Production of 4-Ene-3-ketosteroids in Corynebacterium glutamicum
Abstract
:1. Introduction
2. Results
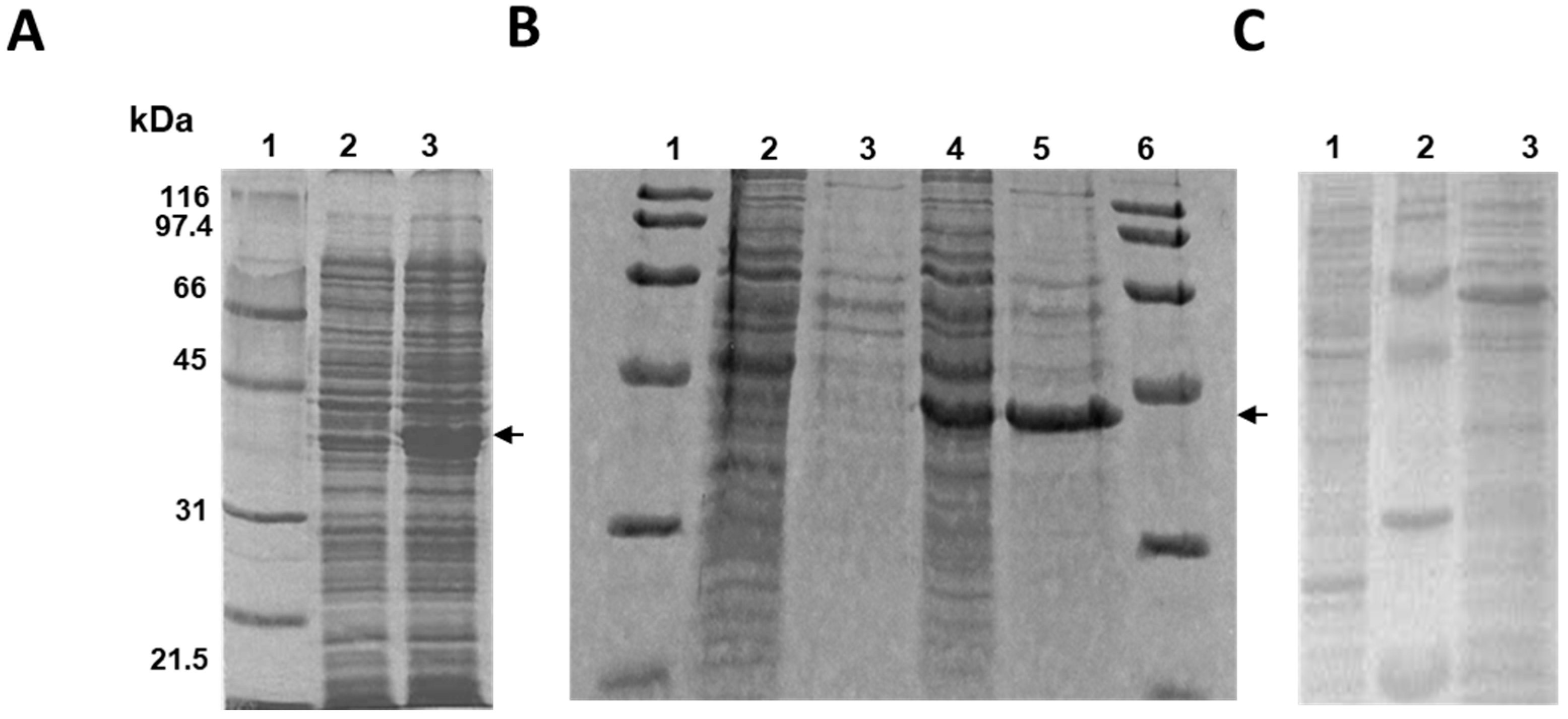
2.1. Cloning and Expression of the MSMEG_5228 Gene in Different Bacteria
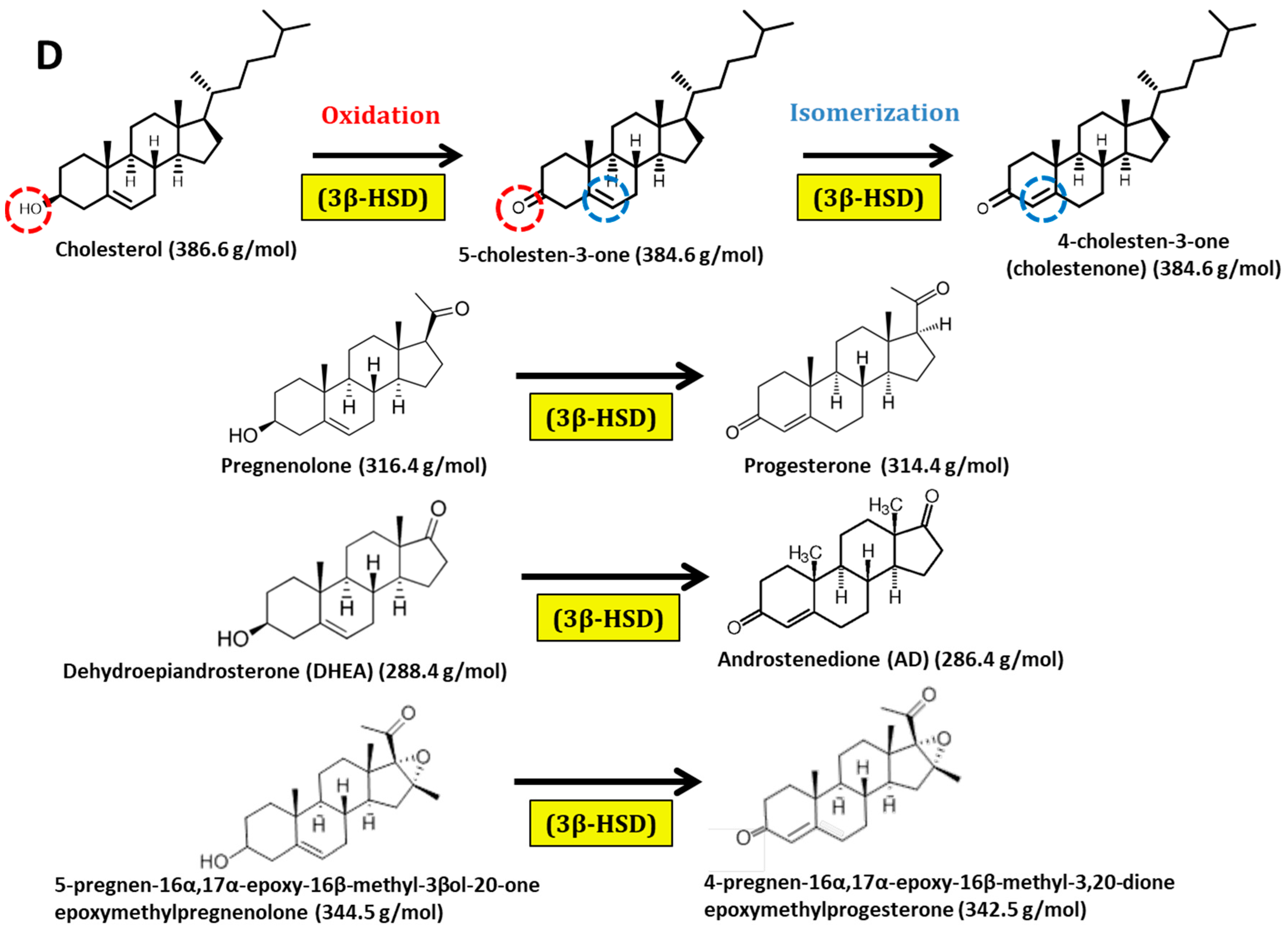
2.2. Biotransformation of Different Steroids by C. glutamicum R31 (pCGL5228)
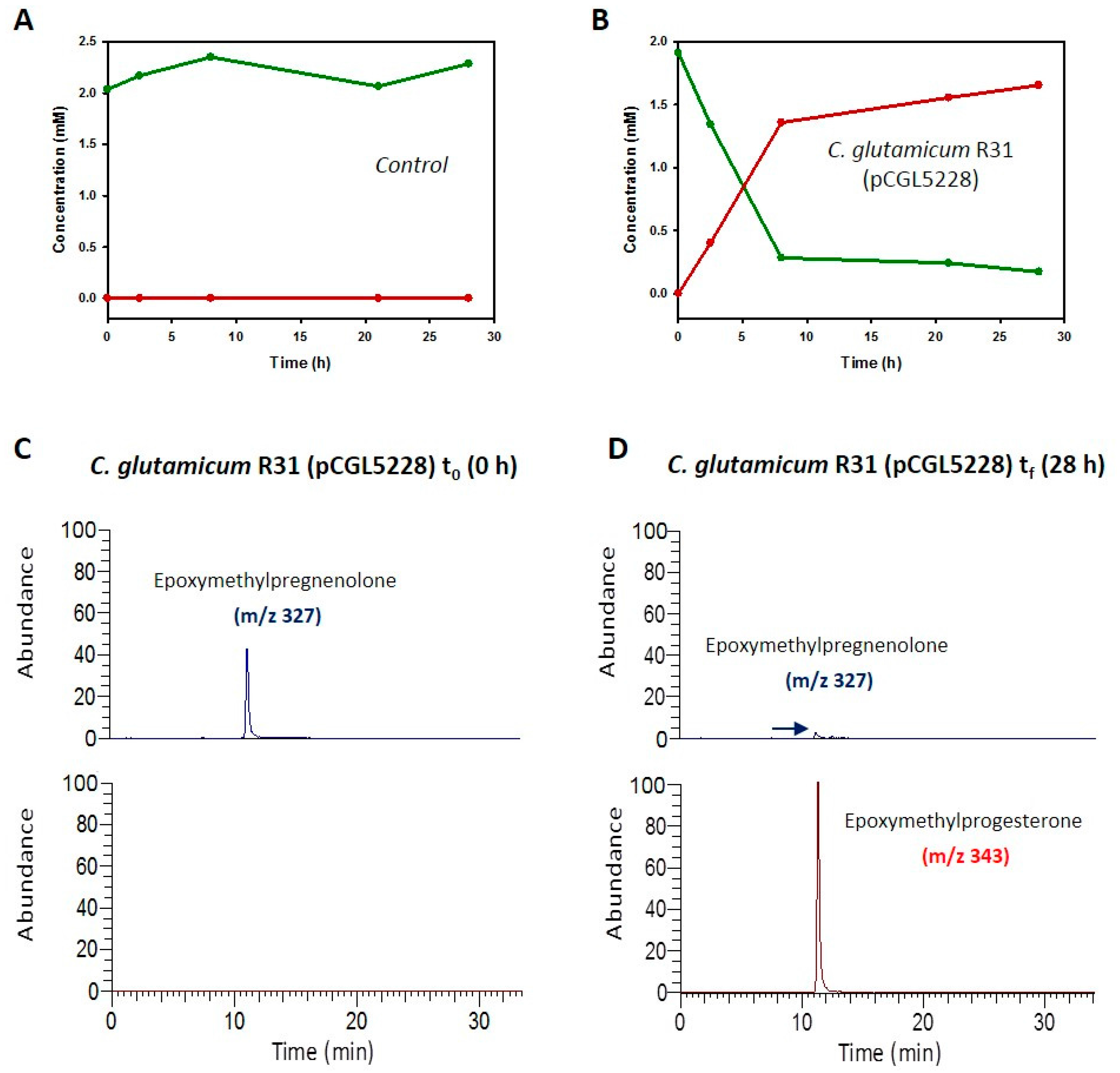
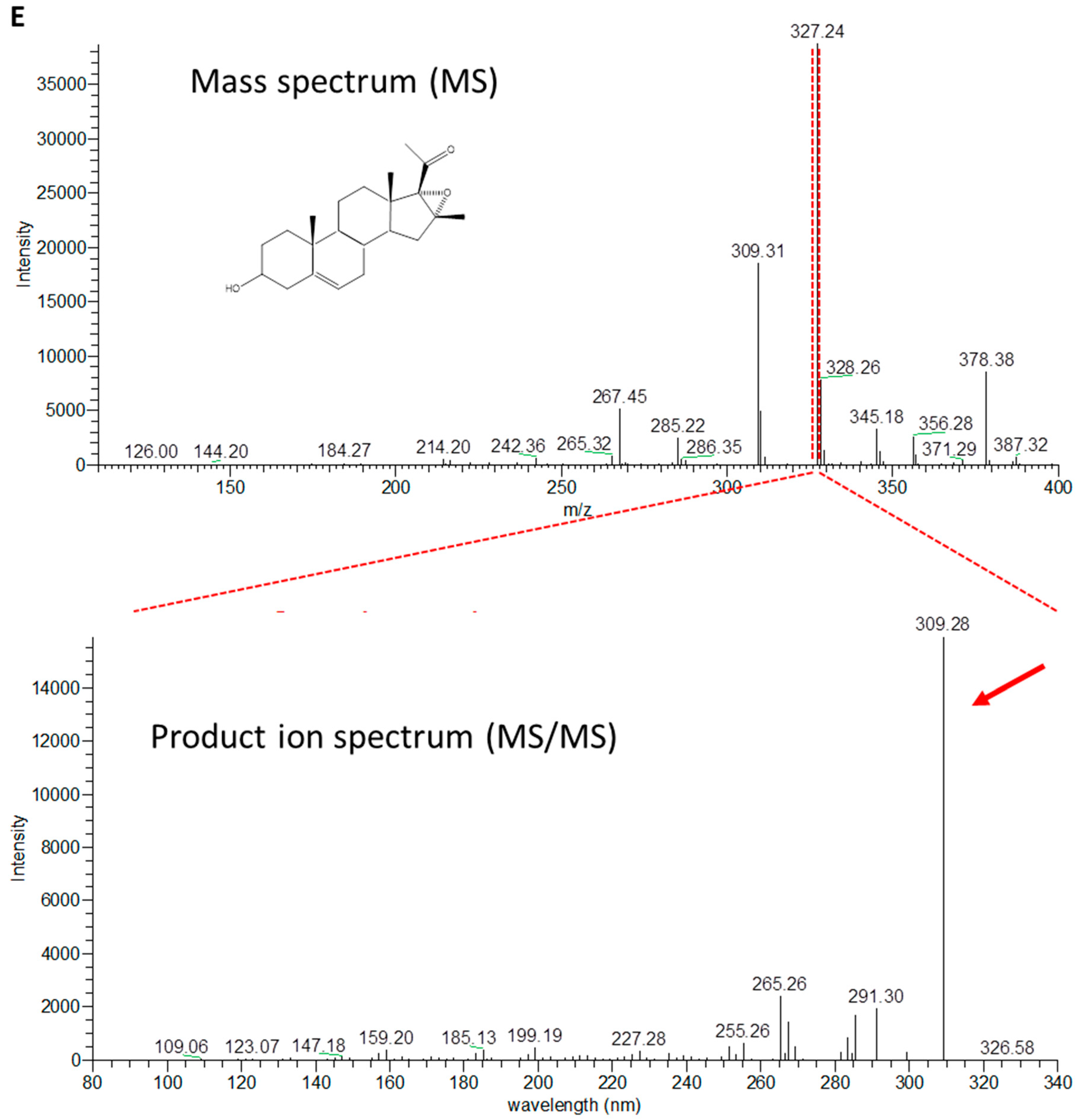
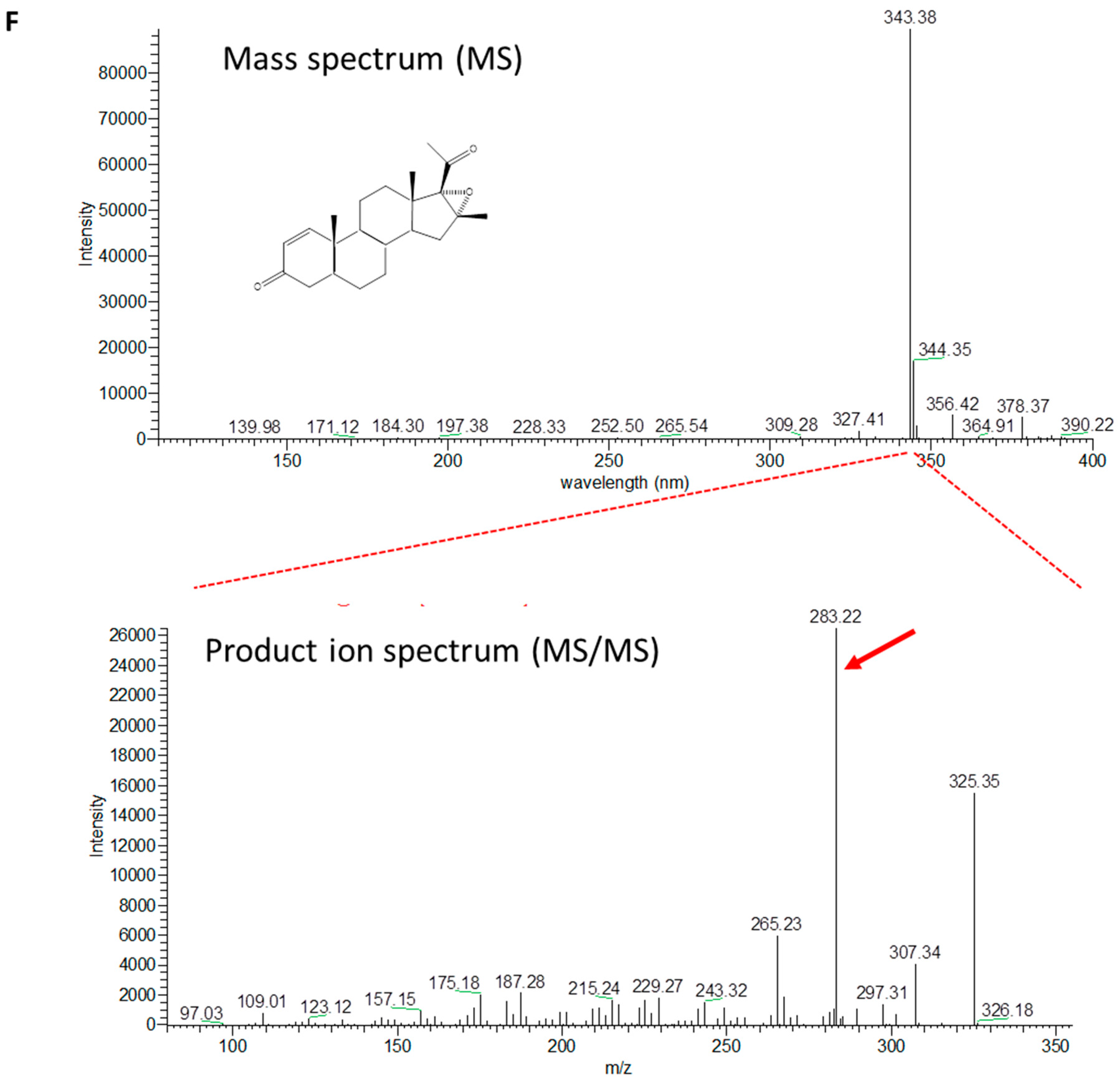
2.3. Production of 4-Pregnen-16α,17α-Epoxy-16β-Methyl-3,20-Dione by C. glutamicum (pCGL5228)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains and Culture Conditions
4.3. Expression of the MSMEG_5228 Protein in R. erythropolis IGTS8, E. coli BL21 and C. glutamicum R31
4.4. Resting Cell Assay for Steroid Biotransformation Using C. glutamicum R31 (pCGL5228)
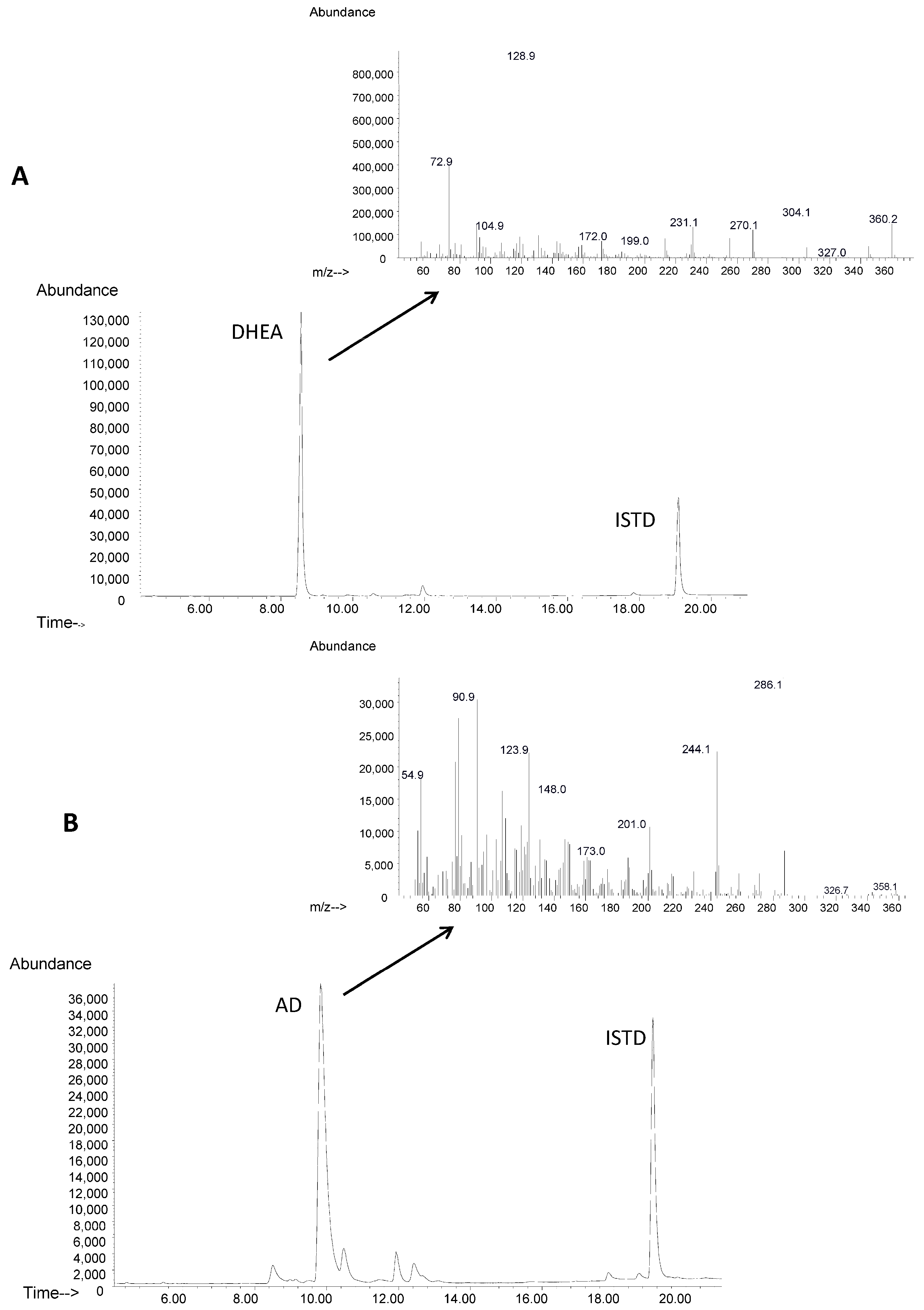
4.5. GC/MS Analysis: Identification and Quantification of DHEA and AD
4.6. LC/MS Analysis: Identification and Quantification of Epoxymethylpregnenolone and Epoxymethylprogesterone
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kalinowski, J.; Bathe, B.; Bartels, D.; Bischoff, N.; Bott, M.; Burkovski, A.; Dusch, N.; Eggeling, L.; Eikmanns, B.J.; Gaigalat, L.; et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 2003, 104, 5–25. [Google Scholar] [CrossRef]
- Ikeda, M.; Nakagawa, S. The Corynebacterium glutamicum genome, features and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 2003, 62, 99–109. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, V. Chassis organism from Corynebacterium glutamicum, the way towards biotechnological domestication of Corynebacteria. Biotechnol. J. 2015, 10, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Unthan, S.; Baumgart, M.; Radek, A.; Herbst, M.; Siebert, D.; Brühl, N.; Bartsch, A.; Bott, M.; Wiechert, W.; Marin, K.; et al. Chassis organism from Corynebacterium glutamicum—A topdown approach to identify and delete irrelevant gene clusters. Biotechnol. J. 2014, 10, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Wittman, C. Bio-based production of chemicals, materials and fuels-Corynebacterium glutamicum as versatile cell factory. Curr. Opin. Biotechnol. 2012, 23, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Charney, W.; Nobile, A.; Sutter, D.; Herzog, H.L.; Payne, C.C.; Tully, M.E.; Gentles, M.J.; Hershberg, E.B. Microbiological transformation of steroids—XI, The action of Corynebacterium simplex on non-corticoid steroid substrates. Tetrahedron 1962, 18, 591–596. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Yanga, T.; Xu, M.; Rao, Z. Over-expression of Mycobacterium neoaurum 3-ketosteroid-Δ1-dehydrogenase in Corynebacterium crenatum for efficient bioconversion of 4-androstene-3,17-dione to androst-1,4-diene-3,17-dione. Elec. J. Biotechnol. 2016, 24, 84–90. [Google Scholar] [CrossRef]
- Donova, M.V.; Egorova, O.V. Microbial steroid transformations, current state and prospects. Appl. Microbiol. Biotechnol. 2012, 94, 1423–1447. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; Uhía, I.; Galán, B. Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb. Biotechnol. 2012, 5, 679–699. [Google Scholar] [CrossRef] [PubMed]
- Galán, B.; Uhía, I.; García-Fernández, E.; Martínez, I.; Bahíllo, E.; de la Fuente, J.L.; Barredo, J.L.; Fernández-Cabezón, L.; García, J.L. Mycobacterium smegmatis is a suitable cell factory for the production of steroidic synthons. Microb. Biotechnol. 2017, 10, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Donova, M.V. Steroid Bioconversions. Methods Mol. Biol. 2017, 1645, 1–13. [Google Scholar] [PubMed]
- Kreit, J. Microbial catabolism of sterols, focus on the enzymes that transform the sterol 3β-hydroxy-5-en into 3-keto-4-en. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Uhía, I.; Galán, B.; Morales, V.; García, J.L. Initial step in the catabolism of cholesterol by Mycobacterium smegmatis mc2 155. Environ. Microbiol. 2011, 13, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Hayashi, N.; Choi, K.-P.; Yamamoto, H.; Yamashita, M.; Murooka, Y. Bacterial cholesterol oxidases are able to act as flavoprotein-linked ketosteroid monooxygenases that catalyse the hydroxylation of cholesterol to 4-cholesten-6-ol-3-one. Mol. Microbiol. 1993, 7, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Toyama, M.; Ono, H.; Fujii, I.; Hirayama, N.; Murooka, Y. Separation of the two reactions, oxidation and isomerization, catalyzed by Streptomyces cholesterol oxidase. Protein Eng. 1998, 11, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Mohn, W.W.; van der Geize, R.; Stewart, G.R.; Okamoto, S.; Liu, J.; Dijkhuizen, L.; Eltis, L.D. The actinobacterial mce4 locus encodes a steroid transporter. J. Biol. Chem. 2008, 283, 35368–35374. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, J.; Papavinasasundaram, K.; Galán, B.; Sassetti, C.M.; García, J.L. Unravelling the pleiotropic role of the MceG ATPase in Mycobacterium smegmatis. Environ. Microbiol. 2017, 19, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Piubelli, L.; Molla, G. Cholesterol oxidase, biotechnological applications. FEBS J. 2009, 276, 6857–6870. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Kanwar, S.S. Cholesterol oxidase and its applications. Adv. Microbiol. 2012, 2, 49–65. [Google Scholar] [CrossRef]
- Alexander, D.L.; Fisher, J.F. A convenient synthesis of 7α-hydroxycholest-4-en-3-one by the hydroxypropyl-β-cyclodextrin-facilitated cholesterol oxidase oxidation of 3β,7α-cholest-5-ene-3,7-diol. Steroids 1995, 60, 290–294. [Google Scholar] [CrossRef]
- Labaree, D.; Hoyte, R.M.; Hochberg, R.B. A direct stereoselective synthesis of 7β-hydroxytestosterone. Steroids 1997, 62, 482–486. [Google Scholar] [CrossRef]
- Biellmann, J.F. Resolution of alcohols by cholesterol oxidase from Rhodococcus erythropolis, lack of enantiospecificity of the steroids. Chirality 2001, 13, 34–39. [Google Scholar] [CrossRef]
- Guo, L.-W.; Wilson, W.K.; Pang, J.; Shackleton, C.H.L. Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17α,20-triols, potential steroid metabolites in Smith-LemLi-Opitz syndrome. Steroids 2002, 68, 31–42. [Google Scholar] [CrossRef]
- Yang, X.; Dubnau, E.; Smith, I.; Sampson, N.S. Rv1106c from Mycobacterium tuberculosis is a 3beta-hydroxysteroid dehydrogenase. Biochemistry 2007, 46, 9058–9067. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, S.; Ishizuka, H.; Beppu, T. Cloning, nucleotide sequence, and transcriptional analysis of the NAD(P)-dependent cholesterol dehydrogenase gene from a Nocardia sp. and its hyperexpression in Streptomyces spp. Appl. Environ. Microbiol. 1991, 57, 1386–1393. [Google Scholar] [PubMed]
- Kishi, K.; Watazu, Y.; Katayama, Y.; Okabe, H. The characteristics and applications of recombinant cholesterol dehydrogenase. Biosci. Biotechnol. Biochem. 2000, 64, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, J.; Papavinasasundaram, K.; Galán, B.; Sassetti, C.M.; García, J.L. Molecular and functional analysis of the MCE4 operon in Mycobacterium smegmatis. Environ. Microbiol. 2017, 19, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Snapper, S.B.; Melton, R.E.; Mustafa, S.; Kieser, T.; Jacobs, W.R., Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 1990, 4, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Kilbane, J.J. Desulfurization of coal, the microbial solution. Trends Biotechnol. 1989, 7, 97–101. [Google Scholar] [CrossRef]
- Santamaria, R.; Gil, J.A.; Martin, J.F. High-frequency transformation of Brevibacterium lactofermentum protoplasts by plasmid DNA. J. Bacteriol. 1985, 162, 463–467. [Google Scholar] [PubMed]
- Nakashima, N.; Tamura, T. Isolation and characterization of a rolling-circle-type plasmid from Rhodococcus erythropolis and application of the plasmid to multiple-recombinant-protein expression. Appl. Environ. Microbiol. 2004, 70, 5557–5568. [Google Scholar] [CrossRef] [PubMed]
- Peyret, J.L.; Bayan, N.; Joliff, G.; Gulik-Krzywicki, T.; Mathieu, L.; Schechter, E.; Leblon, G. Characterization of the cspB gene encoding PS2, an ordered surface-layer protein in Corynebacterium glutamicum. Mol. Microbiol. 1993, 9, 97–109. [Google Scholar] [CrossRef] [PubMed]
| Strains | Genotype and/or Description | Source or Reference |
|---|---|---|
| Escherichia coli DH10B | F’, mcrA Δ(mrr hsdRMS-mcrBC) F80dlacDM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK λ rpsL endA1 nupG | Invitrogen |
| Escherichia coli BL21 (DE3) | F−, ompT, hsdSB(rB-mB-), gal, dcm, λDE3 (harboring gene 1 of the RNA polymerase from the phage T7 under the PlacUV5 promoter) | Invitrogen |
| Mycobacterium smegmatis mc2155 | ept-1, mc²6 mutant efficient for electroporation | [28] |
| Rhodococcus erythropolis IGTS8 | Isolated for oil desulfurization using dsz genes | [29] |
| Corynebacterium glutamicum R31 | MeLisR, AecR. Efficient for transformation | [30] |
| Plasmids | ||
| pET29a(+) | Cloning and expression vector, Kmr, oriColE1, T7 promoter | Novagen |
| pET5228 | pET29 derivative containing MSMEG_5288 gene | [13] |
| pTipQC1 | Bifunctional expression vector. PtipA, repAB (pRE2895), MCS type 1, CmR | [31] |
| pCGL0482 | Bifunctional expression vector E. coli/C. glutamicum. PS1 promoter (modified from plasmid pCGL482), CmR | [32] (Gift from X. Meniche) |
| pTip5228 | pTipQC1 harboring the MSMEG_5228 gene cloned in NdeI/HindIII, CmR | This study |
| pCGL5228 | pCGL0482 harboring the MSMEG_5228 gene cloned in XhoI/HpaI, CmR | This study |
| Primer Name | Sequence 1 (5’-3’) | Use 2 |
|---|---|---|
| pTip5228 F | CGCGCATATGGCTGACTCCACCACCGAC | Amplification of MSMEG_5228 for cloning in pTipQC1 (NdeI) |
| pTip5228 R | CGCGAAGCTTCGTGCTGCCCTGAACTAG | Amplification of MSMEG_5228 for cloning in pTipQC1 (HindIII) |
| pCGL5228 F | CGCGCTCGAGATGGCTGACTCCACCACCGACCTC | Amplification of MSMEG_5228 for cloning in pCGL0482 (XhoI) |
| pCGL5228 R | CGCGGTTAACGCCCGTGCTGCCCTGAAC | Amplification of MSMEG_5228 for cloning in pCGL0482 (HpaI) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Fernández, J.; Galán, B.; Felpeto-Santero, C.; Barredo, J.L.; García, J.L. Production of 4-Ene-3-ketosteroids in Corynebacterium glutamicum. Catalysts 2017, 7, 316. https://doi.org/10.3390/catal7110316
García-Fernández J, Galán B, Felpeto-Santero C, Barredo JL, García JL. Production of 4-Ene-3-ketosteroids in Corynebacterium glutamicum. Catalysts. 2017; 7(11):316. https://doi.org/10.3390/catal7110316
Chicago/Turabian StyleGarcía-Fernández, Julia, Beatriz Galán, Carmen Felpeto-Santero, José L. Barredo, and José L. García. 2017. "Production of 4-Ene-3-ketosteroids in Corynebacterium glutamicum" Catalysts 7, no. 11: 316. https://doi.org/10.3390/catal7110316
APA StyleGarcía-Fernández, J., Galán, B., Felpeto-Santero, C., Barredo, J. L., & García, J. L. (2017). Production of 4-Ene-3-ketosteroids in Corynebacterium glutamicum. Catalysts, 7(11), 316. https://doi.org/10.3390/catal7110316




