Design of Efficient Molecular Catalysts for Synthesis of Cyclic Olefin Copolymers (COC) by Copolymerization of Ethylene and α-Olefins with Norbornene or Tetracyclododecene
Abstract
:1. Introduction
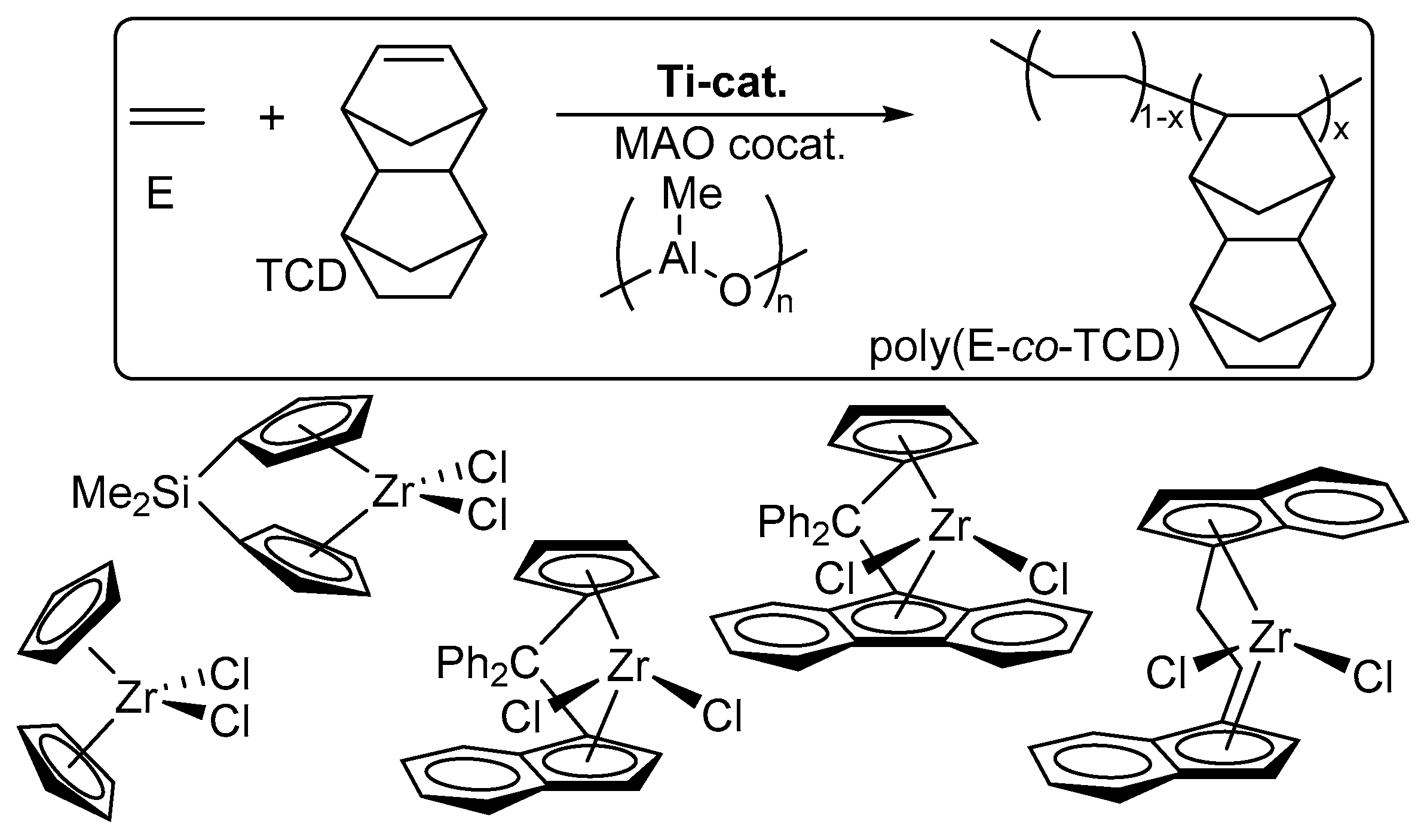
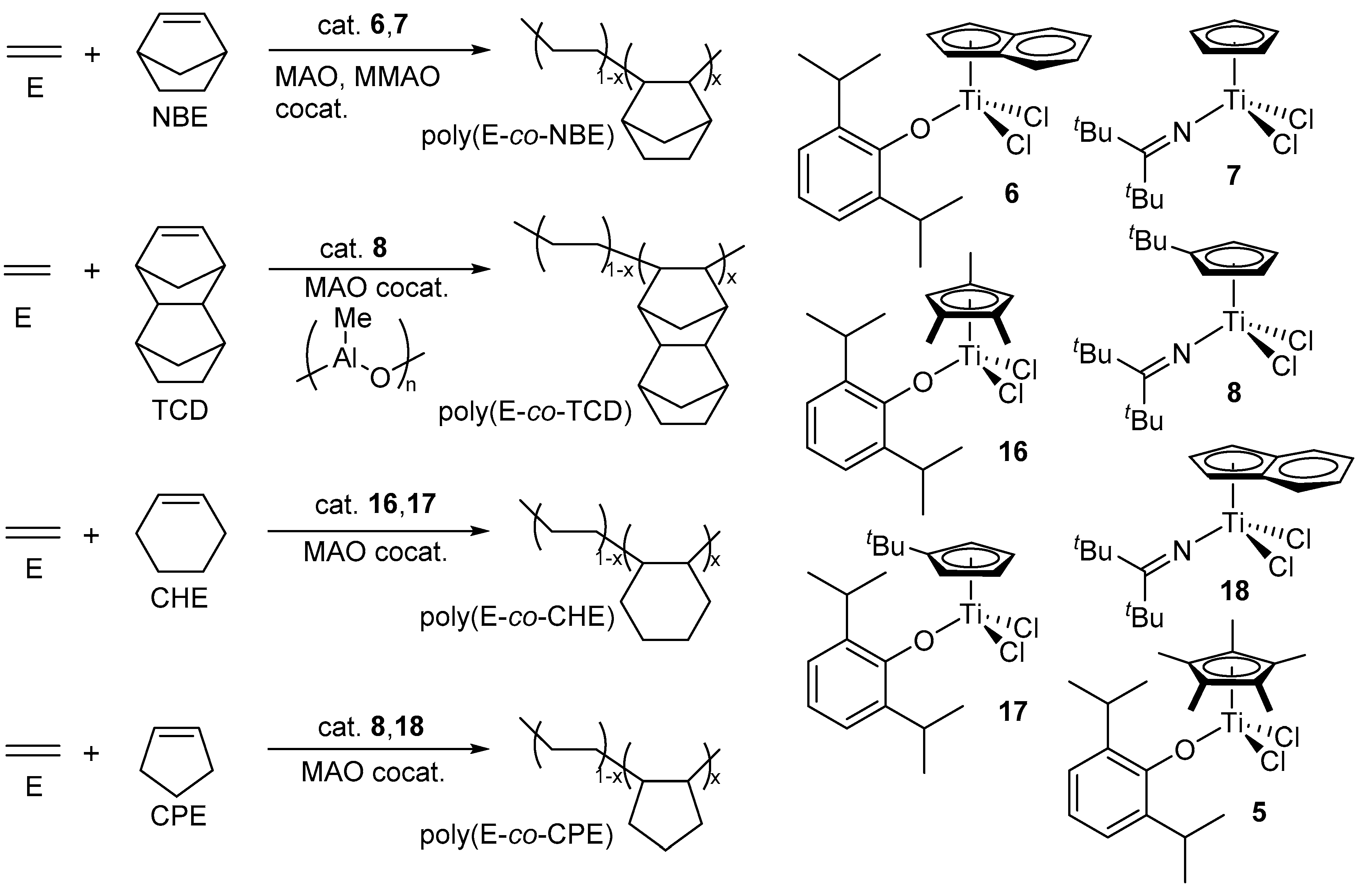
2. Copolymerization of Ethylene with Norbornene (NBE), Tetracyclododecene (TCD) by Titanium and Zirconium Complex Catalysts
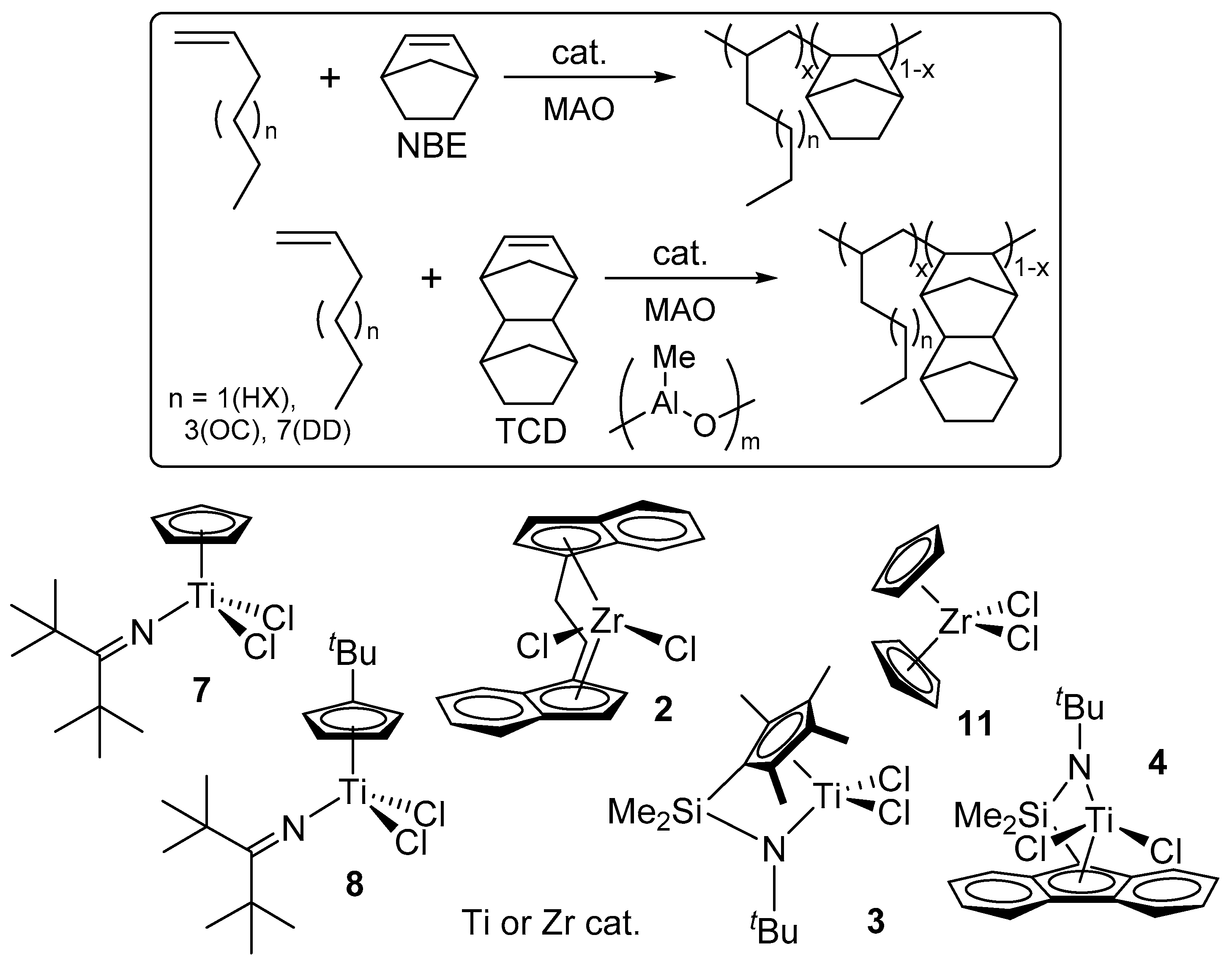
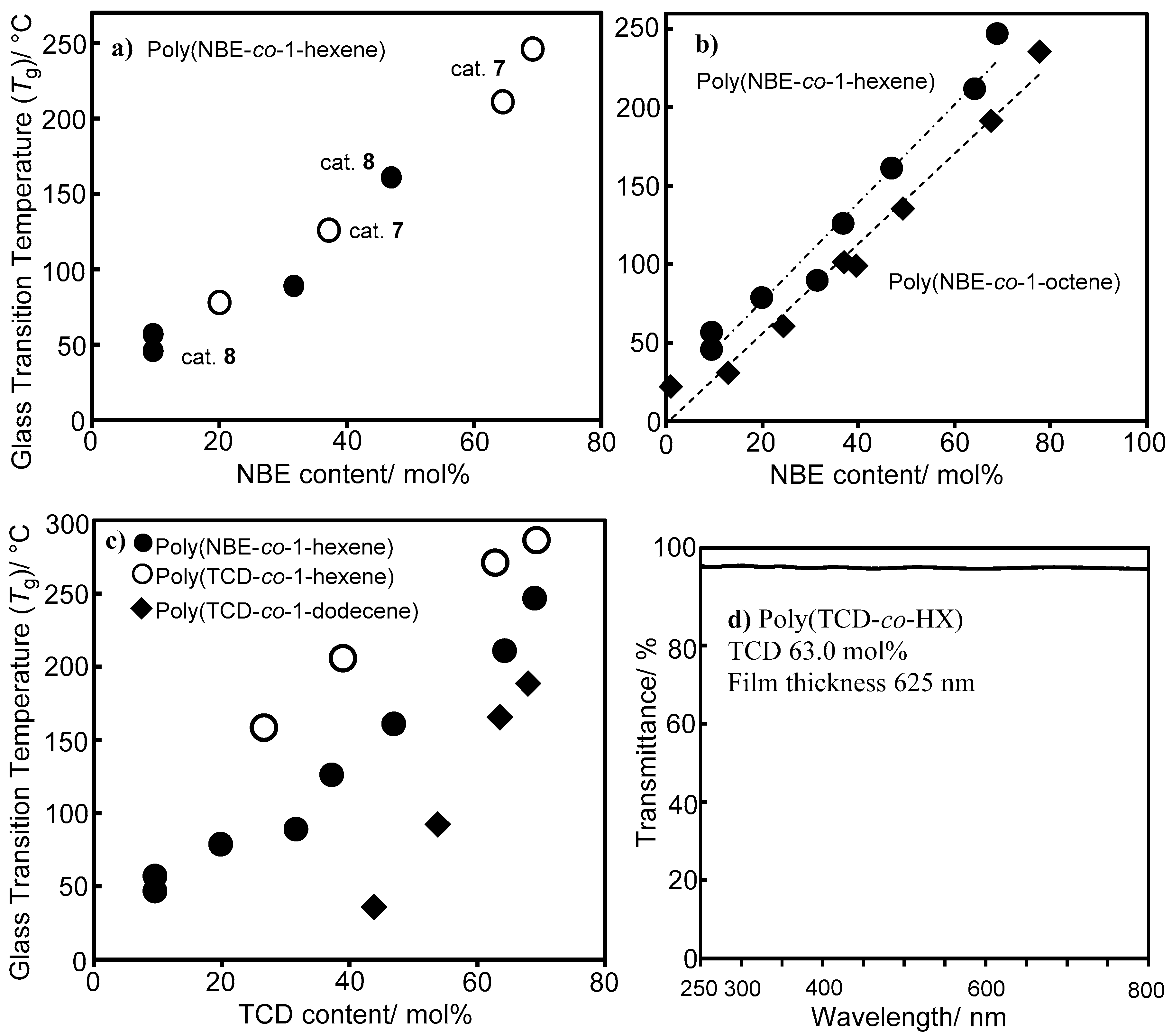
3. Copolymerization of α-Olefin with Norbornene (NBE) or Tetracyclododecene (TCD) by Half-Titanocene Catalysts
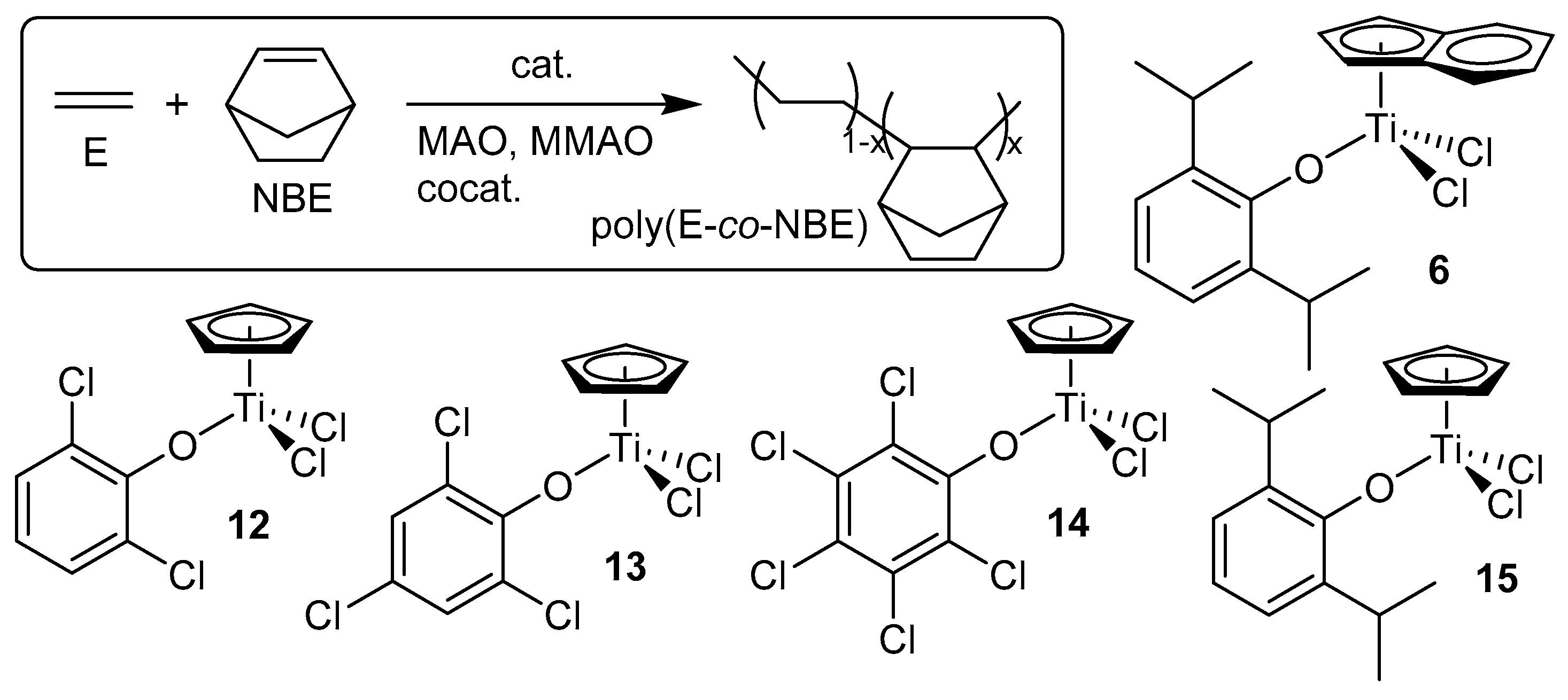
4. Efficient Norbornene (NBE) Incorporation in Ethylene/NBE Copolymerization by Half-Titanocene Catalysts Containing Chlorinated Aryloxo Ligands
5. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Kaminsky, W. Olefinpolymerisation mittels metallocenkatalysatoren. Angew. Makromol. Chem. 1994, 223, 101–120. [Google Scholar] [CrossRef]
- Cherdron, H.; Brekner, M.-J.; Osan, F. Cycloolefin copolymer: A new class of transparent thermoplastics. Angew. Makromol. Chem. 1994, 223, 121–133. [Google Scholar] [CrossRef]
- Kaminsky, W.; Beulich, I.; Arndt-Rosenau, M. Copolymerization of ethene with cyclic and other sterically hindered olefins. Macromol. Symp. 2001, 173, 211–226. [Google Scholar] [CrossRef]
- Dragutan, V.; Streck, R. Catalytic Polymerisation of Cycloolefins—Ionic, Ziegler-Natta and Ring-Opening Metathesis Polymerization. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2000; Volume 131. [Google Scholar]
- Tritto, I.; Boggioni, L.; Ferro, D.R. Metallocene catalyzed ethene- and propene co-norbornene polymerization: Mechanisms from a detailed microstructural analysis. Coord. Chem. Rev. 2006, 250, 212–241. [Google Scholar] [CrossRef]
- Nomura, K. Nonbridged half-titanocenes containing anionic ancillary donor ligands: Promising new catalysts for precise synthesis of cyclic olefin copolymers (COCs). Chin. J. Polym. Sci. 2008, 26, 513–523. [Google Scholar] [CrossRef]
- Li, X.; Hou, Z. Organometallic catalysts for copolymerization of cyclic olefins. Coord. Chem. Rev. 2008, 252, 1842–1869. [Google Scholar] [CrossRef]
- TOPAS. Available online: http://www.topas.com/products/topas-coc-polymers (accessed on 31 October 2016).
- APEL. Available online: http://www.mitsuichem.com/service/functional_polymeric/polymers/apel/ (accessed on 31 October 2016).
- Ruchatz, D.; Fink, G. Ethene-Norbornene copolymerization using homogenous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 1. Kinetics of the ethane-norbornene copolymerization using the [(isopropylidene)(η5-inden-1-ylidene-η5-cyclopentadienyl)]zirconium dichloride/methylaluminoxane catalyst. Macromolecules 1998, 31, 4669–4673. [Google Scholar] [PubMed]
- Ruchatz, D.; Fink, G. Ethene-Norbornene copolymerization using homogenous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 2. Comparative study of different metallocene- and half-sandwich/methylaluminoxane catalysts and analysis of the copolymers by 13C nuclear magnetic resonance spectroscopy. Macromolecules 1998, 31, 4674–4680. [Google Scholar] [PubMed]
- Ruchatz, D.; Fink, G. Ethene-Norbornene copolymerization with homogeneous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 3. Copolymerization parameters and copolymerization diagrams. Macromolecules 1998, 31, 4681–4683. [Google Scholar] [CrossRef] [PubMed]
- Ruchatz, D.; Fink, G. Ethene-Norbornene copolymerization with homogeneous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 4. Development of molecular weights. Macromolecules 1998, 31, 4684–4686. [Google Scholar] [CrossRef] [PubMed]
- Provasoli, A.; Ferro, D.R.; Tritto, I.; Boggioni, L. The conformational characteristics of ethylene-norbornene copolymers and their influence on the 13C NMR spectra. Macromolecules 1999, 32, 6697–6706. [Google Scholar] [CrossRef]
- Tritto, I.; Marestin, C.; Boggioni, L.; Zetta, L.; Provasoli, A.; Ferro, D.R. Ethylene-Norbornene copolymer microstructure. Assessment and advances based on assignments of 13C NMR spectra. Macromolecules 2000, 33, 8931–8944. [Google Scholar] [CrossRef]
- Tritto, I.; Marestin, C.; Boggioni, L.; Sacchi, M.C.; Brintzinger, H.-H.; Ferro, D.R. Stereoregular and stereoirregular alternating ethylene-norbornene copolymers. Macromolecules 2001, 34, 5770–5777. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Jansen, J.C.; Thorshaug, K.; Sacchi, M.C.; Ferro, D.R. Ethylene-Norbornene copolymers from metallocene-based catalysts: Microstructure at tetrad level and reactivity ratios. Macromolecules 2002, 35, 616–623. [Google Scholar] [CrossRef]
- Harrington, B.A.; Crowther, D.J. Stereoregular, alternating ethylene-norbornene copolymers from monocyclopentadienyl catalysts activated with non-coordinating discrete anions. J. Mol. Catal. A: Chem. 1998, 128, 79–84. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Ethylene/Norbornene copolymerizations with titanium CpA catalysts. Macromolecules 1999, 32, 2816–2825. [Google Scholar] [CrossRef]
- Thorshaug, K.; Mendichi, R.; Boggioni, L.; Tritto, I.; Trinkle, S.; Friedrich, C.; Mülhaupt, R. Poly(ethene-co-norbornene) obtained with a constrained geometry catalyst. A study of reaction kinetics and copolymer properties. Macromolecules 2002, 35, 2903–2911. [Google Scholar] [CrossRef]
- Hasan, T.; Ikeda, T.; Shiono, T. Ethene-Norbornene copolymer with high norbornene content produced by ansa-fluorenylamidodimethyltitanium complex using a suitable activator. Macromolecules 2004, 37, 8503–8509. [Google Scholar] [CrossRef]
- Nomura, K.; Tsubota, M.; Fujiki, M. Efficient ethylene/norbornene copolymerization by (aryloxo)(indenyl)titanium(IV) complexes−MAO catalyst system. Macromolecules 2003, 36, 3797–3799. [Google Scholar] [CrossRef]
- Wang, W.; Tanaka, T.; Tsubota, M.; Fujiki, M.; Yamanaka, S.; Nomura, K. Effect of cyclopentadienyl fragment in copolymerization of ethylene with cyclic olefins catalyzed by non-bridged (aryloxo)(cyclopentadienyl)titanium(IV) complexes. Adv. Synth. Catal. 2005, 347, 433–446. [Google Scholar] [CrossRef]
- Nomura, K.; Wang, W.; Fujiki, M.; Liu, J. Notable norbornene (NBE) incorporation in ethylene–NBE copolymerization catalysed by nonbridged half-titanocenes: Better correlation between NBE incorporation and coordination energy. Chem. Commun. 2006, 2659–2661. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Fukuda, H.; Katao, S.; Fujiki, M.; Kim, H.J.; Kim, D.H.; Saeed, I. Olefin polymerization by half-titanocenes containing η2-pyrazolato ligands-MAO catalyst systems. Macromolecules 2011, 44, 1986–1998. [Google Scholar] [CrossRef]
- Apisuk, W.; Trambitas, A.G.; Kitiyanan, B.; Tamm, M.; Nomura, K. Efficient ethylene/norbornene copolymerization by half-titanocenes containing imidazolin-2-iminato ligands and MAO catalyst systems. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 2575–2580. [Google Scholar] [CrossRef]
- Altamura, P.; Grassi, A. Crystalline alternating sequences identified in ethylene-co-norbornene polymers produced by the (η5-C2B9H11)Zr(NEt2)2(NHEt2)-AliBu3 catalyst. Macromolecules 2001, 34, 9197–9200. [Google Scholar] [CrossRef]
- Yoshida, Y.; Saito, J.; Mitani, M.; Takagi, Y.; Matsui, S.; Ishii, S.; Nakano, T.; Kashiwa, N.; Fujita, T. Living ethylene/norbornene copolymerisation catalyzed by titanium complexes having two pyrrolide-imine chelate ligands. Chem. Commun. 2002, 12, 1298–1299. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mohri, J.; Ishii, S.; Mitani, M.; Saito, J.; Matsui, S.; Makio, H.; Nakano, T.; Tanaka, H.; Onda, M.; et al. Living copolymerization of ethylene with norbornene catalyzed by bis(pyrrolide-imine) titanium complexes with MAO. J. Am. Chem. Soc. 2004, 126, 12023–12032. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Dai, K.; Ye, W.-P.; Pan, L.; Li, Y.-S. New titanium complexes with two β-enaminoketonato chelate ligands: Syntheses, structures, and olefin polymerization activities. Organometallics 2004, 23, 1223–1230. [Google Scholar] [CrossRef]
- For example, synthesis of poly(ethylene-co-TCD)s using vanadium catalyst systems [VOCl3, VO(OEt)Cl2-EtAlCl2·Et2AlCl etc.] in the presence of halogenated Al cocatalyst, JP2001-106730; JP2006-22266; JP2008-248171 (Mitsui Chemicals Co.).
- Kaminsky, W.; Bark, A. Copolymerization of ethene and dimethanooctahydronaphthalene with aluminoxane containing catalysts. Polym. Int. 1992, 28, 251–253. [Google Scholar] [CrossRef]
- Kaminsky, W.; Engehausen, R.; Kopf, J. A tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 2273–2275. [Google Scholar] [CrossRef]
- Goodall, B.L.; McIntosh, L.H.; Rhodes, L.F. New catalysts for the polymerization of cyclic olefins. Macromol. Symp. 1995, 89, 421–432. [Google Scholar] [CrossRef]
- Donner, M.; Fernandes, M.; Kaminsky, W. Synthesis of copolymers with sterically hindered and polar monomers. Macromol. Symp. 2006, 236, 193–202. [Google Scholar] [CrossRef]
- JP2006-307194 (Sumitomo Chemical Co., Ltd.); JP 2015-199919 (Mitsui Chemicals Co.).
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.F.; Coates, G.W. Macromolecular Engineering; Matyjaszewski, K., Gnanou, Y., Leibler, L., Eds.; Wiley-VCH: Weinheim, Germany, 2007; Volume 1, pp. 217–247. [Google Scholar]
- Nomura, K.; Liu, J.; Padmanabhan, S.; Kitiyanan, B. Nonbridged half-metallocenes containing anionic ancillary donor ligands: New promising candidates as catalysts for precise olefin polymerization. J. Mol. Catal. A: Chem. 2007, 267, 1–29. [Google Scholar] [CrossRef]
- Metal Catalysts in Olefin Polymerization; Guan, Z. (Ed.) Topics in Organometallic Chemistry 26; Springer: Berlin, Germany, 2009.
- Nomura, K. Half-Titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 8811–8823. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef] [PubMed]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef] [PubMed]
- Delferro, M.; Marks, T.J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Liu, J. Half-Titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef] [PubMed]
- Organometallic Reactions and Polymerization; Osakada, K. (Ed.) the Lecture Notes in Chemistry 85; Springer: Berlin, Germany, 2014.
- McInnis, J.P.; Delferro, M.; Marks, T.J. Multinuclear group 4 catalysis: Olefin polymerization pathways modified by strong metal-metal cooperative effects. Acc. Chem. Res. 2014, 47, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Randoll, S.; Bannenberg, T.; Herdtweck, E. Titanium complexes with imidazolin-2-iminato ligands. Chem. Commun. 2004, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Randoll, S.; Herdtweck, E.; Kleigrewe, N.; Kehr, G.; Erker, G.; Rieger, B. Imidazolin-2-iminato titanium complexes: Synthesis, structure and use in ethylene polymerization catalysis. Dalton Trans. 2006, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Fukuda, H.; Apisuk, W.; Trambitas, A.G.; Kitiyanan, B.; Tamm, M. Ethylene copolymerization by half-titanocenes containing imidazolin-2-iminato ligands-MAO catalyst systems. J. Mol. Catal. A: Chem. 2012, 363–364, 501–511. [Google Scholar] [CrossRef]
- Nomura, K.; Patamma, S.; Matsuda, H.; Katao, S.; Tsutsumi, K.; Fukuda, H. Synthesis of half-titanocenes containing 1,3-imidazolidin-2-iminato ligands of type, Cp*TiCl2[1,3-R2(CH2N)2C=N]: Highly active catalyst precursors in ethylene (co)polymerization. RSC Adv. 2015, 5, 64503–64513. [Google Scholar] [CrossRef]
- Apisuk, W.; Ito, H.; Nomura, K. Efficient synthesis of cyclic olefin copolymers with high glass transition temperatures by ethylene copolymerization with tetracyclododecene (TCD) using (tert-BuC5H4)TiCl2(N=CtBu2)-MAO catalyst. J. Polym. Sci. Part A: Polym. Chem. 2016, 54, 2662–2667. [Google Scholar] [CrossRef]
- Zhao, W.; Nomura, K. Copolymerizations of norbornene, tetracyclododecene with α-olefins by half-titanocene catalysts: Efficient synthesis of highly transparent, thermal resistance polymers. Macromolecules 2016, 49, 59–70. [Google Scholar] [CrossRef]
- Henschke, O.; Köller, F.; Arnold, M. Polyolefins with high glass transition temperatures. Makromol. Rapid Commun. 1997, 18, 617–623. [Google Scholar] [CrossRef]
- Boggioni, L.; Bertini, F.; Zannoni, G.; Tritto, I.; Carbone, P.; Ragazzi, M.; Ferro, D.R. Propene-Norbornene copolymers: Synthesis and analysis of polymer structure by 13C NMR spectroscopy and ab initio chemical shift computations. Macromolecules 2003, 36, 882–890. [Google Scholar] [CrossRef]
- Boggioni, L.; Tritto, I.; Ragazzi, M.; Carbone, P.; Ferro, D.R. Propene-Norbornene copolymers: Synthesis and microstructure. Macromol. Symp. 2004, 218, 39–50. [Google Scholar] [CrossRef]
- Jung, H.Y.; Hong, S.-D.; Jung, M.W.; Lee, H.; Park, Y.-W. Norbornene copolymerization with α-olefins using methylene-bridged ansa-zirconocene. Polyhedron 2005, 24, 1269–1273. [Google Scholar] [CrossRef]
- Vanegas, M.E.; Quijada, R.; Galland, G.B. Syndiotactic poly(propene-co-norbornene): Synthesis and properties at low norbornene incorporation. Polymer 2010, 51, 4627–4631. [Google Scholar] [CrossRef]
- Hasan, T.; Ikeda, T.; Shiono, T. Random Copolymerization of propene and norbornene with ansa-fluorenylamidodimethyltitanium-based catalysts. Macromolecules 2005, 38, 1071–1074. [Google Scholar] [CrossRef]
- Cai, Z.; Nakayama, Y.; Shiono, T. Living Random Copolymerization of Propylene and norbornene with ansa-fluorenylamidodimethyltitanium complex: Synthesis of novel syndiotactic polypropylene-b-poly(propylene-ran-norbornene). Macromolecules 2006, 39, 2031–2033. [Google Scholar] [CrossRef]
- Shiono, T.; Sugimoto, M.; Hasan, T.; Cai, Z.; Ikeda, T. Random copolymerization of norbornene with higher 1-alkene with ansa-fluorenylamidodimethyltitanium catalyst. Macromolecules 2008, 41, 8292–8294. [Google Scholar] [CrossRef]
- Cai, Z.; Harada, R.; Nakayama, Y.; Shiono, T. Highly active living random copolymerization of norbornene and 1-alkene with ansa-fluorenylamidodimethyltitanium derivative: Substituent effects on fluorenyl ligand. Macromolecules 2010, 43, 4527–4531. [Google Scholar] [CrossRef]
- Hasan, T.; Nishii, K.; Shiono, T.; Ikeda, T. Living polymerization of norbornene via vinyl addition with ansa-fluorenylamidodimethyltitanium complex. Macromolecules 2002, 35, 8933–8935. [Google Scholar] [CrossRef]
- Hasan, T.; Ikeda, T.; Shiono, T. Highly efficient Ti-based catalyst systems for vinyl addition polymerization of norbornene. Macromolecules 2004, 37, 7432–7436. [Google Scholar] [CrossRef]
- Hasan, T.; Ioku, A.; Nishii, K.; Shiono, T.; Ikeda, T. Syndiospecific living polymerization of propene with [t-BuNSiMe2Flu]TiMe2 using MAO as cocatalyst. Macromolecules 2001, 34, 3142–3145. [Google Scholar] [CrossRef]
- Li, X.; Nishiura, M.; Mori, K.; Mashiko, T.; Hou, Z. Cationic scandium aminobenzyl complexes. Synthesis, structure and unprecedented catalysis of copolymerization of 1-hexene and dicyclopentadiene. Chem. Commun. 2007, 4137–4139. [Google Scholar] [CrossRef] [PubMed]
- Suhm, J.; Schneider, M.J.; Mülhaupt, R. Temperature dependence of copolymerization parameters in ethene/1-octene copolymerization using homogeneous rac-Me2Si(2-MeBenz[e]Ind)2ZrCl2/MAO catalyst. J. Polym. Sci. Part A: Polym. Chem. 1997, 35, 735–740. [Google Scholar] [CrossRef]
- Suhm, J.; Schneider, M.J.; Mülhaupt, R. Influence of metallocene structures on ethene copolymerization with 1-butene and 1-octene. J. Mol. Catal. A: Chem. 1998, 128, 215–227. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, Q.; Tsutsumi, K.; Nomura, K. Efficient norbornene (NBE) incorporation in ethylene/NBE copolymerization by half-titanocene catalysts containing chlorinated aryloxo ligands. Organometallics 2016, 35, 1895–1905. [Google Scholar] [CrossRef]
- Nomura, K.; Oya, K.; Imanishi, Y. Ethylene/α-olefin copolymerization by various nonbridged (cyclopentadienyl)(aryloxy) titanium(IV) complexes-MAO catalyst system. J. Mol. Catal. A: Chem. 2001, 174, 127–140. [Google Scholar] [CrossRef]
- Wang, W.; Fujiki, M.; Nomura, K. Copolymerization of ethylene with cyclohexene (CHE) catalyzed by nonbridged half-titanocenes containing aryloxo ligand: Notable effect of both cyclopentadienyl and anionic donor ligand for efficient CHE incorporation. J. Am. Chem. Soc. 2005, 128, 4582–4583. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nomura, K. Highly efficient ethylene/cyclopentene copolymerization with exclusive 1,2-cyclopentene incorporation by (cyclopentadienyl)(ketimide) titanium(IV) complexes-MAO catalysts. Adv. Synth. Catal. 2007, 349, 2235–2240. [Google Scholar] [CrossRef]
- Nomura, K.; Itagaki, K.; Fujiki, M. Efficient incorporation of 2-methyl-1-pentene in copolymerization of ethylene with 2-methyl-1-pentene catalyzed by nonbridged half-titanocenes. Macromolecules 2005, 38, 2053–2055. [Google Scholar] [CrossRef]


| Cat. (μmol) | Temp./°C | Time/min | E/atm | NBE b/M | [NBE]0/[E]0 c | Activity d | Mn e × 10−4 | Mw/Mn e | NBE f/mol% | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (0.10) | 25 | 10 | 4 | 0.2 | 0.41 | 28,860 | 23.1 | 2.02 | 10.8 | 24 |
| 1 (0.10) | 25 | 10 | 4 | 1.0 | 2.04 | 4860 | 22.9 | 2.37 | 29.5 | 24 |
| 3 (0.50) | 25 | 10 | 4 | 0.2 | 0.41 | 2460 | 21.1 | 1.88 | 9.6 | 24 |
| 3 (0.50) | 25 | 10 | 4 | 1.0 | 2.04 | 2000 | 12.8 | 2.15 | 26.5 | 24 |
| 5 (0.2) | 25 | 10 | 4 | 0.2 | 0.41 | 6540 | 57.9 | 1.61 | 8.2 | 24 |
| 5 (0.2) | 25 | 10 | 4 | 1.0 | 2.04 | 2640 | 29.6 | 1.46 | 21.7 | 24 |
| 6 (0.2) | 25 | 10 | 4 | 0.2 | 0.41 | 10,500 | 14.6 | 1.56 | 14.0 | 24 |
| 6 (0.5) | 25 | 10 | 4 | 1.0 | 2.04 | 2300 | 5.87 | 1.82 | 35.2 | 24 |
| 7 (0.02) | 25 | 10 | 4 | 0.2 | 0.41 | 21,600 | 70.6 | 1.85 | 17.8 | 24 |
| 7 (0.02) | 25 | 10 | 4 | 1.0 | 2.04 | 40,200 | 71.9 | 2.92 | 40.7 | 24 |
| 7 (0.02) | 25 | 20 | 4 | 1.0 | 2.04 | 60,150 | 53.4 | 2.11 | 41.5 | 24 |
| 7 (0.02) | 25 | 30 | 4 | 1.0 | 2.04 | 59,700 | 61.3 | 2.18 | 41.0 | 24 |
| 7 (0.02) g | 25 | 10 | 4 | 1.0 | 2.04 | 42,300 | 35.5 | 2.42 | 41.8 | 24 |
| 7 (0.02) h | 25 | 10 | 4 | 1.0 | 2.04 | 50,400 | 35.1 | 2.31 | 42.9 | 24 |
| 7 (0.01) i | 25 | 10 | 2 | 5.0 | 20.6 | 85,800 | 34.0 | 2.00 | 65.8 | 24 |
| 7 (0.01) i | 25 | 10 | 2 | 10.0 | 41.2 | 31,500 | 44.4 | 2.01 | 73.5 | 24 |
| 7 (0.02) | 40 | 10 | 4 | 1.0 | 2.45 | 48,900 | 62.0 | 2.37 | 45.9 | 24 |
| 7 (0.02) | 60 | 10 | 4 | 1.0 | 3.02 | 194,000 | 47.5 | 2.20 | 51.2 | 24 |
| 7 (0.02) | 80 | 10 | 4 | 1.0 | 3.94 | 133,000 | 33.8 | 2.34 | 61.7 | 24 |
| 8 (0.01) | 25 | 10 | 4 | 1.0 | 2.04 | 68,400 | 62.4 | 2.78 | 38.2 | 6 |
| 8 (0.10) g | 25 | 10 | 2 | 5.0 | 20.6 | 4980 | 14.2 | 1.94 | 64.8 | 6 |
| 9 (0.20) | 25 | 10 | 4 | 1.0 | 2.04 | 6180 | 108 | 2.53 | 31.4 | 26 |
| 9 (0.20) | 80 | 10 | 4 | 1.0 | 3.94 | 5780 | 80.0 | 2.35 | 36.9 | 26 |
| 9 (1.0) | 50 | 10 | 2 | 5.0 | 27.3 | 1820 | 13.8 | 1.85 | 55.5 | 26 |
| 10 (0.25) | 25 | 10 | 5 | 0.5 | 0.82 | 5170 | 126 | 2.32 | 37.2 | 26 |
| 10 (0.25) | 25 | 10 | 5 | 1.0 | 1.63 | 3470 | 89.2 | 2.63 | 43.9 | 25 |
| Ti/μmol | TCD b/mol/L | temp./°C | Activity c | Mn d × 10−5 | Mw/Mn d | Tg e (Tm e)/°C | TCD f/mol% |
|---|---|---|---|---|---|---|---|
| 3 (0.05) | 1.0 | 25 | 13,900 | 14.3 | 1.58 | 56 | - |
| 7 (0.4) | 1.0 | 25 | 1510 | 2.33 | 1.56 | - | - |
| 7 (0.8) | 2.0 | 25 | 1650 | 1.92 | 1.41 | 150 | |
| 8 (0.02) | 1.0 | 25 | 43,700 | 5.88 | 1.60 | 108 | 25.6 |
| 8 (0.02) | 2.0 | 25 | 23,900 | 6.38 | 1.50 | 153 | 32.8 |
| 8 (0.02) | 2.0 | 40 | 27,800 | 6.43 | 1.67 | 170 | 33.5 g |
| 8 (0.02) | 2.0 | 60 | 33,300 | 6.53 | 1.72 | 177 | 35.3 |
| 8 (0.02) | 3.0 | 25 | 16,800 | 6.43 | 1.61 | 171 | 33.6 g |
| 8 (0.02) | 4.0 | 60 | 22,400 | 6.08 | 1.61 | 203 | 36.7 |
| 9 (0.2) | 1.0 | 25 | 6680 | 4.68 | 1.77 | 160 (108) g | - |
| Catalyst (µmol) | MAO/mmol | α-olefin | CO | CO Feed ratio b/% | Time/min | Activity c | Mn d × 10−4 | Mw/Mn d | CO cont. e/mol % | Tg f /°C |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 (0.04) | 3.0 | HX | NBE | 12 | 30 | 7500 | 4.18 | 1.64 | 20.0 | 78 |
| 7 (0.04) | 3.0 | HX | NBE | 21 | 30 | 9620 | 3.61 | 1.58 | 37.2 | 126 |
| 7 (0.03) | 3.0 | HX | NBE | 44 | 60 | 12,900 | 4.09 | 1.72 | 64.5 | 211 |
| 7 (0.02) | 3.0 | HX | NBE | 57 | 60 | 12,500 | 3.94 | 1.61 | 69.2 | 246 |
| 7 (0.10) | 3.0 | OC | NBE | 14 | 60 | 6140 | 4.57 | 1.67 | 13.0 | 31 |
| 7 (0.05) | 3.0 | OC | NBE | 25 | 20 | 8240 | 3.33 | 1.63 | 39.6 | 99 |
| 7 (0.03) | 3.0 | OC | NBE | 50 | 60 | 7890 | 4.18 | 1.67 | 67.5 | 192 |
| 7 (0.03) | 3.0 | OC | NBE | 63 | 60 | 6220 | 4.79 | 3.24 | 77.6 | 235 |
| 7 (0.10) | 3.0 | DD | NBE | 32 | 60 | 4490 | 3.84 | 1.67 | 37.3 | 56 |
| 7 (0.03) | 3.0 | DD | NBE | 59 | 60 | 4580 | 5.42 | 1.75 | 76.1 | 157 |
| 7 (0.03) | 3.0 | DD | NBE | 70 | 60 | 4150 | 8.26 | 3.19 | 84.1 | 212 |
| 8 (0.20) | 3.0 | OC | NBE | 50 | 60 | 1720 | 6.49 | 1.69 | 37.0 | 102 |
| 8 (0.20) | 3.0 | OC | NBE | 63 | 60 | 930 | 6.34 | 1.68 | 49.2 | 135 |
| 8 (0.30) | 3.0 | DD | NBE | 59 | 60 | 1370 | 6.68 | 1.76 | 43.0 | 64 |
| 8 (0.20) | 3.0 | DD | NBE | 70 | 60 | 1650 | 6.61 | 1.64 | 55.0 | 100 |
| 2 (20) | 10 | HX | TCD | 75 | 10 | 49 | 0.73 | 1.18 | - | - |
| 2 (20) | 10 | OC | TCD | 75 | 20 | 26 | 0.76 | 1.22 | - | - |
| 2 (20) | 10 | DD | TCD | 75 | 20 | 21.5 | 0.71 | 1.25 | - | - |
| 11 (20) | 10 | HX | TCD | 75 | 120 | 1.0 | 1.4 | 1.27 | - | 98 |
| 11 (20) | 10 | OC | TCD | 75 | 120 | 0.9 | 1.5 | 1.28 | - | 83 |
| 3 (10) | 6.0 | HX | TCD | 50 | 30 | 31 | 5.3 | 1.41 | - | 9.8 |
| 3 (10) | 10 | OC | TCD | 75 | 60 | 6 | 3.6 | 1.37 | - | 6.3 |
| 7 (0.20) | 1.0 | HX | TCD | 50 | 15 | 4590 | 17.6 | 1.70 | 41.6 | 205 |
| 7 (0.20) | 1.5 | HX | TCD | 75 | 15 | 2870 | 12.2 | 1.76 | 62.8 | 271 |
| 7 (0.30) | 1.5 | OC | TCD | 75 | 15 | 2030 | 9.9 | 1.93 | 55.5 | 235 |
| 7 (0.40) | 1.0 | OC | TCD | 83 | 15 | 1410 | 1.03 | 1.74 | 67.6 | 259 |
| 7 (0.40) | 1.5 | DD | TCD | 75 | 15 | 1830 | 13.0 | 1.67 | 60.5 | 165 |
| 8 (0.50) | 1.0 | HX | TCD | 50 | 15 | 307 | - | - | - | - |
| 8 (0.60) | 1.5 | OC | TCD | 75 | 15 | 120 | - | - | - | - |
| 8 (1.0) | 1.5 | DD | TCD | 75 | 15 | 62 | - | - | - | - |
| Catalyst (µmol) | MAO/mmol | α-olefin | TCD Feed Ratio b/% | temp./°C | Activity c | Mn d × 10−4 | Mw/Mn d | TCD cont. e/mol % | Tg f/°C |
|---|---|---|---|---|---|---|---|---|---|
| 7 (0.20) | 1.0 | HX | 50 | 25 | 4590 | 17.6 | 1.70 | 41.6 | 205 |
| 7 (0.20) | 1.0 | HX | 75 | 25 | 2560 | 1.42 | 1.66 | - | - |
| 7 (0.20) | 1.5 | HX | 75 | 25 | 2870 | 12.2 | 1.76 | 62.8 | 271 |
| 7 (0.20) | 1.5 | HX | 75 | 50 | 2550 | 1.18 | 1.73 | - | 282 |
| 7 (0.20) | 1.5 | HX | 75 | 70 | 1670 | 1.01 | 1.71 | - | 289 |
| 7 (0.20) | 1.0 | OC | 50 | 25 | 4210 | 1.83 | 1.61 | 44.4 | 172 |
| 7 (0.30) | 1.5 | OC | 75 | 25 | 2030 | 9.9 | 1.93 | 55.5 | 235 |
| 7 (0.40) | 1.0 | OC | 83 | 25 | 1410 | 1.03 | 1.74 | 67.6 | 259 |
| 7 (0.40) | 1.5 | DD | 75 | 25 | 1830 | 13.0 | 1.67 | 60.5 | 165 |
| Cat. (μmol) | NBE Feed/mol/L | Temp./°C | Activity b | Tg c/°C | Mn d × 10−4 | Mw/Mn d | NBE cont. e/mol % |
|---|---|---|---|---|---|---|---|
| 12 (0.3) | 1.0 | 25 | 2470 | 153 | 5.47 | 1.55 | 49.6 |
| 12 (0.5) | 2.0 | 25 | 2010 | 164 | 6.78 | 1.63 | - |
| 12 (0.5) | 2.0 | 40 | 2390 | 176 | 5.60 | 1.56 | - |
| 13 (0.3) | 1.0 | 25 | 3090 | 142 | 7.41 | 1.50 | 45.3 |
| 13 (0.3) | 1.0 | 40 | 4140 | 151 | 6.50 | 1.37 | - |
| 13 (0.3) | 1.0 | 60 | 3930 | 160 | 2.75 | 1.79 | - |
| 13 (0.5) f | 2.0 | 25 | 3060 | 156 | 8.25 | 1.37 | 53.3 |
| 13 (0.3) | 2.0 | 50 | 2350 | 176 | 3.50 | 1.65 | 58.8 |
| 14 (0.3) | 1.0 | 25 | 2320 | 128 | 6.01 | 1.49 | 42.3 |
| 14 (0.6) f | 2.0 | 25 | 2030 | 150 | 6.94 | 1.40 | - |
| 15 (2.5) | 2.0 | 25 | 319 | - | bimodal | - | |
| 6 (2.0) | 2.0 | 25 | 492 | 114 | 2.52 | 1.29 | - |
| 7 (0.04) | 2.0 | 25 | 20,400 | 127 | 57.3 | 1.78 | - |
| CGC (3) (0.10) | 1.0 | 25 | 4860 | - | 22.9 | 2.37 | 29.5 |
| DSBI (1) (0.50) | 1.0 | 25 | 2000 | - | 12.8 | 2.15 | 26.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Nomura, K. Design of Efficient Molecular Catalysts for Synthesis of Cyclic Olefin Copolymers (COC) by Copolymerization of Ethylene and α-Olefins with Norbornene or Tetracyclododecene. Catalysts 2016, 6, 175. https://doi.org/10.3390/catal6110175
Zhao W, Nomura K. Design of Efficient Molecular Catalysts for Synthesis of Cyclic Olefin Copolymers (COC) by Copolymerization of Ethylene and α-Olefins with Norbornene or Tetracyclododecene. Catalysts. 2016; 6(11):175. https://doi.org/10.3390/catal6110175
Chicago/Turabian StyleZhao, Weizhen, and Kotohiro Nomura. 2016. "Design of Efficient Molecular Catalysts for Synthesis of Cyclic Olefin Copolymers (COC) by Copolymerization of Ethylene and α-Olefins with Norbornene or Tetracyclododecene" Catalysts 6, no. 11: 175. https://doi.org/10.3390/catal6110175
APA StyleZhao, W., & Nomura, K. (2016). Design of Efficient Molecular Catalysts for Synthesis of Cyclic Olefin Copolymers (COC) by Copolymerization of Ethylene and α-Olefins with Norbornene or Tetracyclododecene. Catalysts, 6(11), 175. https://doi.org/10.3390/catal6110175







