Carbon Supported Oxide-Rich Pd-Cu Bimetallic Electrocatalysts for Ethanol Electrooxidation in Alkaline Media Enhanced by Cu/CuOx
Abstract
:1. Introduction
2. Results and Discussion
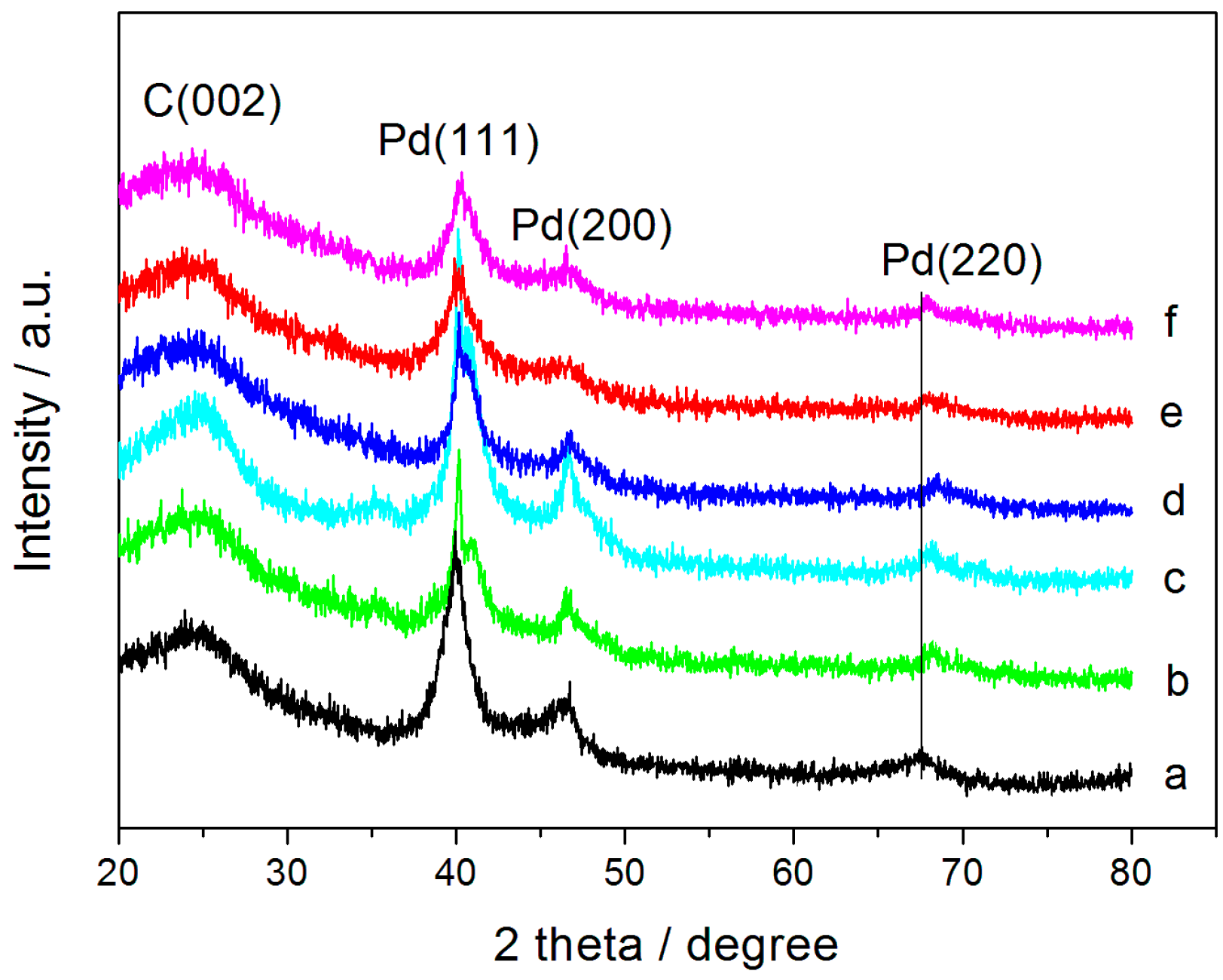
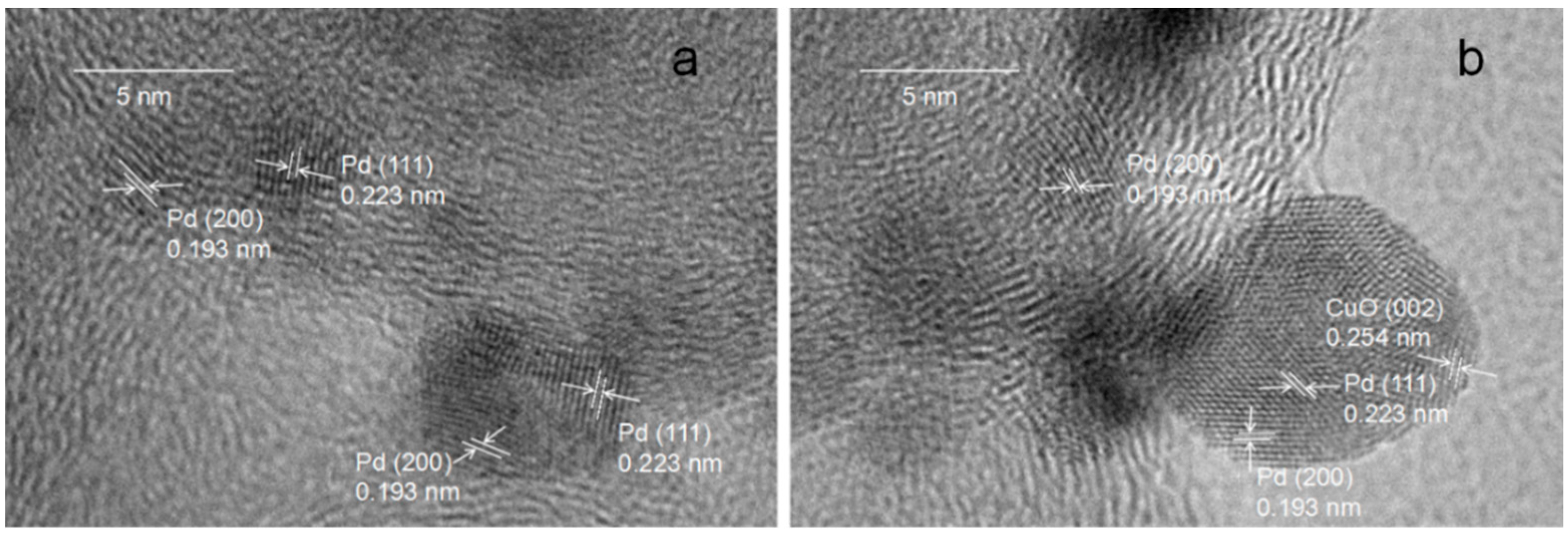
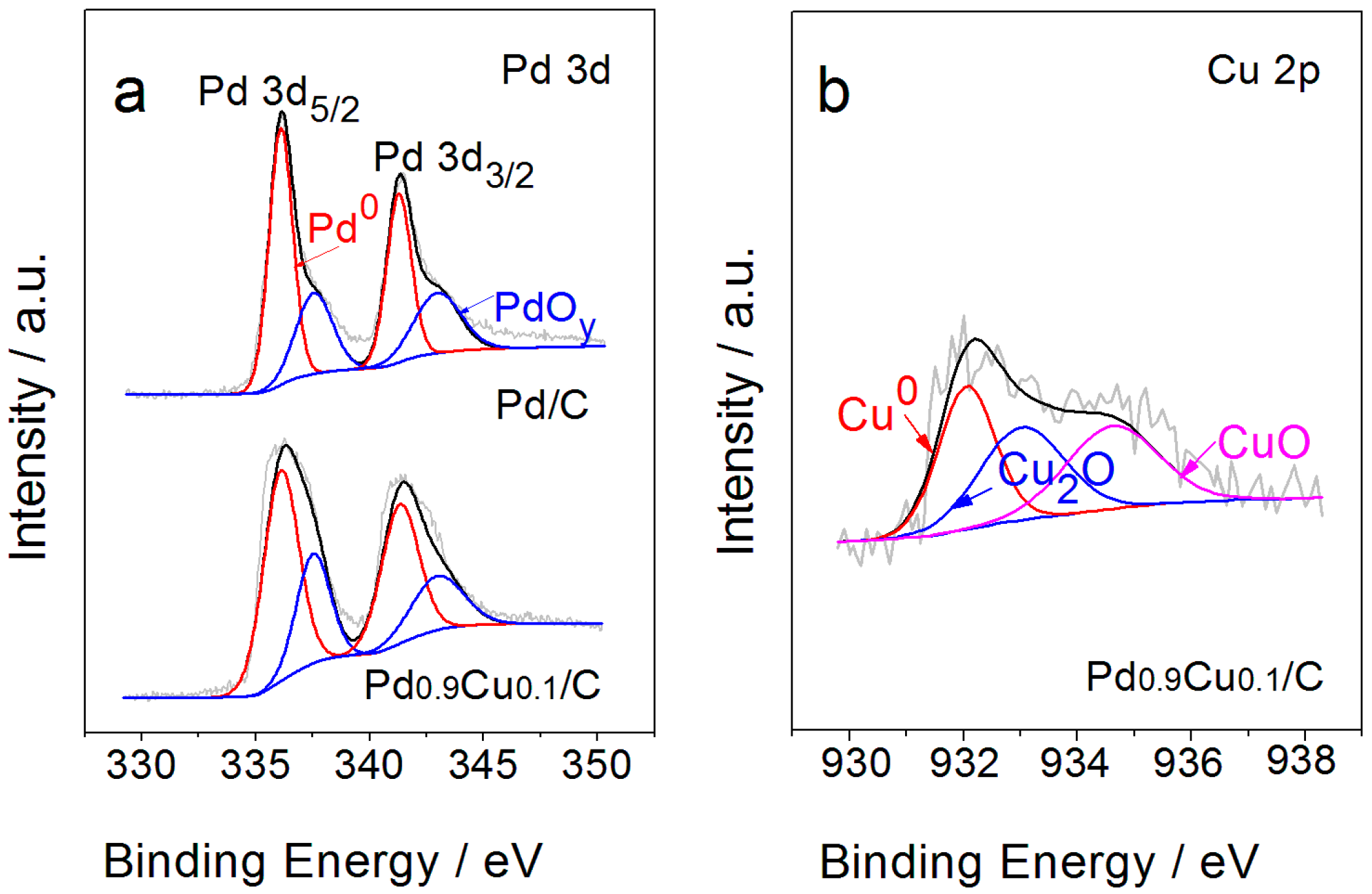
2.1. Materials Characterization
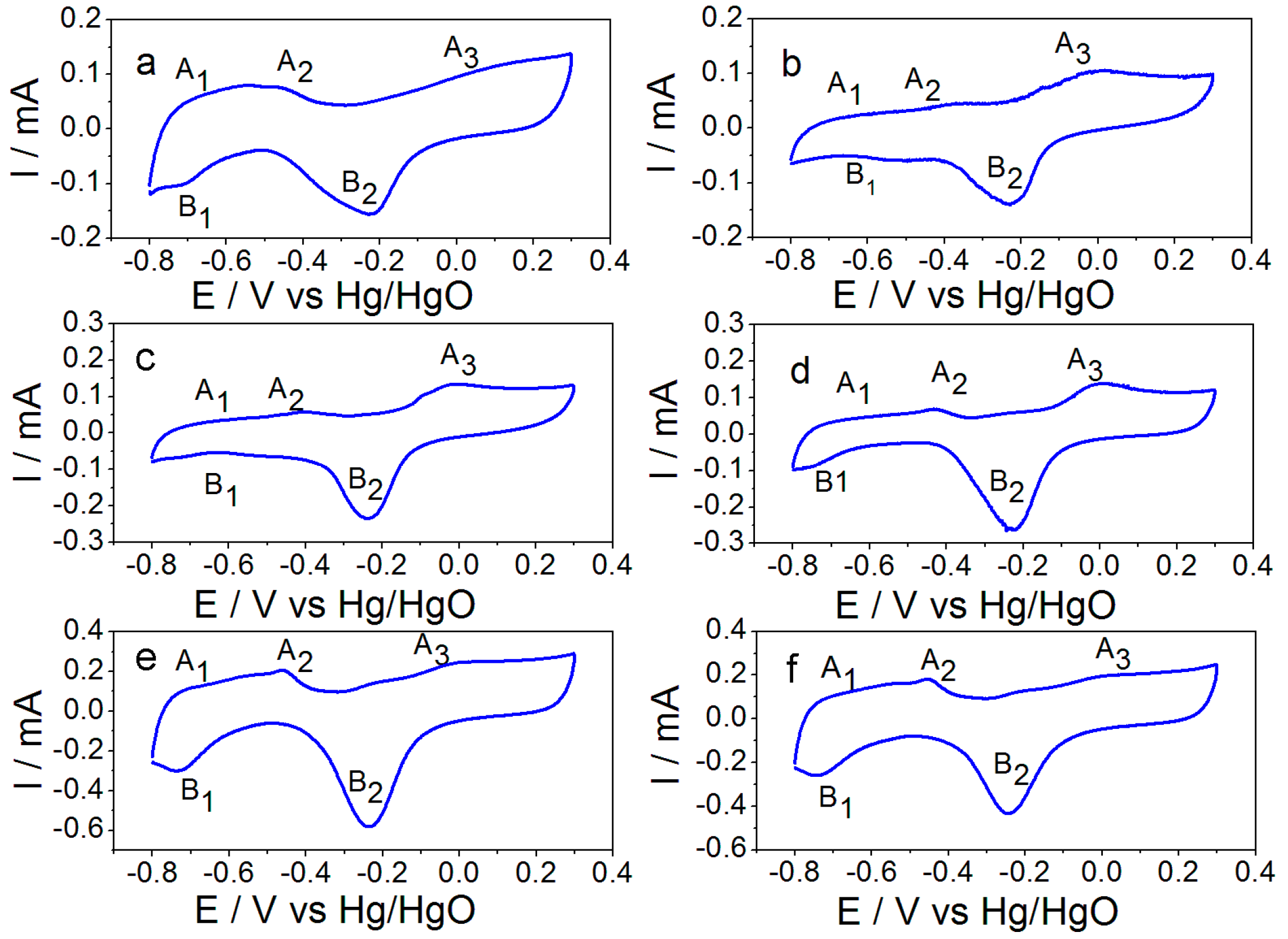
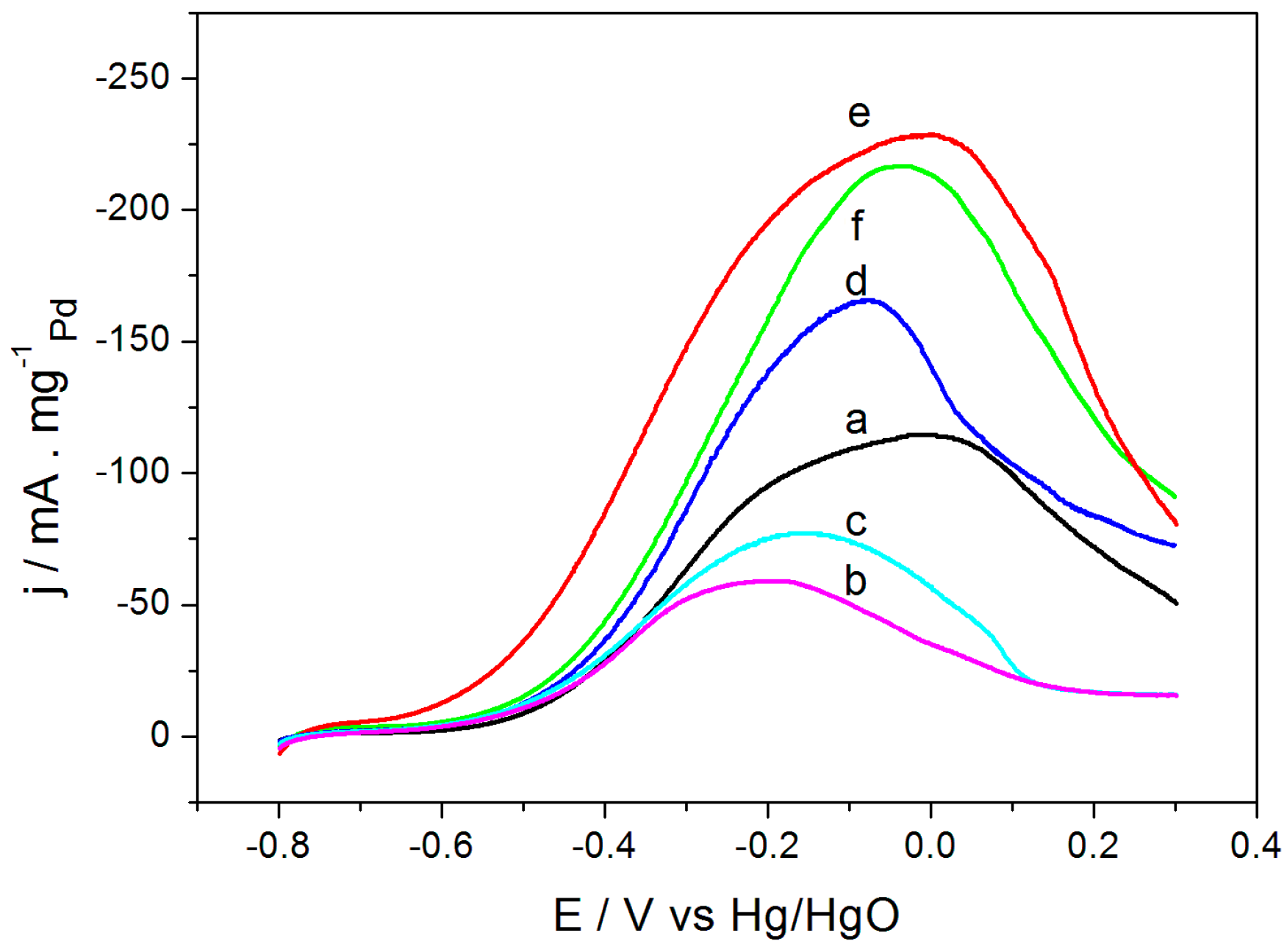
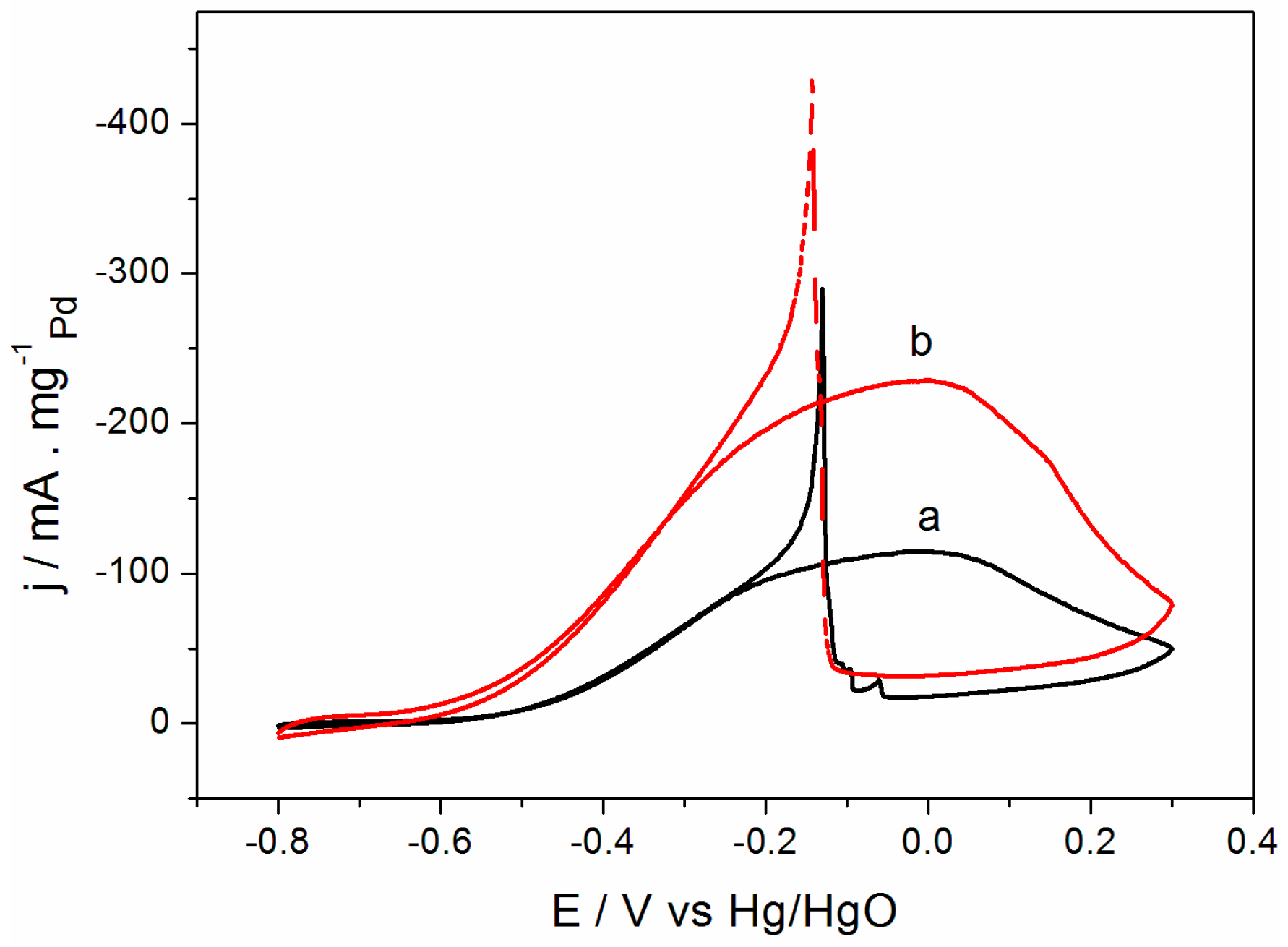
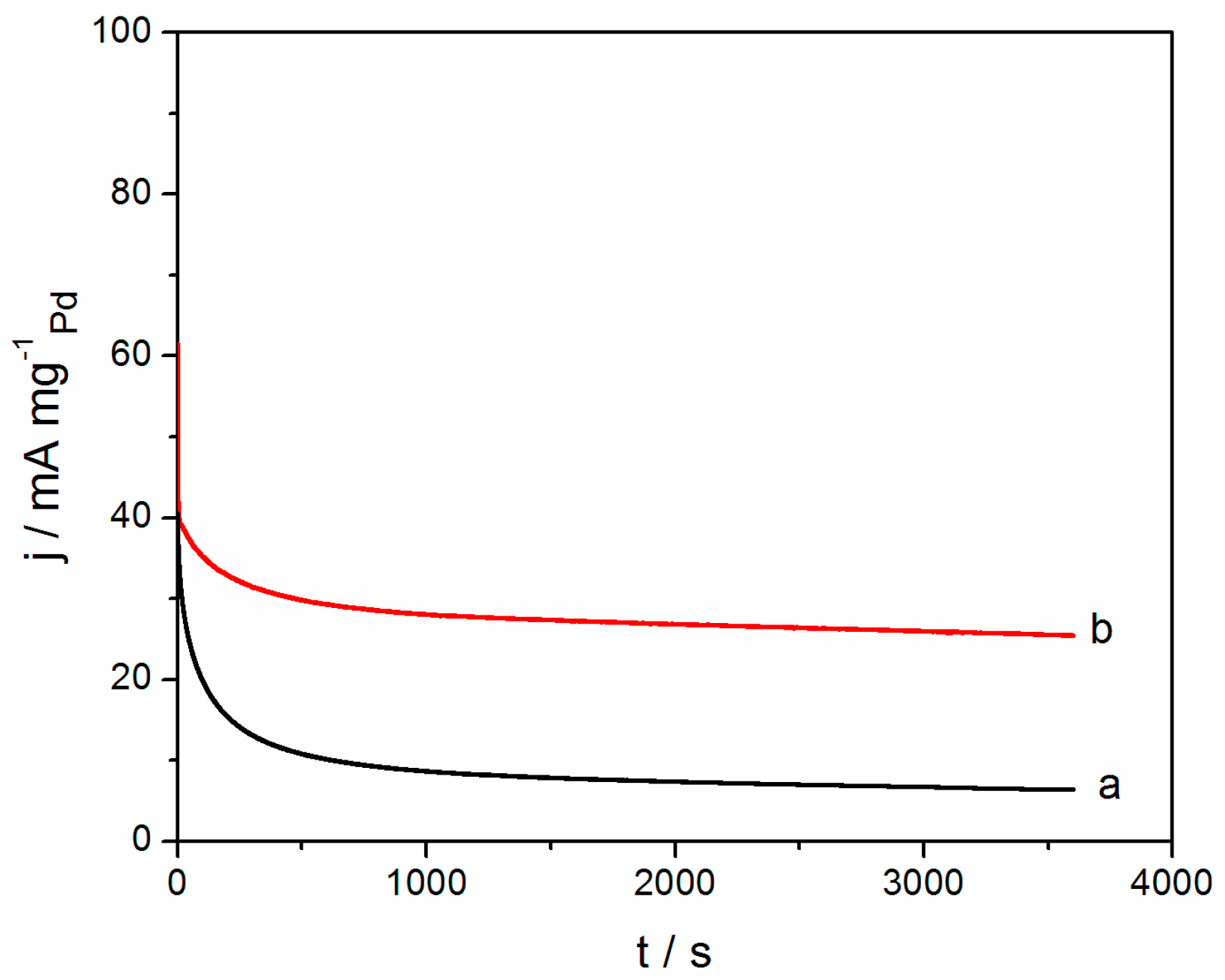
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Chemical Reagents
3.2. Preparation of the Catalysts
3.3. Preparation of the Working Electrode
3.4. Materials Characterization
3.5. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells: Principles, types, fuels, and applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. Handbook of Fuel Cells: Fundamentals, Technology, Applications; John Wiley & Sons Inc.: New York, NY, USA, 2003. [Google Scholar]
- U.S. Department of Energy. Basic Research Needs for the Hydrogen Economy; U.S. Department of Energy: Washington, DC, USA, 2004.
- Zhang, Q.L.; Zheng, J.N.; Xu, T.Q.; Wang, A.J.; Wei, J.; Chen, J.R.; Feng, J.J. Simple one-pot preparation of Pd-on-Cu nanocrystals supported on reduced graphene oxide for enhanced ethanol electrooxidation. Electrochim. Acta 2014, 132, 551–560. [Google Scholar] [CrossRef]
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Sources 2008, 182, 124–132. [Google Scholar] [CrossRef]
- Ribeiro, J.; dos Anjos, D.M.; Léger, J.M.; Hahn, F.; Olivi, P.; de Andrade, A.R.; Tremiliosi-Filho, G.; Kokoh, K.B. Effect of W on PtSn/C catalysts for ethanol electrooxidation. J. Appl. Electrochem. 2008, 38, 653–662. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; Shen, P. Thermodynamic and kinetic considerations for ethanol electrooxidation in direct ethanol fuel cells. Chin. J. Catal. 2007, 28, 752–754. [Google Scholar] [CrossRef]
- Giz, M.J.; Camara, G.A. The ethanol electrooxidation reaction at Pt (111): The effect of ethanol concentration. J. Electroanal. Chem. 2009, 625, 117–122. [Google Scholar] [CrossRef]
- Camara, G.A.; Iwasita, T. Parallel pathways of ethanol oxidation: The effect of ethanol concentration. J. Electroanal. Chem. 2005, 578, 315–321. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, Z.; Zhuang, J.; Wang, X. Seed displacement, epitaxial synthesis of Rh/Pt bimetallic ultrathin nanowires for highly selective oxidizing ethanol to CO2. Chem. Mater. 2010, 22, 2395–2402. [Google Scholar] [CrossRef]
- Freitas, R.G.; Pereira, E.C.; Christensen, P.A. The selective oxidation of ethanol to CO2 at Ptpc/Ir/Pt metallic multilayer nanostructured electrodes. Electrochem. Commun. 2011, 13, 1147–1150. [Google Scholar] [CrossRef]
- Zeller, G. Electrolytic oxidation of ethyl alcohol to acetic acid. Trans. Electrochem. Soc. 1947, 92, 335–342. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Tian, J.; Wang, F.; Zhan, L. Methanol electro-oxidation on Ni@Pd core-shell nanoparticles supported on multi-walled carbon nanotubes in alkaline media. Int. J. Hydrogen Energy 2010, 35, 3249–3257. [Google Scholar] [CrossRef]
- Zhou, W.J.; Song, S.Q.; Li, W.Z.; Sun, G.Q.; Xin, Q.; Kontou, S.; Poulianitis, K.; Tsiakaras, P. Pt-based anode catalysts for direct ethanol fuel cells. Solid State Ionics 2004, 175, 797–803. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Gonzalez, E.R. Ethanol electro-oxidation on carbon-supported Pt-Ru, Pt-Rh and Pt-Ru-Rh nanoparticles. Electrochim. Acta 2008, 53, 2963–2971. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Zhan, L.; Ou, S.; Tian, J. High electrocatalytic activity of PtRu nanoparticles supported on starch-functionalized multi-walled carbon nanotubes for ethanol oxidation. J. Mater. Chem. 2011, 21, 4257–4263. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. 2007, 9, 2654–2675. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.K.; Xu, C. Alcohol oxidation on nanocrystalline oxide Pd/C promoted electrocatalysts. Electrochem. Commun. 2006, 8, 184–188. [Google Scholar] [CrossRef]
- Ma, L.; Chu, D.; Chen, R. Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Int. J. Hydrogen Energy 2012, 37, 11185–11194. [Google Scholar] [CrossRef]
- Rao, V.; Hariyanto; Cremers, C.; Stimming, U. Investigation of the ethanol electro-oxidation in alkaline membrane electrode assembly by differential electrochemical mass spectrometry. Fuel Cells 2007, 7, 417–423. [Google Scholar] [CrossRef]
- Zheng, H.T.; Li, Y.; Chen, S.; Shen, P.K. Effect of support on the activity of Pd electrocatalyst for ethanol oxidation. J. Power Sources 2006, 163, 371–375. [Google Scholar] [CrossRef]
- Ye, J.; Liu, J.; Xu, C.; Jiang, S.P.; Tong, Y. Electrooxidation of 2-propanol on Pt, Pd and Au in alkaline medium. Electrochem. Commun. 2007, 9, 2760–2763. [Google Scholar] [CrossRef]
- Xu, C.; Shen, P.K.; Liu, Y. Ethanol electrooxidation on Pt/C and Pd/C catalysts promoted with oxide. J. Power Sources 2007, 164, 527–531. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Law, H.M.; Nguyen, H.T.; Kristian, N.; Wang, S.; Chan, S.H.; Wang, X. Enhancement effect of Ag for Pd/C towards the ethanol electro-oxidation in alkaline media. Appl. Catal. B 2009, 91, 507–515. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Yang, S.; Ding, B.; Song, X. Au@Pd core-shell nanobricks with concave structures and their catalysis of ethanol oxidation. ChemSusChem 2013, 6, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B.; Li, Y.S. Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells. J. Power Sources 2010, 195, 1001–1006. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Yin, J.; Liu, M.; Dong, Q.; Su, Y. Synthesis and electrocatalytic alcohol oxidation performance of Pd-Co bimetallic nanoparticles supported on graphene. Int. J. Hydrogen Energy 2014, 39, 1325–1335. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Wang, Q.; Zeng, F.; Kuang, Y. Reduced graphene oxide supported palladium-silver bimetallic nanoparticles for ethanol electro-oxidation in alkaline media. J. Mater. Sci. 2012, 47, 2188–2194. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Sun, J.; Kou, T.; Zhao, C. Ultrafine nanoporous Cu-Pd alloys with superior catalytic activities towards electro-oxidation of methanol and ethanol in alkaline media. RSC Adv. 2012, 2, 11820–11828. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, F.F.; Yang, Y.Y.; Cai, W.B. Carbon supported Pd-Ni-P nanoalloy as an efficient catalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2013, 243, 369–373. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, P.S.; Mandal, K.; Bhattacharjee, D.; Dasgupta, S.; Bhattacharya, S.K. Improved catalysis of room temperature synthesized Pd-Cu alloy nanoparticles for anodic oxidation of ethanol in alkaline media. Electrochim. Acta 2015, 154, 447–455. [Google Scholar] [CrossRef]
- Dutta, A.; Datta, J. Energy efficient role of Ni/NiO in PdNi nano catalyst used in alkaline DEFC. J. Mater. Chem. A 2014, 2, 3237–3250. [Google Scholar] [CrossRef]
- Rahim, M.A.A.; Hameed, R.M.A.; Khalil, M.W. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Jiang, X.; Koizumi, N.; Guo, X.; Song, C. Bimetallic Pd-Cu catalysts for selective CO2 hydrogenation to methanol. Appl. Catal. B 2015, 170, 173–185. [Google Scholar] [CrossRef]
- Behmenyar, G.; Akın, A.N. Investigation of carbon supported Pd-Cu nanoparticles as anode catalysts for direct borohydride fuel cell. J. Power Sources 2014, 249, 239–246. [Google Scholar] [CrossRef]
- Yang, L.; Hu, C.; Wang, J.; Yang, Z.; Cuo, Y.; Bai, Z.; Wang, K. Facile synthesis of hollow palladium/copper alloyed nanocubes for formic acid oxidation. Chem. Commun. 2011, 47, 8581–8583. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, K.; Zhang, H.; Dong, Y.; Wang, T.; He, D. Low temperature CO catalytic oxidation over supported Pd-Cu catalysts calcined at different temperatures. Chem. Eng. J. 2014, 242, 10–18. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Lin, R.; Wei, X. Electro-catalytic performance of Pd decorated Cu nanowires catalyst for the methanol oxidation. Int. J. Hydrogen Energy 2015, 40, 2621–2630. [Google Scholar] [CrossRef]
- Dong, Q.; Zhao, Y.; Han, X.; Wang, Y.; Liu, M.; Li, Y. Pd/Cu bimetallic nanoparticles supported on graphene nanosheets: Facile synthesis and application as novel electrocatalyst for ethanol oxidation in alkaline media. Int. J. Hydrogen Energy 2014, 39, 14669–14679. [Google Scholar] [CrossRef]
- Na, H.Y.; Zhang, L.; Qiu, H.X.; Wu, T.; Chen, M.X.; Yang, N.; Li, L.Z.; Xing, F.B.; Gao, J.P. A two step method to synthesize palladium-copper nanoparticles on reduced graphene oxide and their extremely high electrocatalytic activity for the electrooxidation of methanol and ethanol. J. Power Sources 2015, 288, 160–167. [Google Scholar] [CrossRef]
- Noborikawa, J.; Lau, J.; Ta, J.; Hu, S.; Scudiero, L.; Derakhshan, S.; Ha, S.; Haan, J.L. Palladium-copper electrocatalyst for promotion of oxidation of formate and ethanol in alkaline media. Electrochim. Acta 2014, 137, 654–660. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Wang, L.; Liu, Z.; Chen, W. PdxCu100−x networks: An active and durable electrocatalyst for ethanol oxidation in alkaline medium. J. Mater. Chem. A 2014, 2, 20933–20938. [Google Scholar] [CrossRef]
- Mao, H.; Huang, T.; Yu, A. Surface palladium rich CuxPdy/carbon catalysts for methanol and ethanol oxidation in alkaline media. Electrochim. Acta 2015, 174, 1–7. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, G.; Bai, Z.; Hang, R.; Tang, B.; Zhang, Z.; Wang, X. High activity of carbon nanotubes supported binary and ternary Pd-based catalysts for methanol, ethanol and formic acid electro-oxidation. J. Power Sources 2013, 242, 610–620. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Cai, K.; Zhang, H.; Lu, Z.; Li, T.; Zuo, Y.; Han, H. Clean synthesis of an economical 3D nanochain network of PdCu alloy with enhanced electrocatalytic performance towards ethanol oxidation. Chem. Eur. J. 2015, 21, 17779–17785. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, Y.; Chen, Z.; Wang, D.; Li, Y. Bimetallic Pd-Cu nanocrystals and their tunable catalytic properties. Chem. Commun. 2014, 50, 4588–4591. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Y.; Wang, J.; Geng, H.; Qiu, H. Nanoporous PdCu alloy for formic acid electro-oxidation. J. Power Sources 2012, 199, 124–131. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Wan, Y.; Jiang, J.; Zhao, X.S. Dendritic Pt-Cu bimetallic nanocrystals with a high electrocatalytic activity toward methanol oxidation. Mater. Chem. Phys. 2012, 132, 244–247. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Zhu, D.; Xie, A.; Shen, Y. A novel porous CuO nanorod/rGO composite as a high stability anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 1833–1839. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, H. Three-dimensional CuO microflowers as anode materials for Li-ion batteries. Ceram. Int. 2015, 41, 8257–8260. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F.; Cao, J.; Liu, Y.; Wang, B. Enhanced electrochemical performance of Cu2O-modified Li4Ti5O12 anode material for lithium-ion batteries. Ionics 2015, 21, 2155–2160. [Google Scholar] [CrossRef]
- Pan, L.; Tang, J.; Wang, F. Facile synthesis of nanoscaled α-Fe2O3, CuO and CuO/Fe2O3 hybrid oxides and their electrocatalytic and photocatalytic properties. Cent. Eur. J. Chem. 2013, 11, 763–773. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide-CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B 2016, 181, 352–362. [Google Scholar] [CrossRef]
- Yue, G.H.; Zhang, Y.; Zhang, X.Q.; Wang, C.G.; Zhao, Y.C.; Peng, D.L. Synthesis of Cu2O mesocrystal and its application in photocatalysis. Appl. Phys. A 2015, 118, 763–767. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, P.; Yan, Z.; Ren, N. Room temperature photoluminescence properties of CuO nanowire arrays. Opt. Mater. 2015, 42, 544–547. [Google Scholar] [CrossRef]
- Garuthara, R.; Siripala, W. Photoluminescence characterization of polycrystalline n-type Cu2O films. J. Lumin. 2006, 121, 173–178. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, P.F.; Yang, Z.J. CuO nanoparticles supported on carbon microspheres as electrode material for supercapacitors. Ionics 2015, 21, 185–190. [Google Scholar] [CrossRef]
- Park, H.; Han, T.H. Facile hybridization of graphene oxide and Cu2O for high-performance electrochemical supercapacitors. Macromol. Res. 2014, 22, 809–812. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Liu, J.; Feng, H.; Qian, D.; Peng, S.; Jiang, J.; Liu, Y. One-step solution-phase synthesis of a novel RGO-Cu2O-TiO2 ternary nanocomposite with excellent cycling stability for supercapacitors. J. Alloys Compd. 2013, 581, 303–307. [Google Scholar] [CrossRef]
- Sharma, J.K.; Akhtar, M.S.; Ameen, S.; Srivastava, P.; Singh, G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compd. 2015, 632, 321–325. [Google Scholar] [CrossRef]
- Soundaram, N.; Chandramohan, R.; Valanarasu, S.; Thomas, R.; Kathalingam, A. Studies on SILAR deposited Cu2O and ZnO films for solar cell applications. J. Mater. Sci. Mater. Electron. 2015, 26, 5030–5036. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.W.; Kim, J.H.; Lee, J.H.; Lee, J.S.; Kim, S.S. Importance of the nanograin size on the H2S-sensing properties of ZnO-CuO composite nanofibers. Sens. Actuators B 2015, 214, 111–116. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly monodisperse Cu2O and CuO nanospheres: Preparation and applications for sensitive gas sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Zhang, S.; Wang, W.; Chen, Z. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles decorated carbon spheres. Sens. Actuators B 2015, 211, 385–391. [Google Scholar] [CrossRef]
- Gao, P.; Liu, D. Petal-like CuO nanostructures prepared by a simple wet chemical method, and their application to non-enzymatic amperometric determination of hydrogen peroxide. Microchim. Acta 2015, 182, 1231–1239. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, Y.; Zhang, Y.; Weng, W.; Li, S. Carbon quantum dots/octahedral Cu2O nanocomposites for non-enzymatic glucose and hydrogen peroxide amperometric sensor. Sens. Actuators B 2015, 206, 735–743. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Yin, Z.; Song, Q. One-step self-assembled synthesis of CuO with tunable hierarchical structures and their electrocatalytic properties for nitrite oxidation in aqueous media. J. Colloid Interface Sci. 2013, 396, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, H.; Wang, R.; Wang, H.; Lv, W.; Ji, S. Ultrathin willow-like CuO nanoflakes as an efficient catalyst for electro-oxidation of hydrazine. J. Power Sources 2015, 289, 22–25. [Google Scholar]
- Liu, X.; Cui, S.; Sun, Z.; Du, P. Copper oxide nanomaterials synthesized from simple copper salts as active catalysts for electrocatalytic water oxidation. Electrochim. Acta 2015, 160, 202–208. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W.; Chen, X. Highly enhanced electrocatalytic oxidation of glucose on Cu(OH)2/CuO nanotube arrays modified copper electrode. J. Solid State Electrochem. 2012, 16, 2877–2881. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Huang, X.; Wang, H.; Zheng, Y.; Lin, L.; Wang, S.; Chen, S.; Jin, Y. Cupric oxide nanowires assembled by nanoparticles in situ with enhancing electrocatalytic oxidation of ascorbic acid. Appl. Surf. Sci. 2014, 292, 291–296. [Google Scholar] [CrossRef]
- Yan, X.Y.; Tong, X.L.; Zhang, Y.F.; Han, X.D.; Wang, Y.Y.; Jin, G.Q.; Qin, Y.; Guo, X.Y. Cuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chem. Commun. 2012, 48, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Felix, S.; Kollu, P.; Raghupathy, B.P.C.; Jeong, S.K.; Grace, A.N. Electrocatalytic activity of Cu2O nanocubes based electrode for glucose oxidation. J. Chem. Sci. 2014, 126, 25–32. [Google Scholar] [CrossRef]
- Rostami, H.; Rostami, A.A.; Omrani, A. An electrochemical method to prepare of Pd/Cu2O/MWCNT nanostructure as an anode electrocatalyst for alkaline direct ethanol fuel cells. Electrochim. Acta 2016, 194, 431–440. [Google Scholar] [CrossRef]
- Cheng, W.H. Reaction and XRD studies on Cu based methanol decomposition catalysts: Role of constituents and development of high-activity multicomponent catalysts. Appl. Catal. A 1995, 130, 13–30. [Google Scholar] [CrossRef]
- Sieben, J.M.; Alvarez, A.E.; Comignani, V.; Duarte, M.M.E. Methanol and ethanol oxidation on carbon supported nanostructured Cu core Pt–Pd shell electrocatalysts synthesized via redox displacement. Int. J. Hydrogen Energy 2014, 39, 11547–11556. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, J.Y.; Chen, W.; Han, M.; Gan, L.M. Physical and electrochemical characterizations of microwave-assisted polyol preparation of carbon-supported PtRu nanoparticles. Langmuir 2004, 20, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Y.; Wu, H.; Chen, W. Nano-PtPd cubes on graphene exhibit enhanced activity and durability in methanol electrooxidation after CO stripping-cleaning. J. Phys. Chem. C 2013, 117, 2926–2938. [Google Scholar] [CrossRef]
- Wen, W.; Li, C.; Li, W.; Tian, Y. Carbon-supported Pd–Cr electrocatalysts for the electrooxidation of formic acid that demonstrate high activity and stability. Electrochim. Acta 2013, 109, 201–206. [Google Scholar] [CrossRef]
- Kim, K.S.; Gossmann, A.F.; Winograd, N. X-ray photoelectron spectroscopic studies of palladium oxides and the palladium-oxygen electrode. Anal. Chem. 1974, 46, 197–200. [Google Scholar] [CrossRef]
- Ding, K.; Li, Y.; Zhao, Y.; Liu, L.; Gu, H.; Liu, L.; Qiu, S.; He, C.; Liu, J.; Wang, Q.; et al. Dry-grinding synthesized multi-walled carbon nanotubes supported PdO catalyst for ethanol oxidation reaction. Electrochim. Acta 2014, 149, 186–192. [Google Scholar] [CrossRef]
- Mao, H.; Wang, L.; Zhu, P.; Xu, Q.; Li, Q. Carbon-supported PdSn–SnO2 catalyst for ethanol electro-oxidation in alkaline media. Int. J. Hydrogen Energy 2014, 39, 17583–17588. [Google Scholar] [CrossRef]
- Du, C.; Chen, M.; Wang, W.; Yin, G. Nanoporous PdNi alloy nanowires as highly active catalysts for the electro-oxidation of formic acid. ACS Appl. Mater. Interfaces 2010, 3, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wan, L.; Ma, Y.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Crystalline palladium-cobalt alloy nanoassemblies with enhanced activity and stability for the formic acid oxidation reaction. Appl. Catal. B 2013, 138, 229–235. [Google Scholar] [CrossRef]
- Yu, B.; Wen, W.; Li, W.; Yang, Y.; Hou, D.; Liu, C. Fabrication of high performance carbon-supported ternary Pd-Cu-Fe electrocatalysts for formic acid electrooxidation via partly galvanic sacrifice of tunable binary Cu-Fe alloy templates. Electrochim. Acta 2016, 196, 223–230. [Google Scholar] [CrossRef]
- Yang, G.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Preparation of carbon supported Pd-P catalyst with high content of element phosphorus and its electrocatalytic performance for formic acid oxidation. Electrochem. Commun. 2010, 12, 492–495. [Google Scholar] [CrossRef]
- Wakisaka, M.; Mitsui, S.; Hirose, Y.; Kawashima, K.; Uchida, H.; Watanabe, M. Electronic structures of Pt-Co and Pt-Ru alloys for CO-tolerant anode catalysts in polymer electrolyte fuel cells studied by EC-XPS. J. Phys. Chem. B 2006, 110, 23489–23496. [Google Scholar] [CrossRef] [PubMed]
- Paixão, T.R.L.C.; Bertotti, M. Development of a breath alcohol sensor using a copper electrode in an alkaline medium. J. Electroanal. Chem. 2004, 571, 101–109. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Scott, K. Performance of carbon nanofiber supported Pd-Ni catalysts for electro-oxidation of ethanol in alkaline medium. J. Power Sources 2010, 195, 5246–5251. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Elaboration of Cu-Pd films by coelectrodeposition: Application to nitrate electroreduction. J. Phys. Chem. C 2008, 113, 290–297. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Qin, Y.H.; Yang, H.H.; Zhang, X.S.; Li, P.; Ma, C.A. Effect of carbon nanofibers microstructure on electrocatalytic activities of Pd electrocatalysts for ethanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2010, 35, 7667–7674. [Google Scholar] [CrossRef]
- Li, W.; Fan, F.R.F.; Bard, A.J. The application of scanning electrochemical microscopy to the discovery of Pd-W electrocatalysts for the oxygen reduction reaction that demonstrate high activity, stability, and methanol tolerance. J. Solid State Electrochem. 2012, 16, 2563–2568. [Google Scholar] [CrossRef]

| Catalysts | Negative Shift | Morphology | Support | Ref. |
|---|---|---|---|---|
| Pd-on-Cu 1 | 35 mV | Pd-on-Cu nanocrystals | reduced graphene oxide | [4] |
| Pd-Cu | - | ultrafine nanoporous Cu-Pd alloys | carbon | [29] |
| Pd-Cu 2 | 15 mV | Pd-Cu alloy nanoparticles | carbon | [31] |
| Pd/Cu 2 | 138 mV | bimetallic nanoparticles | graphene nanosheets | [39] |
| Pd-Cu | - | Pd-Cu nanoparticles | reduced graphene oxide | [40] |
| Pd-Cu 2 | 55 mV | Pd-Cu nanoparticles | carbon | [41] |
| Pd-Cu 2 | 63 mV | PdxCu100−x networks | no support | [42] |
| Pd-Cu 2 | 79 mV | surface palladium rich CuxPdy | carbon | [43] |
| Pd-Cu 3 | 38 mV | Pd-Cu alloying | carbon nanotubes | [44] |
| Pd-Cu 2 | 30 mV | 3D nanochain network Pd-Cu alloy | no support | [45] |
| Pd-Cu 2 | 88 mV | monodisperse Pd-Cu bimetallic nanocrystals | no support | [46] |
| Electrode | Pd:Cu | Pd:Cu:O | Metal Pd:Cu | Pd(0):Pd(II) | Cu(0):Cu(I):Cu(II) |
|---|---|---|---|---|---|
| Pd/C | Pd | Pd0.75:O0.25 | Pd | Pd(0)0.66:Pd(II)0.34 | - |
| Pd0.9Cu0.1/C | Pd0.92:Cu0.08 | Pd0.68:Cu0.06:O0.26 | Pd0.96:Cu0.04 | Pd(0)0.63:Pd(II)0.37 | Cu(0)0.26:Cu(I)0.49:Cu(II)0.25 |
| Electrode | Onset Potential (V) | 1.0 M KOH + 1.0 M C2H5OH | Pd Cu Atomic Ratios | ||
|---|---|---|---|---|---|
| - | - | If (mA·mg−1Pd) | Ib (mA·mg−1Pd) | If/Ib | by ICP-AES |
| Pd/C | −552.5 mV | 114.51 | 287.74 | 0.40 | Pd |
| Pd0.95Cu0.05/C | −621.2 mV | 230.93 | - | - | Pd0.94Cu0.06 |
| Pd0.9Cu0.1/C | −704.8 mV | 229.61 | 428.3 | 0.54 | Pd0.90Cu0.10 |
| Pd0.8Cu0.2/C | −618.7 mV | 164.94 | - | - | Pd0.81Cu0.19 |
| Pd0.7Cu0.3/C | −600.7 mV | 77.93 | - | - | Pd0.71Cu0.29 |
| Pd0.6Cu0.4/C | −586.9 mV | 55.85 | - | - | Pd0.61Cu0.39 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Liu, T.; Li, W.; Zhang, C.; Zhang, D.; Pang, Z. Carbon Supported Oxide-Rich Pd-Cu Bimetallic Electrocatalysts for Ethanol Electrooxidation in Alkaline Media Enhanced by Cu/CuOx. Catalysts 2016, 6, 62. https://doi.org/10.3390/catal6050062
Guo Z, Liu T, Li W, Zhang C, Zhang D, Pang Z. Carbon Supported Oxide-Rich Pd-Cu Bimetallic Electrocatalysts for Ethanol Electrooxidation in Alkaline Media Enhanced by Cu/CuOx. Catalysts. 2016; 6(5):62. https://doi.org/10.3390/catal6050062
Chicago/Turabian StyleGuo, Zengfeng, Tengfei Liu, Wenpeng Li, Cai Zhang, Dong Zhang, and Zongjie Pang. 2016. "Carbon Supported Oxide-Rich Pd-Cu Bimetallic Electrocatalysts for Ethanol Electrooxidation in Alkaline Media Enhanced by Cu/CuOx" Catalysts 6, no. 5: 62. https://doi.org/10.3390/catal6050062
APA StyleGuo, Z., Liu, T., Li, W., Zhang, C., Zhang, D., & Pang, Z. (2016). Carbon Supported Oxide-Rich Pd-Cu Bimetallic Electrocatalysts for Ethanol Electrooxidation in Alkaline Media Enhanced by Cu/CuOx. Catalysts, 6(5), 62. https://doi.org/10.3390/catal6050062