Low-Temperature Catalytic Performance of Ni-Cu/Al2O3 Catalysts for Gasoline Reforming to Produce Hydrogen Applied in Spark Ignition Engines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural, Structural, and Morphological Characterizations
2.1.1. Textural Characterizations
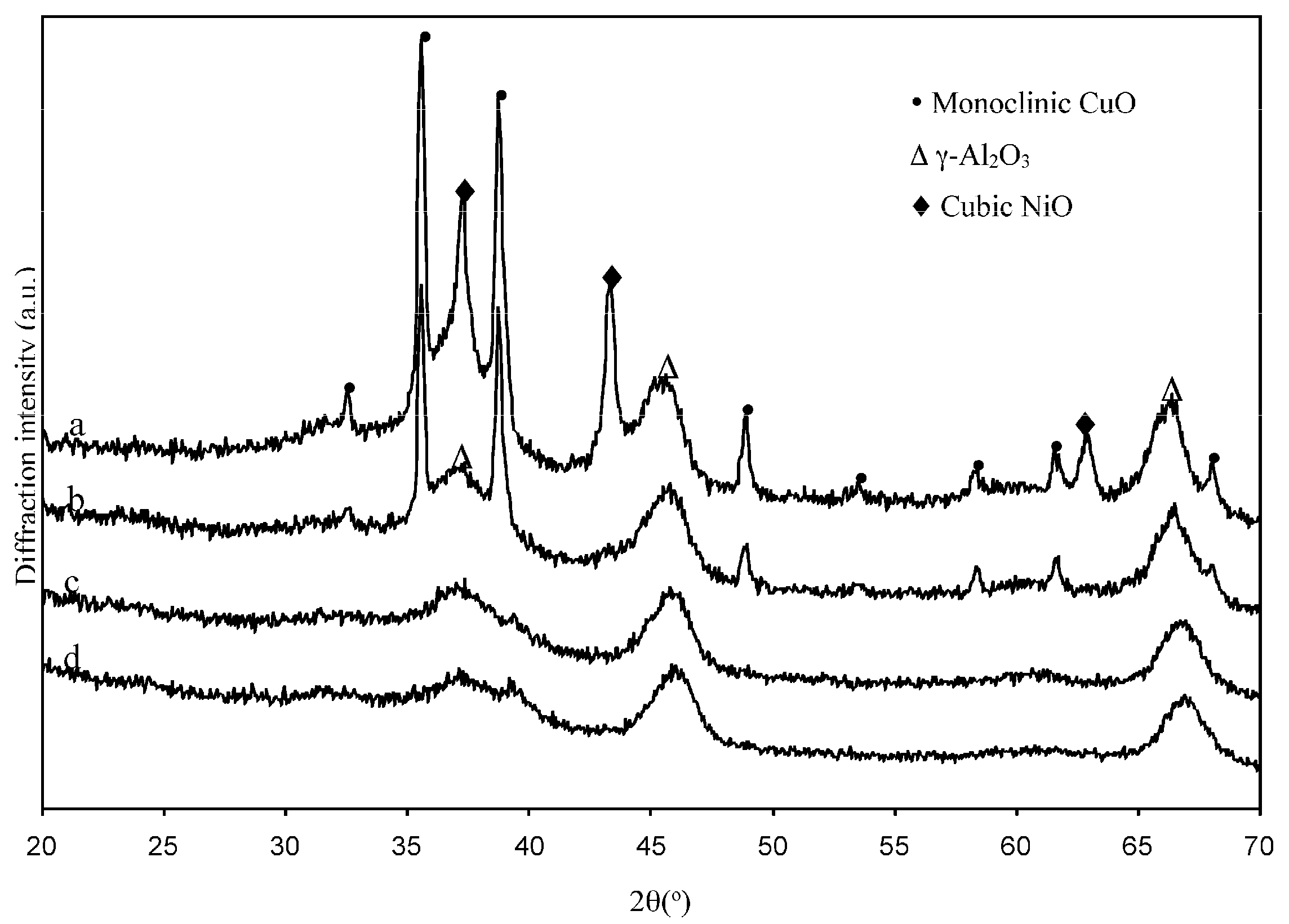
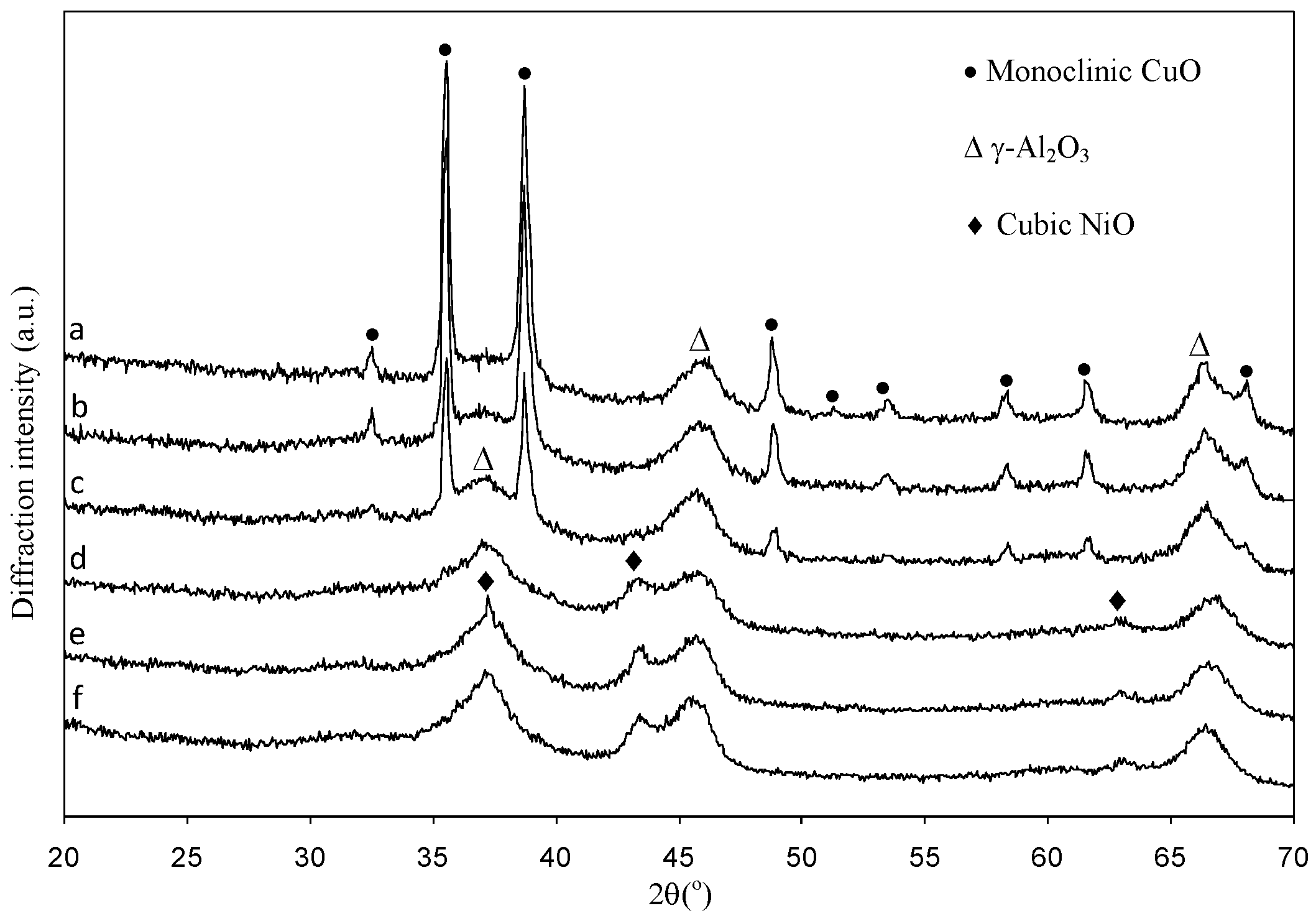
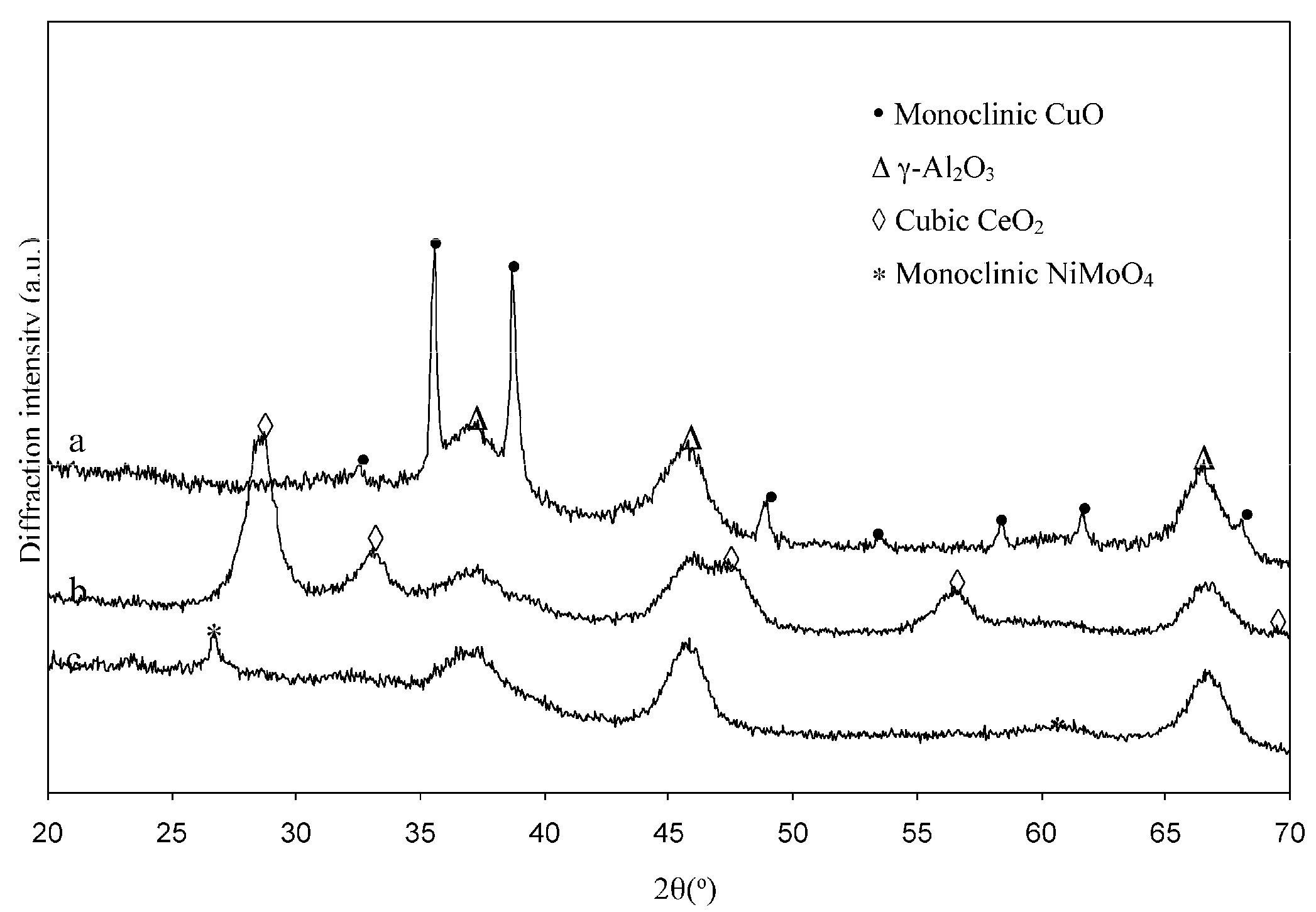
2.1.2. Structural Characterizations
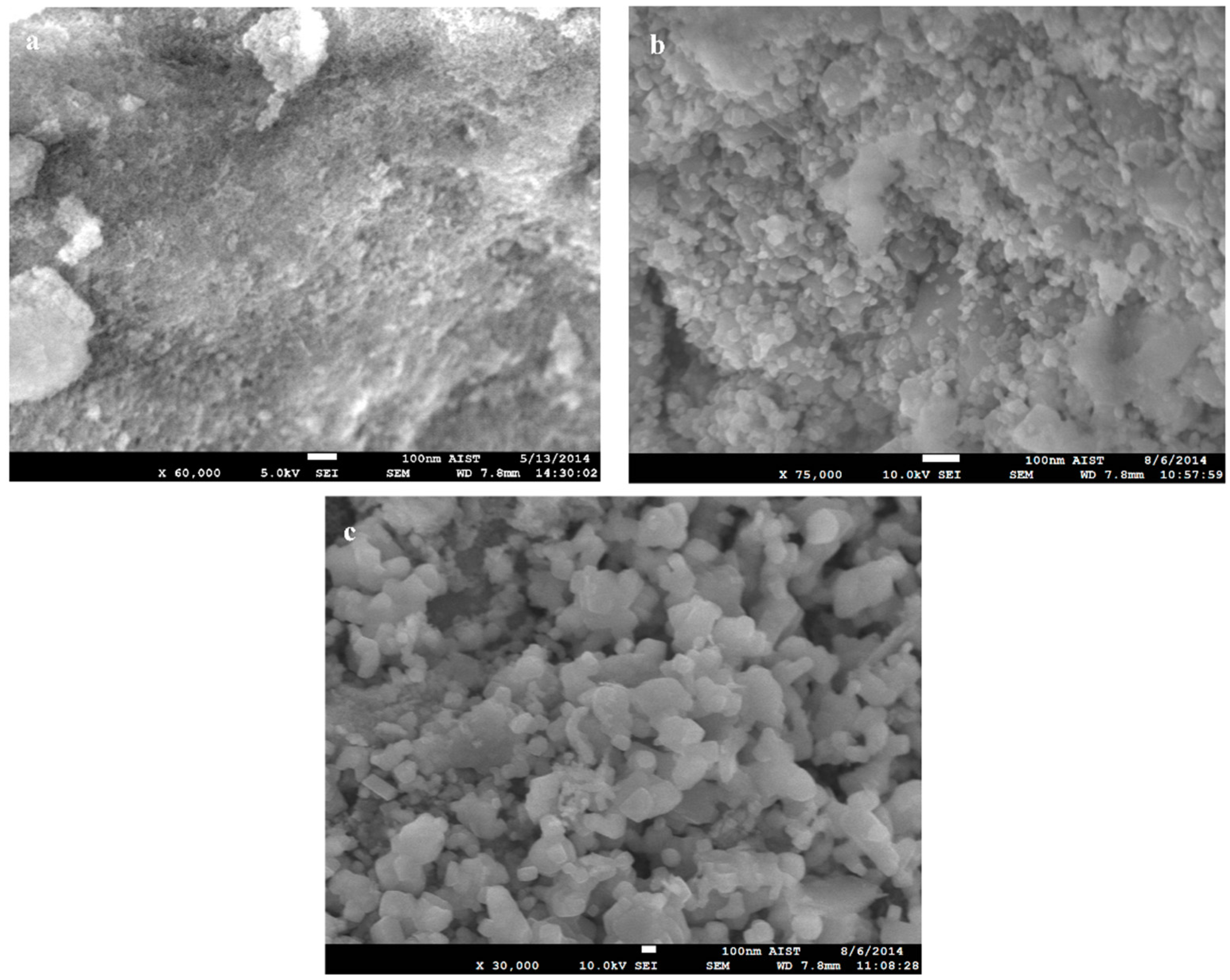
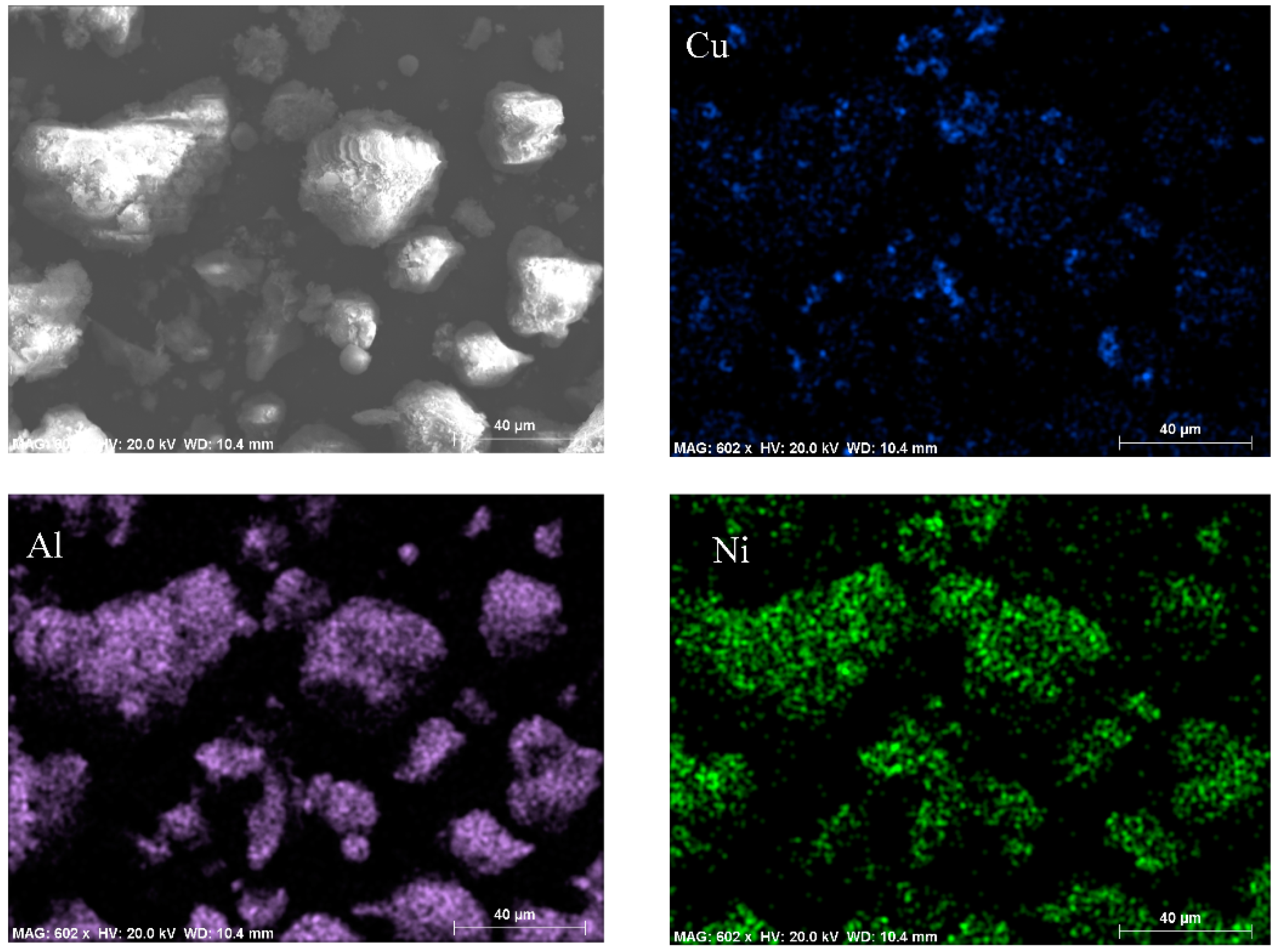
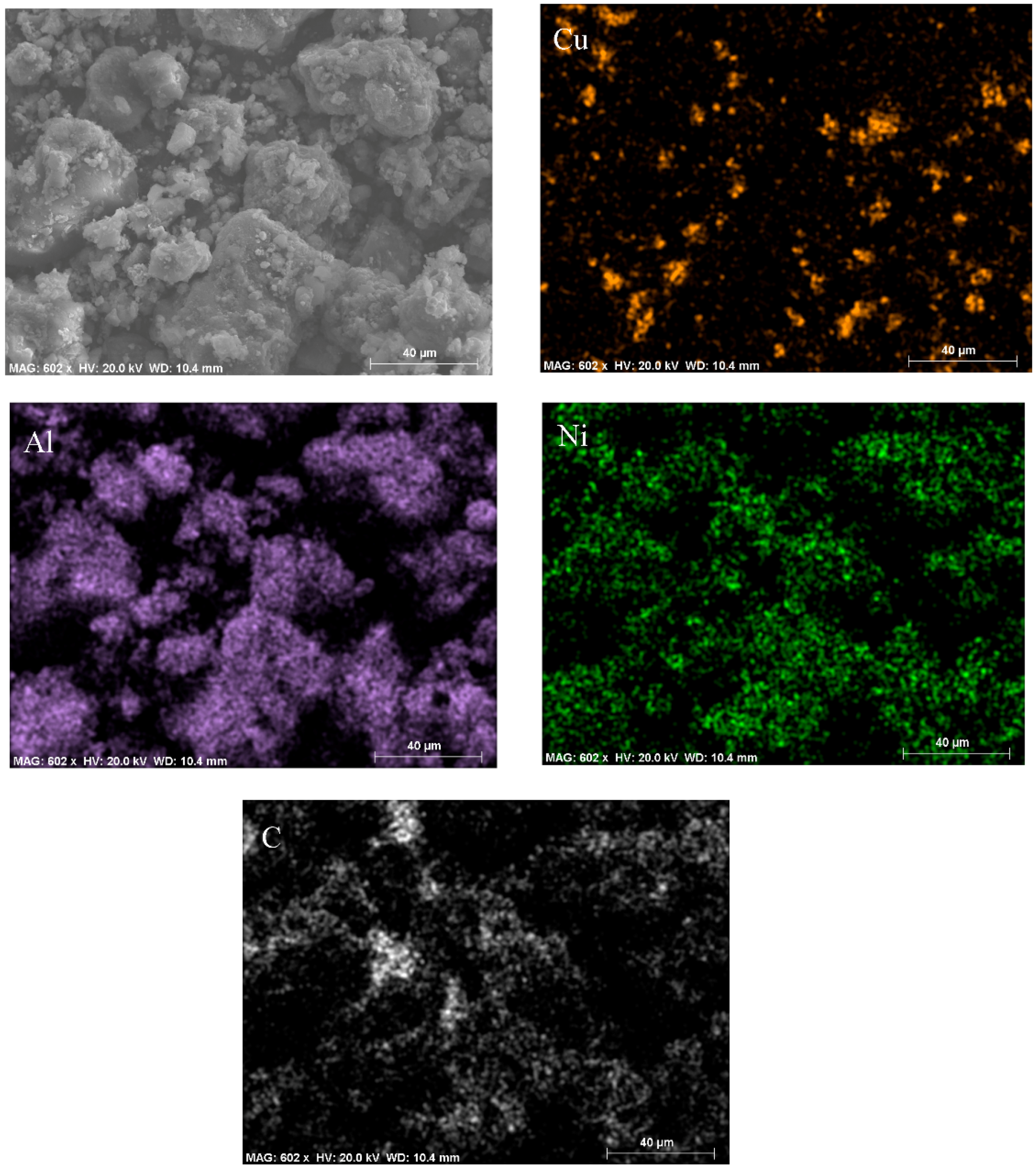
2.1.3. Morphology Characterizations
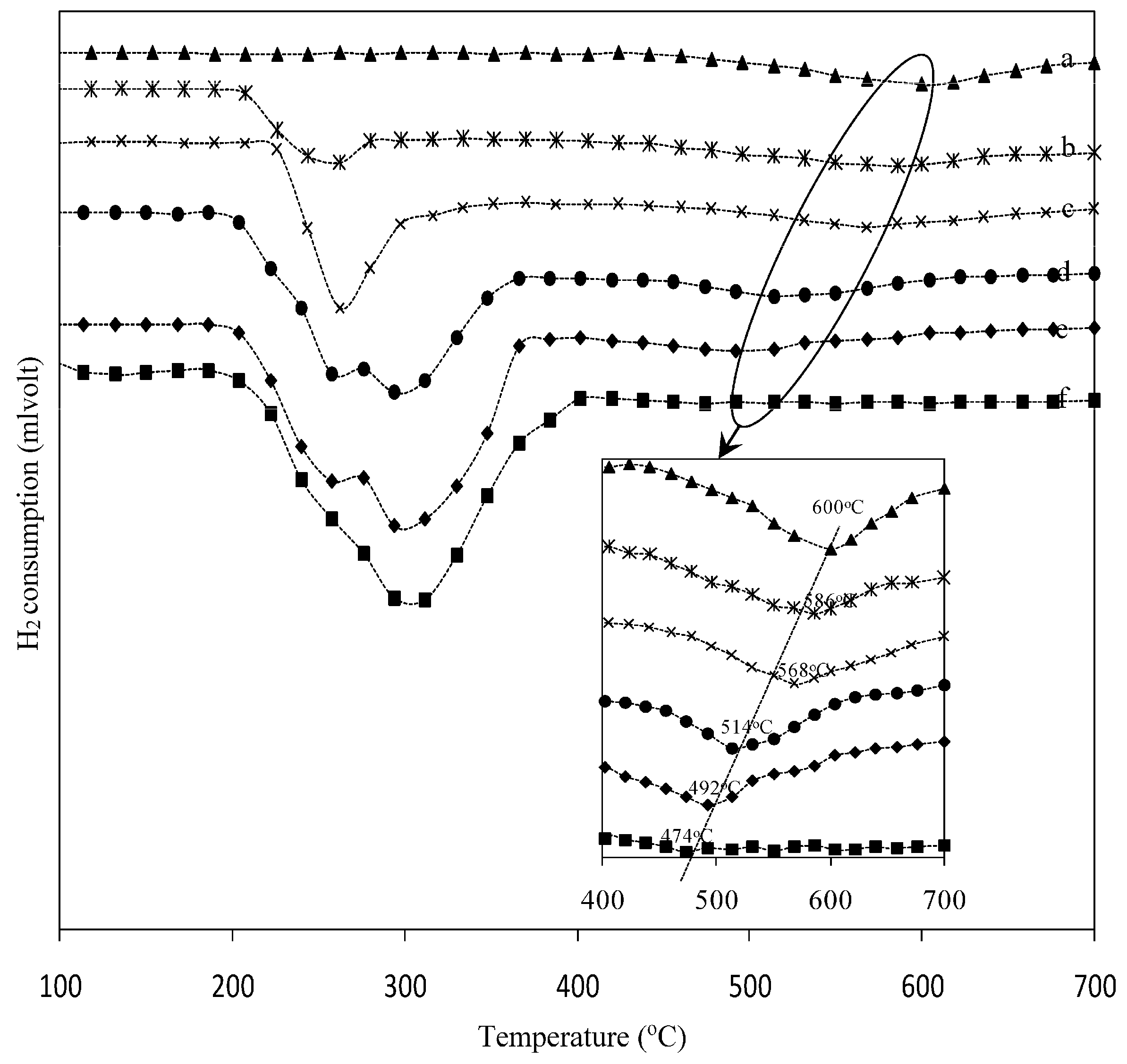
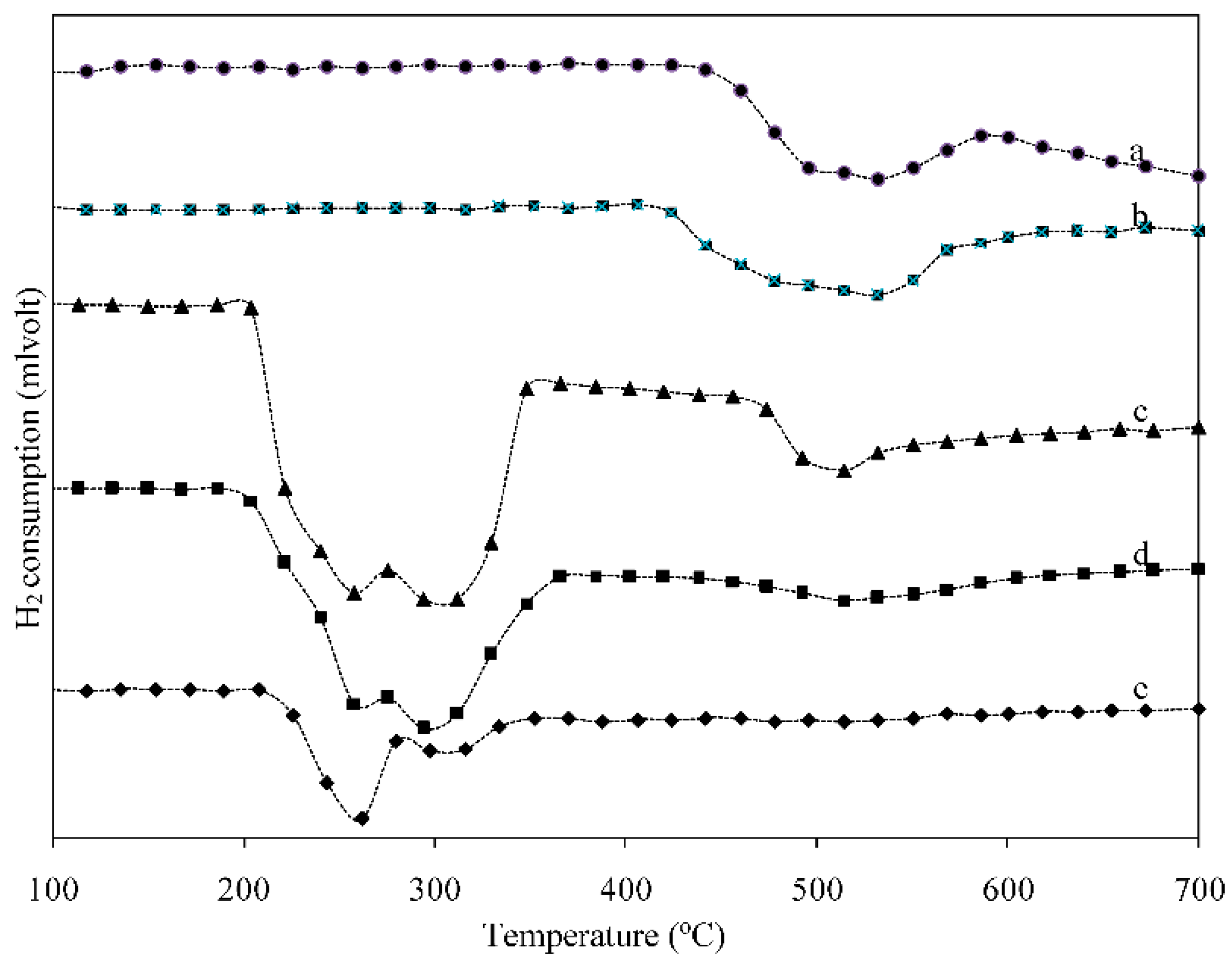
2.2. Temperature Program Reduction (TPR)
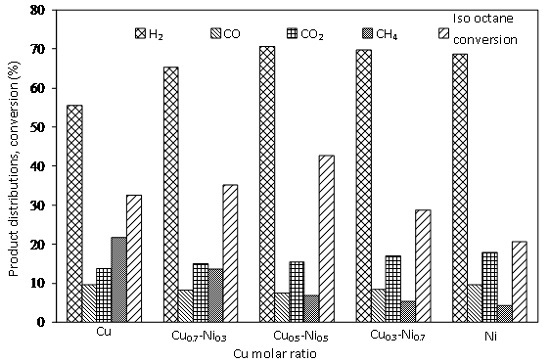
2.3. Catalytic Activity
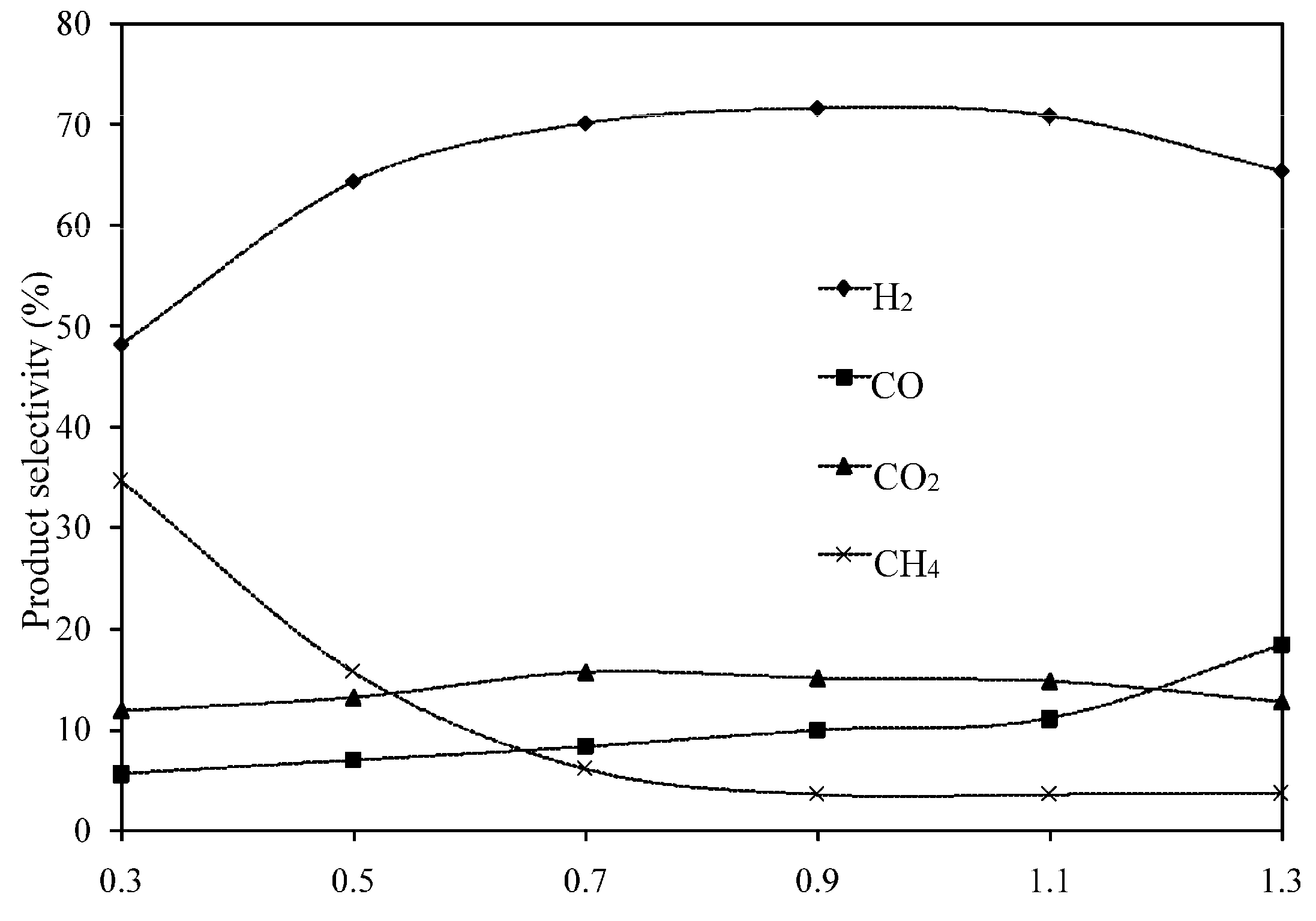
2.3.1. Effect of Steam/Carbon Molar Ratios
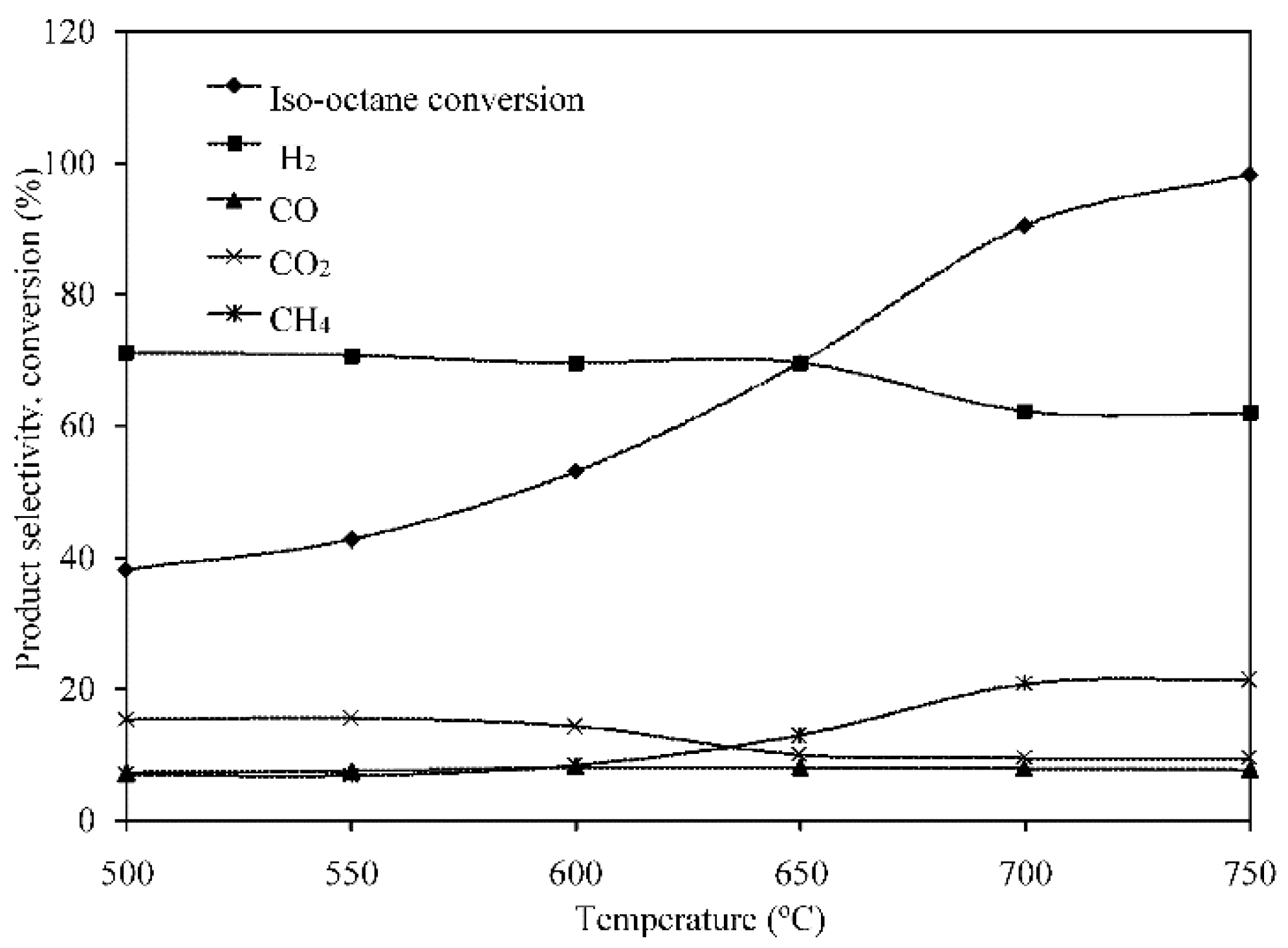
2.3.2. Effect of Temperature on Reformation Composition
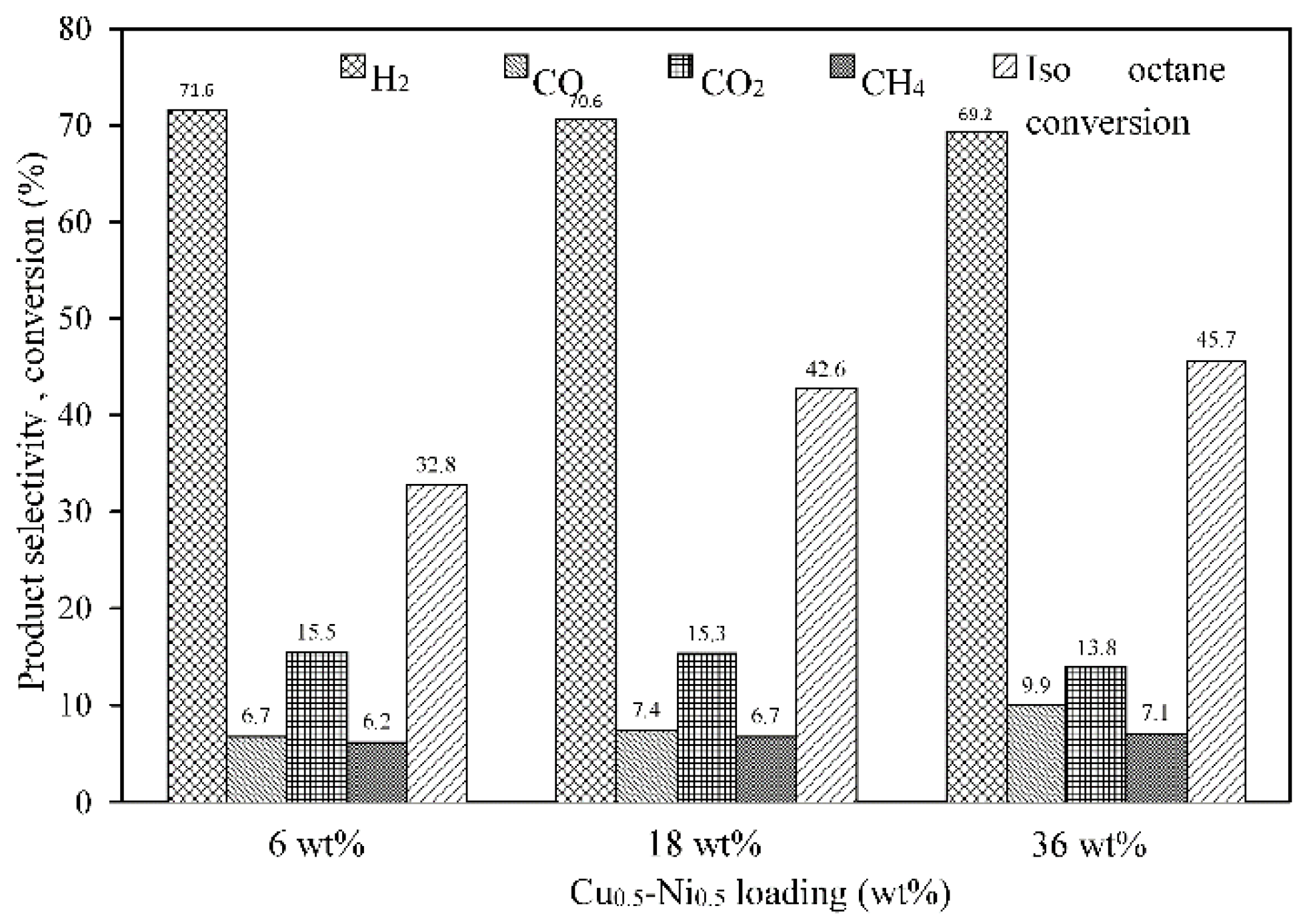
2.3.3. Effect of Ni-Cu Loading on γ-Al2O3 Support
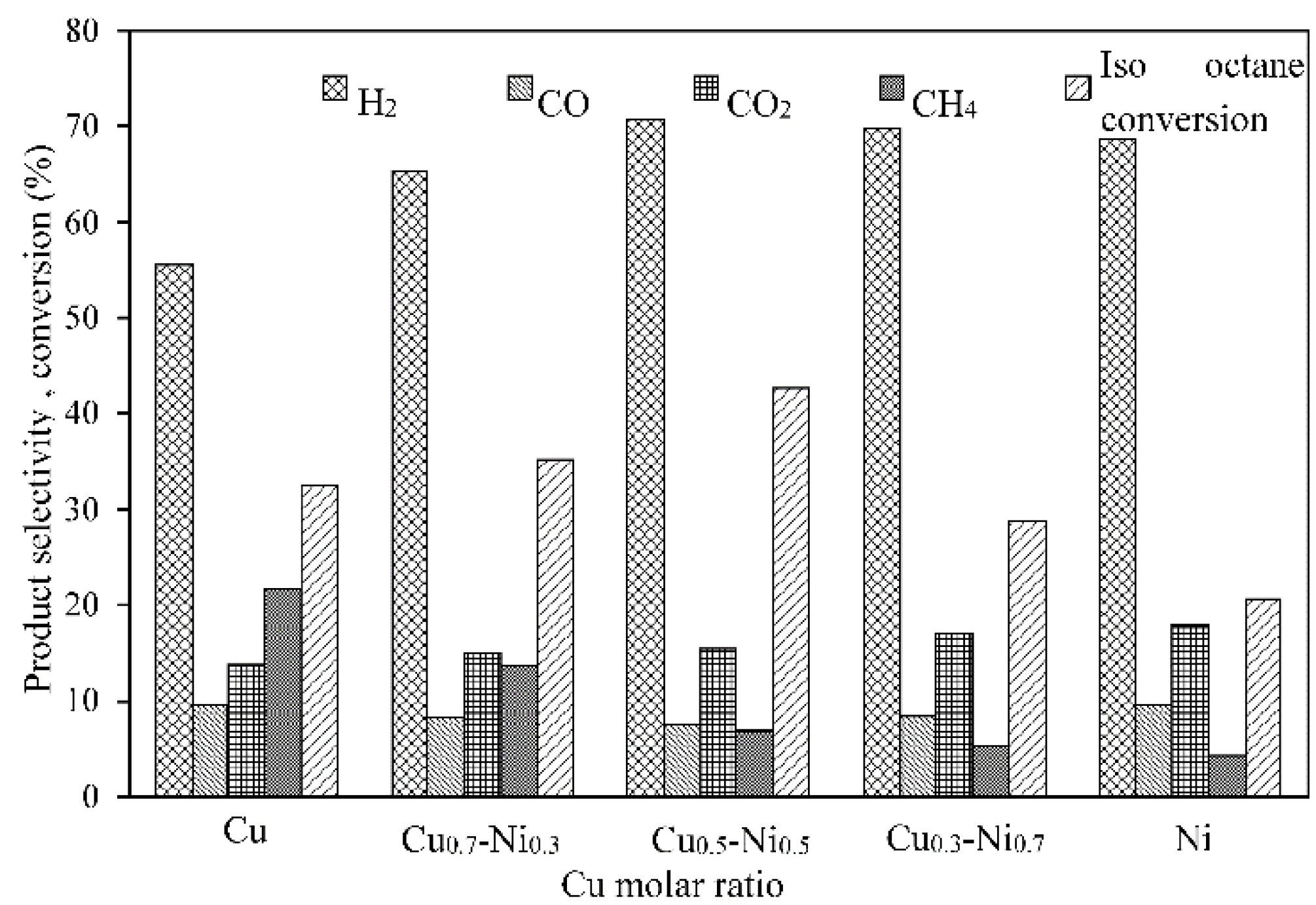
2.3.4. Effect on Catalytic Performance
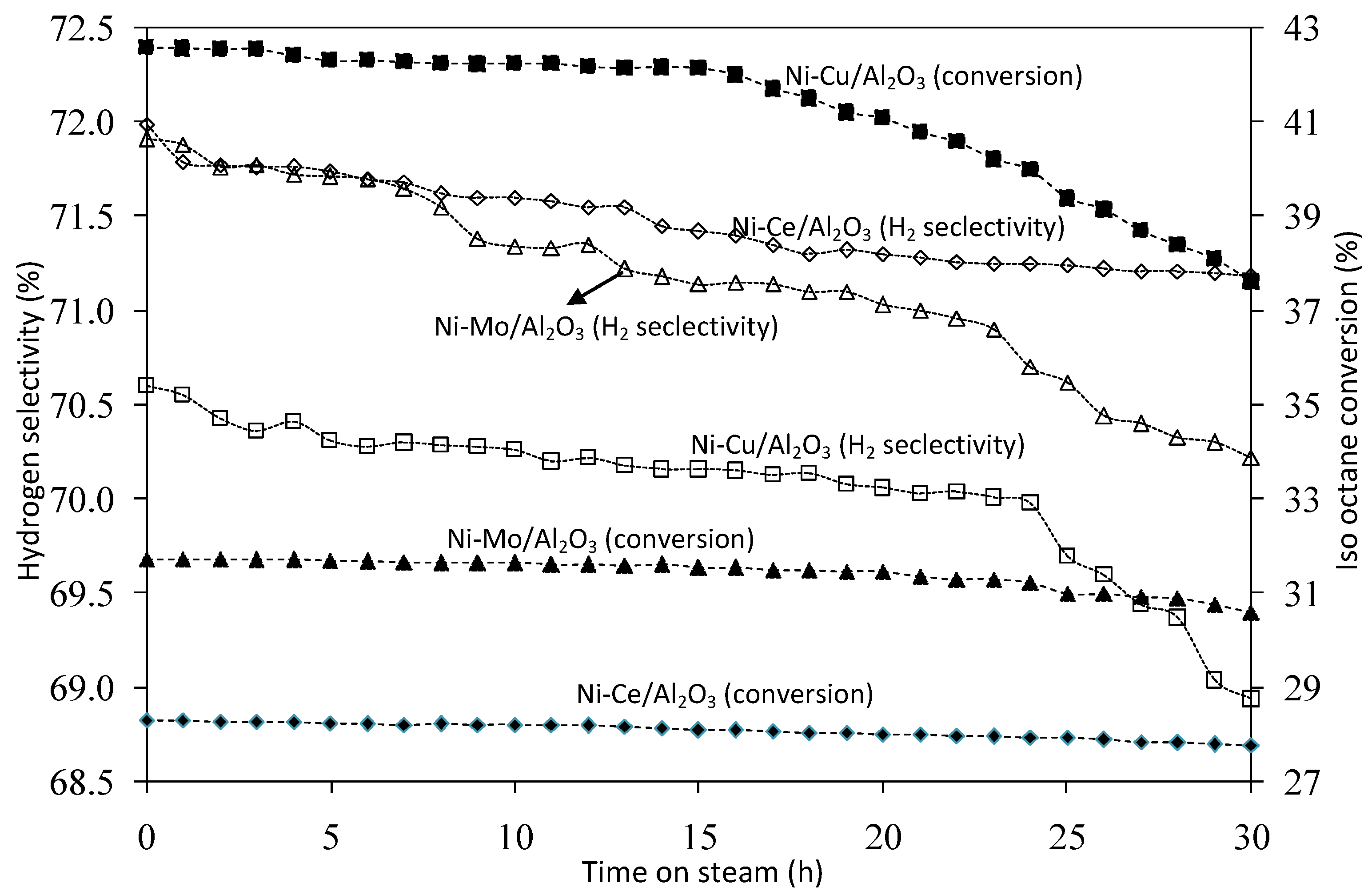
2.3.5. Stability Testing
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, R.O.; Hayes, S.V.; Allan, A.B.; Anderson, R.W.P.; Walker, E.J. The hydrogen engine: Combustion knock and the related Dame velocity. Trans. Eng. Inst. Canada 1958, 2, 143–148. [Google Scholar] [CrossRef]
- Tsolakis, A.; Megaritis, A.; Wyszynski, M.L. Application of exhaust gas fuel reforming in compression ignition engines fuelled by diesel and biodiesel fuel mixtures. Energy Fuels 2003, 17, 1464–1473. [Google Scholar] [CrossRef]
- Jamal, Y.; Wyszynski, M.L. On-board generation of hydrogen rich fuels—A review. Int. J. Hydrogen Energy 1994, 19, 557–572. [Google Scholar] [CrossRef]
- Allenby, S.; Chang, W.C.; Megaritis, A.; Wyszynski, M.L. Hydrogen enrichment: A way to maintain combustion stability in a natural gas fuelled engine with exhaust gas recirculation, the potential of fuel reforming. Proc. Inst. Mech. Eng. 2001, 215, 405–417. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ramesh, A.; Nagalingam, B. Use of hydrogen to enhance the performance of a vegetable oil fuelled compression ignition engine. Int. J. Hydrogen Energy 2003, 28, 1143–1154. [Google Scholar]
- Ji, C.; Wang, S. Effect of hydrogen addition on combustion and emissions performance of a spark ignition gasoline engine at lean conditions. Int. J. Hydrogen Energy 2009, 34, 7823–7834. [Google Scholar] [CrossRef]
- Le Anh, T.; Nguyen Duc, K.; Tran Thi Thu, H.; Cao Van, T. Improving Performance and Reducing Pollution Emissions of a Carburetor Gasoline Engine by Adding HHO Gas into the Intake Manifold; SAE Technical Paper 2013-01-0104; SAE International: Bangkok, Thailand, 2013. [Google Scholar]
- Bade Shrestha, S.; LeBlanc, G.; Balan, G.; de Souza, M. A Before Treatment Method for Reduction of Emissions in Diesel Engines; SAE Technical Paper Series No. 2000-01-2791; SAE International: Paris, France, 2000. [Google Scholar]
- Sogaard, C.; Schramm, J.; Jensen, T.K. Reduction of UHC Emissions from Natural Gas Fired SI-Engine-Production and Application of Steam Reforming Natural Gas; SAE Technical Paper Series No. 2000-01-2823; SAE International: Paris, France, 2000. [Google Scholar]
- Kirwan, J.E.; Quader, A.A.; Grieve, M.J. Advanced Engine Management Using On-Board Gasoline Partial Oxidation Reforming for Meeting Super-ULEV (SULEV) Emissions Standards; SAE Technical Paper Series No. 1999-01-2927; SAE International: Costa Mesa, CA, USA, 1999. [Google Scholar]
- Tsolakis, A.; Megaritis, A.; Wyszynski, M.L. Effects of reformed EGR on the diesel engine smoke-NOx emissions trade-off. In Proceedings of the 9th Research Symposium 2003, School of Engineering, The University of Birmingham, Birmingham, UK, 2003.
- Kratzel, T.; Pantow, E.; Eichert, H. Modeling of hydrogen combustion: Turbulent Jame acceleration and detonation. Int. J. Hydrogen Energy 1996, 21, 407–414. [Google Scholar] [CrossRef]
- Vezirogcu, T.N. Hydrogen movement and the next action: Fossil fuel industry and sustainability economics. Int. J. Hydrogen Energy 1997, 22, 551–556. [Google Scholar] [CrossRef]
- Vandenborre, H.; Sierens, R. Greenbus: A hydrogen fueled city bus. Int. J. Hydrogen Energy 1996, 21, 521–524. [Google Scholar] [CrossRef]
- Ladommatos, N.; Abdelhalim, S.M.; Zhao, H.; Hu, Z. Effects of EGR on Heat Release in Diesel Combustion; SAE Technical Paper Series No. 980184; SAE International: Detroit, MI, USA, 1998. [Google Scholar]
- Krumplet, M.; Krause, T.R.; Carter, J.D.; Kopasz, J.P.; Ahmed, S. Fuel Processing for Fuel Cell Systems in Transportation and Portable Power Applications. Catal. Today 2002, 77, 3–16. [Google Scholar] [CrossRef]
- Cunha, A.F.; Wu, Y.-J.; Santos, J.C.; Rodrigues, A.E. Sorption Enhanced Steam Reforming of ethanol on Hydrotalcite-like compounds impregnated with active copper. Chem. Eng. Res. Des. 2013, 91, 581–592. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M. Catalytic combustion of butane on Ru/γ-Al2O3 catalysts. Appl. Catal. B 2009, 89, 22–32. [Google Scholar] [CrossRef]
- Cunha, A.F.; Wu, Y.J.; Santos, J.C.; Rodrigues, A.E. Steam reforming of ethanol on copper catalysts derived from hydrotalcite-like materials. Ind. Eng. Chem. Res. 2012, 51, 13132–13143. [Google Scholar] [CrossRef]
- Praveen, K.; Cheekatamarla, C.M. Finnerty, Reforming catalysts for hydrogen generation in fuel cell applications. J. Power Sources 2006, 160, 490–499. [Google Scholar]
- Wang, L.; Murata, K.; Inaba, M. Control of the product ratio of CO2/(CO+CO2) and inhibition of catalyst deactivation for steam reforming of gasoline to produce hydrogen. Appl. Catal. B 2004, 48, 243–248. [Google Scholar] [CrossRef]
- Wang, L.; Murata, K.; Inaba, M. Steam reforming of gasoline promoted by partial oxidation reaction on novel bimetallic Ni-based catalysts to generate hydrogen for fuel cell-powered automobile applications. J. Power Sources 2005, 145, 707–711. [Google Scholar] [CrossRef]
- Wang, L.; Murata, K.; Matsumura, Y.; Inaba, M. Lower-Temperature Catalytic Performance of Bimetallic Ni-Re/Al2O3 Catalyst for Gasoline Reforming to Produce Hydrogen with the Inhibition of Methane Formation. Energy Fuels 2006, 20, 1377–1381. [Google Scholar] [CrossRef]
- Wang, L.; Murata1, K.; Inaba, M. Development of novel highly active and sulphur-tolerant catalysts for steam reforming of liquid hydrocarbons to produce hydrogen. Appl. Catal. A 2004, 257, 43–47. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Maa, R.; Wu, D. Characterization of alumina-supported Ni and Ni-Pd catalysts for partial oxidation and steam reforming of hydrocarbons. Appl. Catal. A: 2003, 243, 251–259. [Google Scholar] [CrossRef]
- Trimm, D.L.; Adesina, A.A.; Cant, N.W. The conversion of gasoline to hydrogen for on-board vehicle applications. Catal. Today 2004, 17, 93–95. [Google Scholar] [CrossRef]
- Navarro, R.M.; Álvarez-Galván, M.C.A.; Rosa, F.; Fierro, J.L.G. Hydrogen production by oxidative reforming of hexadecane over Ni and Pt catalysts supported on Ce/La-doped Al2O3. Appl. Catal. A 2006, 297, 60–72. [Google Scholar] [CrossRef]
- Wang, X.; Pan, X.; Lin, R.; Kou, S.; Zou, W.; Ma, J.X. Steam reforming of dimethyl ether over Cu–Ni/γ-Al2O3 bi-functional catalyst prepared by deposition–precipitation method. Int. J. Hydrogen Energy 2010, 35, 4060–4068. [Google Scholar] [CrossRef]
- Vizcaino, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts. Int. J. Hydrogen Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, W.Y. Effect of second metals and Cu content on catalyst performance of Ni-Cu/SiO2 in the hydrodechlorination of 1,1,2-trichloroethane into vinyl chloride monomer. J. Mol. Catal. A 2001, 174, 193–204. [Google Scholar] [CrossRef]
- Huang, T.; Yu, T.; Jhao, S. Weighting Variation of Water-Gas Shift in Steam Reforming of Methane over Supported Ni and Ni-Cu Catalysts. Ind. Eng. Chem. Res. 2006, 45, 150–156. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Flytzani-Stephanopoulos, M. Low-temperature water-gas shift reaction over Cu- and Ni-loaded cerium oxide catalysts. Appl. Catal. B 2000, 27, 179–191. [Google Scholar] [CrossRef]
- Cunha, A.F.; Wu, Y.J.; Alvarado, F.A.D.; Santos, J.C.; Vaidya, P.D.; Rodrigues, A.E. Steam reforming of ethanol on a Ni/Al2O3 catalyst coupled with a hydrotalcite-like sorbent in a multilayer pattern for CO2 uptake. Can. J. Chem. Eng. 2012, 90, 1514–1526. [Google Scholar] [CrossRef]
- Brito, J.; Laine, J.; Pratt, K.C. Temperature programmed reduction of Ni-Mo oxides. J. Mater. Sci. 1989, 24, 425–431. [Google Scholar] [CrossRef]
- Cordeo, R.L.; Agudo, A.L. Effect of water extraction on the surface properties of Mo/Al2O3 and NiMo/Al2O3 hydrotreating catalysts. Appl. Catal. A 2000, 202, 23–35. [Google Scholar] [CrossRef]
- Hou, Z.Y.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/γ-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A 2003. [Google Scholar] [CrossRef]
- Hu, C.W.; Yao, J.; Yang, H.Q.; Chen, Y.; Tian, A.M. On the inhomogeneity of low nickel loading methanation catalyst. J. Catal. 1997, 166, 1–7. [Google Scholar] [CrossRef]
- Rynkowski, J.M.; Paryjczak, T.; Lenik, M. On the nature of oxidic nickel phases in NiO/γ-Al2O3 catalysts. Appl. Catal. A 1993, 106, 73–82. [Google Scholar] [CrossRef]
- Fierro, G.; Jacono, M.L.; Inversi, M.; Porta, P.; Lavecchia, R.; Cioci, F. A Study of Anomalous Temperature-Programmed Reduction Profiles of Cu2O, CuO, and CuO-ZnO Catalysts. J. Catal. 1994, 148, 709–721. [Google Scholar] [CrossRef]
- Luo, M.-F.; Ma, J.-M.; Lu, J.-Q.; Song, Y.-P.; Wang, Y.-J. High-surface area CuO-CeO2 catalysts prepared by a surfactant-templated method for low-temperature CO oxidation. J. Catal. 2007, 246, 52–59. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2: H Embedding and Kinetic Effects in the Formation of Suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef] [PubMed]
- Hierl, R.; Knozinger, H.; Urbach, H. Surface properties and reduction behavior of calcined CuO/Al2O3 and CuO-NiO/Al2O3 catalyst. J. Catal. 1981, 69, 475–486. [Google Scholar] [CrossRef]
- Gonzalez, O.; Lujano, J.; Pietri, E.; Goldwasser, M.R. New Co-Ni catalyst systems used for methane dry reforming based on supported catalysts over an INT-MM1 mesoporous material and a perovskite-like oxide precursor LaCo0.4Ni0.6O3. Catal. Today 2005, 107–108, 436–443. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Yu, J.; Wu, T.; Wang, G. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and sol-gel methods. Appl. Catal. A 2003, 239, 87–94. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, K.H.; Ihm, S.K. The characteristics of wet air oxidation of phenol over CuO/Al2O3 catalysts: Effect of copper loading. Chemosphere 2007, 68, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Ertl, G.; Knozinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; VCH: Weinheim, Germany, 1997; p. 274. [Google Scholar]
- Borowiecki, T.; Gac, W.; Denis, A. Effects of small MoO3 additions on the properties of nickel catalysts for the steam reforming of hydrocarbons III. Reduction of Ni-Mo/Al2O3 catalysts. Appl. Catal. A 2004, 270, 27–36. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.-S.; Jung, U.H.; Yoon, W.L. CeO2 Promoted Ni/Al2O3 Catalyst in Combined Steam and Carbon Dioxide Reforming of Methane for Gas to Liquid (GTL) Process. Catal. Lett. 2009, 130, 217. [Google Scholar] [CrossRef]
- Smolakova, L.; Kout, M.; Koudelkova, E.; Capek, L. Effect of Calcination Temperature on the Structure and Catalytic Performance of the Ni/Al2O3 and Ni-Ce/Al2O3 Catalysts in Oxidative Dehydrogenation of Ethane. Ind. Eng. Chem. Res. 2015, 54, 12730–12740. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Kariya Masaru Ichikawa, N. Production of hydrogen-rich gas via reforming of iso-octane over Ni-Mn and Rh-Ce bimetallic catalysts using spray pulsed reactor. Catal. Lett. 2005, 100, 17–25. [Google Scholar] [CrossRef]
- Savage, P.E. Mechanisms and kinetics models for hydrocarbon pyrolysis. J. Anal. Appl. Pyrolysis 2000, 54, 109–126. [Google Scholar] [CrossRef]
- Ming, Q.; Healey, T.; Allen, L.; Irving, P. Steam reforming of hydrocarbon fuels. Catal. Today 2002, 77, 51–64. [Google Scholar] [CrossRef]
- Marino, F.; Boveri, M.; Baronetti, G.; Laborde, M. Hydrogen production from steam reforming of bioethanol using Cu/Ni/K/γ-Al2O3 catalysts. Effect of Ni. Int. J. Hydrogen Energy 2001, 26, 665–668. [Google Scholar] [CrossRef]
- Al-Musa, A.; Al-Saleh, M.; Ioakeimidis, Z.C.; Ouzounidou, M.; Yentekakis, I.V.; Konsolakis, M.; Marnellos, G.E. Hydrogen production by iso-octane steam reforming over Cu catalysts supported on rare earth oxides (REOs). Int. J. Hydrogen Energy 2014, 39, 1350–1363. [Google Scholar] [CrossRef]
- Wang, L.; Murata, K.; Inaba, M. Highly efficient conversion of gasoline into hydrogen on Al2O3-supported Ni-based catalysts: Catalyst stability enhancement by modification with W. Appl. Catal. A 2009, 358, 264–268. [Google Scholar] [CrossRef]
- Youn, M.H.; Seo, J.G.; Kim, P.; Song, I.K. Role and effect of molybdenum on the performance of Ni-Mo/Al2O3 catalysts in the hydrogen production by auto-thermal reforming of ethanol. J. Mol. Catal. A 2007, 261, 276–281. [Google Scholar] [CrossRef]
- Kawabata, T.; Matsuoka, H.; Shishido, T.; Li, D.L.; Tian, Y.; Sano, T.; Takehira, K. Steam reforming of dimethyl ether over ZSM-5 coupled with Cu/ZnO/Al2O3 catalyst prepared by homogeneous precipitation. Appl. Catal. A 2006, 308, 82–90. [Google Scholar] [CrossRef]
| Sample | SBET (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| 18 wt. % Ni/γ-Al2O3 | 152.8 | 0.22 | 2.2 |
| 18wt. %Ni0.9-Cu0.1/γ-Al2O3 | 153.1 | 0.22 | 2.3 |
| 18wt. %Ni0.7-Cu0.3/γ-Al2O3 | 154.4 | 0.23 | 3.1 |
| 18wt. %Ni0.5-Cu0.5/γ-Al2O3 | 156.8 | 0.24 | 4.6 |
| 18wt. %Ni0.3-Cu0.7/γ-Al2O3 | 160.4 | 0.28 | 5.1 |
| 18 wt. % Cu/γ-Al2O3 | 165.9 | 0.29 | 5.8 |
| 18 wt. % Ni0.5-Mo0.5/γ-Al2O3 | 154.7 | 0.23 | 3.1 |
| 18 wt. % Ni0.5-Ce0.5/γ-Al2O3 | 171.4 | 0.31 | 6.6 |
| 6wt. %Ni0.5-Cu0.5/γ-Al2O3 | 197.2 | 0.35 | 7.4 |
| 36 wt. % Ni0.5-Cu0.5/γ-Al2O3 | 105.4 | 0.17 | 1.6 |
| Sample | Temperature (°C) | H2 Consumption (mmol·g−1) | |||
|---|---|---|---|---|---|
| T°C (Initial) | Peak I | Peak II | Peak III | ||
| 18 wt. % Ni/γ-Al2O3 | 400 | - | - | 600 | 1.67 |
| 18 wt. % Ni0.9-Cu0.1/γ-Al2O3 | 208 | 260 | - | 586 | 2.23 |
| 18 wt. % Ni0.7-Cu0.3/γ-Al2O3 | 220 | 262 | - | 568 | 3.31 |
| 18 wt. % Ni0.5-Cu0.5/γ-Al2O3 | 185 | 258 | 295 | 514 | 4.75 |
| 18 wt. % Ni0.3-Cu0.7/γ-Al2O3 | 180 | 258 | 300 | 492 | 4.83 |
| 18 wt. % Cu/γ-Al2O3 | 185 | 295 | - | 474 | 4.39 |
| 18 wt. % Ni0.5-Mo0.5/γ-Al2O3 | 425 | - | 532 | - | 3.68 |
| 18 wt. % Ni0.5-Ce0.5/γ-Al2O3 | 350 | - | 535 | - | 3.73 |
| 6 wt. % Ni0.5-Cu0.5/γ-Al2O3 | 210 | 262 | 316 | - | 2.84 |
| 36 wt. % Ni0.5-Cu0.5/γ-Al2O3 | 205 | 258 | 312 | 514 | 3.81 |
| Catalysts | Conversion (%) | Product Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| iso-Octane | Water | H2 | CO | CO2 | CH4 | |
| 18 wt. % Ni0.5-Cu0.5/γ-Al2O3 | 42.6 | 12.2 | 70.6 | 7.4 | 15.3 | 6.7 |
| 18 wt. % Ni0.5-Mo0.5/γ-Al2O3 | 31.7 | 9.7 | 71.9 | 7.1 | 15.6 | 5.4 |
| 18 wt. % Ni0.5-Ce0.5/γ-Al2O3 | 28.3 | 8.2 | 72.0 | 7.6 | 16.1 | 4.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuan, L.A.; Luong, N.T.; Ishihara, K.N. Low-Temperature Catalytic Performance of Ni-Cu/Al2O3 Catalysts for Gasoline Reforming to Produce Hydrogen Applied in Spark Ignition Engines. Catalysts 2016, 6, 45. https://doi.org/10.3390/catal6030045
Tuan LA, Luong NT, Ishihara KN. Low-Temperature Catalytic Performance of Ni-Cu/Al2O3 Catalysts for Gasoline Reforming to Produce Hydrogen Applied in Spark Ignition Engines. Catalysts. 2016; 6(3):45. https://doi.org/10.3390/catal6030045
Chicago/Turabian StyleTuan, Le Anh, Nguyen The Luong, and Keiichi N. Ishihara. 2016. "Low-Temperature Catalytic Performance of Ni-Cu/Al2O3 Catalysts for Gasoline Reforming to Produce Hydrogen Applied in Spark Ignition Engines" Catalysts 6, no. 3: 45. https://doi.org/10.3390/catal6030045
APA StyleTuan, L. A., Luong, N. T., & Ishihara, K. N. (2016). Low-Temperature Catalytic Performance of Ni-Cu/Al2O3 Catalysts for Gasoline Reforming to Produce Hydrogen Applied in Spark Ignition Engines. Catalysts, 6(3), 45. https://doi.org/10.3390/catal6030045