Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants
Abstract
:1. Introduction
2. Results and Discussion
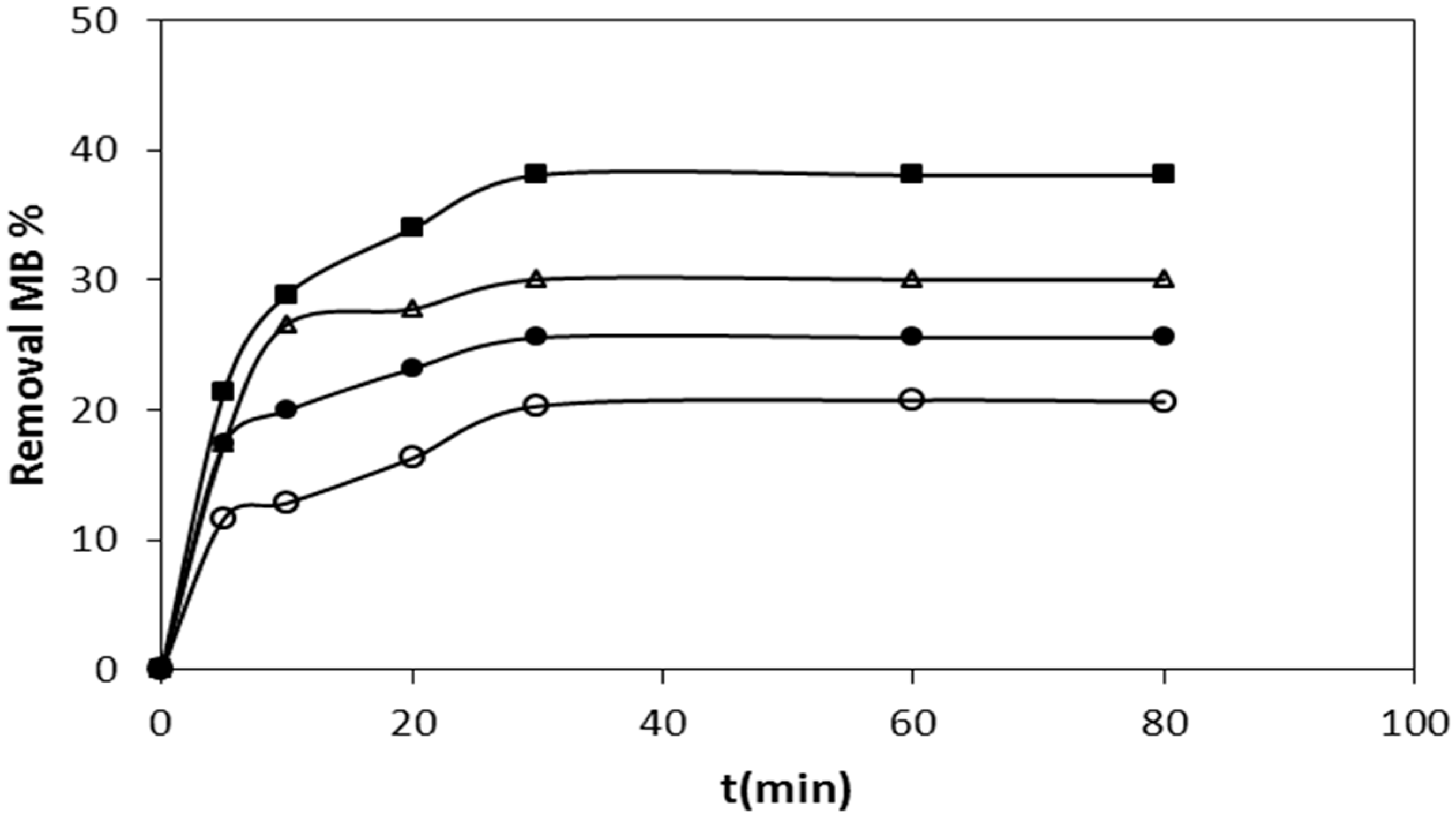
2.1. Adsorption Studies
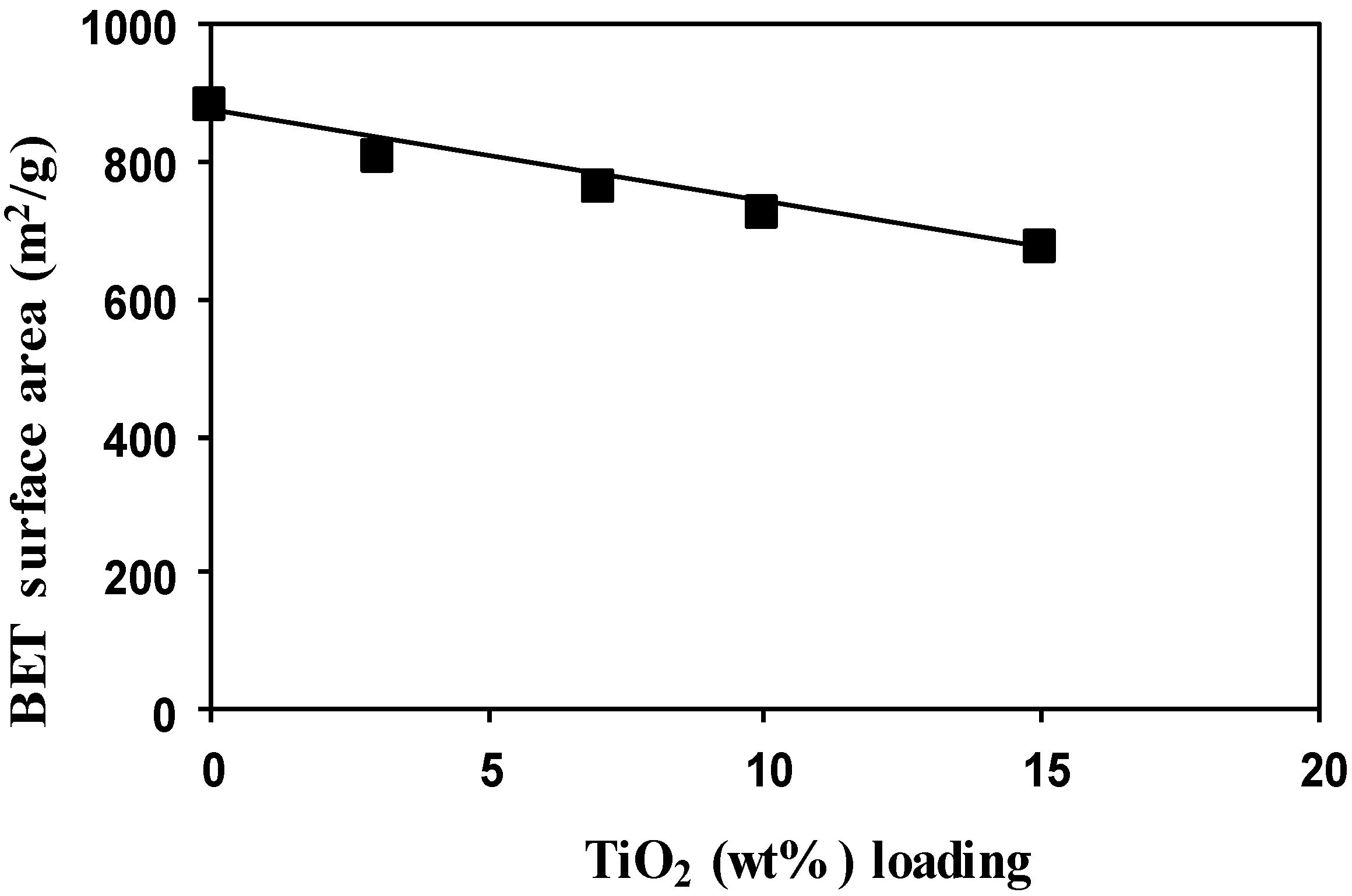
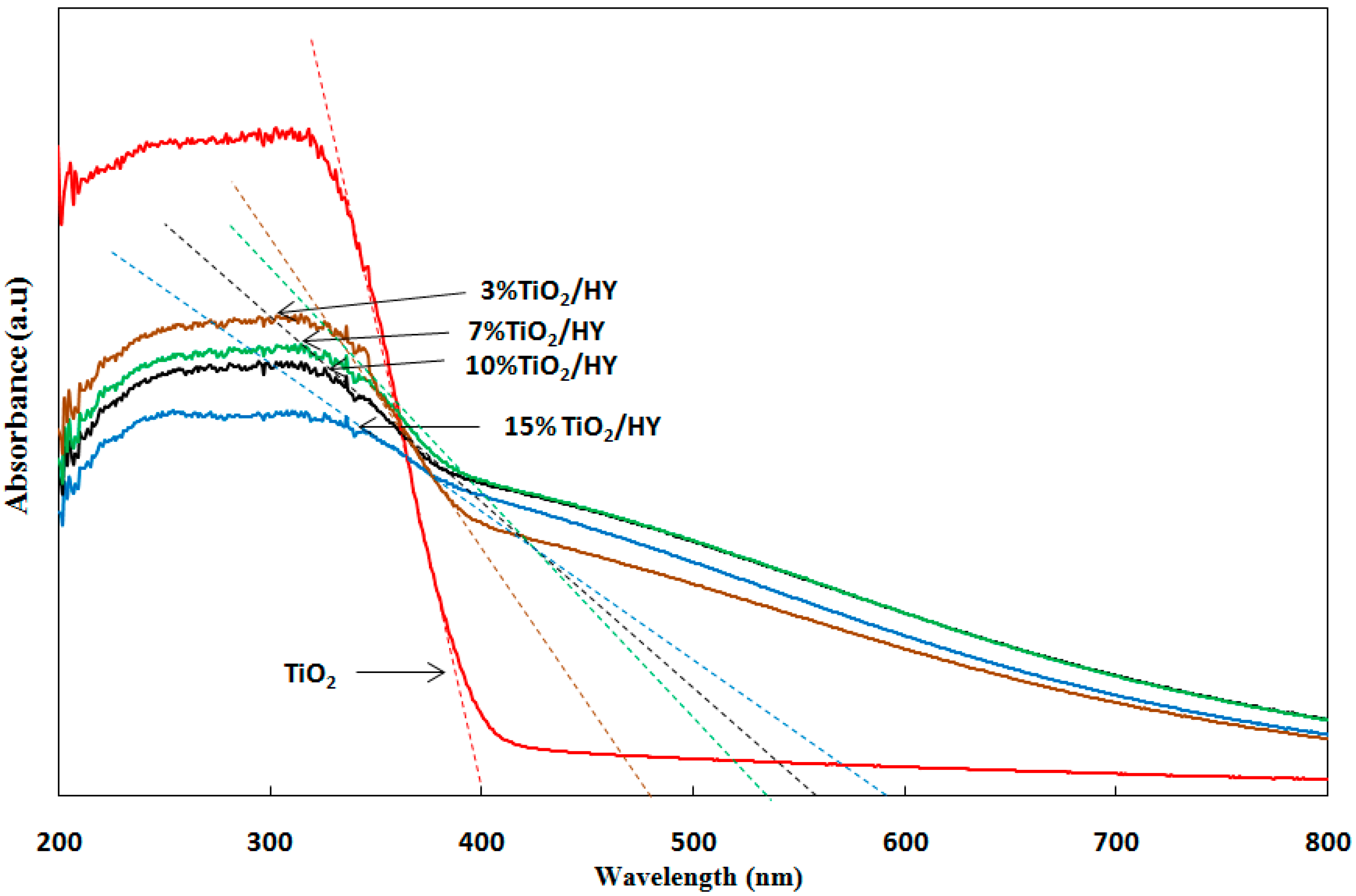
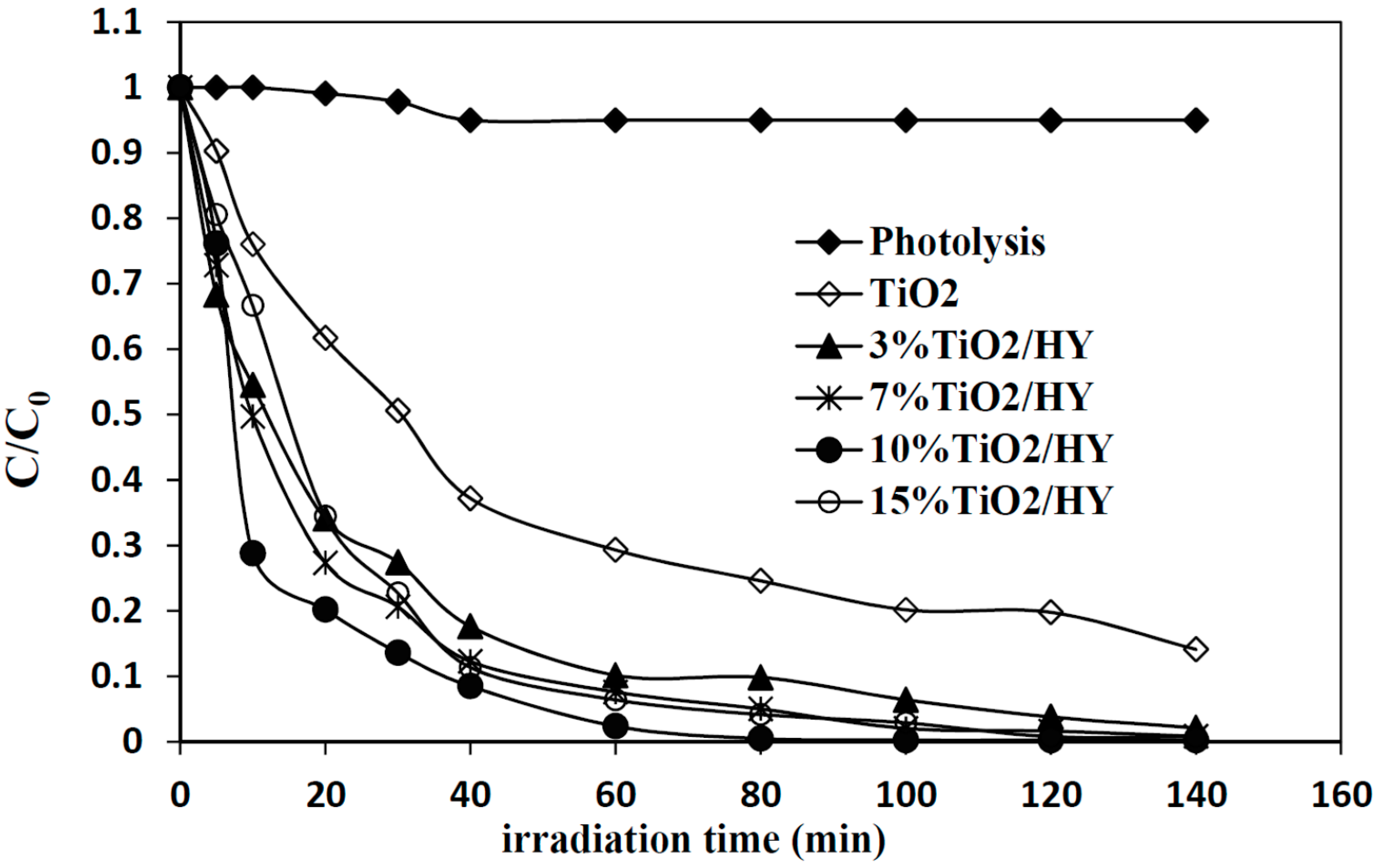
2.2. Effect of TiO2 Loading on HY Zeolite
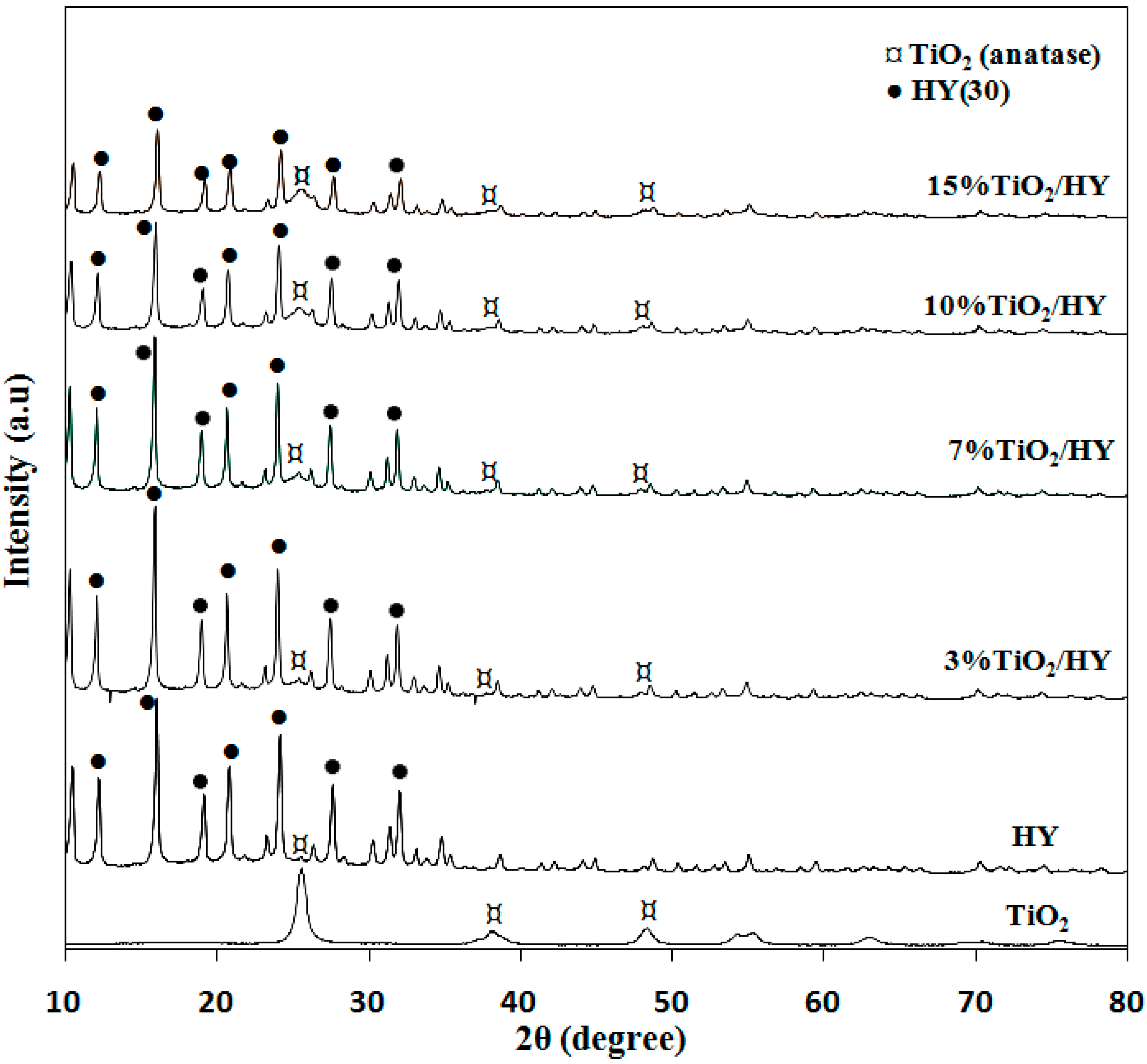
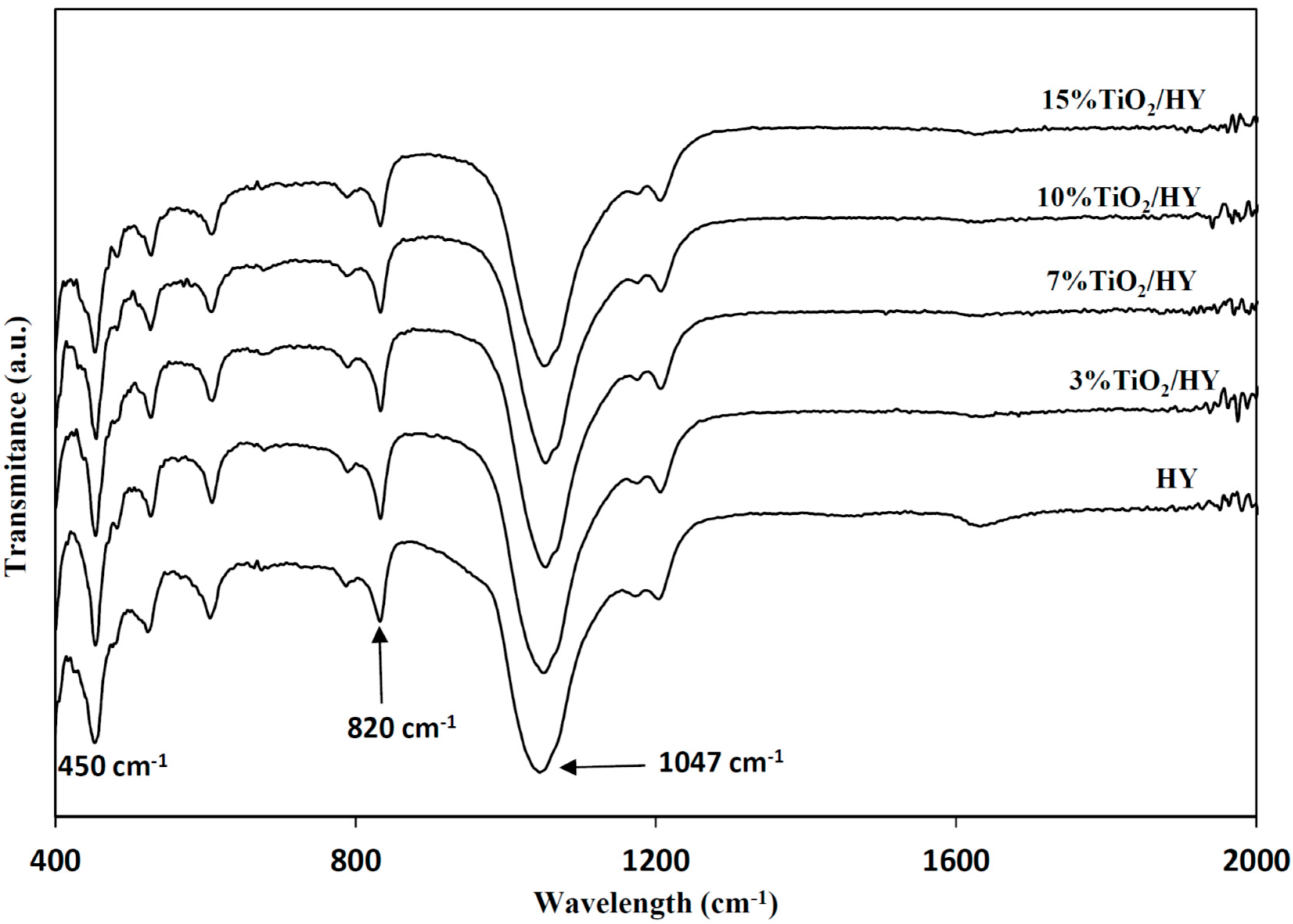
2.2.1. Characterization of the Photocatalysts
2.2.2. Evaluation of the Photocatalytic Activity
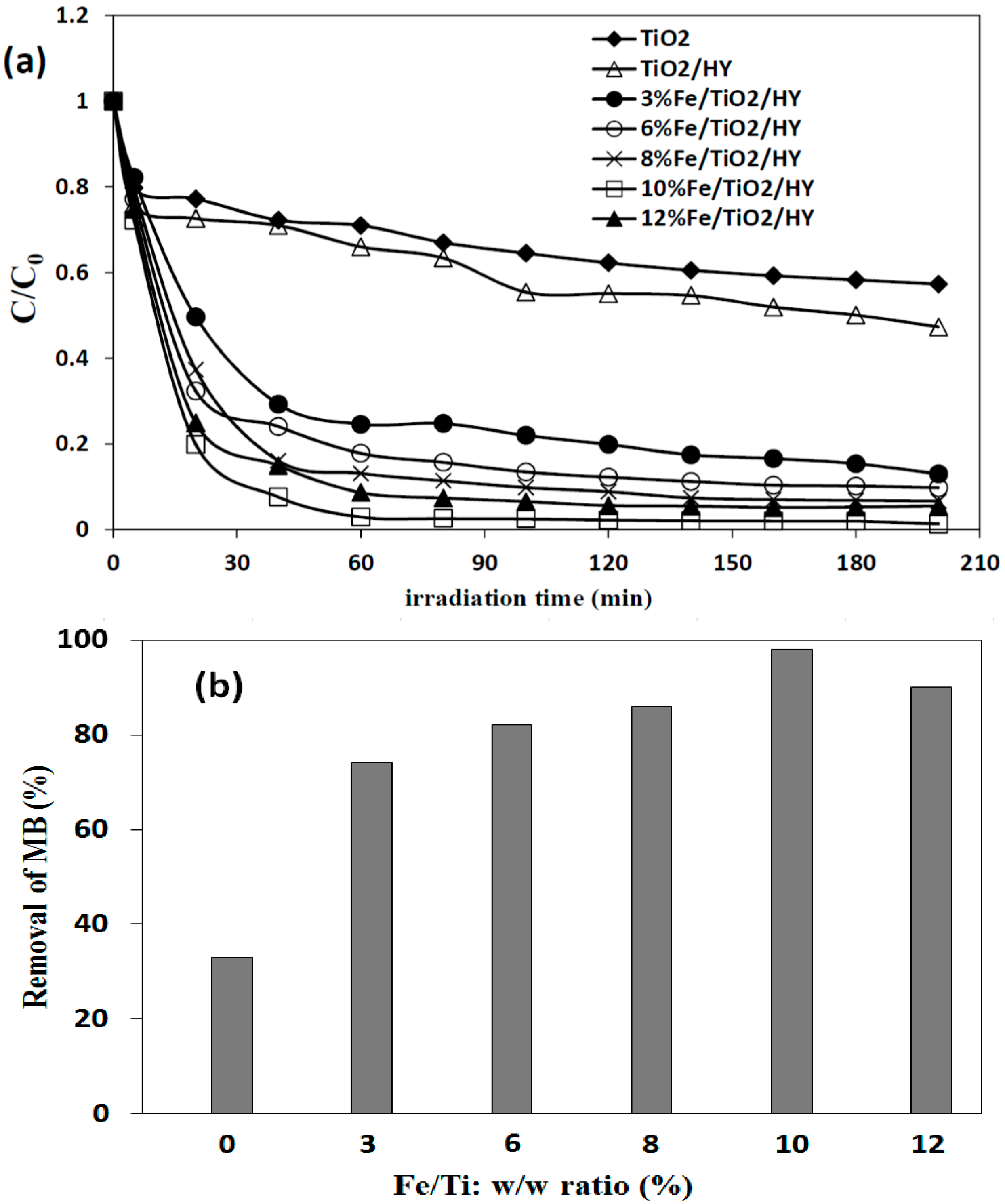
2.3. Effect of Fe Doping onto TiO2/HY Zeolite
2.3.1. Characterization of the Photocatalysts
2.3.2. Photocatalytic Studies
3. Materials and Experimental Details
3.1. Materials
3.2. Photocatalyst Preparation
- (1)
- TiO2/zeolite: titanium(IV) tetraisopropoxide, TIPT (Aldrich, 97%, Darmstadt, Germany), was added to the zeolite-suspended isopropanol solution and the mixture was stirred for 2 h. Water was added to the resulting slurry to hydrolyze titanium(IV) tetraisopropoxide adsorbed on zeolite particles and then the resulting slurry was left under continuous magnetic stirring until a gel was obtained. The gel was dried at 90 °C for 12 h and calcinated at 450 °C for 3 h to give rise to TiO2 supported on zeolites. Finally, the catalysts were ground into a fine powder and stored in the dark. The amount of TiO2 loaded on zeolite was in the range from 0 to 15 wt%, namely, 3, 7, 10 and 15 wt% TiO2 on HY.
- (2)
- Fe-doped TiO2/zeolite: HY zeolite powders (specific surface area = 880 m2 g−1) were dispersed in 10-fold distilled water and heated up to 70 °C. Subsequently, TIPT solution and Fe (NO3)3·9H2O solution (the nominal weight ratios of Fe/Ti were 3.0, 6.0, 8.0, 10, 12% w/w, respectively) were added dropwise into the beaker and the pH of the suspension solution was adjusted to a value of about 3. The system was vigorously stirred at 70 °C for 4 h. After ageing for 12 h, the products were repeatedly washed with deionized water and then dried at 80 °C for 2 h. These samples were finally calcined at 450 °C for 3 h in a muffle furnace and then the photocatalysts were obtained. For the purpose of comparative photocatalysis study, a reference sample of titanium (IV) dioxide, TiO2 was also prepared using the same procedure except for the addition of zeolite.
3.3. Characterization of Photocatalyst
3.4. Adsorption Study
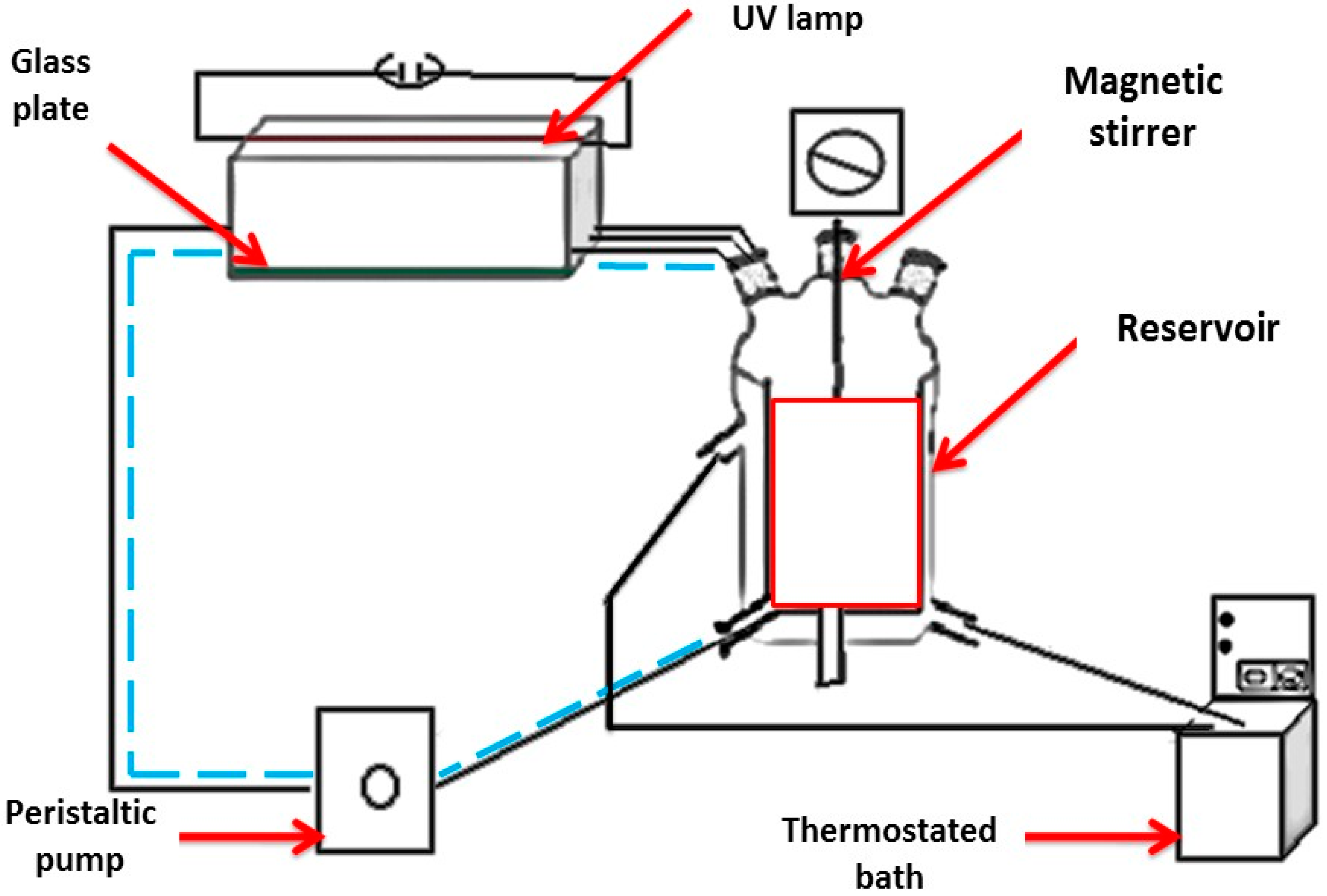
3.5. Photocatalysis Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. “Lest we forget you—Methylene blue…”. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.K. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dyes Pigments 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Toxicity of imine-iminium dyes and pigments: Electron transfer, radicals, oxidative stress and other physiological effects. J. Appl. Toxicol. 2014, 34, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.; Deebika, B.; Balu, K. Decolourization of aqueous dye solutions by a novel adsorbent: Application of statistical designs and surface plots for the optimization and regression analysis. J. Hazard. Mater. 2005, 122, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hamal, D.B.; Klabunde, K. Synthesis, characterization, and visible light activity of new nanoparticles photocatalysis based on silver, carbon, and sulfur-doped TiO2. J. Colloid Interface Sci. 2007, 311, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Nawi, M.A.; Jawad, A.H.; Sabar, S.; Wan Ngah, W.S. Immobilized bilayer TiO2/chitosan system for the removal of phenol under irradiation by a 45 Watt compact fluorescent lamp. Desalination 2011, 280, 288–296. [Google Scholar] [CrossRef]
- Caratto, V.; Locardi, F.; Alberti, S.; Villa, S.; Sanguineti, E.; Martinelli, A.; Balbi, T.; Canesi, L.; Ferretti, M. Different sol-gel prepartion of iron-doped TiO2 nanoparticles: Characterization, photocatalytic and cytotoxicity. J. Sol.-Gel Sci. Technol. 2016, 80, 152–159. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, W.; Gao, L. Anatase TiO2 nanoparticles immobilized on ZnO tetrapods as a highly efficient and easily recyclable photocatalyst. Appl. Catal. B 2007, 76, 168–173. [Google Scholar] [CrossRef]
- Andronic, L.; Duta, A. TiO2 thin films for dyes photodegradation. Thin Solid Films 2007, 515, 6294–6297. [Google Scholar] [CrossRef]
- Myilsamy, M.; Mahalakshmi, M.; Subha, N.; Rajabhuvaneswari, A.; Murugesan, V. Visible light responsive mesoporous graphene-Eu2O3/TiO2 nanocomposites for the efficient photocatalytic degradation of 4-chlorophenol. RSC Adv. 2016, 6, 35024–35035. [Google Scholar] [CrossRef]
- Izadyar, S.; Fatemi, S. Fabrication of X Zeolite Based Modified Nano TiO2 Photocatalytic Paper for Removal of VOC Pollutants under Visible Light. Ind. Eng. Chem. Res. 2013, 52, 10961–10968. [Google Scholar] [CrossRef]
- Zhu, J.F.; Deng, Z.G.; Chen, F.; Zhang, J.L.; Chen, H.J.; Anpo, M.; Huang, J.Z.; Zhang, L.Z. Structures and magnetic properties of p-type Mn: TiO2 dilute magnetic semiconductor thin films. Appl. Catal. B 2006, 62, 329–335. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Maeda, K.; Shimodaira, Y.; Lee, B.; Teramura, K.; Lu, D.; Kobayashi, H.; Domen, K. Studies on TiNxOyFz as a Visible-Light-Responsive Photocatalyst. J. Phys. Chem. C 2007, 111, 18264–18270. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Teixeira, V.O.; Portinha, A.; Magalhaes, A.; Coutinho, P.; Tavares, C.J.; Newton, R. Iron-doped photocatalytic TiO2 sputtered coatings on plastics for self-cleaning applications. Mater. Sci. Eng. B 2007, 138, 144–150. [Google Scholar] [CrossRef]
- Gota, K.R.; Suresh, S. Preparation and its application of TiO2/ZrO2 and TiO2/Fe photocatalysts: A perspective study. Asian J. Chem. 2014, 26, 7087–7101. [Google Scholar]
- Zhang, Y.; Fan, W.; Du, H.Q.; Zhao, Y.W.; Song, R.G.; Xiang, N. Microstructure and photocatalytic property of TiO2 and Fe3+:TiO2 films produced by micro-arc oxidation. Surf. Coat. Technol. 2017, 315, 196–204. [Google Scholar] [CrossRef]
- Sunada, F.; Heller, A. Effects of water, salt water, and silicone over coating of the TiO2 photocatalyst on the rates and products of photocatalytic oxidation of liquid 3-octanol and 3-octanone. Environ. Sci. Technol. 1998, 32, 282–288. [Google Scholar] [CrossRef]
- Shi, J.; Kuwahara, Y.; An, T.; Yamashita, H. The fabrication of TiO2 supported on slag-made calcium silicate as low-cost photocatalyst with high adsorption ability for the degradation of dye pollutants in water. Catal. Today 2017, 281, 21–28. [Google Scholar] [CrossRef]
- Strauss, M.; Pastorello, M.; Sigoli, F.A.; de Souza e Silva, J.M.; Mazali, I.O. Singular effect of crystallite size on the charge carrier generation and photocatalytic activity of nano-TiO2. Appl. Surf. Sci. 2014, 319, 151–157. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Lou, L. Photodegradation of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J. Photochem. Photobiol. A Chem. 2004, 163, 281–287. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, W.; Liu, W. Enhanced photocatalytic activity of supported TiO2: Dispersing effect of SiO2. J. Photochem. Photobiol. A Chem. 1999, 122, 57–60. [Google Scholar] [CrossRef]
- Hermann, J.; Matos, J.; Didier, J.; Guillard, C.; Laine, J.; Malato, S.; Blanco, J. Solar photocatalytic degradation of 4-chlorophenol using the synergistic effect between titania and activated carbon in aqueous suspension. Catal. Today 1999, 54, 255–265. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; Ben Zina, M.; Da Costa, P.; Galvez, M.E. Heterogeneous TiO2–Fe-plate catalyst for the discoloration and mineralization of aqueous solutions of cationic and anionic dyes. Desalination Water Treat. 2016, 57, 13505–13517. [Google Scholar] [CrossRef]
- Ooka, C.; Yoshida, H.; Suzuki, K.; Hattori, T. Highly hydrophobic TiO2 pillared clay for photocatalytic degradation of organic compounds in water. Microporous Mesoporous Mater. 2004, 67, 143–150. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.; Ke, Q.; Yang, Y.; Yuan, J. Photocatalytic degradation of cationic azo dye by TiO2/bentonite nanocomposite. J. Photochem. Photobiol. A Chem. 2002, 149, 169–174. [Google Scholar] [CrossRef]
- Kang, M.G.; Park, H.S.; Kim, K.J. Effect of improved crystallinity of titanium silicalite-2 on photodecomposition of simple aromatic hydrocarbons. J. Photochem. Photobiol. A Chem. 2002, 149, 175–181. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Sand supported mixed-phase TiO2 photocatalysts for water decontamination applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar] [CrossRef]
- Luo, M.; Bowden, D.; Brimblecombe, P. Removal of Dyes from Water Using a TiO2 Photocatalyst Supported on Black Sand. Water Air Soil Pollut. 2009, 198, 233–241. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Kawi, S.; Ray, M.B. Photocatalytic degradation of orange II by TiO2 catalysts supported on adsorbents. Catal. Today 2004, 98, 431–439. [Google Scholar] [CrossRef]
- Suwarnkar, M.B.; Kadam, A.N.; Khade, G.V.; Gavade, N.L.; Garadkar, K.M. Modification of TiO2 nanoparticles by HZSM-5 for the enhancement in photodegradation of Acid Green 25. J. Mater. Sci. Mater. Electron. 2016, 27, 843–851. [Google Scholar] [CrossRef]
- Ilinoiu, E.C.; Pode, R.; Manea, F.; Colar, L.A.; Jakab, A.; Orha, C.; Ratiu, C.; Lazau, C.; Sfarloaga, P. Photocatalytic activity of a nitrogen-doped TiO2 modified zeolite in the degradation of Reactive Yellow 125 azo dye. J. Taiwan Inst. Chem. Eng. 2013, 44, 270–278. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Mahalakshmi, M.; Vishnu Priya, S.; Arabindoo, B.; Palanichamy, M.; Murugesan, M. Photocatalytic degradation of aqueous propoxur solution using TiO2 and Hβ zeolite-supported TiO2. J. Hazard. Mater. 2009, 161, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Xie, S.; Liu, S.; Xu, L. Physical and chemical regeneration of zeolitic adsorbents for dye removal in wastewater treatment. Chemosphere 2006, 65, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef] [PubMed]
- Nezamzadeh-Ejhieh, A.; Karimi-Shamsabadi, M. Comparison of photocatalytic efficiency of supported CuO onto microand nano particles of zeolite X in photodecolorization of methylene blue and methyl orange aqueous mixture. Appl. Catal. A Gen. 2014, 477, 83–92. [Google Scholar] [CrossRef]
- Miaoliang, H.; Xu, C.; Wu, Z.; Huang, Y.; Lin, J.; Wu, J. Photocatalytic discolorization of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dyes Pigments 2008, 77, 327–334. [Google Scholar]
- Lazau, C.; Ratiu, C.; Orha, C.; Pode, R.; Manea, F. Photocatalytic activity of undoped and Ag-doped TiO2-supported zeolite for humic acid degradation and mineralization. Mater. Res. Bull. 2011, 46, 1916–1921. [Google Scholar] [CrossRef]
- Khatamian, M.; Hashemian, S.; Yavari, A.; Saket, M. Preparation of metal ion (Fe3+ and Ni2+) doped TiO2 nanoparticles supported on ZSM-5 zeolite and investigation of its photocatalytic activity. Mater. Sci. Eng. B 2012, 177, 1623–1627. [Google Scholar] [CrossRef]
- Kim, Y.; Yoon, M. TiO2/Y-Zeolite encapsulating intramolecular charge transfer molecules: A new photocatalyst for photoreduction of methyl orange in aqueous medium. J. Mol. Catal. A Chem. 2001, 168, 257–263. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Shankar, M.V.; Cheralathan, K.K.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Enhanced photocatalytic activity for the destruction of monocrotophos pesticide by TiO2/Hβ. J. Mol. Catal. A Chem. 2004, 223, 195–200. [Google Scholar] [CrossRef]
- Hassan, H.; Hameed, B.H. Oxidative decolorization of Acid Red 1 solutions by Fe–zeolite Y type catalyst. Desalination 2011, 276, 45–52. [Google Scholar] [CrossRef]
- Petkowicz, D.I.; Brambilla, R.; Radtke, C.; Da Silva, C.; Da Rocha, Z.N.; Pergher, S.B.C.; Dos Santos, J.H.Z. Photodegradation of methylene blue by in situ generated titania supported on a NaA zeolite. Appl. Catal. A Gen. 2009, 357, 125–134. [Google Scholar] [CrossRef]
- Cheng-Cai, W.; Chung-Kung, L.; Meng-Du, L.; Lain-Chuen, J. Photocatalytic degradation of C.I. Basic Violet 10 using TiO2 catalysts supported by Y zeolite: An investigation of the effects of operational parameters. Dyes Pigments 2008, 76, 817–824. [Google Scholar]
- Aramendía, M.A.; Colmenares, J.C.; López-Fernández, S.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Screening of different zeolite-based catalysts for gas-phase selective photooxidation of propan-2-ol. Catal. Today 2007, 129, 102–109. [Google Scholar] [CrossRef]
- Liu, X.; Iu, K.K.; Thomas, J.K. Encapsulation of TiO2 in zeolite Y. Chem. Phys. Lett. 1992, 195, 163–168. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ichihashi, Y.; Fujii, Y.; Honda, M. Photocatalytic Reduction of CO2 with H2O on Titanium Oxides Anchored within Micropores of Zeolites: Effects of the Structure of the Active Sites and the Addition of Pt. J. Phys. Chem. B 1997, 101, 2632–2636. [Google Scholar] [CrossRef]
- Cheralathan, K.K.; Sudarsan Kumar, I.; Palanichamy, M.; Murugesan, V.J. Liquid phase alkylation of phenol with 4-hydroxybutan-2-one in the presence of modified zeolite HBEA. Appl. Catal. A Gen. 2003, 241, 247–260. [Google Scholar] [CrossRef]
- Durga Kumari, V.; Subrahmanyam, M.; Subba Rao, K.V.; Ratnamala, A.; Noorjahan, M.; Tanaka, K. An easy and efficient use of TiO2 supported HZM-5 and TiO2+HZSM-5 zeolite combinate in the photodegradation of aqueous phenol and p-chlorophenol. Appl. Catal. A Gen. 2002, 234, 155–165. [Google Scholar] [CrossRef]
- Zheng, S.; Gao, L.; Zhang, Q.; Zhang, W.; Guo, J. Preparation, characterization and photocatalytic properties of singly and doubly titania-modified mesoporous silicate MCM-41 by varying titanium precursors. J. Mater. Chem. 2001, 11, 578–583. [Google Scholar] [CrossRef]
- Babic, B.; Zarubic, A.; Minovic Arsic, T.; Pantic, J.; Jokic, B.; Abazovic, N.; Matovic, B. Iron doped anatase for application in photocatalysis. Eur. Ceram. Soc. 2016, 36, 2991–2996. [Google Scholar] [CrossRef]
- Sasikala, R.; Shirole, A.R.; Sudarsan, V.; Jagannath; Sudakar, C.; Naik, R.; Rao, R.; Bharadwaj, S.R. Enhanced photocatalytic activity of indium and nitrogen co-doped TiO2–Pd nanocomposites for hydrogen generation. Appl. Catal. A Gen. 2010, 377, 47–54. [Google Scholar] [CrossRef]
- Phanikrishna Sharma, M.V.; Lalitha, K.; Durgakumari, V.; Subrahmanyam, M. Solar photocatalytic mineralization of isoproturon over TiO2/HY composite systems. Sol. Energy Mater. Sol. Cells 2008, 92, 332–342. [Google Scholar] [CrossRef]
- Qamara, M.; Merzougui, B.; Anjum, D.; Hakeem, A.S.; Yamani, Z.H.; Bahnemann, D. Synthesis and photocatalytic activity of mesoporous nanocrystalline Fe-doped titanium Dioxide. Catal. Today 2014, 230, 158–165. [Google Scholar] [CrossRef]
- Lei, J.; Li, X.; Li, W.; Sun, F.; Lu, D.; Lin, Y. Photocatalytic degradation of methyl orange on arrayed porous iron-doped anatase TiO2. J. Solid State Electrochem. 2012, 16, 625–632. [Google Scholar] [CrossRef]
- Navio, J.A.; Testa, J.J.; Djedjeian, P.; Padron, J.R.; Rodriguez, D.; Litter, M.I. Iron doped titania powders prepared by a sol–gel method. Part II: Photocatalytic properties. Appl. Catal. A 1999, 178, 191–203. [Google Scholar] [CrossRef]
- Tong, T.Z.; Zhang, J.L.; Tian, B.Z.; Chen, F.; He, D.N. Preparation of Fe3+-doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.A.; Navio, J.A. Photocatalytic properties of iron-doped titania semiconductors. J. Photochem. Photobiol. A 1996, 98, 171–181. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Sankararaman, S.; Balakrishna, K.M.; Varghese, T. Enhanced visible light photocatalysis using TiO2/phthalocyanine nanocomposites for the degradation of selected industrial dyes. J. Alloys Compd. 2017, 720, 541–549. [Google Scholar] [CrossRef]
- Miranda, L.D.L.; Bellato, C.R.; Milagres, J.A.L.; Moura, L.G.; Mounteer, A.H.; de Almeida, M.F. Hydrotalcite TiO2 magnetic iron oxide intercalated with the anionic surfactant dodecylsulfate in the photocatalytic degradation of methylene blue dye. J. Environ. Manag. 2015, 156, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, B.; Mu, J.; Zhang, M.; Zhang, P.; Zhang, Z.; Wang, J.; Zhang, X.; Sun, Y.; Shao, C.; et al. Iron phthalocyanine/TiO2 nanofiber heterostructures with enhanced visible photocatalytic activity assisted with H2O2. J. Hazard. Mater. 2012, 219–220, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mesgaria, Z.; Gharagozloub, M.; Khosravic, A.; Gharanjig, K. Synthesis, characterization and evaluation of efficiency of new hybrid Pc/Fe-TiO2 nanocomposite as photocatalyst for decolorization of methyl orange using visible light irradiation. Appl. Catal. A Gen. 2012, 411–412, 139–145. [Google Scholar] [CrossRef]
- Shende, T.P.; Bhanvase, B.A.; Rathod, A.P.; Pinjari, D.V.; Sonawane, S.H. Sonochemical synthesis of Graphene-Ce-TiO2 and Graphene-Fe-TiO2 ternary hybrid photocatalyst nanocomposite and its application in degradation of Crystal Violet Dye. Ultrason. Sonochem. 2018, 41, 582–589. [Google Scholar] [CrossRef]
- Rosu, M.C.; Coros, M.; Pogacean, F.; Magerusan, L.; Socaci, C.; Turza, A.; Pruneanu, S. Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 2017, 70, 13–20. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Yu, Y.; He, H. TiO2/HZSM-5 nano-composite photocatalyst: HCl treatment of NaZSM-5 promotes photocatalytic degradation of methyl orange. Chem. Eng. J. 2010, 163, 62–67. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Li, Y. Synthesis and characteristics of natural zeolite supported Fe3+-TiO2 photocatalysts. Appl. Surf. Sci. 2011, 257, 6873–6877. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foura, G.; Chouchou, N.; Soualah, A.; Kouachi, K.; Guidotti, M.; Robert, D. Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants. Catalysts 2017, 7, 344. https://doi.org/10.3390/catal7110344
Foura G, Chouchou N, Soualah A, Kouachi K, Guidotti M, Robert D. Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants. Catalysts. 2017; 7(11):344. https://doi.org/10.3390/catal7110344
Chicago/Turabian StyleFoura, Ghania, Nawel Chouchou, Ahcène Soualah, Kahina Kouachi, Matteo Guidotti, and Didier Robert. 2017. "Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants" Catalysts 7, no. 11: 344. https://doi.org/10.3390/catal7110344
APA StyleFoura, G., Chouchou, N., Soualah, A., Kouachi, K., Guidotti, M., & Robert, D. (2017). Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants. Catalysts, 7(11), 344. https://doi.org/10.3390/catal7110344