpy)2(H2O)2]2+. The catalytic mechanism of cis-[Ru(bpy)2(H2O)2]2+ is believed to involve a water nucleophilic attack on the RuV=O species produced in a series of proton-coupled electron transfer reactions, which occur upon oxidation [18]. cis-[Ru(bpy)2(H2O)2]2+ and various products of its oxidation have been studied by density functional theory (DFT), EPR, and X-ray Absorption Spectroscopy, allowing for the establishment of their geometric and electronic structure [19].
Here, we report our study of water oxidation at acidic conditions, using [Ru(bpy)2(H2O)2]2+ as a catalyst upon addition of CeIV. It was found that for the more catalytically active cis isomer, the rate of O2 evolution increases non-linearly with complex concentration. This effect is ascribed to the formation of a di-Ru complex of [Ru(bpy)2(H2O)2]2+. EPR signals of the cis-[RuV(bpy)2(O)(OH)]2+ species and cis,cis-[RuIII(bpy)2(H2O)ORuIV(bpy)2(OH)]4+ dimer resulting from its reactivity are reported. DFT calculations were used to show that dimer formation is energetically favorable. While on a much slower time scale, dimer formation during catalysis has previously been reported for [Ru(tpy)(bpy)(H2O)]2+ [13,14] and so a comparison of the two compounds is provided.
2. Results
2.1. Oxygen Evolution Measurements
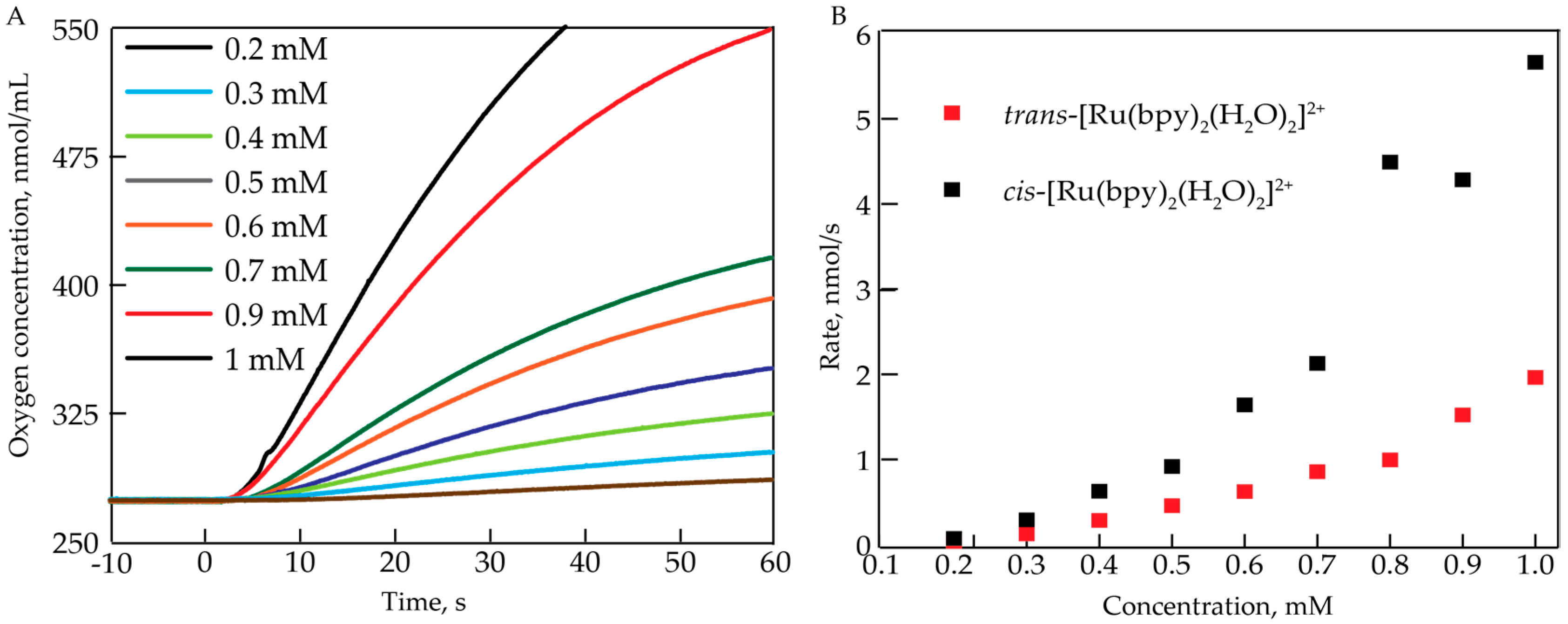
The oxygen evolving activity of cis-[Ru(bpy)2(H2O)2]2+ was assessed at acidic pH = 1 (0.1 M HNO3) using a Clark electrode. To induce O2 evolution, an excess (20 equiv.) of CeIV oxidant was added. Figure 1A shows the resulting O2 evolution profiles for different concentrations (0.2–1 mM) of cis-[Ru(bpy)2(H2O)2]2+. There was a delay between injecting CeIV and an increase in O2 concentration which is greater at lower concentrations of [Ru(bpy)2(H2O)2]2+ (0.2–0.4 mM). Additionally, it is evident that at given conditions (20 equiv. CeIV, in 0.1 M HNO3), the rate of oxygen evolution increased non-linearly with complex concentration. The initial rate was determined within the first 15 s of the oxygen evolution, and was plotted against the concentration of cis-[Ru(bpy)2(H2O)2]2+ (Figure 1B). Oxygen evolution measurements show that the rate of oxygen production had a second order dependence on the concentration of the complex. These results indicate that there could be a secondary concentration-dependent process occurring upon the addition of CeIV.
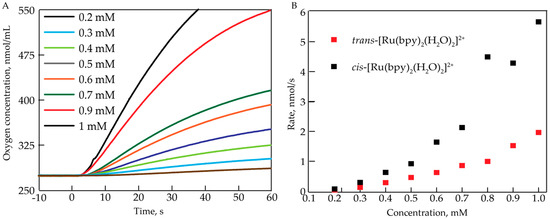
Figure 1.
(A) Oxygen evolution profiles for different concentrations of cis-[Ru(bpy)2(H2O)2]2+ in 0.1 M HNO3. 20 equiv. of CeIV were added at t = 0 s; (B) Initial rate (determined between 5 and 10 s) of O2 evolution as a function of the complex concentration for cis-[Ru(bpy)2(H2O)2]2+ and trans-[Ru(bpy)2(H2O)2]2+.
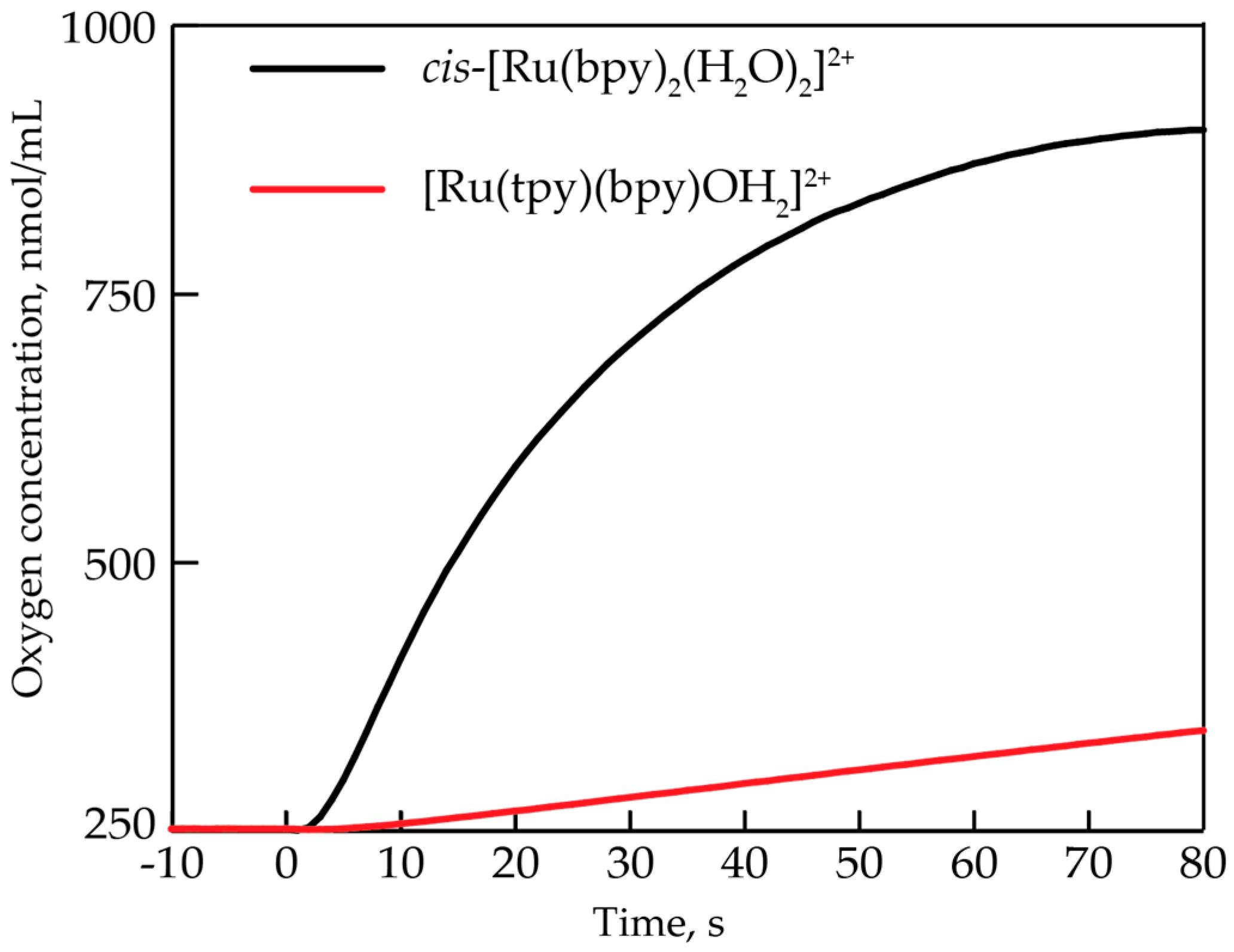
Figure 2 compares the oxygen evolution profiles at a 0.7 mM concentration of cis-[Ru(bpy)2(H2O)2]2+ and [Ru(tpy)(bpy)(H2O)]2+, which is one of the most extensively characterized single-site water oxidation catalysts. It is clear that the two seemingly similar single-site catalysts have significantly different catalytic activity.
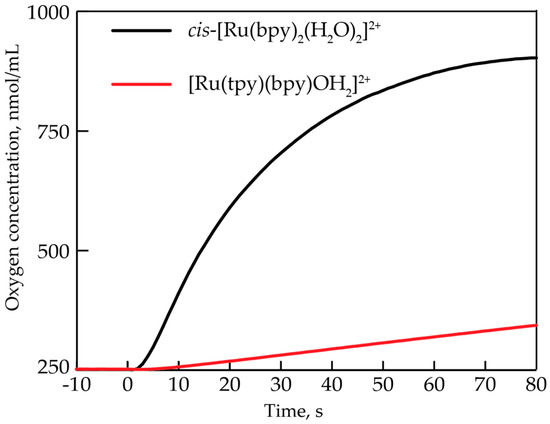
Figure 2.
Oxygen evolution profiles of cis-[Ru(bpy)2(H2O)2]2+ (black) and [Ru(tpy)(bpy)(H2O)]2+ upon the addition of 20 equiv. CeIV. Conditions: room temperature, 0.7 mM concentration of Ru complex, 0.1 M HNO3 (pH = 1).
2.2. Electron Paramagnetic Resonance Measurements
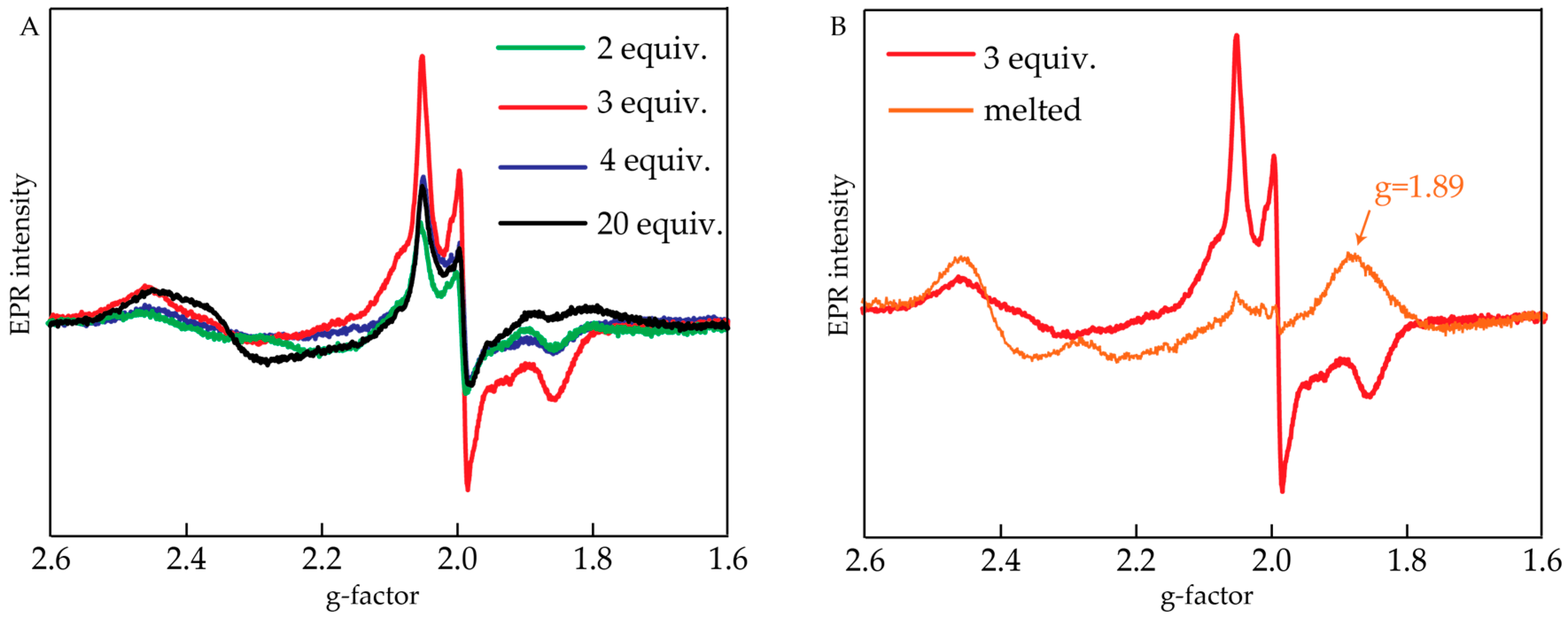
Studies using EPR were done to identify the products of the reaction. Oxidized samples were frozen within 30 s after the addition of CeIV, and their EPR spectra were measured. The samples were then melted at room temperature for 2 min, refrozen and another set of EPR spectra was obtained. Figure 3A shows EPR spectra obtained after oxidizing 200 μL of 1 mM [Ru(bpy)2(H2O)2]2+ with 2–20 equiv. of CeIV and freezing it within 30 s. There was a rhombic EPR signal observed with gxx = 2.05, gyy = 1.99 and gzz = 1.85. Its maximum intensity was observed upon adding 3 equiv. of CeIV. This signal is characteristic for intermediates containing a RuV=O fragment [20] and has been previously reported for cis-[Ru(bpy)2(H2O)2]2+ [19].
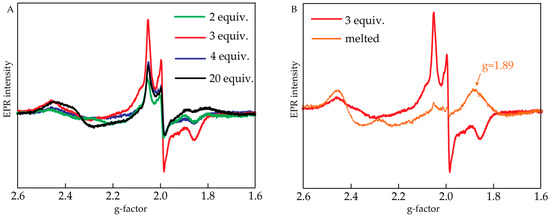
Figure 3.
(A) Electron paramagnetic resonance (EPR) spectra of cis-[Ru(bpy)2(H2O)2]2+ upon addition of 2, 3, 4 or 20 equiv. of CeIV and immediate freezing of the sample; (B) EPR spectrum of cis-[Ru(bpy)2(H2O)2]2+ + 3 equiv. of CeIV before and after melting for 2 min. Conditions: 20 K, 1 mM cis-[Ru(bpy)2(H2O)2]2+, 0.1 M HNO3 (pH = 1).
The sample that was oxidized with 3 equiv. of CeIV was melted for 2 min and then refrozen. The resulting EPR spectrum compared to the one before melting is shown in Figure 3B. First, it is seen that the signal related to RuV=O has decreased significantly. A new signal appeared at g = 1.89, which is characteristic of the so-called “blue dimer” (cis,cis-[RuIII(bpy)2(H2O)ORuIV(bpy)2(OH)]4+) complex [21,22,23]. Same EPR signal was obtained in catalytic mixture produced by adding 20 equiv. of CeIV (data not shown). This indicates that after short time and at high concentration of the initial catalyst, a di-Ru complex was formed under the conditions of water oxidation.
2.3. Density Functional Theory Calculations
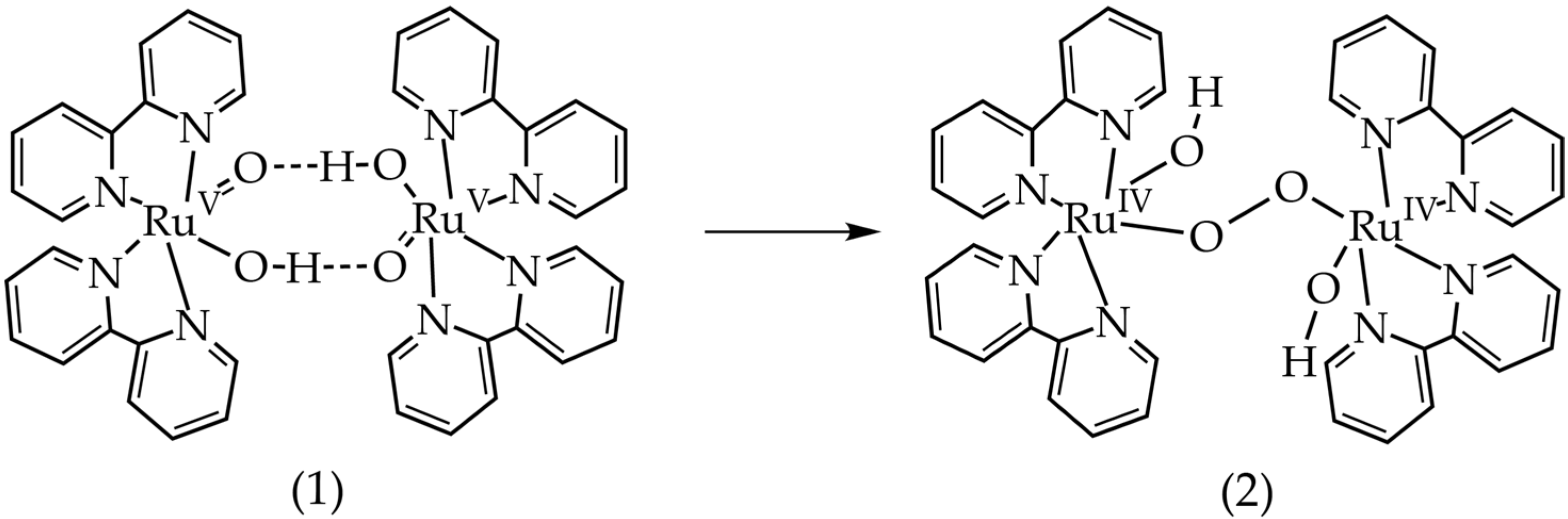
In order to rationalize the difference in the kinetic behavior in the trans and cis isomers, DFT calculations were done to analyze the intermediates of the water oxidation catalysis, Figure 4 and Figure 5. DFT results show that the short-lived [(bpy)2RuV=O,OH]2+ intermediate detected by EPR was accessible by oxidation with CeIV at predicted +1.4 V, which is in good agreement with reported experimental redox potential of +1.3 V, Table 1. To analyze pathways for dimerization and radical coupling proposed for some of Ru-based catalysts [24], we computed a ∆G = −0.27 eV for dimer formation from two [(bpy)2RuV=O,OH]2+ molecules (Figure 4). This dimer appears to be stabilized by two hydrogen bonds, which is only possible for the cis isomer of the complex (Figure 4). Formation of the peroxo-bridged dimer with O–O bond distance of ~1.34 Å has a ∆G = −0.33 eV, Table 1. After oxygen is expelled from this peroxo-bridged intermediate, there is a possibility of the μ-oxo-bridge formation between two Ru centers, Table 1. The overall reaction leading from the two [(bpy)2RuV=O,OH]2+ molecules to oxygen (O2) and blue dimer in the oxidation state BD [III,III] has a significant driving force of −1.55 eV, Table 1.
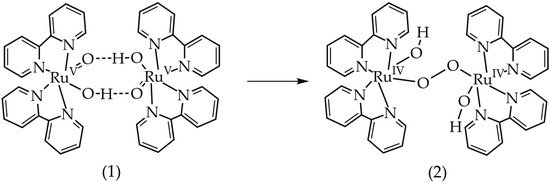
Figure 4.
Proposed path with formation of H-bond-stabilized dimer of [(bpy)2RuV=O,OH]2+. The starting compound, (1), can only be formed by the cis isomer, which could explain the different rates of oxygen production of the two isomers. The optimized geometry of the initial state, (1), as well as the product, (2), were found.
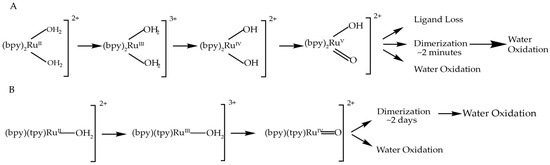
Figure 5.
(A) Reaction pathway for cis-[RuII(bpy)2(H2O)2]2+ up to RuV, the highest measured oxidation state, and possible paths it follows from there; (B) Reaction pathway for cis-[RuII(bpy)2(H2O)2]2+ up to RuIV, the highest measured oxidation state, and possible paths it could follow from there.

Table 1.
Summary of DFT calculations on the intermediates of cis-[Ru(bpy)2(H2O)2]2+. Comparison between the calculated redox potential and experimentally reports redox potential is provided, and ΔG for possible dimer formation is shown.
From the available experimental observables, we currently cannot determine the relative contributions of the two catalytic mechanisms: catalysis via radical coupling in the dimer of the [(bpy)2RuV=O,OH]2+ versus catalysis derived by the reactivity of the blue dimer, which can be formed in significant amounts after a single turnover. However, it is clear that the trans isomer of the [Ru(bpy)2(H2O)2]2+ will be less susceptible to both the dimer formation and the transformation into blue dimer complex.
3. Discussion
3.1. Oxygen Evolution by cis-[Ru(bpy)2(H2O)2]2+ with DFT and EPR Characterization
The initial report indicated that cis-[Ru(bpy)2(H2O)2]2+ is unstable, and thus water oxidation is preceded by ligand loss and the formation of RuO2 [17]. More recent studies have shown a surprising fact: cis-[Ru(bpy)2(H2O)2]2+ is more active under catalytic conditions, with a turnover number(TON) = 4, than trans-[Ru(bpy)2(H2O)2]2+, with a TON = 1. The two complexes showed a significantly different behavior, meaning that, counter to what was proposed in [17]—cis-[Ru(bpy)2(H2O)2]2+ does not evolve oxygen via ligand loss preceding the formation of RuO2. If the oxidation of water was occurring due to formation of RuO2, the lower catalytic activity of the trans isomer could be explained by the higher stability of the ligands in the trans isomer relative to the cis isomer [14]. The TON for cis-[Ru(bpy)2(H2O)2]2+, however, was shown to be higher than both RuO2, indicating a need for another explanation. If (as proposed in [13]) the oxidation of water by cis-[Ru(bpy)2(H2O)2]2+ is accomplished purely by water nucleophilic attack on oxidized form of cis-[Ru(bpy)2(H2O)2]2+, however, the rate of oxygen evolution should be linear in the concentration of cis-[Ru(bpy)2(H2O)2]2+. Our oxygen evolution measurements, however, have shown that the O2 evolution rate has second order dependence on concentration of cis-[Ru(bpy)2(H2O)2]2+.
Second-order behavior shows that there could be a reaction in the oxidation cycle with a dependence on the concentration of cis-[Ru(bpy)2(H2O)2]2+. A possible mechanism that would explain a second-order reaction is radical coupling between two RuV=O species with the formation of O–O bond (2RuV=O,OH→HO-RuIV-O-O-RuIV-OH), Figure 4. Counter to what was seen with cis-[Ru(bpy)2(H2O)2]2+, oxygen evolution shows that the rate of oxygen production for trans-[Ru(bpy)2(H2O)2]2+ is linear in the same concentration range. This indicates that the two isomers are following different reaction mechanisms when oxidized. The requirement for radical coupling to occur is reaching the RuV=O complex in the catalytic cycle [24]. While at higher pH, it was shown that only the cis isomer is stable in the RuV=O configuration, both are able to reach RuV at pH 1.0 [17] , where this experiment occurred. If the radical coupling mechanism is taking place for the cis-isomer and not the trans-isomer, it would explain the difference in catalytic activity, as this pathway corresponds to a high rate of oxygen production. The difference in behavior of the cis and trans isomers means that the radical coupling mechanism (if it is occurring) has to be proceeded by a reaction involving both coordinated oxygens.
It could be that the cis-isomer of the complex is forming the blue dimer, either instead of or after the radical coupling pathway. In combination with the linear dependence of the rate of oxygen production on concentration, this process gives a second-order dependence. With EPR analysis, we have seen that cis-[Ru(bpy)2(H2O)2]2+ is likely forming the so-called “blue dimer” upon adding oxidizing agent. “Blue dimer” is known to be able to oxidize water and in fact was the first designed water oxidation catalyst [8]. The formation of a new catalytically active, di-Ru complex would explain the non-linear dependence of the O2 evolution rate on concentration. Our oxygen evolution measurements also show that-due to linear dependence on concentration-dimers are not being formed by trans-[Ru(bpy)2(H2O)2]2+. The oxygen evolution rate of the “blue dimer” complex is about 4.3 nM/s after adding 20 equiv. of CeIV to 0.1 mM of the complex in 0.1 M HNO3 [26]. For the cis-[Ru(bpy)2(H2O)2]2+, oxygen evolution rates range from 0.1 to 5.8 nM/s across the concentration range 0.2–1 mM. So, cis-[Ru(bpy)2(H2O)2]2+ and blue dimer have comparable oxygen evolution rates at the same oxidation conditions. As the percent of the initial complex converted to blue dimer is unknown, the exact comparison of rates is not possible. More detailed structural studies would need to be done in order to distinguish between formation of H-bonded dimer of [(bpy)2RuV=O,OH]2+ and formation of blue dimer.
3.2. Comparison to Other Single-Site Ru WOC’s
Most single-site Ru WOC’s contain polypyridine ligands and one or more water molecules coordinated to the Ru center. [Ru(tpy)(bpy)(H2O)]2+ is a typical representative of this class of catalysts, and has been extensively studied. Highly oxidized intermediates (containing RuIV=O fragment) are produced via proton-coupled electron transfer (PCET) which requires the presence of a ligand that can be deprotonated (usually water). The derivatives of this complex that do not contain water (or other ligands capable of PCET), such as [Ru(tpy)(bpy)Cl]2+ and [Ru(tpy)(bpy)I]2+ have been studied. It was demonstrated that the Ru-Cl bond is retained under oxidative conditions, and thus [Ru(tpy)(bpy)Cl]2+ is not a water oxidation catalyst [25]. [Ru(tpy)(bpy)I]2+ however loses the iodide ligand upon oxidation and forms the same intermediate as the parent complex via PCET, namely [(tpy)(bpy)RuIV=O]2+ [25,27]. This implies at least one Ru-H2O fragment is required in order for the complex to be an active catalyst.
It has been proposed that [Ru(tpy)(bpy)(H2O)]2+ is capable of forming an intermediate with a high oxidation state, [(tpy)(bpy)RuV=O]3+, and that its reaction with water is the rate-limiting step; however, there is no direct experimental proof that it reaches this oxidation state. Attempts to spectroscopically demonstrate the presence of the RuV=O intermediate have been unsuccessful, and it has been confirmed by use of X-ray spectroscopy and EPR that in catalytic steady state, the majority of species present are [(tpy)(bpy)RuIV=O]2+ [25]. No RuV=O species has been directly observed so far for [Ru(tpy)(bpy)(H2O)]2+ or other single-site Ru complexes containing only one water molecule.
[Ru(bpy)2(H2O)2]2+, in contrast to [Ru(tpy)(bpy)(H2O)]2+, has 2 water molecules coordinated to Ru center. This makes the formation of RuV=O fragment possible via PCET, which was observed using EPR spectroscopy in this and previous work [16]. This fact once again demonstrates that the oxidation of Ru center to RuV via PCET is possible only in the presence of at least 2 ligands capable of deprotonation (i.e., water).
DFT simulations have been performed on [Ru(tpy)(bpy)(H2O)]2+ [25]. They show that the RuV=O intermediate is much less energetically accessible in [Ru(tpy)(bpy)(H2O)]2 than in [Ru(bpy)2(H2O)2]2+ (Table 2), thus supporting the experimental data.

Table 2.
Summary of DFT calculations on the intermediates of [Ru(tpy)(bpy)(H2O)]2+. Comparison between the calculated redox potential and experimentally reports redox potential is provided. Each calculation included two explicit water molecules. Results show a much higher potential for the RuV=O intermediate as compared to [Ru(bpy)2(H2O)]2+.
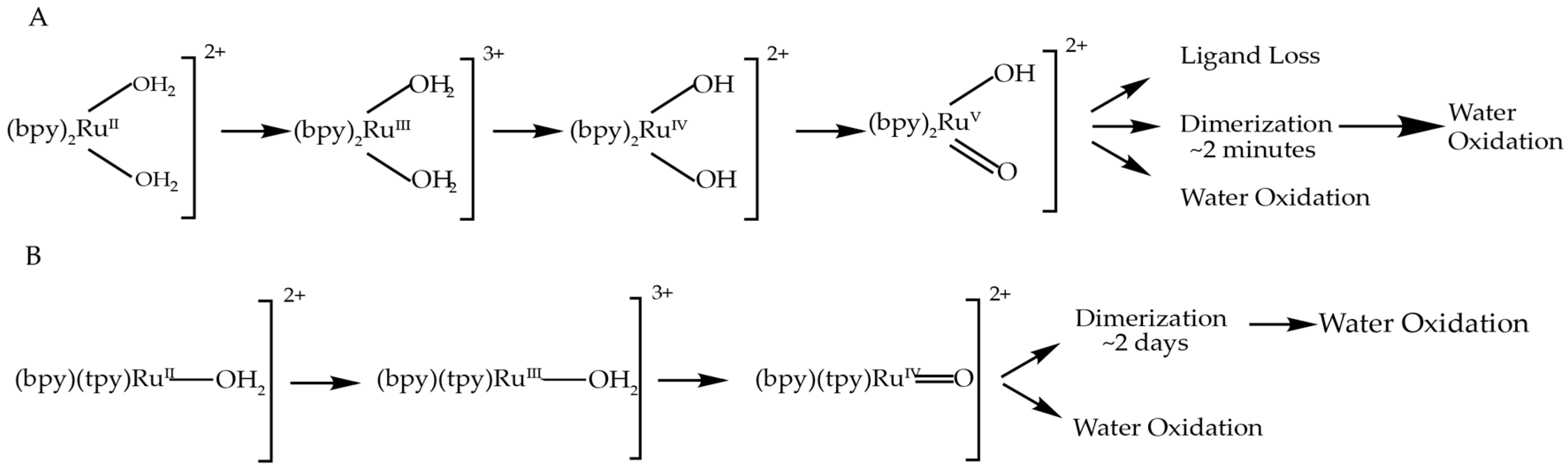
The formation of catalytically active dimers under catalytic conditions has been observed not only for cis-[Ru(bpy)2(H2O)2]2+, but also for [Ru(tpy)(bpy)(H2O)]2+ and similar complexes [13,14]. It was demonstrated that after prolonged oxidation of [Ru(tpy)(bpy)(H2O)]2+ via bulk electrolysis at 1.64 V (vs. normal hydrogen electrode), a significant part of it is irreversibly converted into a more stable di-nuclear complex that is also capable of oxidizing water. The difference, however, is in the timescale of the dimerization effect: in the case of cis-[Ru(bpy)2(H2O)2]2+, the EPR signal characteristic to the di-Ru complex is observed within minutes, while for example [Ru(tpy)(5,5′-H2-bpmy)(H2O)]2+ shows ~25% conversion to its corresponding di-nuclear complex after 2 days of bulk electrolysis. The formation of di-nuclear complexes from [Ru(tpy)(bpy)(H2O)]2+ also requires the loss of bpy ligand by one of the complexes, while cis-[Ru(bpy)2(H2O)2]2+ does not need to lose a ligand. Figure 4 shows the pathways of both catalysts and products of their oxidation, up to the highest oxidation state measure for each, and the possible outcomes from there.
It is also interesting to note that the activities of [Ru(tpy)(bpy)(H2O)]2+ and [Ru(bpy)2(H2O)2]2+ are drastically different: it has been previously reported that [Ru(bpy)2(H2O)2]2+ is able to do only a few turnovers (TON = 4 for cis isomer), while [Ru(tpy)(bpy)(H2O)]2+ demonstrates TON on the order of 1000 [31]. It could be explained by the difference in stability of tpy and bpy ligands (the first one has 3 nitrogen atoms coordinated to Ru center while another has only 2); the detailed analysis of stability of these complexes is, however, a subject reserved for a separate study. Despite the similarity in the structure of the two, [Ru(bpy)2(H2O)2]2+ is a much more active WOC than [Ru(tpy)(bpy)(H2O)]2+, but with a much shorter lifetime.
4. Materials and Methods
Aqueous solutions were prepared using ultrapure (Type 1) water (resistivity 18.2 MΩ·cm at 25 °C) from a Q-POD unit of Milli-Q integral water purification system (Millipore, Billerica, MA, USA). Solvents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification.
To prepare cis-[Ru(bpy)2(H2O)2]2+, 200 mg (0.38 mmol) of cis-[Ru(bpy)2Cl2]·2H2O was dissolved in 30 mL of EtOH. After dissolving, 130 mg (0.76 mmol) of AgNO3 was added. The mixture was refluxed for 30 min. The deposited AgCl was then separated by filtration. The solution’s volume was reduced by rotary evaporation, after which 20 mL of H2O was added. A solution of 125 mg of NH4PF6 in 3 mL H2O was added, immediately forming crystals. The resulting crystals were filtered and washed twice with ethanol and diethyl ether.
X-band EPR measurements were performed on an EMX X-band spectrometer equipped with an X-Band CW microwave bridge (Bruker, Billerica, MA, USA). Samples were oxidized with ammonium cerium nitrate (CeIV) and frozen within 30 s in liquid nitrogen. During EPR measurements sample temperature was maintained at 20 K using a closed cycle cryostat (ColdEdge Technologies, Allentown, PA, USA). Spectrometer conditions were as follows: microwave frequency 9.47 GHz; field modulation amplitude 10 G at 100 kHz, microwave power 31.7 mW. Measurements were performed on the same day in the same conditions, in order to allow comparison of signal intensities.
Oxygen evolution was measured with Clark type polarographic oxygen electrode with an Oxygraph System (Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK). Borosilicate vessel was filled with 600 μL solution of the complex at pH = 1 (in 0.1 M nitric acid) and constantly stirred. CeIV dissolved in nitric acid at pH = 1 was added to the chamber and oxygen concentration was recorded as a function of time. Calibration was performed by measuring signal in O2-saturated deionized water and then adding sodium dithionite (oxygen-depleting agent). Drop in the signal was set equal to the solubility of oxygen in water at room temperature (262 µmol/L).
Density functional theory calculations were performed at the UB3LYP level of theory, with the DGDZVP basis set for the ruthenium atoms, with all other atoms using the 6-31G* basis set. All molecules were modelled in water using the Conductor Polarized Continuum Model (CPCM) solvation model. Additionally, two explicit water molecules were included. All redox potentials were calculated using the DFT calculated free energies of the products minus the reactants. From this value, 4.44 V was subtracted to account for the NHE voltage. The free energy of solvation for H+ was taken to be −11.64 eV.
Acknowledgments
This material is based upon work supported by the U.S. Department of Energy, Office of Sciences, Office of Basic Energy Sciences under grant number DE-FG02-10ER16184 (Yulia Pushkar) Access to EPR was provided by the Amy Instrumentation Facility, Department of Chemistry, Purdue University, under the supervision of Michael Everly.
Author Contributions
Darren Erdman, Yuliana Pineda-Galvan and Yulia Pushkar performed the experiments, analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Hull, J.F.; Balcells, D.; Blakemore, J.D.; Incarvito, C.D.; Eisenstein, O.; Brudvig, G.W.; Crabtree, R.H. Highly active and robust Cp* iridium complexes for catalytic water oxidation. J. Am. Chem. Soc. 2009, 131, 8730–8731. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, J.D.; Schley, N.D.; Balcells, D.; Hull, J.F.; Olack, G.W.; Incarvito, C.D.; Eisenstein, O.; Brudvig, G.W.; Crabtree, R.H. Half-sandwich iridium complexes for homogeneous water-oxidation catalysis. J. Am. Chem. Soc. 2010, 132, 16017–16029. [Google Scholar] [CrossRef] [PubMed]
- Wasylenko, D.J.; Palmer, R.D.; Berlinguette, C.P. Homogeneous water oxidation catalysts containing a single metal site. Chem. Commun. 2013, 49, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Zong, R.; Thummel, R.P. A new family of Ru complexes for water oxidation. J. Am. Chem. Soc. 2005, 127, 12802–12803. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.J.; Jurss, J.W.; Templeton, J.L.; Meyer, T.J. One site is enough. Catalytic water oxidation by [Ru(tpy)(bpm)(OH2)]2+ and [Ru(tpy)(bpz)(OH2)]2+. J. Am. Chem. Soc. 2008, 130, 16462–16463. [Google Scholar] [CrossRef] [PubMed]
- Kaveevivitchai, N.; Zong, R.; Tseng, H.W.; Chitta, R.; Thummel, R.P. Further observations on water oxidation catalyzed by mononuclear Ru(II) complexes. Inorg. Chem. 2012, 51, 2930–2939. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Tajima, S.; Komia, M.; Yamazakia, H. Highly active and tunable catalysts for O2 evolution from water based on mononuclear ruthenium(II) monoaquo complexe. Dalt. Trans. 2011, 40, 3802–3804. [Google Scholar] [CrossRef] [PubMed]
- Gersten, S.W.; Samuels, G.J.; Meyer, T.J. Catalytic oxidation of water by an oxo-bridged ruthenium dimer. J. Am. Chem. Soc. 1982, 104, 4029–4030. [Google Scholar] [CrossRef]
- Wada, T.; Tsuge, K.; Tanaka, K. Electrochemical oxidation of water to dioxygen catalyzed by the oxidized form of the bis(ruthenium-hydroxo) complex in H2O. Angew. Chemie Int. Ed. 2000, 39, 1479–1482. [Google Scholar] [CrossRef]
- Sander, A.C.; Maji, S.; Francàs, L.; Böhnisch, T.; Dechert, S.; Llobet, A.; Meyer, F. Highly efficient binuclear ruthenium catalyst for water oxidation. ChemSusChem 2015, 8, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Tseng, H.W.; Zong, R.; Wang, D.; Thummel, R. Preparation and study of a family of dinuclear Ru(II) complexes that catalyze the decomposition of water. Inorg. Chem. 2008, 47, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Limburg, B.; Bouwman, E.; Bonnet, S. Molecular water oxidation catalysts based on transition metals and their decomposition pathways. Coord. Chem. Rev. 2012, 256, 1451–1467. [Google Scholar] [CrossRef]
- López, I.; Ertem, M.Z.; Maji, S.; Benet-Buchholz, J.; Keidel, A.; Kuhlmann, U.; Hildebrandt, P.; Cramer, C.J.; Batista, V.S.; Llobet, A. A self-improved water-oxidation catalyst: Is one site really enough? Angew. Chemie Int. Ed. 2014, 126, 209–213. [Google Scholar] [CrossRef]
- López, I.; Maji, S.; Benet-Buchholz, J.; Llobet, A. Oxo-bridge scenario behind single-site water-oxidation catalysts. Inorg. Chem. 2015, 54, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Tsubonouchi, Y.; Lin, S.; Parent, A.R.; Brudvig, G.W.; Sakai, K. Light-induced water oxidation catalyzed by an oxido-bridged triruthenium complex with a Ru–O–Ru–O–Ru motif. Chem. Commun. 2016, 52, 8018–8021. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.; Wilson, S.R.; Hodgson, D.J.; Meyer, T.J. Cis-trans photoisomerization in Ru(bpy)2(OH2)22+. Crystal structure of trans-[Ru(bpy)2(OH2)(OH)](ClO4)2. J. Am. Chem. Soc. 1980, 102, 600–607. [Google Scholar] [CrossRef]
- Dobson, J.C.; Meyer, T.J. Redox properties and ligand loss chemistry in aqua/hydroxo/oxo complexes derived from cis-and trans-[(bpy)2RuII(OH2)2]2+. Inorg. Chem. 1988, 27, 3283–3291. [Google Scholar] [CrossRef]
- Sala, X.; Ertem, M.Z.; Vigara, L.; Todorova, T.K.; Chen, W.; Rocha, R.C.; Aquilante, F.; Cramer, C.J.; Gagliardi, L.; Llobet, A. The cis-[RuII(bpy)2(H2O)2]2+ water-oxidation catalyst revisited. Angew. Chemie Int. Ed. 2010, 49, 7745–7747. [Google Scholar] [CrossRef] [PubMed]
- Planas, N.; Vigara, L.; Cady, C.; Miró, P.; Huang, P.; Hammarström, L.; Styring, S.; Leidel, N.; Dau, H.; Haumann, M.; et al. Electronic structure of oxidized complexes derived from cis-[RuII(bpy)2(H2O)2]2+ and its photoisomerization mechanism. Inorg. Chem. 2011, 50, 11134–11142. [Google Scholar] [CrossRef] [PubMed]
- Dengel, A.C.; Griffith, W.P. Studies on transition-metal oxo and nitrido complexes. 12. Synthesis, spectroscopic properties, and reactions of stable ruthenium(V) and osmium(V) oxo complexes containing α-hydroxy carboxylate and α-amino carboxylate ligands. Inorg. Chem. 1991, 30, 869–871. [Google Scholar] [CrossRef]
- Lei, Y.; Hurst, J.K. Dynamical investigations of the catalytic mechanisms of water oxidation by the [(bpy)2Ru(OH2)]2O4+ ion. Inorganica Chim. Acta 1994, 226, 179–185. [Google Scholar] [CrossRef]
- Moonshiram, D.; Alperovich, I.; Concepcion, J.J.; Meyer, T.J.; Pushkar, Y. Experimental demonstration of radicaloid character in a RuV=O intermediate in catalytic water oxidation. Proc. Natl. Acad. Sci. 2013, 110, 3765–3770. [Google Scholar] [CrossRef] [PubMed]
- Moonshiram, D.; Jurss, J.W.; Concepcion, J.J.; Zakharova, T.; Alperovich, I.; Meyer, T.J.; Pushkar, Y. Structure and electronic configurations of the intermediates of water oxidation in blue ruthenium dimer catalysis. J. Am. Chem. Soc. 2012, 134, 4625–4636. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Bozoglian, F.; Mandal, S.; Stewart, B.; Privalov, T.; Llobet, A.; Sun, L. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 2012, 4, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Pushkar, Y.; Moonshiram, D.; Purohit, V.; Yan, L.; Alperovich, I. Spectroscopic analysis of catalytic water oxidation by [RuII(bpy)(tpy)H2O]2+ suggests that RuV=O is not a rate-limiting intermediate. J. Am. Chem. Soc. 2014, 136, 11938–11945. [Google Scholar] [CrossRef] [PubMed]
- Moonshiram, D.; Purohit, V.; Concepcion, J.J.; Meyer, T.J.; Pushkar, Y. Mechanism of catalytic water oxidation by the ruthenium blue dimer catalyst: Comparative study in D2O versus H2O. Materials 2013, 6, 392–409. [Google Scholar] [CrossRef]
- Yan, L.; Zong, R.; Pushkar, Y. Unexpected ligand lability in condition of water oxidation catalysis. J. Catal. 2015, 330, 255–260. [Google Scholar] [CrossRef]
- Takeuchi, K.J.; Thompson, M.S.; Pipes, D.W.; Meyer, T.J. Redox and spectral properties of monooxo polypyridyl complexes of ruthenium and osmium in aqueous media. Inorg. Chem. 1984, 23, 1845–1851. [Google Scholar] [CrossRef]
- Wasylenko, D.J.; Ganesamoorthy, C.; Kolvisto, B.D.; Henderson, M.A.; Berllnguette, C.P. Insight into water oxidation by mononuclear polypyridyl Ru catalysts. Inorg. Chem. 2010, 49, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Sanguantrakun, N.; Schulze, B.; Schubert, U.S.; Berlinguette, C.P.; Wasylenko, D.J.; Ganesamoorthy, C.C.; Kolvisto, B.D.; Henderson, M.A.; Berllnguette, C.P.; et al. Unraveling the roles of the acid medium, experimental probes, and terminal oxidant, (NH4)2[Ce(NO3)6], in the study of a homogeneous water oxidation catalyst. J. Am. Chem. Soc. 2010, 49, 2202–2209. [Google Scholar]
- Concepcion, J.J.; Jurss, J.W.; Norris, M.R.; Chen, Z.; Templeton, J.L.; Meyer, T.J. Catalytic water oxidation by single-site ruthenium catalysts. Inorg. Chem. 2010, 49, 1277–1279. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).